Abstract
Heme regulatory motifs (HRMs) are found in a variety of proteins with diverse biological functions. In heme oxygenase-2 (HO2), heme binds to the HRMs and is readily transferred to the catalytic site in the core of the protein. To further define this heme transfer mechanism, we evaluated the ability of GAPDH, a known heme chaperone, to transfer heme to the HRMs and/or the catalytic core of HO2. Our results indicate GAPDH and HO2 form a complex in vitro. We have followed heme insertion at both sites by fluorescence quenching in HEK293 cells with HO2 reporter constructs. Upon mutation of residues essential for heme binding at each site in our reporter construct, we found that HO2 binds heme at the core and the HRMs in live cells and that heme delivery to HO2 is dependent on the presence of GAPDH that is competent for heme binding. In sum, GAPDH is involved in heme delivery to HO2 but, surprisingly, not to a specific site on HO2. Our results thus emphasize the importance of heme binding to both the core and the HRMs and the interplay of HO2 with the heme pool via GAPDH to maintain cellular heme homeostasis.
Keywords: chaperone, GAPDH, glyceraldehyde-3-phosphate dehydrogenase, heme oxygenase-2, heme regulatory motifs, heme trafficking
Introduction
Heme is a vital cofactor whose concentrations must be maintained at optimal levels in living organisms because its surplus and shortage are toxic. A means by which cellular heme levels are regulated is via a degradation pathway. Humans utilize heme oxygenase (HO) to catalyze the degradation of heme in a conversion that requires cytochrome P450 reductase (CPR), NADPH, and O2 to yield biliverdin, iron, and CO. While the HO catalytic mechanism has been well characterized, further work is needed to understand how heme homeostasis is maintained by balancing degradation with other processes, including uptake, export, and biosynthesis (Chambers et al. 2021; Donegan et al. 2019; Reddi and Hamza 2016; Swenson et al. 2020). Central to that balance are the factors that determine when and how heme is delivered to HO.
Detailing the process of heme delivery to heme oxygenase-2 (HO2), the constitutively active isoform of HO, is particularly relevant to the study of heme homeostasis. HO2 and the inducible isoform of HO, heme oxygenase-1 (HO1), both bind heme at a conserved His residue in the central catalytic core of the proteins (Bianchetti et al. 2007; Schuller et al. 1999), and HO1 and HO2 degrade heme with similar catalytic efficiencies (Maines et al. 1986). However, the function and stability of HO2 is particularly sensitive to variations in cellular heme levels. We have recently demonstrated that, within the cell, HO2 binds and sequesters heme without degrading it, suggesting a novel role for HO2 in regulating heme availability by acting as a buffer or reservoir of heme below a critical threshold at which heme degradation initiates (Hanna et al. 2022). Then, under heme-deficient conditions when heme degradation is unnecessary or perhaps deleterious, apo-HO2 is destabilized and targeted for degradation by the lysosome through chaperone-mediated autophagy (Liu et al. 2020).
In addition to the sensitivity of its core region to heme bioavailability, HO2 can bind up to two more molecules of heme at each of its heme regulatory motifs (HRMs), which can potentially enhance the responsiveness of HO2 to changes in heme levels. These HRMs (Zhang and Guarente 1995), are located in the C-terminal “tail” of HO2, an unstructured region that is situated between its core and membrane interacting region. Centered at Cys265-Pro266 and Cys282-Pro283, the two HRM sites act as a thiol/disulfide redox switch, in which the thiolates of Cys265 and Cys282 bind Fe3+-heme, albeit with weaker affinity than the core, upon reduction of a disulfide bond (Bagai et al. 2015; Fleischhacker et al. 2015; Yi and Ragsdale 2007; Yi et al. 2009). We have recently demonstrated that heme bound to the HRMs can be transferred to the core and that heme can also equilibrate between the core and the tail (Fleischhacker et al. 2020) (Figure 1), suggesting that all three heme binding sites of HO2 can be in equilibrium with the cellular heme pool.
Figure 1:

Heme delivery to and heme transfer within HO2. GAPDH is proposed to deliver heme to apo-HO2 (top). Once heme is bound to HO2, it can be transferred between the catalytic core region (with ligation via His45, red) and the C-terminal HRMs (centered at Cys265 and Cys282, blue) of HO2. A linear representation of HO2 (bottom) indicates residues involved in heme binding.
HO2 thus appears to play a key role in heme trafficking and homeostasis, but critical details are missing regarding heme delivery to HO2, preventing full comprehension of the complex role of this protein in regulating and responding to heme levels. We therefore hypothesized that HO2 might interact with a heme chaperone such as glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Sweeny et al. 2018) to acquire its heme (Figure 1). GAPDH has been shown to deliver heme to soluble guanylyl cyclase (Dai et al. 2020), inducible nitric oxide synthase (Chakravarti et al. 2010), hemoglobin and myoglobin (Tupta et al. 2022), and indoleamine dioxygenase and tryptophan dioxygenase (Biswas et al. 2022). We therefore developed several HO2 reporter constructs that allowed us to follow heme insertion by fluorescence quenching. Here, we demonstrate that heme delivery to both the core and the HRMs of HO2 is dependent on the presence of GAPDH and its ability to bind heme.
Results
GAPDH interacts with HO2
We used fluorescence polarization methods to probe whether purified GAPDH could form a direct complex with previously characterized truncated, soluble HO2 constructs (Figure 1) (Fleischhacker et al. 2015; Yi and Ragsdale 2007): HO2 (1–248) that spans the core region, HO2 (213–288) that spans the HRM-containing region, and HO2 (1–288) that spans both regions. A set concentration of fluorescein isothiocyanate (FITC)-labeled HO2 proteins (1 µM) was titrated with increasing amounts of GAPDH and their binding was assessed by measuring the level of residual fluorescence polarization, which increases upon protein complex formation because of the complex having a slower tumbling rate (Sarkar et al. 2015). Figure 2 shows that GAPDH bound to all three HO2 proteins at micromolar concentrations; GAPDH binding was saturable within this range for the full-length and 1–248 fragment. HO2 (1–288) displayed the highest affinity toward GAPDH (EC50 = 0.2 ± 0.1 μM), followed by HO2 (1–248) (EC50 = 1.7 ± 0.4 μM), whereas HO2 (213–288) had notably lower GAPDH binding affinity. Together, our studies show that HO2 can form a direct complex with GAPDH, and its affinity toward GAPDH is influenced by both N- and C-terminal structural elements.
Figure 2:
Binding interaction of HO2 and its fragments with GAPDH. The panels show the change in residual polarized fluorescence of FITC-labeled HO2 protein constructs during titration with GAPDH. (A) FITC-labeled full-length HO2 (1–288). (B) FITC-labeled HO2 (1–248). (C) FITC-labeled HO2 (213–288). Points are the mean ± SD for three samples and are representative of three independent experiments
Design and characterization of HO2 reporter constructs to monitor heme insertion
Our objective was to incorporate the TC motif, CCPGCC (Adams et al. 2002; Griffin et al. 1998; Martin et al. 2005), into HO2 near each type of heme binding site (the catalytic core and the HRMs) to report on site-specific heme acquisition. A biarsenical indicator dye, FlAsH, binds to the TC motif and becomes fluorescent. However, the FlAsH fluorescence is quenched when heme binds to the FlAsH-labeled, TC motif-containing protein, as demonstrated with another heme-binding protein soluble guanylyl cyclase (Hoffmann et al. 2011). Here, we incorporated TC motifs into HO2 (1–248) that spans the core region, HO2 (213–288) that spans the HRM-containing region, and HO2 (1–288) that spans both regions (Figure 1) and characterized our reporter constructs in vitro.
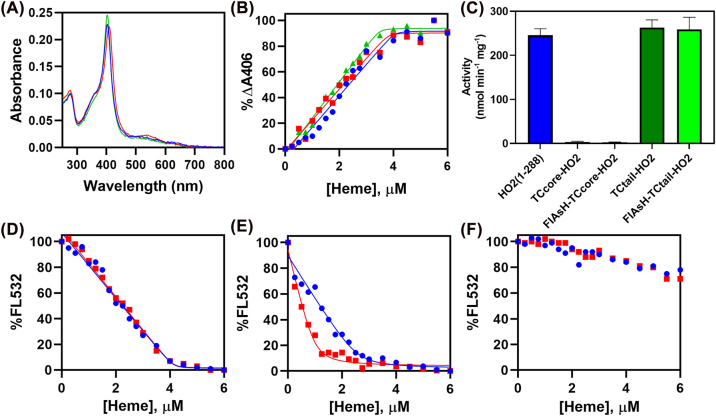
We identified three potential locations within the core region to change a series of amino acids to CCPGCC. Specifically, we looked for loop regions with a proline (D90HPAFA95, Q126CPKAA131, and K176LPSTG182, as indicated in Figure 3A) to introduce the TC motif. While mutation of either D90HPAFA95 or Q126CPKAA131 to CCPGCC yielded protein that no longer bound heme, the change of K176LPSTG182 to CCPGCC did not significantly alter the structure of the core region, as judged by CD spectroscopy (Figure 3B), or its ability to bind heme (Figure 3C, D). Therefore, we proceeded with this variant, which we will refer to as TCcore-HO2. A red shift of 3–4 nm was observed in the Soret maximum of the heme-bound form of TCcore-HO2 (1–248) with and without FlAsH bound; the only other difference in the spectra was appearance of the peak centered at 510 nm due to the binding of FlAsH to the TC motif of TCcore-HO2 (1–248) (Adams and Tsien 2008). TCcore-HO2 (1–248), with and without FlAsH bound, was then titrated with heme and the change in absorbance of the Soret peak was monitored (Figure 3D). The resulting data fit to a KD of 3.6 nM, the reported binding affinity of HO2 (1–248) (Carter et al. 2017), demonstrating that the affinity of the protein for heme is not significantly altered by the incorporation of the TC motif or by FlAsH binding to the incorporated TC motif. Further, the fluorescence of FlAsH-bound TCcore-HO2 (1–248) was quenched upon titration with heme as expected, and, as with the absorbance titration, the data fit to a KD of 3.6 nM (Figure 3E).
Figure 3:
Effect of the TC motif on heme binding to the HO2 catalytic core, HO2 (1–248), and HRM-containing tail, HO2 (213–288). (A) The loop region (K176-G181, teal) adjacent to the distal helix was substituted with a TC motif in TCcore-HO2, as indicated on the structure of the catalytic core of heme-bound HO2 (PDB code 2RGZ). Two other regions, indicated in yellow, were also changed to a TC motif, but those variants were not investigated further. (B) Overlay of the CD spectra of HO2 (1–248) (blue) and TCcore-HO2 (1–248) (red). (C) Spectra of the heme-bound forms of HO2 (1–248) (blue), TCcore-HO2 (1–248) (red), and FlAsH bound TCcore-HO2 (1–248) (green). (D) Difference titrations of HO2 (1–248) (blue), TCcore-HO2 (1–248) (red), and FlAsH-bound TCcore-HO2 (1–248) (green) with heme. The percent of the total difference in absorbance at 406 nm was plotted and fit to a KD of 3.6 nM. (E) The difference in absorbance of FlAsH bound TCcore-HO2 (1–248) upon titration with heme (green) is plotted against the percent of total fluorescence intensity at 532 nm after excitation at 485 nm as heme was added to the protein (orange). (F–H) Same as panels (A–C) except with C282A HO2 (213–288) (blue), TCtail-HO2 (213–288) (red), and FlAsH-bound TCtail-HO2 (213–288) (green). The change in fluorescence of FlAsH bound TCtail-HO2 (213–288) is plotted in orange in H.
The TC motif was incorporated into the HRM-containing region at HRM2, which is centered at C282P283. Because of the very weak affinity (KD = 0.9 μM) (Fleischhacker et al. 2015), we expect this HRM to only bind heme under conditions of heme excess. Replacement of S281CPFRT286 with CCPGCC created what we will refer to as TCtail-HO2. The maximum absorbance of the visible spectrum of the heme-bound form of TCtail-HO2 (213–288) with and without FlAsH bound matched that of C282A HO2 (213–288) (Figure 3F); again, the only other difference was the peak at 510 nm due to the bound FlAsH (Adams and Tsien 2008). Following the increase in the Soret peak of the absorption spectrum, heme titration of TCtail-HO2 (213–288), with and without FlAsH bound (Figure 3G), fit to a KD of 55 nM, which is the reported value of C282A HO2 (213–288) (Fleischhacker et al. 2020), demonstrating that neither incorporation of the TC motif nor FlAsH alter affinity of the protein for heme. Further, the fluorescence of FlAsH-bound TCtail-HO2 (213–288) was quenched upon titration with heme according to a KD of 55 nM (Figure 3H).
The insertion of a TCtail and the binding of FlAsH to the HRM region was not expected to affect heme binding to the core given that heme binding to the core or tail region is independent of the other (Fleischhacker et al. 2015). Indeed, heme titrations of wild type, TCcore, and TCtail versions of HO2 (1–288) yielded a change in the Soret peak that fit to a KD of 3.6 nM (Figure 4B), identical to that shown above for HO2 (1–248). As for HO2 (1–248), a red shift of 3–4 nm was again observed in the Soret maximum of the heme-bound TCcore-HO2 (1–288) but not for TCtail-HO2 (1–288) (Figure 4A).
Figure 4:
Characterization of TC motif-containing HO2 (1–288). (A) Spectra of the heme-bound forms of HO2 (1–288) (blue), TCcore-HO2 (1–288) (red), and TCtail-HO2 (1–288) (green). (B) Difference titrations of HO2 (1–288) (blue), TCcore-HO2 (1–288) (red), and TCtail-HO2 (1–288) (green) with heme. The percent of the total difference in absorbance at 406 nm was plotted and fit to a KD of 3.6 nM. (C) Steady state activity of HO2 (1–288) as compared to the activity of TCcore-HO2 (1–288) and TCtail-HO2 (1–288) with and without FlAsH bound. (D) The change in the percent of total fluorescence intensity at 532 nm after excitation at 485 nm as heme was added to TCcore-G163D/C265A/C282A-HO2 (1–288) (blue) and TCtail-G163D/C265A-HO2 (1–288) (red). Data was fit to a KD of 3.6 nM. (E) The change in the percent of total fluorescence intensity at 532 nm after excitation at 485 nm as heme was added to TCcore-H45W/G159W-HO2 (1–288) (blue) and TCtail-H45W/G159W-HO2 (1–288) (red). Both curves were fit to a KD of 55 nM. (F) The change in the percent of total fluorescence intensity at 532 nm after excitation at 485 nm as heme was added to TCcore-H45W/G159W/C265A/C282A-HO2 (1–288) (blue) and TCtail-H45W/G159W/C265A-HO2 (1–288) (red).
Given that incorporation of the TC motif into the core shifted the Soret spectrum of both TCcore-HO2 (1–248) and TCcore-HO2 (1–288), we tested their catalytic activities. We observed a 100-fold decrease in steady state activity (Figure 4C), indicating that incorporation of TCcore in the loop region between the distal helix and helix A6 of HO2 may have disrupted the hydrogen bonding network in the active site and/or flexibility of the distal helix. Similar minor changes in heme binding affinity and Soret maxima have been noted for HO1 and HO2 with mutations in key active site glycine residues that dramatically affect catalytic activity (Liu et al. 1999; Liu et al. 2020). Alternatively, incorporation of the TC motif could prevent CPR from binding to HO2 given that the FMN binding domain of CPR interacts with the distal helix while its FAD binding domain interacts with helix A6 (Spencer et al. 2014; Sugishima et al. 2014). While more studies would be needed to confirm either hypothesis, our results suggest the reporter constructs will effectively monitor heme binding, our intended objective for this study.
We next tested whether incorporation of a TC motif near each type of heme binding site in HO2 would report on site-specific heme acquisition. To answer this question, we created variants of TCcore- or TCtail-HO2 (1–288) that would eliminate heme binding to a specific site and monitored heme binding to the FlAsH-labeled proteins by fluorescence quenching. We used the C265A/C282A variant to eliminate heme binding to the HRMs (Fleischhacker et al. 2015; Yi and Ragsdale 2007) and the H45W/G159W variant to eliminate heme binding to the core (Fleischhacker et al. 2020). In addition, we used the G163D variant, which retains wild type heme binding affinity but is inactive (Liu et al. 2020). Surprisingly, the fluorescence of FlAsH bound to the core (Figure 4D) or to the tail (Figure 4E) is quenched by heme binding to either site. The difference in these heme titrations is likely because TCcore-H45W/G159W-HO2 (1–288) retains heme binding to both HRMs; however, in TCtail-H45W/G159W-HO2 (1–288), heme can only bind to Cys265 (Fleischhacker et al. 2015) since Cys282 is inactivated by the incorporation of the TC motif. Further, the fluorescence of FlAsH bound to TCcore or to TCtail is relatively steady upon heme titration of proteins with mutations in all the heme binding sites (Figure 4F), but some quenching is observed once heme levels are above the protein concentration (∼2 μM), suggesting some non-specific association of heme with HO2. In sum, the TC motif containing HO2 constructs efficiently report on heme acquisition, but the assays do not give site-specific information regarding heme binding without the use of additional mutations to inactivate selected heme binding sites.
Cellular heme delivery to HO2 correlates with the GAPDH expression level and relies on the heme binding ability of GAPDH
We tested the involvement of GAPDH in heme delivery to HO2 using an established mammalian cell culture based strategy (Dai et al. 2020). Briefly, we first determined whether decreasing cellular GAPDH expression levels by siRNA knockdown would inhibit heme acquisition by HO2. If affirmative, we then tried to rescue the inhibition by transiently expressing siRNA-resistant forms of WT GAPDH or H53A GAPDH, a variant that is unable to bind heme. If only exogenous WT GAPDH would support normal HO2 heme acquisition in the knockdown cells, we scored HO2 as having GAPDH-dependent heme delivery.
In our experiments, we utilized the TC-HO2 constructs to monitor heme incorporation in real time. In these assays, quenching of FlAsH fluorescence signifies heme binding to HO2. Briefly, HEK293 cells were initially heme-depleted by culturing with the heme biosynthesis inhibitor succinyl acetone (SA) for 72 h before being transiently transfected to express the various TC-HO2 constructs. This ensures the expression and accumulation of heme-free (apo) TC-HO2 proteins in cells. After 24 h, we labeled the expressed apo-TC-HO2 proteins with the FlAsH reagent. Heme was then added to the cell cultures in heme-depleted media without SA, and we assessed heme insertion into the FlAsH-labeled apo-TC-HO2 proteins by monitoring changes in cellular fluorescence emission, which declines if heme binds to either the core or the tail of HO2.
We first tested the three TC-HO2 proteins with TC motifs in the catalytic core. Results for the simplest of these (TCcore), which has no additional mutations besides the TC insertion, are shown in Figure 5. Cells expressing FlAsH-apo-TCcore-HO2 that received no heme (vehicle negative control) maintained a steady fluorescence intensity over the 2 h time course, indicating their heme level remained unchanged (Figure 5A). In contrast, the cells that received heme showed a steady loss in FlAsH fluorescence, indicating heme was incorporated into the FlAsH-labeled apo-TCcore-HO2 over the same period (Figure 5A). In the GAPDH knockdown cells, there was comparatively less fluorescence loss after the heme addition (Figure 5B); however, cells receiving scrambled siRNA experienced a decrease in fluorescence after heme addition. Thus, a diminished GAPDH expression correlated with poor heme delivery to the apo-TCcore-HO2. Notably, normal heme delivery to this protein was restored in the knockdown cells by co-expression of WT HA-GAPDH, but not by co-expression of the heme binding-defective HA-GAPDH H53A variant (Figure 5C). Our results indicate that cellular heme delivery to apo-TCcore-HO2 depends on the heme-binding ability of GAPDH.
Figure 5:
Heme delivery to apo-TCcore-HO2 proteins in living cells and the importance of GAPDH. The apo-TCcore-HO2 protein (A–C) or the G163D/C265A/C282A (D–F) or the H45W/G159W (G–I) variants of the TCcore-HO2 protein was expressed in heme-deficient HEK293 cells and FlAsH-labeled. The change in fluorescence intensity versus time was monitored after adding vehiclHO2 ( buffer or 5 µM heme to the cell cultures, and a decrease in FlAsH fluorescence intensity indicates heme incorporation. In this Figure, “siGAPDH” indicates heme was added to cells transfected with siGAPDH RNA; “scramble RNA” indicates heme was added to cells transfected with scramble RNA; “HA-GAPDH H53A” indicates heme was added to cells transfected with siGAPDH RNA and siRNA resistant HA-GAPDH H53A; and “HA-GAPDH WT” indicates heme was added to cells transfected with siGAPDH RNA and siRNA resistant HA-GAPDH. Kinetic traces are the mean ± SD of three wells and are representative of two independent experiments.
We next tested two other FlAsH-labeled TC-HO2 core proteins with the same strategy: (i) TCcore-G163D/C265A/C282A, which binds heme only at the core and is catalytically inactive, and (ii) TCcore-H45W/G159W, which only binds heme at the HRMs. As shown in Figure 5, the results for both constructs were like those we observed with the TCcore-HO2 construct. The results indicate that heme delivery to both heme binding sites relies on proper levels and the heme binding ability of the cellular GAPDH. Overall, these results are consistent with our in vitro studies that indicated a FlAsH TC label located in the HO2 core can detect heme binding in the HO2 core or tail and further confirm that, in living cells, heme binding to the HRMs of HO2 can occur independent of any heme binding in the HO2 core site.
We then tested the three TC-HO2 proteins that contain a TC motif at the tail. Results for TCtail, which has no additional mutations, are shown in Figure 6. The results were very similar to those observed with the TCcore-HO2 protein in Figure 5, further supporting that cellular heme delivery to HO2 depends on the GAPDH expression level and its ability to bind heme. We then examined cellular heme delivery to the two TCtail-HO2 variants that bind heme only at the core (TCtail-G163D/C265A) or at the tail (TCtail-H45W/G159W). The results with both constructs clearly demonstrated heme binding to either the HO2 core or tail, and further confirmed that cell heme delivery to apo-TC-HO2 is GAPDH-dependent and relies on the heme binding ability of GAPDH.
Figure 6:
Heme delivery to apo-TCtail-HO2 proteins in living cells and the importance of GAPDH. The apo-TCtail-HO2 protein (A–C) or the G163D/C265A (D–F) or the H45W/G159W (G–I) variants of the TCtail-HO2 protein was expressed in heme-deficient HEK293 cells and FlAsH-labeled. The change in fluorescence intensity versus time was monitored after adding vehicle buffer or 5 µM heme to the cell cultures, and a decrease in FlAsH fluorescence intensity indicates heme incorporation. In this Figure, “siGAPDH” indicates heme was added to cells transfected with siGAPDH RNA; “scramble RNA” indicates heme was added to cells transfected with scramble RNA; “HA-GAPDH H53A” indicates heme was added to cells transfected with siGAPDH RNA and siRNA resistant HA-GAPDH H53A; and “HA-GAPDH WT” indicates heme was added to cells transfected with siGAPDH RNA and siRNA resistant HA-GAPDH. Kinetic traces are the mean ± SD of three wells and are representative of two independent experiments.
Discussion
Our results demonstrate that cellular heme delivery to HO2, like six other proteins (soluble guanylyl cyclase, inducible nitric oxide synthase, hemoglobin, myoglobin, indoleamine dioxygenase and tryptophan dioxygenase) is GAPDH-dependent. In most of these systems, HSP90 is also involved in heme transfer, presumably to catalyze local unfolding of the heme binding site (Biswas et al. 2022). Heme acquisition by HO2 both correlates with the GAPDH expression level and relies on the specific ability of GAPDH to bind intracellular heme. The siRNA knockdown of GAPDH and siRNA-resistant GAPDH expression rescue approaches (Dai et al. 2020) helped to obtain a reduced background of native GAPDH expression. In turn, this approach provided a sensitive test for GAPDH-dependent heme delivery. We have previously demonstrated that repressing GAPDH expression does not indirectly diminish cellular heme delivery by impacting other functions of GAPDH such as its dehydrogenase activity for cell glycolysis (Chakravarti et al. 2010; Dai et al. 2020; Sweeny et al. 2018). Therefore, these experiments report on the direct delivery of heme by GAPDH to target proteins. Our in cellulo results, along with our demonstration that HO2 and GAPDH form a complex in vitro, thus support the hypothesis that GAPDH functions to deliver heme to apo-HO2. The potential involvement of HSP90 still needs to be explored.
While GAPDH is involved in heme delivery to HO2, it does not appear to deliver heme specifically to the catalytic core or to the HRM-containing tail of HO2. Using variants of the TCcore-HO2 and TCtail-HO2 that bind heme only in the catalytic core or only at the HRMs, our cellular experiments suggest that heme is delivered to either site in a GAPDH-dependent process. Further, our in vitro experiments with purified proteins suggests that truncated proteins HO2 (1–248), which lacks the tail, and HO2 (213–288), lacking the core, also associate with GAPDH. However, HO2 (1–288), which spans both regions of the protein, displayed higher affinity toward GAPDH than either of the truncated forms. Thus, we hypothesize that GAPDH may need to contact both regions of HO2 for optimal binding, but future studies will focus on obtaining the residue-specific information needed to define the protein-protein interaction interface.
Our reporter constructs unfortunately did not give site-specific information regarding heme binding without the use of additional mutations in selected heme binding sites, so we were unable to determine if there was any order or preference of binding. However, our experiments using the H45W/G159W variant of our reporter constructs provide the first direct in cellulo evidence that heme binds to the HRMs. As discussed previously (Fleischhacker et al. 2018, 2022), in vitro comparisons of the cellular heme levels and measurements of the heme binding affinity of the HRMs suggest that the HRM centered at Cys265-Pro266 is likely to bind heme in vivo when not in a disulfide bond, but in vivo binding had not been previously reported. Thus, the HRMs could still serve as HO2’s link to the cellular heme pool, but further experiments would be needed to confirm that heme transfer from the HRMs to the core is functionally relevant in live cells.
Also significant in our cellular experiments using reporter constructs is the demonstration that HO2 binds and apparently retains heme at the core. The fluorescence of FlAsH-bound TCcore-HO2, TCtail-HO2, and G163D/C265A TCtail-HO2 reporter constructs exhibited similar fluorescence quenching profiles in live cells, reflecting the comparable heme affinities of the core and tail. However, while TCtail-HO2 can also degrade heme, TCcore-HO2 and the G163D/C265A and H45W/G159W variants of TCtail-HO2 cannot. Yet we do not observe differences in the degree to which the fluorescence is quenched. Thus, we may be observing the ability of HO2 to bind and sequester heme without degrading it, as reported previously (Hanna et al. 2022). Alternatively, the levels of heme in the experiment may not have reached the critical threshold when heme degradation is initiated, or perhaps heme degradation intermediates or products could quench the fluorescence of protein-bound FlAsH.
The siRNA knockdown and siRNA-resistant GAPDH expression rescue method has been used to identify several heme proteins that use GAPDH as a heme delivery partner. However, while the other identified GAPDH target proteins require heme for catalytic activity or gas binding, HO2 represents a new type of GAPDH target that binds heme for sequestration or degradation. We are thus interested in using the same approach to investigate heme delivery to a wide variety of other heme proteins, e.g., other HRM-containing proteins and HO1, to define the types of heme binding sites that GAPDH targets. In sum, our results emphasize the importance of heme binding to both the core and the HRMs of HO2 as well as the interplay of HO2 and GAPDH with the heme pool to maintain cellular heme homeostasis.
Materials and methods
Protein expression and purification
As described previously (Fleischhacker et al. 2015), the soluble form of human HO2 spanning residues 1–248 [HO2 (1–248]) was expressed from pET28a and the soluble form of human HO2 spanning residues 213–288 [HO2 (213–288)] (wild type or the C282A variant) was expressed from a modified version of expression vector pMCSG7 (Stols et al. 2002) containing the protein GB1 domain (vector generously provided by W. C. Brown of the High-throughput Protein Lab in the Center for Structural Biology at the University of Michigan). HO2 point mutations were generated by site-directed mutagenesis using the Q5 site-directed mutagenesis kit (New England Biolabs Inc., Ispwich, MA) in a pMSCG10 (Eschenfeldt et al. 2009) vector harboring HO2 (1–288) described previously (Kochert et al. 2019). However, due to poor protein expression (data not shown), HO2 was subcloned using the HiFi DNA Assembly Cloning kit (New England Biolabs Inc.) into NdeI and EcoRI sites of a pET28a vector in which the thrombin cleavage site had been changed to a tobacco etch virus (TEV) protease cleavage site using the Q5 site-directed mutagenesis kit (New England Biolabs, Inc). HO2 (1–248) containing a tetracysteine (TC) motif was constructed from HO2 (1–288) containing the TC motif by deleting amino acids 249–288 using the Q5 site-directed mutagenesis kit (New England Biolabs Inc.). All proteins were expressed in BL21 (λDE3) cells using growth conditions described previously (Bagai et al. 2015). Proteins were purified on a Ni-nitrilotriacetic acid affinity column (Qiagen, Hilden, Germany) before the affinity tag was removed by treatment with either thrombin (Bagai et al. 2015) or TEV protease (Spencer et al. 2014) followed by a second Ni-nitrilotriacetic acid affinity column.
Fluorescence polarization measurements
FITC labeling of purified HO2 proteins and residual fluorescence polarization measurements were performed at room temperature using methods described previously (Dai et al. 2020). Curves were fitted using following equation:
| (1) |
CD spectroscopy
CD spectra of HO2 proteins (5 μM in 50 mM potassium phosphate buffer, pH 8.0) were acquired on a J-1500 CD Spectrometer (JASCO, Easton, MD). Each spectrum was acquired with five scans in a cuvette with a path length of 0.1 cm.
FlAsH labeling of proteins
HO2 proteins containing a TC motif were labeled with FlAsH as described previously (Pan et al. 2016) with minor modifications. Briefly, HO2 protein (50 μM in 50 mM potassium phosphate buffer, pH 8.0) was reduced with 1 mM tris(2-carboxyethyl)phosphine in an anaerobic chamber for 30 min. FlAsH-EDT2 (Cayman Chemical, Ann Arbor, MI or Toronto Research Chemicals, Toronto, ON) was added to a final concentration of 75 μM for 1 h at room temperature while protected from light. Excess FlAsH-EDT2 was removed by applying the mixture to either a PD-10 column (Cytiva, Buckinghamshire, UK) or Micro BioSpin 6 columns (Bio-Rad, Hercules, CA).
Heme titrations
A heme stock was freshly prepared in 50 mM potassium phosphate buffer, pH 8.0 with 15% dimethyl sulfoxide and 0.1 M NaOH. The stock was passed through a 22 μm filter to remove insoluble matter, and the concentration was determined in 0.1 M NaOH using an extinction coefficient of 58.4 mM−1 cm−1 at 385 nm (Dawson et al. 1969). Varying concentrations of heme were added to protein (∼2 μM) in Eppendorf tubes and left at room temperature for 1 h. The protein-heme samples were transferred to either a clear bottom 96-well plate for absorbance readings and/or a black bottom 96-well plate for fluorescence readings. For absorbance readings, samples without protein (at varying heme concentrations) were also transferred to the 96-well plate. Samples were read after a 1 h incubation at room temperature on a Safire 2 multi-detection plate reader (Tecan, Mannedorf, Switzerland). For absorbance readings, the difference in absorbance between the protein-heme and heme-only samples at the indicated wavelength were calculated, converted to a percent total difference in absorbance, plotted as a function of the heme concentration, and fit using a tight binding (quadratic) equation in GraphPad Prism 8 as described previously (Yi and Ragsdale 2007), setting KD to the indicated value. For fluorescence readings, the excitation wavelength was set to 485 nm, the emission at 532 nm, converted to a percent total observed difference in fluorescence, was plotted as a function of the heme concentration, and the resulting data was fit using a tight binding (quadratic) equation GraphPad Prism 8 as described previously (Yi and Ragsdale 2007), setting KD to the indicated value. For FlAsH-labeled samples, samples were kept under minimal light. For samples in which heme-binding to an HRM was monitored, all work was conducted in an anaerobic chamber using protein that had been treated with tris(2-carboxyethyl)phosphine to reduce any disulfide bonds (Yi and Ragsdale 2007).
Heme oxygenase activity assays
Steady-state activity of HO was measured as described previously (Spencer et al. 2014). Briefly, a reaction containing 0.1–1 μM HO, 15 μM heme, 1 μM biliverdin reductase, 0.25 mg/ml BSA, 20 units of catalase, and 5 μM truncated CPR in 50 mM Tris (pH 8.0), 50 mM KCl was incubated for 2 min at 37 °C. The reaction was initiated by the addition of 1 μl of 100 mm NADPH. Activity was monitored by following the increase in absorbance at 468 nm due to bilirubin formation. Using a difference extinction coefficient of 43 mM−1 cm−1, activity was calculated as nmol of bilirubin formed/min/mg of HO. A truncated, soluble form of CPR lacking the N-terminal amino acids (residues 1–66) that act as the membrane anchor and biliverdin reductase were purified as described (Spencer et al. 2014).
Plasmid constructs and mutagenesis
Full-length human HO2 (amino acids 1–316) cDNA was subcloned into a pcDNA3.1 (+) vector using HindIII and XhoI restriction sites (Liu et al. 2020; Yi et al. 2009). HO2 point mutations were generated by site-directed mutagenesis using the Q5 site-directed mutagenesis kit (New England Biolabs Inc.). pcDNA3.1 plasmids encoding HA-tagged human GAPDH WT or H53A that are resistant to GAPDH siRNA (Dharmacon, Lafayette, CO) were reported previously (Dai et al. 2020).
Cell culture and transient transfection
HEK293 cells were cultured on fluorescent 96-well plates as described elsewhere (Dai et al. 2020). TC-HO2 and HA-GAPDH WT and mutant proteins were transfected into HEK293 cells using a method reported previously (Dai et al. 2020). GAPDH knockdown was performed using GAPDH siRNA according to a method reported previously (Dai et al. 2020). In some cases, the heme biosynthesis inhibitor SA was added at 400 µM 72 h prior to transfection to enable cell accumulation of apo-TC-HO2, and then SA was removed after FlAsH labeling.
Monitoring heme insertion into HO2 in cells
TC-HO2 proteins expressed in HEK293 cells were labeled with the TC-FlAsH In-Cell Tetra Cysteine Tag Detection Kit (Invitrogen, Waltham, MA) using a method reported previously (Dai et al. 2020). Heme insertion into FlAsH-labeled TC-HO2 proteins expressed in cells was monitored at 37 °C on an iD5 max plate reader instrument (Molecular Devices, San Jose, CA) as reported previously (Dai et al. 2020).
Acknowledgements
We thank Prof. Zhan Chen and lab members for assistance with CD experiments, Dr. Anindita Sarkar for helpful discussion and valuable feedback, and Claire Maiocco for assistance with method development.
Footnotes
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: This work was supported by National Institutes of Health Grants R01GM130624 (to D.J.S.), R01-GM123513 (to S.W.R.) and R35-GM141758 (to S.W.R.).
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
- Adams S.R., Campbell R.E., Gross L.A., Martin B.R., Walkup G.K., Yao Y., Llopis J., Tsien R.Y. New biarsenical ligands and tetracysteine motifs for protein labeling in vitro and in vivo: synthesis and biological applications. J. Am. Chem. Soc. 2002;124:6063–6076. doi: 10.1021/ja017687n. [DOI] [PubMed] [Google Scholar]
- Adams S.R., Tsien R.Y. Preparation of the membrane-permeant biarsenicals FlAsH-EDT2 and ReAsH-EDT2 for fluorescent labeling of tetracysteine-tagged proteins. Nat. Protoc. 2008;3:1527–1534. doi: 10.1038/nprot.2008.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagai I., Sarangi R., Fleischhacker A.S., Sharma A., Hoffman B.M., Zuiderweg E.R.P., Ragsdale S.W. Spectroscopic studies reveal that the heme regulatory motifs of heme oxygenase-2 are dynamically disordered and exhibit redox-dependent interaction with heme. Biochemistry. 2015;54:2693–2708. doi: 10.1021/bi501489r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchetti C.M., Yi L., Ragsdale S.W., Phillips G.N. Comparison of apo- and heme-bound crystal structures of a truncated human heme oxygenase-2. J. Biol. Chem. 2007;282:37624–37631. doi: 10.1074/jbc.m707396200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas P., Dai Y., Stuehr D.J. Indoleamine dioxygenase and tryptophan dioxygenase activities are regulated through GAPDH- and Hsp90-dependent control of their heme levels. Free Radic. Biol. Med. 2022;180:179–190. doi: 10.1016/j.freeradbiomed.2022.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter E.L., Ramirez Y., Ragsdale S.W. The heme-regulatory motif of nuclear receptor Rev-erb beta is a key mediator of heme and redox signaling in circadian rhythm maintenance and metabolism. J. Biol. Chem. 2017;292:11280–11299. doi: 10.1074/jbc.m117.783118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarti R., Aulak K.S., Fox P.L., Stuehr D.J. GAPDH regulates cellular heme insertion into inducible nitric oxide synthase. Proc. Natl. Acad. Sci. U.S.A. 2010;107:18004–18009. doi: 10.1073/pnas.1008133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers I.G., Willoughby M.M., Hamza I., Reddi A.R. One ring to bring them all and in the darkness bind them: the trafficking of heme without deliverers. Biochim. Biophys. Acta Mol. Cell Res. 2021;1868:118881. doi: 10.1016/j.bbamcr.2020.118881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y., Sweeny E.A., Schlanger S., Ghosh A., Stuehr D.J. GAPDH delivers heme to soluble guanylyl cyclase. J. Biol. Chem. 2020;295:8145–8154. doi: 10.1074/jbc.ra120.013802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson R.M.C., Elliott D.C., Elliott W.H., Jones K.M. Data for biochemical research . 2nd ed. Oxford, UK: Oxford University Press; 1969. [Google Scholar]
- Donegan R.K., Moore C.M., Hanna D.A., Reddi A.R. Handling heme: the mechanisms underlying the movement of heme within and between cells. Free Radic. Biol. Med. 2019;133:88–100. doi: 10.1016/j.freeradbiomed.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenfeldt W.H., Stols L., Millard C.S., Joachimiak A., Donnelly M.I. In: High throughput protein expression and purification . Doyle S.A., editor. Humana Press; Humana Totowa, NJ: 2009. A family of LIC vectors for high-throughput cloning and purification of proteins; pp. 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischhacker A.S., Carter E.L., Ragsdale S.W. Redox regulation of heme oxygenase-2 and the transcription factor, Rev-Erb, through heme regulatory motifs. Antioxid. Redox Signaling. 2018;29:1841–1857. doi: 10.1089/ars.2017.7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischhacker A.S., Gunawan A.L., Kochert B.A., Liu L., Wales T.E., Borowy M.C., Engen J.R., Ragsdale S.W. The heme-regulatory motifs of heme oxygenase-2 contribute to the transfer of heme to the catalytic site for degradation. J. Biol. Chem. 2020;295:5177–5191. doi: 10.1074/jbc.ra120.012803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischhacker A.S., Sarkar A., Liu L., Ragsdale S.W. Regulation of protein function and degradation by heme, heme responsive motifs, and CO. Crit. Rev. Biochem. Mol. Biol. 2022;57:16–47. doi: 10.1080/10409238.2021.1961674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischhacker A.S., Sharma A., Choi M., Spencer A.M., Bagai I., Hoffman B.M., Ragsdale S.W. The C-terminal heme regulatory motifs of heme oxygenase-2 are redox-regulated heme binding sites. Biochemistry. 2015;54:2709–2718. doi: 10.1021/acs.biochem.5b00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin B.A., Adams S.R., Tsien R.Y. Specific covalent labeling of recombinant protein molecules inside live cells. Science. 1998;281:269–272. doi: 10.1126/science.281.5374.269. [DOI] [PubMed] [Google Scholar]
- Hanna D.A., Moore C.M., Liu L., Yuan X.J., Dominic I.M., Fleischhacker A.S., Hamza I., Ragsdale S.W., Reddi A.R. Heme oxygenase-2 (HO-2) binds and buffers labile ferric heme in human embryonic kidney cells. J. Biol. Chem. 2022;298:101549. doi: 10.1016/j.jbc.2021.101549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann L.S., Schmidt P.M., Keim Y., Hoffmann C., Schmidt H., Stasch J.P. Fluorescence dequenching makes haem-free soluble guanylate cyclase detectable in living cells. PLoS One. 2011;6:e23596. doi: 10.1371/journal.pone.0023596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochert B.A., Fleischhacker A.S., Wales T.E., Becker D.F., Engen J.R., Ragsdale S.W. Dynamic and structural differences between heme oxygenase-1 and-2 are due to differences in their C-terminal regions. J. Biol. Chem. 2019;294:8259–8272. doi: 10.1074/jbc.ra119.008592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Dumbrepatil A.B., Fleischhacker A.S., Marsh E.N.G., Ragsdale S.W. Heme oxygenase-2 is post-translationally regulated by heme occupancy in the catalytic site. J. Biol. Chem. 2020;295:17227–17240. doi: 10.1074/jbc.ra120.014919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Moenne-Loccoz P., Hildebrand D.P., Wilks A., Loehr T.M., Mauk A.G., de Montellano P.R.O. Replacement of the proximal histidine iron ligand by a cysteine or tyrosine converts heme oxygenase to an oxidase. Biochemistry. 1999;38:3733–3743. doi: 10.1021/bi982707s. [DOI] [PubMed] [Google Scholar]
- Maines M.D., Trakshel G.M., Kutty R.K. Characterization of two constitutive forms of rat-liver microsomal heme oxygenase: only one molecular-species of the enzyme is inducible. J. Biol. Chem. 1986;261:411–419. doi: 10.1016/s0021-9258(17)42488-4. [DOI] [PubMed] [Google Scholar]
- Martin B.R., Giepmans B.N.G., Adams S.R., Tsien R.Y. Mammalian cell-based optimization of the biarsenical-binding tetracysteine motif for improved fluorescence and affinity. Nat. Biotechnol. 2005;23:1308–1314. doi: 10.1038/nbt1136. [DOI] [PubMed] [Google Scholar]
- Pan J., Zhang X.X., Yuan H., Xu Q.M., Zhang H.J., Zhou Y.J., Huang Z.X., Tan X.S. The molecular mechanism of heme loss from oxidized soluble guanylate cyclase induced by conformational change. Biochim. Biophys. Acta Proteins Proteomics. 2016;1864:488–500. doi: 10.1016/j.bbapap.2016.02.012. [DOI] [PubMed] [Google Scholar]
- Reddi A.R., Hamza I. Heme mobilization in animals: a metallolipid’s journey. Accounts Chem. Res. 2016;49:1104–1110. doi: 10.1021/acs.accounts.5b00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A., Dai Y., Haque M.M., Seeger F., Ghosh A., Garcin E.D., Montfort W.R., Hazen S.L., Misra S., Stuehr D.J. Heat shock protein 90 associates with the Per-Arnt-Sim domain of heme-free soluble guanylate cyclase: implications for enzyme maturation. J. Biol. Chem. 2015;290:21615–21628. doi: 10.1074/jbc.m115.645515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuller D.J., Wilks A., de Montellano P.R.O., Poulos T.L. Crystal structure of human heme oxygenase-1. Nat. Struct. Biol. 1999;6:860–867. doi: 10.1038/12319. [DOI] [PubMed] [Google Scholar]
- Spencer A.L.M., Bagai I., Becker D.F., Zuiderweg E.R.P., Ragsdale S.W. Protein/protein interactions in the mammalian heme degradation pathway: heme oxygenase-2, cytochrome P450 reductase, and biliverdin reductase. J. Biol. Chem. 2014;289:29836–29858. doi: 10.1074/jbc.m114.582783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stols L., Gu M.Y., Dieckman L., Raffen R., Collart F.R., Donnelly M.I. A new vector for high-throughput, ligation-independent cloning encoding a tobacco etch virus protease cleavage site. Protein Expr. Purif. 2002;25:8–15. doi: 10.1006/prep.2001.1603. [DOI] [PubMed] [Google Scholar]
- Sugishima M., Sato H., Higashimoto Y., Harada J., Wada K., Fukuyama K., Noguchi M. Structural basis for the electron transfer from an open form of NADPH-cytochrome P450 oxidoreductase to heme oxygenase. Proc. Natl. Acad. Sci. U.S.A. 2014;111:2524–2529. doi: 10.1073/pnas.1322034111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeny E.A., Singh A.B., Chakravarti R., Martinez-Guzman O., Saini A., Haque M.M., Garee G., Dans P.D., Hannibal L., Reddi A.R., et al. Glyceraldehyde 3-phosphate dehydrogenase is a chaperone that allocates labile heme in cells. J. Biol. Chem. 2018;293:14557–14568. doi: 10.1074/jbc.ra118.004169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson S.A., Moore C.M., Marcero J.R., Medlock A.E., Reddi A.R., Khalimonchuk O. From synthesis to utilization: the ins and outs of mitochondrial heme. Cells. 2020;9:579. doi: 10.3390/cells9030579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupta B., Stuehr E., Sumi M.P., Sweeny E.A., Smith B., Stuehr D.J., Ghosh A. GAPDH is involved in the heme-maturation of myoglobin and hemoglobin. FASEB J. 2022;36:e22099. doi: 10.1096/fj.202101237rr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi L., Jenkins P.M., Leichert L.I., Jakob U., Martens J.R., Ragsdale S.W. Heme regulatory motifs in heme oxygenase-2 form a thiol/disulfide redox switch that responds to the cellular redox state. J. Biol. Chem. 2009;284:20556–20561. doi: 10.1074/jbc.m109.015651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi L., Ragsdale S.W. Evidence that the heme regulatory motifs in heme oxygenase-2 serve as a thiol/disulfide redox switch regulating heme binding. J. Biol. Chem. 2007;282:21056–21067. doi: 10.1074/jbc.m700664200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Guarente L. Heme binds to a short sequence that serves a regulatory function in diverse proteins. EMBO J. 1995;14:313–320. doi: 10.1002/j.1460-2075.1995.tb07005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]