ABSTRACT
Aims/Introduction
To evaluate the impact of the COVID‐19 pandemic on the glycemic control, eating habits, and body composition of people with diabetes mellitus; to identify the determinants of worsening glycemic control in people with diabetes mellitus.
Materials and methods
This retrospective, longitudinal observational study was performed in outpatients with diabetes mellitus who visited our hospital between April 2019 and March 2020 (pre‐COVID‐19 period) and continued for follow up from April 2020 to March 2021 (COVID‐19 period). We compared the glycemic control, nutritional intakes, and body composition of people with diabetes mellitus between the two periods. The changes in the HbA1c values (ΔHbA1c) and other study variables were compared between the two periods. Logistic regression analysis was performed to identify the factors associated with the increase of HbA1c levels.
Results
A significant increase of HbA1c was observed during the COVID‐19 period. The percent fat mass (FM) also increased, while the percent skeletal muscle mass (SMM) decreased during the COVID‐19 period. After adjustments for age and sex, the ΔBMI (OR:2.33), ΔFM (OR:1.45), and ΔSMM (OR:0.51) were identified as being associated with elevated levels of HbA1c.
Conclusions
The COVID‐19 pandemic had a negative impact on the glycemic control and body composition of people with diabetes mellitus. The increased body weight and FM and decreased SMM observed during the pandemic were associated with poor glycemic control in people with diabetes mellitus.
Keywords: COVID‐19, Diabetes, Glycemic control
The COVID‐19 pandemic had a negative impact on the glycemic control and body composition of people with diabetes mellitus. The increased body weight and fat mass and decreased skeletal muscle mass observed during the pandemic were associated with poor glycemic control in people with diabetes mellitus.
INTRODUCTION
The WHO declared coronavirus disease‐2019 (COVID‐19), a disease caused by the severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), a global pandemic in early 2020 1 . As of June 2022, more than 536 million cases of SARS‐CoV‐2 infections and more than 6 million deaths from the disease have been reported worldwide 2 . SARS‐CoV‐2 infects people of all age groups, but individuals aged over 65 years and those with comorbidities such as diabetes mellitus, chronic respiratory disease, and cardiovascular disease are at a higher risk of developing severe disease 3 . Several systematic reviews and meta‐analyses conducted to evaluate the prognosis of COVID‐19 in people with diabetes mellitus have reported an approximately two‐ to three‐fold higher risk of mortality from COVID‐19 in people with diabetes mellitus compared with those without diabetes mellitus 4 , 5 . A recent study reported the existence of an association of elevated hemoglobin A1C (HbA1c) levels with inflammation, hypercoagulability, and low oxygen saturation, and also with a higher mortality rate in COVID‐19 patients 6 . Therefore, maintaining optimal glycemic control is crucial in people with diabetes mellitus during the COVID‐19 pandemic.
In Japan, the first ever case of COVID‐19 was confirmed on January 15, 2020 7 . Japan declared a state of emergency, although request‐based, in major cities including Tokyo, from April 7 to May 25, 2020 and from January 8 to March 21, 2021. COVID‐19 and its preventive measures implemented to stop the spread transmission of COVID‐19 had greatly changed people's daily routines and annual events, denying them the opportunities to enjoy eating and drinking in groups, to exercise daily, or to commute or travel. Previous studies have confirmed that various lifestyle habits, such as eating habits, sedentary behaviors, and physical activity, were influenced by the COVID‐19 pandemic 8 , 9 , 10 . These changes could potentially have both positive and negative effects on people with diabetes mellitus. Moreover, a previous study using a nationwide claims database in Japan reported a transient decline in the number of physician visits by people with chronic diseases, including those with diabetes mellitus, decreasing immediately after the COVID‐19 outbreak and returning to the baseline by a month later 11 . On the other hand, a decrease in the adherence rates to treatments, such as medications, medical nutritional therapy, and physical activity, among people with diabetes mellitus during the COVID‐19 pandemic has been reported 12 , 13 , 14 . Several studies have reported a worsening of glycemic control and weight gain in people with diabetes mellitus during the COVID‐19 outbreak 15 , 16 , whereas others found no worsening of the glycemic control in people with diabetes mellitus during the COVID‐19 pandemic 17 , 18 . A previous study that investigated the changes in the dietary habits of people with type 2 diabetes during the COVID‐19 pandemic reported an increase in the intakes of vegetables, sugary foods, and snacks during the pandemic 19 . Most of these previous studies were conducted on a relatively small number of participants over short study periods, and focused primarily on the first wave of the COVID‐19 pandemic, and failed to evaluate the food habits and body composition of the participants. Therefore, we investigated the impact of the COVID‐19 pandemic and the state of emergency on the glycemic control, eating habits, and body composition of people with diabetes mellitus by comparing the variables between the period from April 2020 to March 2021 and the same period of the previous year. We also attempted to identify the determinants of worsening of the glycemic control during the pandemic in people with diabetes mellitus.
MATERIAL AND METHODS
Study design and participants
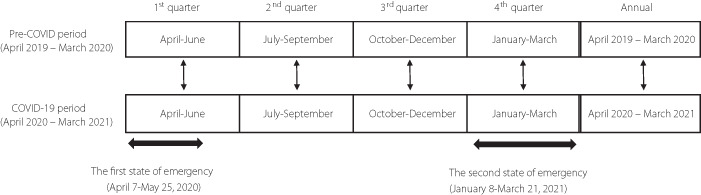
We performed a retrospective, longitudinal observational study in outpatients with diabetes mellitus who visited the University of Tokyo hospital between April 2019 and March 2020 and who were followed up from April 2020 to March 2021. In Japan, the first state of emergency for the COVID‐19 pandemic was enforced from April 7 to May 25, 2020, and the second from January 8 to March 21, 2021. In order to determine the influence of the COVID‐19 pandemic on the study endpoints, we categorized the period from April 2019 to March 2020 as the pre‐COVID‐19 period and the period from April 2020 to March 2021 as the COVID‐19 period. Furthermore, we divided each period into quarters (1st quarter: April–June; 2nd quarter: July–September; 3rd quarter: October–December; 4th quarter: January–March). The values in each quarter were compared for each participant. The mean values of the variables of glycemic control, anthropometric variables, and nutritional intakes were calculated for the entire period of 1 year of the pre‐COVID‐19 and COVID19 periods and for each quarter. The changes in the HbA1c values (ΔHbA1c) were compared between the pre‐COVID‐19 and COVID‐19 periods. The same method was applied to evaluate the changes in the anthropometric variables (Δbody mass index [BMI], Δfat mass, and Δskeletal muscle mass), and also the changes in the nutritional intakes (Δenergy intake, Δprotein intake, Δfat intake, Δcarbohydrate intake, and Δalcohol intake). We investigated the impact of the COVID‐19 pandemic and a state of emergency on the glycemic control, eating habits, and body composition of people with diabetes mellitus by comparing the variables between the corresponding quarters of the pre‐COVID‐19 and COVID‐19 periods (Figure 1). We also attempted to identify the determinants of the worsening of glycemic control.
Figure 1.
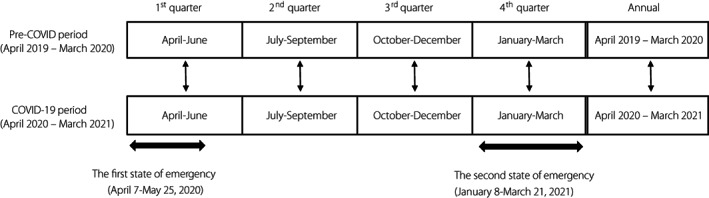
COVID‐19, coronavirus disease 2019.
The inclusion criterion was people with diabetes mellitus who had undergone medical examination and nutritional assessments, including dietary surveys and anthropometry, once or more during the pre‐COVID‐19 and COVID‐19 periods. The exclusion criteria were as follows: (1) under 18 years old, (2) pregnant women, (3) history of metabolic surgery, (4) new‐onset diabetes, (5) consumption of a special diet, and (6) history of treatment for diabetic ketoacidosis and hyperosmolar hyperglycemic syndrome during the study periods. Ethics approval for the study was obtained from the Research Ethics Committee of the University of Tokyo Hospital (2021068NI), in accordance with the principles of the Declaration of Helsinki; every effort was made to ensure patient anonymity.
Clinical and laboratory data
Clinical data such as the age, sex, antidiabetic medication history, HbA1c, blood glucose, and laboratory data were collected from electronic medical records maintained at the University of Tokyo hospital.
Anthropometric data
The body height, weight, and composition of the participants were measured at every visit. The BMI was calculated as the body weight (in kilograms) divided by the body height (in meters) squared. Body composition was measured using a Bioelectrical Impedance Analysis system (InBody 720, InBody Japan) that measures the body weight, skeletal muscle mass, appendicular muscle mass, trunk muscle mass, and fat mass of participants. The system was used to measure separately the impedance in the participants' right arm, left arm, trunk, right leg, and left leg at six different frequencies (1, 5, 50, 250, 500, and 1,000 kHz) for each body segment. Body composition variables determined were the percent fat mass, percent skeletal muscle mass, percent upper limb mass, percent trunk muscle mass, and percent lower limb muscle mass divided by the body weight.
Nutritional data
Dietary intakes were evaluated using a 24 h dietary recall by a dietitian. Nutritional intakes were calculated using the ‘Asuken’ website (http://www.asken.jp/). Users selected dishes from a list of approximately 100,000 dishes and foods, and input the serving sizes that they consumed. The nutrient intakes were calculated on the basis of the weight before cooking using the Standard Tables of Food Composition in Japan 2015 20 . For processed foods, the nutrient content data provided by the manufacturer were used.
Statistical analysis
Descriptive statistics are shown by mean ± standard deviation (SD) for continuous variables and the number of cases (proportion) for categorical variables. Distributional assumption of normality in continuous variables was assessed using the Shapiro–Wilk test. Mean differences were compared between pre‐COVID‐19 and COVID‐19 periods by paired t‐test for variables with normal distribution and by Wilcoxon's signed rank test for variables with non‐normal distribution. The mean variation of the HbA1c was calculated as the ‘annual mean HbA1c during the COVID‐19 period (%) ‐ annual mean HbA1c in the pre‐COVID period (%)’, and it was 0.07%. The participants were divided into two groups according to the mean variation of the HbA1c in the pre‐COVID‐19 period and during the COVID‐19 period of 0.07%: those with a > 0.07% increase of the HbA1c value were classified into the Worsen group and the remaining participants were classified into the Steady group. Comparisons between groups were performed by Student's t‐test for continuous variables with a normal distribution or the Mann–Whitney U‐test for continuous variables with a non‐normal distribution, and by the chi‐square test for categorical variables. Logistic regression analysis was performed to explore the factors associated with an increase of the HbA1c. To determine if the observed associations were independent of age and sex, a multivariate logistic regression analysis was performed using age and sex as the covariates 21 , 22 . A value of P < 0.05 was considered as being indicative of statistical significance. All statistical analyses were performed using SPSS, version 28.
RESULTS
Participants' characteristics
The basic characteristics of the study participants are presented in Table 1. A total of 408 participants were included in this study, including 239 men (58.6%) and 169 women (41.4%), with a mean age of 60.5 ± 12.9 years. People with type 2 diabetes mellitus were predominant in this study (96.8%). Seventy‐five participants (18.4%) were receiving insulin therapy, 44.4% were receiving biguanides, and 44.9% were receiving dipeptidyl‐peptidase 4 (DPP‐4) inhibitors.
Table 1.
Basic characteristics of this study
| Variable | Data (n = 408) |
|---|---|
| Age (years) | 60.5 ± 12.9 |
| Male/Female, n, (%) | 239/169 (58.6/41.4) |
| Diabetes type | |
| Type 1 diabetes n, (%) | 13 (3.2) |
| Type 2 diabetes n, (%) | 395 (96.8) |
| Medication | |
| Insulin use, n, (%) | 75 (18.4) |
| Sulphonylurea, n, (%) | 44 (10.8) |
| Glinides, n, (%) | 33 (8.1) |
| α‐Glucosidase inhibitors, n, (%) | 65 (15.9) |
| Thiazolidinediones, n, (%) | 46 (11.3) |
| Biguanides, n, (%) | 181 (44.4) |
| DPP‐4 inhibitors, n, (%) | 183 (44.9) |
| SGLT‐2 inhibitors, n, (%) | 119 (29.2) |
| GLP‐1 receptor agonist, n, (%) | 51 (12.5) |
Data are expressed as mean ± SD, number (%).
DPP‐4, dipeptidyl‐peptidase‐IV; SGLT‐2, sodium‐glucose transporter‐2; GLP‐1, glucagon‐like peptide 1 receptor.
Effects of the COVID‐19 pandemic on the clinical and laboratory variables
Comparison of the changes in the clinical and laboratory variables during the pre‐COVID‐19 and COVID‐19 periods are shown in Table 2. The HbA1c showed a significantly greater increase in the 2nd, 3rd, and 4th quarters of the COVID‐19 period compared with the corresponding quarters of the pre‐COVID‐19 period. The annual mean HbA1c was also significantly higher in the COVID‐19 period (the HbA1c increased from 7.12 ± 0.90% to 7.19 ± 1.01%, P = 0.010). No significant changes of the annual mean total cholesterol and triglyceride levels were observed in the COVID‐19 period. While the annual means of the serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma‐glutamyl transpeptidase (γ‐GTP) remained unchanged during the COVID‐19 period, the serum ALT was significantly higher in the 2nd quarter, and the serum γ‐GTP was significantly lower in the 4th quarter of the COVID‐19 period. Systolic blood pressure was significantly higher in the 1st and 2nd quarters of the COVID‐19 period. The annual mean systolic blood pressure was also significantly higher in the COVID‐19 period, while the annual mean diastolic blood pressure remained unchanged during the COVID‐19 period.
Table 2.
Comparison of the clinical and laboratory variables, the body composition, and the nutritional intakes between the pre‐COVID‐19 and COVID‐19 periods
| Period | 1st quarter (April–June) | 2nd quarter (July–September) | 3rd quarter (October–December) | 4th quarter (January–March) | Annual | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| mean ± SD | P | mean ± SD | P | mean ± SD | P | mean ± SD | P | mean ± SD | P | ||
| Clinical and laboratory variables | |||||||||||
| HbA1c (%) | Pre‐COVID‐19 | 7.18 ± 0.93 | 0.317 | 7.04 ± 0.91 | <0.001* | 7.13 ± 1.01 | 0.009 | 7.20 ± 0.99 | <0.001 | 7.12 ± 0.90 | 0.010 |
| COVID‐19 | 7.22 ± 1.10 | 7.16 ± 1.08 | 7.19 ± 1.11 | 7.28 ± 1.11 | 7.19 ± 1.01 | ||||||
| Total cholesterol (mg/dL) | Pre‐COVID‐19 | 192.73 ± 34.72 | 0.929 | 190.65 ± 37.09 | 0.023 | 189.17 ± 34.14 | 0.009 | 193.18 ± 36.41 | 0.504 | 192.75 ± 32.32 | 0.968 |
| COVID‐19 | 191.65 ± 36.85 | 186.09 ± 36.33 | 192.08 ± 35.29 | 191.37 ± 33.77 | 190.99 ± 31.75 | ||||||
| Triglycerides (mg/dL) | Pre‐COVID‐19 | 171.42 ± 187.78 | 0.823 | 171.81 ± 157.23 | 0.291 | 161.33 ± 131.18 | 0.184 | 170.26 ± 162.67 | 0.786 | 167.75 ± 142.34 | 0.325 |
| COVID‐19 | 170.14 ± 193.47 | 172.65 ± 198.12 | 156.63 ± 124.26 | 157.75 ± 101.74 | 164.16 ± 135.88 | ||||||
| AST (IU/L) | Pre‐COVID‐19 | 26.54 ± 12.87 | 0.690 | 27.22 ± 15.14 | 0.051 | 27.21 ± 14.60 | 0.767 | 27.49 ± 13.81 | 0.070 | 27.55 ± 14.22 | 0.722 |
| COVID‐19 | 26.88 ± 13.64 | 28.64 ± 17.29 | 27.26 ± 14.96 | 26.70 ± 16.40 | 28.04 ± 15.68 | ||||||
| ALT (IU/L) | Pre‐COVID‐19 | 30.92 ± 21.10 | 0.690 | 30.76 ± 22.19 | 0.020 | 31.13 ± 23.74 | 0.202 | 31.49 ± 23.18 | 0.799 | 31.50 ± 21.95 | 0.167 |
| COVID‐19 | 31.18 ± 22.52 | 32.53 ± 24.74 | 31.36 ± 22.56 | 30.91 ± 26.09 | 32.24 ± 24.12 | ||||||
| γ‐GTP (IU/L) | Pre‐COVID‐19 | 47.97 ± 49.03 | 0.746 | 49.10 ± 52.15 | 0.645 | 49.37 ± 56.90 | 0.330 | 49.66 ± 56.08 | 0.020 | 49.07 ± 51.84 | 0.412 |
| COVID‐19 | 48.12 ± 59.02 | 54.37 ± 82.57 | 47.69 ± 51.41 | 46.03 ± 57.57 | 49.71 ± 62.31 | ||||||
| Systolic blood pressure (mmHg) | Pre‐COVID‐19 | 135.36 ± 18.52 | 0.023 | 134.15 ± 18.00 | 0.024 | 135.67 ± 18.28 | 0.063 | 136.96 ± 17.75 | 0.320 | 135.50 ± 15.98 | 0.020 |
| COVID‐19 | 137.71 ± 18.29 | 136.14 ± 17.61 | 137.41 ± 18.11 | 136.02 ± 16.35 | 136.61 ± 16.09 | ||||||
| Diastolic blood pressure (mmHg) | Pre‐COVID‐19 | 77.65 ± 13.21 | 0.311 | 77.57 ± 11.87 | 0.721 | 76.57 ± 12.33 | 940 | 78.18 ± 12.44 | 0.023 | 77.50 ± 11.16 | 0.464 |
| COVID‐19 | 78.35 ± 12.01 | 77.35 ± 11.82 | 76.61 ± 12.78 | 76.81 ± 12.42 | 77.24 ± 11.38 | ||||||
| Body composition | |||||||||||
| BMI (kg/m2) | Pre‐COVID‐19 | 28.61 ± 6.48 | 0.122 | 28.47 ± 6.48 | 0.538 | 28.65 ± 6.44 | 0.225 | 28.36 ± 6.35 | 0.909 | 28.49 ± 6.31 | 0.756 |
| COVID‐19 | 28.48 ± 6.39 | 28.41 ± 6.56 | 28.71 ± 6.58 | 28.38 ± 6.49 | 28.48 ± 6.38 | ||||||
| Fat mass (%) | Pre‐COVID‐19 | 33.79 ± 9.44 | 0.025 | 33.76 ± 9.94 | <0.001 | 34.70 ± 9.41 | 0.002 | 34.30 ± 9.53 | <0.001 | 34.59 ± 9.52 | <0.001 |
| COVID‐19 | 34.14 ± 9.28 | 34.21 ± 10.01 | 35.07 ± 9.53 | 34.86 ± 9.45 | 34.95 ± 9.53 | ||||||
| Skeletal muscle mass (%) | Pre‐COVID‐19 | 36.16 ± 5.38 | 0.003 | 36.14 ± 5.70 | <0.001 | 35.63 ± 5.39 | <0.001 | 35.89 ± 5.47 | <0.001 | 35.65 ± 5.45 | <0.001 |
| COVID‐19 | 35.91 ± 5.31 | 35.83 ± 5.73 | 35.40 ± 5.48 | 35.54 ± 5.46 | 35.41 ± 5.46 | ||||||
| Upper limb muscle mass (%) | Pre‐COVID‐19 | 7.07 ± 1.12 | 0.613 | 7.05 ± 1.21 | 0.007 | 6.97 ± 1.10 | 0.863 | 7.05 ± 1.13 | 0.006 | 6.95 ± 1.14 | 0.134 |
| COVID‐19 | 7.06 ± 1.11 | 7.01 ± 1.21 | 6.97 ± 1.15 | 7.01 ± 1.14 | 6.94 ± 1.15 | ||||||
| Trunk muscle mass (%) | Pre‐COVID‐19 | 29.49 ± 3.85 | 0.948 | 29.46 ± 4.13 | 0.290 | 29.24 ± 3.80 | 0.736 | 29.56 ± 3.88 | 0.031 | 29.25 ± 3.89 | 0.548 |
| COVID‐19 | 29.49 ± 3.85 | 29.39 ± 4.14 | 29.22 ± 3.89 | 29.44 ± 3.85 | 29.23 ± 3.92 | ||||||
| Lower limb muscle mass (%) | Pre‐COVID‐19 | 20.75 ± 3.34 | 0.001 | 20.84 ± 3.51 | 0.004 | 20.38 ± 3.38 | <0.001 | 20.41 ± 3.41 | <0.001 | 20.43 ± 3.37 | <0.001 |
| COVID‐19 | 20.54 ± 3.26 | 20.68 ± 3.55 | 20.19 ± 3.39 | 20.22 ± 3.37 | 20.26 ± 3.34 | ||||||
| Nutritional intakes | |||||||||||
| Energy (kcal) | Pre‐COVID‐19 | 1793.43 ± 520.95 | 0.638 | 1768.30 ± 449.15 | 0.795 | 1729.58 ± 460.46 | 0.243 | 1747.71 ± 463.36 | 0.096 | 1732.67 ± 391.36 | 0.262 |
| COVID‐19 | 1817.49 ± 497.39 | 1769.29 ± 441.76 | 1762.33 ± 449.49 | 1714.00 ± 470.92 | 1750.20 ± 393.97 | ||||||
| Protein (% Energy) | Pre‐COVID‐19 | 15.38 ± 3.44 | 0.011 | 15.14 ± 3.02 | 0.242 | 15.09 ± 3.54 | 0.021 | 14.90 ± 3.22 | 0.236 | 15.24 ± 2.65 | 0.996 |
| COVID‐19 | 14.71 ± 2.91 | 15.05 ± 3.26 | 15.50 ± 3.65 | 15.35 ± 3.58 | 15.24 ± 2.61 | ||||||
| Fat (% Energy) | Pre‐COVID‐19 | 29.93 ± 7.53 | 0.980 | 29.77 ± 7.65 | 0.636 | 29.37 ± 7.38 | 0.029 | 29.06 ± 8.07 | 0.358 | 29.62 ± 5.99 | 0.172 |
| COVID‐19 | 29.87 ± 7.33 | 30.04 ± 7.69 | 30.64 ± 7.80 | 29.66 ± 7.40 | 29.87 ± 5.93 | ||||||
| Carbohydrate (% Energy) | Pre‐COVID‐19 | 51.15 ± 9.96 | 0.099 | 51.89 ± 8.89 | 0.817 | 52.45 ± 8.77 | 0.008 | 52.53 ± 9.07 | 0.115 | 51.53 ± 7.6 | 0.717 |
| COVID‐19 | 52.37 ± 7.97 | 51.75 ± 9.05 | 50.76 ± 8.17 | 51.53 ± 8.07 | 51.4 ± 7.17 | ||||||
| Alcohol (g) | Pre‐COVID‐19 | 13.94 ± 28.80 | 0.076 | 10.19 ± 21.02 | 0.749 | 8.32 ± 14.42 | 0.047 | 9.86 ± 18.25 | 0.479 | 9.52 ± 19.66 | 0.042 |
| COVID‐19 | 10.65 ± 25.75 | 8.91 ± 17.10 | 7.79 ± 16.19 | 9.49 ± 17.22 | 8.81 ± 19.03 | ||||||
Data are expressed as mean ± SD.*P‐values were calculated by Wilcoxon's signed rank test; **P‐values were calculated by paired t‐test.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; COVID‐19, coronavirus disease 2019; γ‐GTP, gamma‐glutamyl transpeptidase; HbA1c, hemoglobin A1c.
Effects of the COVID‐19 pandemic on the body composition
Comparisons of the body composition during the pre‐COVID‐19 and COVID‐19 periods are shown in Table 2. There was no significant difference in the BMI between the two periods. The percent of fat mass values during the 1 year period, as well as all in the quarters, were significantly higher in the COVID‐19 period. In contrast, the values of the percent skeletal muscle mass during the 1 year period, as well as in all the quarters, were significantly lower during the COVID‐19 period. Consistent with this, the values of the percent lower limb muscle mass in the 1 year period, as well as in all the quarters, were significantly lower in the COVID‐19 period. The values of the percent upper limb and trunk muscle masses were significantly lower in some quarters, whereas the annual mean percent muscle mass remained unchanged, in the COVID‐19 period.
Effects of the COVID‐19 pandemic on nutritional intake
Comparisons of the nutritional intakes in the pre‐COVID‐19 and COVID‐19 periods are shown in Table 2. The energy intake remained unchanged throughout the study period. While the energy intake ratios (%E) as protein, fat, and carbohydrate were significantly changed in several quarters, there were no significant differences in the annual means of the values between the pre‐COVID‐19 and COVID‐19 periods. On the other hand, a significant decrease of the alcohol intake level was observed in the 3rd quarter of the COVID‐19 period compared with the pre‐COVID‐19 period. The annual mean alcohol intake level was also significantly lower in the COVID‐19 period.
The factors associated with worsening of the glycemic control during the COVID‐19 pandemic
Comparison of the clinical characteristics and variations of the body composition and nutritional intakes between participants in whom relatively stable glycemic control was maintained during the COVID‐19 period (Steady group) and participants in whom the glucose control worsened (Worsen group) is shown in Table 3. In the Worsen group, the mean values of the BMI and percent fat mass in the pre‐COVID‐19 period was higher than those in the Steady group (P = 0.048, P = 0.042). Significant differences were also observed in the ΔBMI, Δfat mass, and Δskeletal muscle mass between the two groups (P < 0.001, respectively).
Table 3.
Comparison of the clinical characteristics and variations of the body composition and nutritional intakes between participants in whom a relatively stable glucose control was maintained (Steady) and participants in whom the glucose control worsened (Worsen) during the COVID‐19 period
| Steady (n = 208) | Worsen (n = 200) | P value | |
|---|---|---|---|
| Age | 61.49 ± 12.86 | 59.47 ± 12.8 | 0.124 |
| Male (%) | 129 (62.0) | 110 (55.0) | 0.160 |
| Type 2 diabetes n, (%) | 202 (97.1) | 193 (96.5) | 0.784 |
| Insulin use (%) | 38 (18.3) | 37 (18.5) | 1.00 |
| Mean pre‐COVID‐19 BMI (kg/m2) | 27.81 ± 5.74 | 29.20 ± 6.80 | 0.048 |
| Mean pre‐COVID‐19 fat mass (%) | 33.65 ± 9.66 | 35.57 ± 9.29 | 0.042 |
| Mean pre‐COVID‐19 skeletal muscle mass (%) | 36.15 ± 5.49 | 35.13 ± 5.38 | 0.058 |
| ΔBMI (kg/m2) | −0.41 ± 1.33 | 0.39 ± 0.92 | <0.001 |
| Δfat mass (%) | −0.28 ± 2.34 | 1.02 ± 1.78 | <0.001 |
| Δskeletal muscle mass (%) | 0.09 ± 1.23 | −0.59 ± 0.97 | <0.001 |
| Δenergy (kcal) | −14.42 ± 323.96 | 50.76 ± 390.09 | 0.096 |
| Δprotein (% Energy) | 0.09 ± 2.94 | −0.09 ± 2.44 | 0.524 |
| Δfat (% Energy) | 0.14 ± 6.9 | 0.36 ± 6.33 | 0.732 |
| Δcarbohydrate (% Energy) | −0.2 ± 6.88 | −0.06 ± 7.25 | 0.975 |
| Δalcohol (g) | −0.74 ± 14.48 | −0.69 ± 12.13 | 0.739 |
Data are expressed as mean ± SD.
*P‐values were calculated by Mann–Whitney U‐test.
BMI, body mass index; COVID‐19, coronavirus disease 2019.
The results of the logistic regression analysis are shown in Table 4. The mean values of the BMI and percent fat mass, the ΔBMI, and Δfat mass in the COVID‐19 period were associated with increasing odds ratios (OR) of HbA1c elevation, while the Δskeletal muscle mass during the period was associated with a reduced OR of HbA1c elevation. We next conducted a multivariate analysis with adjustments for potential confounding variables (age and sex) 21 , 22 , which identified ΔBMI (adjusted OR: 2.33, 95% confidence interval [CI]: 1.78–3.05), Δfat mass (adjusted OR:1.45, 95% CI: 1.27–1.65), and Δskeletal muscle mass (adjusted OR: 0.51, 95% CI: 0.41–0.65) as significant factors of HbA1c elevation (P < 0.001, respectively).
Table 4.
Logistic regression analysis to determine the associated factors for increase of the HbA1c
| Odds ratio (95% CI) | P | Adjusted odds ratio (95% CI) | P* | ||||
|---|---|---|---|---|---|---|---|
| Age | 0.99 | (0.97–1.00 | 0.114 | ||||
| Sex (Female) | 1.34 | (0.90–1.98) | 0.151 | ||||
| Insulin use (%) | 1.02 | (0.62–1.68) | 0.952 | ||||
| Mean pre‐COVID‐19 BMI (kg/m2) | 1.03 | (1.00–1.07) | 0.027 | 1.03 | (0.99 | 1.06) | 0.117 |
| Mean pre‐COVID‐19 fat mass (%) | 1.02 | (1.00–1.04) | 0.042 | 0.99 | (0.97 | 1.01) | 0.212 |
| Mean pre‐COVID‐19 skeletal muscle mass (%) | 1.01 | (0.97–1.04) | 0.733 | ||||
| ΔBMI (kg/m2) | 2.30 | (1.77–2.99) | <0.001 | 2.33 | (1.78–3.05) | <0.001 | |
| Δfat mass (%) | 1.44 | (1.27–1.63) | <0.001 | 1.45 | (1.27–1.65) | <0.001 | |
| Δskeletal muscle mass (%) | 0.53 | (0.42–0.66) | <0.001 | 0.51 | (0.41–0.65) | <0.001 | |
| Δenergy (kcal) | 1.00 | (1.00–1.00) | 0.068 | ||||
| Δprotein (% Energy) | 0.98 | (0.91–1.05) | 0.523 | ||||
| Δfat (% Energy) | 1.00 | (0.98–1.04) | 0.731 | ||||
| Δcarbohydrate (% Energy) | 1.00 | (0.98–1.03) | 0.850 | ||||
| Δalcohol (g) | 1.00 | (0.99–1.02) | 0.971 | ||||
*Adjusted for the age and sex.
BMI, body mass index; CI, confidence interval; COVID‐19, coronavirus disease 2019; HbA1c, hemoglobin A1c.
DISCUSSION
COVID‐19 still continues to impact the world, since the first case of infection was confirmed in December 2019 in China, and many countries have repeatedly introduced lockdowns and other such measures to curb the spread of the infection. In this study, we investigated the impact of the COVID‐19 pandemic on the glycemic control, eating habits, and body composition of people with diabetes mellitus by comparing the changes in the variables between the COVID‐19 pandemic period in the fiscal year 2020, which included the first and second periods of emergency declaration in Japan, with those in the previous year in Japan. Furthermore, we divided each year into quarters and compared the changes in the corresponding quarters of the previous year, so as to take into account the effects of the season and duration of implementation of a state of emergency. To the best of our knowledge, this is the first longitudinal study investigating the effects of the COVID‐19 pandemic in people with diabetes mellitus.
The present study revealed a significant increase of the HbA1c level in people with diabetes mellitus during the COVID‐19 pandemic; the changes in the body weight and percent fat and skeletal muscle masses, and not the absolute values of these variables, were identified as independent associated factors for elevated levels of HbA1c, after adjustments for age and sex. Considering that there were no significant differences in the nutritional intakes, these changes in the percent fat and skeletal muscle masses during the COVID‐19 period could have been responsible for the decreased physical activity. Several studies reported decreased physical activity of the lower extremities, such as decreased daily step counts, reduced walking time, and increased sedentary time during the COVID‐19 pandemic 8 , 19 , 23 . Due to the significant decrease of the percent lower limb muscle mass observed in this study, we speculate that people with diabetes mellitus who stayed at home spent less time doing aerobic exercises, such as walking, cycling, and jogging. Maintaining physical activity under the new normal is likely to improve the glycemic control in people with diabetes mellitus.
In this study, the fat mass was increased and the skeletal muscle mass decreased during the COVID‐19 pandemic, suggesting the risk of progression of sarcopenic obesity in people with diabetes mellitus. Sarcopenic obesity, defined as a combination of high fat mass and low muscle mass, is associated with accelerated functional decline, and increased risks of cardiometabolic disease and mortality 24 . Since sarcopenic obesity increases with age 25 , exercise and physical activity should be recommended for elderly people with diabetes mellitus during the COVID‐19 pandemic.
Although various restrictions, such as against holding events, traveling, and dining and drinking at restaurants, lasted for only 2 months of the first and second emergency periods, the increase in HbA1c and percent fat mass and the decrease in percent skeletal muscle mass persisted throughout the COVID‐19 period. These results suggest that people with diabetes mellitus continued social distancing and stay‐at‐home practices even after the state of emergency was lifted. Consistent with this, the decrease of serum γ‐GTP, the most common traditional biomarker of excessive alcohol intake or alcohol‐induced liver injury 26 was observed from October to March during the COVID‐19 period. Alcohol intake also decreased significantly during the COVID‐19 period, particularly from October to December, when people most often go out for drinks, such as year‐end parties and business drinks parties. According to the Family Income and Expenditure Survey in Japan, alcohol consumption in restaurants in December 2020 was 81.7% lower compared with that in the same month of the previous year 27 . Thus, improved γ‐GTP from January to March could have resulted from the decrease in alcohol consumption at the end of the year due to the loss of social drinking opportunities on account of the COVID‐19 preventive measures in force during that time. People with diabetes mellitus are generally provided with instructions on how to reduce their susceptibility to infections. Moreover, several studies have reported that people with diabetes mellitus are at a higher risk of developing severe illness associated with COVID‐19 3 , 28 , and various organizations have alerted people with diabetes mellitus to take precautions against contracting COVID‐19 29 , 30 . Thus, it is possible that people with diabetes mellitus continued to adopt the preventive measures spontaneously.
In regard to the dietary intakes, there were no changes during the COVID‐19 period in this study, although previous studies conducted in non‐diabetic people reported a change in eating habits 9 , 10 , 31 . Our study participants could have maintained their dietary patterns during the COVID‐19 pandemic as they were people with diabetes mellitus who had already received instructions on therapeutic nutritional modifications. Further studies are needed to examine the impact of the COVID‐19 pandemic on the eating habits of people with diabetes mellitus.
There were some limitations of this study. First, as this study was a single‐center retrospective study conducted at a university hospital, our results may not reliably represent the general Japanese diabetic population. Second, due to the observational study design, we were unable to examine the influences of some factors such as smoking, education level, and income. Third, several other clinical variables that could affect the glycemic control were not assessed in this study (e.g., duration of diabetes, comorbidities). These confounding factors could have influenced the presented results. Fourth, because of the relatively high BMI of the participants in this study, our results may not reliably represent the situation in the general Japanese diabetic population.
In conclusion, our study revealed a negative impact of the COVID‐19 pandemic on the glycemic control and body composition in people with diabetes mellitus for a year. The present study also showed that the increase of body weight and fat mass and the decrease of the skeletal muscle mass during the pandemic were associated with poor glycemic control, independent of the age and sex, in people with diabetes mellitus.
FUNDING INFORMATION
This study was supported by JSPS KAKENHI Grant‐in‐Aid for Encouragement of Scientists (M.S., 2022). The funder played no role in the design, analysis, or preparation of this article.
DISCLOSURES
The authors have no conflicts of interest to declare.
Approval of the research protocol: Ethics approval for the study was obtained from the Research Ethics Committee of the University of Tokyo Hospital (2021068NI), in accordance with the principles of the Declaration of Helsinki; every effort was made to ensure patient anonymity.
Informed Consent: The need for informed consent was waived owing to the retrospective nature of the investigation and the opt‐out method of inclusion.
Approval date of Registry and the Registration No. of the study/trial: N/A.
Animal Studies: N/A.
ACKNOWLEDGMENT
We thank all the staff members of the Department of Diabetes and Metabolic Diseases, the Department of Healthcare and Information Management, and the Department of Clinical Nutrition Therapy, The University of Tokyo Hospital, for their excellent assistance.
Mika Sawada and Kanako Ohkuma are contributed equally to this work.
REFERENCES
- 1. World Health Organization . Coronavirus disease (COVID‐19) pandemic. Available at: https://www.who.int/europe/emergencies/situations/covid‐19.
- 2. World Health Organization . WHO Coronavirus Disease (COVID‐19) Dashboard. Available at: https://covid19.who.int/.
- 3. Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID‐19 cases: A systematic literature review and meta‐analysis. J Infect 2020; 81: e16–e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tian W, Jiang W, Yao J, et al. Predictors of mortality in hospitalized COVID‐19 patients: A systematic review and meta‐analysis. J Med Virol 2020; 92: 1875–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nandy K, Salunke A, Pathak SK, et al. Coronavirus disease (COVID‐19): A systematic review and meta‐analysis to evaluate the impact of various comorbidities on serious events. Diabetes Metab Syndr 2020; 14: 1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang Z, Du Z, Zhu F. Glycosylated hemoglobin is associated with systemic inflammation, hypercoagulability, and prognosis of COVID‐19 patients. Diabetes Res Clin Pract 2020; 164: 108214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ministry of Health, Labour, and Welfare Japan . Available at: https://www.mhlw.go.jp/stf/newpage_08906.html.
- 8. Castaneda‐Babarro A, Arbillaga‐Etxarri A, Gutierrez‐Santamaria B, et al. Physical activity change during COVID‐19 confinement. Int J Environ Res Public Health 2020; 17: 6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Di Renzo L, Gualtieri P, Pivari F, et al. Eating habits and lifestyle changes during COVID‐19 lockdown: An Italian survey. J Transl Med 2020; 18: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ruiz‐Roso MB, de Carvalho PP, Matilla‐Escalante DC, et al. Changes of physical activity and ultra‐processed food consumption in adolescents from different countries during Covid‐19 pandemic: An observational study. Nutrients 2020; 12: 2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Osawa I, Goto T, Asami Y, et al. Physician visits and medication prescriptions for major chronic diseases during the COVID‐19 pandemic in Japan: Retrospective cohort study. BMJ Open 2021; 11: e050938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abate HK, Ferede YM, Mekonnen CK. Adherence to physical exercise recommendations among type 2 diabetes patients during the COVID‐19 pandemic. Int J Afr Nurs Sci 2022; 16: 100407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Asheq A, Ashames A, Al‐Tabakha M, et al. Medication adherence in type 2 diabetes mellitus patients during Covid‐19 pandemic: A cross‐sectional study from The United Arab Emirates. F1000Res 2021; 10: 435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kumari N, Prakash V, Roy SS, et al. Impact of SARS‐CoV‐2 pandemic on Glycaemic control, metabolic status, treatment adherence, quality of life in diabetes mellitus patients in tertiary Care Hospital of Eastern India. Maedica (Bucur) 2022; 17: 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Biamonte E, Pegoraro F, Carrone F, et al. Weight change and glycemic control in type 2 diabetes patients during COVID‐19 pandemic: The lockdown effect. Endocrine 2021; 72: 604–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karatas S, Yesim T, Beysel S. Impact of lockdown COVID‐19 on metabolic control in type 2 diabetes mellitus and healthy people. Prim Care Diabetes 2021; 15: 424–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Onmez A, Gamsizkan Z, Ozdemir S, et al. The effect of COVID‐19 lockdown on glycemic control in patients with type 2 diabetes mellitus in Turkey. Diabetes Metab Syndr 2020; 14: 1963–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. D'Onofrio L, Pieralice S, Maddaloni E, et al. Effects of the COVID‐19 lockdown on glycaemic control in subjects with type 2 diabetes: The glycalock study. Diabetes Obes Metab 2021; 23: 1624–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ruiz‐Roso MB, Knott‐Torcal C, Matilla‐Escalante DC, et al. COVID‐19 lockdown and changes of the dietary pattern and physical activity habits in a cohort of patients with type 2 diabetes mellitus. Nutrients 2020; 12: 2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ministry of Education, Culture, Sports, Science and Technology of Japan . Standard Tables of Food Composition in Japan 2015. Available at: https://www.mext.go.jp/a_menu/syokuhinseibun/1365297.htm.
- 21. Guo SS, Zeller C, Chumlea WC, et al. Aging, body composition, and lifestyle: The Fels longitudinal study. Am J Clin Nutr 1999; 70: 405–411. [DOI] [PubMed] [Google Scholar]
- 22. Janssen I, Heymsfield SB, Wang ZM, et al. Skeletal muscle mass and distribution in 468 men and women aged 18‐88 yr. J Appl Physiol 1985; 2000: 81–88. [DOI] [PubMed] [Google Scholar]
- 23. Nagata S, Adachi HM, Hanibuchi T, et al. Relationships among changes in walking and sedentary behaviors, individual attributes, changes in work situation, and anxiety during the COVID‐19 pandemic in Japan. Prev Med Rep 2021; 24: 101640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roh E, Choi KM. Health consequences of sarcopenic obesity: A narrative review. Front Endocrinol (Lausanne) 2020; 11: 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Batsis JA, Villareal DT. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol 2018; 14: 513–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Conigrave KM, Davies P, Haber P, et al. Traditional markers of excessive alcohol use. Addiction 2003; 98(Suppl 2): 31–43. [DOI] [PubMed] [Google Scholar]
- 27. Income Family and Survey Expenditure. Use Classification ‐ Yearly Average of Monthly Receipts and Disbursements per Household. Food (Alcoholic beverages ‐‐ Meals outside the home). Available at: https://www.e‐stat.go.jp/en/stat‐search/files?page=1&layout=datalist&toukei=00200561&tstat=000000330001&cycle=7&year=20200&month=0&tclass1=000000330001&tclass2=000000330004&tclass3=000000330006&result_back=1&tclass4val=0.
- 28. Team CC‐R. Preliminary Estimates of the Prevalence of Selected Underlying Health Conditions Among Patients with Coronavirus Disease . United States, February 12‐march 28, 2020. MMWR Morb Mortal Wkly Rep 2019; 2020: 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. The Japan Diabetes Society . Diabetes and COVID‐19 (Q&A). Available at: http://www.jds.or.jp/modules/important/index.php?content_id=137.
- 30. The American Diabetes Association . How COVID‐19 Impacts People with Diabetes. Available at: https://www.diabetes.org/coronavirus‐covid‐19/how‐coronavirus‐impacts‐people‐with‐diabetes.
- 31. Gallo LA, Gallo TF, Young SL, et al. The impact of isolation measures due to COVID‐19 on energy intake and physical activity levels in Australian university students. Nutrients 2020; 1865: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]


