Abstract
Periodontal ligament (PDL) cells are a promising tool for periodontal regeneration therapy. Achieving a sufficient number of PDL cells is essential to PDL regeneration. In our study, appropriate flow shear stress (FSS, 1–6 dyn/cm2) promotes the proliferation of PDL cells. FSS remodels cytoskeleton and focal adhesion in a duration-dependent manner. FSS induces PDL cells to form the actin cap within 10 min, flattens the nuclei, and increases the nuclear pore size, which promotes nuclear translocation of Yes-associated protein (YAP). FSS activates p38, which plays a dual function in YAP regulation. p38 regulates the phosphorylation of Akt and cofilin, as well as induced F-actin polymerization to induce YAP activity. In addition, p38 inhibits pLATS and consecutively regulates angiomotin (AMOT) and YAP phosphorylation. AMOT competitively binds to F-actin and YAP to participate in FSS-mediated YAP nuclear translocation and cell proliferation. Taken collectively, our results provide mechanistic insights into the role of p38-AMOT-YAP in FSS-mediated PDL cells proliferation and indicate potential applications in dental regenerative medicine.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-022-04591-w.
Keywords: Mechanotransduction, Nuclear-cytoplasmic translocation, Nuclear changes, LINC, Biomechanics
Introduction
Periodontal diseases are globally prevalent diseases which affect up to 90% of the population [1]. Periodontitis causes chronic inflammation in gingiva, bones, and periodontal ligaments. It is a major cause of tooth loss in adults, and it negatively affects digestion, nutrition, and diet habits, as well as causes systemic complications [2]. Clinicians have introduced several new approaches to regenerate the lost periodontal tissues, including guided tissue regeneration (GTR), growth factor-based therapies, and bone grafts [3]. Most of these methods are cell-based approaches. As such, the seed cells, the periodontal ligament (PDL) cells are essential to periodontal regeneration. PDL cells’ regenerative potential has already been examined in large animal models, and their clinical outcome has been reported [4]. However, for their limitation of the tissue resource and the proliferation capability, the development of effective methods to expand PDL cells for periodontal regeneration is critical.
PDL is located between the alveolar bone and the cementum, and it continuously senses mechanical forces, such as tensile stress, compressive stress, and shear stress during orthodontic tooth movement (OTM) or mastication. PDL cells sense and respond to mechanical stresses which is essential for tissue homeostasis and remodeling [5, 6]. PDL is considered a type of porous tissue that contains an extensive vascular network, crisscrossed fibers, and interstitial fluid. The interstitial fluid flow squeezes by the mechanical stresses and produces FSS on PDL cells. Shear stress is regarded as a main type of stress during mastication and OTM [7, 8]. Although FSS plays an important role in periodontal tissues remodeling, it is still a challenge to obtain accurate data on FSS in vivo due to the thickness of PDL (0.15–0.38 mm) and the lack of proper technique to study the FSS of PDL. In vitro experiments have also indicated that FSS can regulate the biological behavior of PDL cells [9]. In Qi’s work, they used increasing fluid shear stress in fixed increments (3, 6, 9, 12, 15 dyn/cm2) for 6 h significantly increased the proliferation rate and osteogenic differentiation of PDL cells [10]. Our previous study indicated long duration FSS of 6 dyn/cm2, such as 12 or 24 h, inhibited proliferation of PDL cells. 2 or 4 h of 6 dyn/cm2 promoted proliferation of PDL cells [11, 12]. Based on the previous works and our studies, FSS could be clean and efficient strategy to promote the cell proliferation. However, the underlying mechanism is still unclear.
YAP is the co-transcription factor of the Hippo pathway which play an important role in cell proliferation, migration, and differentiation [13]. In addition, YAP has mechanotransduction properties. Cells gather information on their microenvironment through transmembrane proteins, such as integrins, that can restructure the cytoskeleton. Many signaling proteins, including ROCK and non-muscle myosin II, regulate cytoskeletal tension, which ultimately activates YAP. YAP translocate to the nucleus and bind to TEA domain transcription factors (TEAD), another transcription factor, to promote downstream gene expression [14]. Belgardt et al. reported that cytoskeletal tension could regulate zyxin, which mediated the nuclear transport of YAP in human PDL fibroblasts [15]. In another study of an animal model of tooth movement, Gli1 + cells in the PDL could respond to force and regulate bone remodeling by YAP activation [16]. However, whether YAP participates in shear stress-mediated PDL cell proliferation and the upstream regulators are far to reveal.
In this study, we report that 1–6 dyn/cm2 of FSS promoted but 9 dyn/cm2 of FSS inhibited cell proliferation. FSS form the actin cap within 10 min and presses the nuclei, which induces nuclear transport of YAP to induce proliferation. The integrity of cytoskeleton and linker of the nucleoskeleton and cytoskeleton (LINC) contributed to nuclear flatten which involved in YAP activity. Furthermore, p38 played dual roles in YAP regulation, and it promoted actin filament polymerization by activating pAkt and pcofilin. In addition, p38 inhibited pLATS, which was required for YAP activity. LATS and AMOT could regulate each other. FSS affected AMOT, which competitively bind to F-actin and YAP, thereby inducing FSS-mediated cell proliferation. In summary, our results provide new insights on PDL cell proliferation as a feasible approach of PDL tissue regeneration. And the mechanistic observation on p38-AMOT-YAP mechanotransduction shed light to tooth movement and dentofacial orthopedics.
Results
FSS promoted proliferation and rearranged the cytoskeleton and nuclear shape of PDL cells
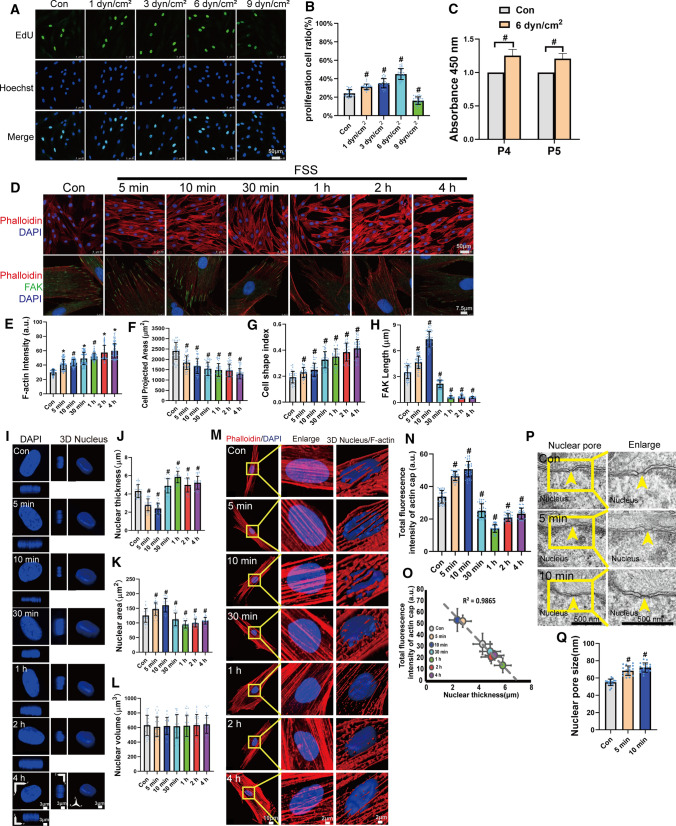
To investigate the effect of FSS on the proliferation of PDL cells, we quantified EdU-positive PDL cells by 1, 3, 6, and 9 dyn/cm2 of FSS for 4 h. In our previous study, 12 h of FSS would inhibit cell proliferation and 4 h of FSS could promote endogenous growth factor expression [11]. In this study, 1–6 dyn/cm2 of FSS promoted cell proliferation but 9 dyn/cm2 of FSS inhibited cell proliferation. Furthermore, 6 dyn/cm2 of FSS demonstrated the highest effect, increasing the number of EdU-positive cells from 24.41% ± 4.01% to 43.13% ± 6.03% (Fig. 1A and B). We selected 6 dyn/cm2 of FSS in our subsequent experiments. We passaged PDL cells which exposed to 6 dyn/cm2 of FSS for two generations. Interestingly, the cells maintained higher viability comparing with the static control (Fig. 1C). To investigate the effect of FSS on PDL cytoskeleton remodeling, PDL cells were exposed to 6 dyn/cm2 of FSS for 5 min, 10 min, 30 min, 1 h, 2 h, and 4 h. FSS increased the density of F-actin filaments (Fig. 1D and E). The projected areas decreased and cell shape index increased in a time-dependent manner (Fig. 1F and G), indicating that FSS could change the cell shape to smaller and rounder cells. Next, we studied FSS-mediated cell adhesion. Interestingly, FSS seemed to regulate FAK and paxillin in a two-phase model. It increased the length of FAK and paxillin after 5–10 min. However, these parameters declined after more than 30 min of FSS (Figs. 1D, H and S1).
Fig. 1.
FSS promoted proliferation, rearranged the cytoskeleton, and changed the nuclear shape of PDL cells. A PDL Cells were subjected to 1 dyn/cm2, 3 dyn/cm2, 6 dyn/cm2, and 9 dyn/cm2 of FSS for 4 h. Cells cultured under static conditions served as the control. EdU-positive (green) cells and nuclei (blue) were imaged. Scale bars = 50 μm. B Quantification of the percentage of EdU-positive cells in each group. (n = 29, 29, 31, 30, 30 roi, respectively). C PDL cells were subjected to FSS for 4 h and cultured for two passages (P4 and P5). Cell viability was quantified and is represented as mean ± s.d. (n ≥ 3). D PDL cells subjected to FSS at 6 dyn/cm2 for 5 min, 10 min, 30 min, 1 h, 2 h, or 4 h were immunostained with rhodamine phalloidin (F-actin, red), FAK (green), and DAPI (blue). Quantification of cell density of F-actin (E) (n = 135, 135, 134, 135, 134, 134, 135 roi, respectively), cell projected areas (F) (n = 38, 35, 35, 44, 52, 45, 40 roi, respectively), cell shape index (CSI = 4π x area/perimeter2) (G) (n = 38, 26, 29, 31, 28, 26, 40 roi, respectively) in static or sheared PDL cells. H Quantification of FAK length (n = 47, 50, 50, 48, 50, 50 50 roi, respectively) in static or sheared PDL cells examined in (D). I PDL cells under static conditions or subjected to FSS at 6 dyn/cm2 for 5 min, 10 min, 30 min, 1 h, 2 h, or 4 h. Nuclear morphology of DAPI-stained nuclei (blue) indicates 3D nuclear shape, where maximum intensity projection onto the XY-plane was performed using upper hemispheres of the 3D reconstructed nuclei. The cross-sectional side view was captured along the XZ-plane and YZ-plane crossing the center of the nucleus. Scale bars = 3 μm. Nuclear thickness (J) and the projected nuclear area (K) onto the XY-plane was shown. The volume of the 3D reconstructed nuclei (L). (n = 29 roi per condition). M Actin cap was reorganized by FSS. Representative images of F-actin organization in the apical region of the nucleus were given. Scale bars = 10 μm. Yellow boxes indicate the regions that were shown as a magnified view. Scale bars = 2 μm. 3D rendering illustrate indicated the presence of the perinuclear actin cap. Scale bars = 2 μm. N Quantification of total fluorescence intensity of actin cap in static or sheared PDL cells (n = 39 roi per condition). O Total fluorescence intensity of actin cap versus nuclear thickness for the conditions in (N) and (J). The dashed line shows a linear fit to the data (R2, squared correlation coefficient). P TEM images of nuclear pores of PDL cells under static conditions or subjected to FSS for 5 min or 10 min. Yellow arrows indicated nuclear pores. Scale bars = 500 nm. Q Quantification of Nuclear pore size (n = 18 roi per condition nuclear pores from ≥ 10 cells per condition) in static or sheared PDL cells examined in (P). Bar represents mean ± s.d. *p < 0.05 and #p < 0.01; NS not significant
When FSS was loaded on PDL cells for 5 min to 4 h, the nuclear thickness declined within 10 min and increased after 30 min. The nuclear area enhanced within 10 min of FSS and depressed after 30 min (Fig. 1I–K). However, the nuclear volume did not change by FSS (Fig. 1L), indicating that within 10 min of FSS the nuclei became flatter but after 30 min they achieved a more stereoscopic shape. The actin cap increased within 10 min and decreased after 30 min (Fig. 1M and N), and the total fluorescence intensity of actin cap and the nuclear thickness showed a high correlation (Fig. 1O), demonstrating that FSS promoted actin cap expansion and compressed nuclei. We further investigated the effect within 10 min of FSS on the nuclear pore. The TEM results indicated that 5–10 min of FSS increased the nuclear pore size (Fig. 1P and Q). To understand deeply what is happening in terms of nuclear reorganization, we measured the chromatin condensation parameter (CCP) in PDL cells loaded or unloaded FSS with DAPI-stained nuclei. We found that the CCP in PDL cells was significantly elevated (Fig. S2).
YAP involved in FSS-induced PDL cell proliferation
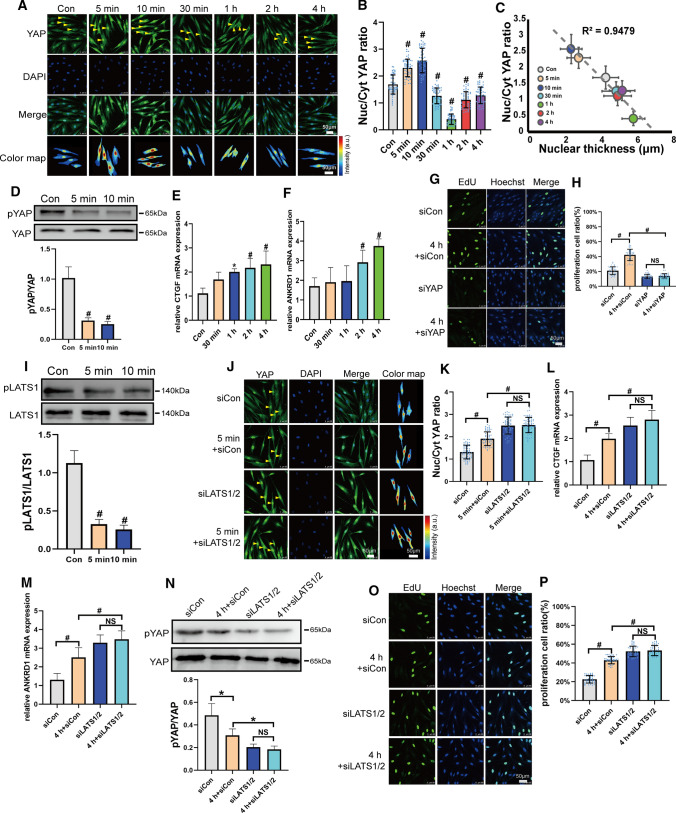
YAP is a mechanotransducer and plays an important role in cell proliferation. We investigated their role in FSS-induced proliferation in PDL cells. As shown in Fig. 2A and B, YAP showed a dynamic translocation by the duration of FSS. They accumulated in the nuclei in response to FSS within 5–10 min. However, YAP was excluded from the nuclei after 30 min, 1 h, 2 h, or 4 h of FSS. The N/C of the YAP ratio was highly correlated with the nuclear thickness, which indicated that the nuclear shape changes affected YAP nuclear localization (Fig. 2C). The nuclear export inhibitor (LMB) blocked 1 h of FSS-induced YAP export (Fig. S3), which showed that nuclear pore export was essential for FSS-induced YAP transport. Ser127 of YAP, a key target of LATS1/2, was downregulated by FSS from 5 min to 4 h (Figs. 2D and S4). The YAP target genes, such as connective tissue growth factor (CTGF) and ankyrin repeat domain 1 (ANKRD1), were promoted by 30 min–4 h of FSS (Fig. 2E and F), indicating that the downstream genes were continually activated by FSS. It indicated that although the FSS-induced localization of YAP was dynamic, the activation of downstream genes and their depressed phosphorylation continually maintained. When we knocked down YAP, FSS-induced cell proliferation was blocked (Figs. 2G, H and S5A). These results demonstrated that FSS-regulated YAP played a key role in FSS-induced PDL cell proliferation.
Fig. 2.
YAP involved in FSS-induced proliferation in PDL cells. A PDL cells under static conditions or after FSS at 6 dyn/cm2 for 5 min, 10 min, 30 min, 1 h, 2 h, or 4 h were fixed and immunostained with anti-YAP (green) antibody together with DAPI (blue). Yellow arrowheads of color maps showing YAP intensity for the conditions measured, respectively. Scale bars = 50 μm. B Quantification of nuclear relative to cytoplasmic fluorescent intensity of YAP in static or FSS. (n = 55 roi per condition). C Nuc/Cyt YAP ratio versus nuclear thickness for the conditions in (B) and Fig. 1J. The dashed line shows a linear fit to the data (R2, squared correlation coefficient). D Phosphorylated YAP on Ser127 expression on static or FSS (6 dyn/cm2, 5 min, 10 min) in PDL cells. pYAP/total YAP intensity ratio is shown on lower panel (n ≥ 3). E and F qPCR of CTGF (E) and ANKRD1 (F) gene expression under static conditions or FSS (n ≥ 3). G PDL Cells transfected with control siRNA or YAP siRNAs and subjected to FSS (6 dyn/cm2, 4 h). Representative image of EdU-positive cells in each group was given. Scale bars = 50 μm. H Quantification of the percentage of EdU-positive cells in each group was shown. (n = 41 roi per condition). I pLATS1 (Thr1079) expression of static or sheared (6 dyn/cm2, 5 min, 10 min) were detected by Western blotting and pLATS1/total LATS1 intensity were presented at lower panel (n ≥ 3). PDL Cells transfected with control siRNA or LATS1/2 siRNAs were subjected to FSS (6 dyn/cm2, 5 min) and YAP (green) localization (J), quantification of nuclear relative to cytoplasmic fluorescent intensity (K) (n = 65 roi per condition), CTGF (L), ANKRD1 (M) gene expression (n ≥ 3), pYAP expression (N) (n ≥ 3) and (O, P) EdU results (n = 42 roi per condition) were presented. Bar represents mean ± s.d. *p < 0.05 and #p < 0.01; NS not significant
LATS1/2 are the upstream kinases that regulate the activation of YAP in the Hippo pathway. pLATS1 decreased by 6 dyn/cm2 of FSS from 5 min to 4 h (Figs. 2I and S6). When LATS1/2 were silenced, YAP nuclear localization, as well as CTGF and ANKRD1 gene expression, was enhanced, even in control PDL cells, and FSS could not regulate their levels (Figs. 2J–M and S5B). The pYAP level was inhibited by knockdown of LATS1/2 and failed to respond to FSS (Fig. 2N). These data indicate that FSS could regulate LATS1/2 activity, and therefore modulated the localization and activation of YAP. When LATS1/2 were silenced, even in control cells, FSS failed to promote cell proliferation (Fig. 2O and P). These results demonstrated that LATS involved in FSS-induced YAP regulation and played a role in FSS-induced PDL cell proliferation.
p38 regulated FSS-induced cell proliferation by regulating LATS and YAP in PDL cells
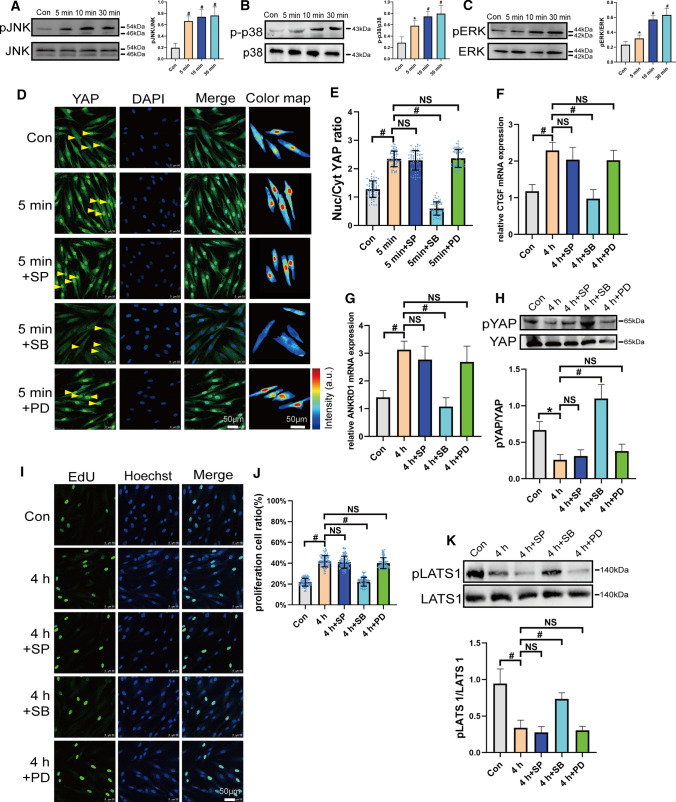
Recently, the MAPK signaling pathway has been reported to regulate YAP activity. In our study, 6 dyn/cm2 of FSS could rapidly activate ERK, p38, and JNK within 5–30 min. (Fig. 3A–C). When PDL cells were individually pretreated with ERK, p38, and JNK inhibitors, the p38 inhibitor, but not JNK and ERK inhibitors, decreased FSS-induced YAP nuclear aggregation (Fig. 3D and E). pYAP expression, which was decreased by FSS, was rescued by the p38 inhibitor, but not inhibitor of JNK or ERK (Fig. 3F). A similar trend was found for the expression of CTGF and ANKRD1 (Fig. 3G and H). We further examined the role of p38 and found that FSS-induced proliferation was significantly disrupted by the p38 inhibitor, but not JNK and ERK inhibitors (Fig. 3I and J). As FSS downregulated pLATS1, we found only the p38 inhibitor could rescue pLATS1 expression under FSS (Fig. 3K). It exhibited that p38 regulated FSS-induced cell proliferation by depressed LATS phosphorylation and promoted YAP activity.
Fig. 3.
p38 regulated FSS-induced cell proliferation by regulating LATS and YAP in PDL cells. A–C 6 dyn/cm2 of FSS was loaded on PDL cells as the duration shown in figures. pJNK/JNK, p-p38/p38, pERK1/2/ERK expressions were detected by Western blotting. (n ≥ 3). D–K PDL cells preincubated with DMSO, JNK pathway inhibitor SP600125 (SP, 10 μm, 2 h), p38 pathway inhibitor SB203580 (SB, 10 μm, 2 h), or ERK pathway inhibitor PD98059 (PD, 10 μm, 2 h) and subjected to FSS. YAP localization (green) (D), quantification of nuclear relative to cytoplasmic fluorescent intensity of YAP (E) (n = 64 roi per condition), pYAP(Ser127) (n ≥ 3) (F), CTGF (G), ANKRD1 (H), mRNA expression (n ≥ 3) and EdU results (n = 113, 107, 111, 109, 108 roi, respectively) (I, J), and pLATS1 (Thr1079) (n ≥ 3) (K). Bar represents mean ± s.d. *p < 0.05 and #p < 0.01; NS not significant
Cytoskeleton and LINC regulated FSS-induced YAP localization in PDL cells
It is known that YAP is highly regulated by cytoskeleton. To investigate the role of cytoskeleton in FSS-induced YAP localization, we used different cytoskeleton inhibitors, cytochalasin D (Cyto D), a disruptor of actin filament polymerization, Y27632, a blocker of Rho kinase, and blebbistatin (Bleb), an inhibitor for non-muscle myosin-II ATPase, to disturb different aspects of the cytoskeleton. The results indicated that the impaired actin filaments accorded with nuclear expulsion of YAP under FSS. On the contrary, when cells were incubated with jasplakinolide (Jasp), a reagent which promotes actin polymerization, the FSS-induced nuclear localization of YAP increased (Fig. 4A and B), indicating that the integrity of actin filaments played an important role in FSS-induced YAP localization. These cytoskeleton reagents also regulated nuclear shape. Cytoskeletal inhibitors impaired enhanced the nuclear height and depressed the nuclear area. However, jasplakinolide had the opposite effects (Fig. 4C–F). LINC complex is known to transfer forces from the cytoplasmic cytoskeleton to the nucleus. When we silenced nesprin1 which connected actin to the LINC, FSS-induced F-actin formation and YAP nuclear localization were impeded (Figs. 4G, H and S5E). Interestingly, FSS-promoted F-actin and paxillin were also declined when nesprin1 was knocked down (Figs. 4I and S7). Silencing nesprin1 abolished FSS increased actin cap and nuclear flattening (Fig. 4J–O). It demonstrated that LINC was vital to FSS transduction from the cytoskeleton to the nucleus. It was involved in FSS-induced YAP transport.
Fig. 4.
Cytoskeleton and LINC involve in FSS-induced YAP localization in PDL cells. (A) PDL cells preincubated with DMSO, actin inhibitor cytochalasin D (cyto D, 1 μm, 1 h), myosin II inhibitor Blebbistatin (Bleb, 50 μm, 2 h), Rho kinases (ROCK) inhibitor Y27632 (Y27632, 10 μm, 2 h), or actin stabilizer Jasplakinolide (Jasp, 50 nM, 2 h) were under static or subjected to FSS (6 dyn/cm2, 5 min) and were immunostained with anti-YAP (green) antibody and rhodamine phalloidin (red). Scale bars = 50 μm. B Quantification of nuclear relative to cytoplasmic fluorescent intensity of YAP in static or sheared PDL cells examined in (A) (n = 65, 65, 65, 65, 65, 64 roi, respectively). C–F Nuclear morphology changed by FSS and cytoskeleton inhibitors. Scale bars = 3 μm. Quantification of nuclear thickness (D), nuclear area (E), and volume (F) (n = 34 roi per condition). G–O PDL Cells transfected with control siRNA or Nesprin1 siRNAs were under static conditions or subjected to FSS (6 dyn/cm2, 5 min). Immunostained YAP (green) (G), quantification of nuclear fluorescence intensity of YAP relative to that of cytoplasm (H) (n = 70 roi per condition), quantification of F-actin fluorescence intensity (I) (n = 45 roi per condition), F-actin organization in the apical region of the nucleus and quantification of total fluorescence intensity of actin cap (J and K) (n = 36 roi per condition), nuclear morphology (L), nuclear thickness (M), nuclear area (N) and volume (O) (n = 34 roi per condition). Bar represents mean ± s.d. *p < 0.05 and #p < 0.01; NS not significant
p38 affected Akt/cofilin and LATS to regulate FSS-induced F-actin polymerization in PDL cells
Akt and cofilin are key factors that regulate actin polymerization. Our results indicated that pAkt/Akt increased by 5–30 min of FSS and Akt expression was unchanged during this period (Fig. 5A). Furthermore, the levels of pcofilin/cofilin also upregulated by 5–30 min of FSS, and the cofilin level did not affect (Fig. 5B). When cofilin was silenced, FSS upregulated YAP nuclear localization, and downstream gene expression was further increased with the same trend as F-actin formation (Figs. 5C–G, S5C, and S8). FSS-induced pcofilin expression was restrained when we used the Akt inhibitor MK2206 and the PI3K inhibitor LY294002 (Figs. 5H and S9). When PDL cells were preincubated with the p38 inhibitor and loaded with 6 dyn/cm2 of FSS, pAkt/Akt, and pcofilin/cofilin, the levels of F-actin decreased compared with the control (Figs. 5I–L and S10). It indicated that p38 was vital to actin polymerization. To demonstrate the relationship of LATS1 and Akt, MK-2206 was incubated with PDL cells to inhibit Akt activity. The results indicated that pLATS1/LATS1 expressions, which were decreased by FSS, were rescued by the Akt inhibitor (Fig. 5M). On the contrary, knocking down LATS1/2 barely affected FSS-mediated Akt activity (Fig. S11). As p38 could inhibit the pLATS level, the F-actin density increased when we silenced LATS1/2 and it did not response to FSS (Fig. 5N and O). When LATS1/2 were knocked down, it showed that actin cap increased even in control group and FSS could not enhance it (Fig. 5P and Q). Silencing LATS1/2 further increased the FSS-induced nuclear flattening which did not response to FSS regulation (Fig. 5R–U). Our results indicated that FSS activated p38 and subsequently activated Akt which phosphorylated cofilin to promote F-action polymerization. Activation of the Akt involved in depression of phosphorylation of LATS. LATS which inhibited by p38 negatively regulated the F-actin, actin cap formation, and nuclear flattening. Thereby, the FSS-induced polymerization of F-actin increased YAP nuclear translocation.
Fig. 5.
p38 affected Akt/cofilin and LATS to regulate FSS-induced F-actin polymerization in PDL cells. A and B 6 dyn/cm2 of FSS loaded on PDL cells for 5 min, 10 min, and 30 min. pAkt, Akt (A), pcofilin and cofilin (B) protein expressions were detected by Western blotting (n ≥ 3). C–G PDL cells transfected with control siRNA or cofilin siRNAs and under static conditions or subjected to FSS (6 dyn/cm2, 5 min). Immunostaining YAP (green) (C), quantification of nuclear relative to cytoplasmic fluorescent intensity of YAP (D) (n = 65 roi per condition), CTGF (E), ANKRD1 (F), mRNA expression (n ≥ 3), F-actin intensity (G) (n = 41 roi per condition). H pcofilin/cofilin expressions were detected when cells were incubated with DMSO or Akt inhibitor MK-2206 (2.5 μm, 24 h) under static conditions or subjected to FSS (6 dyn/cm2, 5 min) (n ≥ 3). I–L PDL cells preincubated with DMSO or p38 pathway inhibitor SB203580 (10 μm, 2 h) and under static conditions or subjected to FSS (6 dyn/cm2, 5 min). pAkt (I), pcofilin (J) (n ≥ 3), and F-actin intensity (K and L) (n = 109,109,106 roi, respectively). M pLATS1/LATS1 expressions were detected when cells were incubated with DMSO or Akt inhibitor MK-2206 (2.5 μm, 24 h) under static conditions or subjected to FSS (6 dyn/cm2, 5 min) (n ≥ 3). N–U PDL cells transfected with control siRNA or LATS1/2 siRNAs and under static conditions or subjected to FSS (6 dyn/cm2, 5 min). F-actin intensity (N and O) (n = 101,102, 103,104 roi, respectively), actin cap (P and Q) (n = 36 roi per condition), nuclear morphology (R), quantification of nuclear thickness (S), nuclear area (T), and volume (U) (n = 33 roi per condition). Bar represents mean ± s.d. *p < 0.05 and #p < 0.01; NS not significant
AMOT negatively regulated FSS-induced YAP transport in PDL cells
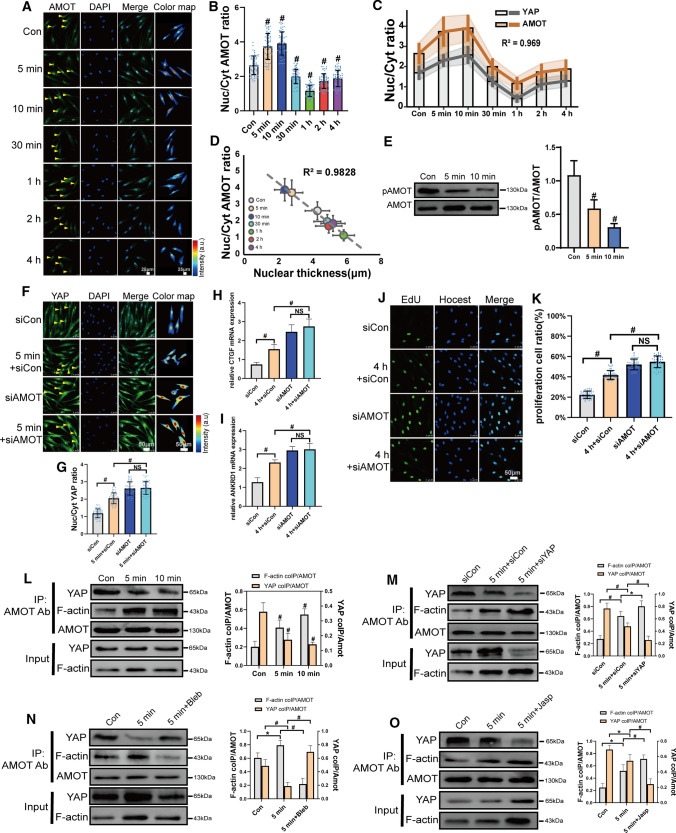
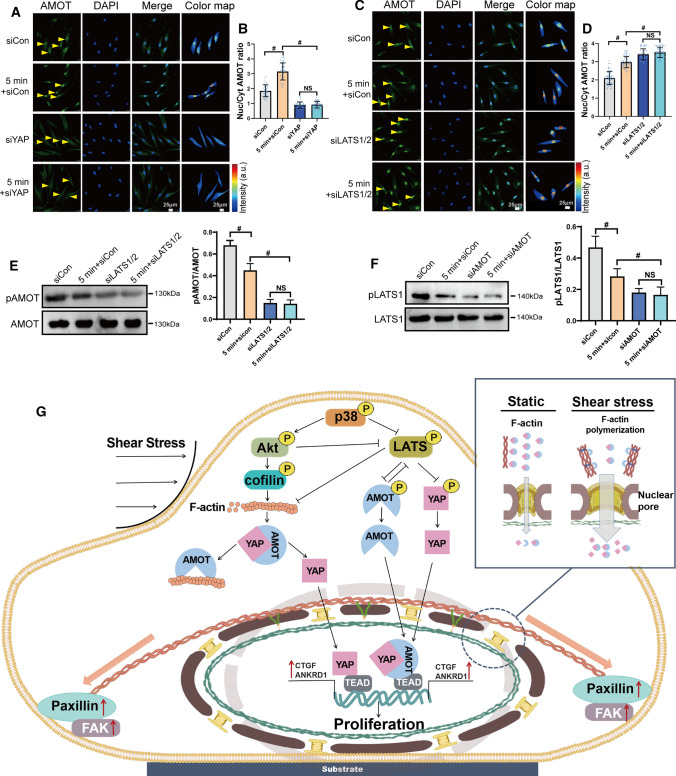
It has been reported that the AMOT family can interact with several Hippo pathway components and regulate YAP activity. Interestingly, our results showed that AMOT was transported into the nuclei, and the pattern was similar to that of FSS-induced YAP localization (Fig. 6A–C). AMOT N/C ratio was high relative to nuclear thickness (Fig. 6D). FSS inhibited phosphorylation of AMOT within 5 to 10 min (Fig. 6E). When we silenced AMOT, YAP nuclear localization was increased, and it did not respond to FSS (Figs. 6F, G and S5D). The downstream genes, such as CTGF and ANKRD1, showed the same trend (Fig. 6H and I). The knockdown of AMOT promoted FSS-induced proliferation of PDL cells. FSS accelerated proliferation was not regulated by FSS when AMOT was silenced (Fig. 6J and K). It indicated that AMOT was a negative regulator and required for FSS-induced YAP nuclear localization and cell proliferation. To study the underlying mechanism, we investigated the interaction of AMOT with F-actin and YAP. Our results showed that FSS could increase AMOT binding with F-actin and decrease AMOT-YAP interactions (Fig. 6L). When we silenced YAP, AMOT-F-actin interactions was further enhanced under FSS (Fig. 6M). When we inhibited F-actin polymerization by blebbistatin, AMOT-YAP interactions were promoted (Fig. 6N). When jasplakinolide was used, AMOT-YAP interactions decreased under FSS (Fig. 6O). Our results demonstrate that AMOT could competitively bind with F-actin and YAP. The polymerization of F-actin promoted the interaction of AMOT with F-actin. To further investigate the regulation mechanism of AMOT, we found that siYAP depressed the FSS-mediated nuclear localization of AMOT (Fig. 7A and B). When we silenced the YAP-negative regulator, LATS1/2, AMOT nuclear localization was increased and did not respond to FSS (Fig. 7C and D). siLATS1/2 also decreased their phosphorylation (Fig. 7E), indicating that LATS1/2 could regulate pAMOT and FSS-induced nuclear localization. AMOT also could regulate the phosphorylation of LATS1. When we silenced AMOT, FSS-inhibited phosphorylation was further declined, and it was no longer regulated by FSS (Fig. 7F). Our results demonstrate that FSS inhibited pLATS1, which affected pAMOT and their nuclear localization. AMOT could phosphorylate LATS1. The nuclear localization of AMOT partially depended on YAP nuclear transport (Fig. 7G).
Fig. 6.
Angiomotin competitively bond with YAP and F-actin and involved in FSS-induced proliferation in PDL cells. PDL cells under static or exposed to 6 dyn/cm2 of FSS for 5 min, 10 min, 30 min, 1 h, 2 h, or 4 h. Immunostaining of AMOT (green) (A) and quantification of nuclear relative to cytoplasmic fluorescent intensity of AMOT (B) (n = 63 roi per condition), C Nuc/Cyt ratio of YAP and AMOT by FSS (R2, squared correlation coefficient). D Nuc/Cyt AMOT ratio versus nuclear thickness. E FSS (6 dyn/cm2, 5 min, 10 min) inhibited pAMOT (Ser175) expression (n ≥ 3). F–K PDL cells transfected with control siRNA or AMOT siRNAs and subjected to FSS (6 dyn/cm2, 5 min or 4 h). YAP nuclear localization (F and G) (n = 68 roi per condition), CTGF (H), ANKRD1 (I), expression and EdU results (J, K) (n = 41, 41, 42, 41 roi, respectively). L–O PDL cells were kept under static conditions or subjected to FSS (6 dyn/cm2, 5 min,10 min) (L). PDL cells transfected with control siRNA or YAP siRNAs were kept under static conditions or subjected to FSS (6 dyn/cm2, 5 min) (M). PDL cells were preincubated with DMSO, myosin II inhibitor Blebbistatin (Bleb, 50 μm, 2 h) (N), or actin stabilizer Jasplakinolide (Jasp, 50 nM, 2 h) (O) and were kept under static conditions or subjected to FSS (6 dyn/cm2, 5 min). Lysates of PDL cells were immunoprecipitated with anti-AMOT antibody. Coprecipitated endogenous YAP and F-actin were detected by immunoblotting with anti-YAP and anti-F-actin antibody. YAP and F-actin immunoprecipitated with anti-AMOT antibody (IP: AMOT Ab) in static or sheared PDL cells were analyzed. The relative intensity was calculated by the intensity of the band of immunoprecipitated YAP or F-actin by anti-AMOT antibody in the static or sheared PDL cells. Bar represents mean ± s.d. *p < 0.05 and #p < 0.01; NS not significant
Fig. 7.
YAP and LATS regulated FSS-induced AMOT phosphorylation and localization. PDL cells transfected with control siRNA or YAP siRNAs (A, B) or LATS1/2 siRNAs (C, D) and subjected to FSS (6 dyn/cm2, 5 min). Immunostaining of AMOT and quantification of nuclear relative to cytoplasmic fluorescent intensity of AMOT (B, n = 43 roi per condition) (D, n = 63 roi per condition). E When LATS1/2 were knocked down, FSS-depressed pAMOT (Ser175) expression was further declined (n ≥ 3). F When AMOT was knocked down, FSS-depressed pLATS1 expression was further decreased (n ≥ 3). G A schematic representation of how FSS regulated p38 and further affected YAP nuclear translocation to promoted proliferation in PDL cells. Bar represents mean ± s.d. *p < 0.05 and #p < 0.01; NS not significant
Discussion
Periodontitis is a highly prevalent disease worldwide, which eventually causes tooth loss. PDL cells are the main source of seed cells in PDL regeneration. However, the shortage of PDL cells greatly restricts their clinical application. As such, it is critical to identify new ways in which PDL cells can be expanded. In this study, we demonstrated that 1–6 dyn/cm2 of FSS promoted cell proliferation. This approach does not require exogenous growth factors which means it is safe, economical, and easily applicable in the culture of PDL cells.
To investigate the mechanism of FSS-mediated cell proliferation, we first examined the changes in the shape of cells and nuclei. Cell shape can affect cell behavior, such as the proliferation, adhesion, and differentiation of cells [17]. In this study, FSS reorganized F-actin, rendering cells as rounder and smaller. Focal adhesions (FAs) are structures that connect the cytoskeleton to the ECM [18]. As the most important FA proteins, paxillin and FAK bind to the cytoplasmic tails of integrin and localize near the plasma membrane [19]. Paxillin and FAK appeared at the distal tip of cells after 5–10 min of FSS, indicating that PDL cells can aggregate FA proteins to form large FAs and resist FSS. However, when FSS lasted for more than 30 min, PDL cells could no longer form large FAs. The cell shape and projected areas were decreased by FSS, which might be the reason for the transport of YAP to cytoplasm by 30 min of FSS, consistent with a previous study that reported ECM detachment to cause YAP nuclear export [20]. Our results demonstrate that FA assembly is a dynamic process that can adapt to mechanical stress in PDL cells.
Recent studies have shown that the role of the nucleus as a mechanosensitive organelle whereby physical deformations induced by forces mediate to the nuclear envelope and regulate nuclear and cellular functions. It has been reported that force-induced nuclear flattening can increase nuclear pore permeability and promote YAP nuclear import [21]. In this study, the actin cap increased by 10 min of FSS and decreased by 30 min of FSS. Furthermore, the nuclear thickness was correlated with the actin cap formation, indicating that FSS promoted the actin cap to flatten the nuclei, which further increased the nuclear pore size, consistent with a previous study [21]. As such, the nuclear localization of YAP correlated with the nuclear thickness. The nuclear flattening by 10 min of FSS contributed to FSS-mediated YAP nuclear transport. However, 30 min to 4 h of FSS, especially 1 h of FSS, depressed the formation of actin cap and increased the nuclear thickness. It contributed to YAP cytoplasmic transport. Several studies indicate that forces acting on the nucleus can modulate chromatin structure and trigger conformational changes of key proteins, thereby activating or repressing mechanoresponsive genes [22, 23]. Promita’s work indicated that the topographic constraints significantly squeezed the nuclei, increased chromatin condensation, and promoted cell proliferation [24]. In our study, FSS increased chromatin condensation during 5 min–4 h. It indicated that FSS might regulate nuclear deformation which affect chromatin condensation, and thereby regulate cell proliferation.
YAP is a transcription cofactor that have essential roles in the proliferation, differentiation, development, and apoptosis of cells. The mechanical forces, which are generated by extracellular matrix rigidity, cell strain, and cell shape, control nuclear translocation of YAP [25, 26]. Our results indicate that FSS regulated the localization of YAP in a time dependent. 10 min of FSS promoted the nuclear transport of YAP, whereas 30 min–4 h of FSS promoted its cytoplasmic transport. Similar results were reported that YAP accumulated in nuclei after 10 min of 15 dyn/cm2 of FSS, but expelled after 6 h or 24 h of FSS in endothelial cells [27]. However, the downstream target genes CTGF and ANKRD1 were continually activated by FSS. Besides, FSS-mediated YAP export was transported through nuclear pores which proved by nuclear export inhibitor LMB. When YAP was silenced, FSS-mediated cell proliferation was inhibited, indicating that YAP is critical for PDL cell proliferation.
LATS1/2 are important negative regulators of the Hippo pathway which phosphorylate YAP and inhibit their nuclear accumulation. Mechanical stress regulates YAP expression in LATS1/2-dependent [28] and -independent [29, 30] pathways. Our results indicate that FSS inhibited pLATS1/LATS1 expressions. The knockdown of LATS1/2 increased FSS-mediated YAP nuclear localization and activity, as well as downstream target gene expression. When LATS1/2 was silenced in PDL cells, cell proliferation increased even in static condition and did respond to FSS. As such, FSS inhibited phosphorylates of LATS which regulated YAP activity and cell proliferation.
The MAPK pathway, including ERK, JNK, and p38, controls a wide array of physiological processes and involves in the regulation of YAP [31]. Guan et al. showed that osmotic stress-induced YAP cytoplasmic translocation was p38 dependent, but Hippo independent [32]. The disturbed flow induced YAP/TAZ activation by enhancing JNK activity in endothelial cells [33]. It has been reported that ERK, JNK, and p38 could increase Ajuba family proteins binding with LATS which inhibited pLATS to increase YAP activity [34–36]. In our study, FSS activated ERK, JNK, and p38; however, only p38 was involved in FSS-mediated YAP nuclear localization and activity. And p38 plays the dual role in FSS-regulated YAP. On one hand, FSS-induced p38 inhibited pLATS and pYAP expressions. On the other hand, p38 activated pAkt which is responsible for the F-actin polymerization.
Increasing studies have demonstrated that mechanical stress can generate intracellular tension and F-actin remodeling, both of which associate with YAP activity [37]. The disruption of F-actin or myosin II sequesters YAP in the cytoplasm [20, 26]. Our results indicate that disrupting cytoskeleton integrity prevented FSS-mediated YAP nuclear localization. However, promoting F-actin polymerization increased FSS-mediated YAP nuclear transport. The disruption of F-actin increased the nuclear thickness, which may explain why YAP was transported to the cytoplasm. The LINC complex connects to the nucleoskeleton and the cytoskeleton, where it transmits forces to the nucleus [38]. When LINC was silenced, the FSS-mediated F-actin restructuring and actin cap decreased, whereas the nuclear thickness increased and the nuclear height was not regulated by FSS. It demonstrated that LINC as the nuclear-cytoskeletal coupling was critical for FSS-induced YAP regulation.
Akt is a critical mediator of actin reorganization in different types of cells [39]. FSS could increase pAkt/Akt, as well as the inactive form of cofilin (pcofilin). Phosphorylation on Ser3 deactivates cofilin and inhibits its ability to sever and depolymerize F-actin [40]. The Akt inhibitor, MK-2206, could de-phosphorylated FSS-induced pcofilin, indicating that FSS regulated F-actin polymerization by activating Akt following cofilin inhibition. Knockdown of cofilin could increase YAP nuclear transport and downstream gene expression, consistent with a previous study [22]. The inhibition of p38 decreased FSS-induced phosphorylation of Akt and cofilin, as well as F-actin density, indicating that p38 is the upstream regulator of F-actin polymerization. These results are consistent with an earlier study that p38 mediated VEGF-induced cofilin phosphorylation and stress fiber formation [41]. p38 could promote F-actin accumulation and regulate YAP activity in drosophila and mammalian cells [34]. However, Liu et al. showed that p38 and JNK exerted their actions downstream of F-actin polymerization through mechanical tension in AT2 cells [42]. Besides distribute to F-actin remodeling, p38 could activate Akt to inhibit pLATS. However, LATS does not affect FSS-induced activation of Akt. These results are consisted with Xuan's study that activation of the PI3K/Akt pathway inhibits pMST1 and pLATS1, increasing nuclear YAP accumulation and proliferation [43]. Knocking down of LATS increased F-actin polymerization which also reported [44]. Thereby, it showed the dual functions of p38 to promote F-actin polymerization and inhibit pLATS to regulate YAP activity.
The AMOT family is reported to regulate YAP in a controversial manner. AMOT negatively regulated FSS-promoted YAP nuclear entry in endothelial cells [27] and facilitated YAP nuclear transport in ductal cells [45]. Interestingly, AMOT showed the same nuclear transport trend as YAP under FSS in our study. When we silenced AMOT, YAP nuclear entry increased. When YAP was silenced, AMOT did not respond to FSS anymore, indicating that AMOT may be a negative regulator, binding to YAP and entering nuclei. Our data indicate that AMOT competed with YAP for binding to F-actin. FSS accelerated F-actin and recruited more AMOT to bind with F-actin, thereby promoting YAP release from AMOT. Recent studies have shown the decrease of AMOT phosphorylation level was a key event that directs AMOT to enter the nucleus [46, 47]. In our study, we found that AMOT’s phosphorylation state was downregulated by FSS from 5 to 10 min which promoted AMOT to localize in the nucleus. Knocking down of LATS depresses pAMOT and promotes AMOT nuclear import. The nuclear pore complex (NPC) can allow molecules smaller than ~ 70 kDa to passively diffuse across the central part of the pore [48]. The YAP and AMOT complex, which is larger than 190 KDa, declined YAP nuclear transport. The disruption of F-actin increased YAP and AMOT interactions which might explain the relationship of F-actin with YAP. LATS1/2 and AMOT were dual-directional regulation which consistent with a previous study [49, 50].
In this study, we report that FSS could promote PDL cell proliferation via p38-AMOT-YAP. FSS activated p38, which inhibited pLATS and upregulated YAP activity. Besides, FSS-induced p38 expression also activated Akt and pcofilin, which promoted actin cap and F-actin polymerization, flattened nuclei and increased nuclear pore size to increased YAP nuclear translocation. F-actin recruited AMOT for binding and released YAP from the YAP-AMOT complex. Our study demonstrates that FSS as a promising tool for PDL cell expansion. It also sheds light on the molecular mechanism of YAP mechanotransduction, which will benefit dental regeneration in the future.
Materials and methods
Cell culture
Primary human PDL cells were obtained from healthy donors (18–25 years of age) undergoing third molar extractions due to orthodontic indications at the Hospital of Beihang University. The study conformed to the Declaration of Helsinki, and all procedures were approved by the Beihang University Ethical Committee. The PDL was scraped from the middle third of the tooth roots to avoid intermixing gingivae and dental pulp. PDL tissues were cut into pieces and digested with 3 mg/mL collagenase I (Sigma-Aldrich, St. Louis, MO, USA) at 37 °C for 1 h. After centrifuging, the cells were resuspended in high Dulbecco’s modified Eagle’s medium (high DMEM, Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS, Gibco) and 0.1 mg/ml penicillin/streptomycin (P/S, Amresco, Solon, OH, USA). Cells were cultured in a humidified atmosphere of 5% CO2 in air at 37 °C, and the medium was changed every 2–3 days. Cells were characterized as vimentin positive and cytokeratin negative by immunolocalization. Cells of passage 3–6 were used in this study.
Mechanical strain devices
A parallel plate flow chamber applied FSS to PDL cells (Fig. S11). Cells cultured on glass slides were exposed to steady laminar shear flow when they reached 80–90% confluence. The mechanical device was placed in a humidified atmosphere of 5% CO2 in air at 37 °C. Shear stress (τ, dyn/cm2) was calculated as follows: τ = 6μQ/a2b, where μ is the viscosity of the perfusate (poise), Q is the flow volume (ml/s), and a and b are the cross-sectional dimensions of the flow path (cm). PDL cells were exposed to 1, 3, 6, and 9 dyn/cm2 of FSS.
Immunofluorescent staining
Human PDL cells were fixed with 4% paraformaldehyde in PBS for 10 min, permeabilized with 0.2% Triton X-100 in PBS for 7 min, and blocked with 5% BSA in PBS for 2 h, all at room temperature (RT). Thereafter, cells were incubated with primary antibodies diluted in PBS containing 5% BSA overnight at 4 °C, followed by Alexa Fluor 488-labeled secondary antibodies. Details of the antibodies are given in Table S1. To visualize F-actin and cell nuclei, cells were stained with Texas red isothiocyanate-conjugated phalloidin (Thermo Fischer Scientific, Waltham, MA, USA) (1:1000) and 4′,6-diamidino-2 phenylindole (DAPI, Sigma-Aldrich) (1:2000), respectively. Images were captured with a confocal microscope (TCS-SP8, Leica Microsystems, Vienna, Austria). Images were analyzed by NIH ImageJ software. Z-stack confocal 3D images were obtained with a confocal laser-scanning microscope (Andor Dragonfly 500, Leica, England) with a separation interval of 0.15 μm. Z-stack images were analyzed using IMARIS software.
Calculation of the chromatin condensation parameter (CCP)
To assess chromatin condensation, constructs were incubated in 4% paraformaldehyde for 10 min at room temperature to fix cells, followed by washing and permeabilization with 0.2% Triton X-100 in PBS for 7 min. Nuclei were visualized by DAPI staining and scanned across their mid-section using a confocal microscope (TCS-SP8, Leica Microsystems, Vienna, Austria). To calculate the chromatin condensation parameter (CCP), a gradient-based Sobel edge detection algorithm was employed using MATLAB to measure the edge density for individual nuclei.
Transmission electron microscopy
For transmission electron microscopy (TEM) experiments, PDL cells were exposed to 6 dyn/cm2 of FSS for 5 or 10 min and then fixed with 2.5% glutaraldehyde and 1% paraformaldehyde for 1 h at RT. Cells were post-fixed with 1% osmium tetroxide in 0.8% K4Fe(CN)6 for 1 h at RT in the dark, followed by rinsing with 0.1 M phosphate buffer, dehydration in an acetone series (50%, 70%, 90%, 96%, and 100%; 5 min each), embedment in Epon 812, and polymerization for 48 h at 60 °C. Ultrathin 60-nm-thick sections were obtained using a UC7 ultramicrotome (Leica Microsystems). Sections were stained with 5% uranyl acetate for 10 min and lead citrate for 10 min. Sections were collected on 200-mesh grids and examined by TEM (Hitachi, 7065B, Tokyo, Japan) operating at 80 kV, and images were acquired with a charge-coupled device camera (SC1000; Gatan, Pleasanton, CA, USA). Nuclear pores with visible nuclear baskets were selected. The nuclear pore length was measured from one side of the double bilayer, at the point where the nuclear basket starts, to the other side of the double bilayer where the nuclear basket ends.
EdU (5-Ethynyl-2′-deoxyuridine) staining
Human PDL cells were exposed to FSS for 4 h and cultured in high DMEM supplemented with 10% FBS and P/S for 16 h. Cells cultured for 20 h under static conditions served as the control. Cells were incubated with high DMEM medium containing 10 μm EdU for 4 h. Thereafter, the medium was removed, and cells were fixed with 4% paraformaldehyde for 10 min at RT. Cells were rinsed twice with 3% bovine serum albumin in PBS and permeabilized with 0.5% Triton X-100 for 20 min. Cells were incubated with iClick reaction cocktail for 30 min at RT according to the instructions of the iClick EdU Andy Fluor 488 Imaging kit (ABP Biosciences, Rockville, MD, USA). Nuclei were stained with Hoechst 33,342. Images were captured with a confocal microscope (TCS-SP8, Leica Microsystems, Vienna, Austria). Cell proliferation was expressed as the ratio of EdU-positive cells to total cells.
Cell viability assay
The Cell Counting Kit-8 (CCK-8) kit (Beyotime Biotechnology, Shanghai, China) was used to assess cell viability. PDL cells were incubated for 2 h in high-DMEM containing 10% CCK-8 reagent. Absorbance values at 450 nm were obtained with a microplate reader (Thermo Fischer Scientific).
Quantitative real-time polymerase chain reaction
Total RNA was isolated from PDL cells with TRIZOL reagent (Thermo Fisher Scientific) according to the manufacturer’s protocol. In brief, 2 μg of RNA was reverse transcribed into cDNA. Real-time PCR was carried out with iQ SYBR Green supermix (Takara, Tokyo, Japan) in the Bio-Rad iCycler system (Bio-Rad Laboratories, Melville, NY, USA). All reactions were predenatured at 95 °C for 3 min, followed by 40 cycles at 95 °C for 10 s and 56 °C for 34 s. Relative mRNA expression was calculated and normalized to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Relative gene expression was determined by the 2−ΔΔCT method. Primers sequences are given in Table S2.
Western blotting
After washing with prechilled PBS, cells were lysed in RIPA buffer (Beyotime Biotechnology) supplemented with phenylmethyl sulfonyl fluoride (Beyotime Biotechnology) and a protease inhibitor cocktail (Beyotime Biotechnology). Lysates were centrifuged at 15,000×g and 4 °C to obtain cell extracts, which were combined with loading buffer and boiled for 5 min. Protein quantification was performed using the BCA protein assay kit (CwBio, Jiangsu, China). Equivalent amounts of cell lysates were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and proteins were transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA, USA). Membranes were blocked with 5% BSA in triethanolamine-buffered saline solution (TBS-T) for 2 h at RT, incubated with primary antibodies diluted in 5% BSA in TBST overnight at 4 °C, and then incubated with secondary HRP-conjugated antibodies for 2 h at RT. Enhanced chemiluminescence (Applygen, Beijing, China) was used to detect the target proteins. ImageJ software was used for densitometric analysis. Details on the antibodies are given in Table S1.
Co-immunoprecipitation (Co-IP)
PDL cells were rinsed with prechilled PBS and lysed in NP-40 lysis buffer (Beyotime Biotechnology) containing phenylmethylsulfonyl fluoride and a protease inhibitor cocktail at 4 °C. Supernatants were incubated with a monoclonal anti-AMOT antibody for 1 h at 4 °C, followed by incubation with Dynabeads Protein A (Thermo Fisher Scientific) for 2 h. Precipitates were washed thrice with prechilled PBS. The immunoprecipitates and aliquots of the total cell extracts were subjected to Western blotting with the indicated antibodies. Antibodies were diluted as recommended by the manufacturers, and target proteins were detected by HRP-conjugated secondary antibodies and enhanced chemiluminescence. ImageJ software was used for densitometric analysis. Details on the antibodies are given in Table S1.
siRNA transfection and drug treatment
Small interfering RNAs (siRNAs) targeting YAP, LATS1/2, cofilin, AMOT, and Nesprin1 were designed and synthesized by GenePharma Corporation (Shanghai, China). Details on the siRNA sequences are given in Table S3. PDL cells were transfected with Lipo3000 transfection reagent (Thermo Fisher Scientific) according to the manufacturer’s instructions. Cells transfected with a scrambled non-sense siRNA served as the control (siCon). The knockdown efficiency was confirmed by qPCR or Western blotting (Fig. S4).
PDL cells were preincubated with the p38 inhibitor SB203580 (SB, 10 μm, Santa Cruz Biotechnology, Sanat Cruz, CA, USA), ERK1/2 inhibitor PD98059 (PD, 10 μm, Beyotime Biotechnology), JNK inhibitor SP600125 (SP, 10 μM, Beyotime Biotechnology), actin inhibitor cytochalasin D (cyto D, 1 μm, Meilunbio, Dalian, China), myosin inhibitor blebbistatin (Bleb, 50 μm, Meilunbio), Rho kinase (ROCK) inhibitor Y27632 (10 μm, Merck Millipore, Burlington, MA, USA), actin stabilizer jasplakinolide (Jasp, 50 nM, Merck Millipore), Akt inhibitor MK-2206 (2.5 μm, Selleck, Houston, TX, USA), PI3K inhibitor LY-294002 (2 μm, Sigma-Aldrich), or nuclear export inhibitor leptomycin B (LMB, 20 nM, Beyotime Biotechnology) and exposed to FSS.
Statistical analysis
All experiments were performed in at least three biological replicates. In all figures, measurements are presented as mean ± standard deviation (SD). Statistical comparisons were carried out using SPSS 13.0 statistical software (SPSS Inc., Chicago, IL, USA) with two-tailed Student’s t tests when two cases were compared and with one-way analysis of variance (ANOVA; Tukey or Dunnett) tests when more than two cases were analyzed. When data did not meet normality criteria, equivalent non-parametric tests were used. Data were considered statistically significant if *p values < 0.05, #p values < 0.01. Comparisons that were not statistically significant are designated NS or are not shown. Pearson’s correlation was analyzed, and R2 were displayed. Details on sample numbers are given in figure legends.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
LSZ and YBF designed research; QSS, JN, XYC, YXS, and LSZ performed research; LXY, ZJY and CYL analyzed data; LSZ and YBF wrote the paper.
Funding
This work was supported by National Natural Science Foundation of China [Grant Numbers 11972067, 32171310, 11827803, 11421202, U20A20390], the 111 Project [Grant Number B13003].
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
Declarations
Conflict of interest
The authors declare that there are no conflicts of interest regarding the material discussed in the manuscript.
Ethical approval
The study conformed to the Declaration of Helsinki, and all procedures were approved by the Beihang University Ethical Committee (BM20180013).
Consent for publication
All authors have read and approved the final version of the manuscript and agreed to its publication in Cellular and Molecular Life Sciences.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qiusheng Shi and Lisha Zheng contributed equally to this work.
Contributor Information
Lisha Zheng, Email: lishazheng@buaa.edu.cn.
Yubo Fan, Email: yubofan@buaa.edu.cn.
References
- 1.Pihlstrom BL, Michalowicz BS, Johnson NW (2005) Periodontal diseases. Lancet 366:1809–1820. 10.1016/S0140-6736(05)67728-8 [DOI] [PubMed] [Google Scholar]
- 2.Kinane DF, Stathopoulou PG, Papapanou PN (2017) Periodontal diseases. Nat Rev Dis Primers 22:17038–17049. 10.1038/nrdp.2017.38 [DOI] [PubMed] [Google Scholar]
- 3.Chen FM, Sun HH, Lu H, Yu Q (2012) Stem cell-delivery therapeutics for periodontal tissue regeneration. Biomaterials 33:6320–6344. 10.1016/j.biomaterials.2012.05.048 [DOI] [PubMed] [Google Scholar]
- 4.Iwata T, Yamato M, Zhang Z et al (2010) Validation of human periodontal ligament-derived cells as a reliable source for cytotherapeutic use. J Clin Periodontol 37:1088–1099. 10.1111/j.1600-051X.2010.01597.x [DOI] [PubMed] [Google Scholar]
- 5.Nakdilok K, Langsa-ard S, Krisanaprakornkit S et al (2020) Enhancement of human periodontal ligament by preapplication of orthodontic loading. Am J Orthod Dentofacial Orthop 157:186–193. 10.1016/j.ajodo.2019.03.019 [DOI] [PubMed] [Google Scholar]
- 6.Palumbo R, Gaetano C, Melillo G et al (2000) Shear stress downregulation of platelet-derived growth factor receptor-β and matrix metalloprotease-2 is associated with inhibition of smooth muscle cell invasion and migration. Circulation 102:225–230. 10.1161/01.cir.102.2.225 [DOI] [PubMed] [Google Scholar]
- 7.Kim SG, Viechnicki B, Kim S et al (2011) Engineering of a periodontal ligament construct: cell and fibre alignment induced by shear stress. J Clin Periodontol 38:1130–1136. 10.1111/j.1600-051X.2011.01790.x [DOI] [PubMed] [Google Scholar]
- 8.Zheng LS, Huang Y, Song W et al (2012) Fluid shear stress regulates metalloproteinase-1 and 2 in human periodontal ligament cells: involvement of extracellular signal-regulated kinase (ERK) and p38 signaling pathways. J Biomech 45:2368–2375. 10.1016/j.jbiomech.2012.07.013 [DOI] [PubMed] [Google Scholar]
- 9.Tang M, Peng ZL, Mai ZH et al (2014) Fluid shear stress stimulates osteogenic differentiation of human periodontal ligament cells via the extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase signaling pathways. J Periodontol 85:1806–1813. 10.1902/jop.2014.140244 [DOI] [PubMed] [Google Scholar]
- 10.Qi L, Zhang Y (2014) The microRNA 132 regulates fluid shear stress-induced differentiation in periodontal ligament cells through mTOR signaling pathway. Cell Physiol Biochem 33:433–445. 10.1159/000358624 [DOI] [PubMed] [Google Scholar]
- 11.Zheng LS, Shi QS, Na J et al (2019) Platelet-derived growth factor receptor-alpha and beta are involved in fluid shear stress regulated cell migration in human periodontal ligament cells. Cell Mol Bioeng 12:85–97. 10.1007/s12195-018-0546-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng LS, Chen LP, Chen YC et al (2016) The effects of fluid shear stress on proliferation and osteogenesis of human periodontal ligament cells. J Biomech 49:572–579. 10.1016/j.jbiomech.2016.01.034 [DOI] [PubMed] [Google Scholar]
- 13.Zanconato F, Cordenonsi M, Piccolo S (2019) YAP and TAZ: a signalling hub of the tumour microenvironment. Nat Rev Cancer 19:454–464. 10.1038/s41568-019-0168-y [DOI] [PubMed] [Google Scholar]
- 14.Totaro A, Panciera T, Piccolo S (2018) YAP/TAZ upstream signals and downstream responses. Nat Cell Biol 20:888–899. 10.1038/s41556-018-0142-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belgardt E, Steinberg T, Husari A et al (2020) Force-responsive Zyxin modulation in periodontal ligament cells is regulated by YAP rather than TAZ. Cell Signal 72:109662–109672. 10.1016/j.cellsig.2020.109662 [DOI] [PubMed] [Google Scholar]
- 16.Liu AQ, Zhang LS, Chen J et al (2020) Mechanosensing by Gli1(+) cells contributes to the orthodontic force-induced bone remodelling. Cell Prolif 53:e12810. 10.1111/cpr.12810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen CS, Mrksich M, Huang S et al (1997) Geometric control of cell life and death. Science 276:1425–1428. 10.1126/science.276.5317.1425 [DOI] [PubMed] [Google Scholar]
- 18.Case LB, Waterman CM (2015) Integration of actin dynamics and cell adhesion by a three-dimensional, mechanosensitive molecular clutch. Nat Cell Biol 17:955–963. 10.1038/ncb3191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanchanawong P, Shtengel G, Pasapera AM et al (2010) Nanoscale architecture of integrin-based cell adhesions. Nature 468:580–584. 10.1038/nature09621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao B, Li L, Wang L et al (2012) Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev 26:54–68. 10.1101/gad.173435.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elosegui-Artola A, Andreu I, Beedle Amy EM et al (2017) Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell 171:1397–1410. 10.1016/j.cell.2017.10.008 [DOI] [PubMed] [Google Scholar]
- 22.Soumya G, Nimi M, Apurva S et al (2012) Role of actin dependent nuclear deformation in regulating early gene expression. PLoS One 7:e53031. 10.1371/journal.pone.0053031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miroshnikova YA, Nava MM, WickstrM SA (2017) Emerging roles of mechanical forces in chromatin regulation. J Cell Sci 130:2243–2250. 10.1242/jcs.202192 [DOI] [PubMed] [Google Scholar]
- 24.Promita B, Brenton LC, Mark A (2020) Effect of substrate topography on the regulation of human corneal stromal cells. Colloids Surf B Biointerfaces 190:110971–110981. 10.1016/j.colsurfb.2020.110971 [DOI] [PubMed] [Google Scholar]
- 25.Aragona M, Panciera T, Manfrin A et al (2013) A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 154:1047–1059. 10.1016/j.cell.2013.07.042 [DOI] [PubMed] [Google Scholar]
- 26.Wada K, Itoga K, Okano T et al (2011) Hippo pathway regulation by cell morphology and stress fibers. Development 138:3907–3914. 10.1242/dev.070987 [DOI] [PubMed] [Google Scholar]
- 27.Nakajima H, Yamamoto K, Agarwala S et al (2017) Flow-dependent endothelial YAP regulation contributes to vessel maintenance. Dev Cell 40:523–536. 10.1016/j.devcel.2017.02.019 [DOI] [PubMed] [Google Scholar]
- 28.Codelia VA, Sun GP, Irvine KD (2014) Regulation of YAP by mechanical strain through JNK and Hippo signaling. Curr Biol 24:2012–2017. 10.1016/j.cub.2014.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calvo F, Ege N, Grande-Garcia A et al (2013) Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol 15:637–646. 10.1038/ncb2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dupont S, Morsut L, Aragona M et al (2011) Role of YAP/TAZ in mechanotransduction. Nature 474:179–183. 10.1038/nature10137 [DOI] [PubMed] [Google Scholar]
- 31.Johnson GL, Lapadat R (2002) Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298:1911–1912. 10.1126/science.1072682 [DOI] [PubMed] [Google Scholar]
- 32.Lin KC, Moroishi T, Meng ZP et al (2017) Regulation of Hippo pathway transcription factor TEAD by p38 MAPK-induced cytoplasmic translocation. Nat Cell Biol 19:996–1002. 10.1038/ncb3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L, Luo JY, Li BC et al (2016) Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature 540:579–582. 10.1038/nature20602 [DOI] [PubMed] [Google Scholar]
- 34.Huang DS, Li XJ, Sun L et al (2016) Regulation of Hippo signalling by p38 signalling. J Mol Cell Biol 8:328–337. 10.1093/jmcb/mjw036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun GP, Irvine KD (2013) Ajuba family proteins link JNK to Hippo signaling. Sci Signal 292:ra81. 10.1126/scisignal.2004324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reddy BVVG, Irvine KD (2013) Regulation of Hippo signaling by EGFR-MAPK signaling through Ajuba family proteins. Dev Cell 24:459–471. 10.1016/j.devcel.2013.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seo J, Kim J (2018) Regulation of Hippo signaling by actin remodeling. BMB Rep 51:151–156. 10.5483/bmbrep.2018.51.3.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crisp M, Liu Q, Roux K et al (2006) Coupling of the nucleus and cytoplasm: role of the LINC complex. J Cell Biol 172:41–53. 10.1083/jcb.200509124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xue GD, Hemmings BA (2013) PKB/Akt-dependent regulation of cell motility. J Natl Cancer Inst 105:393–404. 10.1093/jnci/djs648 [DOI] [PubMed] [Google Scholar]
- 40.Xu J, Huang Y, Zhao JM et al (2021) Cofilin: a promising protein implicated in cancer metastasis and apoptosis. Front Cell Dev Biol 9:599065–599075. 10.3389/fcell.2021.599065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kobayashi M, Nishita M, Mishima T et al (2006) MAPKAPK-2-mediated LIM-kinase activation is critical for VEGF-induced actin remodeling and cell migration. EMBO J 25:713–726. 10.1038/sj.emboj.7600973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Z, Wu HJ, Jiang KW et al (2016) MAPK-mediated YAP activation controls mechanical-tension-induced pulmonary alveolar regeneration. Cell Rep 16:1810–1819. 10.1016/j.celrep.2016.07.020 [DOI] [PubMed] [Google Scholar]
- 43.Qian X, He LL, Hao M et al (2021) YAP mediates the interaction between the Hippo and PI3K/Akt pathways in mesangial cell proliferation in diabetic nephropathy. Acta Diabetol 58:47–62. 10.1007/s00592-020-01582-w [DOI] [PubMed] [Google Scholar]
- 44.Dai XM, She PL, Chi FT et al (2013) Phosphorylation of angiomotin by LATS1/2 kinases inhibits F-actin binding, cell migration, and angiogenesis. J Biol Chem 288:34041–34051. 10.1074/jbc.M113.518019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yi CL, Shen ZW, Stemmer-Rachamimov A et al (2013) The p130 isoform of angiomotin is required for YAP-Mediated hepatic epithelial cell proliferation and tumorigenesis. Sci Signal 6:ra77. 10.1126/scisignal.2004060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moleirinho S, Hoxha S, Mandati V et al (2017) Regulation of localization and function of the transcriptional co-activator YAP by angiomotin. Elife 6:e23966. 10.7554/eLife.23966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang PH, Schaffer DV, Kumar S (2020) Angiomotin links ROCK and YAP signaling in mechanosensitive differentiation of neural stem cells. Mol Biol Cell 31:386–396. 10.1091/mbc.E19-11-0602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boeri L, Albani D, Raimondi MT et al (2019) Mechanical regulation of nucleocytoplasmic translocation in mesenchymal stem cells: characterization and methods for investigation. Biophys Rev 11:817–831. 10.1007/s12551-019-00594-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mana-Capelli S, McCollum D (2018) Angiomotins stimulate LATS kinase autophosphorylation and act as scaffolds that promote Hippo signaling. J Biol Chem 293:18230–18241. 10.1074/jbc.RA118.004187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mana-Capelli S, Paramasivam M, Dutta S et al (2014) Angiomotins link F-actin architecture to Hippo pathway signaling. Mol Biol Cell 25:1676–1685. 10.1091/mbc.E13-11-0701 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.