Abstract

The low electron count Pt(II) complexes [Pt(NHC′)(NHC)][BArF] (where NHC is a N-heterocyclic carbene ligand and NHC′ its metalated form) react with tertiary hydrogermanes HGeR3 at room temperature to generate the 14-electron platinum(II) germyl derivatives [Pt(GeR3)(NHC)2][BArF]. Low-temperature NMR studies allowed us to detect and characterize spectroscopically some of the σ-GeH intermediates [Pt(η2-HGeR3)(NHC′)(NHC)][BArF] that evolve into the platinum-germyl species. One of these compounds has been characterized by X-ray diffraction studies, and the interaction of the H–Ge bond with the platinum center has been analyzed in detail by computational methods, which suggest that the main contribution is the donation of the H–Ge to a σ*(Pt–C) orbital, but backdonation from the platinum to the σ*(Ge–H) orbital is significant. Primary and secondary hydrogermanes also produce the corresponding platinum-germyl complexes, a result that contrasts with the reactivity observed with primary silanes, in which carbon–silicon bond-forming reactions have been reported. According to density functional theory calculations, the formation of Pt–Ge/C–H bonds is both kinetically and thermodynamically preferred over the competitive reaction pathway leading to Pt–H/C–Ge bonds.
Short abstract
Cyclometalated Pt(II) complexes interact with hydrogermanes to form σ-GeH complexes. Theoretical calculations suggest that the GeH → Pt interactions fall between classical η2 and η1 coordination modes. These compounds are thermally unstable, evolving through activation of the Ge−H bond to form platinum-germyl species in a process that involves the formation of C−H/Pt−Ge bonds. DFT studies point to a σ-CAM mechanism in which the competitive reaction pathway leading to C−Ge/Pt−H bonds is both kinetically and thermodynamically less favored.
Introduction
The interaction of E–H bonds (E = H, B, Si, Ge,···) with transition metals leading to σ-EH complexes is considered the first step toward the cleavage of the E–H bond to forge two new M–E and M–H bonds through a formal oxidative addition process. In the last decades, our understanding of this type of interaction has reached a high degree of knowledge, thanks to the spectroscopic observation, crystallographic isolation, and computational analyses of a great number of species of this type, particularly regarding those systems with dihydrogen and hydrosilanes, as well as with hydroboranes.1 Obviously, this has been particularly driven by the participation of this type of compounds in catalytic hydrogenation, hydrosilylation, and hydroboration reactions. On the contrary, the number of σ-GeH complexes spectroscopically observed is very limited,2 and only very few have been characterized by X-ray diffraction methods.3 Besides, all of these complexes are neutral derivatives, but, to the best of our knowledge, no cationic systems have been reported. This might be due, at least in part, to the highly reactive nature of cationic σ-GeH complexes (similar to their σ-SiH counterparts), which are prone to the heterolytic cleavage of the Ge–H bond even by weak nucleophiles.1h In a series of recent contributions by our group, we have been able to isolate and characterize, by spectroscopic means and X-ray diffraction studies, σ-EH cationic platinum complexes of dihydrogen, silanes, and boranes.4 We have observed that these species are thermally unstable and evolve through E–H bond cleavage. Intriguingly, these processes result, in subsequent steps, in C–H/Pt–Si or C–Si/Pt–H coupling reactions for silanes (depending on the nature of the silane, Scheme 1A)4b and in reversible C–B/Pt–H coupling events (finally leading to the thermodynamically more stable C–H/Pt–B species, Scheme 1B).4a Considering all of the above and the increasing interest in the use of organo-germanium compounds,5 in this contribution, we have explored the reactivity of cationic Pt(II) complexes [Pt(NHC′)(NHC)][BArF] toward hydrogermanes, which have led to the successful isolation of the corresponding σ-GeH derivatives.
Scheme 1. Reactions of Cyclometalated Complex [Pt(ItBuiPr′)(ItBuiPr)][BArF] with Hydrosilanes (A) and Hydroboranes (B).
Results and Discussion
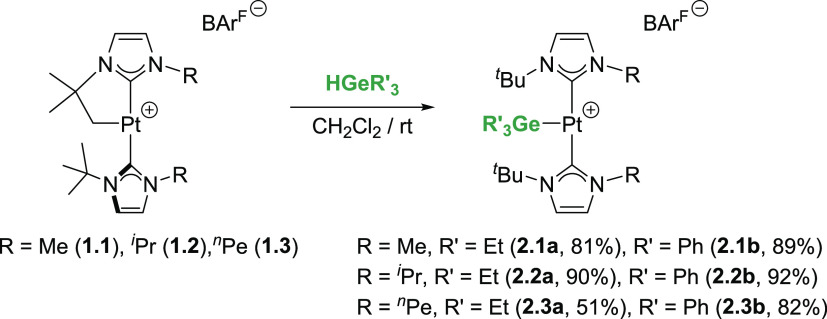
Following our previous procedure for the reaction of complexes [Pt(NHC′)(NHC)][BArF] (1.1–1.3) with hydrosilanes,4b,4c we have investigated their reactivity toward tertiary, secondary, and primary hydrogermanes. Initially, we focused our attention on tertiary hydrogermanes, since the parent hydrosilanes led to the formation of the corresponding platinum-silyl derivatives [Pt(SiR3)(NHC)2][BArF].4c In the same way, the reaction of complexes [Pt(NHC′)(NHC)][BArF] with HGeEt3 or HGePh3 resulted in the generation of the germyl complexes [Pt(GeR3)(NHC)2][BArF] through the formal C–H and Pt–Ge bond-forming processes (Scheme 2). The reaction proceeds smoothly at room temperature in dichloromethane, although the reaction times vary depending on the hydrogermane used and the N-heterocyclic carbene ligands. Thus, very fast reactions are observed with HGePh3 (typically less than 10 min), whereas longer times are required for completion in the case of HGeEt3 (ca. 18–24 h). On the other hand, no reaction takes place with tertiary hydrogermanes and the IMes derivative [Pt(IMes*′)(IMes*)][BArF], 1.4 (see Scheme 3 above). The 1H and 13C{1H} NMR of the products revealed a highly symmetrical environment, in agreement with the presence of two chemically equivalent NHCs. It is worth mentioning that the CH2 carbon atoms of the Et3Ge- fragment exhibit coupling to 195Pt nucleus in the 13C{1H} NMR (2JPt,C = 118.7 (2.1a), 118.5 (2.2a) and 155.0 (2.3a) Hz), providing evidence for the direct bonding of the platinum and germyl moieties. Definite proof of the structure of these compounds came from X-ray diffraction studies of complexes 2.1a, 2.1b, 2.2b, and 2.3b (Figure 1). Similar to the silyl derivatives, these compounds show a T-shaped structure in which the two NHCs are mutually trans and the germyl fragment occupies the third coordination site in trans to the formal vacant site. Agostic interactions,6 if present, should be weak, with the closest carbon to platinum atoms at 3.502(2) (2.1a), 3.086(3) (2.1b), 3.193(4) (2.2b), and 3.265(4) (2.3b) Å. The platinum–germanium bond distances (2.3938(6), 2.1a; 2.3953(5), 2.1b; 2.3881(5), 2.2b; and 2.4075(6) Å, 2.3b) fall between those reported for platinum(II) germyl complexes.3g,7
Scheme 2. Reaction of Complexes [Pt(NHC′)(NHC)][BArF] (1.1–1.3) with Tertiary Hydrogermanes.
Scheme 3. Reactions of Complexes 1.2 (A) and 1.4 (B) with Primary and Secondary Hydrogermanes.
Figure 1.
Thermal ellipsoid depiction of the cationic fragments of complexes 2.1a, 2.1b, 2.2b, and 2.3b (hydrogens omitted for clarity). Thermal ellipsoids at 30% probability. Selected bond distances (Å) and angles (°): 2.1a: Pt1-Ge1, 2.3938(6); Pt1-C1, 2.029(6); Pt1-C9, 2.020(6); and C1-Pt1-C9, 172.1(2). 2.1b: Pt1-Ge1, 2.3953(5); Pt1-C1, 2.031(3); Pt1-C9, 2.028(3); and C1-Pt1-C9, 169.0(1). 2.2b: Pt1-Ge1, 2.3881(5); Pt1-C1, 2.015(3); Pt1-C11, 2.027(3); and C1-Pt1-C11, 167.5(1). 2.3b: Pt1-Ge1, 2.4075(6); Pt1-C1, 2.029(4); Pt1-C13, 2.041(4); and C1-Pt1-C9, 172.8(2).
The reaction of complexes 1.1–1.4 with primary and secondary hydrogermanes has also been explored. It is worth recalling at this point that the reaction of complexes 1.2 and 1.4 with primary silanes RSiH3 follows a different outcome than that involving tertiary silanes. Previously, we have reported that a double Si–H bond activation takes place involving the initial formation of C–Si and Pt–H bonds (first Si–H bond activation), followed by a cyclometalation event that forges a new Pt–Si bond with extrusion of H2 (second Si–H activation).4b However, the reaction of primary germane nBuGeH3 with the cyclometalated complexes 1.2 and 1.4 yielded the platinum-germyl complexes [Pt(GeH2nBu)(NHC)2][BArF] (2.2c and 2.4c), with no signs of formation of the products arising from C–Ge bond forming (Scheme 3).8 At variance with the silyl derivatives, for which carbon–silicon products are observed, leading to two distinct NHCs (Scheme 1A), the germyl derivatives 2.2c and 2.4c exhibit a highly symmetrical environment in their 1H and 13C{1H} NMR spectra, in agreement with the presence of two equivalent NHC ligands. In addition, a signal at 2.59 (2.2c) ppm with a coupling constant to 195Pt of ca. 190 Hz integrating for two protons has been assigned to the GeH2nBu moiety.9 Nevertheless, the reaction of complex 1.3 with nBuGeH3 follows a slightly different outcome. The 1H NMR of the crude reaction mixture shows the formation of two main products in ca. 2:1 ratio (Scheme 4 and Figure S42). The major compound is symmetrical, and, according to an alternative synthetic route (see below), the signals correspond to the platinum-germyl complex 2.3c. The other species is unsymmetrical, based on the resonances for two different NHC ligands. In addition, a signal at 2.95 ppm with a JPt,H of 200 Hz (similar to that of complex 2.2c, 188 Hz), with an integral value for one proton, suggests the presence of a Pt–Ge–H fragment. These data, together with the detection of H2 evolution, point to the possible formation of the carbon–germanium coupled product 3.3c. Unfortunately, we have not been able to isolate this complex. Nevertheless, as mentioned above, complex 2.3c can be obtained in pure form by a sequential process that involves hydrogenation of starting material 1.3, leading to hydride 4, followed by addition of nBuGeH3 (with concomitant release of H2, as shown in Scheme 4).
Scheme 4. Reaction of Cyclometalated Complex 1.3 and Hydride 4 with Primary and Secondary Hydrogermanes.

Interestingly, this lack of selectivity is not observed in the reaction of 1.3 and the secondary hydrogermane Ph2GeH2 (Scheme 4). In this case, the only product observed is the platinum-germyl complex [Pt(GeHPh2)(ItBunPe)2][BArF], 2.3d (see the Supporting Information). This result hints at small differences in the energetic barriers, leading to either C–H/Pt–Ge or C–Ge/Pt–H bond-forming processes (see below) and suggests that sterics may be relevant in directing the reaction in one direction or another. Similarly, the reaction between complex 1.4 and diphenylgermane Ph2GeH2 leads exclusively to the platinum-germyl complex 2.4d (Scheme 3B). As in the previous reactions, the 1H NMR of 2.4d is very simple as a consequence of the high symmetry of the molecule. The most characteristic resonance in the 1H NMR is that of the GeH proton that appears at 3.45 ppm with a large 2JPt,H of 207 Hz, which is considerably larger than that found in related hydrogermyl-platinum complexes (ca. 50–65 Hz).3f,3g,7j The X-ray structure of this compound is depicted in Figure 2. Bond distances and angles are in the range of those described before, and 2.4d can be described as a low electron count Pt(II) species, since the closest carbon–platinum distance of 3.841(7) Å (corresponding Pt–H = 3.05 Å) is too long for an agostic interaction to be present.6
Figure 2.

Thermal ellipsoid depiction of the cationic fragment of complex 2.4d (hydrogen, except H1, and some carbon atoms omitted for clarity). Thermal ellipsoids at 30% probability. Selected bond distances (Å) and angles (°): Pt1-Ge1, 2.36248(6); Ge1-H1, 1.45(3); Pt1-C1, 2.025(4); Pt1-C24, 2.038(4); and C1-Pt1-C24, 167.1(2).
To look for possible intermediates in the reaction of complex 1.2 with hydrogermanes R3GeH (R = Et, Ph) and nBuGeH3 (particularly the formation of σ-GeH complexes), low-temperature NMR studies were carried out. First, we analyzed the reaction of 1.2 and Et3GeH in CD2Cl2 from −80 °C to room temperature. Similar to the reactions with Et3SiH, a clean reaction takes place, leading to the nonclassical σ-GeH complex 1.2·HGeEt3 (Scheme 5). The most significant NMR resonance in the 1H NMR spectrum is that of the bridging Pt–H–Ge proton, which appears at −5.36 ppm (−60 °C) with a 1JPt,H of 400 Hz. Both the chemical shift and coupling constant are similar to those observed in the related σ-SiH and σ-BH complexes 1.2·HSiEt3 (δ −4.90; 1JPt,H = 396 Hz) and 1.2·HBpin (δ −3.95; 1JPt,H = 357 Hz) but significantly different to those observed in agostic Pt···H···Ge interactions (δ 0.43, 1JPt,H = 785 Hz).3g Like 1.2·HSiEt3 and 1.2·HBpin, the diastereotopic protons of the Pt–CH2 fragment resonate at 2.23 and 2.06 ppm with reduced coupling constants to 195Pt (66 and 94 Hz at −10 °C) with respect to the cyclometalated starting material 1.2 (2JPt,H = 109 Hz). This smaller value is consistent with the presence of a weak ligand trans to the CH2–Pt fragment.10 The integrity of complex 1.2·HGeEt3 is maintained at temperatures below 15 °C, but it slowly evolves over a period of 24 h at room temperature to the germyl complex 2.2a. Analogous results were obtained in the reaction of 1.2 and Ph3GeH. At low temperatures, formation of 1.2·HGePh3 is characterized by a 1H NMR spectrum, with a signal in the hydride region at −4.68 ppm (1JPt,H = 396 Hz, at −80 °C, cf. 1.2·HSiPh3 analogue resonates at −4.00 ppm with 1JPt,H = 410 Hz) (see the Supporting Information). Complex 1.2·HGePh3 is considerably less stable, and it starts rearranging into 2.2b at −15 °C, being fully transformed within a few minutes at 15 °C. This different behavior with respect to Et3GeH is very likely a consequence of the increased electronegativity of the Ph groups compared to Et fragments, which lowers the energy of the σ*(Ge–H) molecular orbital, allowing a more efficient backdonation from the platinum atom.1a,2d,3b,11 These results are in good agreement with those observed for Ph3SiH and Et3SiH.4c
Scheme 5. Low-Temperature NMR Experiments between Complex 1.2 and Hydrogermanes.
Finally, the case of nBuGeH3 was examined. The different behavior of this hydrogermane in comparison to the parent hydrosilane nBuSiH3 is somewhat puzzling. One possibility is that a reversible C–Ge/Pt–H coupling reaction is taking place similar to that observed in the reaction of tricoordinated boranes HBR2 with complex 1.2 (Scheme 1B):4a a C–B bond coupling process occurs in a first step, but this reaction is reversible, leading eventually to the thermodynamically favored platinum-boryl complex Pt–BR2. Therefore, to look for similar potential intermediates, low-temperature NMR studies were undertaken. Upon mixing 1.2 with nBuGeH3 at −40 °C, the exclusive formation of the σ-GeH complex was detected. As in the previous systems, the bridging Pt–H–Ge proton resonates at −5.59 ppm (1JPt,H = 416 Hz), whereas the terminal GeH protons appear as broad signals at 4.81 and 4.52 ppm, that is, downfield shifted with respect to free nBuGeH3 (ca. 3.5 ppm). Then, upon increasing the temperature to 0 °C, the final product 2.2c begins to form without detecting any other intermediates during the course of the transformation. Thus, the platinum-germyl complex is, very likely, both kinetically and thermodynamically favored over the products deriving from C–Ge bond coupling. As detailed below, density functional theory (DFT) calculations support this hypothesis.
With all of the information extracted from the NMR studies, it becomes clear that complex 1.2·HGeEt3 appears to be the most stable of the three systems studied and should be the most accessible to be crystallized for X-ray diffraction studies. Indeed, we obtained crystals suitable for X-ray analysis of complex 1.2·HGeEt3 by slow diffusion of a concentrated solution in CH2Cl2 into pentane at −20 °C. Figure 3 shows the thermal ellipsoid representation of complex 1.2·HGeEt3. The metrical parameters are very similar to those observed for the parent 1.2·HSiEt3. The cation contains the two NHC ligands in trans, one of which is cyclometalated, and the fourth coordination site is occupied by the hydrogermane. The Ge–H bond distance of 1.78(4) Å is elongated with respect to free germanes (1.53 Å)3a,3b and in the range for other complexes having η2-GeH type interactions. The Pt–H bond length is rather short (1.41(4) Å) (1.58(3) for the related 1.2·HSiEt3), although caution should be taken considering the drawbacks of X-ray diffraction to locate hydrogen atoms. Interestingly, the Pt···Ge bond separation is 2.6468(8) Å, longer than the sum of covalent radii of germanium and platinum (2.44 Å)12 and longer than that observed for the platinum-germyl complexes 2.1a, 2.1b, 2.2b, and 2.3b. Another remarkable metrical parameter is the angle defined by the Pt···H···Ge atoms of 111(2)°, which is wider than that observed for the silane derivative (103(2)°). All of the data point to a bonding scenario halfway between a η2- and η1-type. To shed light into the coordination mode of the germane in 1.2·HGeEt3, the nature of the bonding interaction between the cationic platinum complex 1.2 and the germane HGeEt3 has been analyzed in detail.
Figure 3.
Thermal ellipsoid (left) and DFT-calculated (right) representations of 1.2·HGeEt3. Thermal ellipsoids are set to 30% probability. BArF anion and hydrogen atoms (except H27) are omitted for clarity. Selected bond distances [Å] and angles [°]: Experimental [Theoretical]: Pt2-C20, 2.041(3) [2.062]; Pt2-C35, 2.012(2) [2.024]; Pt2-C47, 2.089(3) [2.102]; Pt2-H27, 1.41(4) [1.695]; Ge2-H27, 1.78(4) [1.786]; Pt2···Ge2, 2.6468(8) [2.659]; Pt2-H27-Ge2, 111(2) [99.6]; and C47-Pt2-H27, 172.1 [168.4], C20-Pt2-C35, 167.8 (1) [167.7].
To this end, the energy decomposition analysis (EDA) in combination with the natural orbital for chemical valence (NOCV) method was applied at the relativistic and dispersion-corrected ZORA–BP86-D3/TZ2P//RI-BP86-D3/def2-TZVPP level. This methodology has been chosen to enable a direct comparison with the data recently reported by us for its silicon counterpart σ-SiH complex 1.2·HSiEt3.13 Once again, it is confirmed that the main contribution to the total interaction (ΔEint) between the [Pt]+ and HGeEt3 fragments in complex 1.2·HGeEt3 comes from the electrostatic attractions (ΔEelstat), which represent ca. 57% of the total attractive interactions, and are almost twice as strong as the orbital interactions (ΔEorb), contributing ca. 32% to the total bonding. The partitioning of the ΔEorb into pairwise orbital contributions by means of the NOCV method indicates that two main interactions dominate the total orbital interactions, namely, the donation from the σ(Ge–H) molecular orbital to a vacant σ*(Pt–C) (denoted ΔEorb(1), Figure 4) and the backdonation from a doubly occupied d atomic orbital of the transition metal to the vacant σ*(Ge–H) molecular orbital (denoted ΔEorb(2)). Similar to 1.2·HSiEt3, the σ(Ge–H) σ*(Pt–C) interaction is significantly stronger (ΔEorb(1) = −40.8 kcal mol–1) than the d(Pt) → σ*(Ge–H) backdonation (ΔEorb(2) = −17.6 kcal mol–1, Figure 4). These values are rather similar to those reported for the analogous platinum σ-SiH complex (ΔEorb(1) = −41.8 kcal mol–1 and ΔEorb(2) = −20.7),13 thus indicating that the donor and acceptor abilities of the σ(Ge–H) bond strongly resembles those of σ(Si–H) bonds. Despite that, it becomes evident that the backdonation in this germane complex is substantial, which suggests a coordination mode intermediate between the extreme situations represented by η1 (i.e., no backdonation) and η2 bonding modes.
Figure 4.
Deformation densities and associated molecular orbitals of the most important orbital interactions, ΔEorb(1) and ΔEorb(2), in complex 1.2·HGeEt3. The color code used to represent the flow of charge is red→blue. All data were computed at the ZORA–BP86-D3/TZ2P//RI-BP86-D3/def2-TZVPP level. Results from EDA-NOCV (in kcal mol–1): ΔEPauli = 185.4; ΔEelstat = −137.4; ΔEorb = −78.1; ΔEdisp = −26.2; and ΔEint = −56.3 (see the Supporting Information for a description of each term).
The reaction between 1.2 and nBuGeH3 was chosen as a representative process to be studied computationally14 to explore the mechanism by which these germyl complexes are formed. Following our previous studies on σ-SiH and σ-BH complexes, different pathways can be envisaged, starting from σ-GeH complex 1.2·HGeH2nBu. On the one hand, hydride transfer from the germane to the CH2 moiety would forge C–H and Pt–Ge bonds, giving rise to the aforementioned germyl species (C–H bond formation pathway). On the other hand, this hydride could instead form a Pt–H bond with concomitant formation of a C–Ge linkage (C–Ge bond formation pathway), which might account for the observation of complexes like 3.3c (Scheme 4). Additionally, the relatively small volume of the NHC fragments allows cis(4c,15) or trans geometries of these ligands around the metal center. Thus, DFT calculations were carried out for each mechanistic pathway (i.e., C–H or C–Ge coupling) considering both isomers.
First, the C–H bond pathway was calculated based on the experimental selective formation of the platinum-germyl complexes and the lack of observed intermediates during low-temperature NMR experiments. Whereas the process involving the NHC ligands in a trans geometry requires overcoming a kinetic barrier of 27.7 kcal mol–1 for the oxidative addition of the germane (Figure S48), analysis of the analogous reaction involving the NHC ligands in a cis arrangement gave lower energy barriers (Figure 5). As expected, germane coordination to give 1.2·HGeH2nBu is thermodynamically favored, since the σ-GeH complex is 3.0 kcal mol–1 more stable than 1.2 (energy reference). This species exhibits Pt–H and Pt···Ge distances of 1.78 and 2.77 Å, respectively, and a Pt–H–Ge angle of 107.8°, in line with the metrics mentioned above for similar σ-GeH species. Trans-cis isomerization from 1.2 through TS1 (12.9 kcal mol–1) yields cyclometalated Pt(II) complex 1.2-cis (11.0 kcal mol–1).4a,16 In this isomerization process, the CNHC–Pt–CNHC contracts from 176.4 to 112.5°. From this point, nBuGeH3 can bind the metal center to form the σ complex 1.2·HGeH2nBu-cis, 3.6 kcal mol–1 above the origin. Contrary to that observed for σ-BH complexes,4a the cis σ-GeH species did not clearly exhibit an orientation of the germane molecule that would place the Ge or H atoms close to the CH2 moiety. Formation of the C–H/Pt–Ge bonds takes place through TS2 (23.1 kcal mol–1 barrier), which involves H transfer (Pt–H = 1.60 Å, Pt···Ge = 2.55 Å, Ge···H = 2.08 Å, Pt–H–Ge = 86.6°) to the methylene unit and formation of germyl species 2.2c-cis (4.9 kcal mol–1 above the energy reference). This transition state gives the cis isomer of the observed product in a concerted fashion through a σ-CAM (σ-complex assisted metathesis) mechanism,17 similar to that observed for σ-BH complexes.4a,4f Thus, the orientation of the NHC ligands in the complex has a direct impact on the nature and energy barriers of the resulting mechanism for the formation of germyl species (i.e., oxidative addition for a trans arrangement vs σ-CAM for a cis geometry). From 2.2c-cis, cis-trans isomerization occurs via TS3 (8.7 kcal mol–1), leading to germyl complex 2.2c (−14.8 kcal mol–1). However, this complex possesses both NHC ligands almost coplanar (dihedral angle = 15.0°), and a different isomer with a wider dihedral angle (42.8°) between both carbene ligands was found to be more stable (−18.8 kcal mol–1).
Figure 5.
Computed Gibbs energy profile in dichloromethane for the C–H coupling pathway (NHCs in cis) upon reaction between 1.2 and nBuGeH3. Gibbs energies computed at 298 K are given in kcal mol–1. The Gibbs energy of 1.2 + nBuGeH3 has been taken as zero-energy.
Next, the C–Ge bond formation pathways were calculated. The mechanism for the system possessing both NHC ligands in a cis arrangement is displayed in Figure 6 (the analogous C–Ge coupling pathway involving both NHC ligands in trans requires a barrier of 27.4 kcal mol–1, see Figure S46). While the first isomerization part is identical to that shown in Figure 5, TS4 (22.2 kcal mol–1 energy barrier, 25.2 kcal mol–1 overall) describes the C–Ge and Pt–H coupling in a concerted step (σ-CAM process). Whereas the Pt–H (1.62 Å) and Pt···Ge (2.69 Å) distances are similar to those observed in TS2, the Ge···H distance is much longer (2.55 Å). Indeed, the geometry of TS4 and the energy of Int A-cis (14.6 kcal mol–1) indicate that TS4 is a late transition state. Species Int A-cis exhibits an agostic interaction6a (Pt–H = 1.89 Å, Pt–H–C = 105.7°), stabilizing the vacant position on platinum through one of the C–H bonds of the methylene group. From this geometry, all attempts to cleave the C–Ge bond leading to a cis germyl species 2.2c-cis (Figure 5) have proven futile, given that the σ-GeH complex was observed instead, in line with our observations of σ-BH complexes.4a Therefore, cleaving the C–Ge bond from a structure with both NHC ligands in a trans geometry was calculated instead (Figure 7, C–Ge cleavage pathway). Thus, isomerization through TS5 (20.2 kcal mol–1) is necessary, giving complex Int A, 5.8 kcal mol–1 above the energy reference. Unlike Int A-cis, this species does not possess an agostic interaction. From here, a concerted process can take place in which the hydride ligand bound to platinum is transferred to the CH2 moiety at the same time the germyl fragment forms a bond with the metal center. Nevertheless, this step is too energy-demanding (TS6, 47.4 kcal mol–1) and not feasible under the reaction conditions experimentally employed, which might explain why these C–Ge coupling species are observed in some cases like 3.3c (Scheme 4). Indeed, this alternative pathway was also explored by DFT methods (Figure 7, H2 extrusion pathway), which would first proceed via TS7 (17.9 kcal mol–1), where the C–H bond of the agostic interaction in Int A-cis is replaced by one of the Ge–H bonds, yielding Int A-cis′ (1.8 kcal mol–1). This complex exhibits shorter contacts of the germane fragment with the metal compared to that observed in 1.2·HGeH2nBu, according to key bonding metrics [Pt–H–Ge = 99.5° (vs 107.8°), Pt···Ge = 2.62 Å (vs 2.77 Å), Ge–H = 1.74 Å (vs 1.65 Å)]. This might be due to the reduced steric bulk around the germane moiety since one of the NHC ligands is now trans to it. From this geometry, Pt–Ge and H–H bond formation occurs through a σ-CAM process in TS8 (7.7 kcal mol–1). Then, 3.2c-cis·H2 (5.4 kcal mol–1) is obtained, which exhibits a 6-membered germametalacycle and a coordinated H2 molecule (H–H = 0.85 Å). Finally, dihydrogen dissociation directly leads to a trans geometry of both carbene ligands (3.2c, −9.5 kcal mol–1), analogous to that observed for C–Si coupling products.4b A similar H2 extrusion mechanism involving both NHC ligands in a trans arrangement gave higher energy barriers compared to the cis pathway (see Figure S47).
Figure 6.
Computed Gibbs energy profile in dichloromethane for the C–Ge coupling pathway (NHCs in cis) upon reaction between 1.2 and nBuGeH3. Gibbs energies computed at 298 K are given in kcal mol–1. The Gibbs energy of 1.2 + nBuGeH3 has been taken as zero-energy.
Figure 7.
Computed Gibbs energy profile in dichloromethane for the processes that might take place after C–Ge bond formation (NHCs in cis), namely, H2 extrusion (left) or C–Ge bond cleavage (right). Gibbs energies computed at 298 K are given in kcal mol–1. The Gibbs energy of 1.2 + nBuGeH3 has been taken as zero-energy.
In light of the aforementioned energy profiles, it is reasonable to propose that the formation of the cationic Pt(II) germyl complexes experimentally obtained can occur through a mechanism in which both NHC ligands are in a cis geometry, given the lower barriers computed for both C–H and C–Ge coupling compared to their trans analogues. In particular, the C–H bond formation pathway (Figure 5) involving NHC ligands in cis seems to be the most likely mechanism, given that it best describes the evolution of the system along the reaction coordinate, with the lowest kinetic barrier (23.1 kcal mol–1) of the computed profiles. Then, although C–Ge coupling seems energetically feasible as well (Figure 6), it is higher in energy (25.2 kcal mol–1), which might explain why no intermediates possessing C–Ge bonds are observed (unlike C–B coupling processes using the same Pt system).4a Nonetheless, this energy difference is relatively small, and if some C–Ge product is formed, it can enter the H2 extrusion pathway (with smaller kinetic barriers, Figure 7), leading to the corresponding germametalacycle. This small energy gap might then account for the experimentally detected amounts of complex 3.3c (where isopropyl has been replaced by neopentyl). A summary of the highest kinetic barriers for all of the computed reaction pathways can be found in Table 1.
Table 1. Summary of the Highest Kinetic Barriers for the Computed Reaction Mechanismsa.
| mechanism | C–H coupling (cis) | C–H coupling (trans) | C–Ge coupling (cis) | C–Ge coupling (trans) | C–Ge cleavage (cis) | C–Ge cleavage (trans) | H2 extrusion (cis) | H2 extrusion (trans) |
|---|---|---|---|---|---|---|---|---|
| ΔG (kcal mol–1) | 23.1 | 27.7 | 25.2 | 27.4 | not found (gives back 2.2c-cis) | 50.4 | 20.9 | 25.6 |
NB: C–Ge and H2 extrusion pathways are subsequent steps following the C–Ge coupling process.
Finally, to gain some information about the reactivity of some of these platinum-germyl complexes, exchange reactions with silanes have been carried out. To this aim, complexes 2.1a,b were treated with a slight excess of Ph2SiH2 or PhSiH3 (Scheme 6). The reaction proceeds smoothly at room temperature, yielding the platinum-silyl complexes [Pt(SiHPhR)(ItBuMe)2][BArF] (R = Ph, 5; R = H, 6). The process is rather fast for triphenylgermyl derivative 2.1b (full conversion in less than 1 h), whereas it is slow for triethylgermyl-complex 2.1a (full conversion after 24 h). Both reactions are very clean and serve as an alternative way to obtain this type of product without producing C–Si coupling products (as in Scheme 1A). No exchange was observed when Ph3SiH was used, suggesting (at least in part) that sterics play an important role in the process. Another peculiarity of the reaction is that this behavior appears to be opposite to previous exchange processes in which the metal-germyl compounds are more stable than the corresponding silyl derivatives, and thus they can be synthesized by exchange reactions from the silyl derivatives. This effect has been attributed to the higher ease of cleavage of Ge–H bonds in comparison to Si–H bonds.3b,18 However, other factors such as steric constraints and the strength of the Pt–E (E = Si, Ge) bond generated should be taken into consideration to explain our results.
Scheme 6. Exchange Reactions of Complexes 2.1a,b and Hydrosilanes.
Conclusions
The platinum-cyclometalated complexes [Pt(NHC′)(NHC)][BArF] react with primary, secondary, and tertiary germanes to generate the platinum-germyl derivatives [Pt(GeR3)(NHC)2][BArF], arising from C–H/Pt–Ge reaction pathways. At variance with reactions with primary silanes, the competitive process leading to C–Ge/Pt–H bonds is not observed when primary or secondary germanes are used, with the exception of the reaction of complex 1.3 with nBuGeH3, in which mixtures of compounds seem to be formed in different ratios. DFT calculations suggest that the mechanism involves a σ-CAM process through a trans-to-cis isomerization of the NHCs in which the barriers computed for the formation C–H/Pt–Ge bonds are lower (ca. 2 kcal mol–1) than those for C–Ge/Pt–H bonds. The relatively small energetic difference might explain why in the case reaction of complex 1.3 with nBuGeH3 both types of processes are observed. Low-temperature NMR studies allowed us to detect σ-GeH complexes [Pt(η2-HGeR3)(NHC′)(NHC)][BArF] as intermediates, one of which was sufficiently stable to be characterized by X-ray diffraction studies. The solid-state structure of complex [Pt(HGeEt3)(ItBuiPr′)(ItBuiPr)][BArF] indicates that the coordination mode of the Ge–H fragment falls between η2 and η1, which is consistent with the significant backdonation from the cationic platinum fragment into the σ*(Ge–H) molecular orbital suggested by the EDA-NOCV method. The results shown in this contribution are, therefore, complementary to those previously reported for hydrosilanes (in which irreversible formation of C–Si bonds are observed with primary silanes) and tricoordinated hydroboranes (with processes involving reversible formation of C–B bonds).
Acknowledgments
This work was supported by the Spanish MCIN/AEI/10.13039/501100011033 (Grants PID2019-109312GB-I00, PID2019-106184GB-I00, and RED2018-102387-T), European FEDER funds, and the Junta de Andalucía (project P20_00513). C.J.L.-G. thanks for a Margarita Salas grant financed by the European Union-NextGenerationEU, Ministry of Universities and Recovery, Transformation and Resilience Plan, through a call from University of Oviedo (Grant MU-21-UP2021-030 53307942). The use of the computational facilities of the Supercomputing Center of Galicia (CESGA) is gratefully acknowledged.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.inorgchem.2c03186.
Synthetic and experimental procedures, characterization of new compounds, X-ray crystallographic details, and computational methods (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- a Whited M. T.; Taylor B. L. H. Metal/organosilicon complexes: structure, reactivity, and considerations for catalysis. Comments Inorg. Chem. 2020, 40, 217–276. 10.1080/02603594.2020.1737026. [DOI] [Google Scholar]; b Corey J. Y. Reactions of hydrosilanes with transition metal complexes. Chem. Rev. 2016, 116, 11291–11435. 10.1021/acs.chemrev.5b00559. [DOI] [PubMed] [Google Scholar]; c Riddlestone I. M.; Abdalla J. A. B.; Aldridge S. Coordination and activation of E–H Bonds (E = B, Al, Ga) at transition metal centers. Adv. Organomet. Chem. 2015, 63, 1–38. [Google Scholar]; d Corey J. Y. Reactions of hydrosilanes with transition metal complexes and characterization of the products. Chem. Rev. 2011, 111, 863–1071. 10.1021/cr900359c. [DOI] [PubMed] [Google Scholar]; e Pandey K. K. Transition metal σ-borane complexes. Coord. Chem. Rev. 2009, 253, 37–55. 10.1016/j.ccr.2007.11.026. [DOI] [Google Scholar]; f Alcaraz G.; Grellier M.; Sabo-Etienne S. Bis σ-bond dihydrogen and borane ruthenium complexes: bonding nature, catalytic applications, and reversible hydrogen release. Acc. Chem. Res. 2009, 42, 1640–1649. 10.1021/ar900091a. [DOI] [PubMed] [Google Scholar]; g Nikonov G. I. Recent advances in nonclassical interligand Si···H interactions. Adv. Organomet. Chem. 2005, 53, 217–309. [Google Scholar]; h Kubas G. J. Heterolytic splitting of H–H, Si–H, and other σ bonds on electrophilic metal centers. Adv. Inorg. Chem. 2004, 56, 127–177. [Google Scholar]
- a Handzlik J.; Kochel A.; Szymańska-Buzar T. H–Ge bond activation by tungsten carbonyls: An experimental and theoretical study. Polyhedron 2012, 31, 622–637. 10.1016/j.poly.2011.10.040. [DOI] [Google Scholar]; b Zyder M.; Szymańska-Buzar T. Activation of the Ge–H bond of Et3GeH in photochemical reaction with molybdenum(0) carbonyl complexes and hydrogermylation of norbornadiene. J. Organomet. Chem. 2009, 694, 2110–2113. 10.1016/j.jorganchem.2009.02.013. [DOI] [Google Scholar]; c Sabo-Etienne S.; Hernandez M.; Chung G.; Chaudret B.; Castel A. Substitution reactions of coordinated dihydrogen by weakly coordinating ligands: preparation of RuH2(N2)2(PCy3)2 and RuH2(η2-H2)(HEPh3)(PCy3)2 (E = Si, Ge). New J. Chem. 1994, 18, 175–177. [Google Scholar]; d Lichtenberger D. L.; Rai-Chaudhuri A. Electronic structure factors of Ge–H bond activation by transition metals. Photoelectron spectra of [Mn(η5-C5H5)(CO)2(HGePh3)], [Mn(η5-C5H4Me)(CO)2(HGePh3)], and [Mn(η5-C5Me5)(CO)2(HGePh3)]. J. Chem. Soc., Dalton Trans. 1990, 2161–2166. 10.1039/DT9900002161. [DOI] [Google Scholar]; e Garré F.; Colomer E.; Corriu R. J. P.; Vioux A. Stereochemistry of the insertion of manganese into Si–H and Ge–H Bonds. Complexes containing a two-electron, three-center Mn···H···Si (or Ge) interaction. Organometallics 1984, 3, 1272–1278. 10.1021/om00086a022. [DOI] [Google Scholar]
- a Smart K. A.; Mothes-Martin E.; Vendier L.; Perutz R. N.; Grellier M.; Sabo-Etienne S. A ruthenium dihydrogen germylene complex and the catalytic synthesis of digermoxane. Organometallics 2015, 34, 4158–4163. 10.1021/acs.organomet.5b00570. [DOI] [Google Scholar]; b Vincent J. L.; Luo S.; Scott B. L.; Butcher R.; Unkefer C. J.; Burns C. J.; Kubas G. J.; Lledós A.; Maseras F.; Tomàs J. Oxidative addition of germanes and silanes, EH4-nPhn (E = Si, Ge; n = 0-3), to Mo(CO)(diphosphine)2. The first structurally characterized germane σ complex. Organometallics 2003, 22, 5307–5323. 10.1021/om030569j. [DOI] [Google Scholar]; For “agostic” (mono- and di-nuclear) complexes see:; c Takaya J.; Iwasawa N. Synthesis, structure, and reactivity of a mononuclear η2-(Ge–H)-palladium(0) complex bearing a PGeP-pincer-type germyl ligand: reactivity differences between silicon and germanium. Eur. J. Inorg. Chem. 2018, 2018, 5012–5018. 10.1002/ejic.201801257. [DOI] [Google Scholar]; d Mobarok M. H.; McDonald R.; Ferguson M. J.; Cowie M. Germyl- and germylene-bridged complexes of Rh/Ir and subsequent chemistry of a bridging germylene group. Inorg. Chem. 2012, 51, 4020–4034. 10.1021/ic2021269. [DOI] [PubMed] [Google Scholar]; e Zyder M.; Kochel A.; Handzlik J.; Szymańska-Buzar T. Photochemical reaction of Mo(CO)6 with Et2GeH2: NMR and DFT studies of reaction products; crystal structure of a novel complex [{Mo(μ-η2-H-GeEt2)(CO)4}2]. Organometallics 2009, 28, 5857–5865. 10.1021/om900299k. [DOI] [Google Scholar]; f Tanabe M.; Ishikawa N.; Osakada K. Preparation and structure of a new dipalladium complex with bridging diphenylgermyl ligands. Diverse reactivities of Pd(PCy3)2 and Pt(PCy3)2 toward Ph2GeH2. Organometallics 2006, 25, 796–798. 10.1021/om050981u. [DOI] [Google Scholar]; g Braddock-Wilking J.; Corey J. Y.; White C.; Xu H.; Rath N. P. Reaction of diphenylgermane with (Ph3P)2Pt(η2-C2H4): generation of mono- and dinuclear complexes containing Pt–Ge bonds. X-ray crystal structure determination of [(Ph3P)Pt(μ-η2-H-GePh2)]2. Organometallics 2005, 24, 4113–4115. 10.1021/om050392o. [DOI] [Google Scholar]
- a Ríos P.; Martín-de la Calle R.; Vidossich P.; Fernández-de-Córdova F. J.; Lledós A.; Conejero S. Reversible carbon–boron bond formation at platinum centers through σ-BH complexes. Chem. Sci. 2021, 12, 1647–1655. 10.1039/D0SC05522K. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ríos P.; Fouilloux H.; Díez J.; Vidossich P.; Lledós A.; Conejero S. σ-Silane platinum(II) complexes as intermediates in C–Si bond- coupling processes. Chem. - Eur. J. 2019, 25, 11346–11355. 10.1002/chem.201902226. [DOI] [PubMed] [Google Scholar]; c Ríos P.; Fouilloux H.; Vidossich P.; Díez J.; Lledós A.; Conejero S. Isolation of a cationic platinum(II) σ-silane complex. Angew. Chem., Int. Ed. 2018, 57, 3217–3221. 10.1002/anie.201712791. [DOI] [PubMed] [Google Scholar]; d Ríos P.; Díez J.; López-Serrano J.; Rodríguez A.; Conejero S. Cationic platinum(II) σ-SiH complexes in carbon dioxide hydrosilation. Chem. - Eur. J. 2016, 22, 16791–16795. 10.1002/chem.201603524. [DOI] [PubMed] [Google Scholar]; e Rivada-Wheelaghan O.; Roselló-Merino M.; Ortuño M. A.; Vidossich P.; Gutiérrez-Puebla E.; Lledós A.; Conejero S. Reactivity of coordinatively unsaturated bis(N-heterocyclic carbene) Pt(II) complexes toward H2. Crystal structure of a 14-electron Pt(II) hydride complex. Inorg. Chem. 2014, 53, 4257–4268. 10.1021/ic500705t. [DOI] [PubMed] [Google Scholar]; f Ríos P.; Fernández-de-Córdova F. J.; Borge J.; Curado N.; Lledós A.; Conejero S. Ligand effects in carbon-boron coupling processes mediated by σ-BH platinum complexes. Eur. J. Inorg. Chem. 2021, 2021, 3528–3539. 10.1002/ejic.202100428. [DOI] [Google Scholar]
- See for example:; a Selmani A.; Schoetz M. D.; Queen A. E.; Schoenebeck F. Modularity in the Csp3 space–alkyl germanes as orthogonal molecular handles for chemoselective diversification. ACS Catal. 2022, 12, 4833–4839. 10.1021/acscatal.2c00852. [DOI] [Google Scholar]; b Dahiya A.; Schoenebeck F. Direct C–H dehydrogenative germylation of terminal alkynes with hydrogermanes. Org. Lett. 2022, 24, 2728–2732. 10.1021/acs.orglett.2c00840. [DOI] [PubMed] [Google Scholar]; c Xu Q.-H.; Wei L.-P.; Xiao B. Alkyl-GeMe3: neutral metalloid radical precursors upon visible light photocatalysis. Angew. Chem., Int. Ed. 2022, 61, e202115592. [DOI] [PubMed] [Google Scholar]; d Luo Y.; Xu B.; Lv L.; Li Z. Copper-catalyzed three-component germyl peroxidation of alkenes. Org. Lett. 2022, 24, 2425–2430. 10.1021/acs.orglett.2c00698. [DOI] [PubMed] [Google Scholar]; e Su P.-F.; Wang K.; Peng X.; Pang X.; Guo P.; Shu X.-Z. Nickel-catalyzed reductive C–Ge coupling of aryl/alkenyl electrophiles with chlorogermanes. Angew. Chem., Int. Ed. 2021, 60, 26571–2657. 10.1002/anie.202112876. [DOI] [PubMed] [Google Scholar]; f Kojima K.; Uchida S.; Kinoshita H.; Miura K. Synthesis of polysubstituted germoles and benzogermoles using a substoichiometric amount of diisobutylaluminum hydride. Org. Lett. 2021, 23, 4598–4602. 10.1021/acs.orglett.1c01314. [DOI] [PubMed] [Google Scholar]; g Fricke C.; Schoenebeck F. Organogermanes as orthogonal coupling partners in synthesis and catalysis. Acc. Chem. Res. 2020, 53, 2715–2725. 10.1021/acs.accounts.0c00527. [DOI] [PubMed] [Google Scholar]; h Sherborne G. J.; Gevondian A. G.; Funes-Ardoiz I.; Dahiya A.; Fricke C.; Schoenebeck F. Modular and selective arylation of aryl germanes (C–GeEt3) over C–Bpin, C–SiR3 and halogens enabled by light-activated gold catalysis. Angew. Chem., Int. Ed. 2020, 59, 15543–15548. 10.1002/anie.202005066. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Liang H.; Ji Y.-X.; Wang R.-H.; Zhang Z.-H.; Zhang B. Visible-light-initiated manganese-catalyzed E-selective hydrosilylation and hydrogermylation of alkynes. Org. Lett. 2019, 21, 2750–2754. 10.1021/acs.orglett.9b00701. [DOI] [PubMed] [Google Scholar]; j Xue W.; Mao W.; Zhang L.; Oestreich M. Mechanistic dichotomy of magnesium- and zinc-based germanium nucleophiles in the C(sp3)–Ge cross-coupling with alkyl electrophiles. Angew. Chem., Int. Ed. 2019, 58, 6440–6443. 10.1002/anie.201901860. [DOI] [PubMed] [Google Scholar]; k Keess S.; Oestreich M. Access to fully alkylated germanes by B(C6F5)3-catalyzed transfer hydrogermylation of alkenes. Org. Lett. 2017, 19, 1898–1901. 10.1021/acs.orglett.7b00672. [DOI] [PubMed] [Google Scholar]
- a Brookhart M.; Green M. L. H.; Parkin G. Agostic interactions in transition metal compounds. Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 6908–6914. 10.1073/pnas.0610747104. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Brookhart M.; Green M. L. H. Carbon–hydrogen transition metal bonds. J. Organomet. Chem. 1983, 250, 395–408. 10.1016/0022-328X(83)85065-7. [DOI] [Google Scholar]
- a Karimi M.; Tabei E. S.; Fayad R.; Saber M. R.; Danilov E. O.; Jones C.; Castellano F. N.; Gabbaï F. P. Photodriven elimination of chlorine from germanium and platinum in a dinuclear PtII→GeIV complex. Angew. Chem., Int. Ed. 2021, 60, 22352–22358. 10.1002/anie.202107485. [DOI] [PubMed] [Google Scholar]; b Karimi M.; Gabbäi F. P. Photoreductive elimination of PhCl across the dinuclear core of a [GePt]VI complex. Organometallics 2022, 41, 642–648. 10.1021/acs.organomet.2c00004. [DOI] [Google Scholar]; c Cabeza J. A.; Fernández I.; Fernández-Colinas J. M.; García-Álvarez P.; Laglera-Gándara C. J. A germylene supported by two 2-pyrrolylphosphane groups as precursor to PGeP pincer square-planar group 10 metal(II) and T-shaped Gold(I) complexes. Chem. - Eur. J. 2019, 25, 12423–12430. 10.1002/chem.201902784. [DOI] [PubMed] [Google Scholar]; d Álvarez-Rodríguez L.; Brugos J.; Cabeza J. A.; García-Álvarez P.; Pérez-Carreño E. Diphosphanegermylene to Nickel, Palladium, and Platinum complexes containing germyl PGeP pincer ligands. Chem. - Eur. J. 2017, 23, 15107–15115. 10.1002/chem.201702629. [DOI] [PubMed] [Google Scholar]; e Herrmann R.; Wittwer P.; Braun T. Platinum complexes bearing a tripodal germyl ligand. Eur. J. Inorg. Chem. 2016, 2016, 4898–4905. 10.1002/ejic.201600652. [DOI] [Google Scholar]; f Nakata N.; Sekizawa N.; Ishii A. Cationic dinuclear platinum and palladium complexes with bridging hydrogermylene and hydrido ligands. Chem. Commun. 2015, 51, 10111–10114. 10.1039/C5CC03062E. [DOI] [PubMed] [Google Scholar]; g Arii H.; Watanabe N.; Mochida K.; Kawashima T. Steric effect of a phosphane ligand on the equilibrium of Ge–Ge bond formation at a platinum center. Eur. J. Inorg. Chem. 2012, 2012, 4791–4794. 10.1002/ejic.201200782. [DOI] [Google Scholar]; h Tanabe M.; Deguchi T.; Osakada K. Chemical properties of tetragermaplatinacyclopentane. Insertion of an alkyne into a Pt–Ge bond and silylation Caused by H2SiPh2. Organometallics 2011, 30, 3386–3391. 10.1021/om200275w. [DOI] [Google Scholar]; i Nakata N.; Fukazawa S.; Ishii A. Synthesis and crystal structures of the first stable mononuclear dihydrogermyl(hydrido) platinum(II) complexes. Organometallics 2009, 28, 534–538. 10.1021/om800809n. [DOI] [Google Scholar]; j Arii H.; Nanjo M.; Mochida K. Characterization of mononuclear and dinuclear germylplatinum complexes and Ge-Ge bond formation at the platinum center. Organometallics 2008, 27, 4147–4151. 10.1021/om800267k. [DOI] [Google Scholar]; k Usui Y.; Fukushima T.; Nanjo M.; Mochida K.; Akasaka K.; Kudo T.; Komiya S. Synthesis and kaleidoscopic reactivities of bis(tritolylgermyl)bis(dimethylphenylphosphine)platinum(II). Chem. Lett. 2006, 35, 810–811. 10.1246/cl.2006.810. [DOI] [Google Scholar]
- The reaction of complex 1.1 with GeH3nBu initially leads to a complex that appears to be the platinum germyl species [Pt(GeH2nBu)(ItBuMe)2][BArF]. However, this compound decomposed both in solution as well as during the purification process to a very complex mixture of compounds.
- The signal for the GeH2 protons in complex 2.4c is masked by the resonances of the CH3 groups of the IMes* ligand, and therefore no coupling constant to 195Pt could be obtained.
- Ortuño M. A.; Conejero S.; Lledós A. True and masked three-coordinate T-shaped platinum(II) intermediates. Beilstein J. Org. Chem. 2013, 9, 1352–1382. 10.3762/bjoc.9.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrady G. S.; Sirsch P.; Chatterton N. P.; Ostermann A.; Gatti C.; Altmannshofer S.; Herz V.; Eickerling G.; Scherer W. Nature of the bonding in metal-silane σ-complexes. Inorg. Chem. 2009, 48, 1588–1598. 10.1021/ic8019777. [DOI] [PubMed] [Google Scholar]
- Pyykkö P.; Atsumi M. Molecular single-bond covalent radii for elements 1–118. Chem. - Eur. J. 2009, 15, 186–197. 10.1002/chem.200800987. [DOI] [PubMed] [Google Scholar]
- Ríos P.; Conejero S.; Fernández I. Bonding situation of σ-E-H complexes in transition metal and main group compounds. Chem. - Eur. J. 2022, 28, e202201920 10.1002/chem.202201920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calculations were carried out using the M06 functional in dichloromethane (SMD). Further details and references can be found in the Supporting Information file.
- Fortman G. C.; Scott N. M.; Linden A.; Stevens E. D.; Dorta R.; Nolan S. P. Unusual reactivities of N-heterocyclic carbenes upon coordination to the platinum(II)–dimethyl moiety. Chem. Commun. 2010, 46, 1050–1052. 10.1039/b920482b. [DOI] [PubMed] [Google Scholar]
- cis-trans isomerization without the H–E substrate bound gives lower energy barriers. See reference 4a for some examples of isomerization of σ-BH complexes.
- a Perutz R. N.; Sabo-Etienne S.; Weller A. S. Metathesis by partner interchange in σ-bond ligands: expanding applications of the σ-CAM mechanism. Angew. Chem., Int. Ed. 2022, 61, e202111462 10.1002/anie.202111462. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Perutz R. N.; Sabo-Etienne S. The σ-CAM mechanism: σ complexes as the basis of σ-bond metathesis at late-transition-metal centers. Angew. Chem., Int. Ed. 2007, 46, 2578–2592. 10.1002/anie.200603224. [DOI] [PubMed] [Google Scholar]
- Kubas G. J. Dihydrogen complexes as prototypes for the coordination chemistry of saturated molecules. Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 6901–6907. 10.1073/pnas.0609707104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.