Abstract
Purpose
Whether COVID-19 reduces male fertility remains requires further investigation. This meta-analysis and systematic review evaluated the impact of COVID-19 on male fertility.
Materials and Methods
The literature in PubMed, Embase, MEDLINE, Web of Science, and Cochrane Library up to January 01, 2022 was systematically searched, and a meta-analysis was conducted to investigate the effect of COVID-19 on male fertility. Totally 17 studies with a total of 1,627 patients and 1,535 control subjects were included in our meta-analysis.
Results
Regarding sperm quality, COVID-19 decreased the total sperm count (p=0.012), sperm concentration (p=0.001), total motility (p=0.001), progressive sperm motility (p=0.048), and viability (p=0.031). Subgroup analyses showed that different control group populations did not change the results. It was found that during the illness stage of COVID-19, semen volume decreased, and during the recovery stage of COVID-19, sperm concentration and total motility decreased <90 days. We found that sperm concentration and total motility decreased during recovery for ≥90 days. Fever because of COVID-19 significantly reduced sperm concentration and progressive sperm motility, and COVID-19 without fever ≥90 days, the sperm total motility and progressive sperm motility decreased. Regarding disease severity, the moderate type of COVID-19 significantly reduced sperm total motility, but not the mild type. Regarding sex hormones, COVID-19 increased prolactin and estradiol. Subgroup analyses showed that during the illness stage, COVID-19 decreased testosterone (T) levels and increased luteinizing hormone levels. A potential publication bias may have existed in our meta-analysis.
Conclusions
COVID-19 in men significantly reduced sperm quality and caused sex hormone disruption. COVID-19 had long-term effects on sperm quality, especially on sperm concentration and total motility. It is critical to conduct larger multicenter studies to determine the consequences of COVID-19 on male fertility.
Keywords: COVID-19, Fertility, Male, SARS-CoV-2, Sex hormone, Sperm
INTRODUCTION
The SARS-CoV-2 virus infection caused coronavirus disease 2019 (COVID-19) has spread rapidly and led to a global pandemic in 2019 [1], causing more than 200 million infections and millions of deaths [2]. This ongoing pandemic is a significant challenge to healthcare systems and damages economic development and social stability [3,4]. Many researchers have conducted studies on the biological features of COVID-19, and many studies have elucidated the pathogenesis and mechanisms of this pandemic disease after its onset.
SARS-CoV-2 virus is a single-strand RNA virus that infects host cells mainly by using angiotensin-converting enzyme 2 (ACE-2) or transmembrane serine protease 2 (TMPRSS2) as receptors, which are widely expressed in various systems and make these tissues prone to infection [5,6]. Some researchers have verified that ACE-2 is expressed in Leydig cells, Sertoli cells, and the germ line, which are closely related to sperm development [7]. Therefore, the level of ACE-2 in the testes indicates that SARS-CoV-2 virus attacks not only the respiratory system, but also the reproductive system. Such studies may explain why SARS-CoV-2 is present in the testes. Li et al [8] tested 38 semen samples from patients using reverse transcription-polymerase chain reaction (RT-PCR), and 6 samples (from patients in the acute and recovery stages of infection) were positive for COVID-19. A recent study performed by Delaroche et al [9] indicated that among 32 COVID-19 patients, the semen from one patient tested positive for the virus. Several studies have indicated that COVID-19 may impair the testes and quality of sperm and sex hormones [10,11].
Donders et al [12] demonstrated that sperm were damaged after COVID-19 but recovered over time. Ruan et al [13] found that sperm quality decreased in COVID-19 patients, and was significantly decreased in patients with longer recovery times. However, the level of sex hormones did not change. The effect of COVID-19 on sperm quality and changes in sex hormones over time remains inconclusive. Therefore, we conducted a more comprehensive meta-analysis to evaluate the effects and recovery time from COVID-19 on sperm quality and sex hormones, and how the disease affects male fertility.
MATERIALS AND METHODS
This systematic review and Meta-snalysis was registered in the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY) with registration number INPLASY202210110.
1. Literature search
Meta-analysis and systemic review were conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines [14]. A comprehensive literature search was conducted in PubMed, Embase, MEDLINE, Web of Science, and Cochrane Library up to January 01, 2022. The search terms were as follows: (“SARS-CoV-2” OR “COVID-19” OR “Severe acute respiratory syndrome-coronavirus 2” OR “2019-nCoV” OR “Coronavirus”) AND (“Sperm” OR “Semen” OR “Sperm quality” OR “Seminal” OR “Testes” OR “Testicular” OR “Male fertility” OR “follicle-stimulating hormone” OR “FSH” OR “luteinizing hormone” OR “LH” OR “prolactin” OR “PRL” OR “Testosterone” OR “T” OR “oestradiol” OR “estradiol” OR “E2”).
2. Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) the study must contain information on the relationship between COVID-19 and male fertility; (2) the study must have available data to estimate the relative risk and 95% confidence interval (CI) and the study must include a COVID-19 negative control group; (3) the study must include human male subjects; and (4) the study’s sperm parameters must have been assessed according to the World Health Organization 2010 guidelines [15]. The exclusion criteria were as follows: (1) review articles, case reports, and basic science reports; (2) duplicated studies; (3) insufficient raw data; and (4) raw data that were not transformed into mean±standard deviation (SD).
3. Data extraction
Two investigators extracted data independently following the inclusion and exclusion criteria and reached a consensus on each data point. The data extracted included author name, year, country, design, population, sample size, main outcome, mean±SD and 95% CI, and median (min–max) or median (interquartile range [IQR]).
4. Quality assessment
The Newcastle–Ottawa scale (NOS) [16] was used to assess the quality of case-control studies and scores of 0–3, 4–6, and 7–8 were assigned for low, moderate, and high quality, respectively. The Agency for Health Care Research and Quality (AHRQ) statement was used to assess a cross-sectional study [16,17], and we scored the number of “yes” statements less than 4 as low, 5–7 as moderate, and 8–11 as high-quality research.
5. Statistical analysis
We used the standard mean difference (SMD) and its corresponding 95% CI to assess the effect of COVID-19 on sperm quality and sex hormone levels. p<0.05 was considered statistically significant. Data are presented as median (min–max) or median (IQR) and were transformed into mean±SD according to Luo et al [18] and Wan et al [19].
We used the Q-test to evaluate heterogeneity among different studies, and p<0.05 was recognized as statistically significant. We quantified this inconsistency using the I2 statistic. When heterogeneity was not significant, a fixed effects model was used; otherwise, a random effects model was chosen.
6. Publication bias assessment
Funnel plots, Begg’s test, and Egger’s test were used to detect possible publication biases. Sensitivity analyses were performed to assess the possible causes of heterogeneity in the results.
7. Subgroup analyses
Differences in the populations of the control group may result in different baselines of fertility. To investigate the influence of COVID-19 on patients compared with different control group populations (healthy population, or with the patients themselves before and after infection with COVID-19), subgroup analyses were conducted based on the control group population.
To evaluate the potential effects of age, different stages, with or without fever, and the disease severity of COVID-19 on male fertility, the following investigation was conducted. We performed subgroup analyses based on the effects of age (≥50 y, and <50 y), different stages (period of illness stage, recovery time <90 d, and recovery time ≥90 d), with or without fever, and different disease severities of COVID-19 on sperm quality and sex hormones. To evaluate the association between COVID-19 infection and male fertility purely as rather than the association between COVID-19 infection as a febrile illness and male fertility, we performed subgroup analyses based on different stages among without fever infected individuals. According to the included studies, the mild symptomatic defined as mild symptoms without radiographic features (outpatient treatment), and moderate disease was defined as fever, respiratory symptoms (SpO2 <93%, requiring hospitalization for oxygen therapy) and radiographic features, and the severe cases met one of the following three criteria: (1) dyspnea, with a respiratory rate ≥30 times/min, (2) oxygen saturation ≤93% at rest, or (3) PaO2/FiO2 ≤300 mmHg. All meta-analyses were performed using the STATA software (Version 12.0; Stata Corporation, College Station, TX, USA).
RESULTS
1. Study characteristics
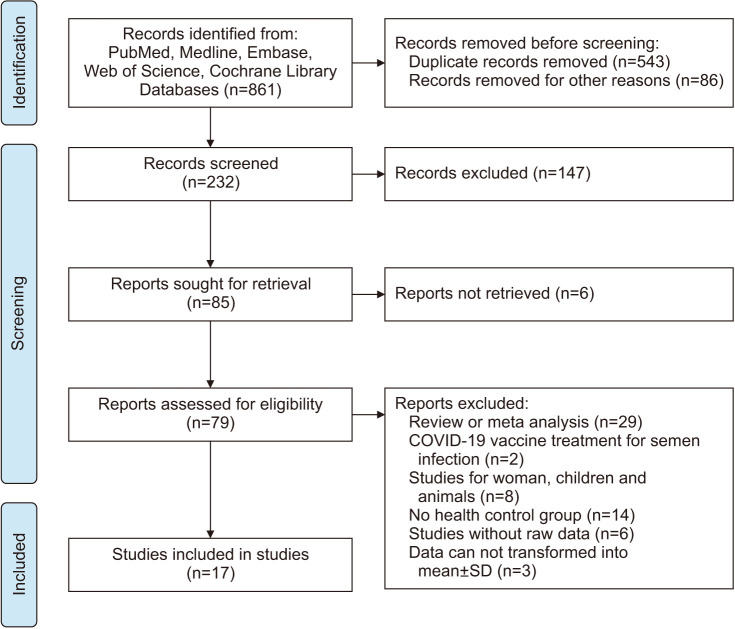
Based on the search strategy, we identified 861 potential studies in the databases (Fig. 1). After excluding duplicate publications, 232 studies were included in the meta-analysis. After the records were screened for retrieval, 79 studies were considered eligible. Among the 79 studies, 62 articles were excluded: 29 studies were reviews or meta-analyses; 2 articles were about COVID-19 vaccine treatment for semen infection; 8 studies were on women, children, or animals; 14 studies did not have a healthy control group; 6 studies did not present raw data; and 3 articles provided raw data that could not be transformed into the mean±SD. Ultimately, 17 studies were included in our meta-analysis, with a total of 1,627 patients and 1,535 healthy or COVID-19-negative controls. Detailed information on the 17 studies is listed in Table 1[13,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35].
Fig. 1. Flowchart of the literature selection.
Table 1. General characteristics of the studies included in the meta-analysis.
| Main author | Year | Country | Study design | Fertility of population | Time points of semen or sex hormones tested (n) and age (y, mean±SD or range) | Outcome |
|---|---|---|---|---|---|---|
| Temiz [20] | 2021 | Turkey | Cross-sectional | Fertile men | Infection stage (10): 38±8.28 Recovery time <90 d (10): 37±8.69 Healthy control (10): 36.64±9.63 |
Total sperm count, progressive sperm motility, total motility, FSH, LH, T, PRL |
| Holtmann [21] | 2020 | Germany | Cohort | Fertile men | Recovery time <90 d (16): unclear Healthy control (14): 33.4±13.1 |
Semen volume, sperm concentration, total sperm count, progressive sperm motility, total motility |
| Li [22] | 2020 | China | Cross-sectional | Fertile men | Infection stage (23): 40.5±5.9 Health control (22): 40.8±8.5 |
Sperm concentration |
| Rafiee [23] | 2021 | Iran | Cohort | Normal sperm men | Infection stage (200): 36.1±4.1 Recovery time ≥90 d (100): 36.1±4.1 Self-COVID-19 negative control (200): 36.1±4.1 |
Semen volume, sperm concentration, total sperm count, normal morphology |
| Pazir [24] | 2021 | Turkey | Cohort | Normal sperm men | Recovery time ≥90 d (24): 34.7±6.4 Self-COVID-19 negative control (24): 34.7±6.4 |
Semen volume, sperm concentration, progressive sperm motility, total motile sperm count, total motility |
| Gul [25] | 2021 | Turkey | Cross-sectional | Fertile men | Recovery time ≥90 d (29): 31.21±5.48 Self-COVID-19 negative control (29): 31.21±5.48 |
Semen volume, sperm concentration, total sperm count, progressive sperm motility, total motility, FSH, LH, PRL, T |
| Koç [26] | 2021 | Turkey | Cohort | Infertile men without azoospermia | Infection stage (21): 32±6.3 Self-COVID-19 negative control (21): 32±6.3 |
Semen volume, sperm concentration, total sperm count, progressive sperm motility, nonprogressive sperm motility, total motility, immotile sperm, normal morphology, FSH, LH, T |
| Ma [27] | 2021 | China | Cross-sectional | Reproductive-aged men | Semen: Recovery time <90 d (3): 39 (35.0–44.0) Healthy control (3): 39 (35.0–42.0) Sex hormones: Recovery time <90 d (119) :39 (35.0–44.0) Healthy control (173): 39 (35.0–42.0) |
Semen volume, sperm concentration, total sperm count, progressive sperm motility, nonprogressive sperm motility; immotile sperm, normal morphology, viability, FSH, LH, T, T/LH |
| Ruan [13] | 2021 | China | Cross-sectional | Semen volume more than 1.5 mL, fertile men | Recovery time <90 d (35): 31.89±5.43 Recovery time ≥90 d (20): 29.7±4.93 Healthy control (145): 30.69±4.36 |
Semen volume, sperm concentration, total sperm count, progressive sperm motility, total sperm motility |
| Guo [28] | 2021 | China | Case-control | Reproductive-aged men | Recovery time <90 d (55): 26.0 (22.0–34.0) Recovery time ≥90 d (9): unclear Healthy control (50): 26.5 (25.0–34.0) |
Semen volume, sperm concentration, total sperm count, progressive sperm motility, sperm motility, vitality, FSH, LH, T, PRL, E2 |
| Erbay [29] | 2021 | Turkey | Cross-sectional | Sexually active men | Recovery time more than 90 d (26/43): mild, 30.04±4.8/moderate, 31.06±4.2 Self-COVID-19 negative control (26/43): mild, 30.04±4.8/moderate, 31.06±4.2 |
Semen volume, sperm concentration, total sperm count, progressive sperm motility, vitality |
| Xu [30] | 2021 | China | Cross-sectional | Unclear | Recovery time <90 d (39): 60 (46.5–65.5) Healthy control (22): 62 (52–68.75) |
FSH, LH, T, PRL, E2 |
| Karkin [31] | 2021 | Turkey | Cross-sectional | Fertile men | Recovery time ≥90 d (70): 52.33±13.51 Self-COVID-19 negative control (70): 52.33±13.51 |
T |
| Okçelik [32] | 2021 | Turkey | Cross-sectional | Men 18–50 y | Infection stage (24): 35.5±9.85 Healthy control (20): 35.5±9.85 |
FSH, LH, T |
| Salonia [33] | 2021 | Italy | Cohort | Men >18 y | Infection stage (286): 58 (49–66) Healthy control (281): 46 (35–52) |
FSH, LH, T, E2 |
| Saad [34] | 2022 | Egypt | Case-control | Sexually active men | Recovery time <90 d (107): 32.66±4.83 Healthy control (90): 32.76±5.02 |
T |
| Cinislioglu [35] | 2022 | Turkey | Cohort | Unclear | Infection stage (358): 64.9±11.6 Healthy control (92): 67.2±13.6 |
FSH, LH, T |
FSH: follicle-stimulating hormone, LH: luteinizing hormone, T: testosterone, PRL: prolactin, E2: estradiol.
2. Study quality
All included studies were of high quality. The details of the quality assessment are shown in Supplement Table 1 and Supplement Table 2.
3. Effects of COVID-19 on sperm quality and sex hormones
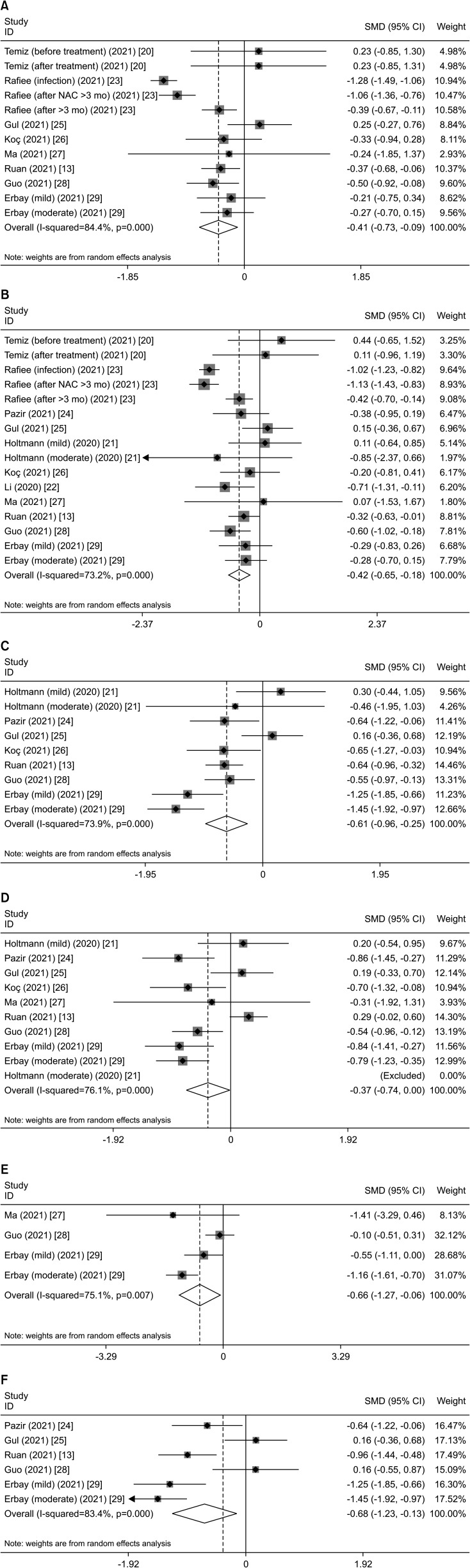
Regarding sperm quality, the combined results showed that COVID-19 decreased the total sperm count (SMD, -0.411; 95% CI, -0.730 to -0.091; p=0.012) (Table 2, Fig. 2A), sperm concentration (SMD, -0.416; 95% CI, -0.652 to -0.180; p=0.001) (Table 2, Fig. 2B), total motility (SMD, -0.605; 95% CI, -0.963 to -0.247; p=0.001) (Table 2, Fig. 2C), progressive sperm motility (SMD, -0.372; 95% CI, -0.740 to -0.003; p=0.048) (Table 2, Fig. 2D), and viability (SMD, -0.665; 95% CI, -1.268 to -0.061; p=0.031) (Table 2, Fig. 2E). However, the combined results showed that COVID-19 did not affect semen volume, nonprogressive sperm motility, immotility, and normal morphology.
Table 2. Effects of COVID-19 on sperm quality and sex hormones.
| All patients vs. healthy individuals | No. | p-valuea | SMD (95% CI) | I2 (%) | p-valueb | p-valuec |
|---|---|---|---|---|---|---|
| Semen volume (mL) | 9 | 0.931 | 0.023 (-0.502 to 0.549) | 94.8 | 0.903 | 0.630 |
| Total sperm count (×106) | 8 | 0.012 | -0.411 (-0.730 to -0.091) | 84.4 | 0.903 | 0.011 |
| Sperm concentration (×106/mL) | 11 | 0.001 | -0.416 (-0.652 to -0.180) | 73.2 | 0.528 | 0.015 |
| Total motility (%) | 7 | 0.001 | -0.605 (-0.963 to -0.247) | 73.9 | 0.677 | 0.702 |
| Progressive sperm motility (%) | 8 | 0.048 | -0.372 (-0.740 to -0.003) | 76.1 | >0.999 | 0.382 |
| Nonprogressive sperm motility (%) | 2 | 0.788 | -0.078 (-0.647 to -0.491) | 0.0 | 0.317 | - |
| Immotile sperm (%) | 3 | 0.166 | 0.310 (-0.129 to 0.749) | 25.3 | >0.999 | 0.890 |
| Normal morphology (%) | 3 | 0.312 | -0.508 (-1.493 to 0.477) | 97.3 | 0.807 | 0.695 |
| Viability (%) | 3 | 0.031 | -0.665 (-1.268 to -0.061) | 75.1 | 0.497 | 0.672 |
| Serum FSH (IU/L or mIU/mL) | 8 | 0.764 | 0.032 (-0.179 to 0.244) | 68.2 | 0.458 | 0.720 |
| Serum LH (IU/L or mIU/mL) | 8 | 0.121 | 0.360 (-0.095 to 0.814) | 93.4 | 0.805 | 0.435 |
| Serum PRL (ng/mL) | 4 | 0.022 | 0.305 (0.045 to 0.566) | 5.9 | >0.999 | 0.725 |
| Serum T (ng/mL or ng/dL) | 10 | 0.119 | -0.486 (-1.098 to 0.125) | 97.1 | 0.788 | 0.152 |
| Serum E2 (pg/mL) | 3 | 0.001 | 0.652 (0.254 to 1.049) | 71.9 | 0.602 | 0.620 |
No.: number of studies, SMD: standard mean difference, CI: confidence interval, FSH: follicle-stimulating hormone, LH: luteinizing hormone, PRL: prolactin, T: testosterone, E2: estradiol, -: not available.
ap-value of effect. bp-value of Begg’s test for publication bias. cp-value of Egger’s test for publication bias.
The bold values indicate statistical significance (p<0.05).
Fig. 2. Representative forest plots of effects of COVID-19 on sperm quality. (A) Total sperm count in the overall analysis. (B) Sperm concentration in the overall analysis. (C) Total motility in the overall analysis. (D) Progressive sperm motility in the overall analysis. (E) Viability in the overall analysis. (F) Total motility in subgroup analysis of recovery time more than 90 days. SMD: standard mean difference, CI: confidence interval, NAC: N-acetyl cysteine.
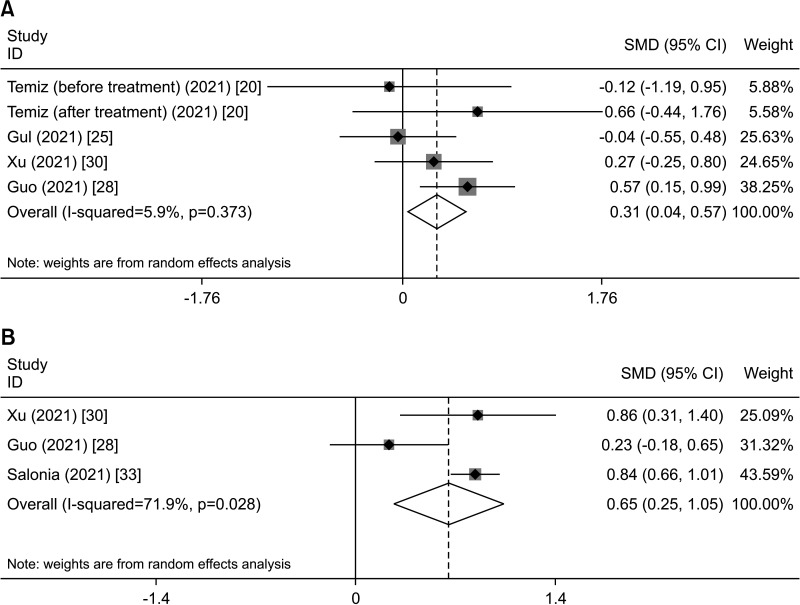
The combined results showed that COVID-19 increased prolactin (PRL) levels (SMD, 0.305; 95% CI, 0.045 to 0.566; p=0.022) (Table 2, Fig 3A) and estradiol (E2) levels (SMD, 0.652; 95% CI, 0.254 to 1.049; p=0.001) (Table 2, Fig. 3B). However, the combined results showed that COVID-19 did not affect follicle-stimulating hormone (FSH), luteinizing hormone (LH), or testosterone (T).
Fig. 3. Representative forest plots of effects of COVID-19 on sex hormones. (A) Serum PRL in the overall analysis. (B) Serum E2 in the overall analysis. SMD: standard mean difference, CI: confidence interval, PRL: prolactin, E2: estradiol.
Subgroup analyses were conducted based on age. The results showed that serum PRL were increased in <50 years old COVID-19 patients, and serum E2 were increased in ≥50 years old COVID-19 patients. The serum T were decreased in ≥50 years old COVID-19 patients. Regarding sperm quality, all included patients <50 years old and not suitable for the age-based subgroup analyses. The results of subgroup analyses are presented in Table 3.
Table 3. Subgroup analysis of effects of age of COVID-19 on sex hormones.
| Variable | Subgroup (y) | No. | p-valuea | SMD (95% CI) | I2 (%) | p-valueb | p-valuec |
|---|---|---|---|---|---|---|---|
| Serum FSH (IU/L or mIU/mL) | <50 | 5 | 0.897 | 0.011 (-0.154 to 0.176) | 0.0 | 0.142 | 0.200 |
| ≥50 | 3 | 0.920 | -0.026 (-0.523 to 0.472) | 90.5 | 0.602 | 0.741 | |
| Serum LH (IU/L or mIU/mL) | <50 | 5 | 0.235 | 0.433 (-0.310 to 1.176) | 93.1 | >0.999 | 0.010 |
| ≥50 | 3 | 0.452 | 0.239 (-0.384 to 0.861) | 93.9 | 0.602 | 0.571 | |
| Serum PRL (ng/mL) | <50 | 3 | 0.039 | 0.316 (0.015 to 0.616) | 29.1 | >0.999 | 0.483 |
| ≥50 | 1 | - | - | - | - | - | |
| Serum T (ng/mL or ng/dL) | <50 | 6 | 0.241 | -0.144 (-0.386 to 0.097) | 55.4 | 0.851 | 0.501 |
| ≥50 | 4 | 0.045 | -1.006 (-1.989 to -0.24) | 97.7 | 0.174 | 0.067 | |
| Serum E2 (pg/mL) | <50 | 1 | - | - | - | - | - |
| ≥50 | 2 | <0.001 | 0.838 (0.674 to 1.001) | 0.0 | 0.317 | - |
No.: number of studies, SMD: standard mean difference, CI: confidence interval, FSH: follicle-stimulating hormone, LH: luteinizing hormone, PRL: prolactin, T: testosterone, E2: estradiol, -: not available.
ap-value of effect. bp-value of Begg’s test for publication bias. cp-value of Egger’s test for publication bias.
The bold values indicate statistical significance (p<0.05).
Subgroup analyses were conducted on the control group. The results showed that sperm count and sperm concentration were both decreased in COVID-19 patients regardless of whether the healthy population or the patients themselves were negative for COVID-19. Regarding sex hormones, there were insufficient data for the subgroup analyses. The results of subgroup analyses are presented in Table 4.
Table 4. Subgroup analysis of effects of different control groups of COVID-19 on sperm quality and sex hormones.
| Variable | Subgroup | No. | p-valuea | SMD (95% CI) | I2 (%) | p-valueb | p-valuec |
|---|---|---|---|---|---|---|---|
| Volume (mL) | Healthy control | 4 | 0.068 | -0.022 (-0.47 to 0.02) | 25.3 | 0.652 | 0.868 |
| Self control | 5 | 0.604 | 0.256 (-0.71 to 1.22) | 97.7 | 0.573 | 0.502 | |
| Total sperm count (×106) | Healthy control | 5 | 0.001 | -0.315 (-0.508 to -0.123) | 0.0 | 0.099 | 0.069 |
| Self control | 3 | 0.023 | -0.597 (-1.112 to -0.081) | 91.6 | 0.327 | 0.153 | |
| Sperm concentration (×106/mL) | Healthy control | 7 | <0.001 | -0.339 (-0.516 to -0.162) | 0.0 | 0.180 | 0.323 |
| Self control | 4 | 0.005 | -0.547 (-0.932 to -0.161) | 85.6 | 0.348 | 0.107 | |
| Total motility (%) | Healthy control | 3 | 0.001 | -0.734 (-1.181 to -0.287) | 75.0 | 0.851 | 0.841 |
| Self control | 4 | 0.204 | -0.356 (-0.905 to 0.193) | 63.9 | 0.602 | 0.224 | |
| Progressive sperm motility (%) | Healthy control | 5 | 0.173 | -0.336 (-0.821 to 0.148) | 79.8 | 0.851 | 0.586 |
| Self control | 3 | 0.197 | -0.441 (-0.740 to 0.229) | 75.4 | 0.602 | 0.249 | |
| Nonprogressive sperm motility (%) | Healthy control | 1 | - | - | - | - | - |
| Self control | 1 | - | - | - | - | - | |
| Immotile sperm (%) | Healthy control | 2 | 0.420 | -0.027 (-0.647 to 0.593) | 0.0 | 0.117 | 0.033 |
| Self control | 1 | - | - | - | - | - | |
| Normal morphology (%) | Healthy control | 1 | - | - | - | - | - |
| Self control | 2 | 0.308 | -0.558 (-1.630 to 0.514) | 98.0 | 0.734 | 0.483 | |
| Viability (%) | Healthy control | 3 | 0.031 | -0.665 (-1.268 to -0.061) | 75.1 | 0.497 | 0.672 |
| Self control | 0 | - | - | - | - | - | |
| Serum FSH (IU/L or mIU/mL) | Healthy control | 6 | 0.905 | 0.015 (-0.238 to 0.269) | 72.2 | 0.573 | 0.684 |
| Self control | 2 | 0.659 | 0.088 (-0.304 to 0.480) | 0.0 | 0.317 | - | |
| Serum LH (IU/L or mIU/mL) | Healthy control | 6 | 0.110 | 0.440 (-0.099 to 0.978) | 95.0 | 0.851 | 0.663 |
| Self control | 2 | 0.654 | 0.090 (-0.302 to 0.482) | 0.0 | 0.317 | - | |
| Serum PRL (ng/mL) | Healthy control | 3 | 0.006 | 0.424 (0.122 to 0.726) | 0.0 | 0.497 | 0.571 |
| Self control | 1 | - | - | - | - | - | |
| Serum T (ng/mL or ng/dL) | Healthy control | 8 | 0.124 | -0.211 (-0.827 to 0.405) | 97.7 | 0.322 | 0.212 |
| Self control | 2 | 0.502 | -0.547 (-1.243 to 0.150) | 57.7 | 0.317 | - | |
| Serum E2 (pg/mL) | Healthy control | 3 | 0.001 | 0.652 (0.254 to 1.049) | 71.9 | 0.602 | 0.620 |
| Self control | 0 | - | - | - | - | - |
No.: number of studies, SMD: standard mean difference, CI: confidence interval, FSH: follicle-stimulating hormone, LH: luteinizing hormone, PRL: prolactin, T: testosterone, E2: estradiol, -: not available.
ap-value of effect. bp-value of Begg’s test for publication bias. cp-value of Egger’s test for publication bias.
The bold values indicate statistical significance (p<0.05).
4. Effects of difference stages of COVID-19 on sperm quality and sex hormones
We conducted a subgroup analysis to investigate the effects of different stages of COVID-19 on sperm quality and sex hormones. The results showed that during the illness stage of COVID-19, the infection decreased seminal volume (SMD, -0.755; 95% CI, -0.948 to -0.562; p<0.001) (Table 5). During the recovery stage of COVID-19 <90 days, sperm concentration (SMD, -0.271; 95% CI, -0.508 to -0.035; p=0.025) (Table 5) and total motility (SMD, -0.413; 95% CI, -0.664 to -0.163; p=0.001) (Table 5) decreased.
Table 5. Subgroup analysis of effects of different stages of COVID-19 on sperm quality and sex hormones.
| Variable | Subgroup | No. | p-valuea | SMD (95% CI) | I2 (%) | p-valueb | p-valuec |
|---|---|---|---|---|---|---|---|
| Volume (mL) | Period of illness stage | 2 | <0.001 | -0.755 (-0.948 to -0.562) | 0.0 | 0.317 | - |
| Recovery <90 d | 4 | 0.771 | -0.036 (-0.281 to 0.208) | 0.0 | 0.624 | 0.803 | |
| Recovery ≥90 d | 6 | 0.539 | 0.222 (-0.487 to 0.931) | 95.0 | >0.999 | 0.389 | |
| Total sperm count (×106) | Period of illness stage | 3 | 0.243 | -0.543 (-1.455 to 0.369) | 88.2 | 0.117 | 0.031 |
| Recovery <90 d | 4 | 0.162 | -0.180 (-0.432 to 0.072) | 0.0 | 0.497 | 0.472 | |
| Recovery ≥90 d | 5 | 0.074 | -0.327 (-0.686 to 0.032) | 79.9 | 0.099 | 0.099 | |
| Sperm concentration (×106/mL) | Period of illness stage | 4 | 0.127 | -0.465 (-1.064 to 0.133) | 80.5 | 0.042 | 0.068 |
| Recovery <90 d | 5 | 0.025 | -0.271 (-0.508 to -0.035) | 0.0 | 0.851 | 0.566 | |
| Recovery ≥90 d | 6 | 0.013 | -0.389 (-0.698 to -0.081) | 74.4 | 0.322 | 0.105 | |
| Total motility (%) | Period of illness stage | 1 | - | - | - | - | - |
| Recovery <90 d | 3 | 0.001 | -0.413 (-0.664 to -0.163) | 47.1 | >0.999 | 0.596 | |
| Recovery ≥90 d | 5 | 0.015 | -0.680 (-1.231 to -0.130) | 83.4 | 0.348 | 0.375 | |
| Progressive sperm motility (%) | Period of illness stage | 1 | - | - | - | - | - |
| Recovery <90 d | 4 | 0.884 | -0.054 (-0.773 to 0.666) | 83.1 | 0.491 | 0.979 | |
| Recovery ≥90 d | 5 | 0.086 | -0.363 (-0.779 to 0.052) | 72.0 | 0.348 | 0.645 | |
| Nonprogressive sperm motility (%) | Period of illness stage | 1 | - | - | - | - | - |
| Recovery <90 d | 1 | - | - | - | - | - | |
| Recovery ≥90 d | - | - | - | - | - | - | |
| Immotile sperm (%) | Period of illness stage | 1 | - | - | - | - | - |
| Recovery <90 d | 2 | 0.930 | -0.027 (-0.647 to 0593) | 0.0 | 0.117 | 0.033 | |
| Recovery ≥90 d | - | - | - | - | - | - | |
| Normal morphology (%) | Period of illness stage | 2 | 0.147 | -1.132 (-2.660 to 0.397) | 95.4 | 0.317 | - |
| Recovery <90 d | 2 | 0.969 | -0.007 (-0.380 to 0.365) | 0.0 | 0.317 | - | |
| Recovery ≥90 d | 2 | 0.963 | 0.004 (-0.184 to 0.193) | 0.0 | 0.297 | - | |
| Viability (%) | Period of illness stage | - | - | - | - | - | - |
| Recovery <90 d | 2 | 0.142 | -0.282 (-0.659 to 0.094) | 31.3 | 0.317 | - | |
| Recovery ≥90 d | 2 | 0.206 | -0.509 (-1.297 to 0.280) | 82.8 | 0.117 | 0.026 | |
| Serum FSH (IU/L or mIU/mL) | Period of illness stage | 4 | 0.303 | 0.175 (-0.158 to 0.507) | 76.4 | >0.999 | 0.789 |
| Recovery <90 d | 2 | 0.358 | -0.302 (-0.946 to 0.342) | 80.2 | 0.317 | - | |
| Recovery ≥90 d | 1 | - | - | - | - | - | |
| Serum LH (IU/L or mIU/mL) | Period of illness stage | 4 | 0.049 | 0.417 (0.002 to 0.833) | 84.9 | >0.999 | 0.946 |
| Recovery <90 d | 2 | 0.659 | 0.464 (-1.594 to 2.522) | 98.0 | 0.317 | - | |
| Recovery ≥90 d | 1 | - | - | - | - | - | |
| Serum PRL (ng/mL) | Period of illness stage | 1 | - | - | - | - | - |
| Recovery <90 d | 2 | 0.089 | 0.395 (-0.060 to 0.850) | 0.0 | 0.317 | - | |
| Recovery ≥90 d | 1 | - | - | - | - | - | |
| Serum T (ng/mL or ng/dL) | Period of illness stage | 4 | 0.038 | -1.021 (-1.986 to -0.055) | 96.9 | 0.497 | 0.204 |
| Recovery <90 d | 3 | 0.274 | -0.190 (-0.532 to 0.151) | 71.3 | 0.602 | 0.954 | |
| Recovery ≥90 d | 2 | 0.397 | -0.231 (-0.765 to 0.304) | 67.5 | 0.317 | - | |
| Serum E2 (pg/mL) | Period of illness stage | 1 | - | - | - | - | - |
| Recovery <90 d | 1 | - | - | - | - | - | |
| Recovery ≥90 d | - | - | - | - | - | - |
No.: number of studies, SMD: standard mean difference, CI: confidence interval, FSH: follicle-stimulating hormone, LH: luteinizing hormone, PRL: prolactin, T: testosterone, E2: estradiol, -: not available.
ap-value of effect. bp-value of Begg’s test for publication bias. cp-value of Egger’s test for publication bias.
The bold values indicate statistical significance (p<0.05).
During the recovery stage of COVID-19 >90 days, sperm concentration (SMD, -0.389; 95% CI, -0.698 to -0.081; p=0.013) (Table 5) and total motility (SMD, -0.680; 95% CI, -1.231 to -0.130; p=0.015) decreased (Table 5, Fig. 2F).
Regarding sex hormones, the subgroup analysis showed that during the illness stage of COVID-19, the LH level increased (SMD, 0.417; 95% CI, 0.002 to 0.833; p=0.049) (Table 5), and the T level decreased (SMD, -1.021; 95% CI, -1.986 to -0.055; p=0.038) (Table 5). In the recovery stage, COVID-19 did not affect sex hormone levels. However, studies on the effect of different stages of COVID-19 on sex hormones are limited. Therefore, these results should be interpreted cautiously.
5. Effects of COVID-19 with or without fever on sperm quality and sex hormones
Subgroup analysis was performed to investigate the effects of COVID-19 with or without fever on sperm quality and sex hormones. The results showed that COVID-19 with fever significantly reduced sperm concentration (SMD, -0.457; 95% CI, -0.847 to -0.067; p=0.022) and progressive sperm motility (SMD, -0.697; 95% CI, -1.229 to -0.164; p=0.010), but that this was not the case in the group without fever. Regarding sex hormones, there were insufficient data for the subgroup analyses. The results of subgroup analyses are shown in Table 6.
Table 6. Subgroup analysis of the effects of COVID-19 with and without fever on sperm quality and sex hormones.
| Variable | Subgroup | No. | p-valuea | SMD (95% CI) | I2 (%) | p-valueb | p-valuec |
|---|---|---|---|---|---|---|---|
| Volume (mL) | With fever | 2 | 0.272 | -0.335 (-0.933 to 0.263) | 34.6 | 0.317 | - |
| Without fever | 2 | 0.760 | 0.092 (-0.498 to 0.682) | 0.0 | 0.317 | - | |
| Total sperm count (×106) | With fever | 1 | - | - | - | - | - |
| Without fever | 1 | - | - | - | - | - | |
| Sperm concentration (×106/mL) | With fever | 4 | 0.022 | -0.457 (-0.847 to -0.067) | 0.0 | 0.497 | 0.486 |
| Without fever | 4 | 0.112 | -0.329 (-0.734 to 0.077) | 0.0 | 0.174 | 0.352 | |
| Total motility (%) | With fever | 3 | 0.085 | -0.817 (-1.746 to 0.111) | 74.2 | 0.117 | 0.187 |
| Without fever | 3 | 0.342 | -0.670 (-2.053 to 0.713) | 83.6 | 0.602 | 0.536 | |
| Progressive sperm motility (%) | With fever | 3 | 0.010 | -0.697 (-1.229 to -0.164) | 25.9 | 0.117 | 0.091 |
| Without fever | 3 | 0.314 | -0.560 (-1.649 to 0.530) | 75.7 | 0.602 | 0.794 |
No.: number of studies, SMD: standard mean difference, CI: confidence interval, -: not available.
ap-value of effect. bp-value of Begg’s test for publication bias. cp-value of Egger’s test for publication bias.
The bold values indicate statistical significance (p<0.05).
We performed subgroup analyses based on different stages among without fever infected individuals. The results showed that COVID-19 without fever >90 days, the sperm total motility and progressive sperm motility decreased. Regarding sex hormones, there was insufficient data for the subgroup analyses. The results of subgroup analyses are shown in Table 7.
Table 7. Subgroup analysis of the effects of COVID-19 without fever on sperm quality.
| Variable | Subgroup | No. | p-valuea | SMD (95% CI) | I2 (%) | p-valueb | p-valuec | |
|---|---|---|---|---|---|---|---|---|
| Volume (mL) | Without fever | ≥90 d | 2 | 0.760 | 0.092 (-0.498 to 0.682) | 0.0 | 0.317 | - |
| <90 d | 0 | - | - | - | - | - | ||
| Sperm concentration (×106/mL) | Without fever | ≥90 d | 2 | 0.299 | -0.331 (-0.955,0.294) | 0.0 | 0.317 | - |
| <90 d | 2 | 0.513 | -0.270 (-1.079,0.539) | 54.4 | 0.317 | - | ||
| Total motility (%) | Without fever | ≥90 d | 2 | 0.045 | -1.304 (-2.577,-0.032) | 66.3 | 0.317 | - |
| <90 d | 1 | - | - | - | - | - | ||
| Progressive sperm motility (%) | Without fever | ≥90 d | 2 | 0.002 | -1.089 (-1.764,-0.414) | 25.0 | 0.317 | - |
| <90 d | 1 | - | - | - | - | - | ||
No.: number of studies, SMD: standard mean difference, CI: confidence interval, -: not available.
ap-value of effect. bp-value of Begg’s test for publication bias. cp-value of Egger’s test for publication bias.
The bold values indicate statistical significance (p<0.05).
6. Effects of disease severity of COVID-19 on sperm quality and sex hormones
Subgroup analysis was performed to investigate the effects of disease severity of COVID-19 on sperm quality and sex hormones. The results showed that the moderate type of COVID-19 significantly reduced total sperm motility (SMD, -0.953; 95% CI, -1.618 to -0.289; p=0.005), but that this was not the case in the mild type. For sex hormones and the severity of COVID-19, there were insufficient data for subgroup analyses. The results of subgroup analyses are shown in Table 8.
Table 8. Subgroup analysis of the effects of COVID-19 disease severity on sperm quality and sex hormones.
| Variable | Subgroup | No. | p-valuea | SMD (95% CI) | I2 (%) | p-valueb | p-valuec |
|---|---|---|---|---|---|---|---|
| Volume (mL) | Mild type | 3 | 0.331 | -0.188 (-0.568 to 0.192) | 0.0 | 0.602 | 0.745 |
| Moderate type | 3 | 0.182 | -0.402 (-0.991 to 0.188) | 64.0 | 0.602 | 0.644 | |
| Total sperm count (×106) | Mild type | 2 | 0.274 | -0.247 (-0.690 to 0.196) | 0.0 | 0.317 | - |
| Moderate type | 2 | 0.161 | -0.217 (-0.520 to 0.086) | 0.0 | 0.317 | - | |
| Sperm concentration (×106/mL) | Mild type | 3 | 0.578 | -0.108 (-0.488 to 0.273) | 0.0 | 0.602 | 0.156 |
| Moderate type | 3 | 0.067 | -0.277 (-0.447 to 0.021) | 0.0 | 0.602 | 0.143 | |
| Total motility (%) | Mild type | 3 | 0.261 | -0.523 (-1.434 to 0.388) | 80.4 | 0.602 | 0.476 |
| Moderate type | 3 | 0.005 | -0.953 (-1.618 to -0.289) | 69.1 | 0.602 | 0.877 | |
| Progressive sperm motility (%) | Mild type | 3 | 0.574 | -0.205 (-0.918 to 0.508) | 69.1 | 0.602 | 0.074 |
| Moderate type | 3 | 0.516 | -0.309 (-1.242 to 0.624) | 89.1 | 0.317 | - |
No.: number of studies, SMD: standard mean difference, CI: confidence interval, -: not available.
ap-value of effect. bp-value of Begg’s test for publication bias. cp-value of Egger’s test for publication bias.
7. Sensitivity analyses
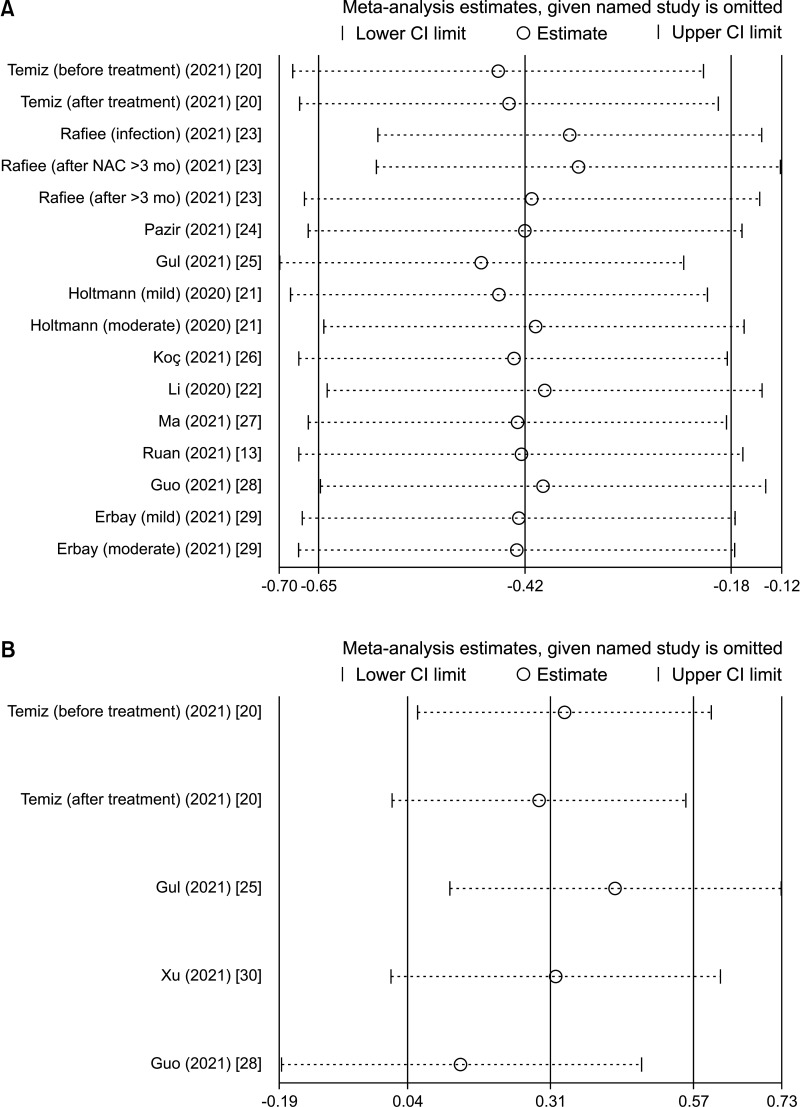
Sensitivity analyses of sperm quality (volume, sperm concentration [Fig. 4A], total sperm count, progressive sperm motility, non-progressive sperm motility, total motility, immotility, normal morphology, viability) and sex hormones (FSH, LH, PRL [Fig. 4B], T, and E2) were performed. The results showed that no single study influenced the total effects, indicating the robustness of the results of this study.
Fig. 4. Representative images of sensitivity analysis. (A) Sensitivity analysis of sperm concentration in overall analysis. (B) Sensitivity analysis of PRL in overall analysis. CI: confidence interval, NAC: N-acetyl cysteine, PRL: prolactin.
8. Publication bias
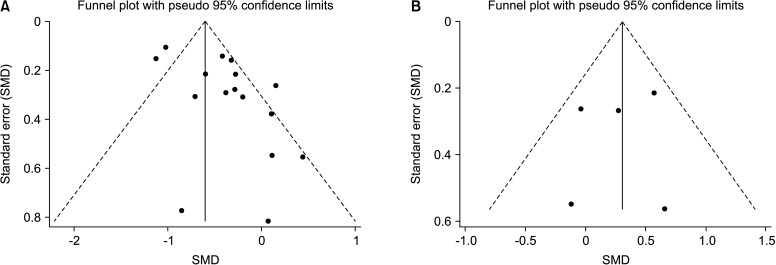
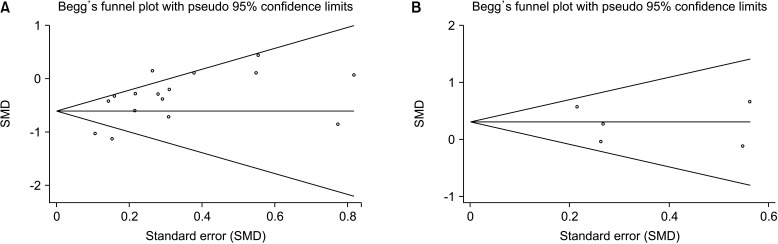
Funnel plots (Fig. 5) and Begg’s test (Fig. 6) did not provide evidence of publication bias for sperm quality (volume, sperm concentration, total sperm count, progressive sperm motility, nonprogressive sperm motility, total motility, immotility, normal morphology, and viability) and sex hormones (FSH, LH, PRL, T, and E2) (Table 2). In the subgroup analysis, Begg’s test and Egger’s test showed that publication bias existed in some results. These results indicated that a potential publication bias may exist in our meta-analysis.
Fig. 5. Representative images of funnel plot of publication bias. (A) Funnel plot of sperm concentration in overall analysis. (B) Funnel plot of PRL in overall analysis. SMD: standard mean difference, PRL: prolactin.
Fig. 6. Representative images of Begg’s funnel plot of publication bias. (A) Funnel plot of sperm concentration in overall analysis. (B) Funnel plot of PRL in overall analysis. SMD: standard mean difference, PRL: prolactin.
DISCUSSION
This meta-analysis investigated the association between SARS-CoV-2 infection and male fertility, and included 17 studies with a total of 1,627 patients and 1,535 healthy or COVID-19-negative controls. Overall, this study found that COVID-19 significantly reduced sperm quality and caused sex hormone disruption. Differences in the control populations may result in different baselines of fertility. Subgroup analyses were conducted on the basis of the control group population. And found that compared with the healthy population or with before and after COVID-19, the same results were seen, that COVID-19 could reduce sperm quality.
Subgroup analyses found that COVID-19 significantly caused sex hormone disruption in ≥50 or <50 years old. Because all the included patients with sperm quality examination were <50 years old and the data not suitable for the age-based subgroup analyses. And we found that at a recovery time of <90 days, COVID-19 significantly reduced sperm concentration and total motility. In a recovery time of ≥90 days, COVID-19 still significantly reduced the sperm concentration and total motility. Moreover, this study found that COVID-19 with fever was more likely to significantly reduce sperm concentration and progressive sperm motility than in cases without fever. To evaluate the association between COVID-19 infection and male fertility purely as rather than the association between COVID-19 infection as a febrile illness and male fertility, we performed subgroup analyses in without fever patients and found that COVID-19 without fever ≥90 days, the sperm total motility and progressive sperm motility decreased. The moderate type of COVID-19 significantly reduced total sperm motility compared to the mild type. Our meta-analysis revealed that the different symptoms and disease severity of COVID-19 had different effects on sperm quality. Because sufficient data on sex hormones was not available to include, data on sperm quality were limited. Therefore, our meta-analysis revealed that COVID-19 had long-term effects on sperm quality and sex hormone levels, mainly during the illness stage. These results remind us that more attention should be paid to the fertility of COVID-19 patients, especially for patients with high disease severity.
The 2019 SARS-CoV-2 outbreak has spread worldwide. The mortality rate of this infectious disease has been reported to be 1% to 4% in many countries, revealing the severity and pathogenicity of SARS-CoV-2 [21]. Although effective vaccines have been developed and many people have been vaccinated, controlling COVID-19 is still a challenge because of the emergence of new variants of SARS-CoV-2 [36]. An overwhelming amount of research has been published regarding COVID-19, but the multiple sequelae of SARS-CoV-2 infection are still poorly understood.
Therefore, we conducted this meta-analysis to investigate the effects of SARS-CoV-2 on male fertility during infection. A meta-analysis conducted by Tiwari et al [37] verified that COVID-19 impaired sperm quality and sex hormones. However, the study did not reveal a relationship between recovery time, symptoms, disease severity, and changes in sperm parameters. An increasing number of studies have confirmed that SARS-CoV-2 can impair the male reproductive system and have far-reaching effects on sperm quality of patients and their children [10]. The conclusions regarding the effects of SARS-CoV-2 on sperm quality and sex hormones are inconsistent. Our meta-analysis showed that COVID-19 significantly reduced sperm quality and caused sex hormone disruption.
Mechanistically, SARS-CoV-2 needs a receptor (ACE-2 or TMPRSS2) to enter the cell, but their expression is low in testicular tissue [38,39]; therefore, the incidence of SARS-CoV-2 entering testis tissues is low. Therefore, the prevailing hypothesis is that patients with mild illness and those in the recovery stage test negative for SARS-CoV-2. Previous studies have shown that patients tested positive for SARS-CoV-2 in their semen with small sample sizes; however, semen samples were vulnerable to contamination during collection [9,38,39]. In addition, in autopsied testicular samples of two men who died from COVID-19, SARS-CoV-2 was detected in testicular and Leydig cells [40].
Numerous studies have reported the effects of COVID-19 on sperm quality. We found that patients with fever and moderate COVID-19 were more likely to have reduced sperm quality than those without fever or mid-type COVID-19. Fever interferes with the physiology of normal reproduction, and fever of more than 39℃ for ≥3 days can lead to severe impairment of sperm quality [41]. Most COVID-19 patients have fever; therefore, increased testicular temperature may be one of the mechanisms through which SARS-CoV-2 impairs male reproduction. We performed subgroup analyses to evaluate the association between COVID-19 infection and male fertility in without fever patients and found that COVID-19 without fever ≥90 days, the sperm total motility and progressive sperm motility decreased. This results indicates that there are some other mechanisms that play a role in the biological processes that SARS-CoV-2 impairs sperm quality. Donders et al [12] found a strong correlation between anti-SARS-CoV-2 serum antibodies and sperm parameters. In autopsied samples, immunoglobulin (Ig) G antibodies were present in the seminiferous tubules, and increased concentrations of CD68+ and CD3+ were found in testicular tissue [22]. Therefore, the immune response may be one of the mechanisms through which COVID-19 impairs sperm quality. Another hypothesis is that the cytokine storm induced by COVID-19 is the mechanism of impaired sperm quality [42,43,44]. The effects of COVID-19 on sperm quality are complex and may be affected by multiple mechanisms.
It is well known that the recovery of sperm parameters to the basal level is a lengthy process of approximately 3 months. Therefore, we defined 90 days as the cut-off to study the immediate impact and long-term influence of COVID-19 on sperm quality. Our study revealed that COVID-19 had long-term effects on sperm quality, especially on sperm concentration and motility. The severity and duration of the abnormalities and the exact recovery time are unknown, and the effect of COVID-19 on future children is unknown.
In men, spermatogenesis is regulated by FSH, LH, and T, which are connected to the hypothalamus, pituitary gland, and gonad axis. Our results showed that, in terms of infection status, the effect of COVID-19 decreased T levels, expecially in the older men, and increased LH levels. These findings indicate primary hypogonadism. In the recovery stage, COVID-19 had no effect on T and LH levels, which may be due to the immunity, regulatory abilities, and compensatory capacities of the body overcoming the infection. In our study, we did not find that COVID-19 decreased FSH levels. Data on the effects of COVID-19 on sex hormones is limited. Therefore, these results should be interpreted cautiously. Many studies have verified that SARS-CoV-2 leads to the disturbance of sex hormones and that COVID-19 patients have lower T concentrations than healthy men [33,45,46,47]. T cells are primarily produced by Leydig cells and are stimulated by LH. Inflammatory cell infiltration has been observed in the testes of patients with severe COVID-19 [22,48,49], which demonstrated that in the acute phase, cytokines may lead to Leydig cell damage and decrease the response to LH stimulation [50]. Another possible explanation for lower T concentrations is that inflammatory mediators restrain the gonadal axis, decrease the testicular response, and increase the clearance of T during the acute stage of the disease [51,52]. In recent decades, studies have verified that T cells have a multitude of biological functions in the reproductive system and play an important anti-inflammatory role in preserving the number of regulatory T cells and CD8+ T cells [53,54]. Low T predicts an adverse clinical condition that may be due to the immune function of T deficiency. It has been shown that COVID-19 patients with lower T levels were more likely to have an increased risk of hospital admission, ventilator use, or death compared to those with normal T levels [46,47,55]. Additionally, some studies have verified that a worsening clinical stage correlates with lower T levels as well as higher LH levels [47].
Our meta-analysis results also showed that E2 levels were higher in COVID-19 patients, including those in the infection and recovery stages. E2 is an important sex hormone in male reproduction and is converted from T catalyzed by aromatase [56,57]. Aromatase is expressed in the testes, adipose tissue, bone, and brain [57]. During inflammation, aromatase is upregulated in adipose tissue, possibly stimulated by inflammatory cytokines. Thus, the conversion of T to E2 increases, resulting indecreasing T and increasing E2 concentrations [58,59]. Therefore, many studies have found that E2 levels are higher in COVID-19 patients than in healthy men and higher in severe COVID-19 patients than in those with milder clinical conditions [55]. In our meta-analysis, PRL levels in COVID-19 patients were higher than those in healthy men, which may be related to having multiple physiological or pathological conditions or medication, but the exact mechanism is still unclear. Therefore, hormone surveillance studies are needed to assess the damage caused and the recovery time of gonadal function from COVID-19 and the long-term consequences of the disease on sex hormone levels.
Low-quality studies were not included, which ensured that our meta-analysis was more rigorous. Funnel plots and Begg’s and Egger’s tests were used to evaluate publication bias, and partial analysis results showed that potential publication bias may have existed in our meta-analysis. Heterogeneity may also have existed in our study. However, we conducted sensitivity analyses, and the results showed that the trend did not change significantly, indicating that our conclusions were reliable.
Some limitations in our study should be mentioned: (1) few studies focused on different disease stages of COVID-19 and limited data on the association between disease stages and male fertility are available; (2) some parameters of sperm quality, such as DNA fragmentation, were not included in our study because few investigations have studied this parameter; (3) different variants of SARS-Cov-2 may have different effects on male fertility; (4) the heterogeneity of ethnicity of the population may exist; (5) technical laboratory measures of seminal alterations and T may not be standardized; and (6) most research of this meta-analysis comes from Asia. There was insufficient data for subgroup analysis by region and ethnicity, and there may be regional and race bias in the research results. In addition, we could not confirm the sex hormone levels measured in all studies early in the morning during fasting. Nonetheless, our meta-analysis consisted only of currently published data to maximize the inclusion of available data. The results of our study should remind clinicians to focus on the reproductive health of COVID-19 patients. Thus, it is crucial to conduct multicenter, larger, and long-term follow-up studies to determine the long-term effects of COVID-19 on male fertility.
CONCLUSIONS
COVID-19 in men significantly reduces sperm quality and causes sex hormone disruption. Infection with COVID-19 had long-term effects on sperm quality, especially sperm concentration and total motility. It is critical to conduct larger multicenter studies to determine the consequences of COVID-19 on male fertility.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Funding: This study was funded by the National Natural Science Funds of China (82171594) and the Zhao Yi-Cheng Medical Science Foundation (ZYYFY2018031).
- Conceptualization: XL, SW.
- Data curation: YP, LL, SN, FZ.
- Formal analysis: SW, AZ.
- Funding acquisition: XL.
- Investigation: SW, AZ.
- Methodology: YP, LL, SN, FZ.
- Project administration: XL, SW.
- Resources: SW, AZ.
- Software: SW, AZ, YP, LL, SN, FZ.
- Supervision: XL.
- Validation: XL, SW.
- Visualization: SW, AZ.
- Writing – original draft: SW, AZ.
- Writing – review & editing: SW, AZ, XL.
Data Sharing Statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.5534/wjmh.220091.
The Agency for Health Care Research and Quality (AHRQ) statement for cross-sectional study quality assessment
Newcastle–Ottawa Scale scores for literature quality assessment
References
- 1.Chauhan S. Comprehensive review of coronavirus disease 2019 (COVID-19) Biomed J. 2020;43:334–340. doi: 10.1016/j.bj.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng L, Liu L. Exploring posttraumatic growth after the COVID-19 pandemic. Tour Manag. 2022;90:104474. doi: 10.1016/j.tourman.2021.104474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19) J Pathol. 2020;251:228–248. doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sacks D, Baxter B, Campbell BCV, Carpenter JS, Cognard C, Dippel D, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke. 2018;13:612–632. doi: 10.1177/1747493018778713. [DOI] [PubMed] [Google Scholar]
- 5.Kumar P, Sah AK, Tripathi G, Kashyap A, Tripathi A, Rao R, et al. Role of ACE2 receptor and the landscape of treatment options from convalescent plasma therapy to the drug repurposing in COVID-19. Mol Cell Biochem. 2021;476:553–574. doi: 10.1007/s11010-020-03924-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verma S, Saksena S, Sadri-Ardekani H. ACE2 receptor expression in testes: implications in coronavirus disease 2019 pathogenesis†. Biol Reprod. 2020;103:449–451. doi: 10.1093/biolre/ioaa080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li D, Jin M, Bao P, Zhao W, Zhang S. Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Netw Open. 2020;3:e208292. doi: 10.1001/jamanetworkopen.2020.8292. Erratum in: JAMA Netw Open 2020;3:e2010845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delaroche L, Bertine M, Oger P, Descamps D, Damond F, Genauzeau E, et al. Evaluation of SARS-CoV-2 in semen, seminal plasma, and spermatozoa pellet of COVID-19 patients in the acute stage of infection. PLoS One. 2021;16:e0260187. doi: 10.1371/journal.pone.0260187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel DP, Hsieh TC. Can fever alone alter sperm parameters after severe acute respiratory syndrome coronavirus 2 infection? Fertil Steril. 2022;117:297. doi: 10.1016/j.fertnstert.2021.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo SP, Hsieh TC, Pastuszak AW, Hotaling JM, Patel DP. Effects of SARS CoV-2, COVID-19, and its vaccines on male sexual health and reproduction: where do we stand? Int J Impot Res. 2022;34:138–144. doi: 10.1038/s41443-021-00483-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donders GGG, Bosmans E, Reumers J, Donders F, Jonckheere J, Salembier G, et al. Sperm quality and absence of SARS-CoV-2 RNA in semen after COVID-19 infection: a prospective, observational study and validation of the SpermCOVID test. Fertil Steril. 2022;117:287–296. doi: 10.1016/j.fertnstert.2021.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruan Y, Hu B, Liu Z, Liu K, Jiang H, Li H, et al. No detection of SARS-CoV-2 from urine, expressed prostatic secretions, and semen in 74 recovered COVID-19 male patients: a perspective and urogenital evaluation. Andrology. 2021;9:99–106. doi: 10.1111/andr.12939. [DOI] [PubMed] [Google Scholar]
- 14.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: WHO; 2010. [Google Scholar]
- 16.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 17.Henriksen K, Dymek C, Harrison MI, Brady PJ, Arnold SB. Challenges and opportunities from the Agency for Healthcare Research and Quality (AHRQ) research summit on improving diagnosis: a proceedings review. Diagnosis (Berl) 2017;4:57–66. doi: 10.1515/dx-2017-0016. [DOI] [PubMed] [Google Scholar]
- 18.Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27:1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 19.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Temiz MZ, Dincer MM, Hacibey I, Yazar RO, Celik C, Kucuk SH, et al. Investigation of SARS-CoV-2 in semen samples and the effects of COVID-19 on male sexual health by using semen analysis and serum male hormone profile: a cross-sectional, pilot study. Andrologia. 2021;53:e13912. doi: 10.1111/and.13912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holtmann N, Edimiris P, Andree M, Doehmen C, Baston-Buest D, Adams O, et al. Assessment of SARS-CoV-2 in human semen-a cohort study. Fertil Steril. 2020;114:233–238. doi: 10.1016/j.fertnstert.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Xiao X, Zhang J, Zafar MI, Wu C, Long Y, et al. Impaired spermatogenesis in COVID-19 patients. EClinicalMedicine. 2020;28:100604. doi: 10.1016/j.eclinm.2020.100604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rafiee B, Bagher Tabei SM. The effect of N-acetyl cysteine consumption on men with abnormal sperm parameters due to positive history of COVID-19 in the last three months. Arch Ital Urol Androl. 2021;93:465–467. doi: 10.4081/aiua.2021.4.465. [DOI] [PubMed] [Google Scholar]
- 24.Pazir Y, Eroglu T, Kose A, Bulut TB, Genc C, Kadihasanoglu M. Impaired semen parameters in patients with confirmed SARS-CoV-2 infection: a prospective cohort study. Andrologia. 2021;53:e14157. doi: 10.1111/and.14157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gul A, Zengin S, Dundar G, Ozturk M. Do SARS-CoV-2 infection (COVID-19) and the medications administered for its treatment impair testicular functions? Urol Int. 2021;105:944–948. doi: 10.1159/000517925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koç E, Keseroğlu BB. Does COVID-19 worsen the semen parameters? Early results of a tertiary healthcare center. Urol Int. 2021;105:743–748. doi: 10.1159/000517276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma L, Xie W, Li D, Shi L, Ye G, Mao Y, et al. Evaluation of sex-related hormones and semen characteristics in reproductive-aged male COVID-19 patients. J Med Virol. 2021;93:456–462. doi: 10.1002/jmv.26259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo TH, Sang MY, Bai S, Ma H, Wan YY, Jiang XH, et al. Semen parameters in men recovered from COVID-19. Asian J Androl. 2021;23:479–483. doi: 10.4103/aja.aja_31_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erbay G, Sanli A, Turel H, Yavuz U, Erdogan A, Karabakan M, et al. Short-term effects of COVID-19 on semen parameters: a multicenter study of 69 cases. Andrology. 2021;9:1060–1065. doi: 10.1111/andr.13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu H, Wang Z, Feng C, Yu W, Chen Y, Zeng X, et al. Effects of SARS-CoV-2 infection on male sex-related hormones in recovering patients. Andrology. 2021;9:107–114. doi: 10.1111/andr.12942. [DOI] [PubMed] [Google Scholar]
- 31.Karkin K, Alma E. Erectile dysfunction and testosterone levels prior to COVID-19 disease: what is the relationship? Arch Ital Urol Androl. 2021;93:460–464. doi: 10.4081/aiua.2021.4.460. [DOI] [PubMed] [Google Scholar]
- 32.Okçelik S. COVID-19 pneumonia causes lower testosterone levels. Andrologia. 2021;53:e13909. doi: 10.1111/and.13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salonia A, Pontillo M, Capogrosso P, Gregori S, Tassara M, Boeri L, et al. Severely low testosterone in males with COVID-19: a case-control study. Andrology. 2021;9:1043–1052. doi: 10.1111/andr.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saad HM, GamalEl Din SF, Elbokl OM, Adel A. Predictive factors of erectile dysfunction in Egyptian individuals after contracting COVID-19: a prospective case-control study. Andrologia. 2022;54:e14308. doi: 10.1111/and.14308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cinislioglu AE, Cinislioglu N, Demirdogen SO, Sam E, Akkas F, Altay MS, et al. The relationship of serum testosterone levels with the clinical course and prognosis of COVID-19 disease in male patients: a prospective study. Andrology. 2022;10:24–33. doi: 10.1111/andr.13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang L, Li Q, Liang Z, Li T, Liu S, Cui Q, et al. The significant immune escape of pseudotyped SARS-CoV-2 variant Omicron. Emerg Microbes Infect. 2022;11:1–5. doi: 10.1080/22221751.2021.2017757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tiwari S, Kc N, Thapa S, Ghimire A, Bijukchhe S, Sah GS, et al. Semen parameters in men recovered from COVID-19: a systematic review and meta-analysis. Middle East Fertil Soc J. 2021;26:44. doi: 10.1186/s43043-021-00089-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anifandis G, Tempest HG, Oliva R, Swanson GM, Simopoulou M, Easley CA, et al. COVID-19 and human reproduction: a pandemic that packs a serious punch. Syst Biol Reprod Med. 2021;67:3–23. doi: 10.1080/19396368.2020.1855271. [DOI] [PubMed] [Google Scholar]
- 39.Pan F, Xiao X, Guo J, Song Y, Li H, Patel DP, et al. No evidence of severe acute respiratory syndrome-coronavirus 2 in semen of males recovering from coronavirus disease 2019. Fertil Steril. 2020;113:1135–1139. doi: 10.1016/j.fertnstert.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perry MJ, Arrington S, Neumann LM, Carrell D, Mores CN. It is currently unknown whether SARS-CoV-2 is viable in semen or whether COVID-19 damages spermatozoa. Andrology. 2021;9:30–32. doi: 10.1111/andr.12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jung A, Schuppe HC, Schill WB. [Fever as etiology of temporary infertility in the man] Hautarzt. 2001;52:1090–1093. doi: 10.1007/s001050170018. German. [DOI] [PubMed] [Google Scholar]
- 42.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020 Jul 28;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teixeira TA, Bernardes FS, Oliveira YC, Hsieh MK, Esteves SC, Duarte-Neto AN, et al. SARS-CoV-2 and multi-organ damage - what men's health specialists should know about the COVID-19 pathophysiology. Int Braz J Urol. 2021;47:637–646. doi: 10.1590/S1677-5538.IBJU.2020.0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Camici M, Zuppi P, Lorenzini P, Scarnecchia L, Pinnetti C, Cicalini S, et al. ReCoVeRi Study Group. Role of testosterone in SARS-CoV-2 infection: a key pathogenic factor and a biomarker for severe pneumonia. Int J Infect Dis. 2021;108:244–251. doi: 10.1016/j.ijid.2021.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Çayan S, Uğuz M, Saylam B, Akbay E. Effect of serum total testosterone and its relationship with other laboratory parameters on the prognosis of coronavirus disease 2019 (COVID-19) in SARS-CoV-2 infected male patients: a cohort study. Aging Male. 2020;23:1493–1503. doi: 10.1080/13685538.2020.1807930. [DOI] [PubMed] [Google Scholar]
- 47.Rastrelli G, Di Stasi V, Inglese F, Beccaria M, Garuti M, Di Costanzo D, et al. Low testosterone levels predict clinical adverse outcomes in SARS-CoV-2 pneumonia patients. Andrology. 2021;9:88–98. doi: 10.1111/andr.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duarte-Neto AN, Teixeira TA, Caldini EG, Kanamura CT, Gomes-Gouvêa MS, Dos Santos ABG, et al. Testicular pathology in fatal COVID-19: a descriptive autopsy study. Andrology. 2022;10:13–23. doi: 10.1111/andr.13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Satturwar S, Fowkes M, Farver C, Wilson AM, Eccher A, Girolami I, et al. Postmortem findings associated with SARS-CoV-2: systematic review and meta-analysis. Am J Surg Pathol. 2021;45:587–603. doi: 10.1097/PAS.0000000000001650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo H, Calkins JH, Sigel MM, Lin T. Interleukin-2 is a potent inhibitor of Leydig cell steroidogenesis. Endocrinology. 1990;127:1234–1239. doi: 10.1210/endo-127-3-1234. [DOI] [PubMed] [Google Scholar]
- 51.Russell SH, Small CJ, Stanley SA, Franks S, Ghatei MA, Bloom SR. The in vitro role of tumour necrosis factor-alpha and interleukin-6 in the hypothalamic-pituitary gonadal axis. J Neuroendocrinol. 2001;13:296–301. doi: 10.1046/j.1365-2826.2001.00632.x. [DOI] [PubMed] [Google Scholar]
- 52.Watanobe H, Hayakawa Y. Hypothalamic interleukin-1 beta and tumor necrosis factor-alpha, but not interleukin-6, mediate the endotoxin-induced suppression of the reproductive axis in rats. Endocrinology. 2003;144:4868–4875. doi: 10.1210/en.2003-0644. [DOI] [PubMed] [Google Scholar]
- 53.Page ST, Plymate SR, Bremner WJ, Matsumoto AM, Hess DL, Lin DW, et al. Effect of medical castration on CD4+ CD25+ T cells, CD8+ T cell IFN-gamma expression, and NK cells: a physiological role for testosterone and/or its metabolites. Am J Physiol Endocrinol Metab. 2006;290:E856–E863. doi: 10.1152/ajpendo.00484.2005. [DOI] [PubMed] [Google Scholar]
- 54.Vignozzi L, Cellai I, Santi R, Lombardelli L, Morelli A, Comeglio P, et al. Antiinflammatory effect of androgen receptor activation in human benign prostatic hyperplasia cells. J Endocrinol. 2012;214:31–43. doi: 10.1530/JOE-12-0142. Erratum in: J Endocrinol 2012;214:239. [DOI] [PubMed] [Google Scholar]
- 55.Dhindsa S, Zhang N, McPhaul MJ, Wu Z, Ghoshal AK, Erlich EC, et al. Association of circulating sex hormones with inflammation and disease severity in patients with COVID-19. JAMA Netw Open. 2021;4:e2111398. doi: 10.1001/jamanetworkopen.2021.11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nelson LR, Bulun SE. Estrogen production and action. J Am Acad Dermatol. 2001;45(3 Suppl):S116–S124. doi: 10.1067/mjd.2001.117432. [DOI] [PubMed] [Google Scholar]
- 57.Stocco C. Tissue physiology and pathology of aromatase. Steroids. 2012;77:27–35. doi: 10.1016/j.steroids.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spratt DI, Morton JR, Kramer RS, Mayo SW, Longcope C, Vary CP. Increases in serum estrogen levels during major illness are caused by increased peripheral aromatization. Am J Physiol Endocrinol Metab. 2006;291:E631–E638. doi: 10.1152/ajpendo.00467.2005. [DOI] [PubMed] [Google Scholar]
- 59.van den Berghe G, Weekers F, Baxter RC, Wouters P, Iranmanesh A, Bouillon R, et al. Five-day pulsatile gonadotropin-releasing hormone administration unveils combined hypothalamic-pituitary-gonadal defects underlying profound hypoandrogenism in men with prolonged critical illness. J Clin Endocrinol Metab. 2001;86:3217–3226. doi: 10.1210/jcem.86.7.7680. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The Agency for Health Care Research and Quality (AHRQ) statement for cross-sectional study quality assessment
Newcastle–Ottawa Scale scores for literature quality assessment
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.