Abstract
Background
To study the pattern and treatment outcome of rectal cancer (RC) with concurrent locoregional recurrence (LR) and distant metastasis (DM) after total mesorectal excision (TME) and to identify patient-, disease-, and treatment-related factors associated with differences in prognosis after concurrent LR and DM.
Methods
RC patients who were diagnosed with concurrent LR and DM after TME from May 2015 to June 2019 were included in our study. All patients received single or multiple treatment modalities under the guidance of multidisciplinary team (MDT) of colorectal cancer in Fudan University Shanghai Cancer Center. The prognostic value of various clinicopathological factors for survival were calculated by Kaplan–Meier curves and Cox regression analyses.
Results
A total of 74 RC patients with concurrent LR and DM who had undergone TME with a median follow-up of 27 months were eligible for analysis. The median survival of the included patients was 34 months, and 30 patients (41%) died. Fifty-nine patients (80%) underwent comprehensive treatments. Patients with oligometastatic disease (OMD) achieved no evidence of disease (NED) status more frequently than those with multiple metastases (P = 0.003). In the univariate analysis, patients achieving NED, diagnosed with OMD and five or less peritoneal metastases tended to have longer survival after LR and DM diagnosis (P < 0.05). In the multivariate analysis, attaining NED status was the only independent factor for survival (hazard ratio (HR), 2.419; P = 0.032). Survival after concurrent LR and DM in the non-NED group was significantly shorter than that in the NED group (median survival, 32 vs. 46 months; HR, 2.7; P = 0.014).
Conclusions
The pattern and treatment outcome of RC with concurrent LR and DM after TME has changed with the development of multiple treatment modalities. Although the prognosis remains poor, pursuing NED status through comprehensive treatments may improve the survival of RC patients with concurrent LR and DM after TME.
Keywords: Locoregional recurrence, Distant metastasis, Rectal cancer, Treatment outcome, NED
Background
Colorectal cancer (CRC) is the third most common cancer worldwide, and its overall 5-year survival rate is approximately 65% [1–3]. Approximately 50% of patients with CRC develop distant metastasis (DM) after curative resection, the most common of which is liver metastases [4, 5]. Rectal cancer (RC) accounts for 29% of all CRCs [2, 6]. Total mesorectal excision (TME) combined with pre-operative or postoperative radiotherapy (RT) or chemoradiotherapy (CRT) significantly reduces the locoregional recurrence (LR) rate in patients with RC to less than 10%, even 5% in some clinical centers [7, 8]. There are about 3% RC patients diagnosed with concurrent LR and DM after TME, which may cause severe disabling symptoms and usually have fatal outcomes [7–12].
For early and locally advanced RC, normative guidelines can be adopted for standardized treatment [7], while no consensus of treatment has been reached for concurrent LR and DM after TME. With the development of different treatment modalities, perioperative chemotherapy, palliative chemotherapy, targeted therapy, RT, radiofrequency ablation (RFA) and surgical resection can be applied singly or multiply [13–17]. However, several key problems have not been solved, including the sequence of local intervention and systemic treatment, the selection of surgical resection or RFA for local treatment, and the evaluation of tumors’ sensitivity to chemotherapy or RT. Thus, the individualized and comprehensive treatment of concurrent LR and DM still needs to be intensively studied.
Here, our study was designed to study the pattern and treatment outcome of RC with concurrent LR and DM after TME and to identify patient-, disease-, and treatment-related factors associated with differences in prognosis after concurrent LR and DM.
Patients and methods
Study design and patients
RC patients who were diagnosed with concurrent LR and DM after TME from May 2015 to June 2019 and fulfilled the following criteria were eligible for study entry: (i) aged from 18 to 80 years old at the time of diagnosis of concurrent LR and DM; (ii) diagnosed with resectable RC (histologically proven rectal adenocarcinoma) and received TME surgery with or without preoperative CRT; (iii) received treatment for LR and DM at Fudan University Shanghai Cancer Center (FUSCC); (iv) had complete medical records since RC diagnosis. After being diagnosed with concurrent LR and DM, all patients received single or multiple treatment modalities, including palliative chemotherapy, RT, RFA and surgical resection with or without preoperative chemotherapy under the guidance of multidisciplinary team (MDT) of CRC at FUSCC.
Patients were followed up regularly according to Chinese guidelines for CRC and ended at date of death or on December 31, 2019. Physical examination and carcinoembryonic antigen (CEA) were performed every 3–6 months for the first 2 years, every 6 months within the third to fifth year, and then annually. Chest/abdominal/pelvis computed tomography was performed annually for up to 5 years, and colonoscopy was performed for proper patients the first year after treatment and repeated in the third year if no advanced adenoma was found and then every 5 years. Clinical and pathological data were collected from electronic medical record system. Data on treatments and follow-up were gathered from surgeons, medical oncologists and radiologists. Patient data were collected prospectively using a standard form by researchers. All the follow up data of 74 patients are complete. The study was reviewed and approved by Institutional Ethics Committees of Fudan University Shanghai Cancer Center. All methods were carried out in accordance with relevant guidelines and regulations. Informed consent was obtained from all subjects and/or their legal guardian(s) before undergoing TME surgery and/ or treatment of recurrent disease. All the patients and/or legal guardians gave their consent that their data was used for this specific study.
Evaluations of LR, DM and NED
LR was defined as radiologic and/or histologic evidence of a tumor within the lesser pelvis or the perineal wound after a macroscopically complete resection. LR location was categorized according to an adapted version of the subdivision proposed by Philipsen et al. [18] into recurrences located at the level of the anastomosis, regional lymph node and pelvic recurrences. DM was defined as radiologic and/or histologic evidence of a tumor in any other area. In this study, oligometastatic disease (OMD) was defined as DM in up to 2 organs or structures including liver, lung and localized lymph node, absence of ascites and peritoneal, bone and central nervous system metastasis. No evidence of disease (NED) status was defined as all LR and DM being grossly resected or ablated and with no sign of remnant disease at one month after surgery. Clear circumferential margin of local recurrence was not mandatory for determination of NED. Two fixed senior radiologists checked all images reported LR and DM.
Statistical analyses
Chi-square tests were used to compare proportions, and Mann–Whitney U tests were used to compare continuous variables. Kaplan–Meier analyses were used to compare overall survival in patients between different groups. Cox regression was used for univariate and multivariate analyses with hazard ratios (HRs) and 95% confidence intervals (CI). Factors that were statistically significant in the univariate analysis were included in the multivariate analysis. P < 0.05 was considered as significant. Data on patients who were alive were censored at date of last contact. Because the aim of the study was to document the pattern and treatment outcome of RC patients with concurrent LR and DM after TME, the starting point for all survival analyses was the date of LR and DM diagnosis. All analyses were performed with SPSS statistical software (version 19.0 for Windows; SPSS Inc, Chicago, IL).
Results
Patients characteristics
Among 8,376 patients with RC received TME surgery at FUSCC from May 2015 to June 2019, a total of 74 patients diagnosed with concurrent LR and DM were included in our study. The diagnostic rate of concurrent LR and DM in RC patients was 0.88%. Median time between date of LR and DM and date of primary tumor diagnosis was 16 months (range, 1 to 60 months) (Fig. 1).
Fig. 1.
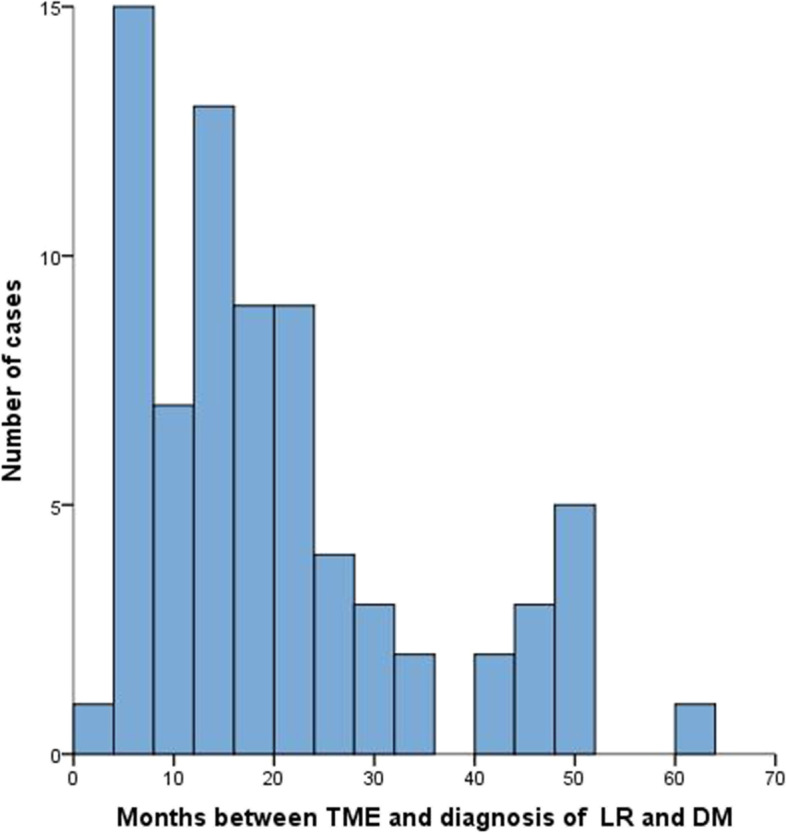
Time of diagnosis of concurrent locoregional recurrence (LR) and distant metastasis (DM) after total mesorectal excision (TME)
The clinicopathological characteristics of 74 eligible patients were summarized in Table 1. Among all patients, half were aged over 60 years old. The primary tumor of 62.2% (46/74) patients were located over 5 cm from the anal verge. In terms of characteristics for the primary tumor, 91.9% (68/74) were diagnosed as T3-4, 60.8% as positive lymph nodes involved and 23.0% as G3 tumors. 74.3% (55/74) had anterior resection and 31.1% (23/74) had preoperative CRT or RT. For the type of local recurrence, 47.3% (35/74) of patients were diagnosed as regional lymph node recurrence while 31.1% (23/74) as anastomotic recurrence and 21.6% (16/74) as undetermined pelvic recurrence. For the type of distant metastasis, 71.6% (53/76) of patients were diagnosed with OMD and 28.4% (21/74) of patients were diagnosed to have metastases in 3 or more organs or in peritoneal. 73.0% and 85.1% of observed LR and DM had occurred within 2 and 3 years.
Table 1.
Baseline characteristics of all eligible patients (n = 74)
| Characteristics | No. (%) |
|---|---|
| Gender | |
| Male | 45 (60.8) |
| Female | 29 (39.2) |
| Age (years) | |
| < 60 | 37 (50.0) |
| ≥ 60 | 37 (50.0) |
| Primary tumor location: distance from the anal verge (cm) | |
| > 5 | 46 (62.2) |
| ≤ 5 | 28 (37.8) |
| Type of local recurrence | |
| Anastomotic recurrence | 23 (31.1) |
| Regional lymph node recurrence | 35 (47.3) |
| Undetermined pelvic recurrence | 16 (21.6) |
| Distant metastasis | |
| Liver/lung/localized lymph node | 53 (71.6) |
| 3 or more organs/structures involved or peritoneal metastases | 21 (28.4) |
| Type of resection of primary tumor | |
| Anterior resection | 55 (74.3) |
| Abdominoperineal resection | 13 (17.6) |
| Others | 6 (8.1) |
| T stage of primary tumor | |
| T1-2 | 6 (8.1) |
| T3 | 41 (55.4) |
| T4 | 27 (36.5) |
| N stage of primary tumor | |
| N0 | 29 (39.2) |
| N1 | 36 (48.6) |
| N2 | 9 (12.2) |
| Tumor grade of primary tumor | |
| G1-2 | 57 (77.0) |
| G3 | 17 (23.0) |
| Preoperative CRT or RT of primary tumor | |
| Yes | 23 (31.1) |
| No | 51 (68.9) |
| Time to recurrence | |
| < 24 months | 52 (70.3) |
| 24–36 months | 10 (13.5) |
| > 36 months | 12 (16.2) |
Treatment modalities
Treatment modalities for 74 RC patients with concurrent LR and DM were listed in Table 2. 70 patients (94.6%) underwent at least one of the local treatments including surgical resection, RT and RFA. 48 patients (64.9%) received systemic treatments such as perioperative chemotherapy and palliative chemotherapy. 59 patients (79.7%) underwent multiple treatments. The results suggested that the vast majority of patients with LR and DM received comprehensive treatment no matter aggressively or palliatively.
Table 2.
Treatment modalities of patients
| Treatment modality | ||||||||||
| Surgical resection | √ | √ | √ | √ | √ | |||||
| Perioperative chemotherapy | √ | √ | ||||||||
| Palliative chemotherapy | √ | √ | √ | √ | ||||||
| Radiotherapy | √ | √ | √ | √ | ||||||
| Radiofrequency ablation | √ | √ | √ | √ | ||||||
| Cases | 8 | 12 | 5 | 16 | 3 | 10 | 9 | 4 | 3 | 4 |
| Percentage (%) | 10.8 | 16.2 | 6.8 | 21.6 | 4.1 | 13.5 | 12.2 | 5.4 | 4.1 | 5.4 |
Survival
During follow-up, 30 patients (40.5%) died and for the remaining 44 patients, median time between date of LR and DM diagnosis and date of last contact was 27 months (range, 17 to 48 months). Median survival after LR and DM diagnosis was 34 months (95% CI, 28.6 to 39.4 months) and three-year survival after LR and DM was estimated at 49.3% (Fig. 2).
Fig. 2.
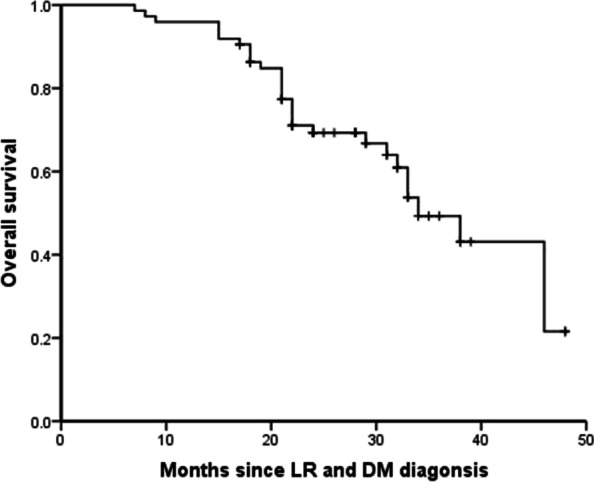
Overall survival of 74 patients after concurrent locoregional recurrence (LR) and distant metastasis (DM) diagnosis
In univariate analysis, type of distant metastasis (HR, 2.464; 95%CI, 1.132–5.362; P = 0.023), number of peritoneal metastases (HR, 2.637; 95%CI, 1.140–2.229; P = 0.023) and NED status (HR, 2.727; 95%CI, 1.229–6.049; P = 0.014) were associated with survival (Table 3). Kaplan–Meier analysis showed that patients achieving NED (P = 0.009), diagnosed with OMD (P = 0.017) and five or less peritoneal metastases (P = 0.017) tended to have longer survival after LR and DM diagnosis (Fig. 3).
Table 3.
Univariate and multivariate Cox regression analysis for survival
| Related factors | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Gender | 0.296 | |||||
| Male | 1.000 | |||||
| Female | 0.658 | 0.300–1.433 | ||||
| Age (years) | 0.333 | |||||
| < 60 | 1.000 | |||||
| ≥ 60 | 0.691 | 0.327–1.460 | ||||
| Primary tumor location: distance from the anal verge (cm) | 0.856 | |||||
| > 5 | 1.000 | |||||
| ≤ 5 | 0.933 | 0.443–1.965 | ||||
| Type of local recurrence | 0.159 | |||||
| Anastomotic recurrence | 1.000 | |||||
| Regional lymph node metastasis | 0.461 | 0.205–1.038 | ||||
| Undetermined pelvic recurrence | 0.565 | 0.182–1.751 | ||||
| Distant metastasis | 0.023 | 0.068 | ||||
| Liver/lung/localized lymph node | 1.000 | 1.000 | ||||
| 3 or more organs/structures involved or peritoneal metastases | 2.464 | 1.132–5.362 | 2.106 | 0.947–4.684 | ||
| Localized abdominal recurrence | 0.060 | |||||
| 0 | 1.000 | |||||
| 1–3 | 0.695 | 0.279–1.734 | ||||
| > 3 | 2.011 | 0.696–5.806 | ||||
| Peritoneal metastases | 0.023 | 0.513 | ||||
| 0–5 | 1.000 | 1.000 | ||||
| > 5 | 2.637 | 1.140–6.099 | 1.380 | 0.526–3.623 | ||
| Type of surgery of primary tumor | 0.268 | |||||
| Anterior resection | 1.000 | |||||
| Abdominoperineal resection | 2.064 | 0.857–4.971 | ||||
| Others | 1.111 | 0.257–4.815 | ||||
| T stage of primary tumor | 0.362 | |||||
| T1-2 | 1.000 | |||||
| T3 | 1.668 | 0.220–12.624 | ||||
| T4 | 0.965 | 0.121–7.668 | ||||
| N stage of primary tumor | 0.424 | |||||
| N0 | 1.000 | |||||
| N1 | 0.979 | 0.441–2.174 | ||||
| N2 | 1.904 | 0.652–5.565 | ||||
| Tumor grade of primary tumor | 0.199 | |||||
| G1-2 | 1.000 | |||||
| G3 | 0.551 | 0.222–1.368 | ||||
| Preoperative treatment of primary tumor | 0.242 | |||||
| Chemoradiotherapy or radiotherapy | 1.000 | |||||
| None | 1.801 | 0.673–4.822 | ||||
| Time to recurrence | 0.233 | |||||
| < 24 months | 1.000 | |||||
| 24–36 months | 3.074 | 0.835–11.315 | ||||
| > 36 months | 1.779 | 0.599–5.290 | ||||
| No evidence of disease | 0.014 | 0.032 | ||||
| NED | 1.000 | 1.000 | ||||
| Non-NED | 2.727 | 1.229–6.049 | 2.419 | 1.078–5.427 | ||
Fig. 3.
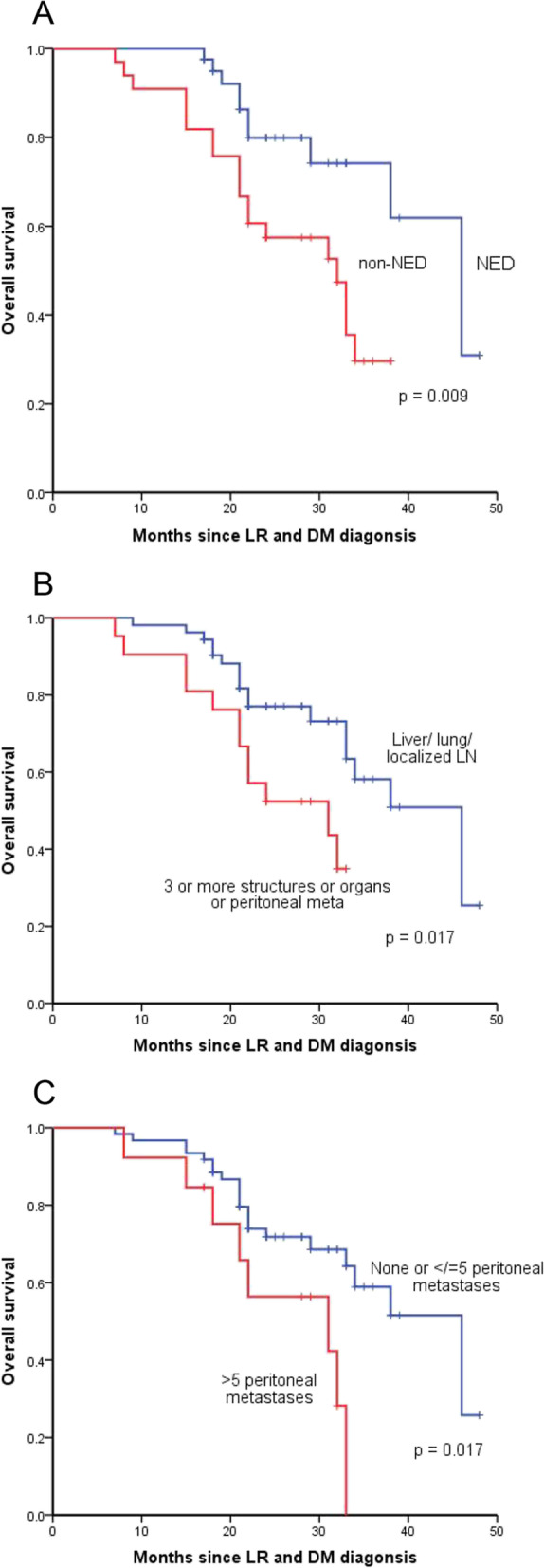
Kaplan–Meier analysis of overall survival in 74 patients after locoregional recurrence (LR) and distant metastasis (DM) according to no evidence of disease (NED) status (A), type of DM (B) and number of peritoneal metastases (C)
After multiple variables adjustment in the Cox proportional hazards regression model, number of peritoneal metastases lost its statistically significance (HR, 1.380; 95%CI, 0.526–3.623; P = 0.513) and type of distant metastasis was marginal statistically significant for predicting survival (HR, 2.106; 95%CI, 0.947–4.684; P = 0.068) (Table 3). NED status was the only independent factor for survival after LR and DM diagnosis (HR, 2.419; 95%CI, 1.078–5.427; P = 0.032) (Table 3).
NED status
The relationship between clinicopathological features and NED status was then analyzed (Table 4). The type of distant metastasis (P = 0.003), number of localized abdominal recurrence (P = 0.005), number of peritoneal metastases were all significantly related with NED status (P = 0.001). Thus, patients with OMD can achieve NED status more frequently.
Table 4.
Association of NED status and clinicopathological features in 74 eligible patients
| Characteristics | NED, n (%) (n = 41) |
Non-NED, n (%) (n = 33) |
χ2 | P value |
|---|---|---|---|---|
| Gender | ||||
| Male | 25 (33.8) | 20 (27.0%) | 0.001 | 0.974 |
| Female | 16 (21.6) | 13 (17.6%) | ||
| Age (years) | ||||
| < 60 | 17 (23.0) | 20 (27.0%) | 2.680 | 0.102 |
| > / = 60 | 24 (32.4) | 13 (17.6%) | ||
| Primary tumor location: distance from the anal verge (cm) | ||||
| > 5 | 26 (35.1) | 20 (27.0%) | 0.061 | 0.804 |
| ≤ 5 | 15 (20.3) | 13 (17.6%) | ||
| Type of local recurrence | ||||
| Anastomotic recurrence | 10 (13.5) | 13 (17.6%) | 2.115 | 0.347 |
| Regional lymph node metastasis | 22 (29.7) | 13 (17.6%) | ||
| Undetermined pelvic recurrence | 9 (12.2) | 7 (9.5%) | ||
| Distant metastasis | ||||
| Liver/lung/localized lymph node | 35 (47.3) | 18 (24.3%) | 8.525 | 0.003 |
| 3 or more organs/structures involved or peritoneal metastases | 6 (8.1) | 15 (20.3%) | ||
| Localized abdominal recurrence | ||||
| None | 10 (13.5) | 4 (5.4%) | 10.636 | 0.005 |
| < / = 3 | 29 (39.2) | 18 (24.3%) | ||
| > 3 | 2 (2.7) | 11 (14.9%) | ||
| Peritoneal metastases | ||||
| None or < / = 5 | 39 (52.7) | 22 (29.7%) | 10.223 | 0.001 |
| > 5 | 2 (2.7) | 11 (14.9%) | ||
| T stage of primary tumor | ||||
| T1-2 | 3 (4.1) | 3 (4.1%) | 1.161 | 0.560 |
| T3 | 25 (33.8) | 16 (21.6%) | ||
| T4 | 13 (17.6) | 14 (18.9%) | ||
| N stage of primary tumor | ||||
| N0 | 17 (23.0) | 12 (16.2%) | 2.920 | 0.232 |
| N1 | 17 (23.0) | 19 (25.7%) | ||
| N2 | 7 (9.5) | 2 (2.7%) | ||
| Tumor grade of primary tumor | ||||
| G1-2 | 30 (40.5) | 27 (36.5%) | 0.773 | 0.379 |
| G3 | 11 (14.9) | 6 (8.1%) | ||
| Preoperative treatment of primary tumor | ||||
| Chemoradiotherapy or radiotherapy | 16 (21.6) | 7 (9.5%) | 2.708 | 0.100 |
| None | 25 (33.8) | 26 (35.1%) | ||
| Treatment modality | ||||
| Single treatment | 8 (10.8) | 7 (9.5%) | 0.033 | 0.857 |
| Multiple treatment | 33 (44.6) | 26 (35.1%) | ||
Further survival analysis showed that 11 patients (26.8%) in NED group and 19 patients (57.6%) in non-NED group died during follow-up (Table 5). Three-year survival after LR and DM was estimated to be 61.8% in NED group and 29.6% in non-NED group. Patients in NED group have longer median survival after LR and DM diagnosis of 46 months (95% CI, 37.5 to 54.5 months), compared with that of 32 months (95% CI, 24.2 to 39.8 months) in non-NED group. Consequently, RC patients with concurrent LR and DM after TME have a poor prognosis, but reaching NED status after treatments can improve patients’ survival.
Table 5.
Survival outcome of NED and non-NED group
| Survival outcome | NED (n = 41) |
Non-NED (n = 33) |
|---|---|---|
| Number of patients followed until death | 11 (26.8%) | 19 (57.6%) |
| 3-year survival rate | 61.8% | 29.6% |
| Median survival time (month) | 46 | 32 |
Discussion
Though the incidence of concurrent LR and DM after TME of rectal cancer is quite low, which is 0.88% in our study, the prognosis of this subset of patients is poor. Our study retrospectively collected the pattern and treatment outcome of 74 RC patients with concurrent LR and DM after TME, to identify patient-, disease-, and treatment-related factors associated with differences in prognosis.
We found that the vast majority of patients with LR and DM received comprehensive treatment no matter aggressively or palliatively. Although the prognosis is still poor, pursuing NED status through comprehensive treatments may improve the survival of RC patients with concurrent LR and DM after TME. There are several possible explanations for this finding.
The first explanation concerns the treatment modalities. With development of multiple treatment modalities and MDT, the pattern and treatment outcome of RC with concurrent LR and DM after TME has changed. Our results indeed showed that the majority of patients (79.7%) underwent multiple treatments no matter aggressively or palliatively. Compared with Dutch trial in 2004 [9], more drugs with better clinical applications, more options for local and systematic treatment and modified therapy with LR and DM can be reached at present. For example, short-term preoperative RT (a total dose of 25 Gy in five fractions over 5 to 7 days) was used at that time, while long-term preoperative RT (a total dose of 45 Gy in 25 fractions over about 5 weeks) is widely used in FUSCC at present. Meanwhile, treatment of metastasis is more aggressive at present [19, 20]. With the development of treatment strategies, the median survival after LR and DM diagnosis was 34 months in our study and the median survival after LR 6.1 months in preoperative RT + TME group and 15.9 months in TME group in Dutch trial [9].
The second explanation concerns the survival. Although RC with concurrent LR and DM after TME has a poor prognosis, many studies have focused on attaining NED status after treatments to improve the overall survival which is also confirmed by our results. Furthermore, we found patients with OMD can achieve NED status after treatments more frequently. Consequently, patients with OMD after TME are the candidates to pursue NED status through upfront curative resection from the initial of the treatment, including CRT and RFA [21, 22].
The third explanation concerns surgical resection which is an important treatment modality to achieve NED status. However, if NED status not achieved, surgical resection of LR still plays an essential role. Due to the limited pelvic space, recurrent tumors are easy to compress other organs, such as ureter and blood vessels, leading to renal insufficiency and lower limb edema, which seriously affects the quality of life and subsequent treatment. Patients undergoing R0 resection have the greatest survival advantage following surgery for recurrent rectal cancer. Meanwhile, there is a survival advantage for R1 over R2 resection [23].
The present study has several limitations. First, the study design was a retrospective single-center trial. Second, this research does not include data for treatment intent. However, in the actual clinical treatment process, we made the choice of curative or palliative treatment intent upon initial diagnosis according to the LR and DM, whether it was resectable, whether it was OMD, the patient’s physical condition, the patient’s own will, and other factors, combined with MDT discussion opinions. Third, we defined OMD as metastasis in up to 2 organs or structures including liver, lung and localized lymph node, without taking the number, size of tumors into account. In ASCO-GI 2020, OMD was defined as up to 5 metastasis, up to 3 metastasis in one organ, up to 3 affected organs, size ≤ 3 cm, absence of ascites and peritoneal, bone and central nervous system metastasis [24]. Thus, it is possible that less patients were counted into OMD status.
Conclusions
In conclusion, our study showed that RC patients with concurrent LR and DM after TME have a poor prognosis. Patients with OMD are the candidates to pursue NED status through multiple treatments including curative resection which may improve the overall survival.
Acknowledgements
We would like to thank the patients and family members who gave their consent on presenting the data in this study, as well as the investigators and research staff involved in this study.
Abbreviations
- CRC
Colorectal cancer
- RC
Rectal cancer
- LR
Locoregional recurrence
- DM
Distant metastasis
- TME
Total mesorectal excision
- MDT
Multidisciplinary team
- OMD
Oligometastatic disease
- NED
No evidence of disease
- HR
Hazard ratio
- RT
Radiotherapy
- CRT
Chemoradiotherapy
- RFA
Radiofrequency ablation
- FUSCC
Fudan University Shanghai Cancer Center
- CEA
Carcinoembryonic antigen
- CI
Confidence interval
Authors’ contributions
J.P. contributed to conception and design; Y.C., S.M., and Y.L. contributed to development of methodology; Y.C., S.M., X.M., Y.L., and X.H. contributed to acquisition of data; Y.C., Y.L., and S.M. contributed to analysis and interpretation of data; Y.C., S.M., Y.L., and X.M. contributed to writing of the manuscript; F.L., S.C., and J.P. contributed to review and revision of the manuscript; J.P. contributed to study supervision. All authors approved the final version of the manuscript, including the authorship list.
Funding
The study was supported by grants from the National Natural Science Foundation of China (U1932145 to JP, 82002946 to YL), Science and Technology Commission of Shanghai Municipality (18401933402 to JP), Fudan University Shanghai Cancer Center Basic and Clinical Translational Research Seed Foundation (YJZZ201802 to SC), and Shanghai Sailing Program (22YF1408800 to SM, 19YF1409500 to YL).
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Ethical approval and consent to participate
The studies involving human participants were reviewed and approved by Institutional Ethics Committees of Fudan University Shanghai Cancer Center. All methods were carried out in accordance with relevant guidelines and regulations. Informed consent was obtained from all subjects and/or their legal guardian(s).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yikuan Chen, Yaqi Li and Shaobo Mo contributed equally to this work.
Contributor Information
Xiaoji Ma, Email: crc_maxiaoji@163.com.
Junjie Peng, Email: pengjj67@hotmail.com.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145–164. doi: 10.3322/caac.21601. [DOI] [PubMed] [Google Scholar]
- 3.Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet (London, England) 2019;394(10207):1467–1480. doi: 10.1016/S0140-6736(19)32319-0. [DOI] [PubMed] [Google Scholar]
- 4.Matsuoka H, Morise Z, Tanaka C, Hayashi T, Ikeda Y, Maeda K, Masumori K, Koide Y, Katsuno H, Tanahashi Y, et al. Repeat hepatectomy with systemic chemotherapy might improve survival of recurrent liver metastasis from colorectal cancer-a retrospective observational study. World J Surg Oncol. 2019;17(1):33. doi: 10.1186/s12957-019-1575-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ren L, Zhu D, Benson AB, 3rd, Nordlinger B, Koehne CH, Delaney CP, Kerr D, Lenz HJ, Fan J, Wang J, et al. Shanghai international consensus on diagnosis and comprehensive treatment of colorectal liver metastases (version 2019) Eur J Surg Oncol. 2020;46(6):955–966. doi: 10.1016/j.ejso.2020.02.019. [DOI] [PubMed] [Google Scholar]
- 6.Gupta N, Kupfer SS, Davis AM. Colorectal Cancer Screening. JAMA. 2019;321(20):2022–2023. doi: 10.1001/jama.2019.4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keller DS, Berho M, Perez RO, Wexner SD, Chand M. The multidisciplinary management of rectal cancer. Nat Rev Gastroenterol Hepatol. 2020;17(7):414–429. doi: 10.1038/s41575-020-0275-y. [DOI] [PubMed] [Google Scholar]
- 8.van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, Rutten HJ, Påhlman L, Glimelius B, van de Velde CJ. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12(6):575–582. doi: 10.1016/S1470-2045(11)70097-3. [DOI] [PubMed] [Google Scholar]
- 9.van den Brink M, Stiggelbout AM, van den Hout WB, Kievit J, Klein Kranenbarg E, Marijnen CA, Nagtegaal ID, Rutten HJ, Wiggers T, van de Velde CJ. Clinical nature and prognosis of locally recurrent rectal cancer after total mesorectal excision with or without preoperative radiotherapy. J Clin Oncol. 2004;22(19):3958–3964. doi: 10.1200/JCO.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 10.Frykholm GJ, Pahlman L, Glimelius B. Treatment of local recurrences of rectal carcinoma. Radiother Oncol. 1995;34(3):185–194. doi: 10.1016/0167-8140(95)01519-M. [DOI] [PubMed] [Google Scholar]
- 11.Holm T, Cedermark B, Rutqvist LE. Local recurrence of rectal adenocarcinoma after 'curative' surgery with and without preoperative radiotherapy. Br J Surg. 1994;81(3):452–455. doi: 10.1002/bjs.1800810344. [DOI] [PubMed] [Google Scholar]
- 12.Wiggers T, de Vries MR, Veeze-Kuypers B. Surgery for local recurrence of rectal carcinoma. Dis Colon Rectum. 1996;39(3):323–328. doi: 10.1007/BF02049476. [DOI] [PubMed] [Google Scholar]
- 13.Akce M, El-Rayes BF. Nonsurgical Management of Rectal Cancer. J Oncol Pract. 2019;15(3):123–131. doi: 10.1200/JOP.18.00769. [DOI] [PubMed] [Google Scholar]
- 14.McCourt M, Armitage J, Monson JR. Rectal cancer. Surgeon. 2009;7(3):162–169. doi: 10.1016/S1479-666X(09)80040-1. [DOI] [PubMed] [Google Scholar]
- 15.Oronsky B, Reid T, Larson C, Knox SJ. Locally advanced rectal cancer: The past, present, and future. Semin Oncol. 2020;47(1):85–92. doi: 10.1053/j.seminoncol.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 16.São Julião GP, Habr-Gama A, Vailati BB, Araujo SEA, Fernandez LM, Perez RO. New Strategies in Rectal Cancer. Surg Clin North Am. 2017;97(3):587–604. doi: 10.1016/j.suc.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Wilkinson N. Management of Rectal Cancer. Surg Clin North Am. 2020;100(3):615–628. doi: 10.1016/j.suc.2020.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Pilipshen SJ, Heilweil M, Quan SH, Sternberg SS, Enker WE. Patterns of pelvic recurrence following definitive resections of rectal cancer. Cancer. 1984;53(6):1354–1362. doi: 10.1002/1097-0142(19840315)53:6<1354::AID-CNCR2820530623>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 19.Holman FA, Bosman SJ, Haddock MG, Gunderson LL, Kusters M, Nieuwenhuijzen GA, van den Berg H, Nelson H, Rutten HJ. Results of a pooled analysis of IOERT containing multimodality treatment for locally recurrent rectal cancer: Results of 565 patients of two major treatment centres. Eur J Surg Oncol. 2017;43(1):107–117. doi: 10.1016/j.ejso.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 20.Harris CA, Solomon MJ, Heriot AG, Sagar PM, Tekkis PP, Dixon L, Pascoe R, Dobbs BR, Frampton CM, Harji DP, et al. The Outcomes and Patterns of Treatment Failure After Surgery for Locally Recurrent Rectal Cancer. Ann Surg. 2016;264(2):323–329. doi: 10.1097/SLA.0000000000001524. [DOI] [PubMed] [Google Scholar]
- 21.Vogl TJ, Eckert R, Naguib NN, Beeres M, Gruber-Rouh T, Nour-Eldin NA. Thermal Ablation of Colorectal Lung Metastases: Retrospective Comparison Among Laser-Induced Thermotherapy, Radiofrequency Ablation, and Microwave Ablation. AJR Am J Roentgenol. 2016;207(6):1340–1349. doi: 10.2214/AJR.15.14401. [DOI] [PubMed] [Google Scholar]
- 22.Winkelmann MT, Clasen S, Pereira PL, Hoffmann R. Local treatment of oligometastatic disease: current role. Br J Radiol. 2019;92(1100):20180835. doi: 10.1259/bjr.20180835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhangu A, Ali SM, Darzi A, Brown G, Tekkis P. Meta-analysis of survival based on resection margin status following surgery for recurrent rectal cancer. Colorectal Dis. 2012;14(12):1457–1466. doi: 10.1111/j.1463-1318.2012.03005.x. [DOI] [PubMed] [Google Scholar]
- 24.Zucchelli G, Moretto R, Rossini D, Lonardi S, Murgioni S, Tonini G, Cupini S, Ratti M, Urbano F, Libertini M, et al. Oligometastatic colorectal cancer: Prognostic implications of tumor load, role of locoregional treatments, and of first-line therapy intensification—A pooled analysis of TRIBE and TRIBE2 studies by GONO. J Clin Oncol. 2020;38(4):12. doi: 10.1200/JCO.2020.38.4_suppl.12. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.


