Abstract
Purpose:
Functional recovery after cataract surgery depends on the anatomical recovery of the eye. This study compared the improvement in visual function parameters after uniocular manual small-incision cataract surgery (MSICS) and phacoemulsification cataract surgery.
Methods:
This study included 310 patients divided randomly into two groups: 155 who received MSICS (MSICS group) and 155 who underwent phacoemulsification (phaco group) for cataract treatment. Outcome measures assessed included vertical and horizontal keratometry reading. The mean corneal astigmatism tear function measured using Schirmer 1 test results were recorded preoperatively, and on postoperative day 1, day 7, and day 30. Optical coherence tomography (OCT) was done to record the average central macular thickness (μm) on day 7 and day 30.
Results:
The mean corneal astigmatism and anterior chamber inflammation were more in the MSICS group than in the phaco group immediately postoperatively. However, no statistically significant difference was found between the groups with respect to corneal sensation, mean corneal astigmatism, tear film function, and visual outcomes on postoperative day 30. Uncorrected visual acuity was better in the phacoemulsification group than in the manual SICS group on postoperative day 1, day 7, and day 30 (P < 0.001).
Conclusion:
Both phacoemulsification cataract surgery and manual small-incision sutureless cataract surgery (MSICS) are safe and effective for visual rehabilitation. Phacoemulsification is the preferred technique where resources are available with the advantages of less mean corneal astigmatism, less anterior chamber inflammation, and better uncorrected visual acuity (UCVA) in the immediate postoperative period.
Keywords: Corneal astigmatism, manual small-incision cataract surgery, phacoemulsification cataract surgery, uncorrected visual acuity
Cataract is defined as a loss of transparency of the natural crystalline lens and is usually an age-related phenomenon. Cataract is the major cause of blindness in India (62.60%) [1] and throughout the world and can only be treated by surgery. Modern cataract surgery techniques such as manual small-incision cataract surgery (MSICS) and phacoemulsification cataract surgery (phaco) offer advantages such as early visual rehabilitation, less induced astigmatism, no suture-related complication, less postoperative inflammation, short-term wound recovery, and allow managed ocular chamber depth during surgery as compared to ECCE. [2,3,4,5]
Initially, cataract surgery was aimed at preventing blindness. Now, it has progressed to being a refractive procedure that aims for postoperative emmetropia and, in effect, the best possible visual outcome and early functional recovery, that is, improvement in visual function (VF) and vision-related quality of life (VRQOL). [6] In recent years, however, there has been increasing recognition of the importance of assessing patients’ satisfaction and views regarding the impact of medical conditions and interventions on quality of life; thus, the quality of life assessment has gained increasing interest and acceptance. [6]
Manual SICS and phacoemulsification techniques have different characteristics in site, size, and depth of the incision. In manual SICS, a straight incision is made on the sclera. It penetrates the cornea at the level of Schwalbe’s line, [4,5] and nucleus delivery is made through a sclerocorneal tunnel, whereas phacoemulsification involves a clear corneal incision, and the tip of the instrument is introduced into the eye through a clear corneal incision, which generates high-frequency waves that break cataract in small pieces and sucked out through the tip. [4,5] As surgical techniques of manual SICS and phacoemulsification are different, anatomical recovery after cataract surgery because of either surgical technique with its effect on change in corneal curvature, corneal sensation, postsurgical inflammation, macular thickness, and tear film function may be different, ultimately affecting the speed of visual rehabilitation. [7,8]
To our knowledge, a combined comparative study of both anatomical and functional recovery after phacoemulsification cataract surgery and manual SICS has not been done in India. Hence, our study aims to compare anatomical and functional recovery in patients undergoing manual SICS and phacoemulsification cataract surgery.
Methods
The study design was conceived and planned at a tertiary eye hospital in Western Maharashtra. This retrospective interventional study was conducted using the records of patients who underwent cataract surgery between December 2012 and November 2014. The approval of the institutional review board was obtained to carry out the study. A total of 310 patients with age-related cataracts, fulfilling the inclusion criteria, who got operated by either surgical technique of manual SICS or phacoemulsification, were identified and included in the study. They were divided equally into two groups: those who underwent SICS (the MSICS group) and those who underwent phacoemulsification (the phaco group). The need for written consent was waived by the board on account of the retrospective nature of the study. The study was conducted in accordance with the principles of the Declaration of Helsinki and its later amendments.
Inclusion criteria
Age: 40–70 years
Male/female
Patients having uncomplicated bilateral immature senile cataract with grade III or less (BCVA in either eye <6/18)
Patient with regular astigmatism
Patient who underwent uncomplicated cataract surgery
Exclusion criteria
Age <40 and >70 years
Grade 4–5 cataracts
Mature, hypermature, complicated, congenital, and traumatic cataract
Patients having immature cataracts associated with other ocular comorbidities, injury, or surgery
Patients having any systemic disorders such as diabetes mellitus and hypertension
The patients underwent MSICS and phacoemulsification as per the procedure described in previous studies. [9,10]
Preoperative examination
A complete preoperative evaluation, including history and general examination, visual acuity assessment and refraction, detailed slit-lamp examination, IOP, lacrimal sac syringing (when required), fundus examination, and IOL power calculation by Carl Zeiss IOLMaster Advanced Technology Version 7.1, was done.
Visual acuity assessment was done using Snellen’s chart (Landolt C chart). Uncorrected visual acuity was recorded preoperatively and postoperative on day 1, day 7, and day 30. The best-corrected visual acuity was recorded only on postoperative day 30. Automated keratometry was performed using Carl Zeiss IOLMaster Advanced Technology Version 7.1.
Vertical and horizontal keratometry reading and mean corneal astigmatism were recorded preoperatively and on postoperative day 1, day 7, and day 30. Only eyes with regular astigmatism (major axis within 15° of 90° or 180°) were included in the study. The evaluation of the corneal curvature preoperatively and postoperatively was done to assess the healing of the wound based on the change in the corneal curvature after surgery by comparing the keratometry value of day 1, day 7, and day 30.
Schirmer test was performed to assess the basal and the reflex secretions of the tears. Corneal sensation was recorded preoperatively and on postoperative day 1, day 7, and 1 month. Optical coherence tomography (OCT) was done using TOPCON 3D OCT-2000 (version 8.00, spectral domain-based OCT) using scan 3D (6.0 × 6.0 mm - 512 × 218 macular cube) with fixation point as the macula to record the average central macular thickness (um) on day 7 and day 30. Central macular thickness (mean thickness at the intersection of six radial scans) was recorded. Preoperative macular thickness was not recorded because of media opacity due to cataract.
All patients were examined on day 1 and followed up on day 7 and day 30. Visual acuity assessment, automated keratometry, Schirmer 1 test without anesthesia, corneal sensation, and OCT to record macular thickness were repeated at every follow-up visit.
Statistical analysis
Data analysis was done by using SPSS (Statistical package for social science) version 19.0 statistical software. Continuous data were expressed as mean ± standard deviation and compared using the Student t test or Mann–Whitney U test. Categoric data were expressed in percentages and compared using the Chi-Square test. Mann–Whitney U test was used for the analysis of anterior chamber inflammation. Fisher’s exact test was used for the analysis of uncorrected visual acuity. Paired t test and two independent sample t test were used to find the significance of various parameters related to corneal curvature, tear film function, and postsurgical inflammation (macular thickness) in the manual SICS group and phacoemulsification group.
Results
The study consisted of 310 patients who underwent cataract surgery. The mean age of the patients in the SICS group was 60.01 ± 7.7 years and in the phaco group was 59.81 ± 7.19 years; 47% of the patients were males. In the SICS group, there was a statistically significant difference in mean vertical and horizontal keratometry when preoperative keratometry values were compared with day 1 keratometry values (P < 0.001), but there was no statistically significant difference in mean vertical and horizontal keratometry values when day 1 keratometry values were compared with day 7 keratometry values and day 7 keratometry values compared with day 30 keratometry values (P > 0.05). In the phaco group, there was a statistically significant difference in mean vertical and horizontal keratometry when preoperative keratometry values were compared with day 1 keratometry values (P < 0.001 and P < 0.026, respectively). There was a minimal statistically significant difference in mean vertical keratometry values when day 1 keratometry values were compared with day 7 keratometry values (P = 0.036) and when day 7 keratometry values were compared with day 30 keratometry values (P = 0.049). There was no statistically significant difference in mean horizontal keratometry values when day 1 keratometry values were compared with day 7 keratometry values and day 7 keratometry values were compared with day 30 keratometry values (P > 0.05).
By Mann–Whitney U test, P < 0.001; thus, there was a statistically significant difference in anterior chamber inflammation between both groups on postoperative day 1, but on day 7 and day 30, there was no statistically significant difference between the two groups. Anterior chamber inflammation was more in the SICS group than in the phaco group on day 1. By using two independent sample t tests with P > 0.05, there was no statistically significant difference between mean central macular thickness on postoperative day 7 and day 30 (intergroup comparison). By using paired t test, P < 0.001; thus, there was a significant difference between mean central macular thickness on day 7 and day 30 in each respective group (intragroup comparison), that is, regardless of the group, a statistically significant mean central macular thickness increased over a period of 1 month. Using two independent sample t test, P > 0.05; thus, there was no significant difference in mean Schirmer 1 test values between the two groups preoperatively and on postoperative day 7 and day 30.
Using Fisher’s exact test, P < 0.001; thus, there was a significant difference in the uncorrected visual acuity between the two groups on postoperative day 1, day 7, and day 30. Uncorrected visual acuity was better in the phaco group than in the SICS group on postoperative day 1, day 7, and day 30 [Fig. 1]. There was no significant difference in the best-corrected visual acuity between the two groups on postoperative day 30 [Fig. 2].
Figure 1.
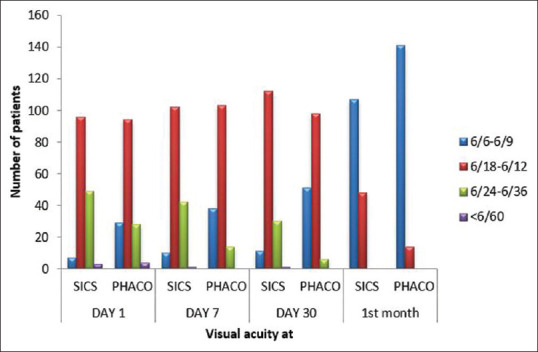
Comparison of postoperative uncorrected visual acuity between the SICS and phaco groupss
Figure 2.
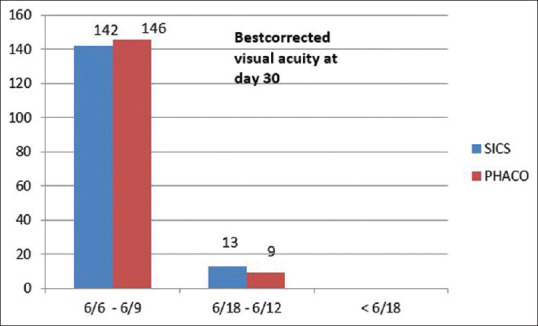
Comparison of best corrected visual acuity between the SICS and phaco groups on postoperative day 30
Discussion
Before the 1980s, cataract surgery aimed to prevent blindness. However, now it has progressed to a refractive procedure that aims for postoperative emmetropia, the best possible visual outcome, and early functional recovery, that is, improvement in visual function (VF) and vision-related quality of life (VRQOL). Functional recovery after cataract surgery depends on the anatomical recovery of the eye.
Anatomical structures that can get affected after cataract surgery are cornea, that is, corneal edema, striate keratopathy, change in corneal curvature, and corneal sensation. There may be a change in intraocular lens position due to the lens capsule contracture (capsular bag contracture), change in anterior chamber depth and content, tear film function, and macular thickness.
In the phacoemulsification and manual SICS group, a variable amount of against the rule astigmatism is produced during wound healing owing to the cornea’s flattening in the vertical meridian. There was no statistically significant difference in preoperative mean vertical and horizontal keratometry between the SICS and phacoemulsification groups. In the manual SICS group, the vertical component of astigmatism was significantly lower postoperatively than preoperatively (P < 0.001), and the horizontal component of astigmatism was significantly more postoperatively than preoperatively (P < 0.001). This was probably the result of the biomechanical effect that may flatten the surgical meridian after wound healing with the meridian of greatest curvature perpendicular to the incision.
It is due to the large incision size (6–6.5 mm) and, to some extent, cauterization. Larger incision size in SICS was associated with more mean astigmatism and less uncorrected visual acuity. Though there was more mean corneal astigmatism and more flattening of vertical curvature of cornea noted postoperatively on day 1 in eyes operated by manual SICS, our study showed that over time, there were no significant changes in the final amount of postoperative mean corneal astigmatism and no change in the vertical and horizontal meridian in the SICS group [Table 1]. It is consistent with the results reported by Khan et al. (2010). [11]
Table 1.
Intergroup comparison of mean astigmatism: Preoperative and on postoperative day 1, day 7, and day 30
| Surgery | n | Mean | SD | P |
|---|---|---|---|---|
| PREOP | ||||
| SICS | 155 | −0.89 | 0.72 | 0.018 |
| Phaco | 155 | −0.72 | 0.48 | |
| DAY1 | ||||
| SICS | 150 | −1.73 | 1.13 | <0.001 |
| Phaco | 152 | −1.04 | 0.74 | |
| DAY7 | ||||
| SICS | 155 | −1.72 | 1.04 | <0.001 |
| Phaco | 155 | −1.02 | 0.64 | |
| DAY30 | ||||
| SICS | 155 | −1.72 | 0.98 | <0.001 |
| Phaco | 155 | −1.00 | 0.60 |
*values of mean in diopters
In the phacoemulsification group, the vertical component of astigmatism was significantly lower postoperatively than preoperatively (P < 0.001), and the horizontal component of astigmatism was more postoperatively than preoperatively (P = 0.026). This was probably the result of corneal relaxation due to an operative incision in that meridian. It is consistent with results reported by Sušić et al. (2007). [12] This change in the vertical and horizontal components of astigmatism was less in the phacoemulsification group if compared with the change noted in the SICS group.
Our study showed that in the phacoemulsification group, there was a significant change in vertical curvature of the cornea postoperatively over the course of time, that is, a significant change in vertical curvature of the cornea was noted when postoperative day 1 vertical keratometry value was compared with that of postoperative day 7 vertical keratometry value (P = 0.036) and postoperative day 7 vertical keratometry value compared with that of day 30 vertical keratometry value (P = 0.049). This change in the vertical curvature of the cornea may be because clear corneal incisions affect the cornea’s curvature differently, and wound healing of clear corneal incision itself can cause wound healing and flattening of the vertical curvature of the cornea. Immediately after the completion of corneal incision, wound edges swell because of the imbibition of fluid by injured corneal lamellae, [13] which resolves after that. However, a pronounced transient postoperative corneal swelling lasting approximately 2 weeks is sometimes noted after phacoemulsification surgery, and corneal swelling becomes stable after 2 weeks [14] These changes in the vertical curvature of the cornea can also be because of hydration of clear corneal incision done for good apposition of the inner wound at the end of the surgery.
Lesser mean corneal astigmatism was noted in the phacoemulsification group. This may be because the incision was smaller and triplanar, made more gently in a controllable manner rather than a uniplanar clear corneal one.
There may be a change in spherical equivalent due to early myopic shift due to a change in anterior chamber depth, possibly because of capsulorhexis shrinkage and change in IOL position or IOL optic configuration. [15] It is consistent with the results reported by Victoria de Juan (2013). [14] There was a statistically significant difference in anterior chamber inflammation between both groups on postoperative day 1 (P < 0.001), but there was no statistically significant difference between the two groups on day 7 and day 30 (P > 0.05) Anterior chamber inflammation was more in the SICS group than in the phacoemulsification group on postoperative day 1. It is consistent with the results reported by Gills et al. (1991). [16] Mean anterior chamber cell grade of 2 in the SICS group and grade of 0.5 (SUN Working Group Grading) in the phacoemulsification group were noted on postoperative day 1. This corroborates with the study by Taravati et al. (2012). [17]
Macular thickness
Our study showed that on postoperative day 7, the mean central macular thickness in the MSICS group was 149.36 ± 39.24 mm and that in the phacoemulsification group was 153.59 ± 49.13 mm, with no significant difference (P = 0.403). On postoperative day 30, the mean central macular thickness in the MSICS group was 159.48 ± 44.03 mm and that in the phacoemulsification group was 166.93 ± 50.83 mm, with no significant difference (P = 0.169).
A significant increase in central macular thickness was observed in the manual SICS and phacoemulsification groups on postoperative day 30 (P < 0.001). This result corroborates with the study by Ghosh et al. (2010). [18] This was attributed to possibly more tissue trauma, postoperative inflammation, and iris manipulation associated with manual SICS and the amount of energy used during phacoemulsification cataract surgery (Ferrari et al. 1999) [19] Larger incision size was associated with more severe blood–aqueous barrier breakdown in a study comparing phacoemulsification with SICS and extracapsular cataract extraction (Pande et al. 1996). [20]
A macular thickness change equal to or more than 40 mm has been described as an index of OCT-significant macular edema (Wittpenn et al., 2008) [21] OCT-significant macular edema was noted in 16 patients, eight in each group (5.01%), with no significant difference. There was no statistically significant difference in corneal sensation both preoperatively and on postoperative day 1, day 7, and day 30. A wisp of the cotton-tipped applicator was used to record corneal sensation at the center of the cornea and recorded as normal or absent.
Previous studies such as those by Sitompul et al. (2008) [22] and Jung et al. (2012) [23] reported that corneal sensitivity decreased at the incision site in the phacoemulsification group on days 1, 7, and 15 after surgery. In contrast, in the manual SICS group, no change in corneal sensitivity was noted. Our study showed no change in corneal sensitivity irrespective of the surgery type. This may be because, in our study, corneal sensations were recorded using a wisp of the cotton-tipped applicator. There was no significant difference in mean Schirmer 1 test values preoperatively between the two groups. There were no patients with measurements less than 10 mm wetting of Schirmer’s strip before and after surgery.
Immediately after surgery, irritation may stimulate the inflammatory process and increase tear production. Alfonso et al. [24] found a correlation between irritation and Schirmer test result (without anesthesia). Thus, Schirmer test without anesthesia was performed to provide a summative result of aqueous production by the lacrimal gland and irritation. [13] Because the inflammatory process subsides naturally or after the administration of anti-inflammatory eye drops, aqueous production decreases by day 15.
However, our study showed that in the SICS group, there was no change in tear film function on day 7 (P > 0.05), but the statistically significant increase in mean Schirmer 1 test values, that is, increase in tear production was noted on day 30 (P < 0.001). These results are not explainable. In the phacoemulsification group, the statistically significant decrease in mean Schirmer 1 test values was noted, that is, tear production decreased on day 7 (P = 0.018) but reached its baseline values on day 30. This may be because decreased corneal sensation disrupts the cornea lacrimal gland loop, resulting in reduced tear secretion. [25]
There was a significant difference in the postoperative uncorrected visual acuity between the two groups on day 1, day 7, and day 30 (P < 0.001). The postoperative uncorrected visual acuity (UCVA) of 6/9 or better was observed in 29 patients (18.7%) in the phacoemulsification group as compared to seven patients (4.51%) in the manual SICS group on day 1, in 38 patients (24.51%) in the phacoemulsification group as compared to 10 patients (6.45%) in the manual SICS group on day 7, and in 51 patients (32.9%) in the phacoemulsification group as compared to 11 patients (7.09%) in the manual SICS group on day 30.
Uncorrected visual acuity was better in the phaco group than in the SICS group on postoperative day 1, day 7, and day 30. This result corroborates the studies by Zhang et al. (2013), [26] and Cook et al. (2012). [27] The main reason for the decreased uncorrected visual acuity in the SICS group is more surgery-induced astigmatism, postoperative corneal edema, and more anterior chamber inflammation.
Best-corrected visual acuity
There was no significant difference in the postoperative best-corrected visual acuity between the two groups on day 30 (P > 0.05). The postoperative best-corrected visual acuity (UCVA) of 6/9 or better was observed in 142 patients (91.61%) in the manual SICS group and 146 patients (94.19%) in the phacoemulsification group on day 30 [Table 2]. These results corroborate the study by Cook (2011) [28] and Ruit et al. (2007). [29]
Table 2.
Comparison of best-corrected visual acuity in both the groups on postoperative day 30
| Postoperative bcva day30 | Surgery group | P | |||
|---|---|---|---|---|---|
|
| |||||
| SICS | % | PHACO | % | ||
| 6/6-6/9 | 142 | 91.61 | 146 | 94.19 | 0.357 |
| 6/18-6/12 | 13 | 8.38 | 9 | 5.81 | |
| <6/18 | 0 | 0.00 | 0 | 0.00 | |
| Total | 155 | 100.00 | 155 | 100.00 | |
Overall, our study showed that although mean corneal astigmatism was more in the SICS group than in the phacoemulsification group, over the course of time, there was no significant change in the final amount of postoperative astigmatism in both groups. UCVA of 6/9 or more was better in the phacoemulsification group as compared to the manual SICS group at every follow-up. There was a significant increase in mean central macular thickness in both groups on day 30. However, OCT-significant macular edema was noted in 16 patients, eight in each group (5.01%), with no significant difference. There were no statistically significant differences in corneal sensation and mean Schirmer 1 test values when comparing both groups.
Phacoemulsification is the preferred technique with the advantages of faster rehabilitation, less mean astigmatism, less post-surgical inflammation, and better postoperative uncorrected visual acuity. Phacoemulsification has become the most significant surgical achievement of the present decade. However, it is still not practiced by most surgeons in developing countries, including India, with a cataract backlog. Two important reasons are that the technique has a prolonged and sometimes traumatic learning curve and requires expensive and complex equipment. Despite excellent facilities and skilled surgeons, the developing world is deprived of the visual benefits of cataract surgery because it may not be an affordable technique. MSICS offers a good and cost-effective alternative in this situation. Its relatively smaller incision offers all merits of phacoemulsification with added advantages of broader applicability, better safety, shorter safety learning curve, and lower cost. An approach aimed at optimizing each step of these procedures, such as the use of a new blade for creating the scleral tunnel incision (because repeated use of the same blade or a blunt blade can create a faulty incision that may not self-seal) and the use of chondroitin sulfate based dispersive OVDs to protect the corneal endothelium, would go a long way toward achieving optimal results in both these time tested procedures.
Our study had several limitations. This was a single-center study; therefore, selection bias may affect the results. The study involved only a follow-up period of 30 days, and the difference in the effects of the two surgical techniques over a longer term could not be estimated. Therefore, studies with larger sample sizes and a more extensive study period are required to corroborate the findings of this study. Moreover, preoperative macular thickness values were not recorded because of the presence of significant media opacity in many patients interfering with good quality OCT scans.
Conclusion
In conclusion, phacoemulsification cataract surgery can be the preferred technique, with its relatively small-incision offering the advantages of less mean corneal astigmatism, less post-surgical inflammation, and better uncorrected visual acuity in the immediate post-surgical period.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Verma R, Khanna P, Prinja S, Rajput M, Arora V. The national programme for controle of blindness in India. Australas Med J. 2011;4:1–3. doi: 10.4066/AMJ.2011.505. doi:10.4066/AMJ.2011.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindstrom RL. Cataract surgery and lens implantation. Curr Opin Ophthalmol. 1994;5:1–4. doi: 10.1097/00055735-199402000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Uusifalo RJ, Tarkkanen A. Outcome of small incision cataract surgery. J Cataract Refract Surg. 1998;24:212–21. doi: 10.1016/s0886-3350(98)80202-2. [DOI] [PubMed] [Google Scholar]
- 4.Linebarger EJ, Hardten DR, Shah GK, Lindstrom RL. Phacoemulsification and modern cataract surgery. Surv Ophthalmol. 1999;44:13–47. doi: 10.1016/s0039-6257(99)00085-5. [DOI] [PubMed] [Google Scholar]
- 5.Irawati Y. Jakarta, Indonesia: Department of Ophthalmology, Medical Faculty, University of Indonesia; 2003. Comparison of Effectivity and Safety of ECCE and Manual SICS in Learning Curve [Thesis of Master in Ophthalmology. [Google Scholar]
- 6.Ellwein LB, Fletcher A, Negrel AD, Thulasiraj RD. quality of life assessment in blindness prevention interventions. Int Ophthalmol. 1994;18:263–8. doi: 10.1007/BF00917828. [DOI] [PubMed] [Google Scholar]
- 7.Dick HB, Schwenn O, Krummenauer F, Krist R, Pfeiffer N. Inflammation after sclerocorneal versus clear corneal tunnel phacoemulsification. Ophthalmology. 2000;107:241–7. doi: 10.1016/s0161-6420(99)00082-2. [DOI] [PubMed] [Google Scholar]
- 8.Venkatesh R, Tan CSH, Singh GP, Veena K, Krishnan KT, Ravindran RD. Safety and efficacy of manual small incision cataract surgery for brunescent and black cataracts. Eye. 2009;23:1155–7. doi: 10.1038/eye.2008.190. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad I, Wahab A, Sajjad S, Untoo RA. Visual rehabilitation following manual small incision cataract surgery. https://www.jkscience.org/archive/Volume73/visual.pdf.
- 10. Available from: http://en.wikipedia.org/wiki/Phacoemulsification .
- 11.Khan MT, Jan S, Hussain Z, Karim S, Khalid MK, Mohammad L. Visual outcome and complications of manual sutureless small incision cataract surgery. Pak J Ophthalmol. 2010;261 Available from: https://pjo.org.pk/index.php/pjo/article/view/601 . [Google Scholar]
- 12.Sušić N, Brajković J, Kalauz-Surać I. Analysis of postoperative corneal astigmatism after phacoemulsification. Acta Clin Croat. 2007;46:37–40. [Google Scholar]
- 13.Belmonte C, Acosta MC, Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res. 2004;78:513–25. doi: 10.1016/j.exer.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 14.de Juan V, Herreras JM, Pérez I, Morejón Á, Río-Cristóbal A, Martín R, et al. Refractive stabilization and corneal swelling after cataract surgery. Optometry Vis Sci. 2013;90:31–6. doi: 10.1097/OPX.0b013e318278fc44. [DOI] [PubMed] [Google Scholar]
- 15.Arai M, Ohzuno I, Zako M. Anterior chamber depth after posterior chamber intraocular lens implantation. Acta Ophthalmol. 1994;72:694–7. doi: 10.1111/j.1755-3768.1994.tb04682.x. [DOI] [PubMed] [Google Scholar]
- 16.Gills JP, Sanders DR. Use of small incisions to control induced astigmatism and inflammation following cataract surgery. J Cataract Refract Surg. 1991;17:740–4. doi: 10.1016/s0886-3350(13)80695-5. [DOI] [PubMed] [Google Scholar]
- 17.Taravati P, Lam DL, Leveque T, Van Gelder RN. Van Gelder postcataract surgical inflammation Curr Opin Ophthalmol. 2012;23:12–8. doi: 10.1097/ICU.0b013e32834cd60e. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh S, Roy I, Biswas PN, Maji D, Mondal LK, Mukhopadhyay S, et al. Prospective randomized comparative study of macular thickness following phacoemulsification and manual small incision cataract surgery. Acta Ophthalmologica. 2010;88:e102–6. doi: 10.1111/j.1755-3768.2010.01896.x. [DOI] [PubMed] [Google Scholar]
- 19.Ferrari MT, Cavallo M, Minnino L, Cardascia N. Macular edema induced by phacoemulcification. Doc Ophthalmol. 1999;97:325–7. doi: 10.1023/a:1002142307952. [DOI] [PubMed] [Google Scholar]
- 20.Pande MV, Spalton DJ, Kerr-Muir MG, Marshall J. Postoperative inflammatory response to phacoemulsification and extracapsular cataract surgery:Aqueous flare and cells. J Cataract Refract Surg. 1999;22((Suppl 1)):770–4. doi: 10.1016/s0886-3350(96)80160-x. [DOI] [PubMed] [Google Scholar]
- 21.Wittpenn JR on behalf of the Acular LS for Cystoid Macular Edema (ACME) Study Group. A randomized, masked comparison of topical ketorolac 0.4 plus steroid vs steroid alone in low-risk cataract surgery patients. Am J Ophthalmol. 2008;146:554–60. doi: 10.1016/j.ajo.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 22.Sitompul R, Sancoyo GS, Hutauruk JA, Gondhowiardjo TD. Sensitivity change in cornea and tear layer due to incision difference on cataract surgery with either manual small-incision cataract surgery or phacoemulsification. Cornea. 2008;27((Suppl 1)):S13–8. doi: 10.1097/ICO.0b013e31817f29d8. [DOI] [PubMed] [Google Scholar]
- 23.Oh T, Jung Y, Chang D, Kim J, Kim H. Changes in the tear film and ocular surface after cataract surgery. Jpn J Ophthalmol. 2012;56:113–8. doi: 10.1007/s10384-012-0117-8. [DOI] [PubMed] [Google Scholar]
- 24.Afonso AA, Monroy D, Stern ME, Feuer WJ, Tseng SC, Pflugfelder SC. Correlation of tear fluorescein clearance and Schirmer test scores with ocular irritation symptoms. Ophthalmology. 1999;106:803–10. doi: 10.1016/S0161-6420(99)90170-7. [DOI] [PubMed] [Google Scholar]
- 25.Donnenfeld ED, Solomon K, Perry HD, Doschi SJ, Ehrenhaus M, Solomon R, et al. The effect of the hinge position on the corneal sensation and the dry eye after LASIK. Ophthalmology. 2003;110:1023–9. doi: 10.1016/S0161-6420(03)00100-3. [DOI] [PubMed] [Google Scholar]
- 26.Zhang JY, Feng YF, Cai JQ Phacoemulsification versus manual small-incision cataract surgery for age-related cataract:Meta-analysis of randomized controlled trials. Clin Experiment Ophthalmol. 2013;41:379–86. doi: 10.1111/j.1442-9071.2012.02868.x. [DOI] [PubMed] [Google Scholar]
- 27.Cook C, Carrara H, Myer L. Phaco-emulsification versus manual small-incision cataract surgery in South Africa. S Afr Med J. 2012;102:537–40. doi: 10.7196/samj.5393. [DOI] [PubMed] [Google Scholar]
- 28.Cook C. Cataract surgery in low income countries-phaco or SICS. Paper presented at the Canadian Ophthalmology Society Congress, Vancouver. 2011 [Google Scholar]
- 29.Ruit S, Tabin J, Chang D, Bajracharya L, Kline C, Richheimer W, et al. A prospective randomized clinical trial of phacoemulsification vs. manual suture less small- incision extracapsular cataract surgery. Am J Ophthalmol. 2007;143:32–8. doi: 10.1016/j.ajo.2006.07.023. [DOI] [PubMed] [Google Scholar]


