Abstract
Diabetic neuropathy (DN) remains arguably the most prevalent chronic complication in people with both type 1 and type 2 diabetes, including in youth, despite changes in the current standards of clinical care. Additionally, emerging evidence demonstrates that neuropathy affects a large proportion of people with undiagnosed diabetes and/or prediabetes, as well as those with obesity. Here we summarize the latest epidemiology of DN, recent findings regarding the pathophysiology of the disease, as well as current outcome measures for screening and diagnosis, in research and clinical settings. The authors discuss novel perspectives on the impact of social determinants of health in DN development and management, and the latest evidence on effective therapies, including pharmacological and nonpharmacological therapies for neuropathic pain. Throughout the publication, we identify knowledge gaps and the need for future funding to address these gaps, as well as needs to advocate for a personalized care approach to reduce the burden of DN and optimize quality of life for all affected individuals.
Keywords: Diabetic peripheral neuropathy, autonomic neuropathies, epidemiology, novel risk factors, mechanisms, personalized care implementation
INTRODUCTION
Diabetic neuropathy (DN) affects people in a myriad of ways including loss of sensation, loss of balance, severe pain, foot ulcers and amputations. Individuals with DN experience depression and anxiety, with poor quality of life and poor daily function. DN also affects the autonomic nervous system, with corresponding heart failure and even sudden cardiac death. More than $10 billion of annual healthcare costs are attributed to DN1, underscoring the magnitude of this highly morbid disorder and the associated socioeconomic problems.
Among the various forms of DN, distal symmetric polyneuropathy (DPN) and diabetic autonomic neuropathies, particularly cardiovascular autonomic neuropathy, are by far the most studied, although emerging data highlight the impact of other forms of autonomic neuropathies such as gastrointestinal and urogenital autonomic neuropathies, on healthcare and patients’ reported outcomes. The urogenital autonomic neuropathies are amply covered in a different manuscript in this same issue, to which the reader is referred to.
EPIDEMIOLOGY AND RISK FACTORS
Distal Symmetric Polyneuropathy (DPN)
DPN remains arguably the most prevalent chronic complication in people with both type 1 diabetes (T1D) and type 2 diabetes (T2D), including in youth. The estimated lifetime prevalence exceeds 50% despite changes in the current standards of clinical care over time2-5 with different rates of DPN progression depending on disease duration, population studied, and the DPN definition2, 3, 5, 6 There are also epidemiological differences between DPN in T1D versus T2D, despite no major structural differences in nerve pathology, highlighting an area that deserves further targeted research.5
The Diabetes Control and Complications Trial (DCCT) and its observational follow-up, the Epidemiology of Diabetes Interventions and Complications (EDIC) study, the largest and best phenotyped TID cohort followed for ~ 40 years, demonstrated that despite a low DPN prevalence in early T1D, the prevalence increases steadily over time to ~ 34% after 25 years.7 Similarly, a large cohort of randomly selected individuals with T1D from 16 European countries, the European Insulin-Dependent Diabetes Mellitus Prospective Complications Study (EURODIAB IDDM), reported similar mean DPN prevalence, but with significant DPN trends associated with age, diabetes duration, and hemoglobin A1c, as well as with hypertension and hyperlipidemia.8
Some may argue that neither the EURODIAB nor the DCCT cohorts are representative of contemporary populations, even though the vast majority of EDIC follow-up was performed after the DCCT lessons were implemented into clinical care.9 More recent data reported from 2 large contemporary cohorts of ~ 6,000 people with T1D each, the T1D Exchange in USA and the Scottish Register, demonstrated a prevalence of 11-13% for symptomatic DPN based on the Michigan Neuropathy Screening Instrument (MNSI) questionnaire. These findings however must be considered in the context of solely using symptom as a diagnostic tool, compared to EURODIAB and DCCT, where clinical examinations were also performed.10, 11
DPN prevalence is even higher in people with T2D. Data from several contemporary cohorts report DPN in ~ 20 % of individuals with newly diagnosed diabetes, despite progress in the standards of care, that increased to ~ 50% after 10 or more years.2-6 For instance in a large cohort of >1,500 individuals with screen-detected T2D enrolled in the ADDITION-Denmark Study, DPN prevalence was ~ 13% at baseline with cumulative incidence rates of 10% over 13 years of follow-up.12 Similarly, among a contemporary cohort of 5,047 people with newly diagnosed T2D (mean duration of only four years) participating in the Glycemia Reduction Approaches in Diabetes - A Comparative Effectiveness (GRADE) trial, ~ 21 % of participants presented with DPN at baseline.13 Conversely, a DPN prevalence rate as high as 50% was reported in the Bypass Angioplasty Revascularization Intervention 2 Diabetes (BARI 2D) cohort that included ~ 2400 T2D participants with a mean diabetes duration of ~10 years.14 Furthermore, unexpectedly high DN prevalence (both DPN and autonomic neuropathy) was reported in youth with T1D and especially T2D.2, 15, 16
The burden of DPN in both youth and adults is particularly alarming given the continuous rise in diabetes prevalence in USA and worldwide, including the disproportionately higher rates in minorities.17, 18 Up to 350 million people may develop DN and related comorbidities by 2045.18 The true prevalence is likely higher when asymptomatic DPN is included as only 30% of cases endorse typical DPN symptoms, including pain.5 Therefore, timely identifying and addressing risk factors for DPN are imperative steps to reduce the burden of this devastating complication.
Traditional risk factors for DPN in T1D and T2D include glycemic control, age, diabetes duration, and height.2, 5, 7, 12 Additionally, cardiovascular risk factors (e.g., obesity, hyperlipidemia, hypertension, and smoking) are reported risk factors in several cohorts of both T1D and T2D.2, 5, 10, 19 These findings agree with several clinical studies in the United States, Europe, and Asia demonstrating the metabolic syndrome is a risk factor for DPN.5, 12, 20, 21 Obese individuals with normoglycemia have a higher prevalence of neuropathy versus non-obese individuals, suggesting that obesity alone may be sufficient to induce neuropathy, while glucose variability is also emerging as a potential risk factor for the development of DPN, particularly painful DPN.22
Social determinants of health (SDOH) are emerging as important diabetes complications risk factors, likely due to both the increased risk of diabetes mellitus as well as inadequate glycemic control.23 However, in the USA, data regarding the association between DPN prevalence and sociodemographic characteristics, are limited. Data from the NHANES and the Atherosclerosis Risk in Communities (ARIC) cohorts show that non-Hispanic Blacks were more likely to have DPN on monofilament testing than non-Hispanic Whites, even after controlling for traditional risk factors, suggesting that race may also impact DPN development.24 Yet data examining DPN prevalence and risk factors among racial/ethnic minorities, particularly Black Americans, are limited. Preliminary data from an ongoing study in Flint, Michigan with a predominantly Black, low-income patient population suggests that DPN is common but often underrecognized.25 High prevalence of DPN has also been reported among native populations, including a cohort of Pima Indians in Arizona.26 Although this increased burden among racial/ethnic minorities is likely due to an increased T2D and metabolic syndrome prevalence23, 27, these findings warrant further investigation. Additionally, U.S. racial/ethnic minority populations with DPN have worse diabetic foot ulcer outcomes and higher rates of amputation than non-Hispanic White Americans.28-30 A recent modeling analysis using longitudinal data from the SEARCH for Diabetes in Youth suggested that unmeasured race and ethnicity-associated factors account for predicted DPN disparities in non-White versus White youth and young adults with diabetes, highlighting the need for further research in this area.31 Teasing apart the association between DPN outcomes and race/ethnicity is challenging as it is likely confounded by socioeconomic status.23 Yet, characterizing these relationships are essential to design interventions targeting patient outcomes.
More recently, the T1D Exchange cohort that collected data on several SDOH reported that both lower education and higher rates of public insurance options were associated with DPN in adults with T1D.10 The role of SDOH was confirmed in a Scottish T1D cohort, which found that social deprivation led to a 2.17 higher odds of DPN.11 In terms of psychological factors, both depression and anxiety have been found to be associated with DPN, particularly painful DPN.32, 33
Epidemiology of Pain in DPN
Up to 30% of individuals with diabetes experience painful DPN and neuropathic pain may be the first symptom that prompts people to seek medical care.2, 4, 34, 35 A large community-based study in the U.K reported higher prevalence of painful symptoms (35% vs 23%) and painful DPN (22% vs 13%) in T2D compared to T1D respectively, using the neuropathy symptom and neuropathy disability scores.36 In a recent cross-sectional, hospital-based, multicenter study including ~800 individuals with both T1D and T2D, the reported prevalence of painful DPN was 13% using the grading system of the Neuropathic Pain Special Interest Group of the International Association for the Study of Pain.37 Another recent large cross-sectional study in the Danish Centre for Strategic Research in Type 2 Diabetes cohort reported prevalence rates of DPN and painful DPN of 18% and 10%, respectively, using the MNSI questionnaire and the Douleur Neuropathique en 4 (DN-4) questionnaire.
Risk factors include female sex, age, duration of diabetes, and obesity.38 Additionally, significant associations between painful DPN and psychosocial factors such as smoking, depression, and anxiety are emerging although the directionality is unclear.39
Knowledge Gaps
Understand what drives the high prevalence of DPN despite continuous refinements in the standards of diabetes care, and the progress in diabetes medications and diabetes technologies
Recognize the relative contributions of various risk factors including SDOH, and the effects of modifying risk factors
Understand differences between T1D and T2D DPN
Identify gaps in the implementation of the current best practices
Identify reasons for the high prevalence of DPN in youth
Diabetic Autonomic Neuropathy
The autonomic nervous system regulates many systems and organs through small C-fibers. Thus, diabetic autonomic neuropathy may manifest with a broad spectrum of signs and symptoms depending on the affected target system including cardiovascular autonomic neuropathy (CAN), gastrointestinal neuropathy, urogenital neuropathy, and others.2
Cardiovascular Autonomic Neuropathy
CAN is by far the most studied form of autonomic neuropathy.2, 3, 40 The reported prevalence of CAN over time varies, and is contingent upon several factors including the definition of CAN, the population studied (e.g. observational cohorts vs interventional trials; T1D vs T2D vs prediabetes) and the study design (longitudinal vs cross-sectional).2, 40
For instance, prevalence is very low in individuals with newly diagnosed T1D. This is documented by the primary prevention arm of the DCCT cohort, which similar to DPN, is arguably the best phenotyped cohort for CAN with cardiovascular autonomic reflex tests (CARTs), validated symptoms instruments, and electrocardiogram recordings obtained repeatedly during follow-up.7, 41 In DCCT/EDIC, the prevalence of CAN increased steadily over time to 44% over 23 years of mean follow-up.7 Similarly high prevalence was reported in other T1D cohorts including the EURODIAB IDDM, the Pittsburgh Epidemiology of Diabetes Complications Study, and the STENO T1D study.2, 40 While the argument may be made that these cohorts preceded the changes in standards of diabetes care, studies utilizing the T1D exchange network evaluated the contemporary prevalence of autonomic neuropathy based on the Survey of Autonomic Symptoms (SAS) in T1D adults with >5 years of diabetes duration. Autonomic symptoms were present in 17% of participants who responded to the surveys and more than 70% of those participants experienced moderate to severe symptoms.42
Prevalence as high as 60% has been reported in earlier cohorts of individuals with long-standing T2D.2, 40 Additionally, recent data from the GRADE cohort13 in the United States and the Anglo-Danish-Dutch Study of Intensive Treatment in People With Screen-Detected Diabetes in Primary Care (ADDITION) cohort43 from Denmark demonstrated that up to 13% of individuals with either short duration or newly diagnosed diabetes already have evidence of CAN. CAN was also reported in prediabetes in individuals with impaired glucose tolerance, insulin resistance, and/or the metabolic syndrome.40, 44 Moreover, an unexpectedly high prevalence of CAN was identified among youth with diabetes participating in the SEARCH for Diabetes in youth study.16
As with DPN, there are several traditional risk factors that are associated with CAN in diabetes including older age, longer diabetes duration, poor glucose control, diabetic kidney disease, hypertension, elevated triglycerides, and smoking. However, more recently, glucose variability, psychological factors (depression), and SDOH (lower income and education) are also emerging as less traditional risk factors for CAN in diabetes.42
Gastrointestinal Autonomic Neuropathy
Autonomic dysfunction in the gastrointestinal system may manifest as esophageal dysmotility, gastroparesis, constipation, diarrhea, and fecal incontinence.2, 3 Among gastrointestinal neuropathies, gastroparesis is by far most frequently encountered in clinical practice.
Earlier prevalence data on gastroparesis are sparse.2 The reported cumulative incidence and prevalence of gastroparesis was 9.8 in women and 2.4 in men per 100,000 person-years and 37.8 for women and 9.6 for men per 100,000 person-years respectively in the only large community-based study in the United States.45 The contemporary prevalence rates of confirmed gastroparesis due to T1D or T2D are low2 but likely increasing based on the growing number of gastroparesis-related hospitalizations in the United States. However, given the increased use of many medications that may directly impact the gastrointestinal system motility, such as glucagon like peptide 1 receptor analogs (GLP1-RA), as well as opioids and more recently recreational marijuana, no well-designed studies have evaluated whether these cases are iatrogenic or due to diabetes.
Knowledge Gaps
Identify risk factors driving the heterogeneity in autonomic neuropathy risk, and of various forms of autonomic neuropathy between T1D and T2D
Evaluate the impact of glucose variability on autonomic neuropathy, particularly CAN risk
Understand the current epidemiology of diabetic gastroparesis and determine the percentage attributable to medication use
FACTS: Pathophysiology/ Mechanisms for Diabetic Neuropathy
Diabetes can damage various components of the peripheral nerve, which comprises the cell body (dorsal root ganglion or anterior horn that originate in the spinal cord), its myelinated cell projections, as well as axonal extensions, innervating the periphery. The most common form of nerve injury is a progressive distal-to-proximal peripheral nerve loss that typically presents as sensory predominant.34 Particularly, small unmyelinated nerve fibers, known as C-fibers or “small fibers”, which relay information related to heat discrimination and chemical pain are an early and frequent target of DPN.5 Small, thinly myelinated Aδ fibers that carry signals related to touch, pressure, and cold are also commonly affected.5 Only much later in the course of the disease is there evidence of large myelinated Aβ fiber (“large fiber”) dysfunction, responsible for vibratory and position perception.5 Progressive sensory loss coupled with motor weakness at later disease stages leads to loss of sensation in the feet and predisposes to impaired balance and falls. 2
The mechanisms promoting the onset and progression of DN are complex (Figure 1). Fully understanding these mechanisms is essential for the successful development of disease modifying therapies, a goal that has been elusive so far.
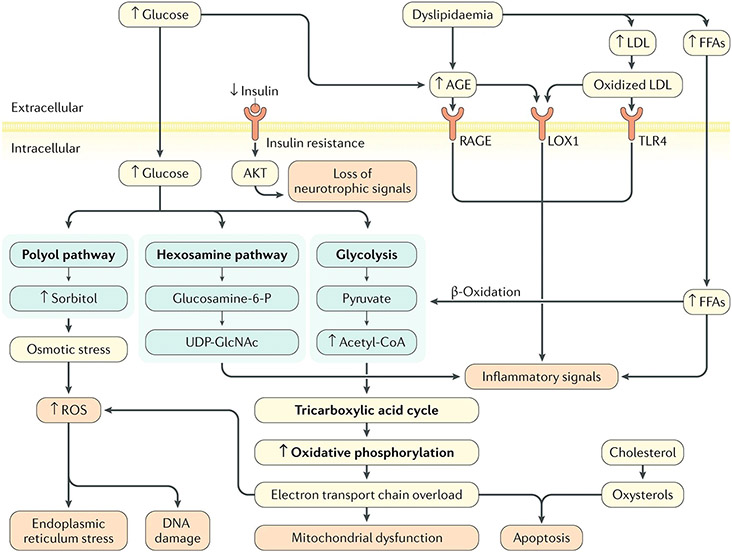
Figure 1:
Diabetic neuropathy pathogenesis. Hyperglycaemia and dyslipidaemia, together with altered insulin signalling, lead to several pathological alterations in neurons, glia and vascular cells that can lead to nerve dysfunction and ultimately, neuropathy, including DNA damage, endoplasmic reticulum stress, mitochondrial dysfunction, neurodegeneration and loss of neurotrophic signalling, and can trigger macrophage activation. The importance of these pathways in the development of neuropathy varies with cell type, disease profile and time, as distinct cell types are more or less susceptible to injury depending on the metabolic impairments. AGE, advanced glycation end-product; FFAs, free fatty acids; Glucosamine-6-P, glucosamine 6-phosphate; LDL, low-density lipoprotein; LOX1, oxidized LDL receptor 1; RAGE, AGE-specific receptor; ROS, reactive oxygen species; TLR4, Toll-like receptor 4; UDP-GlcNAc, uridine diphosphate N-acetylglucosamine.
Reproduced from: Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, Bril V, Russell JW, Viswanathan V. Diabetic neuropathy. Nat Rev Dis Primers. 2019 Jun 13;5(1):42. doi: 10.1038/s41572-019-0097-9. PMID: 31197183; PMCID: PMC7096070.
In the last three decades, DN research, including our own, has focused on glucose and the streptozotocin (STZ)-induced T1D rat as the pre-clinical model of choice. These studies, amply discussed elsewhere,46, 47 provided insights into the molecular mechanisms that drive glucose pathogenesis in DPN which include: 1) the polyol pathway through its key enzyme, the aldose reductase, which results in a series of downstream reactions that decrease sodium–potassium adenosine triphosphatase (ATP) activity, deplete nicotinamide adenine dinucleotide phosphate, and produce reactive oxygen species (ROS); 2) the hexosamine pathway and protein kinase C (PKC) secondary activation generating inflammatory by-products, with subsequent insulin resistance, impairment in growth factor biology, and vasoconstriction of nerve blood vessels; 3) advanced glycation end products (AGEs) that bind the receptors for AGEs (RAGEs), leading to downstream inflammation, ROS accumulation, and decreased nerve blood flow; 4) cyclooxygenase (COX) mainly COX2, with downstream increased ROS leading to reduced nerve blood flow and neuronal dysfunction. Moreover, low-grade inflammation and its downstream impact on several of the pathways described above has emerged as a critical mechanism in the development of DN and painful DPN in several experimental and clinical studies.48-54
Promising results were achieved using aldose reductase inhibitors, PKC inhibitors, or RAGE inhibitors in animal studies, especially in the STZ-induced T1D rat. Unfortunately, singularly targeting each of these pathways either failed to reverse nerve damage in human DPN trials or was too toxic during therapeutic development.2, 5 Additionally, meta-analyses and systematic reviews of large T1D and T2D human trials that included DN outcomes reported that while tight glycemic control reduces DN incidence in T1D, it does not result in complete protection or reversal of disease, and it has a lesser effect in those with T2D.2, 5, 6, 55 Our recent clinical studies support these observations, implicating the metabolic syndrome, including dyslipidemia as major drivers of DPN in prediabetes and T2D, independent of glycemic status.56, 57 All these led to a paradigm shift in the DN field away from models based solely on glucose metabolism and the STZ-induced T1D rodent model towards understanding metabolic drivers in T1D versus T2D and global whole nerve metabolism.21, 55
In a bedside-to-bench approach, recent studies employed non-genetic mouse models of prediabetes and obesity that when placed on a high saturated fat diet, develop features of prediabetes and the metabolic syndrome with dyslipidemia, impaired glucose tolerance, insulin resistance, and neuropathy.58-61 Using integrated lipidomic and transcriptomic profiling, we demonstrated that nerve triglyceride accumulation and triglyceride synthesis are key players in the pathogenesis of neuropathy, and a dietary reversal paradigm, aimed at correcting the lipid profile may be a promising non-pharmacological approach to improve nerve function in prediabetes and T2D.59 Other studies using the same mouse model and in vitro DPN models unveiled the role played by mitochondria function. Under normal conditions, mitochondria use both glucose and lipids to produce ATP. However, in the diabetic environment, excess glucose and lipids disrupt the normal pathways used for their own breakdown, producing excess electron donors that overwhelm mitochondrial capacity, resulting in bioenergetic failure with mitochondrial depolarization, decreased ATP production, impaired mitochondrial trafficking, and accumulation of ROS, leading to inflammation, endoplasmic reticulum stress, apoptosis of neurons, and axonal failure.5, 62 With fewer functional mitochondria in the cell body and along the axons, energy-starved small and large nerve fibers lose their ability to function and undergo degeneration with the axons farthest from the cell body (i.e., those in the feet) and the smallest fibers that regulate pain and dysesthesia being most vulnerable.5, 46, 63 Interestingly, switching saturated fatty acid-rich diets with diets rich in unsaturated fats from plant sources or fish oil improves nerve function63, 64 and prevents axonal mitochondrial dysfunction,63, 65 supporting a beneficial role for unsaturated fats as targeted therapy development for DPN.
Other emerging areas of interest are understanding the changes that occur in whole nerve metabolism, cell-specific changes in the nerve microenvironment during DPN, and how Schwann cell injury may impair energy substrate transfer66 and/or extracellular vesicle secretion.67 An improved understanding of axoglial cross-talk and nerve function will help inform development of future DPN therapies.
Overall, the DN field is moving from a nerve-centric focus on glucose alone to a new era of research centered on understanding the role of additional metabolic and inflammatory factors in DN pathophysiology, which could be instrumental to help develop mechanism-based therapies.
Knowledge Gaps
Develop experimental models that translate well to human disease
Apply a precision approach to identify optimal therapeutic targets
Understand how metabolic factors, other than hyperglycemia alone contribute to DN
Interrogate the direct contribution of Schwann cells, and other cellular components of the nerve, including macrophages to DPN
Understand differences in cellular and molecular mechanisms between DPN and autonomic neuropathies
FACTS: DPN Diagnosis and Outcomes Measures
DPN Diagnosis in Clinical Care:
The hallmark symptoms and signs associated with DPN are the consequence of the progressive damage and loss of the various populations of nerve fibers, each with distinct roles and functions.2, 3 As highlighted in the recent American Diabetes Association Monograph on Diabetic Neuropathy, in diabetes, this process occurs in a specific symmetrical, distal-to-proximal pattern, starting at the tip of the toes and eventually progressing proximally.5 The symptoms and clinical signs associated with DPN follow the same pattern, creating the typical “stocking-and-glove” clinical presentation, an important diagnostic feature.5 A targeted history will unveil specific symptoms that include: a) neuropathic pain, a feature of small fiber damage; b) numbness and tingling without pain, features of small or large fibers damage; c) insensate, numb feet usually associated with more advanced mixed fiber damage.2, 3, 5 In more advanced DPN, individuals present with reduced daily function with poor balance, falls or fractures, and an increased risk for painless injuries that lead to ulcers, infections, and amputations.2, 5 Neuropathic pain in DPN is typically experienced as burning, shooting, electric shock-like or lancinating, usually worse at night, and may be accompanied by dysesthesias such as an exaggerated response to painful stimuli (hyperalgesia) and/or pain evoked by contact with ordinarily unpainful stimuli such as socks, shoes, and bedclothes (allodynia).2, 5
A large fraction of individuals with DPN may be asymptomatic and unaware or reluctant to report their condition.2, 5 Asking specific questions and performing a targeted examination are recommended. A focused examination effectively evaluates small and large fiber function. Small fiber testing includes assessment of pinprick sensation using a sharp object such as a safety pin and temperature threshold sensation with a cold metal object such as a tuning fork in the feet. Large fiber function is assessed using a 128-Hz tuning fork for vibratory sensation and a 10-g monofilament for light-touch pressure on the dorsal aspect of the great toe, and bilateral ankle reflexes (for predominantly large fibers).2, 5 Importantly, the 10-g monofilament alone should not be used to diagnose or exclude DPN as it detects only advanced neuropathy and individuals at increased risk of diabetic foot ulcerations.2, 3 Relying solely on the 10-g monofilament could miss the early stage of the disease which is most amenable to the therapeutic intervention to prevent progression of the disease.2, 3, 5 The clinicians should combine at least two examinations of both small and large fiber nerve function to detect DPN in the clinical practice.2 A comprehensive differential diagnosis is always needed, and readers are referred to published algorithms.5
Knowledge Gaps/ Challenges in Clinical Care Implementation:
Understand the barriers preventing the appropriate implementation of DPN screening and diagnosis in clinical care despite readily available recommended simple tests
Understand why 10-g monofilament testing remains the most used screening test for DPN in primary care despite its low sensitivity and specificity for earlier stages of disease2, 3
Develop innovative educational methods and tools for implementing appropriate DPN diagnosis in the clinical practice.
Outcome Measures in Clinical Research:
As recommended by the Toronto Consensus on Diabetic Neuropathy, an outcome of confirmed DPN requires a combination of symptoms, signs and an abnormality of objective tests.2, 68 While clinical instruments that include any given combination of patient reported symptoms and clinical signs may perform well in large population studies assessing prevalence and incidence rates, different measures are needed for interventional trials. In fact, one of the most critical components for a successful path towards developing effective disease modifying therapies are having access to sensitive and specific outcome measures that correctly capture the natural history of the disease and detect repair in specific nerve fiber populations. This is particularly relevant given that there are currently no approved disease-modifying therapies for any forms of DN, and a very large number of clinical trials evaluating promising experimental targets for these conditions have failed.2, 5
The MNSI, the modified Toronto Clinical Neuropathy Scale, the Neuropathy Disability Score, and the Utah Early Neuropathy Scale are validated clinical instruments that have been used in both observational and interventional studies. Among these, the MNSI has been most consistently used in large cohorts of people with T1D, T2D, the metabolic syndrome and in youth.2 In addition, the Quality of Life in Neurological Disorders (NeuroQOL) and the Norfolk neuropathy instrument, are validated instruments that capture measures of quality of life specific to peripheral neuropathy in several domains including pain, lost/reduced feeling, diffuse sensory-motor symptoms, restrictions in activities of daily living, disruptions in social relationships and emotional distress.2
More objective measures include abnormalities in nerve conduction studies (NCS) and validated measures of small nerve fibers.2 Mild DPN, may be characterized by decrease in sural nerve amplitude or mild reduction in sensory nerve conduction velocity,34 while severe DPN includes NCS motor abnormalities or nonrecordable sensory NCS. NCS may be completely normal in those with primarily small fiber neuropathy and loss of small fibers typically precedes loss of large nerve fibers.34 The gold standard for small fiber neuropathy is assessment of intra-epidermal nerve fiber density (IENFD) measurements by skin punch biopsy, while other measures include quantitative sensory testing for thermal thresholds for either elevated cooling or heat detection thresholds, and emerging corneal confocal microscopy.34
Painful DPN
Painful DPN is associated with depression, insomnia, and poor quality of life.33, 69 Visual analog scale (VAS), Likert scales, and the McGill Pain Questionnaire are used in many clinical studies and are considered as sensitive and validated tools for painful DPN.70
Knowledge Gaps in Clinical Research:
Validate sensitive and specific outcome measures that may correctly capture the natural history of the disease and may detect timely repair in specific nerve fiber populations
Reach consensus among the stake holders in the field to utilize uniform and adequate outcome measures across all interventional and/or observational studies
Incorporate patient reported outcomes including psychological outcomes in DPN studies
Develop reliable biomarkers to be implemented at the point of care
FACTS: Autonomic Neuropathy Diagnosis and Outcomes Measures
Cardiovascular Autonomic Neuropathy
Screening and Diagnosis in Clinical Care
Unlike DPN, individuals presenting with the earliest stages of CAN may be completely asymptomatic, making its early detection challenging. In more advanced stages, people may present with palpitations, or with dizziness, unsteadiness, fainting or syncopal episodes with sudden changes from supine to standing.2 The earliest sign of CAN is reduced heart rate variability (HRV)2, 40 Later people may also present with resting fixed-rate tachycardia, changes in blood pressure (BP) regulation overnight (reverse/non dipping BP), orthostatic hypotension, and sudden premature death.2, 40 CARTs are considered the gold standard which evaluate changes in the heart rate and BP during clinical maneuvers such as deep breathing, standing, and Valsalva.40, 68, 71 Although CARTs could be ordered as part of clinical care, they are seldom used in practice due to inconsistent availability, costs, and perhaps lack of knowledge and understanding by most providers on how to best utilize the information. With the continuous development of several technologies including continuous glucose monitoring (CGM), the detection of CAN may enable their use to prevent hypoglycemia and glucose fluctuations in larger groups of people with diabetes,40 thus reducing arrhythmia risk and other adverse clinical consequences (Figure 2). Additionally, CAN is an important risk for heart failure, now the most prevalent cardiovascular complication in diabetes,72 thus testing for CAN could allow implementation of more aggressive therapy in those most at risk of future complications.72
Figure 2:
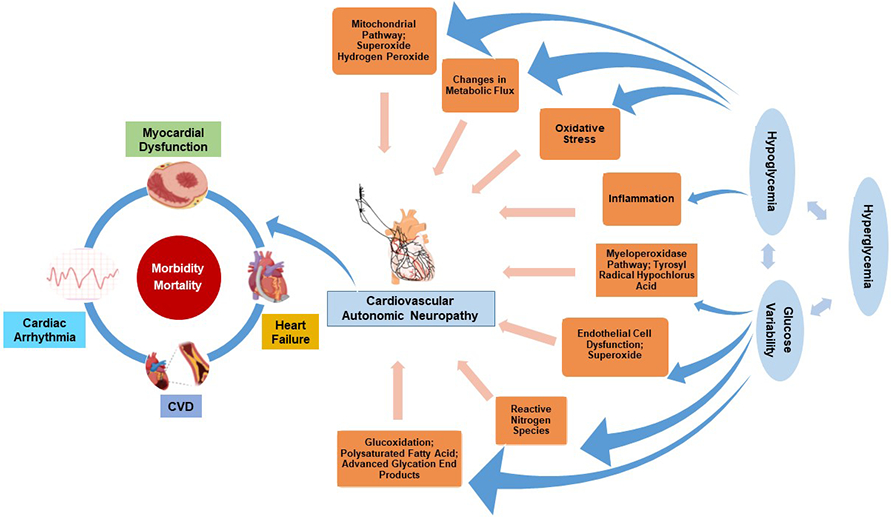
Mechanisms and clinical consequences of cardiovascular autonomic neuropathy (CAN) in diabetes
Knowledge Gaps in Clinical Care
Implement successful providers’ education on how to diagnose and effectively use CAN as a risk stratification tool to promote a personalized medicine approach toward the use of technologies and guideline-directed therapies
Develop reliable CAN biomarkers to be used at the point of care
Outcomes Measures in Clinical Research
The CARTs performed under deep breathing remain the gold standard outcome measure for CAN in research settings, given their refined standardized protocols that can be administered by technicians, as well as their easy scalability in larger cohorts.2, 40 CARTs are the most common CAN outcome measure in both interventional trials, including the DCCT, and observational cohorts such as EDIC. CARTs also allow for CAN staging, with one abnormal test indicative for early/subclinical CAN, while two or more abnormal tests indicative of definite CAN2, 40 although the evidence behind these recommendations is less clear. Recently, a plethora of recording devices including software operating with a “black-box” approach (output of data without reporting the algorithm that calculated the result) have emerged making it difficult to compare data sets and reproducibility across studies, particularly when few age-related normative data sets are available.
Indices of HRV, either in time (e.g. the standard deviation of normal RR intervals -SDNN, the root-mean square of the difference of successive RR intervals -rMSSD), or frequency domain (e.g. low, very low and high frequency power) indices, are emerging as sensitive and specific alternative CAN outcome measures,2, 73 including the HRV indices derived from standard 10-second 12-lead ECG recordings, that are much more feasible and easier to implement in larger and/or longitudinal cohorts.2, 13, 73
Cardiac sympathetic imaging using I-123 MIBG scintigraphy or 11C-HED PET, 24-h BP profiles, muscle sympathetic nerve activity, or baroreflex sensitivity testing to assess cardiac vagal and sympathetic baroreflex function may have higher degrees of sensitivity or specificity based on the hypotheses being tested, and may be used in some research protocols, although these require sophisticated infrastructure, highly trained personnel, and are quite expensive and time consuming.2, 40
Similar to DPN, the value of patient reported outcomes has emerged for autonomic neuropathy, and several surveys were developed and validated over time. While the Autonomic Symptom Profile (ASP), the Composite Autonomic Symptom Scale (COMPASS), or the abbreviated COMPASS-31 are non-invasive and have high sensitivity and specificity, they are quite time-consuming or require complex scoring algorithms.2, 42 More recently, SAS was validated as a brief, specific, and sensitive measurement of symptomatic autonomic neuropathy in diabetes and used in several cohorts including the T1D Exchange.42
Knowledge Gaps Clinical Research
Validate sensitive and specific outcome measures, with reliable intrasubject variability, that may correctly capture the natural history of the disease and may detect timely damage reversal
Reach consensus among the stake holders in the field to utilize uniform and adequate outcome measures across all interventional and/or observational studies, as well as uniform technologies that make the testing algorithms readily available
Incorporate patient reported outcomes including psychological outcomes
Gastroparesis
Diagnosis in Clinical Care
The clinical symptoms of gastroparesis may include early satiety, fullness, bloating, nausea, vomiting, dyspepsia, and abdominal pain. These symptoms are nonspecific and also poorly correlated with severity of gastroparesis and gastric emptying studies.2 Among the clinical signs, individuals with gastroparesis may present with wide glucose fluctuations and frequent unexplained hypoglycemia after meals. Gastric emptying with scintigraphy of digestible solids at 15-minute intervals for four hours after food intake is still considered the gold standard for diagnosis of gastroparesis. Optimization of glucose levels is utmost important before the test to avoid false positives2 as both hypoglycemia and hyperglycemia have direct effects on gastric emptying.74, 75 Scintigraphic gastric emptying studies can be burdensome, time consuming, not readily available, and costly. Recently the use of 13C octanoic acid or an acetate breath test has been FDA approved, providing simpler alternatives.2
Outcomes Measures for Research
The scintigraphic gastric emptying study and 13C octanoic acid or acetate breath test have been widely used in the research setting with the latter being more feasible and available. In addition, collection of full thickness gastric tissue for detecting specific cellular changes associated with diabetic gastroparesis was developed by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Gastroparesis Clinical Research Consortium, although these studies are invasive, require specialized infrastructure and thus may be applicable only in selected situations. Validated questionnaires such as the Patient Assessment of Upper Gastrointestinal Disorders-Symptoms, the Gastroparesis Cardinal Symptom Index-Daily Diary, and the specific gastroparesis questions in the SAS, are widely used to assess the severity of gastroparesis symptoms in clinical research setting.76
DN MANAGEMENT FACTS:
Management of DPN
There is strong evidence that targeting near normal glucose prevents the onset and progression of DPN in T1D.2, 5 However, intensive glucose control does not reverse DPN even in T1D, and the effects of glucose control are less conclusive for DPN in T2D, as highlighted in several cohorts.6 Likely reasons are the presence of several other risk factors and co-morbidities, as well as the complex mechanisms leading to DPN in T2D amply outlined above.
Besides glucose control, lifestyle and behavioral interventions have emerged as promising treatments for DPN prevention or even reversal.5 For instance, Singleton and colleagues reported improvement in small nerve fiber function and reinnervation as assessed by IENFD with a lifestyle intervention comprised of diet and moderate intensity exercise similar to the intervention used in the diabetes prevention program.77 A recent study from the Canadian Study of Longevity in T1D reported that individuals with diabetes who engaged in ≥ 150 minutes physical activity/week had a 12% lower DPN incidence78 providing additional insight on the potential role of lifestyle in the prevention of DPN. Moderate-intensity physical activity delays the onset and progression of DPN in individuals with T2D or prediabetic metabolic syndrome.79 Multimodal aerobic training [moderate-intensity (50% heart rate reserve) or vigorous (75% heart rate reserve) exercise] in a controlled trial improves mobility, balance, and gait outcomes in DPN.80 Additionally, dietary weight loss may help improve symptomatic DPN.81
Painful DPN management includes pharmacological, nutraceuticals, and non-pharmacological options.
Gabapentinoids, serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), and sodium channel blockers are all effective medication classes for the treatment of painful DPN. Few randomized controlled clinical trials report efficacy for opioids including tramadol or tapentadol. These trials were flawed by very high attrition rates and questionable study design.2, 5 Thus, given the evidence of the high risks of addiction, abuse and severe complications including death, compared to the low potential benefits, none of the opioids should be used in the treatment of painful DPN.2, 3 Yet despite these recommendations, the rates of opioid prescriptions for painful DPN remain unacceptably high, and interventions are needed to improve current clinical practice.
Among the nonpharmacological approaches, besides lifestyle interventions discussed above6, a recent large randomized-controlled trial reported substantial pain relief and improvement in quality of life with high-frequency spinal cord stimulation in individuals with refractory painful DPN sustained over 12 months5, 82 that led to FDA approval. However, the lack of a sham arm means that this was an unblinded pain study with a high probability of a large placebo effect. Future more rigorous studies are needed to determine the role of spinal cord stimulation and other devices in the treatment of painful DPN.
CAN Management:
The conclusive data obtained by the DCCT and later during EDIC, as well as evidence from several smaller trials strongly support that tight control of blood glucose implemented early in the disease course is very effective for preventing CAN and slowing its progression in individuals with T1D.2, 7 The evidence for people with T2D continues to evolve.2, 3, 6, 40 Glucose control as part of a multifactorial intervention that targeted hyperglycemia, hypertension, dyslipidemia, and lifestyle, demonstrated a 63% reduction in the rate of progression to CAN in a small T2D cohort participating in the STENO-2 trial.83 Additionally, most recent analyses from the ACCORD trial, reported that after adjusting for multiple other risk factors, intensive glucose treatment reduced CAN risk by 16% and the intensive BP intervention decreased CAN risk by 25% as compared with the standard intervention group.84 Considering that CAN was shown conclusively to predict cardiovascular mortality and cardiovascular events, reducing CAN incidence, would thus have a beneficial effect on the cardiovascular outcomes as well. Lifestyle intervention alone may also be beneficial as suggested by a recent pilot study from Germany that reported that high-intensity exercise training can improve indices of CAN over 12 weeks in overweight individuals with T2D.85 These data are promising, however, we are still in need of larger studies on types and duration of exercise that demonstrate whether improvement in these measures can be sustainable over longer periods time.
Management of orthostatic hypotension involves both behavioral and pharmacological interventions.2, 3, 40 Behavioral supportive measures include avoiding abrupt changes in body position, actions that elevate intra-abdominal and intra-thoracic pressures, or medications that would exacerbate hypotension, as well as raising the head of the bed during sleep, small and frequent meals to minimize postprandial hypotension or physical counter-pressure maneuvers such as leg crossing and squatting. Pharmacological therapy includes midodrine and droxidopa, both FDA approved for the management of orthostatic hypotension, or low dose fludrocortisone for use in individuals who fail non-pharmacological interventions but are limited by side effects.2, 3, 40
Gastroparesis Management:
Dietary changes and_optimizing glucose control with reducing glucose variability may be effective in gastroparesis management.2 Currently available diabetes technologies including sensor-augmented insulin pumps and/or semi closed-loop insulin pumps to improve glucose fluctuations and hypoglycemia, ultimately enhancing the gastric motility.74, 75 Withdrawal of medications that can slow down the gastric emptying, particularly opioids and marijuana, is also important.2 To date, metoclopramide, a prokinetic agent, is the only medication approved by the FDA for the treatment of gastroparesis. However given common extrapyramidal side effects, its use for more than five days is not recommended.2
Knowledge Gaps
Identify personalized prevention strategies using innovative technologies and artificial intelligence methods
Understand why many if not most people are unable or unwilling to engage in lifestyle interventions
Validate the cellular and molecular mechanisms by which lifestyle interventions improve nerve function could accelerate development of novel DPN treatments, beyond diet and exercise
Develop effective disease modifying therapies for DPN, painful DPN, and CAN
Identify sensitive biomarkers for DPN and pain phenotypes in DPN
Implement effective providers’ education on optimal pain management strategies
Build quality improvement initiatives to avoid opioids in people with diabetes and DPN
Build better infrastructure to enable implementation of lifestyle modifications for DPN or CAN
Find adherence mechanisms to lifestyle strategies
Understand the role of psychological factors
Find effective and better tolerated treatments for postural hypotension or true gastroparesis
Engage Pharma interest to develop new therapeutic agents for DN
Develop cost-effective and scientifically sound large-scale pragmatic trials for DN in a real-world setting
Summary:
We present here the most up-to-date facts on prevalence and incidence of various forms of DN, on potential mechanisms behind the development of DN as well as current management options. We also highlight knowledge gaps, current barriers in clinical practice for optimal DN management, including the need for development of disease modifying agents for management of all DN forms.
Acknowledgements:
This manuscript is a summary of the data presented during the plenary Neuropathy lectures and workshops included in the NIDDK/DiaComp funded “Frontiers in Diabetic Complications- From Biology to Technology” Conference, held in May 2022 on the Campus of the University of Michigan, Ann Arbor, Michigan. Financial support for this work was provided by the NIDDK Diabetic Complications Consortium (RRID:SCR_001415, [www.diacomp.org]www.diacomp.org), grants DK076169 and DK115255. RPB was also supported by R01DK107956; U01DK119083; 1U01 DK0945157; R01DK116723, and JDRF Center of Excellence at U of M. BC was supported by grants paid to his institution from the American Academy of Neurology, JDRF, NIDDK (RO1; DK115687), and Veteran Affairs. ME was supported by NINDS 5R25NS089450, NCATS UL1TR002240, NIDDK P30-DK-02926, and NIDDK P30-DK089503. Funding was provided by the National Institutes of Health (NIH) (R01DK130913, 1R24082841) to ELF; Novo Nordisk Foundation (NNF14OC0011633) to ELF, the Nathan and Rose Milstein Research Fund to SAE, and the Neuronetwork for Emerging Therapies at the University of Michigan to SAE and ELF.
Footnotes
Conflict of Interest: LA, KMZ, SAE, ME, and ELF have no conflict of interest, financial or other. BC receives consulting fees from DynaMed, and medical legal consultations including the Vaccine Injury Compensation Program unrelated to the topic of this Comment. RPB consults for Averitas Pharma, Nevro Inc, Roche, and is a member of the Steering Committee of the SOUL Cardiovascular Outcomes Trial funded by Novo Nordisk.
References:
- 1.Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA. The health care costs of diabetic peripheral neuropathy in the US. Diabetes care. 2003;26(6): 1790–1795. [DOI] [PubMed] [Google Scholar]
- 2.Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care. 2017;40(1): 136–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ang L, Cowdin N, Mizokami-Stout K, Pop-Busui R. Update on the Management of Diabetic Neuropathy. Diabetes Spectrum. 2018;31(3): 224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pop-Busui R, Boulton AJM, Sosenko JM. Peripheral and Autonomic Neuropathy in Diabetes. In: Cowie CC, Casagrande SS, Menke A, et al. , eds. Diabetes in America. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases (US); 2018. [PubMed] [Google Scholar]
- 5.Pop-Busui R, Ang L, Boulton AJM, et al. Diagnosis and Treatment of Painful Diabetic Peripheral Neuropathy. Arlington (VA): American Diabetes Association; © 2022 by American Diabetes Association. ; 2022. [PubMed] [Google Scholar]
- 6.Ang L, Jaiswal M, Martin C, Pop-Busui R. Glucose Control and Diabetic Neuropathy: Lessons from Recent Large Clinical Trials. Curr Diab Rep. 2014;14(9): 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braffett BH, Gubitosi-Klug RA, Albers JW, et al. Risk Factors for Diabetic Peripheral Neuropathy and Cardiovascular Autonomic Neuropathy in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes. 2020;69(5): 1000–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tesfaye S, Stevens LK, Stephenson JM, et al. Prevalence of diabetic peripheral neuropathy and its relation to glycaemic control and potential risk factors: the EURODIAB IDDM Complications Study. Diabetologia. 1996;39(11): 1377–1384. [DOI] [PubMed] [Google Scholar]
- 9.The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. New England Journal of Medicine. 1993;329(14): 977–986. [DOI] [PubMed] [Google Scholar]
- 10.Mizokami-Stout KR, Li Z, Foster NC, et al. The contemporary prevalence of diabetic neuropathy in type 1 diabetes: findings from the T1D Exchange. Diabetes care. 2020;43(4): 806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeyam A, McGurnaghan SJ, Blackbourn LA, et al. Diabetic neuropathy is a substantial burden in people with type 1 diabetes and is strongly associated with socioeconomic disadvantage: a population-representative study from Scotland. Diabetes Care. 2020;43(4): 734–742. [DOI] [PubMed] [Google Scholar]
- 12.Andersen ST, Witte DR, Dalsgaard E-M, et al. Risk Factors for Incident Diabetic Polyneuropathy in a Cohort With Screen-Detected Type 2 Diabetes Followed for 13 Years: ADDITION-Denmark. Diabetes Care. 2018;41(5): 1068–1075. [DOI] [PubMed] [Google Scholar]
- 13.Mather KJ, Bebu I, Baker C, et al. Prevalence of microvascular and macrovascular disease in the Glycemia Reduction Approaches in Diabetes - A Comparative Effectiveness (GRADE) Study cohort. Diabetes Res Clin Pract. 2020;165: 108235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pop-Busui R, Lu J, Brooks MM, et al. Impact of glycemic control strategies on the progression of diabetic peripheral neuropathy in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Cohort. Diabetes Care. 2013;36(10): 3208–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Group TS. Risk Factors for Diabetic Peripheral Neuropathy in Adolescents and Young Adults With Type 2 Diabetes: Results From the TODAY Study. Diabetes Care. 2021;45(5): 1065–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dabelea D, Stafford JM, Mayer-Davis EJ, et al. Association of Type 1 Diabetes vs Type 2 Diabetes Diagnosed During Childhood and Adolescence With Complications During Teenage Years and Young Adulthood. JAMA. 2017;317(8): 825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Group* NCCCW. The National Clinical Care Commission Report: Improving Federal Programs That Impact Diabetes Prevention and Care. Annals of Internal Medicine. 2022;175(4): 594–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.International Diabetes Federation. IDF Diabetes Atlas teB. Belgium: 2021. Available at: https://www.diabetesatlas.org. [Google Scholar]
- 19.Tesfaye S, Chaturvedi N, Eaton SE, et al. Vascular risk factors and diabetic neuropathy. New England Journal of Medicine. 2005;352(4): 341–350. [DOI] [PubMed] [Google Scholar]
- 20.Rathmann W, Dickhaus T, Meisinger C, Mielck A. Prevalence of polyneuropathy in pre-diabetes and diabetes is associated with abdominal obesity and macroangiopathy: The MONICA/KORA Augsburg Surveys S2 and S3. Diabetes Care. 2008;31(3): 464. [DOI] [PubMed] [Google Scholar]
- 21.Callaghan BC, Xia R, Banerjee M, et al. Metabolic syndrome components are associated with symptomatic polyneuropathy independent of glycemic status. Diabetes care. 2016;39(5): 801–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu F, Zhao L-h, Su J-b, et al. The relationship between glycemic variability and diabetic peripheral neuropathy in type 2 diabetes with well-controlled HbA1c. Diabetology & Metabolic Syndrome. 2014;6(1): 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill-Briggs F, Adler NE, Berkowitz SA, et al. Social determinants of health and diabetes: a scientific review. Diabetes care. 2021;44(1): 258–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hicks CW, Wang D, Matsushita K, Windham BG, Selvin E. Peripheral neuropathy and all-cause and cardiovascular mortality in US adults: a prospective cohort study. Annals of internal medicine. 2021;174(2): 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.GAGNE A, MARCUS H, DAWOOD T, et al. 461-P: Prevalence and Patient Recognition of Distal Symmetric Neuropathy (DSP) in a Predominantly Low-Income US Patient Population. Diabetes. 2022;71(Supplement_1). [Google Scholar]
- 26.Jaiswal M, Fufaa GD, Martin CL, Pop-Busui R, Nelson RG, Feldman EL. Burden of diabetic peripheral neuropathy in Pima Indians with type 2 diabetes. Diabetes Care. 2016;39(4): e63–e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng YJ, Kanaya AM, Araneta MRG, et al. Prevalence of diabetes by race and ethnicity in the United States, 2011-2016. Jama. 2019;322(24): 2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan T-W, Shih C-D, Concha-Moore KC, et al. Disparities in outcomes of patients admitted with diabetic foot infections. PLoS One. 2019;14(2): e0211481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arya S, Binney Z, Khakharia A, et al. Race and Socioeconomic Status Independently Affect Risk of Major Amputation in Peripheral Artery Disease. J Am Heart Assoc. 2018;7(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan TW, Calhoun EA, Knapp SM, et al. Rates of Diabetes-Related Major Amputations Among Racial and Ethnic Minority Adults Following Medicaid Expansion Under the Patient Protection and Affordable Care Act. JAMA Netw Open. 2022;5(3): e223991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kahkoska AR, Pokaprakarn T, Alexander GR, et al. The impact of racial and ethnic health disparities in diabetes management on clinical outcomes: a reinforcement learning analysis of health inequity among youth and young adults in the SEARCH for diabetes in youth study. Diabetes Care. 2022;45(1): 108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Selvarajah D, Cash T, Sankar A, et al. The contributors of emotional distress in painful diabetic neuropathy. Diab Vasc Dis Res. 2014;11(4): 218–225. [DOI] [PubMed] [Google Scholar]
- 33.Vileikyte L, Leventhal H, Gonzalez JS, et al. Diabetic peripheral neuropathy and depressive symptoms: the association revisited. Diabetes care. 2005;28(10): 2378–2383. [DOI] [PubMed] [Google Scholar]
- 34.Feldman EL, Callaghan BC, Pop-Busui R, et al. Diabetic neuropathy. Nature reviews Disease primers. 2019;5(1): 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tesfaye S, Vileikyte L, Rayman G, et al. Painful diabetic peripheral neuropathy: consensus recommendations on diagnosis, assessment and management. Diabetes/metabolism research and reviews. 2011;27(7): 629–638. [DOI] [PubMed] [Google Scholar]
- 36.Abbott CA, Malik RA, van Ross ERE, Kulkarni J, Boulton AJM. Prevalence and Characteristics of Painful Diabetic Neuropathy in a Large Community-Based Diabetic Population in the U.K. Diabetes Care. 2011;34(10): 2220–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Truini A, Spallone V, Morganti R, et al. A cross-sectional study investigating frequency and features of definitely diagnosed diabetic painful polyneuropathy. PAIN. 2018;159(12): 2658–2666. [DOI] [PubMed] [Google Scholar]
- 38.Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A. Neuropathic pain in diabetes, prediabetes and normal glucose tolerance: the MONICA/KORA Augsburg Surveys S2 and S3. Pain Med. 2009;10(2): 393–400. [DOI] [PubMed] [Google Scholar]
- 39.Gylfadottir SS, Christensen DH, Nicolaisen SK, et al. Diabetic polyneuropathy and pain, prevalence, and patient characteristics: a cross-sectional questionnaire study of 5,514 patients with recently diagnosed type 2 diabetes. Pain. 2020;161(3): 574–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ang L, Dillon B, Mizokami-Stout K, Pop-Busui R. Cardiovascular autonomic neuropathy: A silent killer with long reach. Autonomic Neuroscience. 2020;225: 102646. [DOI] [PubMed] [Google Scholar]
- 41.Pop-Busui R, Low PA, Waberski BH, et al. Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC). Circulation. 2009;119(22): 2886–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mizokami-Stout K, Bailey R, Ang L, et al. Symptomatic diabetic autonomic neuropathy in type 1 diabetes (T1D): Findings from the T1D exchange. J Diabetes Complications. 2022;36(5): 108148. [DOI] [PubMed] [Google Scholar]
- 43.Andersen ST, Witte DR, Fleischer J, et al. Risk Factors for the Presence and Progression of Cardiovascular Autonomic Neuropathy in Type 2 Diabetes: ADDITION-Denmark. Diabetes Care. 2018;41(12): 2586–2594. [DOI] [PubMed] [Google Scholar]
- 44.Stein P, Barzilay J, Domitrovich P, et al. The relationship of heart rate and heart rate variability to non-diabetic fasting glucose levels and the metabolic syndrome: The Cardiovascular Health Study. Diabetic medicine. 2007;24(8): 855–863. [DOI] [PubMed] [Google Scholar]
- 45.Jung HK, Locke III GR, Schleck CD, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136(4): 1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feldman EL, Nave KA, Jensen TS, Bennett DLH. New Horizons in Diabetic Neuropathy: Mechanisms, Bioenergetics, and Pain. Neuron. 2017;93(6): 1296–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dewanjee S, Das S, Das AK, et al. Molecular mechanism of diabetic neuropathy and its pharmacotherapeutic targets. Eur J Pharmacol. 2018;833: 472–523. [DOI] [PubMed] [Google Scholar]
- 48.Pop-Busui R, Ang L, Holmes C, Gallagher K, Feldman EL. Inflammation as a Therapeutic Target for Diabetic Neuropathies. Curr Diab Rep. 2016;16(3): 29–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kellogg AP, Wiggin TD, Larkin DD, Hayes JM, Stevens MJ, Pop-Busui R. Protective Effects of Cyclooxygenase-2 Gene Inactivation Against Peripheral Nerve Dysfunction and Intraepidermal Nerve Fiber Loss in Experimental Diabetes. Diabetes. 2007;56(12): 2997–3005. [DOI] [PubMed] [Google Scholar]
- 50.Baum P, Toyka KV, Bliiher M, Kosacka J, Nowicki M. Inflammatory Mechanisms in the Pathophysiology of Diabetic Peripheral Neuropathy (DN)—New Aspects. International Journal of Molecular Sciences. 2021;22(19): 10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hinder LM, Murdock BJ, Park M, et al. Transcriptional networks of progressive diabetic peripheral neuropathy in the db/db mouse model of type 2 diabetes: An inflammatory story. Exp Neurol. 2018;305: 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herder C, Kannenberg JM, Huth C, et al. Proinflammatory Cytokines Predict the Incidence and Progression of Distal Sensorimotor Polyneuropathy: KORA F4/FF4 Study. Diabetes Care. 2017;40(4): 569–576. [DOI] [PubMed] [Google Scholar]
- 53.Herder C, Lankisch M, Ziegler D, et al. Subclinical Inflammation and Diabetic Polyneuropathy: MONICA/KORA Survey F3 (Augsburg, Germany). Diabetes Care. 2009;32(4): 680–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bäckryd E, Themistocleous A, Larsson A, et al. Hepatocyte growth factor, colony-stimulating factor 1, CD40, and 11 other inflammation-related proteins are associated with pain in diabetic neuropathy: exploration and replication serum data from the Pain in Neuropathy Study. Pain. 2022;163(5): 897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Callaghan BC, Little AA, Feldman EL, Hughes RA. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane database of systematic reviews. 2012(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Callaghan BC, Xia R, Reynolds E, et al. Association Between Metabolic Syndrome Components and Polyneuropathy in an Obese Population. JAMA Neurol. 2016;73(12): 1468–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Christensen DH, Knudsen ST, Gylfadottir SS, et al. Metabolic Factors, Lifestyle Habits, and Possible Polyneuropathy in Early Type 2 Diabetes: A Nationwide Study of 5,249 Patients in the Danish Centre for Strategic Research in Type 2 Diabetes (DD2) Cohort. Diabetes Care. 2020;43(6): 1266–1275. [DOI] [PubMed] [Google Scholar]
- 58.Eid SA, Feldman EL. Advances in diet-induced rodent models of metabolically acquired peripheral neuropathy. Dis Model Mech. 2021;14(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O'Brien PD, Guo K, Eid SA, et al. Integrated lipidomic and transcriptomic analyses identify altered nerve triglycerides in mouse models of prediabetes and type 2 diabetes. Dis Model Mech. 2020;13(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davidson EP, Coppey LJ, Calcutt NA, Oltman CL, Yorek MA. Diet-induced obesity in Sprague-Dawley rats causes microvascular and neural dysfunction. Diabetes Metab Res Rev. 2010;26(4): 306–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guilford BL, Ryals JM, Wright DE. Phenotypic changes in diabetic neuropathy induced by a high-fat diet in diabetic C57BL/6 mice. Exp Diabetes Res. 2011;2011: 848307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rumora AE, Savelieff MG, Sakowski SA, Feldman EL. Disorders of mitochondrial dynamics in peripheral neuropathy: clues from hereditary neuropathy and diabetes. International Review of Neurobiology. 2019;145: 127–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rumora AE, LoGrasso G, Hayes JM, et al. The Divergent Roles of Dietary Saturated and Monounsaturated Fatty Acids on Nerve Function in Murine Models of Obesity. J Neurosci. 2019;39(19): 3770–3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coppey L, Davidson E, Shevalye H, Torres ME, Yorek MA. Effect of dietary oils on peripheral neuropathy-related endpoints in dietary obese rats. Diabetes Metab Syndr Obes. 2018;11: 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sajic M, Rumora AE, Kanhai AA, et al. High Dietary Fat Consumption Impairs Axonal Mitochondrial Function In Vivo. J Neurosci. 2021;41(19): 4321–4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Babetto E, Wong KM, Beirowski B. A glycolytic shift in Schwann cells supports injured axons. Nat Neurosci. 2020;23(10): 1215–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang L, Chopp M, Szalad A, et al. Exosomes Derived From Schwann Cells Ameliorate Peripheral Neuropathy in Type 2 Diabetic Mice. Diabetes. 2020;69(4): 749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tesfaye S, Boulton AJM, Dyck PJ, et al. Diabetic Neuropathies: Update on Definitions, Diagnostic Criteria, Estimation of Severity, and Treatments. Diabetes Care. 2010;33(10): 2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vileikyte L, Rubin RR, Leventhal H. Psychological aspects of diabetic neuropathic foot complications: an overview. Diabetes/metabolism research and reviews. 2004;20(S1): S13–S18. [DOI] [PubMed] [Google Scholar]
- 70.Spallone V, Morganti R, D’amato C, Greco C, Cacciotti L, Marfia G. Validation of DN4 as a screening tool for neuropathic pain in painful diabetic polyneuropathy. Diabetic Medicine. 2012;29(5): 578–585. [DOI] [PubMed] [Google Scholar]
- 71.Pop-Busui R. What Do We Know and We Do Not Know About Cardiovascular Autonomic Neuropathy in Diabetes. J of Cardiovasc Trans Res. 2012;5(4): 463–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pop-Busui R, Januzzi JL, Bruemmer D, et al. Heart Failure: An Underappreciated Complication of Diabetes. A Consensus Report of the American Diabetes Association. Diabetes Care. 2022;45(7): 1670–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pop-Busui R, Backlund JYC, Bebu I, et al. Utility of using electrocardiogram measures of heart rate variability as a measure of cardiovascular autonomic neuropathy in type 1 diabetes patients. Journal of diabetes investigation. 2022;13(1): 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Plummer MP, Jones KL, Cousins CE, et al. Hyperglycemia potentiates the slowing of gastric emptying induced by exogenous GLP-1. Diabetes Care. 2015;38(6): 1123–1129. [DOI] [PubMed] [Google Scholar]
- 75.Russo A, Stevens JE, Chen R, et al. Insulin-induced hypoglycemia accelerates gastric emptying of solids and liquids in long-standing type 1 diabetes. The Journal of Clinical Endocrinology & Metabolism. 2005;90(8): 4489–4495. [DOI] [PubMed] [Google Scholar]
- 76.Revicki D, Camilleri M, Kuo B, Szarka L, McCormack J, Parkman H. Evaluating symptom outcomes in gastroparesis clinical trials: validity and responsiveness of the Gastroparesis Cardinal Symptom Index-Daily Diary (GCSI-DD). Neurogastroenterology & Motility. 2012;24(5): 456–463. [DOI] [PubMed] [Google Scholar]
- 77.Singleton JR, Marcus RL, Lessard MK, Jackson JE, Smith AG. Supervised exercise improves cutaneous reinnervation capacity in metabolic syndrome patients. Ann Neurol. 2015;77(1): 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lewis EJH, Lovblom LE, Lanctot S, et al. The association between physical activity time and neuropathy in longstanding type 1 diabetes: A cross-sectional analysis of the Canadian study of longevity in type 1 diabetes. Journal of Diabetes and its Complications. 2022;36(3): 108134. [DOI] [PubMed] [Google Scholar]
- 79.Kazamel M, Stino AM, Smith AG. Metabolic syndrome and peripheral neuropathy. Muscle Nerve. 2021;63(3): 285–293. [DOI] [PubMed] [Google Scholar]
- 80.Morrison S, Colberg SR, Parson HK, Vinik AI. Exercise improves gait, reaction time and postural stability in older adults with type 2 diabetes and neuropathy. J Diabetes Complications. 2014;28(5): 715–722. [DOI] [PubMed] [Google Scholar]
- 81.The Look ARG. Effects of a long-term lifestyle modification programme on peripheral neuropathy in overweight or obese adults with type 2 diabetes: the Look AHEAD study. Diabetologia. 2017;60(6): 980–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Petersen EA, Stauss TG, Scowcroft JA, et al. Effect of High-frequency (10-kHz) Spinal Cord Stimulation in Patients With Painful Diabetic Neuropathy: A Randomized Clinical Trial. JAMA Neurol. 2021;78(6): 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gæde P, Vedel P, Larsen N, Jensen GVH, Parving H-H, Pedersen O. Multifactorial Intervention and Cardiovascular Disease in Patients with Type 2 Diabetes. New England Journal of Medicine. 2003;348(5): 383–393. [DOI] [PubMed] [Google Scholar]
- 84.Tang Y, Shah H, Bueno Junior CR, et al. Intensive Risk Factor Management and Cardiovascular Autonomic Neuropathy in Type 2 Diabetes: The ACCORD Trial. Diabetes Care. 2021;44(1): 164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bönhof GJ, Strom A, Apostolopoulou M, et al. High-intensity interval training for 12 weeks improves cardiovascular autonomic function but not somatosensory nerve function and structure in overweight men with type 2 diabetes. Diabetologia. 2022;65(6): 1048–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]