Abstract
Background
Long‐time data of peanut allergy over time is sparse. We aimed to study the longitudinal development of sensitization to peanut extract and storage protein allergen molecules and associations with asthma status, airway and systemic inflammation markers.
Methods
The Swedish birth cohort BAMSE followed 4089 participants with questionnaires, clinical investigations and blood sampling between 0 and 24 years. Information on (i) background factors at 2 months, (ii) peanut allergy symptoms and IgE data (ImmunoCAP) at 4, 8, 16, and 24 years, and (iii) IgE to storage proteins, lung function data including exhaled nitric oxide (FENO) as well as systemic inflammatory markers at 24 years of age were collected.
Results
The prevalence of peanut extract sensitization, defined as IgE ≥ 0.35 kUA/L, was 5.4%, 8.0%, 7.5%, and 6.2% at 4, 8, 16, and 24 years of age, respectively. Between 8 and 24 years of age, (33/1565) participants developed IgE‐ab to peanut extract (median 1,4, range 0.7–2.6 kUA/L), and among those 85% were also sensitized to birch. Only six individuals developed sensitization to Ara h 2 (≥0.1 kUA/L) between 8 and 24 years of age, of whom three had an IgE‐ab level between 0.1–0.12 kUA/L. Storage protein sensitization was associated with elevated FENO, blood eosinophils and type 2 inflammation‐related systemic proteins.
Conclusion
Sensitization to peanut extract after 4 years of age is mainly induced by birch cross‐sensitization and IgE to Ara h 2 rarely emerges after eight years of age. Storage protein sensitization is associated with respiratory and systemic inflammation.
Keywords: birth cohort, FENO, molecular allergology, OLINK, peanut allergy, sensitization
The prevalence of peanut extract sensitization, defined as IgE≥0.35 kUA/L, is 5.4%, 8.0%, 7.5%, and 6.2% at 4, 8, 16, and 24 years of age, respectively. Most individuals with peanut sensitization at 24 years of age are sensitized to peanut extract already at 4 and 8 years. Individuals with sensitization to storage protein in peanut and/or tree nut at 24 years of age have higher levels of exhaled nitric oxide and blood eosinophils, both in asthmatics and non‐asthmatics, compared to individuals with other types of sensitization.Abbreviations: Ara h 2, Arachis hypogea 2, peanut allergen molecule; BAMSE, Barn/Children, Allergy, Milieu, Stockholm, Epidemiology; FeNo, exhaled nitric oxide; IgE, immunoglobulin E; kUA/L, kilounits of allergen specific sIgE‐ab per liter; MMP10, Matrix metalloproteinase‐10; NPX, normalized protein expression; TNFRSF11, tumor necrosis factor receptor superfamily 11

Abbreviations
- Ana o3
Anacardium occidental 3, cashew nut allergen molecule
- Ara h 1, Ara h 2, Ara h 3, Ara h 8, Ara h 9
Arachis hypogea 1, 2, etc., peanut allergen molecules
- BMI
body mass index
- Cor a 1, Cor a 9, Cor a 14
Corylus avellana 1, 9, etc., hazelnut allergen molecules
- FENO
fractional exhaled nitric oxide
- FEV1
forced expiratory volume in 1 s (ml)
- FVC
forced vital capacity (ml)
- IgE‐ab
immunoglobulin E antibody
- IL
interleukin
- Jug r 1
Juglans regia 1, walnut allergen molecule
- kUA/l
kilounits of allergen specific sIgE‐ab per liter
- MA
molecular allergology
- MMP10
matrix metalloproteinase‐10
- OAS
oral allergy syndrome
- TNFRSF
tumor necrosis factor receptor superfamily
- TSLP
thymic stromal lymphopoietin
1. BACKGROUND
The development of allergen‐specific Immunoglobulin E antibodies (IgE‐ab) in blood is dynamic throughout childhood and usually precedes development of allergic symptoms. 1 Peanut allergy is a common food allergy, both in adults and children 2 , 3 , 4 and previous studies show that peanut allergy starts early in life and is rarely outgrown. 3 , 5 , 6
Molecular allergology (MA) has led to improved understanding of allergy development. Several peanut allergen molecules have been identified, such as the storage proteins Ara h 1, Ara h 2, Ara h 3, Ara h 6 and the cross‐reacting molecules Ara h 8 (PR‐10) and Ara h 9 (lipid transfer protein). These provide increased accuracy in diagnosing sensitization and predicting symptoms. MA allows to disentangle between primary sensitization and cross‐sensitization, the latter is often of less clinical importance. Studies on peanut allergen sensitization and symptoms are mostly hospital based, cross‐sectional or performed during childhood only, whereas population‐based studies from childhood to adulthood are sparse. 5 , 7 , 8 , 9
Peanut allergy is more often found in individuals with an atopic heredity. 10 , 11 Several risk factors have been considered regarding development of peanut sensitization and the risk of developing peanut allergy. Timing and route of exposure has been discussed and even though the ideal time of oral introduction has not been established, early oral introduction is preferred over delayed and seem to decrease the risk of development of peanut allergy, especially in high‐risk infants with atopic dermatitis or allergy towards egg. 12
Earlier studies have found a correlation between peanut allergy and asthma. 13 , 14 While deaths due to asthma in general are few, the majority of deaths occurred in patients with underlying allergies, especially in young ages where allergy towards peanut/tree nut were among the most common allergen. 15 , 16 Associations between peanut allergy and asthma phenotypes have so far not been studied longitudinally.
1.1. Inflammatory biomarkers and systemic inflammation
Fractional exhaled nitric oxide (FENO) is a biomarker mainly associated with respiratory inflammation. 17 It can be measured by a simple non‐invasive test and is mainly used for evaluating the degree of airway inflammation. FENO is driven by the activation of the Th2 pathway 18 and elevated levels are often found in patients with untreated type 2 inflammation in bronchi and bronchioles, comorbid nasal polyposis, airborne allergy and in patients with an airborne allergy such as to furry animals, house dust mites and pollen 14 , 19 , 20 , 21 and food allergens. 14 FENO has also been found to correspond well to elevated levels of blood eosinophils, often correlated to allergic inflammation. 22
Both peanut allergic individuals with and without asthma 23 have been found to express high levels of FENO even though peanut is primarily a food and not inhalant allergen. 23 , 24 Johnson et al. have reported that asthmatic patients aged 10–35 years, with a sensitization to peanut allergen molecules Ara h 1, h 2 and h 3 or the hazelnut storage proteins Cor a 9 and Cor a 14, were found to express higher levels of FENO than individuals without peanut sensitization, 24 especially in the youngest age group. The explanation for this is not completely understood, neither if this is a sign of airway inflammation or a systemic inflammation, and further studies are needed.
IgE‐mediated allergic reactions cause inflammation and pruritus, usually due to mast cell and blood eosinophil activation and the release of proinflammatory markers. 25 Earlier studies on sera from peanut allergic patients found that peanut sensitized individuals were more prone to express elevated levels of T‐helper cells type 2 (Th2) skewed cytokines and an imbalance between effector and regulatory T cells, in contrast to non‐peanut allergic patients. 26 , 27
Stockfelt et al. utilized a proteomic approach and identified biomarkers related to inflammation and immunological processes that could potentially predict allergy development at an early age 28 but future studies exploring proteomics within the area of asthma and peanut allergy is needed in order to clarify this further.
The BAMSE study is a longitudinal birth cohort with data on early‐life and childhood exposures and participants are followed up to adulthood. 29 This offers novel approaches to study peanut allergy development further.
2. AIM
To study how IgE‐ab sensitization to peanut extract and peanut allergen molecules develops from childhood to adulthood, and how this relates to development of peanut allergy. Additionally, to study how storage protein sensitization relates to asthma phenotypes, lung functions tests such as fractional exhaled nitric oxide (FENO), spirometry and systemic inflammation markers in blood.
3. MATERIAL AND METHODS
3.1. Study design and study population
The BAMSE‐cohort is a population‐based birth cohort consisting of 4089 subjects born 1994–1996 in representative parts of Stockholm. 29 The participants have been followed from birth (median age 2 months) and at 1, 2, 4, 8, 12, 16, and 24 years of age. Background data were collected at time of inclusion (baseline). At follow‐ups, detailed information on symptom of allergic diseases and specific exposures were collected. Clinical investigations with measures of lung function and blood samples were performed at 4, 8, 16, and 24 years of age.
3.2. Sensitization
Blood samples were analyzed with ImmunoCAP System (Thermo Fisher Scientific, Uppsala, Sweden) for IgE‐ab to a mix of common food allergens (fx5®), and if above 0.35 kUA/L the test was further analyzed for specific IgE‐ab to all included allergens (peanut, soy, wheat, milk, egg, and cod). If peanut extract IgE‐ab was positive (≥0.35 kUA/L), further analysis for IgE to peanut allergen molecules (Ara h 1, Ara h 2, Ara h 3, Ara h 8, and Ara h 9) was performed at 8 and 24 years of age: Blood samples at 24 years of age were also analyzed for IgE‐ab to mixes of common tree nuts, fx1® (peanut, hazelnut, brazil nut, almond, coconut) and fx22® (pecan nut, cashew nut, pistachio, walnut), and if above 0.35 kUA/L the test was further analyzed for specific IgE‐ab to all included allergens (peanut, hazelnut, brazil nut, almond, coconut, and pecan nut, cashew nut, pistachio, walnut, respectively). If the sIgE‐ab level to hazelnut was ≥0.35 kUA/l, sIgE‐ab to Cor a 1, Cor a 9 and Cor a 14 were analyzed and if the sIgE‐ab level to walnut or cashew nut was ≥0.35 kUA/l, sIgE‐ab to Jug r 1 and Ana o 3 were analyzed, respectively.
This study includes data from study participants that provided blood samples including specific IgE at the 4‐, 8‐, 16‐, and 24‐year follow‐ups as well as questionnaire data from baseline and at 4, 8, 16, and 24 years. The study populations were (a) all participants who provided blood samples and peanut symptom data at 4, 8, 16, and 24 years for analyses of peanut extract sensitization development over time (n = 1167), and (b) all participants who provided blood samples and peanut symptom data at 8 and 24 years for analyses of IgE‐reactivity to peanut allergen molecules (n = 1565) and (c) participants who provided symptom data and blood samples for IgE analysis at 24 years (n = 2217). Due to the known high cross‐reactivity between peanut and tree nut storage proteins, IgE‐abs to all analyzed storage protein (peanut and tree nuts) were included in the comparative analyses at 24 years of age.
3.3. Proteomic analysis of inflammation related proteins
Expression of inflammation‐related proteins in plasma were analyzed using the Proseek Multiplex Inflammation Panel (version 95302) from Olink Biosciences, Uppsala, Sweden, as previously reported. 30 All 92 proteins analyzed in the panel were included in this study. Values below the lower limit of detection (defined as three standard deviations above background) were used as reported and not replaced by arbitrary values. Protein levels are expressed as Normalized Protein Expression (NPX) units, a relative quantification unit logarithmically related to protein concentration.
3.4. Lung function tests
Spirometry was performed using the Jaeger MasterScreen‐IOS system (Carefusion Technologies, San Diego, CA, USA). 31 Highest values of forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) were used, and the FEV1/FVC ratios were expressed as percentages. Standard deviation scores for FEV1, FVC, and FEV1/FVC were computed taking age, sex, height, and ethnicity into account as previously described. 32 Reversibility was defined as positive if FEV1/FVC was above 12%. Low FEV 1 /FVC was defined as a ratio of FEV1/FVC <0.7.
Fractional Exhaled Nitric oxide (FENO) was measured using Eco Medic instrument system (Eco Medics, Duernten, Switzerland) and the single‐breath technique was used according to the American Thoracic Society and European Respiratory Society guidelines. 33
High FENO at 24 years was defined as FENO >20 parts per billion (ppb).
3.5. Exposure and outcome definitions
Sensitization to peanut extract was defined as IgE‐ab level to peanut extract ≥0.35 kUA/L, levels <0.35 were coded as 0, levels >100 were coded as 101. Lost sensitization was defined as IgE‐sensitization <0.35 kUA/L at later follow‐ups.
Sensitization to peanut allergen molecules Ara h 1, Ara h 2, Ara h 3, Ara h 8, and Ara h 9 was defined as IgE‐ab level ≥0.10 kUA/L, levels <0.1 were coded as 0, >100 as 101.
Storage protein sensitization was defined as participants with a sensitization towards any of the storage molecules Ara h 1, Ara h 2, Ara h 3, Ara h 9, Cor a 9, Cor a 14, Jug r 1, or Ana o 3 with a cutoff level ≥0.10 kUA/L.
Peanut allergy symptoms at 24 years were defined as having specified avoidance of peanut at 24 years and reported specified symptoms at 16 years and/or 24 years, such as unconsciousness, asthma, hoarseness, swelling of face, lips, and eyes, general urticaria, partial urticaria, GI‐symptoms (vomiting, stomach pain), rhino conjunctivitis, oral symptoms consistent with oral allergy syndrome (OAS) or other.
Asthma at 24 years was defined as ever having a doctor's diagnosis of asthma together with symptoms of breathing difficulties in the last 12 months prior to the date of questionnaire at 24 years of age or used asthma medicine occasionally or regularly last 12 months.
Severe asthma at 24 years was defined as fulfilling the asthma at 24 years definition above in combination with at least 2 months usage of both inhaled corticosteroids and inhaled long acting beta2 agonist in the last 12 months and either of the following in the last 12 months due to asthma symptoms: Use of oral cortisone tablets or acute visits to emergency room or perceived impaired daily life or more than 12 episodes of breathing difficulties.
Atopic dermatitis at 24 years was based on clinical examination fulfilling UK Working Party criteria, also called William's criteria, 34 and defined as reporting an itchy rash in the last 12 months prior to the questionnaire at 24 years of age in combination with 3 out of 4 of the following criteria
dry skin last 12 months prior to questionnaire 24
atopic dermatitis onset below age 2 years (based on questionnaire data)
History of flexural atopic dermatitis (at any BAMSE follow‐up)
Personal history of asthma and/or rhinitis (at any BAMSE follow‐up from age 4 years).
Rhinitis at 24 years was defined as prolonged sneezing or a runny or blocked nose without common cold in the last 12 months prior to the date of questionnaire at 24 years of age.
3.6. Statistical analyses
Prevalence rates were presented as numbers and proportions. Chi (2)‐test was used for comparison of dichotomous outcomes and t‐test used to account for group differences in normally distributed continuous outcomes, for example log‐transformed IgE‐levels. p Values of <.05 were considered as significant, allowing rejection of the null hypothesis of no difference. For comparison of median levels of continuous variables, non‐parametric median test was used. Mann‐Whitney U test was used to analyze difference in levels of inflammation‐related proteins based on storage protein sensitization and additionally stratified by asthma. Significance in the protein analyses were based on false discovery rate (FDR) of 5% using the Benjamini–Hochberg procedure 35 but nominal p‐values < .05 are reported.
Statistical analyses were conducted using STATA Statistical Software (15.1 or later).
4. RESULTS
4.1. Study populations and characteristics
From the original BAMSE cohort, 3064 participants (75%) answered the questionnaires at the 24 ‐year follow‐up, and 2270 (56%) participated in the clinical investigation. Blood samples were available at all ages (4, 8, 16, and 24 years) in 1167 participants (defined as study population A) and at both 8 and 24 years together with symptom data in 1565 participants (defined as study population B), see Figure S1.
Among the participants at 24 years of age, 2217 individuals provided blood samples and data about reported symptoms to peanut, defined as study population C, see Figure S1. As earlier described, the BAMSE study population at the 24‐year check‐up consisted of a higher proportion of females and study participants with incomplete blood sampling at every timepoint (4, 8, 16, and 24 years of age) tended to more often have parents from blue collar work occupations, were more exposed to tobacco smoke in early ages and had less atopic heredity as compared to participants who provided blood samples at all clinical follow‐ups. 36
4.2. Peanut extract sensitization
The prevalence of peanut extract IgE‐sensitization over time in study population A was 6.4%, 8.8%, 9.0%, and 7.1% at 4, 8, 16, and 24 years of age, respectively, thus no large differences were seen in peanut extract prevalence between childhood and adulthood, nor in median IgE‐ab levels among sensitized individuals, see Figure 1. Male participants appeared in general to be peanut sensitized to a higher extent than females, although no significant differences were seen at age 4 (7.1% vs. 5.8%, p = .37), at 8 years (9.9% vs. 7.9%, p = .24), at 16 y (10.2% vs. 7.9%, p = .17), and at 24 y (8.6% vs. 5.8%, p = .07). Most individuals with peanut sensitization at 24 years of age were sensitized to peanut extract already at 4 (61%) and 8 (77%) years. The majority of individuals (84%–87%) with de novo peanut sensitization after 4 up to 24 years of age had a concomitant birch pollen sensitization, Figure S2. About half of the peanut extract sensitized individuals reported peanut symptoms (including OAS), with a tendency to increase over time (Figure 1).
FIGURE 1.
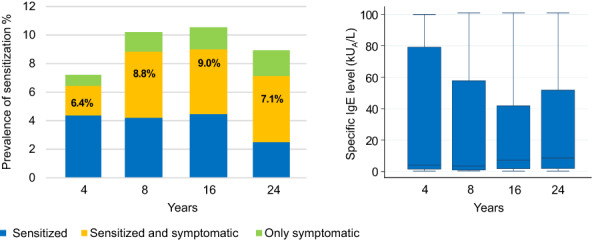
Prevalence of peanut extract sensitization (%), reported peanut intake symptoms and peanut extract IgE levels at 4, 8, 16 and 24 years of age in subgroup A. (Cut off peanut extract IgE ≥0.35 kUA/L). N = 1167.
4.3. Sensitization to peanut allergen molecules at eight and 24 years
The prevalence of sensitization to peanut allergen molecules analyzed in study population B remained stable between 8 and 24 years of age, and most common were IgE‐ab to Ara h 2 and Ara h 8, see Figure 2. Sensitization to Ara h 9 was the least common and the IgE‐ab levels tended to be lower than to the other peanut allergen molecules, Figure 2. Nine participants (14%) lost their Ara h 2 IgE‐sensitization between 8 and 24 years of age, all of them had low Ara h 2 IgE‐ab levels at age 8 (median 0.40, range 0.1–2 kUA/L), Figure 4. Only six individuals (10%) developed Ara h 2 IgE‐ab between 8 and 24 years of age, of which three had an IgE‐ab level between 0.1 and 0.12 kUA/L, and four of them reported peanut symptoms at 24 years of age, Figure 4 and Table S1a.
FIGURE 2.
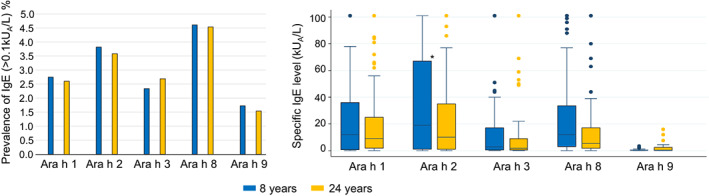
Prevalence of peanut allergen molecules (%) and peanut allergen molecule IgE levels at 8 and 24 years of age in subgroup B. (Cut off ≥0.1 kUA/L). N = 1565.
FIGURE 4.
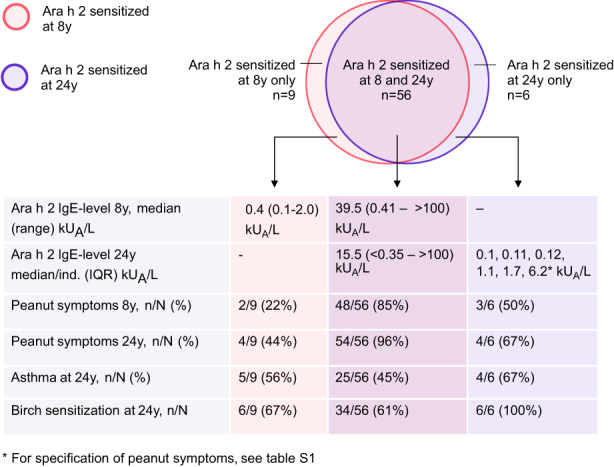
Characteristics of participants in subgroup B with transient, persistent and de novo Ara h 2 sensitization. N = 1565.
4.4. Peanut sensitization and reported peanut allergy up to 24 years
Among participants in study population C who provided blood samples and symptom data at 24 years of age, 133 of 2217 (6.0%) responded to the 24‐year follow‐up questionnaire that they avoided peanut intake, of whom 78 (59%) were peanut extract sensitized, and 72 (54%) Ara h 2 sensitized, Figure 3. The most common specified symptom after peanut intake was OAS, reported by 84 (63%), followed by face swelling and asthma, see Figure 3. Of those who reported peanut avoidance at 24 years of age, 54 individuals reported reactions last 12 months (54/133, 41%). The most common symptom was OAS, reported by 42 (78%) individuals, followed by gastrointestinal problems and face swelling (data not shown). Systemic symptoms such as reported unconsciousness, urticaria and asthma after peanut intake were closely related to presence of peanut Ara h 2 IgE‐ab sensitization, Figure 2. Presenting with only OAS from peanut intake was associated with Ara h 2 IgE‐ab negativity.
FIGURE 3.
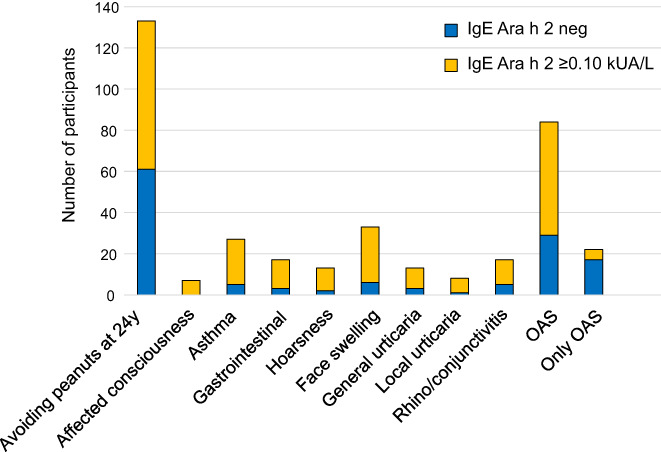
Reported peanut intake symptoms at 24 years of age among Ara h 2 sensitized versus non‐Ara h 2 sensitized participants in subgroup C. N = 2217. *for all specified symptoms p < .01 for difference in proportion sensitized/not sensitized to Ara h 2.
We investigated reported peanut symptom history at 4, 8, 12, and 16 years of age in 98 of 157 individuals in group C categorized as peanut symptomatic at 24 years of age and with complete symptom data from all ages, we saw more frequent Ara h 2 IgE positivity and higher IgE‐values among individuals with early and persistent symptoms. None of the 19 individuals with reported symptoms only at 24 years but not at previous ages had Ara h 2 IgE positivity, peanut symptom history and Ara h 2 IgE status at 24 years of age are presented in Table S1b.
4.5. Storage protein sensitization and allergic phenotype
Individuals in study population C with sensitization to storage protein (n = 123) in peanut and/or tree nut (hazelnut; Cor a 9/Cor a 14, cashew; Ana o 3, walnut; jug r 1) at 24 years of age had a higher prevalence of asthma, 44% (54/123), compared with participants with sensitization to any inhalant or food allergen extract but without storage protein sensitization, 17% (144/860), p < .001. Comparison between the two groups, with and without stratification by asthma, are demonstrated in Table 1. Those who were sensitized to storage proteins were sensitized to a higher number of allergens, had more often atopic dermatitis, had higher median levels of FENO and eosinophils and had a higher use of adrenaline injections compared with non‐storage‐protein sensitized participants. The differences were significant even after stratification for asthma and also significant (except for atopic dermatitis) at comparison of asthmatics without storage protein sensitization versus storage protein sensitized non‐asthmatics, see Table 1. Among asthmatics, those who were sensitized to storage proteins were to a higher extent male. The robustness of the finding of higher FENO levels and higher prevalence of FENO >20 ppb in storage protein sensitized individuals were tested with linear and logistic regression (respectively) adjusted for polysensitization to inhalant and food extracts and asthma. The association remained even after adjustments, see Table S2.
TABLE 1.
Allergic phenotype characteristics in individuals sensitized to common food (Fx5) or inhalant (Phadiatop) allergens, with and without storage protein sensitization, stratified for asthma at 24 years of age
| Characteristics at 24 years n/N (%)/(IQR) | With sensitization but without storage protein sensitization at 24 y, n = 863 no asthma: n = 716 Asthma: n = 144 d | Storage protein sensitized, n = 123 no asthma at 24 y, n = 69 asthma at 24 y, n = 54 | p Value no asthma vs. asthma (storage protein sensitized) | p Value storage protein vs. no storage protein sens | p Value no storage protein + asthma vs. storage protein + no asthma |
|---|---|---|---|---|---|
| Median specific IgE‐level at 8 y, kUA/l (IQR) a | n.a. | 3.6 (0.56–99) | n.a. | ||
| No asthma at 24 y | n.a. | 2.4 (0–44) | .14 | n.a. | n.a. |
| Asthma at 24 y | n.a. | 17 (0.78‐ > 100) | n.a. | ||
| Median specific IgE‐level at 24 y, kUA/l (IQR) a | n.a. | 7.7 (1.3–47) | n.a. | ||
| No asthma at 24 y | n.a. | 4.7 (0.65–18) | .086 | n.a. | n.a. |
| Asthma at 24 y | n.a. | 12 (2.2–71) | n.a. | ||
| Polysens (inhalant and food extract IgE), median n allergens (IQR) a | 2 (1–4) | 6 (4–9) | <.0001 | ||
| No asthma at 24 y | 2 (1–4) | 6 (3–8) | .09 | <.0001 | .001 |
| Asthma at 24 y | 3 (2–5) | 7 (5–10) | <.0001 | ||
| Reported peanut symptoms at 24 y (only OAS excluded) b | 22/863 (2.6) | 66/123 (53.7) | <.0001 | ||
| No asthma at 24 y | 16/716 (2.2) | 35/69 (50.7) | .46 | <.0001 | <.0001 |
| Asthma at 24 y | 6/144 (4.2) | 31/59 (57.4) | <.0001 | ||
| Median BMI at 24 y, kg/m2 (IQR) a | 22.7 (20.7–25.0) | 22.7 (20.7–24.9) | .93 | ||
| No asthma at 24 y | 22.6 (20.6–24.9) | 22.5 (20.8–24.4) | .79 | .99 | .045 |
| Asthma at 24 y | 23.5 (21.1–26.7) | 22.8 (20.7–25.1) | .36 | ||
| Male sex b | 445/863 (51.6) | 69/123 (56.1) | .35 | ||
| No asthma at 24 y | 387/716 (54.1) | 38/69 (55.1) | .80 | .87 | .026 |
| Asthma at 24 y | 56/144 (38.9) | 31/54 (57.4) | .019 | ||
| Severe asthma at 24 y b | 13/862 (1.5) | 8/123 (6.5) | .0003 | ||
| No asthma at 24 y | n.a. | n.a. | n.a. | n.a. | n.a. |
| Asthma at 24 y | 13/144 (9.0) | 8/54 (14.8) | .24 | ||
| AD at 24 y b | 219/860 (25.5) | 58/123 (47.2) | <.0001 | ||
| No asthma at 24 y | 163/714 (22.8) | 28/69 (40.6) | .10 | .001 | .81 |
| Asthma at 24 y | 56/144 (38.9) | 30/54 (55.6) | .035 | ||
| Rhinitis at 24 y b | 633/858 (73.8) | 91/123 (74.0) | .96 | ||
| No asthma at 24 y | 511/712 (71.8) | 45/69 (65.2) | .012 | .25 | .0031 |
| Asthma at 24 y | 120/144 (83.3) | 46/54 (85.2) | .75 | ||
| FENO>20 at 24 y b | 219/733 (29.9) | 66/105 (62.9) | <.0001 | ||
| No asthma at 24 y | 168/602 (27.9) | 34/60 (56.7) | .13 | <.0001 | .014 |
| Asthma at 24 y | 48/128 (37.5) | 32/45 (71.1) | .0001 | ||
| Median FENO level at 24 y, ppb (IQR) a | 13 (10–23) | 25 (14–46) | <.0001 | ||
| No asthma at 24 y | 13 (9–22) | 23.5 (13.5–39.5) | .21 | .003 | .042 |
| Asthma at 24 y | 15 (10–27) | 30 (20–61) | <.0001 | ||
| FEV1/FVC <0.7 at 24 y b | 18/773 (2.3 | 3/108 (2.8) | .77 | ||
| No asthma at 24 y | 10/639 (1.6) | 3/59 (5.1) | .11 | .059 | .78 |
| Asthma at 24 y | 8/131 (6.1) | 0/49 (0) | .077 | ||
| Median FEV1 reversibility c , % (IQR) a | 2.9 (1.4–5.1) | 3.1 (1.0–5.1) | .83 | ||
| No asthma at 24 y | 2.8 (1.3–4.9) | 2.4 (0.5–4.2) | .12 | .57 | .053 |
| Asthma at 24 y | 3.5 (1.5–5.8) | 3.6 (2.1–6.4) | .83 | ||
| Adrenaline use <12 months at 24 y b | 0/861 (0) | 11/123 (8.9) | <.0001 | ||
| No asthma at 24 y | 0/715 (0) | 2/69 (2.9) | .0079 | <.0001 | .040 |
| Asthma at 24 y | 0/144 (0) | 9/54 (16.7) | <.0001 | ||
| Used inhaled corticosteroids <12 months b | 53/860 (6.2) | 16/123 (13.0) | .0054 | ||
| No asthma at 24 y | 10/715 (1.4) | 1/69 (1.5) | <.0001 | .94 | <.0001 |
| Asthma at 24 y | 43/143 (30.1) | 15/41 (27.8) | .75 | ||
| Median blood eosinophil count at 24 y, x109/l (IQR) a | 0.1 (0–0.2) | 0.2 (0.1–0.3) | <.0001 | ||
| No asthma at 24 y | 0.1 (0–0.2) | 0.2 (0.1–0.3) | .095 | <.0001 | .038 |
| Asthma at 24 y | 0.1 (0–0.2) | 0.2 (0.2–0.4) | <.0001 |
Note: N = 2217.
Abbreviation: AD, atopic dermatitis.
Continuous variables: median test (non‐parametric).
Dichotomous variables: chi2 test.
Pre/post β2agonist inhalation.
3 Missing data on asthma.
Bold indicates the significant values.
Since FENO and eosinophil levels among asthmatics and non‐asthmatics sensitized to storage proteins were higher compared with asthmatics sensitized to other allergens, we further explored if this corresponded to differences in plasma levels of inflammation‐related proteins.
4.6. Storage protein sensitization and inflammatory markers
Information from the Proseek multiplex analysis was available for 111 of 123 individuals sensitized to storage proteins and 805 of 863 individuals sensitized to inhalant or food allergen extract without storage protein sensitization. No inflammation‐related plasma protein differed significantly after FDR correction between the two groups, neither overall nor stratified by asthma status. Five proteins had a nominal p‐value < .05 in the storage protein comparison, Table S3. Among these were two members of the tumor necrosis factor receptor superfamily (TNFRSF9 and TNFRSF11), thymic stromal lymphopoietin (TSLP) and matrix metalloproteinase‐10 (MMP10). In the subsequent analysis stratified by asthma status, the MMP10 and TNFSF11 levels were higher among storage sensitized individuals also in the asthma sub‐group with a nominal p‐value < .05, Figure 5A,B.
FIGURE 5.
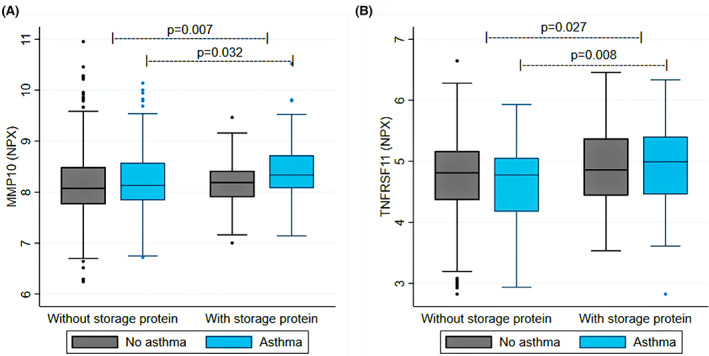
(A, B) Plasma protein level (in NPX) by sensitization and asthma status.
5. DISCUSSION
This is the first report on how peanut sensitization on extract and molecular levels develop from childhood up to adulthood and how they associate with signs of respiratory and systemic inflammation. We conclude that genuine peanut allergy starts early in life and remains up to adulthood, and the prevalence of sensitization to both peanut extract and peanut allergen molecules was stable and did not differ much between early childhood and adulthood, as seen in previous studies. 5 , 37
New sensitization to peanut extract after four years of age was almost exclusively accompanied by birch sensitization. Hence, in this Swedish population, the development of sensitization to peanut extract after four years of age could possibly be explained by birch pollen cross‐sensitization due to a co‐existing birch allergy, which usually is developed in children from school age, with sensitization due to the similarities between the birch allergen Bet v 1 and the peanut allergen molecule Ara h 8 and does not seem to be associated with genuine peanut allergy. This finding is in line with results from previous studies. 38 , 39 , 40 Furthermore, both new sensitization and lost sensitization to storage protein Ara h 2 between 8 and 24 were rare as reported earlier by our group up to teen‐age. 6
Storage protein sensitized individuals had higher levels of exhaled nitric oxide and blood eosinophil cell counts as compared to other sensitized individuals, these result is in line with Johnson et al. 24 who found that storage protein sensitized individuals showed higher FENO (23.2 p vs. 16.7 ppb) compared with non‐storage protein sensitized and by Hughes et al. 23 who found that in individuals with previous asthma or no current medication, the majority still had FENO >35 ppb. However, our findings of elevated FENO in storage protein sensitized individuals independent of asthma and polysensitization is new and has not been shown before. Also, levels of exhaled nitric oxide were higher among non‐asthmatics sensitized to storage protein compared with asthmatics sensitized to other allergens but not any of the storage proteins. This pose important questions for clinicians responsible for these patients. Should treatment with inhaled corticosteroids be recommended, especially for patients with respiratory symptoms at peanut intake? More research is needed to clarify this further. Storage proteins are known for not being airborne 41 but despite that we saw signs of respiratory inflammation (elevated FENO) in storage protein sensitized individuals. Further, we analyzed differences in other markers of systemic inflammation using a predefined inflammation panel of 92 proteins. Levels of TNFRSF11 and MMP10 were higher among storage protein sensitized individuals both overall and among asthmatics, but no proteins differed after adjustment for multiple comparisons. However, the results should be interpreted with caution. TNFRSF11 (also known as receptor activator of NF‐κB ligand, RANK‐L) is a member of the TNF superfamily, a group of cytokines involved in activating the group 2 innate lymphoid cells that initiates and enhances airway inflammation by activation of, for example, eosinophilic cells and mast cells by production of type 2 cytokines. 42 Increased levels of TNFRSF11 have been found in patients with chronic rhinosinusitis with nasal polyps, a disease associated with type 2 inflammation. 43 TSLP is also involved in the activation of group 2 innate lymphoid cells 42 and is known as a key regulator in the pathogenesis of inflammatory airway diseases. 44 ILC2 have been suggested to be involved in the regulatory processes in food allergy and the intestinal eosinophil homeostasis. Also, TSLP have been shown to activate ILC2 in esophageal lymphoid tissue in EoE. 45 MMP10 has been shown to be important in airway remodeling and inflammation by regulating macrophage activity. 46 Increased expression of MMP10 are found in patients with high levels of eosinophils in the bronchial submucosa 47 and in severe asthmatics. 48 These results further support that storage protein sensitization is associated with a more pronounced type 2 inflammation phenotype with both systemic and airway inflammation activity.
As in previous studies we found few individuals with a de novo peanut storage protein sensitization after 8 years of age. 1 , 6 The majority of peanut allergic children developed their sensitization early in life, and new cases with Ara h 2 sensitization were rare and in almost all individuals the IgE‐values were near the lower detection limit.
Our results on the molecular allergen level concerning birch pollen and birch‐related sensitization and reported symptoms after peanut intake are in line with previous results on cross‐reactivity between Ara h 8 and birch Bet v 1. 49 We noticed that co‐existing birch sensitization tended to be more common in de novo peanut extract IgE sensitization, indicating cross‐reactivity rather than onset of genuine peanut allergy. We could also confirm that males were in a higher extent sensitized as found in other studies. 10 , 11
The main strength of our study is the size of the study population and the availability of both clinical and questionnaire data. One limitation is that the classification of food allergy was not done by blinded oral food provocations, which is the golden standard. However, the study size would have made it costly and time consuming. In addition, Ara h 2 sensitization has proved to be a very strong predictor of clinical peanut allergy and one could argue that it would be neither ethical or in the patient's best interest to perform challenges in such a high extent. 50 , 51 The over‐representation of allergic individuals is a common issue in all longitudinal cohort studies and could in our study lead to an overestimation of sensitization prevalence rates and perhaps an underestimation of asymptomatic sensitized individuals. However, previous sensitivity analyses by us show that the selection is of a minor extent compared with many other cohort studies. 36
6. CONCLUSION
In this Swedish population‐based study, the prevalence of sensitization to both peanut extract and peanut allergen molecules was stable and did not differ much between early childhood and adulthood, and the development of IgE‐ab to peanut in older children is mainly seen in individuals with co‐existing birch sensitization. IgE‐ab to Ara h 2 sensitization rarely emerges after eight years of age and seldom disappears once it has developed. However, individuals with initial low IgE‐ab levels to Ara h 2 might outgrow their sensitization. Furthermore, storage protein sensitization was associated with signs of respiratory and systemic type 2 inflammation with elevated exhaled nitric oxide and blood eosinophils, even in patients without a known asthma. However, the proseek analysis revealed no significant differences in inflammation related plasma proteins. Thus, individuals with storage protein sensitization might have a higher risk for more pronounced allergic disease.
AUTHOR CONTRIBUTIONS
All authors have approved the last version before submission. Sandra G. Tedner and Anna Asarnoj participated in design of the study, data analysis, and manuscript writing. Susanna Klevebro performed the statistical analyses related to OLINK proteins, participated in manuscript writing, and critically reviewed data and manuscript. Anna Bergström, Inger Kull, and Erik Melén served as co‐principal investigator (co‐PI) of the BAMSE study, participated in design of the study, critically reviewed data, and revised the manuscript. Niklas Andersson contributed with statistical support, managing of the database, critically reviewed data, and revised the manuscript. Magnus P. Borres, Natalia Ballardini, Marit Westman, Jon R. Konradsen, and Caroline Nilsson critically reviewed data and revised the manuscript. Marianne van Hage was responsible for IgE analyses in the project, critically reviewed data, and revised the manuscript.
FUNDING INFORMATION
This study was supported by grants from the European Research Council (TRIBAL, grant agreement 757919), The King Gustaf V 80th Birthday Foundation, The Swedish Cancer and Allergy Foundation, The Swedish Research Council for Health, Working Life and Welfare (grant agreement 2017‐00526), the Swedish Research Council (grant agreements 2016‐03086; 2018‐02524; 2019‐01060; 2020‐02170), the Swedish Heart‐Lung Foundation, Region Stockholm (ALF project, for cohort and database maintenance and clinical research appointment for Asarnoj), The Konsul Th C Bergh's Foundation, The Swedish Society of Medicine, KI grants, The Hesselman foundation, Swedish Grand lodge of Freemasonry Foundation Barnahuset, Swedish Asthma and Allergy Association, Kerstin Hejdenberg's arresting's scholarship, The Pediatric Research Foundation at Astrid Lindgren Children's Hospital, The Samariten Foundation for Paediatric research, The Sven Jerring Foundation, Swedish Association for Allergology research grant, The Magnus Bergwall foundation and The Crown Princess Lovisa's Foundation. Thermo Fisher Scientific kindly provided reagents for IgE analyses.
CONFLICT OF INTEREST
EM reports Advisory board fees from ALK and AstraZeneca outside the submitted work. MvH has received lecture fees from Thermo Fisher Scientific outside the submitted work. CN reports Advisory board fee from Aimmune Therapeutics, a Nestlé Health Science company outside the submitted work. SK reports Advisory board fee from Novartis and payment for lecture from AstraZeneca outside the submitted work. AA reports Advisory board fees from Aimmune Therapeutics, Nestlé Health Science, Sanofi and Novartis and lecture fees from Orion Pharma, Nestlé and Semper outside the submitted work. ST reports Advisory board fees from ALK and Sanofi outside the submitted work. None of the authors has any conflict of interest in relation to the current study.
Supporting information
Supporting information S1
Figure S1
Figure S2
ACKNOWLEDGMENTS
The authors wish to thank the BAMSE cohort participants and their parents, the study personnel and researchers involved in the BAMSE cohort.
Tedner SG, Klevebro S, Bergström A, et al. Development of sensitization to peanut and storage proteins and relation to markers of airway and systemic inflammation: A 24‐year follow‐up. Allergy. 2023;78:488‐499. doi: 10.1111/all.15568
REFERENCES
- 1. Wickman M, Lupinek C, Andersson N, et al. Detection of IgE reactivity to a handful of allergen molecules in early childhood predicts respiratory allergy in adolescence. EBioMedicine. 2017;26:91‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nwaru BI, Hickstein L, Panesar SS, et al. Prevalence of common food allergies in Europe: a systematic review and meta‐analysis. Allergy. 2014;69(8):992‐1007. [DOI] [PubMed] [Google Scholar]
- 3. Cox A, Sicherer SH. Peanut and tree nut allergy. Chem Immunol Allergy. 2015;101:131‐144. [DOI] [PubMed] [Google Scholar]
- 4. Gupta RS, Warren CM, Smith BM, et al. Prevalence and severity of food allergies among US adults. JAMA Netw Open. 2019;2(1):e185630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arshad SH, Venter C, Roberts G, Dean T, Kurukulaaratchy R. The natural history of peanut sensitization and allergy in a birth cohort. J Allergy Clin Immunol. 2014;134(6):1462‐1463.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Asarnoj A, Hamsten C, Lupinek C, et al. Prediction of peanut allergy in adolescence by early childhood storage protein‐specific IgE signatures: the BAMSE population‐based birth cohort. J Allergy Clin Immunol. 2017;140(2):587‐590.e7. [DOI] [PubMed] [Google Scholar]
- 7. Peters RL, Gurrin LC, Dharmage SC, Koplin JJ, Allen KJ. The natural history of IgE‐mediated food allergy: can skin prick tests and serum‐specific IgE predict the resolution of food allergy? Int J Environ Res Public Health. 2013;10(10):5039‐5061. doi: 10.3390/ijerph10105039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Simons E, Balshaw R, Lefebvre DL, et al. Timing of introduction, sensitization and allergy to highly‐allergenic foods at age 3 years in a general‐population Canadian cohort. J Allergy Clin Immunol Pract. 2019;30(19):166‐175.e10. [DOI] [PubMed] [Google Scholar]
- 9. Alduraywish SA, Lodge CJ, Vicendese D, et al. Sensitization to milk, egg and peanut from birth to 18 years: a longitudinal study of a cohort at risk of allergic disease. Pediatr Allergy Immunol. 2016;27(1):83‐91. doi: 10.1111/pai.12480 [DOI] [PubMed] [Google Scholar]
- 10. Oria MP, Stallings VA, eds. Finding a Path to Safety in Food Allergy: Assessment of the Global Burden, Causes, Prevention, Management, and Public Policy. National Academies Press; 2016. [PubMed] [Google Scholar]
- 11. Sicherer SH, Allen K, Lack G, Taylor SL, Donovan SM, Oria M. Critical issues in food allergy: a National Academies Consensus Report. Pediatrics. 2017;140(2):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Du Toit G, Roberts G, Sayre PH, et al. Identifying infants at high risk of Peanut allergy: the learning early about Peanut allergy (LEAP) screening study. J Allergy Clin Immunol. 2013;131(1):135‐143.e1‐12. doi: 10.1016/j.jaci.2012.09.015 [DOI] [PubMed] [Google Scholar]
- 13. Emons JAM, Gerth van Wijk R. Food allergy and asthma: is there a link? Curr Treat Options Allergy. 2018;5(4):436‐444. doi: 10.1007/s40521-018-0185-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Patelis A, Janson C, Borres MP, Nordvall L, Alving K, Malinovschi A. Aeroallergen and food IgE sensitization and local and systemic inflammation in asthma. Allergy. 2014;69(3):380‐387. doi: 10.1111/all.12345 [DOI] [PubMed] [Google Scholar]
- 15. Bergstrom SE, Boman G, Eriksson L, et al. Asthma mortality among Swedish children and young adults, a 10‐year study. Respir Med. 2008;102(9):1335‐1341. [DOI] [PubMed] [Google Scholar]
- 16. Pouessel G, Turner PJ, Worm M, et al. Food‐induced fatal anaphylaxis: from epidemiological data to general prevention strategies. Clin Exp Allergy. 2018;48(12):1584‐1593. doi: 10.1111/cea.13287 [DOI] [PubMed] [Google Scholar]
- 17. Jang YY, Ahn JY. Evaluation of fractional exhaled nitric oxide in pediatric asthma and allergic rhinitis. Children (Basel). 2020;8(1):3. doi: 10.3390/children8010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cao C, Li W, Hua W, et al. Proteomic analysis of sputum reveals novel biomarkers for various presentations of asthma. J Transl Med. 2017;15(1):171. doi: 10.1186/s12967-017-1264-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Butler CA, Heaney LG. Fractional exhaled nitric oxide and asthma treatment adherence. Curr Opin Allergy Clin Immunol. 2021;21(1):59‐64. doi: 10.1097/ACI.0000000000000704 [DOI] [PubMed] [Google Scholar]
- 20. Ma'pol A, Hashim JH, Norbäck D, Weislander G, Hashim Z, Isa ZM. FeNO level and allergy status among school children in Terengganu. Malaysia J Asthma. 2020;57(8):842‐849. doi: 10.1080/02770903.2019.1614614 [DOI] [PubMed] [Google Scholar]
- 21. Nordlund B, Lundholm C, Ullemar V, van Hage M, Örtqvist AK, Almqvist C. The STOPPA twin study explains the exhaled nitric oxide and asthma link by genetics and sensitization. Twin Res Hum Genet. 2017;20(4):330‐337. doi: 10.1017/thg.2017.35 [DOI] [PubMed] [Google Scholar]
- 22. Kalm‐Stephens P, Malinovschi A, Janson C, Venge P, Nordvall L, Alving K. Concurrence of elevated FeNO and airway hyperresponsiveness in nonasthmatic adolescents. Pediatr Pulmonol. 2020;55(3):571‐579. [DOI] [PubMed] [Google Scholar]
- 23. Hughes JL, Brown T, Edgar JD, Shields MD. Peanut allergy and allergic airways inflammation. Pediatr Allergy Immunol. 2010;21(8):1107‐1113. doi: 10.1111/j.1399-3038.2010.01071.x [DOI] [PubMed] [Google Scholar]
- 24. Johnson J, Malinovschi A, Lidholm J, et al. Sensitization to storage proteins in peanut and hazelnut is associated with higher levels of inflammatory markers in asthma. Clin Mol Allergy. 2020;18:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Justiz Vaillant AA, Vashisht R, Zito PM. Immediate Hypersensitivity Reactions. StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 26. Chiang D, Chen X, Jones SM, et al. Single‐cell profiling of peanut‐responsive T cells in patients with peanut allergy reveals heterogeneous effector T(H)2 subsets. J Allergy Clin Immunol. 2018;141(6):2107‐2120. doi: 10.1016/j.jaci.2017.11.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ruiter B, Smith NP, Monian B, et al. Expansion of the CD4(+) effector T‐cell repertoire characterizes peanut‐allergic patients with heightened clinical sensitivity. J Allergy Clin Immunol. 2020;145(1):270‐282. doi: 10.1016/j.jaci.2019.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stockfelt M, Hong MG, Hesselmar B, et al. Circulating proteins associated with allergy development in infants‐an exploratory analysis. Clin Proteomics. 2021;18(1):11. doi: 10.1186/s12014-021-09318-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wickman M, Kull I, Pershagen G, Nordvall SL. The BAMSE project: presentation of a prospective longitudinal birth cohort study. Pediatr Allergy Immunol. 2002;13(s15):11‐13. [DOI] [PubMed] [Google Scholar]
- 30. Klevebro S, Bjorkander S, Ekstrom S, et al. Inflammation‐related plasma protein levels and association with adiposity measurements in young adults. Sci Rep. 2021;11(1):11391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang G, Hallberg J, Um Bergström P, et al. Assessment of chronic bronchitis and risk factors in young adults: results from BAMSE. Eur Respir J. 2021;57(3):2002120. doi: 10.1183/13993003.02120-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hallberg J, Thunqvist P, Schultz ES, et al. Asthma phenotypes and lung function up to 16 years of age‐the BAMSE cohort. Allergy. 2015;70(6):667‐673. doi: 10.1111/all.12598 [DOI] [PubMed] [Google Scholar]
- 33. American Thoracic Society; European Respiratory Society . ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171(8):912‐930. doi: 10.1164/rccm.200406-710ST [DOI] [PubMed] [Google Scholar]
- 34. Williams HC, Burney PG, Pembroke AC, Hay RJ. Validation of the U.K. diagnostic criteria for atopic dermatitis in a population setting. U.K. diagnostic criteria for atopic dermatitis working party. Br J Dermatol. 1996;135(1):12‐17. [PubMed] [Google Scholar]
- 35. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B Methodol. 1995;57(1):289‐300. [Google Scholar]
- 36. Melen E, Bergstrom A, Kull I, et al. Male sex is strongly associated with IgE‐sensitization to airborne but not food allergens: results up to age 24 years from the BAMSE birth cohort. Clin Transl Allergy. 2020;10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sicherer SH, Sampson HA. Food allergy: a review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol. 2018;141(1):41‐58. doi: 10.1016/j.jaci.2017.11.003 [DOI] [PubMed] [Google Scholar]
- 38. Asarnoj A, Nilsson C, Lidholm J, et al. Peanut component Ara h 8 sensitization and tolerance to peanut. J Allergy Clin Immunol. 2012;130(2):468‐472. [DOI] [PubMed] [Google Scholar]
- 39. Datema MR, Lyons SA, Fernández‐Rivas M, et al. Estimating the risk of severe Peanut allergy using clinical background and IgE sensitization profiles. Front Allergy. 2021;2:670789. doi: 10.3389/falgy.2021.670789 eCollection 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cox AL, Eigenmann PA, Sicherer SH. Clinical relevance of cross‐reactivity in food allergy. J Allergy Clin Immunol Pract. 2021;9(1):82‐99. doi: 10.1016/j.jaip.2020.09.030 [DOI] [PubMed] [Google Scholar]
- 41. Lovén Björkman S, Sederholm U, Ballardini N, et al. Peanuts in the air—clinical and experimental studies. Clin Exp Allergy. 2021;51(4):585‐593. doi: 10.1111/cea.13848 [DOI] [PubMed] [Google Scholar]
- 42. Kato A. Group 2 innate lymphoid cells in airway diseases. Chest. 2019;156(1):141‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ogasawara N, Poposki JA, Klingler AI, et al. Role of RANK‐L as a potential inducer of ILC2‐mediated type 2 inflammation in chronic rhinosinusitis with nasal polyps. Mucosal Immunol. 2020;13(1):86‐95. doi: 10.1038/s41385-019-0215-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hong H, Liao S, Chen F, Yang Q, Wang DY. Role of IL‐25, IL‐33, and TSLP in triggering united airway diseases toward type 2 inflammation. Allergy. 2020;75(11):2794‐2804. doi: 10.1111/all.14526 [DOI] [PubMed] [Google Scholar]
- 45. Sahiner UM, Layhadi JA, Golebski K, et al. Innate lymphoid cells: the missing part of a puzzle in food allergy. Allergy. 2021;76(7):2002‐2016. [DOI] [PubMed] [Google Scholar]
- 46. Wight TN, Frevert CW, Debley JS, Reeves SR, Parks WC, Ziegler SF. Interplay of extracellular matrix and leukocytes in lung inflammation. Cell Immunol. 2017;312:1‐14. doi: 10.1016/j.cellimm.2016.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kuo C‐HS, Pavlidis S, Zhu J, et al. Contribution of airway eosinophils in airway wall remodeling in asthma: role ofMMP‐10andMET. Allergy. 2019;74(6):1102‐1112. [DOI] [PubMed] [Google Scholar]
- 48. Eguíluz‐Gracia I, Malmstrom K, Dheyauldeen SA, et al. Monocytes accumulate in the airways of children with fatal asthma. Clin Exp Allergy. 2018;48(12):1631‐1639. doi: 10.1111/cea.13265 [DOI] [PubMed] [Google Scholar]
- 49. Ballmer‐Weber BK, Lidholm J, Fernandez‐Rivas M, et al. IgE recognition patterns in peanut allergy are age dependent: perspectives of the EuroPrevall study. Allergy. 2015;70(4):391‐407. doi: 10.1111/all.12574 [DOI] [PubMed] [Google Scholar]
- 50. Hemmings O, Du Toit G, Radulovic S, Lack G, Santos AF. Ara h 2 is the dominant peanut allergen despite similarities with Ara h 6. J Allergy Clin Immunol. 2020;146(3):621‐630.e5. doi: 10.1016/j.jaci.2020.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Keet C, Plesa M, Szelag D, et al. Ara h 2‐specific IgE is superior to whole peanut extract‐based serology or skin prick test for diagnosis of peanut allergy in infancy. J Allergy Clin Immunol. 2021;147(3):977‐983.e2. doi: 10.1016/j.jaci.2020.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information S1
Figure S1
Figure S2


