ABSTRACT
BACKGROUND:
Tranexamic acid (TXA) has been shown to reduce intraoperative bleeding and the need for post-operative allogenic blood transfusion requirement in surgery. In our randomized controlled study, we aimed to evaluate the effect of pre-operative 15 mg/kg intravenous TXA on total blood loss (TBL), hidden blood loss (HBL), and transfusion requirement in elderly patient group with intertrochanteric femoral fracture (ITFF) and treated with proximal femoral nailing (PFN).
METHODS:
Patients diagnosed with ITFFs (AO types 31-A1 and 31-A2) and treated using closed reduction and PFN was divided into two groups in our prospective randomized study. Group 1 (TXA group) was administered 15 mg/kg of TXA 15 min before the incision was made, after anesthesia was given, in the form of an IV infusion in 100 cc of saline. Group 2 (control group) was given only 100 cc of isotonic saline. The primary outcome of the study was TBL. The secondary outcomes were the number of transfusions, HBL, and the surgical (intraoperative) blood loss during the operative procedure and post-operative complications. The outcome values were compared between two groups.
RESULTS:
One hundred and two patients (51 patients in each group) were included in our study. There were no statistically significant differences between the two groups in terms of their demographic characteristics and their pre-operative hemoglobin and hematocrit values. The mean TBL was statistically lower in the TXA group than in the control group (684.6±370.1 ml vs. 971.2±505.3 ml, respectively; p=0.002). The amount of intraoperative blood loss was not significantly different between two groups (102.4±59.3 ml in the TXA group vs. 112.7±90.1 ml in the control group, p=0.67). However, the mean estimated HBL was significantly lower in the TXA group than in the control group (582.3±341.2 ml vs. 857.8±493.1 ml, respectively; p=0.002). The post-operative blood transfusion rate and transfusion unit were found to be significantly lower in the TXA group than in the control group (8% vs. 23.5%, respectively [p=0.033], and 6 U vs. 15 U, respectively [p=0.04]). Both medical and surgical post-operative complications were found to be similar for two groups.
CONCLUSION:
Single dose of TXA significantly reduces TBL, HBL, and the need for blood transfusions following PFN in elderly patients with ITFFs, while it does not increase the risk of DVT or thromboembolic events.
Keywords: Blood loss, blood transfusion, intertrochanteric fracture, proximal femoral nailing, tranexamic acid
INTRODUCTION
Hip fracture surgery in elderly patients may result in total blood loss (TBL) of 1500–2200 milliliters.[1,2] This amount of blood loss may cause problems related to anemia in the elderly and fragile patient group, who may require a blood transfusion.[3,4] Allogenic blood transfusion carries some risks, including prolonged hospitalization, delayed mobilization, increased risk of infection and deep vein thrombosis (DVT), allergic reactions, and transfusion-related complications.[5–9] Several methods are used in orthopedic surgery to reduce TBL and the need for allogenic blood transfusion due to blood loss. These methods include hypotensive anesthesia, surgical tourniquet application, minimally invasive surgery, the cell saver method, and the use of computer-assisted navigation.[9] However, each method has its own complications.
Tranexamic acid (TXA) has been shown to decrease the amount of bleeding during the surgery and the need for post-operative allogenic blood transfusion and is frequently used in total hip and knee replacement surgery.[10,11] In recent years, its use in hip fractures has become widespread. Few previous studies have investigated the use of TXA with proximal femoral nailing (PFN) for the treatment of intertrochanteric femoral fracture (ITFF) in elderly population, and there is no consensus on the dosage.[1,9,12–14]
Our hypothesis was that a pre-operative single dose of TXA infusion could reduce TBL and transfusion requirement in elderly patients who will undergo PFN for ITFF. In our randomized controlled study, we aimed to evaluate the effect of pre-operative 15 mg/kg intravenous TXA on TBL and transfusion requirement in elderly patient group with ITFF and treated with PFN.
MATERIALS AND METHODS
This single-center randomized controlled clinical study was approved following an institutional review board (no. 94–2022). All patients signed their written informed consent to participate in the study. The study was registered on the Clinical Trials official website (www.clinicaltrials.gov; ID NCT05359172).
Patients diagnosed with intertrochanteric fractures (AO types 31-A1 and 31-A2) who presented to the emergency department of our institution between April 1, 2021, and April 30, 2022, were eligible for this study. Inclusion criteria were as follows: (1) Patients aged ≥65 years with intertrochanteric fracture, (2) patients who were treated with closed reduction and PFN, and (3) the time from injury to admission to hospital was ≤8 h. Patients were excluded if they showed any of the following: (1) Previous surgery on the same hip, (2) fracture requiring open reduction, (3) any contraindications for the use of TXA (e.g., allergy, previous thromboembolic event, creatinine clearance <30 ml/min, and postmenopausal hormonal therapy), (4) a history of any thromboembolic events, (5) current use of anticoagulant therapy, such as direct thrombin inhibitors, Vitamin K antagonists, direct factor X-a inhibitors, or antiplatelet drugs, (6) disseminated intravascular coagulation, (7) hepatic or renal disease resulting in coagulation dysfunction, and (8) multiple fractures.
Patients who presented to the emergency department of our hospital were assigned sequential numbers and were then randomized using the www.randomizer.org website. Group 1 (the TXA group) was administered 15 mg/kg of TXA 15 min before the incision was made, after anesthesia was given, in the form of an IV infusion in 100 cc of saline. Group 2 (the control group) was given only 100 cc of isotonic saline. During surgery, 1.5 ml/kg/h of crystalloid fluids was administered to the patients. A change in basal heart rate and blood pressure of more than 20% was considered to be related to hypovolemia, and in this case, a 10 ml/kg bolus of crystalloid solution was administered. A pre-operative blood transfusion was administered in cases of Hb <8 g/dl, suspected myocardial ischemia, and hemorrhagic shock. In cases of Hb <8 g/dl or Hb <10 g/dl and symptoms of hypovolemia (dizziness, orthostatic hypotension, and tachycardia), they were accepted as blood transfusion indication in the post-operative period.
Two grams of the cefazolin were given intravenously before the operation as a prophylaxis. All PFN procedures were performed with the patient in the supine position on a fracture table. The fragments of the fracture were identified using an image intensifier. All the operations were performed using the same PFN (Tasarim Med® PN1 PFNA Nail). The surgical procedures were applied according to the manufacturer’s manual. Electrocautery and an aspirator were routinely used during the surgical procedure in all patients. Fascia, dermis, and skin closure were performed for the surgical closure of the layers. No suction drain was used. DVT prophylaxis was applied to the patients using 4000 IU enoxaparin and started at the time of hospitalization. The dose of the day before the surgery was applied 12 h before the surgery and, and the patients were also provided with antiembolic stockings.
The patients were monitored for any clinical symptoms of thromboembolic events. Tenderness of the calf, dyspnea, sudden onset of chest pain, and neurological deficit were taken as clinical signs of thromboembolic events. If case of any concerns, we performed radiologic investigations and consulted with the appropriate department. We did not routinely perform Doppler ultrasound examination for DVT.
The patients’ total blood volume was calculated using Nadler’s formulae.[15]
Female blood volume (L)= height (m)3 × 0.356 + weight (kg) × 0.033 + 0.183
Male blood volume (L) = height (m)3 × 0.356 + weight (kg) × 0.032 + 0.604
Pre-operative hematocrit (HCT) was defined as the HCT on the morning of the day of surgery. Post-operative HCT was defined as post-operative 2nd day HCT. Gross formula was used to calculate the patients’ estimated TBL.[16]
Estimated TBL (L)=blood volume × (Hctpreop − Hctpostop)/([Hctpreop + Hctpostop]/2)
After the operation, the total amount of intraoperative blood loss was determined by subtracting the irrigation fluids from the total amount of liquid in the bag of the aspirator and adding the weight of the blood on the gauzes (the difference between the wet and the dry weight of the gauzes). Hidden blood loss (HBL) was computed following surgery.
HBL=estimated TBL–visible blood loss + transfusion blood
Daily hemogram and laboratory follow-ups were conducted for the first 3 post-operative days to determine any additional transfusion requirements and treatment needs. Patients were also evaluated in the first 3 months following the operation for any complications. Patients were asked to admit the hospital at the 3rd, 6th, and 12th post-operative weeks for post-operative follow-up examinations. Patients were noted for any medical and surgical post-operative complications.
The primary outcome of the study was TBL. The secondary outcomes were the number of transfusions, the surgical (intraoperative), and HBL during PFN.
A total of 102 patients, 51 in each group, were required to give the study a power of at least 80% at a two-sided type I error of 5% and an effect size of 0.5. We included 10% more patients to account for protocol violations and patients who failed follow-up and to ensure adequate power for analysis (55 patients in each group). SPSS (Statistical Package for the Social Sciences) program was used to conduct the statistical analysis (version 22.0, IBM Corp., Armonk, NY, USA). The Kolmogorov-Smirnov test was used to test the distribution of continuous variables. The independent samples t-test was used to analyze normally distributed continuous variables, while the Mann–Whitney U-test was used to analyze non-normally distributed variables. Categorical variables were evaluated using the Chi-square test. P<0.05 was considered to be statistically significant.
RESULTS
One hundred and fifty-six patients with intertrochanteric femur fracture presented to our emergency department. The study included 110 patients who met the inclusion and exclusion criteria. Patients were randomized to the TXA group (n=55) and the control group (n=55). After randomizing the patients, four patients required open reduction and four patients died during follow-up period, and these patients were excluded from the study. As a result, the final analysis included 51 patients from each group (Fig. 1). No patients were lost to follow-up except for the patients who died in the first 3 months.
Figure 1.
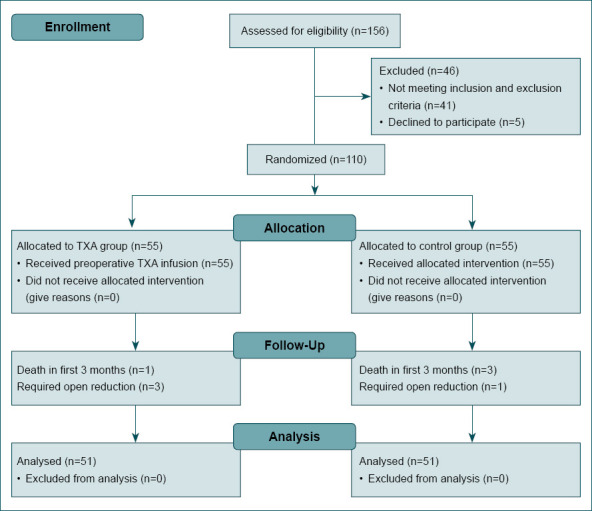
Flow diagram of the study.
The pre-operative hemoglobin and HCT values as well as their demographic characteristics did not differ statistically significant (Table 1). The mean operation time, pre-operative hospital stay, and total hospital stay were similar for the two groups.
Table 1.
Demographic characteristics of the patients included in the study
| Tranexamic acid group | Control group | p-values | |
|---|---|---|---|
| Age (years) | 76.0±18.3 | 79.8±10.5 | 0.89 |
| Gender (female/male) | 25/26 | 32/19 | 0.16 |
| Height (cm) | 166.1±7.2 | 164.9±7.4 | 0.42 |
| Weight (kg) | 74.8±9.5 | 76.1±16 | 0.61 |
| BMI | 27.1±3.9 | 27.9±5.4 | 0.44 |
| AO Classifications 31A1/31A2 | 23/28 | 27/24 | 0.43 |
| Side (right/left) | 26/25 | 23/28 | 0.55 |
| Preop Hemoglobin level (g/dl) | 11.5±1.7 | 11.0±1.4 | 0.66 |
| Preop Hematocrit level (%) | 34.9±4.8 | 33.9±4.1 | 0.31 |
| ASA Classification (I/II/III/IV) | 6/41/4/0 | 8/39/5/0 | 0.80 |
| Preop hospital stay (days) | 2.7±1.6 | 2.4±1.0 | 0.22 |
| Total hospital stay (days) | 7.4±4.4 | 7.5±4.1 | 0.95 |
| Operation time (min) | 92.4±27.5 | 91.9±27.6 | 0.15 |
BMI: Body Mass Index; AO: Arbeitsgemein schaft für Osteosynthesefragen; ASA: American Society of Anesthesiologists; cm: Centimeter; g/dl: Gram/deciliter; min: Minute.
The mean estimated total blood volume of the patients was similar in the two groups (4454.8±495.5 ml vs. 4446.9±701.1 ml, p=0.95). Comparing the TXA group to the control group, the mean TBL was statistically lower in the TXA group (684.6±370.1 ml vs. 971.2±505.3 ml, respectively; p=0.002). The average post-operative hemoglobin and HCT level at the 2nd post-operative day were significantly lower in the control group than in the TXA group (hemoglobin: 9.9±1.4 g/dl vs. 8.9±1.4, respectively [p<0.001]; HCT: 29.9±4.0% vs. 26.9±4.1%, respectively [p<0.001]). The amount of intraoperative blood loss did not differ significantly between the two groups (102.4±59.3 ml in the TXA group vs. 112.7±90.1 ml in the control group, p=0.67). However, the mean estimated HBL was significantly lower in the TXA group than in the control group (582.3±341.2 ml vs. 857.8±493.1 ml, respectively; p=0.002). The post-operative blood transfusion rate and transfusion unit were found to be significantly lower in the TXA group than in the control group (8% vs. 23.5%, respectively [p=0.033], and 6 U vs. 15 U, respectively [p=0.04] [Table 2]).
Table 2.
Comparison of the clinical outcome values of the Tranexamic acid and control group
| Tranexamic acid group | Control group | p-values | |
|---|---|---|---|
| Preop Hemoglobin level (g/dl) | 11.5±1.7 | 11.0±1.4 | 0.66 |
| Preop Hematocrit level (%) | 34.9±4.8 | 33.9±4.1 | 0.31 |
| Hemoglobin level (g/dl) postop day 2 | 9.9±1.4 | 8.9±1.4 | <0.001 |
| Hematocrit level (%) postop day 2 | 29.9±4.0 | 26.9±4.1 | <0.001 |
| Estimated total blood volume (militers) | 4454.8±495.5 | 4446.9±701.1 | 0.95 |
| Estimated total blood loss (mililiters) | 684.6±370.1 | 971.2±505.3 | 0.002 |
| Peroperative blood loss (mililiters) | 102.4±59.3 | 112.7±90.1 | 0.67 |
| Estimated hidden blood loss (mililiters) | 582.3±341.2 | 857.8±493.1 | 0.002 |
| Blood transfusion rate (yes/no) (%) | 4/47 (8%) | 12/39 (24%) | 0.033 |
| Blood transfusion unit (U) | 6 | 15 | 0.04 |
| Intraoperative fluid infusion (ml) | 1304±462.6 | 1325.5±423.7 | 0.12 |
cm: Centimeter; g/dl: Gram/deciliter; min: Minute; U: Unit; ml: Mililiter.
Three patients in the TXA group and two patients in the control group had DVT, and one patient in each group experienced minor pulmonary embolism. There was no statistically significant difference between the two groups for both DVT and pulmonary embolism. Both medical and surgical post-operative complications were found to be similar for two groups (Table 3).
Table 3.
Complications in the tranexamic acid group and control group
| Tranexamic acid group (n=51) | Control group (n=51) | p-values* | |
|---|---|---|---|
| Surgical complications | |||
| Hematoma | 1 | 2 | 1 |
| Surgical site infection | 1 | 2 | 1 |
| Implant failure | 1 | 0 | 1 |
| Medical complications | |||
| Deep vein thrombosis | 3 | 2 | 1 |
| Pulmonary embolism | 1 | 1 | 1 |
| Myocard infarctus | 0 | 0 | |
| Cerebrovascular thrombosis | 1 | 2 | 1 |
| Respiratory infection | 2 | 3 | 1 |
| Renal failure | 0 | 1 | 1 |
| Urinary tract infection | 0 | 1 | 1 |
Fisher’s exact test.
DISCUSSION
TXA is one of the agents used to reduce blood loss and the need for allogenic blood transfusion in orthopedic surgery, including total hip and knee replacement surgery, trauma surgery, and spinal surgery.[10,11,17] Few previous studies have investigated the effect of TXA on total and HBL in osteosynthesis of intertrochanteric fractures in elderly patients using PFN and during post-operative follow-up. Although surgery on these patients is generally performed with minimally invasive techniques, there may be significant blood loss that requires allogenic blood transfusion. Therefore, it is important to reduce blood loss in this fragile patient group without causing any complications. Tengberg et al.[1] estimated that there could be a TBL of up to 2100 ml in patients who undergo PFN with a diagnosis of ITFF. Five recent studies determined that TXA significantly reduced the TBL in PFN.[1,9,12–14] Zhou et al.[14] and Luo et al.[9] found that pre-operative blood loss was significantly lower in patients who were given intravenous TXA. In contrast, Lei et al.[13] found that TXA did not significantly reduce pre-operative blood loss during PFN for ITFF. In our study, it was determined that a single dose of TXA reduced pre-operative blood loss in patients with ITFFs who underwent PFN with a minimally invasive method, but the difference was not found to be significant.
The results of our study showed that approximately 88% of TBL during PFN was HBL. Intraoperative bleeding has been found to account for 12–15% of TBL in the present study. Therefore, HBL following PFN for ITFF is an important issue, and few studies in the literature have addressed this topic. The causes of post-operative occult blood loss include bleeding from the gastrointestinal tract after surgery, bleeding due to the opening of the femoral intramedullary canal, capillary vascular injury due to reaming of the femur, and bleeding from the operation site into the soft tissues.[6,18–20] Zhou et al.[14] and Tian et al.[12] found that HBL was significantly less in patients who had received TXA. Our study showed that HBL was approximately 275 ml less in the TXA group, and the difference was statistically significant. We suggest that another reason for HBL to the intramedullary canal and soft tissues in the post-operative period may be due to the removal of blood clots formed at the fracture site during nailing and the anticoagulant treatments applied to patients for the prophylaxis of thromboembolism.
Post-operative allogenic blood transfusion may cause complications, including prolonged hospital stay, increased risk of infection, an increase in the total cost of treatment, delay in rehabilitation of the patient, and transfusion-related complications.[9,21,22] TXA has been shown to significantly reduce the need for postoperative allogeneic blood transfusion in patients with hip fractures.[23–25] Luo et al.[9] concluded that TXA infusion could prevent 1.5 U of blood transfusion in every five patients as a result of reducing the total number of blood transfusions. The results of the present study support the findings reported in the literature (blood transfusion rate: 8% vs. 24%). Our study indicated that nine units of blood transfusion could be prevented in every 50 patients using a single pre-operative dose of TXA infusion.
There is no consensus in the literature regarding the dose and frequency of administration of TXA in elderly patients with intertrochanteric fractures. Reported applications in elderly patient group who underwent PFN for ITFF have included the following: 1 g pre-operative and 3 g post-operative intravenous infusion;[1] 15 mg/kg pre-operative and post-operative 3rd h intravenous administration;[9] pre-operative, intraoperative, and post-operative systemic infusion,[26] 1 g pre-operative single-dose intravenous infusion,[14] and topical injections into the surgical field.[27] Different doses and routes of administration for TXA have not been shown to significantly increase the complication risk in randomized controlled trials or in meta-analysis of the use of TXA in orthopedic surgery for healthy patients.[28–30]
The literature shows no consensus about the increased risk of thromboembolic events following TXA application. While Zufferey et al.[31] stated that TXA increased the risk of DVT, Weng et al.[32] found that the risk of DVT did not increase after TXA application. In our study, we compared the TXA and the control groups for medical and surgical complications. No significant difference was found between the groups in terms of either thromboembolic or other complications. In the light of the findings of the present study, it can be reported that a single dose of TXA infusion was safe and did not increase the risk of thromboembolic or systemic complications.
Our study has several limitations. First, our study has small number of patients. Second, the main outcomes – TBV, TBL, and HBL – were calculated using patients’ height, weight, and pre-operative/post-operative hemoglobin and HCT values using blood calculation formulas. These are only estimated values. Any changes in a patient’s height and weight may affect these calculations. Pre-operative IV rehydration, intravenous drugs, and oral fluids were not taken into account. Only intraoperative and post-operative fluids could be standardized. Therefore, hemoglobin and HCT values and blood volume calculations may have been affected. Third, not all patients were routinely screened for thromboembolic events, and subclinical thromboembolic events may not have been detected in the present study. Despite the limitations of our study, composing a homogeneous patient group by administering a single dose of TXA to elderly patients with a diagnosis of ITFF who were operated on with a minimally invasive surgical method using a single type of implant is the strength of our study. Considering that the literature contains few level one studies on the use of TXA in ITFFs in elderly patients, the fact that our study was a randomized controlled trial is another strength of this study.
Conclusion
A single dose of TXA significantly reduces TBL, HBL, and the need for blood transfusions following PFN in elderly patients with ITFFs, while it does not increase the risk of DVT or thromboembolic events. TXA can be safely used to reduce bleeding and the need for blood transfusion in elderly patients with intertrochanteric fractures.
Footnotes
Ethics Committee Approval: This study was approved by the Haseki Training and Research Hospital Clinical Research Ethics Committee (Date: 25.05.2022, Decision No: 94-2022).
Peer-review: Internally peer-reviewed.
Authorship Contributions: Concept: M.Ek.; Design: M.Ek., M.O., E.G.; Supervision: M.Ek., M.Y.; Resource: M.Ek., M.O., M.Er., E.K.; Materials: E.K., S.Ö.S.; Data: M.Ek., M.O., E.G.; Analysis: M.Ek., M.Er., E.K., S.Ö.S.; Literature search: M.Ek., M.Er., M.Y.; Writing: M.Ek., M.Er., S.Ö.S., M.Y.; Critical revision: M.Er., M.Ek., E.K., S.Ö.S., M.Y.
Conflict of Interest: None declared.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Tengberg PT, Foss NB, Palm H, Kallemose T, Troelsen A. Tranexamic acid reduces blood loss in patients with extracapsular fractures of the hip: Results of a randomised controlled trial. Bone Joint J. 2016;98:B:747–53. doi: 10.1302/0301-620X.98B6.36645. [DOI] [PubMed] [Google Scholar]
- 2.Foss NB, Kehlet H. Hidden blood loss after surgery for hip fracture. J Bone Joint Surg Br. 2006;88:1053–9. doi: 10.1302/0301-620X.88B8.17534. [DOI] [PubMed] [Google Scholar]
- 3.Swain DG, Nightingale PG, Patel JV. Blood transfusion requirements in femoral neck fracture. Injury. 2000;31:7–10. doi: 10.1016/s0020-1383(99)00191-6. [DOI] [PubMed] [Google Scholar]
- 4.Sharrock NE. Fractured femur in the elderly: Intensive perioperative care is warranted. Br J Anaesth. 2000;84:139–40. doi: 10.1093/oxfordjournals.bja.a013392. [DOI] [PubMed] [Google Scholar]
- 5.Foss NB, Kristensen MT, Kehlet H. Anaemia impedes functional mobility after hip fracture surgery. Age Ageing. 2008;37:173–8. doi: 10.1093/ageing/afm161. [DOI] [PubMed] [Google Scholar]
- 6.Foss NB, Kristensen MT, Jensen PS, Palm H, Krasheninnikoff M, Kehlet H. The effects of liberal versus restrictive transfusion thresholds on ambulation after hip fracture surgery. Transfusion. 2009;49:227–34. doi: 10.1111/j.1537-2995.2008.01967.x. [DOI] [PubMed] [Google Scholar]
- 7.Vochteloo AJ, Van Der Burg BL, Mertens BJ, Niggebrugge AH, De Vries MR, Tuinebreijer WE, et al. Outcome in hip fracture patients related to anemia at admission and allogeneic blood transfusion: An analysis of 1262 surgically treated patients. BMC Musculoskelet Disord. 2011;12:262. doi: 10.1186/1471-2474-12-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willems JM, De Craen AJ, Nelissen RG, Van Luijt PA, Westendorp RG, Blauw GJ. Haemoglobin predicts length of hospital stay after hip fracture surgery in older patients. Maturitas. 2012;72:225–8. doi: 10.1016/j.maturitas.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Luo X, He S, Lin Z, Li Z, Huang C, Li Q. Efficacy and safety of tranexamic acid for controlling bleeding during surgical treatment of intertrochanteric fragility fracture with proximal femoral nail anti-rotation: A randomized controlled trial. Indian J Orthop. 2019;53:263–9. doi: 10.4103/ortho.IJOrtho_401_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang F, Wu D, Ma G, Yin Z, Wang Q. The use of tranexamic acid to reduce blood loss and transfusion in major orthopedic surgery: A meta-analysis. J Surg Res. 2014;186:318–27. doi: 10.1016/j.jss.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 11.Poeran J, Rasul R, Suzuki S, Danninger T, Mazumdar M, Opperer M, et al. Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: Retrospective analysis of effectiveness and safety. BMJ. 2014;349:g4829. doi: 10.1136/bmj.g4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian S, Shen Z, Liu Y, Zhang Y, Peng A. The effect of tranexamic acid on hidden bleeding in older intertrochanteric fracture patients treated with PFNA. Injury. 2018;49:680–4. doi: 10.1016/j.injury.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 13.Lei J, Zhang B, Cong Y, Zhuang Y, Wei X, Fu Y, et al. Tranexamic acid reduces hidden blood loss in the treatment of intertrochanteric fractures with PFNA: A single-center randomized controlled trial. J Orthop Surg Res. 2017;12:124. doi: 10.1186/s13018-017-0625-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou XD, Zhang Y, Jiang LF, Zhang JJ, Zhou D, Wu LD, et al. Efficacy and safety of tranexamic acid in intertrochanteric fractures: A single-blind randomized controlled trial. Orthop Surg. 2019;11:635–42. doi: 10.1111/os.12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nadler SB, Hidalgo JU, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51:224–32. [PubMed] [Google Scholar]
- 16.Gross JB. Estimating allowable blood loss: Corrected for dilution. Anesthesiology. 1983;58:277–80. doi: 10.1097/00000542-198303000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Ng W, Jerath A, Wasowicz M. Tranexamic acid: A clinical review. Anaesthesiol Intensive Ther. 2015;47:339–50. doi: 10.5603/AIT.a2015.0011. [DOI] [PubMed] [Google Scholar]
- 18.Bao N, Zhou L, Cong Y, Guo T, Fan W, Chang Z, et al. Free fatty acids are responsible for the hidden blood loss in total hip and knee arthroplasty. Med Hypotheses. 2013;81:104–7. doi: 10.1016/j.mehy.2013.03.038. [DOI] [PubMed] [Google Scholar]
- 19.Smith GH, Tsang J, Molyneux SG, White TO. The hidden blood loss after hip fracture. Injury. 2011;42:133–5. doi: 10.1016/j.injury.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 20.Millar NL, Deakin AH, Millar LL, Kinnimonth AW, Picard F. Blood loss following total knee replacement in the morbidly obese: Effects of computer navigation. Knee. 2011;18:108–12. doi: 10.1016/j.knee.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Vincent JL, Baron JF, Reinhart K, Gattinoni L, Thijs L, Webb A, et al. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288:1499–507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- 22.Carson JL, Altman DG, Duff A, Noveck H, Weinstein MP, Sonnenberg FA, et al. Risk of bacterial infection associated with allogeneic blood transfusion among patients undergoing hip fracture repair. Transfusion. 1999;39:694–700. doi: 10.1046/j.1537-2995.1999.39070694.x. [DOI] [PubMed] [Google Scholar]
- 23.Farrow LS, Smith TO, Ashcroft GP, Myint PK. A systematic review of tranexamic acid in hip fracture surgery. Br J Clin Pharmacol. 2016;82:1458–70. doi: 10.1111/bcp.13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haj-Younes B, Sivakumar BS, Wang M, An VV, Lorentzos P, Adie S. Tranexamic acid in hip fracture surgery: A systematic review and meta-analysis. J Orthop Surg (Hong Kong) 2020;28:2309499019887995. doi: 10.1177/2309499019887995. [DOI] [PubMed] [Google Scholar]
- 25.Xiao C, Zhang S, Long N, Yu W, Jiang Y. Is intravenous tranexamic acid effective and safe during hip fracture surgery?An updated meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg. 2019;139:893–902. doi: 10.1007/s00402-019-03118-6. [DOI] [PubMed] [Google Scholar]
- 26.Chen F, Jiang Z, Li M, Zhu X. Efficacy and safety of perioperative tranexamic acid in elderly patients undergoing trochanteric fracture surgery: A randomised controlled trial. Hong Kong Med J. 2019;25:120–6. doi: 10.12809/hkmj187570. [DOI] [PubMed] [Google Scholar]
- 27.Drakos A, Raoulis V, Karatzios K, Doxariotis N, Kontogeorgakos V, Malizos K, et al. Efficacy of local administration of tranexamic acid for blood salvage in patients undergoing intertrochanteric fracture surgery. J Orthop Trauma. 2016;30:409–14. doi: 10.1097/BOT.0000000000000577. [DOI] [PubMed] [Google Scholar]
- 28.Lin ZX, Woolf SK. Safety, efficacy, and cost-effectiveness of tranexamic acid in orthopedic surgery. Orthopedics. 2016;39:119–30. doi: 10.3928/01477447-20160301-05. [DOI] [PubMed] [Google Scholar]
- 29.Hegde C, Wasnik S, Kulkarni S, Pradhan S, Shetty V. Simultaneous bilateral computer assisted total knee arthroplasty: The effect of intravenous or intraarticular tranexamic acid. J Arthroplasty. 2013;28:1888–91. doi: 10.1016/j.arth.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 30.Hourlier H, Fennema P. Single tranexamic acid dose to reduce perioperative morbidity in primary total hip replacement: A randomised clinical trial. Hip Int. 2014;24:63–8. doi: 10.5301/hipint.5000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zufferey PJ, Miquet M, Quenet S, Martin P, Adam P, Albaladejo P, et al. Tranexamic acid in hip fracture surgery: A randomized controlled trial. Br J Anaesth. 2010;104:23–30. doi: 10.1093/bja/aep314. [DOI] [PubMed] [Google Scholar]
- 32.Weng K, Zhang X, Bi Q, Zhao C. The effectiveness and safety of tranexamic acid in bilateral total knee arthroplasty: A meta-analysis. Medicine (Baltimore) 2016;95:e4960. doi: 10.1097/MD.0000000000004960. [DOI] [PMC free article] [PubMed] [Google Scholar]


