Abstract
Background
Fentanyl is selected to manage pain in critical care patients on mechanical ventilation in the intensive care unit (ICU). However, the usefulness of fentanyl compared with other opioids is unknown. This study examined the evidence for using fentanyl to improve the clinical outcomes of ICU patients, using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system.
Methods
We searched the MEDLINE, Cochrane Central Register of Controlled Trials, and Igaku Chuo Zasshi databases in June 2021. Two independent assessors reviewed studies to identify randomized, controlled trials (RCTs) that compared the intravenous administration of fentanyl and other opioids in mechanically ventilated patients in the ICU. The study quality was assessed using the GRADE system and Cochrane methodology. The primary outcome was mortality. The secondary outcomes were the duration of mechanical ventilation, duration of the ICU stay, incidence of severe adverse events, and incidence of delirium. We integrated outcome data using a random-effects model and showed absolute values and certainty of evidence in the GRADE evidence profile.
Results
Seven RCTs met the study inclusion criteria with 534 patients (251 were treated with fentanyl and 283 with other opioids, including 242 with remifentanil and 41 with morphine). Among 191 participants from 2 RCTs, fentanyl was not associated with mortality (risk ratio [RR], 0.79; 95% confidence interval [CI], 0.24 to 2.60; low-quality evidence). Regarding the secondary outcomes, fentanyl did not shorten the duration of mechanical ventilation (mean difference, 0.49 h; 95% CI, − 0.90 to 1.88; moderate-quality evidence) or the duration of the ICU stay (mean difference, 7.04 h; 95% CI, − 3.27 to 17.35; moderate-quality evidence) compared with other opioids. Fentanyl did not increase the incidence of severe adverse events (RR, 0.98; 95% CI, 0.50 to 1.90; low-quality evidence) or delirium (RR, 1.27; 95% CI, 0.79 to 2.04; low-quality evidence).
Conclusions
Although fentanyl is a frequently administered opioid in the ICU, patients’ outcomes are not different between fentanyl use and use of other opioids. However, the GRADE evaluation provides little certainty to support the results of this systematic review. Therefore, further large RCTs are required to confirm our conclusions.
Trial registration
PROSPERO, CRD42019130648 (https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=130648).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12871-022-01871-7.
Keywords: Fentanyl, Opioid, Mechanical ventilation, Remifentanil, Morphine
Background
Pain management is an important issue for critically ill adults in the intensive care unit (ICU), and inadequate pain management may lead to posttraumatic stress disorder [1] and post-intensive care syndrome [2, 3]. The Pain, Agitation/Sedation, Delirium, Immobility, and Sleep disruption guidelines recommend a continuous infusion of opioids for procedural pain management in critically ill adults [4]. However, the same opioids (i.e., fentanyl, hydromorphone, morphine, and remifentanil) have consistently been recommended since 2013. There has been no systematic review on the use of opioids for pain management in critically ill adults [5]. Therefore, the appropriate analgesic drugs to use remain controversial.
Fentanyl is a potent, selective 4-anilidopiperidine μ-opioid analgesic used for analgesic management in mechanically ventilated patients in the ICU [6, 7]. Among the other available opioids, morphine has common adverse effects, such as histamine release, pruritus, and accumulation of morphine-6-glucuronide, in patients with renal impairment [8]. Additionally, neither alfentanil nor sufentanil is licensed for use in ICU patients in many countries [8]. Hydromorphone also appears to be used for mechanically ventilated patients in the ICU [9], but few studies have reported its usefulness. Moreover, remifentanil has been reported to slightly shorten the duration of mechanical ventilation, the time to extubation after ceasing sedation, and the ICU stay [10]. However, this drug is not commonly administered in the ICU because of its side effects of acute tolerance and hyperalgesia [11]. Therefore, fentanyl is a relatively common choice of opioid used in multinational, randomized, controlled trials (RCTs) [12] and especially in Japanese ICUs [13]. However, no systematic review of fentanyl intervention and no evidence of the benefits of fentanyl have been provided yet.
We hypothesized that fentanyl is associated with better outcomes than other opioids in mechanically ventilated adults in the ICU. Therefore, this meta-analysis evaluated the effects of fentanyl by analyzing the results of previous RCTs that compared fentanyl with other opioids and integrated the outcomes on the basis of the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system.
Materials and methods
This systematic review and meta-analysis were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [14] and the GRADE system [15]. The study was exempt from ethics review and did not require written informed consent. The study was registered with the International Prospective Register of Systematic Reviews (PROSPERO, number CRD42019130648). This systematic review was conducted as part of the revision of the Japanese Clinical Practice Guidelines for Management of Sepsis and Septic Shock 2020 (J-SSCG 2020). The present study was initially conducted in 2019 for the J-SSCG 2020, but we conducted a literature search again in 2021 to avoid double submissions.
Search strategy
We electronically searched the MEDLINE (PubMed), Cochrane Central Register of Controlled Trials (CENTRAL), and Igaku Chuo Zasshi (largest database of Japanese medical journals) databases in June 2021. We used search strategies according to the Cochrane Handbook for Systematic Reviews of Interventions [16]. Additionally, we searched for ongoing trials in ClinicalTrials.gov and the World Health Organization International Clinical Trials Platform Search Portal. The following keywords were used for the search strategy: “acute lung injury,” “critical care,” “multiple organ failure,” “sepsis,” “ventilation, mechanical,” “fentanyl,” “opioid,” and “randomized controlled trial” (Additional file 1). We also carried out a manual search of the reference lists of the identified studies. We limited our search to articles published in Japanese or English.
Study inclusion criteria
We selected RCTs that compared the use of fentanyl versus other opioids in mechanically ventilated adults in the ICU. We included mechanically ventilated adults in the ICU who were intravenously administered analgesia with fentanyl or other opioids. Exclusion criteria were drugs administered only before ICU admission (e.g., during surgery), non-intravenous administration (e.g., intrathecal administration), patients younger than 18 years, crossover design, and articles in languages other than Japanese and English. The outcomes were selected by an independent committee based on a preliminary assessment of the importance of the outcomes, without members of the systematic review, following the GRADE clinical guideline development process. The primary outcome was mortality, which was defined as the longest period over which mortality was assessed in each article. The secondary outcomes were the duration of mechanical ventilation, duration of the ICU stay, incidence of severe adverse events, and incidence of delirium.
Data collection
The titles and abstracts of all of the extracted studies were screened independently and assessed by two authors (H.K., H.F.) according to our inclusion and exclusion criteria. Data elements were extracted to confirm the study eligibility, study design, patients’ demographics, performed interventions, outcomes of interest, statistical methods, and study results. All inconsistencies during data extraction were resolved by a third author (Y.A.) to reach a consensus.
Assessment of risk of bias
The qualities of the included studies were assessed independently by two authors (H.K., H.F.) according to the Cochrane methodology [16]. In case of disagreement, the final decision was made by a third author (Y.A.). The extent of potential bias in the included studies was assessed using the Cochrane “risk of bias” tool in RevMan 5.4 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). We considered the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. We evaluated each methodological quality item as “yes,” “no,” or “unclear” (owing to no or less clear reporting) for each eligible study and created a “risk of bias” summary. We then evaluated the overall validity of each study as a low, intermediate, or high risk of bias.
Data synthesis and statistical analysis
We carried out the statistical analysis using RevMan 5.4. We used a random-effects model for combined data where the assumption that the studies estimated the same underlying treatment effect was reasonable. Forest plots were constructed to display the results of the individual studies and pooled estimates of effect. The pooled dichotomous outcomes are expressed as the risk ratio (RR) and 95% confidence interval (CI). A pooled estimate of the treatment effect was calculated as the mean difference (MD) and 95% CI for continuous variables. Data provided as the median and interquartile range (or range) were converted to the mean and standard deviation, where appropriate, to calculate pooled RRs and MDs [17]. The forest plot displays other types of opioids (remifentanil and morphine) in the control group as subgroups. In a sensitivity analysis, we excluded studies in which the intervention and comparison groups differed in co-interventions (e.g., sedatives other than opioids). Heterogeneity across studies was tested using the I2 statistic. We investigated reporting bias (publication bias) using a funnel plot and visually assessed the funnel plot asymmetry. Finally, we summarized the results in evidence profiles using the GRADE approach. An independent peer review committee reviewed the GRADE evidence profile, and a third party checked each measurement for validity.
Results
Search results
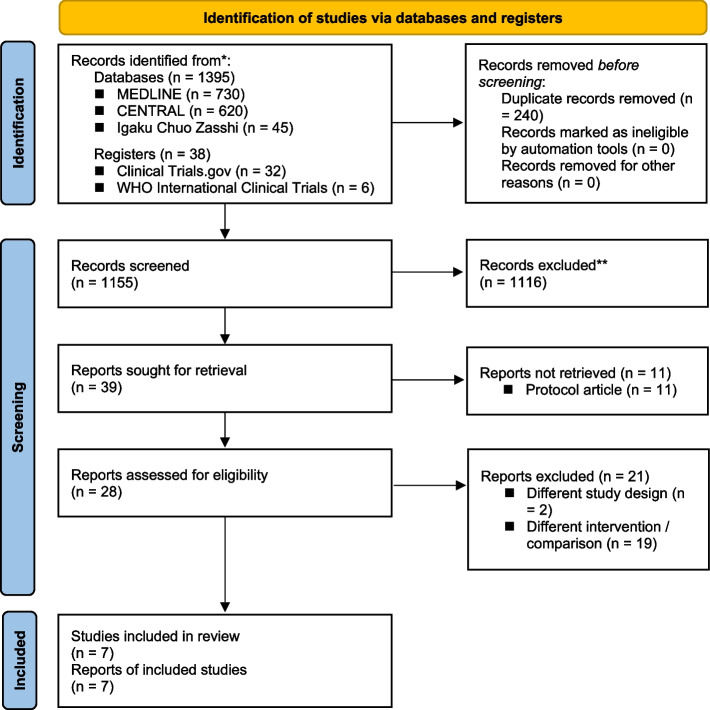
On 22 June 2021, we identified 730, 620, and 45 studies from searches of MEDLINE, CENTRAL, and Igaku Chuo Zasshi, respectively. In addition, as with other sources, we identified 32 studies from ClinicalTrials.gov and 6 studies from World Health Organization International Clinical Trials (Additional file 1). Manual searching of the reference lists did not show any additional publications. Our electronic database search identified 1155 studies, of which 1116 were excluded after applying the inclusion and exclusion criteria. After reviewing the full-text articles, a further 21 studies were excluded. The studies that appeared to meet the inclusion criteria, but were excluded, are summarized in Additional file 2, along with the reasons for exclusion. Therefore, seven studies remained for the systematic review (Fig. 1).
Fig. 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram. CENTRAL, Cochrane Central Register of Controlled Trials; WHO, World Health Organization; MV, mechanical ventilation; ICU, intensive care unit
Design and characteristics of included studies
The seven included studies are shown in Table 1 [18–24]. A total of 534 participants were included, of whom 251 received fentanyl (intervention group) and 283 received other opioids (242 had remifentanil and 41 had morphine; control group). Three studies had two control groups in contrast to the one fentanyl intervention group [19, 20, 23]. Therefore, we used the larger sample size of the two control groups in this study. Because this systematic review showed significant differences in the characteristics of the patients and the indications for sedatives and opioids, details are provided in Additional file 3. Outcomes included in the meta-analysis are listed in Additional file 4 as they appear in the respective literature. In the sensitivity analysis, we excluded two studies in which co-intervention of sedatives differed between the intervention and comparison groups from the analysis [19, 22].
Table 1.
Characteristics of included studies
| Authors | Country | Setting | Study period | Total number of patients randomized | Patients’ conditions | No. of study arms | Interventions | Comparisons | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Cevik et al. [18] | Turkey | Single-center, open-label | September 2007 to May 2008 | 34 | Patients requiring MV and sedation in the ICU | 2 | Fentanyl-midazolam regimen | Remifentanil-midazolam regimen |
• Hemodynamic parameters • Analgesics and sedative dosage • Mean MV timea • Mean time to ICU dischargea • Organ dysfunction • Adverse effecta |
| Karabinis et al. [19] | Six countries in Europe (4 hospitals in Greece, 4 in Spain, 3 in Belgium, 3 in The Netherlands, 2 in Germany, 1 in Austria) | Multi-center, open-label | Not stated (published in 2004) | 161 | Acute, severe neurological insult or injury, or patients who had undergone elective or emergency neurosurgery | 3 | Hypnotic-based treatment (fentanyl) | Remifentanil-based treatment |
• Median time on MVa • Extubation process • Extubation to ICU dischargea • Serious adverse eventsa • Drug-related serious adverse events • Mortalitya |
| Liu et al. [20] | China | Single-center, double-blind | September 2014 to January 2015 | 105 | MV anticipated for > 24 h | 3 | Fentanyl | Remifentanil |
• 28-day mortalitya • Duration of MVa • Length of the ICU staya • Deliriuma • Duration of delirium • Weaning time |
| Muellejans et al. [21] | Five countries, 21 centers (4 centers in Belgium, 8 in Germany, 1 in the Netherlands, 4 in Spain, 4 in the UK) | Multi-center, double-blind | Not stated (published in 2004) | 152 | MV for a further 12 to 72 h | 2 | Fentanyl | Remifentanil |
• Extubation process – extubationa • Extubation – ICU discharge • Drug start—ICU dischargea • Adverse events • Drug-related adverse events • MAP < 50 mmHg • HR < 50 beats/min • Severe adverse eventsa |
| Muellejans et al. [22] | Germany | Single-center, open-label | Not stated (published in 2006) | 80 | MV for > 12 h | 2 | Hypnotic-based sedation with midazolam/fentanyl | Remifentanil-based analgesia and sedation with propofol |
• Any adverse event • Any serious adverse eventa • Deliriuma • Time from arrival in the ICU to extubationa • Time from arrival in the ICU to eligible ICU dischargea • Overall costs |
| Oliver et al. [23] | United States | Single-center, observer-blind | Not stated (published in 2011) | 113 | Scheduled elective cardiac surgery | 3 | Fentanyl and propofol | Morphine and propofol |
• Time to extubationa • First response time • Length of the ICU staya • Length of the hospital stay • ICU direct medical costs |
| Spies et al. [24] | Germany | Two-center, double-blind | December 2005 to June 2008 | 60 | MV for > 24 h | 2 | Fentanyl | Remifentanil |
• Proportion of patients who obtained the target analgesia score • Duration of MVa • Duration of the ICU staya • Duration of the hospital stay • Deliriuma |
MV Mechanical ventilation, ICU Intensive care unit, CABG Coronary artery bypass grafting, PCSU Postcardiac surgical unit, MAP Mean arterial pressure, HR Heart rate
a Outcomes included in the meta-analysis in this study
Effects of interventions
We appraised the certainty of the evidence using the GRADE approach and summarized the results in evidence profiles (Table 2). We found no clinically significant differences in the relative significance and no clinical differences in absolute values for all outcomes. We judged the certainty of evidence for mortality as low, the duration of ventilation and the ICU stay as moderate, and severe adverse events and delirium as low. The reasons for downgrading the certainty of evidence are explained in the footnote to Table 2.
Table 2.
Evidence profiles
| Certainty assessment | No. of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Fentanyl | Other Opioids |
Relative (95% CI) |
Absolute (95% CI) |
||
| Mortality | ||||||||||||
| 2 | Randomized trials | Not serious | Not serious | Not serious | Very serious a | None | 4/72 (5.6%) | 8/119 (6.7%) |
RR 0.79 (0.24 to 2.60) |
14 fewer per 1000 (from 51 fewer to 108 more) |
⨁⨁◯◯ LOW |
CRITICAL |
| Duration of mechanical ventilation | ||||||||||||
| 7 | Randomized trials | Not serious | Serious b | Not serious | Not serious | None | 251 | 283 | - |
MD 0.49 higher (0.9 lower to 1.88 higher) |
⨁⨁⨁◯ MODERATE |
CRITICAL |
| Duration of the ICU stay | ||||||||||||
| 7 | Randomized trials | Not serious | Serious c | Not serious | Not serious | None | 251 | 283 | - |
MD 7.04 higher (3.27 lower to 17.35 higher) |
⨁⨁⨁◯ MODERATE |
CRITICAL |
| Severe adverse events | ||||||||||||
| 4 | Randomized trials | Not serious | Not serious | Not serious | Very serious a | None | 13/173 (7.5%) | 15/255 (5.9%) |
RR 0.98 (0.50 to 1.90) |
1 fewer per 1000 (from 29 fewer to 53 more) |
⨁⨁◯◯ LOW |
CRITICAL |
| Delirium | ||||||||||||
| 3 | Randomized trials | Serious d | Not serious | Not serious | Serious e | None | 30/106 (28.3%) | 23/103 (22.3%) |
RR 1.27 (0.79 to 2.04) |
60 more per 1000 (from 47 fewer to 232 more) |
⨁⨁◯◯ LOW |
CRITICAL |
CI Confidence interval, RR Risk ratio, MD Mean difference, ICU Intensive care unit
a The assessment was downgraded by two levels because it did not meet the optimal information size and the 95% CI spanned 0.75 to 1.25, which was the threshold for judgment
b The grade was downgraded by one level because I2 for heterogeneity was 93%
c The grade was downgraded by one level because I2 for heterogeneity was 93%
d Muellejans et al. (28.9% weight of all results) used different sedatives in the intervention and control groups, and Spies et al.’s study (29.2% weight of all results) was terminated early and downgraded by one level owing to the high risk of bias
e The assessment was downgraded by one level because it did not meet the optimal information size and the 95% CI spanned 1.0 to 1.25, which was the threshold for judgment
Outcome
A forest plot of all outcomes is shown in Additional file 5. Two studies were included [19, 20], and the only opioid used in the control group was remifentanil. Four (4/72) patients in the fentanyl group and eight (8/119) in the other opioids group died, which indicated that fentanyl was not associated with decreased mortality (RR, 0.79; 95% CI, 024 to 2.60). Among the secondary outcomes, the MD for the duration of mechanical ventilation was 0.49 h (95% CI, − 0.90 to 1.88) in 7 RCTs comprising 534 patients. Additionally, the MD for the duration of the ICU stay was 7.04 h (95% CI, − 3.27 to 17.35) in 7 RCTs comprising 534 patients. With regard to the duration of mechanical ventilation and the ICU stay, opioids in the control group were mixed with remifentanil and morphine, but there was no difference in the MD results following a subgroup analysis according to other opioid types. The RR of severe adverse events in 4 RCTs comprising 428 patients was 0.98 (95% CI, 0.50 to 1.90), and the RR of delirium was 1.27 (95% CI, 0.79 to 2.04) in 3 RCTs comprising 209 patients. Additional file 6 shows the sensitivity analysis results, which were similar to those for the primary analysis.
Risk of bias in included studies
The risk of bias assessments for the included studies are summarized in Fig. 2. All domains were a “low risk of bias” in one study [20], but the remaining studies lacked clarity in several domains.
Fig. 2.
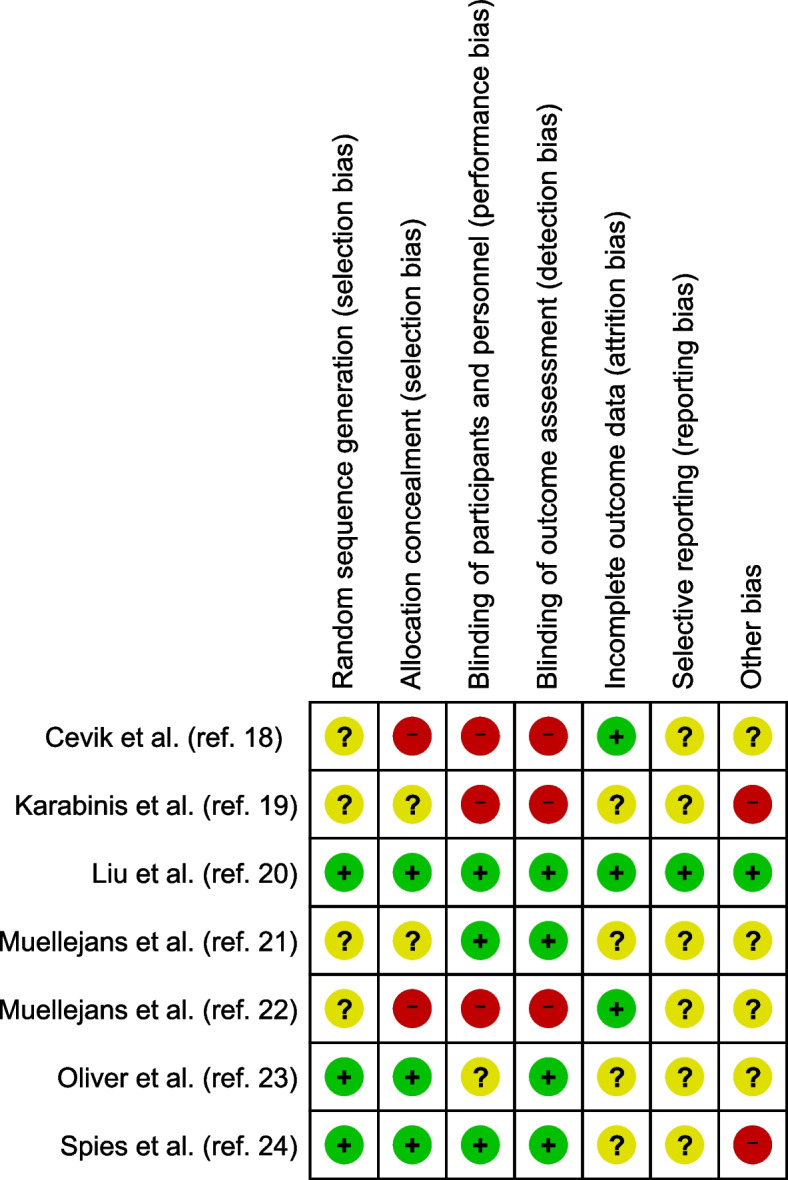
Risk of bias summary. Green circles indicate a low risk of bias, yellow circles indicate an unclear risk, and red circles indicate a high risk
Assessment of reporting bias
We were unable to create a forest plot because fewer than 10 studies were included.
Discussion
This meta-analysis aimed to investigate the effects of fentanyl administration in mechanically ventilated patients in the ICU using the GRADE system. We found that fentanyl was not associated with a decrease in mortality, duration of mechanical ventilation, duration of the ICU stay, incidence of serious adverse events, or incidence of delirium compared with other opioids. The results were also robust in the sensitivity analysis. However, the most common opioid that was compared with fentanyl was remifentanil. Additionally, the GRADE certainty rating of the results was rated as moderate to low, suggesting that the evidence was inadequate, and the results could thus not be confirmed.
To the best of our knowledge, this is the first meta-analysis to examine the effectiveness of fentanyl administration for mechanically ventilated patients in the ICU. Fentanyl is an opioid used for pain relief in mechanically ventilated ICU patients worldwide [6, 7, 12]. Fentanyl is a synthetic opioid with a good profile, but undergoes hepatic metabolism, and thus accumulates in tissues following continuous infusion, resulting in prolonged drug effects [25]. The Pain, Agitation/Sedation, Delirium, Immobility, and Sleep disruption guidelines state that all opioids are equally effective [4]. However, recent reports have indicated differences in postoperative complications among opioids used for postoperative patient-controlled analgesia [26], differences in the rates of maintenance of light sedation in the ICU [27], and differences in opioid-withdrawal syndrome in the ICU [28]. Another important finding of this study is that remifentanil was usually compared with fentanyl in the ICU, and no RCTs compared fentanyl with alfentanil, sufentanil, or hydromorphone. Therefore, the optimal opioid for use in the ICU is controversial, and further research is required.
The current analysis showed no difference in mortality between fentanyl and other opioids. In contrast, however, a recent propensity score-matched cohort study that compared fentanyl and morphine as analgesics in patients with acute respiratory distress syndrome reported a lower mortality with fentanyl [29]. The present meta-analysis showed no significant difference in the duration of mechanical ventilation or the ICU stay between patients treated with fentanyl or other opioids. These results remained similar even after subgroup and sensitivity analyses, which showed no difference between fentanyl and remifentanil. This result is in contrast to a systematic review that showed a slight reduction in the duration of mechanical ventilation and the ICU stay between patients treated with remifentanil and those treated with other opioids [10]. There were no differences in severe complications between the fentanyl and other opioid groups in this study. Although some studies have reported adverse effects of fentanyl in patients in the community [30], there have been few reports of severe complications in the ICU [19, 21, 31]. There was no difference in the rate of delirium between the fentanyl and other opioid groups in our analysis. However, in a relatively small cross-sectional study, fentanyl treatment did not increase the incidence of delirium, but was associated with subsyndromal delirium (defined as a score of between 1 and 3 on the Intensive Care Delirium Screening Checklist) [32]. This inconsistency among studies may be due to the small number of RCTs that compared fentanyl with other opioids, the small sample size, and different patients’ backgrounds and interventions/controls.
Following this systematic review, the independent panel committee of the J-SSCG 2020 decided against issuing a recommendation addressing this clinical question. Fentanyl is currently the only opioid that can be legally administered in Japanese ICUs. The panel committee decided that the results of this review, which did not support the use of fentanyl, might confuse the clinical practice of sepsis management. Because inadequate analgesia is associated with a worse prognosis [1–3], the choice of opioids for patients in the ICU remains an important issue for future consideration.
This study had several limitations. First, this systematic review was conducted as part of the Japanese sepsis guidelines, and experts outside the systematic review team determined the outcomes. Therefore, although opioids in the ICU are sometimes discussed in terms of cost, it was not chosen as an outcome for this study because the cost was judged not to be a critical outcome in the ventilatory management of critically ill patients. Second, the number of included studies was relatively small and the sample size was small. Therefore, a subgroup analysis and sensitivity analysis were conducted, but the robustness of this meta-analysis may be debatable. Third, the dosing protocols of the opioids administered in each study varied, which may have affected the results of this analysis. Therefore, we carefully reviewed the dosing protocols for sedatives and opioids (Additional file 3). The effects of opioids may also vary depending on the patient’s liver and kidney function. However, protocols that adjust drug doses using patients’ assessment scores, such as the Richmond Agitation Sedation Scale, may reduce the differences in drug types. Fourth, the comparison of fentanyl with other opioids was in the short term within hospitalization or 28 days, and no long-term (e.g., > 6 months) comparison has been performed. Opioids may be associated with medium- and long-term outcomes, such as post-intensive care syndrome and chronic pain, but no studies have evaluated these outcomes. Fifth, the present study was initially conducted in 2019 for the J-SSCG 2020, but was re-performed with a more recent literature search in 2021. Therefore, there are slight differences from the PROSPERO registration. An example of these differences is that alfentanil and sufentanil, which are unavailable in Japan, were excluded from the PROSPERO registration in 2019. However, we did not exclude alfentanil and sufentanil from the second search in 2021. A second literature search may have increased the generalizability of the study results. Finally, assessing the certainty of the evidence is subjective, and some researchers may not agree with our assessment. However, the present study was conducted following the methodology of the J-SSCG 2020, which included independent validation of search formulas, an external audit of systematic review work, and a peer review of the GRADE evidence profile by an independent committee. Despite these limitations, we believe that our results, which were obtained using the appropriate procedures and evaluated with the GRADE approach, have important implications for clinicians.
In conclusion, the present study used the GRADE system to examine the effect of fentanyl on mechanically ventilated patients in the ICU. We did not find any significant difference in patients’ outcomes between the use of fentanyl and other opioids. However, remifentanil was usually the only opioid compared with fentanyl, and the GRADE certainty ratings were generally low, indicating a lack of evidence in this area.
Supplementary Information
Additional file 2. Relevant excluded studies and reasons for exclusion.
Additional file 3. Characteristics of included patients, and details of sedatives and opioids.
Additional file 4. Outcomes of included studies.
Additional file 5. Forest plot of all outcomes.
Additional file 6. Forest plot of all outcomes in the sensitivity analysis.
Acknowledgements
We received assistance from the committee of the J-SSCG 2020 collaborators. This organization developed the clinical question to revise the Japanese Guidelines for the Treatment of Sepsis, assessed the importance of outcomes before the systematic review, and conducted a panel meeting on the basis of the systematic review results. The clinical question in this study is included in the Pain, Agitation, Delirium group. We are grateful to Dr. Ryosuke Tsuruta, Dr. Takeshi Suzuki, Dr. Chikashi Takeda, and Dr. Hiroshi Yonekura for their guidance in this group. We also received careful supervision from Dr. Moritoki Egi and Dr. Yuji Ogura, chairpersons of the J-SSCG2020 Special Committee, and Dr. Osamu Nishida and Dr. Hiroshi Tanaka as members of the committee.
Abbreviations
- ICU
Intensive care unit
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- RCTs
Randomized, controlled trials
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- J-SSCG2020
Japanese Clinical Practice Guidelines for Management of Sepsis and Septic Shock 2020
- CENTRAL
Cochrane Central Register of Controlled Trials
Authors’ contributions
YA and MS helped with negotiation, development, revision, and approval of the final version of the manuscript, performance of database search strategies, management of citations, and documentation of searches and search results. HK and NF were mainly responsible for screening the literature obtained from the search formula and extracting data from the literature. HK and NF also helped with the analysis, writing, and approval of the final version of the paper. YS and MD helped with the design, conduction, analysis, writing, and approval of the final version of the manuscript.
Authors’ information
None.
Funding
None.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Myhren H, Ekeberg O, Tøien K, Karlsson S, Stokland O. Posttraumatic stress, anxiety and depression symptoms in patients during the first year post intensive care unit discharge. Crit Care. 2010;14:R14. doi: 10.1186/cc8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, Wunsch H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40:502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 3.Elliott D, Davidson JE, Harvey MA, Bemis-Dougherty A, Hopkins RO, Iwashyna TJ, et al. Exploring the scope of post-intensive care syndrome therapy and care: engagement of non-critical care providers and survivors in a second stakeholders meeting. Crit Care Med. 2014;42:2518–2526. doi: 10.1097/CCM.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 4.Devlin JW, Skrobik Y, Gélinas C, Needham DM, Slooter AJC, Pandharipande PP, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:e825–e873. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 5.Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 6.Soliman HM, Mélot C, Vincent JL. Sedative and analgesic practice in the intensive care unit: the results of a European survey. Br J Anaesth. 2001;87:186–192. doi: 10.1093/bja/87.2.186. [DOI] [PubMed] [Google Scholar]
- 7.Payen J-F, Chanques G, Mantz J, Hercule C, Auriant I, Leguillou J-L, et al. Current practices in sedation and analgesia for mechanically ventilated critically ill patients: a prospective multicenter patient-based study. Anesthesiology. 2007;106:687–95. doi: 10.1097/01.anes.0000264747.09017.da. [DOI] [PubMed] [Google Scholar]
- 8.Wilhelm W, Kreuer S. The place for short-acting opioids: special emphasis on remifentanil. Crit Care. 2008;12(Suppl 3):S5. doi: 10.1186/cc6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wunsch H, Kahn JM, Kramer AA, Rubenfeld GD. Use of intravenous infusion sedation among mechanically ventilated patients in the United States. Crit Care Med. 2009;37:3031–3039. doi: 10.1097/CCM.0b013e3181b02eff. [DOI] [PubMed] [Google Scholar]
- 10.Zhu Y, Wang Y, Du B, Xi X. Could remifentanil reduce duration of mechanical ventilation in comparison with other opioids for mechanically ventilated patients? A systematic review and meta-analysis. Crit Care. 2017;21:206. doi: 10.1186/s13054-017-1789-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu EHY, Tran DHD, Lam SW, Irwin MG. Remifentanil tolerance and hyperalgesia: short-term gain, long-term pain? Anaesthesia. 2016;71:1347–1362. doi: 10.1111/anae.13602. [DOI] [PubMed] [Google Scholar]
- 12.Shehabi Y, Howe BD, Bellomo R, Arabi YM, Bailey M, Bass FE, et al. Early sedation with dexmedetomidine in critically Ill patients. N Engl J Med. 2019;380:2506–2517. doi: 10.1056/NEJMoa1904710. [DOI] [PubMed] [Google Scholar]
- 13.Tsuruta R, Fujita M. Comparison of clinical practice guidelines for the management of pain, agitation, and delirium in critically ill adult patients. Acute Med Surg. 2018;5:207–212. doi: 10.1002/ams2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schünemann HJ, Schünemann AHJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336:1106–1110. doi: 10.1136/bmj.39500.677199.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane. 2022. Available from http://www.training.cochrane.org/handbook.
- 17.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cevik F, Celik M, Clark PM, Macit C. Sedation and analgesia in intensive care: a comparison of fentanyl and remifentanil. Pain Res Treat. 2011;2011:650320. doi: 10.1155/2011/650320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karabinis A, Mandragos K, Stergiopoulos S, Komnos A, Soukup J, Speelberg B, et al. Safety and efficacy of analgesia-based sedation with remifentanil versus standard hypnotic-based regimens in intensive care unit patients with brain injuries: a randomised, controlled trial [ISRCTN50308308] Crit Care. 2004;8:R268–280. doi: 10.1186/cc2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu D, Lyu J, Zhao H, An Y. The influence of analgesic-based sedation protocols on delirium and outcomes in critically ill patients: a randomized controlled trial. PLoS ONE. 2017;12:e0184310. doi: 10.1371/journal.pone.0184310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muellejans B, López A, Cross MH, Bonome C, Morrison L, Kirkham AJT. Remifentanil versus fentanyl for analgesia based sedation to provide patient comfort in the intensive care unit: a randomized, double-blind controlled trial [ISRCTN43755713] Crit Care. 2004;8:R1–11. doi: 10.1186/cc2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muellejans B, Matthey T, Scholpp J, Schill M. Sedation in the intensive care unit with remifentanil/propofol versus midazolam/fentanyl: a randomised, open-label, pharmacoeconomic trial. Crit Care. 2006;10:R91. doi: 10.1186/cc4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oliver WC, Nuttall GA, Murari T, Bauer LK, Johnsrud KH, Hall Long KJ, et al. A prospective, randomized, double-blind trial of 3 regimens for sedation and analgesia after cardiac surgery. J Cardiothorac Vasc Anesth. 2011;25:110–119. doi: 10.1053/j.jvca.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Spies C, Macguill M, Heymann A, Ganea C, Krahne D, Assman A, et al. A prospective, randomized, double-blind, multicenter study comparing remifentanil with fentanyl in mechanically ventilated patients. Intensive Care Med. 2011;37:469–476. doi: 10.1007/s00134-010-2100-5. [DOI] [PubMed] [Google Scholar]
- 25.Battershill AJ, Keating GM. Remifentanil : a review of its analgesic and sedative use in the intensive care unit. Drugs. 2006;66:365–385. doi: 10.2165/00003495-200666030-00013. [DOI] [PubMed] [Google Scholar]
- 26.Yan G, Chen J, Yang G, Duan G, Du Z, Yu Z, et al. Effects of patient-controlled analgesia with hydromorphone or sufentanil on postoperative pulmonary complications in patients undergoing thoracic surgery: a quasi-experimental study. BMC Anesthesiol. 2018;18:192. doi: 10.1186/s12871-018-0657-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aoki Y, Niwa T, Shiko Y, Kawasaki Y, Mimuro S, Doi M, et al. Remifentanil provides an increased proportion of time under light sedation than fentanyl when combined with dexmedetomidine for mechanical ventilation. J Int Med Res. 2021;49:3000605211002683. doi: 10.1177/03000605211002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hyun DG, Huh JW, Hong SB, Koh Y, Lim CM. Iatrogenic opioid withdrawal syndrome in critically ill patients: a retrospective cohort study. J Korean Med Sci. 2020;35:e106. doi: 10.3346/jkms.2020.35.e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu A-M, Shan Z-M, Zhang Z-J, Li H-P. Comparative efficacy of fentanyl and morphine in patients with or at risk for acute respiratory distress syndrome: a propensity score-matched cohort study. Drugs R D. 2021 doi: 10.1007/s40268-021-00338-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DiSalvo P, Cooper G, Tsao J, Romeo M, Laskowski LK, Chesney G, et al. Fentanyl-contaminated cocaine outbreak with laboratory confirmation in New York City in 2019. Am J Emerg Med. 2021;40:103–105. doi: 10.1016/j.ajem.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Devabhakthuni S, Armahizer MJ, Dasta JF, Kane-Gill SL. Analgosedation: a paradigm shift in intensive care unit sedation practice. Ann Pharmacother. 2012;46:530–540. doi: 10.1345/aph.1Q525. [DOI] [PubMed] [Google Scholar]
- 32.Bastos AS, Beccaria LM, da Silva DC, Barbosa TP. Prevalence of delirium in intensive care patients and association with sedoanalgesia, severity and mortality. Rev Gaucha Enferm. 2020;41:e20190068. doi: 10.1590/1983-1447.2020.20190068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 2. Relevant excluded studies and reasons for exclusion.
Additional file 3. Characteristics of included patients, and details of sedatives and opioids.
Additional file 4. Outcomes of included studies.
Additional file 5. Forest plot of all outcomes.
Additional file 6. Forest plot of all outcomes in the sensitivity analysis.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.