Opening Vignette
A 50-year-old man presented at the emergency department with severe dyspnoea and cough productive of yellow sputum. He had a history of chronic smoking, uncontrolled asthma and poor adherence to inhaler treatment. His height was 1.7 m, and his weight was 80 kg. Vital signs were as follows: temperature 38°C, pulse rate 130/min, respiratory rate 40/min, blood pressure 150/90 mmHg and peripheral oxygen saturation 96% (inspired oxygen fraction 50% via Venturi mask). He had accessory muscle use and bilateral polyphonic wheeze. Point-of-care lung ultrasound revealed normal A-pattern bilaterally, normal lung sliding bilaterally and no pleural effusion. Chest X-ray did not show any lung opacities. His breathlessness, tachypnoea and wheezing were not reduced by nebulised salbutamol and ipratropium. He appeared lethargic and could speak only in short phrases. Arterial blood gas measurement showed pH 7.40, partial pressure of carbon dioxide (PaCO2) 40 mmHg, partial pressure of oxygen (PaO2) 75 mmHg and HCO3–24 mmol/L
INTRODUCTION
The patient had acute severe asthma, which is marked by dyspnoea, tachypnoea and respiratory failure. Asthma is a ‘heterogeneous disease defined by the history of respiratory symptoms (e.g., wheeze, shortness of breath, chest tightness, and cough) that vary over time and in intensity, together with variable expiratory airflow limitation’.[1] Acute severe asthma may occur on the background of difficult-to-treat asthma (uncontrolled asthma despite prescription of high-dose inhaler treatment) and severe asthma (uncontrolled asthma despite adequate adherence and inhaler technique).[2] Acute severe asthma may also be known as ‘near-fatal asthma’ and ‘status asthmaticus’.
Acute severe exacerbation of chronic obstructive pulmonary disease (COPD) shares many of the characteristics and management steps with acute severe asthma. Like asthma, COPD involves airflow limitation, though the respiratory symptoms and lung function abnormalities are more persistent in COPD.[3] In some instances, asthma and COPD are hard to distinguish [Table 1], especially if the patient presents for the first time. When in doubt, treat as for asthma rather than for COPD [Table 2]. Complications (e.g., air trapping, pneumothorax, patient–ventilator asynchrony [PVA]) and concurrent conditions (e.g., acute myocardial infarction, acute heart failure, pulmonary embolism, obesity, hypoventilation syndrome) may worsen the clinical presentation of acute severe asthma or COPD. Active checking and treatment of such complications and concurrent conditions would be necessary to achieve optimal outcomes for the patients [Figure 1].
Table 1.
Selected differential diagnosis of wheezing.
| Diagnosis | Key clinical features | Key investigation features |
|---|---|---|
| Asthma | Allergy, eczema, rhinitis | Blood eosinophils >3% (for eosinophilic asthma) |
|
| ||
| Bronchiectasis | Purulent sputum | Chest X-ray: bronchial dilatation and wall thickening |
|
| ||
| Congestive heart failure | History of ischaemic heart disease | Chest X-ray: pulmonary oedema |
|
| ||
| COPD | Chronic smoking | Previous lung function test: reduced post-bronchodilator FEV1/FVC ratio |
|
| ||
| Foreign body inhalation | Alcohol intoxication, reduced consciousness, choking | Chest X-ray: lobar collapse |
|
| ||
| Tuberculosis (endobronchial involvement) | Haemoptysis, weight loss | Chest X-ray: fluffy upper lobe infiltrates |
|
| ||
| Tumour | Haemoptysis, weight loss | Chest X-ray: lung mass |
|
| ||
| Vocal cord mass or paralysis | Hoarse voice, stridor | Endoscopy: mass or paralysis |
COPD: chronic obstructive pulmonary disease, FEV1: forced expiratory volume over the first second, FVC: forced vital capacity
Table 2.
Therapeutic options for acute severe asthma and COPD.
| Condition | Therapeutic options | Example prescription | |
|---|---|---|---|
| Asthma or COPD | Inhaled β-agonists | Four puffs (400 mcg) salbutamol via spacer | |
|
| |||
| Inhaled anti-muscarinic agents | Four puffs (80 mcg) ipratropium via spacer | ||
|
| |||
| Systemic corticosteroids | Intravenous hydrocortisone 100 mg bolus | ||
|
| |||
| Antibiotics for infection | Oral azithromycin 500 mg | ||
|
| |||
| Antivirals for infection | Oral oseltamivir 75 mg | ||
|
| |||
| Invasive mechanical ventilation | Assist-control volume-control mode, inspired oxygen fraction 50%, flow rate 70 L/min, tidal volume 500 ml, rate 10/min, PEEP 5 cmH2O | ||
|
| |||
| Volume repletion (to avoid hypotension when air trapping impedes venous return) | Intravenous normal saline 500 ml over 30 min | ||
|
| |||
| Specific to asthma | Intravenous magnesium | Intravenous magnesium 2 g over 20 min | |
|
| |||
| Specific to COPD | Non-invasive ventilation | Spontaneous-timed mode, inspired oxygen fraction 50%, rate 12/min, IPAP 20 cmH2O, EPAP 5 cmH2O | |
COPD: chronic obstructive pulmonary disease, EPAP: expiratory positive airway pressure, IPAP: inspiratory positive airway pressure, PEEP: positive end-expiratory pressure
Figure 1.
Management of acute severe asthma and chronic obstructive pulmonary disease.
GENERAL APPROACH
Clinical identification
The frontline clinician will be alerted to the presence of acute severe asthma and COPD when a patient presents with severe dyspnoea and wheeze. While such a presentation is hard to miss, the symptoms and signs are not specific to asthma or COPD. Not all that wheezes is asthma (or COPD). Besides bronchospasm, a variety of other conditions can cause dyspnoea and wheezing [Table 1], for instance, upper airway oedema from anaphylaxis and acute bronchial obstruction from foreign body inhalation.
Investigations
Key investigations include point-of-care ultrasound, chest X-ray and arterial blood gas. These may not be available in all care settings (e.g. in a family medicine clinic), but should be performed when frontline clinicians have them. Point-of-care ultrasound for asthma and COPD is lung focused, can be done using a variety of probes (linear, curvilinear, phased array) and is used to exclude complications and concurrent conditions. In the absence of complications and concurrent conditions, lung ultrasound is normal: A-pattern (horizontal reverberation artefacts) [Figure 2], normal lung sliding and no pleural effusion. If pneumothorax is present, A-pattern remains but no lung sliding will be observed. Like ultrasound, chest X-ray is used to exclude complications and concurrent conditions such as pneumothorax, pneumonia, lung collapse (e.g. from mucous plugging) and acute pulmonary oedema (from acute heart failure).
Figure 2.
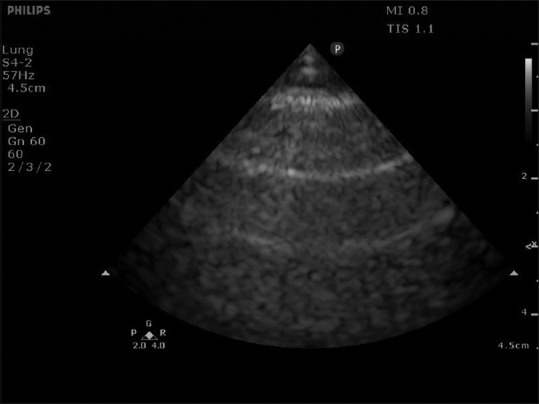
Normal A-pattern of lung ultrasound.
An arterial blood gas is used to check for impending or overt hypercapnic respiratory failure. Given tachypnoea, hypocapnia (arterial partial pressure of carbon dioxide [PaCO2] <35 mmHg) and respiratory alkalosis are expected. PaCO2 in the normal range (35–45 mmHg) in a tachypnoeic patient suggests a large proportion of ineffective ventilation due to air trapping and portends overt respiratory failure. In other words, a numerically normal arterial blood gas should forewarn impending hypercapnic respiratory failure, rather than falsely reassure the clinician.
Treatment
In the clinic or general ward settings, prompt initiation of inhaled bronchodilation is essential [Table 2]. If aerosol spread of infectious agents such as severe acute respiratory syndrome coronavirus-2 is of concern, then nebulisation can be replaced by inhalation of bronchodilators via a spacer device. If bronchospasm precludes adequate inhalation of medication, then parenteral administration of bronchodilators may be considered: subcutaneous or intravenous adrenaline for both asthma and COPD and intravenous magnesium for asthma.[4] Given its lack of efficacy and potential cardiotoxicity, intravenous aminophylline is not recommended for either asthma or COPD exacerbations.[1,5]
Besides bronchodilation, medications should be used to eliminate airway inflammation and infection. These include the use of systemic steroids, antibiotics and antivirals. Given the risk of hypercapnic respiratory failure, and since hyperoxaemia may increase PaCO2 (e.g. due to loss of hypoxic vasoconstriction), oxygen supplementation should target a peripheral oxygen saturation of at least 88%–92%, and not higher than 98%.[1,6,7] Pneumothorax secondary to acute severe asthma or COPD needs to be drained via a chest tube. This should be made to enter the chest wall above the visible liver or spleen as safely as possible under ultrasound guidance. As far as possible, pneumothorax drainage should be done before positive pressure ventilation is provided, as the latter could worsen air leak through a bronchopleural fistula, potentially leading to tension pneumothorax.
Ventilator management
In the emergency department or critical care settings, enhanced respiratory support is usually available. The decision for enhanced respiratory support is based on requirements for positive pressure (to overcome atelectasis), ventilation (i.e. clearance of carbon dioxide or to reduce the work of breathing) and airway protection (e.g. in an obtunded patient). For asthma, benefit of non-invasive ventilation (NIV) is unclear, and a low threshold to intubate should be maintained if NIV is attempted.[8] In contrast, for COPD, NIV should be considered before invasive mechanical ventilation, as the former has the following advantages: reduced sedation needs, preserved airway protective reflexes, avoidance of ventilator-associated pneumonia and decreased mortality. NIV can be used to relieve severe dyspnoea and respiratory muscle fatigue for acute exacerbations of COPD, even if respiratory acidosis is absent.[5] If NIV and invasive mechanical ventilation fail, extracorporeal membrane oxygenation may be considered in expert centres.[6]
NIV can be initiated by selecting the spontaneous-timed (S/T) mode, a back-up respiratory rate of 10 breaths/minute, an inspired oxygen fraction to achieve a peripheral oxygen saturation of at least 88%–92%, an expiratory positive airway pressure (EPAP; equivalent to positive end-expiratory pressure [PEEP]) of 5 cmH2O and an inspiratory positive airway pressure (IPAP) of 20 cmH2O. The difference between the IPAP and EPAP (i.e. the pressure support) determines the tidal volume, and the IPAP can be titrated to achieve a tidal volume of 6–8 ml/kg predicted body weight [Table 3]. Ventilator adjustments can be made every 15–30 min to allow time for changes to take place. Nonetheless, the key to successful NIV is a comfortable mask fit. Start by placing the full face mask (one that covers both the nose and the mouth, but not the whole face) lightly over the patient’s face. Then, as the patient becomes adapted to the mask, create a firm seal between the mask and the patient’s face using head straps. The patient should be kept nil by mouth and should avoid swallowing air. If excessive gastric distension occurs, a nasogastric tube can be inserted, though such a tube can interfere with the mask seal.
Table 3.
Ventilator management for acute severe asthma and COPD.
| Ventilator setting | Initial setting | Adjustment |
|---|---|---|
| Mode | Assist-control volume-control mode | Avoid assist-control pressure-control mode as the volume delivered can vary with changes in airway resistance |
|
| ||
| FIO2 | 50% | Target SpO2 88%-92%, and not higher than 98%, given the risk of hypercapnia |
|
| ||
| Rate | 10 breaths/min | Target pH 7.25-7.45. To lower PaCO2, increase the rate. Do not increase the rate if incomplete exhalation occurs (flow–time curve does not return to baseline at end expiration) |
|
| ||
| Tidal volume | 6 ml/kg PBW | Target pH 7.25-7.45. To lower PaCO2, increase the tidal volume up to 8 ml/kg PBW. Do not increase the tidal volume if incomplete exhalation occurs (flow–time curve does not return to baseline at end expiration) |
|
| ||
| Inspiratory flow | 60 L/min | In the presence of incomplete exhalation, increase the inspiratory flow to decrease inspiratory time and to increase expiratory time |
|
| ||
| PEEP | 5 cmH2O | To avoid aggravating air trapping and to avoid increasing auto-PEEP, the PEEP can be kept at 5 cmH2O |
|
| ||
| Trigger sensitivity | Flow trigger, 2 L/min (the lower the value, the more sensitive is the trigger) | To avoid ineffective triggering, keep the triggering threshold low (i.e. keep the absolute flow trigger value low). Avoid an absolute trigger value <2 L/min, as this may lead to auto-triggering (ventilator triggering without patient’s initiation of a breath) |
For men, PBW (kg) = 50 + (0.91 × [height (cm) − 152.4]). For women, PBW (kg) = 45.5 + (0.91 × [height (cm) − 152.4]). FIO2: inspired oxygen fraction, PaCO2: partial pressure of arterial carbon dioxide, PBW: predicted body weight, PEEP: positive end-expiratory pressure, SpO2: peripheral oxygen saturation.
The invasive mechanical ventilation strategy is similar for asthma and COPD [Table 3] and is aimed at relieving air trapping via expiratory time prolongation. To extend the expiratory time, the inspiratory time needs to be actively shortened. For pressure-controlled ventilation modes, the inspiratory time can be directly shortened. For volume-controlled ventilation modes, the inspiratory time can be indirectly shortened by increasing the inspiratory flow while keeping the tidal volume constant. If spontaneous breathing can be eliminated by deep sedation with or without neuromuscular paralysis, the respiratory rate can be reduced to increase the duration of each breath. Given a fixed inspiratory-to-expiratory time ratio, respiratory rate reduction leads to increased breath duration and prolongation of both inspiratory and expiratory time, which also allows relief of air trapping. Correction of hypercapnia need not be abrupt, and gradual clinical improvement (e.g. reduction of dyspnoea and accessory muscle use) is the therapeutic goal.
Endotracheal administration of inhaled medications needs to be increased compared to conventional doses, given partial deposition within the respiratory circuit. Using an adaptor attached to the inspiratory limb of the ventilator circuit, four puffs (400 mcg) of salbutamol via a metered-dose inhaler provides optimal bronchodilation without excess side effects.[9] Removal of secretions via endotracheal tube suctioning should be done to prevent obstruction by thickened secretions. Auto-PEEP and air trapping should be minimised (Concept 1). During mechanical ventilation, sedation is usually required to maintain patient comfort. Even with sedation, for patients with severe hypercapnia, respiratory drive can be very strong, generating fast and deep spontaneous breaths which disrupt patient–ventilator synchrony (Concept 2). Such a situation may require neuromuscular paralysis to eliminate the effect of a very strong respiratory drive and to achieve patient–ventilator synchrony. Finally, a high peak pressure alarm on the ventilator does not always represent harmful mechanical ventilation (Concept 3).
CONCEPT 1: AUTO-PEEP
Auto-PEEP, also known as intrinsic PEEP, is positive recoil pressure left at the end of expiration. Normally, at end expiration, the static recoil pressure of the respiratory system is zero. However, if time for expiration is inadequate, incomplete lung deflation, air trapping, dynamic hyperinflation (i.e. breath stacking) and a positive recoil pressure at end expiration are observed. Patients at risk for auto-PEEP are those with airway obstruction and expiratory flow limitation, in particular, patients with acute severe asthma and COPD. Auto-PEEP can be detected by inspecting the ventilator graphics: the flow–time curve will not return to the zero baseline at the end expiration. Auto-PEEP can be quantified using the end-expiratory pause function on the ventilator. During the end-expiratory pause manoeuvre, the expiratory valve is closed for 3–5 s during expiration, allowing the alveolar pressure to equilibrate with the airway pressure.
Major complications of auto-PEEP are related to excessive air trapping. Air trapping in alveoli may lead to barotrauma and pneumothorax. More broadly, air trapping in the lungs increases intrathoracic pressure (reducing venous return) and pulmonary vascular resistance (reducing right ventricular output), resulting in hypotension. Pre-existing hypovolemia exacerbates hypotension when air trapping impedes venous return. In a spontaneously breathing patient on mechanical ventilation, at the start of inspiration, the presence of auto-PEEP requires the patient to drop the airway pressure from auto-PEEP to below the externally applied PEEP in order to trigger the ventilator. If auto-PEEP is present, the patient needs to generate a more negative pressure to trigger the ventilator than if auto-PEEP is absent. This results in increased work of breathing or ineffective efforts (ineffective triggering) when a patient is unable to generate sufficient negative pressure.
Treating auto-PEEP includes management of reversible causes, increasing the expiratory time by decreasing respiratory rate, decreasing the inspiratory time and increasing the inspiratory flow rate. Decreasing tidal volume can help by reducing the time needed for complete exhalation, but like decreasing respiratory rate, this can reduce carbon dioxide clearance. For patients in extremis, temporary ventilator disconnection may be necessary to allow passive exhalation, decline of auto-PEEP and improvement of venous return. In a spontaneously breathing patient, if auto-PEEP cannot be eliminated and if ineffective triggering occurs, the work of breathing can be decreased by providing external PEEP (usually set at 75%–80% of auto-PEEP). Appropriate provision of external PEEP narrows the gap between auto-PEEP and the threshold for inspiratory triggering.
CONCEPT 2: PVA
PVA is mismatched coupling between the mechanically delivered breaths (ventilator breaths) and the patient’s own respiratory efforts (neural breaths). PVA can occur with both NIV and invasive ventilation.[10] Mismatched coupling between ventilator and neural breaths can occur at the start of neural inspiration (trigger asynchrony), at the end of neural inspiration (cycling asynchrony) or during neural inspiration (flow asynchrony). Complications include increased duration of mechanical ventilation and mortality.[11] Clinical examination (e.g. patient struggling against the ventilator) and bedside observation of ventilator waveforms are the conventional methods of detecting PVA and aid in management.[12]
For acute severe asthma and COPD, treatment of the underlying bronchospasm and airflow obstruction would help reduce all types of PVA. Ventilator adjustments may be additionally needed in patients with spontaneous breathing. At the start of neural inspiration, ineffective triggering may occur due to inability of the patient’s respiratory effort to overcome auto-PEEP, which can be treated by applying external PEEP set at 75%–80% of auto-PEEP (Concept 1). At the end of neural inspiration, the ventilator breath may be too long for the patient, that is, delayed cycling, which can be treated by decreasing the inspiratory time, increasing the inspiratory flow rate or decreasing the tidal volume on the ventilator. Recurrent PVA refractory to ventilator adjustments is associated with increased mortality,[13] and short-term (24–48 h) administration of neuromuscular paralysis may be needed.
CONCEPT 3: PEAK VERSUS PLATEAU PRESSURE
During mechanical ventilation for severe bronchospasm, one often encounters a high peak pressure alarm. However, a high peak pressure may not mean harmful ventilation. This is because peak airway pressure is the sum of the pressure used to overcome airway resistance and the pressure used to distend the alveoli. The pressure used to overcome airway resistance is high in severe bronchospasm, but it is not harmful. In contrast, excessive pressure exerted beyond the airways can result in alveolar overdistension, which is harmful. To measure the pressure used to distend the alveoli (i.e. the driving pressure), the flow needs to be artificially interrupted using an end-inspiratory pause on the ventilator. During this zero flow state, which lasts a few seconds, the pressure measured by the ventilator equilibrates with the pressure in the alveoli at end inspiration, and this is the plateau pressure. The driving pressure is the difference between the plateau pressure and PEEP.
In the setting of acute severe asthma or COPD, when high peak airway pressures develop, the frontline clinician should measure the plateau pressure. In general, a plateau pressure of less than 30 cmH2O is safe for the patients. If the plateau pressure exceeds 30 cmH2O, then the driving pressure needs to be reduced. For acute severe asthma or COPD, allowing complete exhalation for each breath and avoidance of auto-PEEP take precedence (Concept 1), as this would reduce the distending pressure in the alveoli. Following elimination of auto-PEEP, in the pressure-control mode, the driving pressure can be directly reduced by decreasing the pressure control; in the volume-control mode, the driving pressure can be indirectly reduced by decreasing the tidal volume delivered.
TAKE-HOME MESSAGES
Acute severe asthma is marked by dyspnoea, tachypnoea and respiratory failure. Acute severe exacerbation of COPD shares many of the characteristics and management steps with acute severe asthma.
Key investigations include point-of-care ultrasound, chest X-ray and arterial blood gas. PaCO2 in the normal range (35–45 mmHg) in a severely tachypnoeic patient suggests a large proportion of ineffective ventilation due to air trapping and portends overt respiratory failure.
Management of acute severe asthma and COPD involves (1) opening the obstructed airways, (2) overcoming complications (e.g. air trapping, pneumothorax, PVA) and (3) overcoming concurrent conditions.
In the setting of acute severe asthma or COPD, a high peak pressure during mechanical ventilation may not mean harmful ventilation. When high peak airway pressures develop, the frontline clinician should measure the plateau pressure.
Closing Vignette
Severe acute asthma was recognised. In addition to nebulised bronchodilators, the patient received intravenous magnesium (2 g over 20 min) and intravenous hydrocortisone 100 mg bolus. Given the severe presentation and productive cough, infection could not be confidently excluded and intravenous amoxicillin–clavulanic acid was given. As breathlessness, tachypnoea and wheezing persisted, consideration was given for escalation of respiratory support. The arterial blood gas measurement was not reassuring, as no respiratory alkalosis was present despite tachypnoea. The patient appeared exhausted and his arterial blood gas subsequently showed pH 7.20, PaCO2 63 mmHg and HCO3–24 mmol/L. He was intubated in the emergency department and required deep sedation with neuromuscular paralysis to attain patient-ventilator synchrony. Assist-control volume-control mode of ventilation was chosen, with the following settings: rate 10 breaths/min, flow rate 70 L/min, tidal volume 500 ml (about 7.5 ml/kg predicted body weight), PEEP 5 cmH2O and inspired oxygen fraction 40%. Although the peak pressure reached 45 cmH2O, the plateau pressure was only 15 cmH2O. Arterial blood gas done after 30 min showed pH 7.30, pCO2 50 mmHg, pO2 100 mmHg and HCO3–24 mmol/L. The patient was transferred to the intensive care unit for further management and was successfully extubated after 2 days.
Financial support and sponsorship
Nil.
Conflicts of interest
See KC is the handling editor of the PACC series.
SMC CATEGORY 3B CME PROGRAMME
Online Quiz: https://www.sma.org.sg/cme-programme
Deadline for submission: 6 pm, 16 December 2022
| Question | True | False |
|---|---|---|
| 1. Dyspnoea, tachypnoea, respiratory failure and wheezing are pathognomonic for acute severe asthma. | ||
|
| ||
| 2. Management of acute severe asthma and chronic obstructive pulmonary disease (COPD) requires bronchodilation, overcoming complications like pneumothorax and overcoming concurrent conditions. | ||
|
| ||
| 3. Lung ultrasound can only be performed using the high-frequency linear probe. | ||
|
| ||
| 4. When pneumothorax is present, lung ultrasound would demonstrate an A-pattern (horizontal reverberation artefacts). | ||
|
| ||
| 5. A normal pH and arterial carbon dioxide partial pressure excludes acute severe asthma. | ||
|
| ||
| 6. Delivery of bronchodilators via a spacer device can be used in lieu of nebulised bronchodilators if aerosolisation of infectious agents is a concern. | ||
|
| ||
| 7. Nebulised magnesium is effective for treating acute severe asthma. | ||
|
| ||
| 8. Early and appropriate antibiotics should always be used when treating acute severe asthma. | ||
|
| ||
| 9. When treating acute severe exacerbation of COPD, the target peripheral oxygen saturation can be 92%–96%. | ||
|
| ||
| 10. When pneumothorax is present in a patient with acute severe exacerbation of COPD, urgent endotracheal intubation is required. | ||
|
| ||
| 11. Non-invasive ventilation (NIV) is routinely recommended when patients with acute severe asthma develop hypercapnic respiratory failure. | ||
|
| ||
| 12. NIV is contraindicated in acute severe exacerbation of COPD when the pH is 7.40. | ||
|
| ||
| 13. To relieve air trapping in acute severe asthma, the inspiratory flow should be decreased. | ||
|
| ||
| 14. To minimise development of auto-positive end-expiratory pressure (PEEP), the respiratory rate should be kept high. | ||
|
| ||
| 15. Reduction of respiratory rate is useful for relieving air trapping. | ||
|
| ||
| 16. Volume-controlled rather than pressure-controlled ventilation is preferred for acute severe asthma. | ||
|
| ||
| 17. A goal of mechanical ventilation of acute severe exacerbation of COPD is rapid normalisation of arterial carbondioxide partial pressure. | ||
|
| ||
| 18. Using an adaptor attached to the inspiratory limb of the ventilator circuit, four puffs (400 mcg) of salbutamol via a metered-dose inhaler provides optimal bronchodilation without excess side effects. | ||
|
| ||
| 19. Auto-PEEP occasionally leads to auto-triggering. | ||
|
| ||
| 20. Plateau pressure is measured using an end-inspiratory pause manoeuvre. | ||
REFERENCES
- 1.Reddel HK, Bacharier LB, Bateman ED, Brightling CE, Brusselle GG, Buhl R, et al. Global Initiative for Asthma Strategy 2021:Executive summary and rationale for key changes. Eur Respir J. 2021;59:2102730. doi: 10.1183/13993003.02730-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kui SL, How CH, Koh J. PILL Series. The 'problematic'asthma patient. Singapore Med J. 2015;56:368–71. doi: 10.11622/smedj.2015106. quiz 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roman-Rodriguez M, Kaplan A. GOLD 2021 strategy report:Implications for asthma-COPD overlap. Int J Chron Obstruct Pulmon Dis. 2021;16:1709–15. doi: 10.2147/COPD.S300902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kew KM, Kirtchuk L, Michell CI. Intravenous magnesium sulfate for treating adults with acute asthma in the emergency department. Cochrane Database Syst Rev. 2014 doi: 10.1002/14651858.CD010909.pub2. CD010909. doi:10.1002/14651858. CD010909.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease 2022. [30 Jan 2022]. Available from: https://goldcopd.org/2022-gold-reports-2/
- 6.Le Conte P, Terzi N, Mortamet G, Abroug F, Carteaux G, Charasse C, et al. Management of severe asthma exacerbation:Guidelines from the Societe Francaise de Medecine d'Urgence, the Societe de Reanimation de Langue Francaise and the French Group for Pediatric Intensive Care and Emergencies. Ann Intensive Care. 2019;9:115. doi: 10.1186/s13613-019-0584-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Driscoll BR, Howard LS, Earis J, Mak V. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017;72:ii1–90. doi: 10.1136/thoraxjnl-2016-209729. [DOI] [PubMed] [Google Scholar]
- 8.Holley AD, Boots RJ. Review article:Management of acute severe and near-fatal asthma. Emerg Med Australas. 2009;21:259–68. doi: 10.1111/j.1742-6723.2009.01195.x. [DOI] [PubMed] [Google Scholar]
- 9.Dhand R, Duarte AG, Jubran A, Jenne JW, Fink JB, Fahey PJ, et al. Dose-response to bronchodilator delivered by metered-dose inhaler in ventilator-supported patients. Am J Respir Crit Care Med. 1996;154:388–93. doi: 10.1164/ajrccm.154.2.8756811. [DOI] [PubMed] [Google Scholar]
- 10.Longhini F, Colombo D, Pisani L, Idone F, Chun P, Doorduin J, et al. Efficacy of ventilator waveform observation for detection of patient-ventilator asynchrony during NIV:A multicentre study. ERJ Open Res. 2017;3 doi: 10.1183/23120541.00075-2017. 00075-2017. doi:10.1183/23120541.00075-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanch L, Villagra A, Sales B, Montanya J, Lucangelo U, Luján M, et al. Asynchronies during mechanical ventilation are associated with mortality. Intensive Care Med. 2015;41:633–41. doi: 10.1007/s00134-015-3692-6. [DOI] [PubMed] [Google Scholar]
- 12.See KC, Sahagun J, Cove M, Sum CL, Garcia B, Chanco D, et al. Managing patient-ventilator asynchrony with a twice-daily screening protocol:A retrospective cohort study. Aust Crit Care. 2021;34:539–46. doi: 10.1016/j.aucc.2020.11.008. [DOI] [PubMed] [Google Scholar]
- 13.See KC, Sahagun J, Taculod J. Defining patient-ventilator asynchrony severity according to recurrence. Intensive Care Med. 2020;46:819–22. doi: 10.1007/s00134-020-05974-y. [DOI] [PMC free article] [PubMed] [Google Scholar]



