Abstract
Misalignment of feeding time with the light/dark environment leads to disrupted peripheral circadian clocks and obesity. Conversely, restricting feeding to the active period mitigates metabolic syndrome through mechanisms that remain unknown. Here we show that adipocyte thermogenesis is essential for the healthful metabolic response to time-restricted feeding. Genetic enhancement of adipocyte thermogenesis through ablation of ZFP423 attenuates obesity caused by mistimed high-fat diet feeding through a mechanism involving creatine metabolism. Circadian control of adipocyte creatine metabolism underlies the timing of diet-induced thermogenesis, and enhancement of adipocyte circadian rhythms through overexpression of the clock activator BMAL1 ameliorates metabolic complications during diet-induced obesity. These findings establish creatine-mediated diet-induced thermogenesis as a bioenergetic mechanism driving metabolic benefits during time-restricted feeding.
One-Sentence Summary:
Circadian clock control of adipocyte thermogenesis underlies metabolic benefits during time restricted feeding.
Fundamental to the development of obesity is the imbalance between energy intake and expenditure in the setting of overnutrition. Overconsumption of food is also associated with disrupted endogenous circadian rhythms in meal timing and metabolism (1). Genetic disruption of the molecular circadian clock leads to increased consumption of food during the light period when animals are typically sleeping, and exaggerated diet induced obesity (2). Feeding at the wrong time of day exacerbates diet-induced obesity (3), whereas restricting calorie-dense diet to limited time windows during the normal active period (night for nocturnal rodents and day for humans) improves metabolic health (4, 5). This indicates that misalignment of feeding rhythms with autonomous energetic cycles contributes to diet-induced obesity and metabolic syndrome through mechanisms that remain unknown.
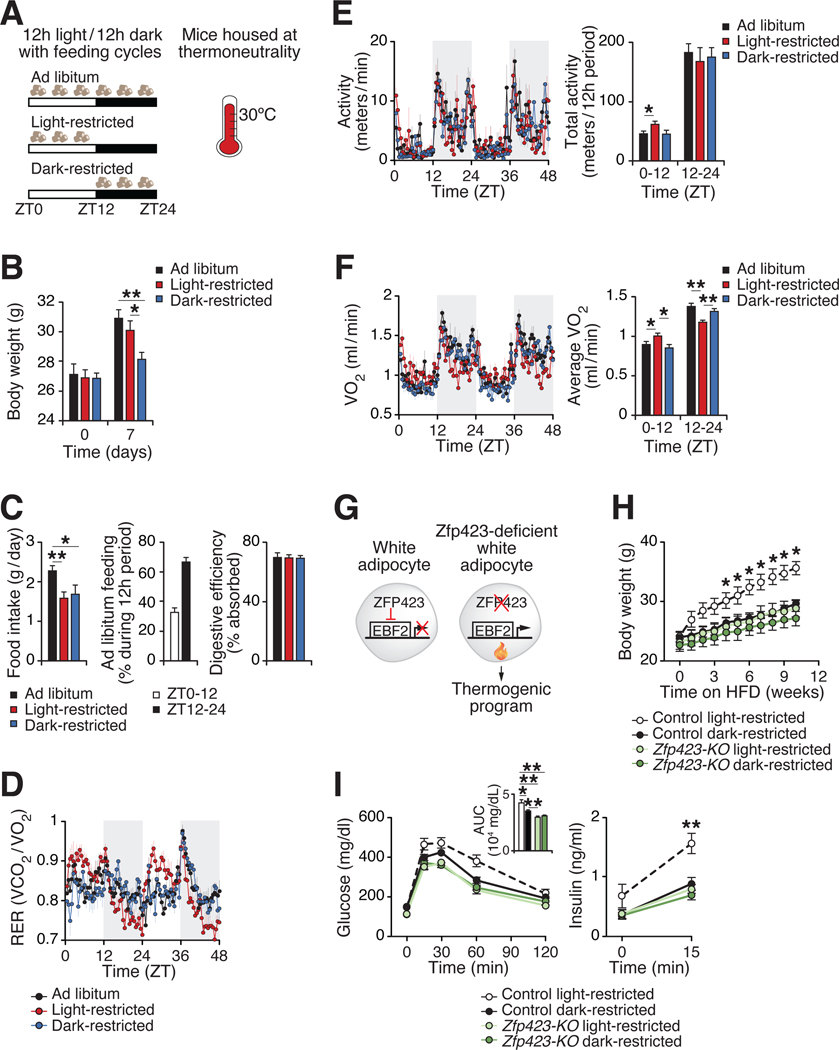
Within as early as one week following the provision of high fat diet (HFD) exclusively during the light/inactive period, mice exhibit increased weight gain compared to isocaloric HFD provided during the dark/active period (3). We sought to determine whether this initial effect of circadian mistimed feeding during time-restricted feeding (TRF) involves altered energy expenditure. To reduce the stimulation of thermogenesis that occurs under standard animal housing temperatures (22–25C) (6), we placed mice at thermoneutrality (30°C). Monitoring mice at thermoneutrality unmasks the contribution of diet-induced thermogenesis to energy balance by amplifying pathways independent of body temperature maintenance that contribute to whole-body metabolic rate (6). We provided HFD to mice ad libitum, restricted to the light period (ZT0–12), or restricted to the dark period (ZT12–24) for one week (Fig. 1A). Mice fed ad libitum HFD displayed increased food intake and weight gain, associated with ~30% feeding extended into the light period as compared to 15–20% on regular chow (Fig. 1B–C) (1). However, mice fed HFD restricted to the light period showed increased weight gain and altered RER rhythms as compared to mice fed HFD restricted to the dark period, despite similar total food intake and digestive efficiency (Fig. 1B–D). Strikingly, mice fed HFD restricted to the light period expended less energy expenditure during the active (dark) period compared to animals fed during the dark period, despite similar activity rhythms (Fig. 1E–F). These results suggest that mismatch between the feeding cycle and the light/dark cycle leads to weight gain through a mechanism involving reduced diet-induced energy expenditure.
Figure 1. Circadian mistiming of feeding promotes obesity through reducing energy expenditure.
(A) Experimental design depicting the timing of high fat diet (HFD) access as Ad libitum, restricted to the light period (ZT0–12; “Light-restricted”) or restricted to the dark period (ZT12–24; “Dark-restricted”) for one week in wildtype male mice at thermoneutrality (30°C). (B) Body weight of mice fed ad libitum, light-restricted, or dark-restricted HFD at day 0 and 7 of HFD (n=5). (C) Average daily food intake of mice, average feeding distribution for ad libitum HFD fed mice from days 5–7 of HFD, and digestive efficiency at days 6–7 of HFD (n=5). (D-F) RER rhythms (D), activity rhythms and average total activity over 12 hours (E), and VO2 rhythms and average VO2 levels over 12 hours (F) during days 5–7 of HFD (n=5). (G) Model of adipocyte-specific deletion of ZFP423 to drive thermogenic programming in white adipocytes. (H) Body weight during isocaloric light-restricted or dark-restricted HFD for 10 weeks in control (Adiponectm-rtTA;Zfp423flox//lox) and Zfp423-KO (Adiponectin-rtTA;TRE-Cre;Zfp423flox//lox) male mice (n=5–6). (I) Glucose tolerance test (GTT), area under curve (AUC) during the GTT, and insulin during the GTT at ZT2 at 10 weeks of HFD (n=5–6). Data are represented as mean ± SEM (*p < 0.05, **p < 0.01, ***p < 0.001).
Insight into the link between overnutrition and circadian disruption originated with the discovery that HFD leads to abnormal circadian behavioral rhythms and inhibition of core clock and metabolic genes in white adipose tissue (WAT) (l). To evaluate whether short-term manipulation of feeding time affects the clock in adipose tissue, we analyzed gene expression at ZT0 and ZT12, corresponding to the peak and trough of Bmall RNA expression, in inguinal WAT (iWAT) and brown adipose tissue (BAT) (l, 7). Mice fed HFD exclusively during the dark period displayed the highest amplitude of core clock gene expression, whereas ad libitum and light-restricted HFD feeding led to reduced expression of clock expression in iWAT and BAT (Fig. S1A–B). Furthermore, expression of uncoupling protein 1 (UCP1) was reduced in iWAT and BAT from light-restricted fed mice. These data suggest that disrupted adipocyte circadian and thermogenic rhythms may underlie the negative metabolic consequences of circadian mistimed feeding.
The adipocyte circadian clock is required for maintenance of thermogenic rhythms in body temperature (7). To determine whether increasing adipocyte thermogenesis can prevent weight gain and improve health during circadian mistimed feeding, we utilized mice with genetically enhanced thermogenesis. Inducible deletion of adipocyte Zfp423 in adult mice represents a more robust thermogenic stimulus as compared to over-expression of the transcriptional thermogenic activators EBF2, PRDM16, or ZFP516, with the strongest activation of thermogenesis occurring at standard housing temperature (22–25 °C) (8). Therefore, we performed TRF in mice with inducible pan adipocyte-specific deletion of the anti-thermogenic transcription factor Zfp423 at room temperature, which leads to widespread accumulation of beige adipocytes in WAT and activation of thermogenesis (Fig. 1G) (9). We found that adipocyte-specific deletion of Zfp423 in adult mice prevented weight gain and improved glucose tolerance during light-restricted isocaloric HFD feeding (Fig. 1H–I, S2). This indicates that enhancing adipocyte thermogenesis is sufficient to improve health during circadian mistimed feeding. Next, we assessed whether adipocyte thermogenesis is required for the metabolic benefits of feeding restricted to the optimal time of day. Inducible overexpression of Zfp423 in all adipocytes in adult mice directs thermogenic adipose tissue to adopt a white adipocyte-like phenotype (9). We found that mice with transgenic expression of Zfp423 specifically in brown and beige (UCP1+) adipocytes gained more weight and had worsened glucose tolerance than control mice when fed isocaloric HFD restricted to the dark period (Fig. S3). These data reveal adipocyte thermogenesis is essential for the healthful benefits during feeding restricted to the optimal circadian time.
ZFP423 acts as a co-repressor of EBF2 activity and inhibits the thermogenic transcriptional program, including the pathway involved in adrenergic activation of UCP1 (9). However, the metabolic mechanisms through which deletion of Zfp423 promotes thermogenesis remains unknown. The major adipocyte thermogenic pathways include proton leak through UCP1, creatine-substrate cycling, calcium-dependent ATP hydrolysis, and lipid cycling (10). To explore the metabolic mechanisms underlying enhanced thermogenesis in Zfp423-knockout (KO) adipocytes, we performed bioenergetic and metabolomics analyses in primary adipocytes differentiated from the stromal vascular fraction (SVF) of the inguinal WAT depot. Zfp423-KO adipocytes displayed increased thermogenic gene expression, increased basal oxygen consumption due to uncoupling, and enhanced norepinephrine-stimulated respiration (Fig. S4, 2A). Isotopic tracing with U-13C-glucose indicated that adipocytes stimulated with norepinephrine have increased glycolytic flux (Fig. 2B), consistent with recent data in brown adipocytes stimulated with a beta-3 adrenergic receptor agonist (11). Zfp423-KO adipocytes also showed increased incorporation of U-13C-glucose into glycolytic intermediates and end products from U-13C-glucose, including M+3 pyruvate and lactate (Fig. 2B). The enhanced glycolytic flux in Zfp423-KO adipocytes mirrored the response to pharmacological activation of the beta-3-adrenergic receptor.
Figure 2. Genetic disinhibition of adipocyte thermogenesis enhances glycolytic flux and creatine metabolism.
(A) Oxygen consumption rate of adipocytes differentiated from inguinal WAT stromal vascular cells from control and adipocyte Zfp423-KO mice. After 3 basal recordings, 100 nM norepinephrine was added onto the cells (n=5). (B) Labeling schematic of U-13C-glucose tracing into glycolysis and the TCA cycle. Isotopic labeling profile of m+6 glucose, m+6 glucose-6-phosphate (G6P), m+3 pyruvate, and m+3 lactate following addition of U-13C-glucose in the presence or absence of 100 nM norepinephrine for 5 hours in differentiated adipocytes from control and adipocyte Zfp423-KO mice (n=6). (C) Heatmap of differentially abundant metabolites (p<0.05) in differentiated adipocytes (n=6). (D) Relative abundance of ATP, ADP, creatine, and phosphocreatine in differentiated adipocytes (n=6). (E) Phosphocreatine to creatine (PCr/Cr) ratio in differentiated adipocytes (left) from control and Zfp423-KO mice and iWAT (right) from 3-month-old male control and adipocyte Zfp423-KO mice after 4 weeks of dox-chow harvested at ZT2 (light period) and ZT14 (dark period) (n=4). Data are represented as mean ± SEM (*p < 0.05, **p < 0.01, ***p < 0.001).
We next performed metabolomic profiling to assess whether steady state metabolites differ following adipocyte-specific ablation of Zfp423. We identified 30 differentially abundant metabolites in Zfp423-KO compared to control adipocytes, including increased levels of creatine, pyruvate, lactate, and carnitine, and decreased levels of phosphocreatine and ATP (Fig. 2C–D). Recent work has demonstrated that elevating creatine synthesis, import, and cycling fuels a futile cycle of mitochondrial ATP turnover in thermogenic cells (12–14). The phosphocreatine/creatine (PCr/Cr) ratio was significantly lowered in Zfp423-KO adipocytes, mirroring the response of adrenergic stimulus with norepinephrine (Fig. 2E). Additionally, the PCr/Cr ratio is reduced in iWAT of ad libitum chow-fed Zfp423-KO mice (Fig. 2E). These data reveal that deletion of Zfp423 fuels adipocyte thermogenesis through increasing uncoupled respiration, enhancing norepinephrine-stimulated respiration, elevating glucose flux into glycolysis, and stimulating creatine cycling. Therefore, the transcriptional program regulated by ZFP423 encompasses a wide range of downstream outputs that control UCPl-dependent and UCPl-independent thermogenic programs.
We found that deletion of Zfp423 in adipocytes leads to a robust increase in creatine cycling and protection from obesity during circadian mistimed feeding. This led us to hypothesize that creatine metabolism may be rhythmically regulated, with enhanced synthesis and/or cycling during the dark period when nocturnal mice eat. Indeed, the PCr/Cr ratio was decreased in adipose tissue during the dark period (ZT14) compared to the light period (ZT2) in ad libitum chow-fed mice (Fig. 2E), indicative of enhanced creatine cycling in vivo during the active/feeding period. Protein levels of creatine kinase B (CKB), the major kinase isoenzyme in the futile creatine cycle in thermogenic fat, were also enriched in iWAT of adipocyte Zfp423-KO mice during ad libitum HFD feeding (Fig. S5) (14).
To uncover adipocyte circadian chromatin architecture underlying timing of metabolic outputs, we performed assay for transposase-accessible chromatin with sequencing (ATAC-seq) in fluorescently-labeled adipocyte nuclei from WAT and BAT isolated every 4 hours throughout the 24 hour day from Adiponectin-Cre;NuTRAPfx/+ mice housed at thermoneutrality (Fig. 3A). We analyzed the subcutaneous iWAT depot and the interscapular BAT depot specifically to identify distinct rhythmic patterns of chromatin regulation in white/beige and brown adipocytes. We identified 83,322 nucleosome-free regions (“peaks”) in WAT and 112,985 peaks in BAT, with the majority of accessible peaks located in intergenic and intronic enhancer regions (79.7% in iWAT, 82.7% in BAT) (Fig. S6A). After filtering out low-count peaks, we performed JTK_cycle analysis using normalized read counts across 25,050 WAT and 42,572 BAT peaks and identified genome-wide oscillations in adipocyte chromatin accessibility (see Methods). In BAT, we identified 16.0% of ATAC-seq peaks, or 6,829 total peaks oscillate with a broad distribution throughout the day, and maximal openness occurring during the dark period (Fig. 3B, Table S1). In iWAT, we found 12.7% of peaks (3,172 peaks total) oscillate with a biphasic pattern of accessibility (Fig. 3B, Table S2). In BAT and iWAT, we found that adipocyte chromatin accessibility exhibits dynamic opening across the light/dark cycle in canonical circadian genes and UCP1 (Fig. 3C–D, S6B–C). Principal component analysis (PCA) of normalized reads at rhythmic peaks highlighted the robust and cyclical 24-hr patterns in chromatin opening in both BAT and iWAT genome-wide (Fig. S6D).
Figure 3. Rhythmic chromatin profiling in adipocytes reveals distinct phases of accessibility in BAT and iWAT.
(A) Following Cre expression, Adiponectin-Cre;NuTRAPfc/+ mice express nuclear membrane labeled with mCherry and biotin and labeling of the translating mRNA polysome complex with EGFP-fused ribosomal protein L10a. Adipocyte nuclei from BAT and iWAT of male Adiponectin-Cre;NuTRAPfc/+ mice housed at thermoneutrality were isolated every 4 hours throughout the 24-hour day (ZT1, 5, 9, 13, 17, 21) by FACS for ATAC-seq analysis. (B) Heatmaps showing rhythmic activation of adipocyte chromatin in BAT and WAT (n=3 per timepoint) and radial histograms showing the phases of maximal accessibility for rhythmic peaks within each 2-hr window. (C) Overlap of genes near oscillating peaks in BAT and WAT. (D) Rhythmic opening of chromatin at the circadian Cryl and thermogenic Ucpl enhancer with location of BMAL1 motifs. (E) Motif enrichment at oscillating peaks separated by phase of maximal accessibility in BAT and WAT throughout the day.
To understand the molecular program underlying the circadian opening of chromatin, we performed motif enrichment analyses at oscillating peaks at a 2-hour resolution and found strong rhythmic chromatin opening in areas occupied by transcription factors including PPARγ, EBF, ESRRβ, CEBP/GR/ARE, and RORγ that regulate bioenergetic, adipogenic, and inflammatory gene networks in both WAT and BAT (Fig. 3E). We also identified BMAL1 motifs at rhythmic accessible regions (± 1 kb) approximate to genes involved in creatine metabolism such as Ckb (P < 0.05 in iWAT), the rate-limiting enzyme in creatine biosynthesis, glycine amidinotransferase (Gatm) (P < 0.05 in BAT), and methionine adenosyltransferase 2A (Mat2a) (P < 0.05 in iWAT), which generates S-adenosylmethionine (SAM) for creatine synthesis (Fig. S6E) (12,14).
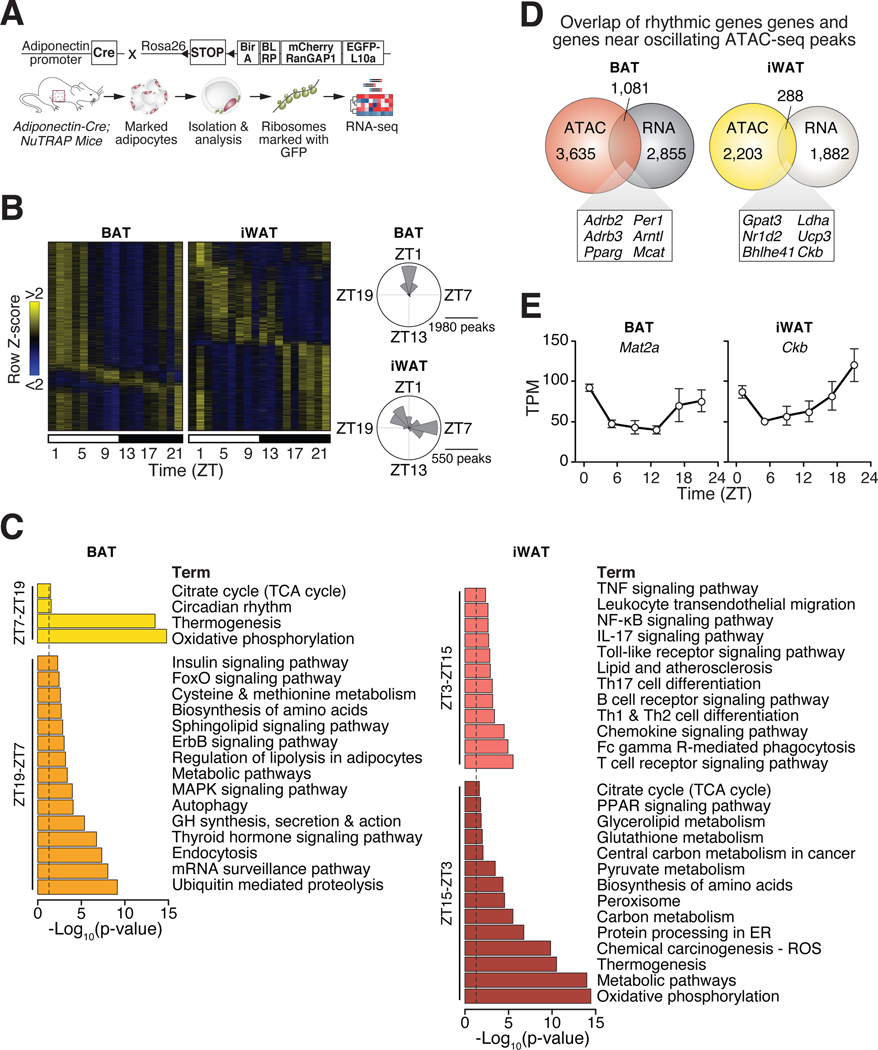
We next investigated whether the genome-wide changes in chromatin accessibility correspond to time-of-day dependent alterations in the adipocyte transcriptome through RNA-sequencing in animals maintained at thermoneutrality. We isolated adipocyte-specific translating RNA from BAT and iWAT of Adiponectin-Cre;NuTRAP mice and performed RNA-sequencing (Fig. 4A). We found expected enrichment of adipocyte- and depot-specific gene expression in BAT and iWAT (Fig. S7). We identified 3,936 oscillating transcripts (28.0% of genes) in BAT by JTK_cycle analysis at an adjusted p < 0.05 and observed one major phase of maximal amplitude during ZT19–7 and a smaller phase in oscillating genes during ZT7–19 (Fig. 4B, Table S3). KEGG pathway analysis revealed enrichment of genes involved in endocrine signaling (thyroid hormone pathway and insulin signaling), metabolism pathways (regulation of lipolysis, sphingolipid signaling, and methionine metabolism), and cellular maintenance pathways (proteolysis and autophagy) during ZT19–7 (Fig. 4C). During the opposite phase of peak rhythmic RNA transcripts (ZT7–19), we identified KEGG pathways related to TCA cycle, thermogenesis, oxidative phosphorylation, and circadian rhythm (Fig. 4C). In iWAT, we identified 2,170 oscillating transcripts (17.3% of genes) through JTK_cycle analysis with an adjusted p < 0.05 and observed two phases of maximal amplitude in oscillating genes, similar to the two major phases in oscillating ATAC-seq peaks (Fig. 4B, 3B, Table S4). During phase ZT3–15, we identified KEGG pathways mostly involved in inflammation (TNF, NFκB, and Toll-like receptor signaling, leukocyte transendothelial migration, and T cell signaling); whereas during phase ZT15–3 we observed several pathways involved in metabolism (oxidative phosphorylation, thermogenesis, pyruvate and carbon metabolism, ROS, and TCA cycle) (Fig. 4C).
Figure 4. Ribosomal RNA profiling reveals diurnal control of adipocyte metabolism.
(A) Adipocyte ribosomal RNA for RNA-sequencing was isolated from BAT and iWAT harvested every 4 hours throughout the 24-hour day (ZT1, 5, 9, 13, 17, 21) from male Adiponectin-Cre;NuTRAPfx/+ mice housed at thermoneutrality. (B) Heatmaps showing rhythmic adipocyte RNA expression in BAT and iWAT (n=3 per timepoint) and radial histograms showing the number of genes whose oscillations peak within each 2-hr window in BAT and iWAT. (C) KEGG pathway analysis of oscillating genes in BAT from ZT7–19 and ZT19–7 and in WAT from ZT3–15 and ZT15–3. (D) Overlap of rhythmic genes identified through RNA-seq and genes near oscillating ATAC-seq peaks. (E) Examples of rhythmic gene expression (shown in transcripts per million “TPM”) identified through RNA-seq in BAT and iWAT (n=3 per timepoint).
Analysis of overlap of rhythmic genes identified through RNA-seq and genes near oscillating ATAC-seq peaks revealed 1,016 common genes in BAT and 285 common genes in iWAT (Fig. 4D, Table S5). Several of these rhythmic genes in BAT are components of the core molecular clock (Arntl, Perl, Per3, Nrldl, Nr1d2), factors involved in adrenergic activation (Adrb2, Adrb3, Crebl), brown adipocyte transcription factors (Ebfl, Ebf3) and metabolic enzymes (Ldha, Pcx, Pdk4). In iWAT, we observed overlap of genes involved in circadian rhythms (Dbp, Ncor2, Nrld2), inflammation (Ccl5, Irf4), and metabolism and oxidative stress (Cs, Ldha, Sodl, Ucp3). In addition, we identified significant overlap in genes involved in creatine metabolism between rhythmic analysis of ATAC-seq and RNA-seq, including Mat2a in BAT (p < 0.01) and Ckb in iWAT (p < 0.01) (Fig. 4E). Collectively, these reveal robust rhythmicity of the adipocyte circadian clock and the creatine metabolic pathway throughout the day regulated at the genomic and transcriptomic levels.
Our rhythmic ATAC-seq and RNA-seq analyses suggest that the adipocyte clock is integrated into BAT and WAT programing at the level of bioenergetic transcription factor activation and/or through CLOCK/BMAL1-dependent activation of creatine metabolism. To test this, we analyzed gene expression and metabolite levels in primary adipocytes lacking Bmall (Bmall-KO). We identified reduced expression of genes involved in creatine metabolism, decreased abundance of creatine, and an increase in the PCr/Cr ratio in Bmall-KO adipocytes (Fig. S8A–C). We observed similar results in vivo in iWAT from ad libitum chow-fed adipocyte-specific Bmall -KO mice (Fig. S8D–G). Interestingly, we also saw significant decreases in the level of SAM and expression of Mat2a in iWAT of adipocyte-specific Bmall-KO mice (Fig. S8F). Creatine synthesis from guanidinoacetate consumes over 40% of all methyl groups (15); therefore, the reduced adipocyte creatine levels in the absence of BMAL1 may be in part due to limited SAM synthesis. Collectively, our genomic and metabolomic findings indicate that the adipocyte molecular clock generates rhythmic cycles of creatine metabolism in alignment with the environmental light/dark cycle.
To evaluate whether clock-controlled rhythms in creatine metabolism underlie enhanced thermogenesis during TRF, we utilized mice lacking BMAL1 specifically in adipocytes (Bmal1-KO). It was previously shown that these mice have increased food intake during the light period and gain more weight during ad-libitum HFD feeding at room temperature (16). We placed adipocyte Bmal1-KO mice at thermoneutrality and provided isocaloric HFD restricted to the dark or light period. As expected, control mice gained more weight during light-restricted feeding compared to dark-restricted feeding (Fig. S9A). However, adipocyte Bmal1-KO mice fed isocaloric HFD restricted to the light or dark period gained equal weight and had similar glucose tolerance as control light-restricted fed mice (Fig. S9A–B). These results reveal that the adipocyte clock is essential for metabolic benefits during TRF to the dark period, perhaps due to decreased creatine cycling. To investigate whether elevating adipose tissue creatine in mice lacking adipocyte BMAL1 can promote diet-induced thermogenesis, we fed control and Bmal1-KO mice HFD supplemented with creatine. Remarkably, creatine supplementation rescued the effects on weight gain, glucose homeostasis, and levels of creatine and SAM in mice lacking a functional adipocyte clock despite no change in food intake or activity (Fig. 5A–C, S9).
Figure 5. The adipocyte clock regulates metabolic health through creatine metabolism.
(A) Body weight of control (Bmalflox/flox) and Bmal1-KO (Adiponectin-Cre;Bmal1flox/flox) male mice fed ad libitum HFD supplemented with 2% creatine for 6 weeks at thermoneutrality (n=5) with average daily food intake and % of feeding during the light period from weeks 5–6 of HFD. (B-C) Average daily activity (B) detected by infrared sensors during week 5–6 of HFD feeding and relative metabolite abundance (C) in iWAT after 6 weeks of HFD (n=5). (D) Experimental design depicting mice with doxycycline-inducible transgenic expression of the clock activator Bmal1 in adipocytes have enhanced amplitude of core clock expression. (E) Body weight of control (Adiponectin-rtTA) and Bmal1-Tg (Adiponectin-rtTA;TRE-Bmal1) mice during 6 weeks of ad libitum HFD feeding at thermoneutrality (n=8). (F) Body composition and adipose tissue weights after 6 weeks of HFD (n=8). (G) VO2 rhythms and average VO2 levels from weeks 5–6 of HFD (n=8). (H) Glucose tolerance test, AUC, and insulin during the GTT at ZT2 at 6 weeks of HFD (n=8). (I- J) Expression of circadian and creatine metabolism genes (I) and the PCr/Cr ratio (J) in iWAT after 6 weeks of HFD (n=8). Data are represented as mean ± SEM (*p < 0.05, **p < 0.01, ***p < 0.001).
HFD feeding markedly dampens clock gene expression rhythms in a tissue-specific manner, particularly in adipose tissue (17). We hypothesize that reduced adipocyte clock function is a significant driver of metabolic defects in HFD-fed mice potentially through disruption of rhythmic creatine metabolism. To test this, we generated mice with inducible adipocyte-specific transgenic expression of BMAL1 (Bmal1-Tg). Constitutive expression of the clock activator BMAL1 in adipocytes is sufficient to enhance clock rhythms through increasing amplitude of expression of core clock genes (Fig. S10A, 4D) (18). When adipocyte Bmal1-Tg mice are placed on HFD at thermoneutrality, they gained less weight than control mice and had significantly increased energy expenditure with similar feeding and activity rhythms (Fig. 4E–G, S10B–C). We also saw improved metabolic health through glucose tolerance testing in adipocyte Bmal1-Tg mice (Fig. 4H). Additionally, adipocyte Bmal1-Tg mice had increased creatine cycling and increased expression of Ckb and Gatm in iWAT (Fig. 4I–J). Therefore, amplifying core molecular clock rhythms in adipocytes is sufficient to enhance energy expenditure and reduce weight gain.
The results provided here establish that misalignment of feeding time with intrinsic cycles of diet-induced adipocyte thermogenesis contribute to metabolic syndrome in the setting of overnutrition. Our analyses build upon advances in the identification of transcriptional regulators of adipose ontogeny that have established a major role for ZPF423 in suppressing thermogenic capacity through inhibition of EBF2 (9, 19). We identify energy dissipation through the creatine futile cycle as a thermogenic mechanism in adipocytes lacking ZFP423.
Our analyses also circumvent a common challenge in bioenergetic experiments through performing studies at thermoneutrality, where adipose tissue energy cycles track with feeding rather than thermal stress. Prior studies show that UCP1 is under control of the core molecular clock (7), consistent with our finding that enhanced thermogenesis during alignment of feeding time with the dark cycle requires an intact adipocyte molecular clock. Our observation that creatine supplementation counters the obesogenic effect of adipocyte clock ablation indicates that the etiology of weight gain caused by mistimed feeding rhythms involves impaired creatine-induced thermogenesis during obesity, when adipocyte clock rhythms are dampened (17). Our analyses establish a primary role for the adipocyte clock in diet-induced thermogenesis since augmentation of adipocyte circadian function is sufficient to attenuate diet-induced obesity.
In settings where humans experience rapid or frequent shifts in feeding schedules due to shiftwork, sleep loss, or exposure to blue light, misalignment between feeding and the endogenous circadian phase of adipose thermogenesis may exacerbate metabolic morbidity. We propose circadian rhythms in adipocyte diet-induced thermogenesis through futile creatine cycling as a major metabolic mechanism through which time-restricted feeding improves metabolic health.
Supplementary Material
Acknowledgements
We thank all members of the Bass, Barish, and Beutler laboratories for helpful discussions. We also thank Joseph Takahashi for kindly providing the TRE-Bmal1 mice. The authors thank the Northwestern University Metabolomics Core, NUSeq Core, and Mouse Histology and Phenotyping Laboratory Core for excellent guidance and assistance with experiments performed here.
Funding:
Research support was from the NIH National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants R01DK127800, R01DK113011, and R01DK090625, and the National Institute on Aging (NIA) grant R01AG065988 and P01AG011412 (to J.B.); NIDDK grant F32DK122675 (to C.H.); NIDDK grant F30DK116481 (to B.J.W.), NIDDK grant F31DK130589 (to N.J.W.), NIDDK grant K99DK124682 to (J.C.), AHA CDA 19CDA34670007 to M.S., and NIDDK grants R01DK104789 and R01DK119163 to (R.K.G.).
Footnotes
Competing interests: The authors declare they have no competing financial interests.
Data and materials availability: Data in this study is publicly available in the GEO repository (GSE181443).
References and Notes
- 1.Kohsaka A. et al. , High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 6, 414–421 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Turek FW et al. , Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308, 1043–1045 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW, Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 17, 2100–2102 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatori M. et al. , Time-Restricted Feeding without Reducing Caloric Intake Prevents Metabolic Diseases in Mice Fed a High-Fat Diet. Cell Metab 15, 848–860 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cienfuegos S. et al. , Effects of 4- and 6-h Time-Restricted Feeding on Weight and Cardiometabolic Health: A Randomized Controlled Trial in Adults with Obesity. Cell Metab 32, 366–378 e363 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J, UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 9, 203–209 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Gerhart-Hines Z. et al. , The nuclear receptor Rev-erbalpha controls circadian thermogenic plasticity. Nature, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shao M, Gupta RK, Transcriptional brakes on the road to adipocyte thermogenesis. Biochim Biophys Acta Mol Cell Biol Lipids 1864, 20–28 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Shao M. et al. , Zfp423 Maintains White Adipocyte Identity through Suppression of the Beige Cell Thermogenic Gene Program. Cell Metab 23, 1167–1184 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chouchani ET, Kazak L, Spiegelman BM, New Advances in Adaptive Thermogenesis: UCP1 and Beyond. Cell Metab 29, 27–37 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Panic V. et al. , Mitochondrial pyruvate carrier is required for optimal brown fat thermogenesis. Elife 9, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kazak L. et al. , Genetic Depletion of Adipocyte Creatine Metabolism Inhibits Diet-Induced Thermogenesis and Drives Obesity. Cell Metab 26, 660–671 e663 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kazak L. et al. , Ablation of adipocyte creatine transport impairs thermogenesis and causes diet-induced obesity. Nat Metab 1, 360–370 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahbani JF et al. , Creatine kinase B controls futile creatine cycling in thermogenic fat. Nature 590, 480–485 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brosnan JT, da Silva RP, Brosnan ME, The metabolic burden of creatine synthesis. Amino Acids 40, 1325–1331 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Paschos GK et al. , Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat Med 18, 1768–1777 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong HK et al. , Requirement for NF-kappaB in maintenance of molecular and behavioral circadian rhythms in mice. Genes Dev 32, 1367–1379 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDearmon EL et al. , Dissecting the functions of the mammalian clock protein BMAL1 by tissue-specific rescue in mice. Science 314, 1304–1308 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seale P. et al. , Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest 121, 96–105 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Acosta-Rodriguez VA, de Groot MHM, Rijo-Ferreira F, Green CB, Takahashi JS, Mice under Caloric Restriction Self-Impose a Temporal Restriction of Food Intake as Revealed by an Automated Feeder System. Cell Metab 26, 267–277 e262 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grobe JL, Comprehensive Assessments of Energy Balance in Mice. Methods Mol Biol 1614, 123–146 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hepler C. et al. , Directing visceral white adipocyte precursors to a thermogenic adipocyte fate improves insulin sensitivity in obese mice. Elife 6, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heinrich P. et al. , Correcting for natural isotope abundance and tracer impurity in MS-, MS/MS- and high-resolution-multiple-tracer-data from stable isotope labeling experiments with IsoCorrectoR. Sci Rep 8, 17910 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roh HC et al. , Simultaneous Transcriptional and Epigenomic Profiling from Specific Cell Types within Heterogeneous Tissues In Vivo. Cell Rep 18, 1048–1061 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corces MR et al. , An improved ATAC-seq protocol reduces background and enables interrogation of frozen tissues. Nat Methods 14, 959–962 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ, Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods 10, 1213–1218 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levine DC et al. , NAD(+) Controls Circadian Reprogramming through PER2 Nuclear Translocation to Counter Aging. Mol Cell 78, 835–849 e837 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hughes ME, Hogenesch JB, Kornacker K, JTK_CYCLE: an efficient nonparametric algorithm for detecting rhythmic components in genome-scale data sets. J Biol Rhythms 25, 372–380 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leporcq C. et al. , TFmotifView: a Webserver for the visualization of transcription factor motifs in genomic regions. Nucleic Acids Res 48, W208–W217 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.