Abstract
Background and Purpose
The impact of the occurrence while sleeping of first unprovoked seizure (FUS) on seizure recurrence in people with FUS is currently unclear. This uncertainty makes it challenging for physicians to determine whether to apply antiseizure medications (ASMs) to people with FUS while sleeping (FUS-S). This study aimed to determine the impact of the occurrence while sleeping of FUS.
Methods
We searched the MEDLINE, Embase, Cochrane Library, Web of Science, and Scopus electronic databases. Among retrieved studies, we selected those that provided information on the number of people with FUS, and relapsed people among these in each instance of FUS-S and FUS when waking (FUS-W). We used a random-effects model for meta-analyses.
Results
Of the 3,582 identified studies, 13 were eligible for systematic review. Seven of these 13 studies were deemed adequate for inclusion in a meta-analysis since they provided information at the time point of 2 years follow-up after FUS. The seven studies were of high quality regarding their risk of bias. When combining these 7 studies, the total sample comprised 1,659 people, of which 626 had FUS-S and 1,033 had FUS-W. The relative risk of seizure recurrence between FUS-S and FUS-W was 1.627. The seizure recurrence rates (SRRs) were 59.8% and 36.5% in the FUS-S and FUS-W groups, respectively.
Conclusions
We verified that the SRR was higher among people with FUS-S than FUS-W. After 2 years of follow-up, the SRR in people with FUS-S was about 60%. It is preferable to initiate an ASM for people with FUS-S.
Systematic review registration
PROSPERO registration number CRD42021266191.
Keywords: meta-analysis, recurrence, prevalence, seizures, epilepsy
INTRODUCTION
In people with a first unprovoked seizure (FUS), seizure recurrence rates (SRRs) exceeding approximately 60% over the next 10 years are consistent with the practical and clinical definition of epilepsy by The International League Against Epilepsy (ILAE).1 Previous studies have found that seizure recurrence risk is high when FUS occurs while sleeping (FUS-S), suggesting that people with this condition will develop epilepsy. The SRRs in children and adults were 53% and 56%, respectively, in previous studies that monitored people with FUS-S for 2 years.2,3 However, because there is currently no comprehensive evidence on SRRs, physicians have difficulty in deciding whether to treat people with FUS-S using antiseizure medication (ASM).
Obtaining the best available evidence by collecting studies that provide information about the SRRs of FUS-S and FUS when waking (FUS-W) may be helpful in deciding whether to treat people with FUS-S using ASM. We therefore gathered studies that provided the relevant information and reviewed them.
This study aimed to determine whether seizure recurrence is more common in FUS-S or in FUS-W, and to determine their SRRs. This systematic review considered the following three questions: Could occurrences while sleeping contribute to higher SRR in people with FUS? What is the pooled estimate for the SRRs of people with FUS-S according to primary pooling studies? Can the integrated SRR be helpful in determining whether to administer ASM therapy to people with FUS-S?
METHODS
Eligibility criteria
This systematic review was performed in accordance with the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 (PRISMA 2020).4 We searched all original studies reporting the SRRs of people with FUS. There was no constraint on the age of research subjects when searching for suitable studies for this systematic review. We also did not impose any language constraints. We retrieved studies that provided information on the number of people with FUS and with seizure recurrence in each case of FUS-S and FUS-W.
The primary outcome measures were the relative risks (RRs) of seizure recurrence between the FUS-S and FUS-W groups and their SRRs. In the FUS-S and FUS-W groups, SRR represents the proportion of people with seizure recurrence. The RRs were determined by dividing the SRR of the FUS-S group by that of the FUS-W group.
The authors have registered this systematic review in PROSPERO (registration number CRD42021266191). The protocol of this systematic review has not been published.
Search strategy and information sources
The following process was used to develop a search strategy: We constructed a straightforward search strategy after reviewing one related study5 identified in a Google search, and two well-known studies2,3 that indicated the impact of sleeping on SRR in people with FUS. We set candidates for search terms by consulting the titles and Abstracts for these three studies, and used their MeSH terms and entry terms in PubMed. Using these terms, we constructed a draft search strategy and tested it by searching the MEDLINE, Embase, Cochrane Library, Web of Science, and Scopus electronic databases. To verify the draft strategy, we tested whether it could find the three studies indicated above2,3,5 and another study dealing with the sleep state of people with FUS.6
On May 20, 2021, we searched the above five databases using the validated draft of the search strategy. While reviewing the searched studies, we updated the search strategy. Four researchers (OY Kwon, TW Yang, YS Kim, and HM Ha) examined the syntaxes, spelling, and overall structures of the updated versions. On August 4, 2021, we finally updated the database search. The final search strategies applied to the five electronic databases are listed below.
MEDLINE
#1. seizures[MeSH Terms] OR epilepsy[MeSH Terms] OR seizure*[tiab]
#2. new[tiab] OR “new onset”[tiab] OR new-onset[tiab] OR onset[tiab] OR first[tiab] OR 1st[tiab] OR single[tiab] OR isolate*[tiab] OR initial[tiab]
#3. recurrence[MeSH Terms] OR prognosis[MeSH Terms] OR recurrence*[tiab] OR recurrent[tiab] OR relapse*[tiab] OR outcome*[tiab] OR prognosis[tiab] OR predictor*[tiab] OR prediction*[tiab]
#4. nocturnal[tiab] OR sleep[tiab] OR night[tiab]
#5. #1 AND #2 AND #3 AND #4
Embase
#1. ‘seizures’/exp OR ‘epilepsy’/exp OR seizure*:ab,ti
#2. new:ab,ti OR ‘new onset’:ab,ti OR new-onset:ab,ti OR onset:ab,ti OR first:ab,ti OR 1st:ab,ti OR single:ab,ti OR isolate*:ab,ti OR initial:ab,ti
#3. ‘recurrence’/exp OR ‘prognosis’/exp OR recurrence*:ab,ti OR recurrent:ab,ti OR relapse*:ab,ti OR outcome*:ab,ti OR prognosis:ab,ti OR predictor*:ab,ti OR prediction*:ab,ti
#4. nocturnal:ab,ti OR sleep:ab,ti OR night:ab,ti
#5. #1 AND #2 AND #3 AND #4 AND [embase]/lim NOT ([embase]/lim AND [medline]/lim)
Cochrane Library
#1. (MeSH descriptor: [seizures] explode all trees) OR (MeSH descriptor: [epilepsy] explode all trees) OR (seizure*:ti,ab,kw)
#2. (new OR “new onset” OR new-onset OR onset OR first OR 1st OR single OR isolate* OR initial):ti,ab,kw
#3. (MeSH descriptor: [recurrence] explode all trees) OR (MeSH descriptor: [prognosis] explode all trees) OR (recurrent OR recurrence* OR relapse* OR outcome* OR prognosis OR predictor* OR prediction*):ti,ab,kw
#4. (nocturnal OR sleep OR night):ti,ab,kw
#5. #1 AND #2 AND #3 AND #4
Web of Science
#1. TS=(seizure OR epilepsy) OR TI=(seizure*)
#2. TI=(new OR “new onset” OR new-onset OR onset OR first OR 1st OR single OR isolate* OR initial)
#3. TS=(recurrence OR prognosis) OR TI=(recurrence* OR recurrent OR relapse* OR outcome* OR prognosis OR predictor* OR prediction*)
#4. TI=(nocturnal OR sleep OR night)
#5. #1 AND #2 AND #3 AND #4
Scopus
#1. INDEXTERMS (seizures) OR INDEXTERMS (epilepsy) OR TITLE-ABS-KEY (seizure*)
#2. TITLE-ABS-KEY (new) OR TITLE-ABS-KEY (“new onset”) OR TITLE-ABS-KEY (new-onset) OR TITLE-ABS-KEY (onset) OR TITLE-ABS-KEY (first) OR TITLE-ABS-KEY (1st) OR TITLE-ABS-KEY (single) OR TITLE-ABS-KEY (isolate*) OR TITLE-ABS-KEY (initial)
#3. INDEXTERMS (recurrence) OR INDEXTERMS (prognosis) OR TITLE-ABS-KEY (recurrence*) OR TITLE-ABS-KEY (recurrent) OR TITLE-ABS-KEY (relapse*) OR TITLE-ABS-KEY (outcome*) OR (prognosis) OR TITLE-ABS-KEY (predictor*) OR TITLE-ABS-KEY (prediction*)
#4. TITLE-ABS-KEY (nocturnal) OR TITLE-ABS-KEY (sleep) OR TITLE-ABS-KEY (night)
#5. #1 AND #2 AND #3 AND #4
Selection process
We conducted a screening and subsequent selection process to determine the studies to be included in this review. To establish a screening consensus, 3 reviewers (OY Kwon, TW Yang, and YS Kim) separately read the titles and Abstracts of the first 100 entries. They addressed inconsistencies until reaching consensuses. These three researchers then independently examined the titles and abstracts of all the articles they had identified to gather potential studies for this review. Disagreements occurred, which were debated to reach consensuses. They referred to full-text articles in the discussions when needed. If necessary, they consulted the third researcher (DH Kim) before making final decisions.
The three reviewers then independently evaluated the full texts of the articles selected through the screening stage. After this process, they finally picked the studies appropriate for inclusion in this review. If there were disagreements, they again decided on inclusion or exclusion through debate. They again conferred with the third researcher (DH Kim) when necessary.
Data collection process
One author (OY Kwon) performed data extraction. Another (TW Yang) double-checked the accuracy of the data to boost its credibility, with debates addressed all of the disagreements made by this author. We entered the integrated data into Excel spreadsheets, and another author (YS Kim) rechecked their accuracy.
Data items
To examine the impact of the occurrence of FUS while sleeping on seizure recurrence, we divided people with FUS into the FUS-S and FUS-W groups. Most studies did not offer data on the FUS that occurred at the time exactly waking up from sleep. Still, some studies presented data about the sleeping and the on-awakening state separately, and some studies showed data on a unified state of sleeping and on-awakening. We judged that on-awakening was also the last sleep process and included that data in the sleep state data.
In this review, the effect sizes were the sample and seizure recurrence event numbers in the FUS-S and FUS-W groups. We identified the effect sizes at each of the six follow-up time points of 6 months and 1, 2, 3, 4, and 5 years. We calculated event numbers by multiplying the sample numbers for the FUS-S and FUS-W groups from the studies for which SRR was reported as a proportion. In the studies that did not provide the number of patients who relapsed at specific time points, event numbers were identified using graph measurements or calculations when the Kaplan-Meyer survival curve or relative risk (RR) was presented.
Besides the effect sizes, we gathered data on bibliography (author, year, and country), study composition (research design, number of hospitals, clinical setting, sleep state definition, and presence of follow-up time points), and subjects (number, age, sex, and confounding cases). We used seizure types, ASM use, and remote symptomatic patients as the confounding cases.
Synthesis methods
For a follow-up time point at which we could collect appropriate data, we performed meta-analyses to obtain the pooled outcomes of RRs of seizure recurrence between the FUS-S and FUS-W groups and the SRRs of each group. In studies where the follow-up period was not precise, or the follow-up period was described as the mean or median, data corresponding to the effect size were extracted and analyzed separately.
Preliminary reviews confirmed that there was variation in the research. The ages of the subjects varied between studies, including children and adults. Some research also defined the sleep state as nighttime rather than actually being asleep. The strictness of inclusion and exclusion criteria also varied among the studies. Some studies excluded ASM use cases and remote symptomatic patients. The authors employed a random-effects model in the meta-analyses due to the suspected clinical variabilities between the previous studies.
The authors used Cochran’s Q test and I-squared (I2) statistics to assess the level and impact of heterogeneity. We arbitrarily adopted an I2 threshold of >75% as an indicator of significant heterogeneity. We also evaluated the evidence for this heterogeneity by observing the localizations and 95% confidence intervals (CIs) in forest plots. All statistical analyses were performed using the ‘meta’ package of R software (version 4.18-0).7,8
We performed subgroup analyses of two pooled estimates of SRR between FUS-S and FUS-W and SRR in FUS-S. These were subgroup analyses conducted between pediatric studies and adult studies, and those between studies that defined sleep state as being asleep and studies defined as nighttime. Cochran’s Q test was used to test subgroup differences (random-effects model) to obtain a Q value for these subgroup analyses. If p<0.05 in the test, there was considered to be no heterogeneity among subgroups.
The sensitivity test for the RR was performed for two aspects identified only in a part of the primary studies but may act as confounding factors. The two sensitivity tests were one that excluded studies that included certain ASM use cases, and the other that excluded a few studies that excluded remote symptomatic patients.
Only seven of the selected studies offered data for the meta-analyses of second-year outcomes. The total number of studies was therefore less than ten, which made it impossible to assess publication bias. As a result, it was difficult to evaluate the small-study effects on outcome reporting bias.
Quality assessment of studies
We used the Newcastle-Ottawa Scale (NOS) to estimate the risks of bias among the selected studies.9 The NOS evaluates each study in three dimensions (selection, comparability, and outcome) and has eight items, with four, one, and three items in the dimensions, respectively. When analyzing each NOS item, evaluators can assign a one-star score if it is of the highest quality. Studies that control for any additional factor may also receive an extra star in the comparability dimension, resulting in a two-star study. The total number of stars determines the quality score on the NOS for a piece of research. As a result, the quality score of each study can range between 0 and 9.
The NOS offers two versions for cohort research and case–control investigations, respectively. We adopted the cohort version because all of the research selected for this review were cohort studies. We considered NOS quality scores of 7–9 as indicating high quality and of 4–6 as indicating moderate quality. An author (OY Kwon) appraised the study quality using the NOS. Another author (TW Yang) reviewed the validity of these decisions to strengthen their reliability. When the latter author raised a dispute, the two reviewers debated and resolved it until a consensus was reached. If the two authors could not reach a consensus through discussion, the third reviewer (YS Kim) determined the quality.
Evaluation of evidence certainty for the outcomes
For the outcomes, one author (OY Kwon) also evaluated the certainty of evidence, and another author (TW Yang) determined the validity. We referred to methods and recommendations described in the Cochrane Handbook for Systematic Reviews of Interventions for the assessment.10 GRADEpro GDT software (2015 version) was used to prepare the findings summary tables.11,12
The studies were evaluated by considering the five evaluation items of GRADE: study limitations, effect consistency, imprecision, indirectness, and publication bias. We assessed the studies that contributed data to the outcome meta-analyses individually and integrated these results.
RESULTS
Selected studies
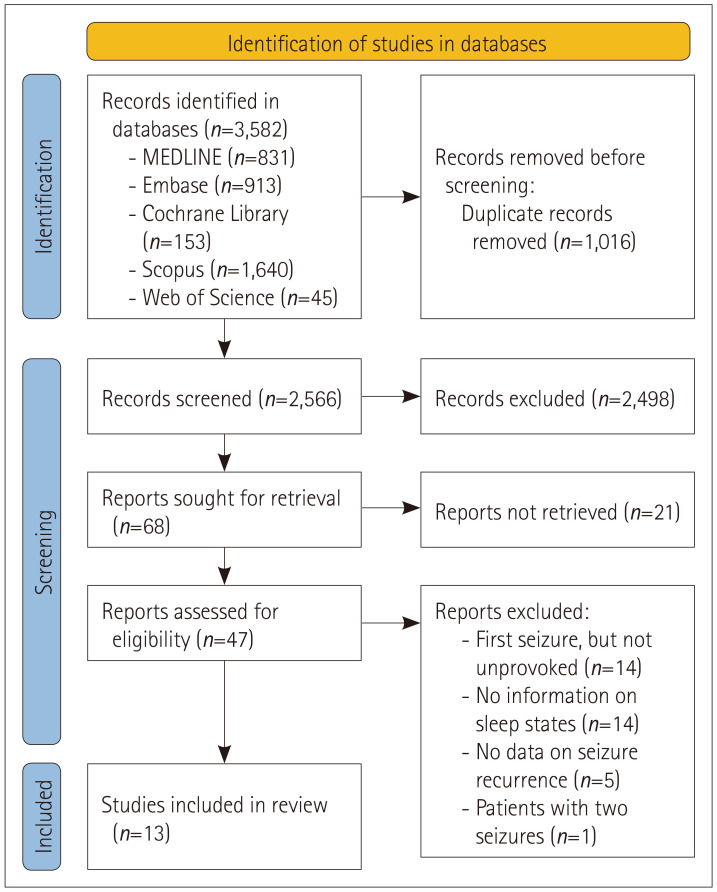
Our database search identified 3,582 studies. EndNote software was used to remove 1,016 duplicate studies, and the remaining 2,566 were included in the subsequent screening process.13 The screening process excluded 2,498 studies, and the full texts of the remaining 68 were found. We excluded 21 ineligible studies and assessed the remaining 47 to determine if they were relevant for inclusion in this systematic review.
We excluded 34 studies for various reasons after reviewing the eligibility of the 47 (Table 1). The reasons for exclusion were as follows (Fig. 1): Subjects with a first seizure were included in 14 studies, but the study subjects did not experience an FUS; instead, they were patients with epilepsy or children with febrile convulsion. There was no information on the sleep state of people with FUS in 14 other studies. There was no information on seizure recurrence in another five studies that analyzed FUS. Finally, the last study analyzed people with seizures twice. Table 1 lists the study characteristics of the contents extracted from the 13 studies, as described in the Methods section. Table 2 presents the SRRs as percentages for the FUS-S and FUS-W groups at the six follow-up time points until 5 years after the occurrences of FUS from the eight primary studies that reported SRRs at specific follow-up time points.
Table 1. Characteristics of the finally selected studies.
| Study | Country | Study design | Number of hospitals | Clinical setting | Sleep-state definition | Follow-up time-point data | Number of participants | Sample size* | Age group | Sex, female | Confounding cases | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Seizure types | ASM use | Remote symptomatic | |||||||||||
| Sathirapanya 20205 | Thailand | R | One | Emergency room | Asleep | No | 414 | 391 | Adults, adolescents | 35.5% | NA | NA | Yes |
| Maia 201714 | Portugal | R | One | Inpatients | Asleep | No | 86 | 86 | Children, adolescents | 61.6% | Various | No | Yes |
| Zhang 201721 | China | R | One | Inpatients | Asleep | Yes | 190 | 190 | Children ≤3 years | 48.9% | NA | NA | Yes |
| Scotoni 200415 | Brazil | P | Two | Emergency room | Asleep | No | 213 | 213 | Children, adolescents | 41.8 % | Excluding some types† | No | No |
| Winckler 200416 | Brazil | P | One | Outpatients | Asleep | Yes | 109 | 109 | Children, adolescents | 35.8% | NA | No | Yes |
| Ramos Lizana 200022 | Spain | P | One | NA | Asleep | Yes | 217 | 217 | Children, adolescents | 38.7% | Various | Yes | Yes |
| Martinovic´ 199717 | Yugoslavia | P | One | NA | Asleep | No | 78 | 50 | Children, adolescents, young adults | 41.0% | GTCs | Yes | No |
| Shinnar 199618 | USA | P | Multiple | NA | Asleep | Yes | 407 | 404 | Children, adolescents, young adults | 42.5% | Excluding some types† | Yes | Yes |
| Bora 199519 | Turkey | P | One | Outpatients | Nighttime | Yes | 147 | 147 | Adults, adolescents | 47.6% | GTCs | Yes | No |
| Shinnar 19932 | USA | P | Multiple | NA | Asleep | Yes | 347 | 327 | Children, adolescents, young adults | 42.9% | NA | Yes | Yes |
| van Donselaar 199120 | Netherlands | P | Multiple | NA | Asleep | Yes | 165 | 165 | Adults, adolescents | 41.2% | Various | No | No |
| Hopkins 19883 | UK | P | One | Outpatients, inpatients, private patients | Nighttime | Yes | 408 | 304 | Adults, adolescents | 40.5% | Excluding some types† | Yes | Yes |
| Camfield 19856 | Canada | R | One | EEG | Asleep | No | 168 | 149 | Children, adolescents | 49.4% | Excluding some types† | NA | Yes |
*Number of patients with sleep information; †Excluding absence seizures, myoclonic seizures, and infantile spasm.
ASM, antiseizure medication; EEG, electroencephalography; GTCs, generalized tonic–clonic seizures; NA, not available; P, prospective; R, retrospective.
Fig. 1. Identification of relevant studies based on the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 (PRISMA 2020). Among 3,582 studies identify by database searching, we removed 1,016 studies that represented duplicated data. There were 68 studies left after screening the remaining 2,566 studies. After excluding 21 studies through a preliminary review, we evaluated whether the remaining 47 were eligible for inclusion in this systematic review by carefully assessing their full texts. We eventually included 13 studies in this review after removing 34 of the 47 articles for various reasons.
Table 2. Seizure recurrence rates (SRRs) in people with first unprovoked seizure (FUS) at six time points during 5 years of follow-up in two groups according to whether the FUS occurred while sleeping (FUS-S) or when waking (FUS-W).
| Study | SRR (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FUS-S | FUS-W | |||||||||||
| 6 months | 1 year | 2 years | 3 years | 4 years | 5 years | 6 months | 1 year | 2 years | 3 years | 4 years | 5 years | |
| Zhang 201721 | NA | NA | NA | NA | NA | 75.5 | NA | NA | NA | NA | NA | 43.3 |
| Winckler 200416 | NA | NA | 69.2 | NA | NA | NA | NA | NA | 43.4 | NA | NA | NA |
| Ramos Lizana 200022 | NA | 59.7 | 74.6 | NA | NA | NA | NA | 38.7 | 49.3 | NA | NA | NA |
| Shinnar 199618 | NA | NA | 49.6 | NA | NA | 52.6 | NA | NA | 31.0 | NA | NA | 36.2 |
| Bora 199519 | 36.5 | 44.4 | 54.0 | 54.0 | NA | NA | 23.8 | 29.8 | 32.1 | 33.3 | NA | NA |
| Shinnar 19932 | 27.8 | 39.2 | 52.6 | NA | 54.6 | NA | 17.8 | 23.0 | 30.0 | NA | 14.8 | NA |
| van Donselaar 199120 | NA | NA | 72.7 | NA | NA | NA | NA | NA | 32.2 | NA | NA | NA |
| Hopkins 19883 | 37.2 | 46.9 | 56.0 | 56.0 | 56.0 | NA | 28.9 | 32.0 | 40.2 | 41.2 | 41.2 | NA |
NA: not available.
Quality assessment of the studies
Supplementary Table 1 (in the online-only Data Supplement) lists the risks of bias of each of the included studies evaluated using the NOS. Nine studies controlled confounding factors by excluding ASM use cases, remote symptomatic patients, or restricted seizure types. We therefore assigned two stars to the “comparability” item of the NOS for the nine studies.3,6,14,15,16,17,18,19,20 We did not assign a star to the “length of follow-up” item for five studies because those studies did not explicitly address the follow-up period. Those five studies provided the median5 or average6,15,17 of the follow-up period of all subjects, or only descriptive information.14 The NOS star scores awarded to 13 studies ranged from 7 to 9 points: 15 received 7 points, 72,6,14,15,17,21,22 received 8 points, and 53,16,18,19,20 received 9 points.
Results of syntheses
SSR data at specific follow-up time points were presented for 8 of the 13 studies (Table 2). At the 2-year follow-up time point, 7 studies2,3,16,18,19,20,22 provided data. The median SRR was 56.0% in the FUS-S group with a range of 49.6%–74.6%, and that in the FUS-W group was 32.2% with a range of 30.0%–49.3% among the seven studies for which data at the 2-year follow-up time point were provided.
At the other time points of 6 months and 1, 3, 4, and 5 years, data were provided by only two to four studies. Seizure recurrence data were reported for three studies at the 6-month time point, for four studies at the 1-year time point, and for two at the 3, 4, and 5-year time points.
Some people with FUS were lost to follow-up in 3 of the 13 included studies.6,14,21 In 1 study, 8 out of 176 subjects had only 1 visit to the hospital and never returned.6 In another study, 17 of the 103 subjects were lost to follow-up,14 while 11 out of 190 individuals were lost during the follow-up period in the third study.21 The seven studies that provided seizure recurrence information at the 2-year follow-up time point for the meta-analyses in the present review had no missing results.
After merging 7 cohort studies that detected SRRs after 2 years of follow-up in both the FUS-S and FUS-W groups, the total number of people with FUS was 1,659: 626 with FUS-S and 1,033 with FUS-W.
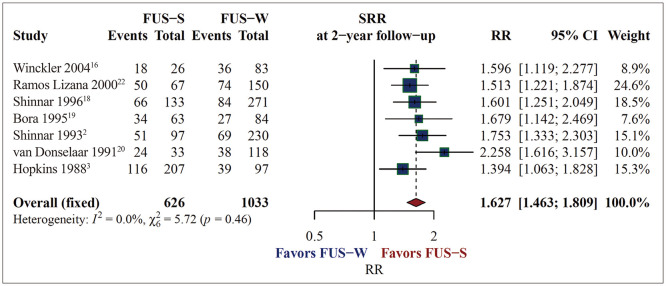
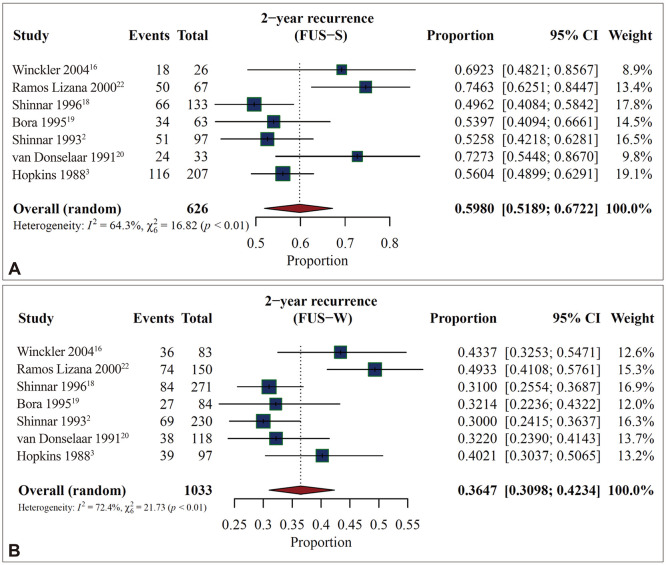
The occurrence of FUS-S was linked to a significant 62.7% increase in the probability of seizure recurrence (RR=1.627, 95% CI=1.463–1.809, p<0.0001, I2=0.0%) (Fig. 2). The SRR was 59.8% (95% CI=51.9–67.2, I2=64.3%) in the FUS-S group and 36.5% (95% CI=31.0–42.3, I2=72.4%) in the FUS-W group (Fig. 3). In one of the seven studies that found SRRs after 2 years of follow-up, both the FUS-S and FUS-W groups exhibited higher SRRs compared with the other studies.22
Fig. 2. Increasing seizure recurrence risk (SRR) after 2 years of follow-up if a first unprovoked seizure (FUS) occurs while sleeping. The total number of patients was 1,659 when combining the 7 cohort studies, with 626 people with the FUS occurring while sleeping (FUS-S) and 1,033 persons with it occurring when waking (FUS-W). The occurrence of FUS-S increased the SRR in people with FUS by 62.7%, according to the relative risk (RR) integrated using a random-effects model (RR=1.627, 95% CI=1.463–1.809, p<0.0001, I2=0.0%). CI, confidence interval.
Fig. 3. Higher seizure recurrence rate (SRR) after 2 years of follow-up in first unprovoked seizures while sleeping (FUS-S) than those while waking (FUS-W). After integrating the seven cohort studies, the SSR was 59.8% in the FUS-S group (A) and 36.5% in the FUS-W group (B). CI, confidence interval.
Based on the 95% CI lines in the forest plot, this study could identify the leading cause of the heterogeneity. The investigators did not place any seizure type restrictions on the subjects selected for the study and described their seizure types as varied. It is therefore reasonable to assume that this study could have had more seizure types with high relapse than other studies. Aside from that, determining the cause of clinical heterogeneity was difficult.
Subgroup analyses
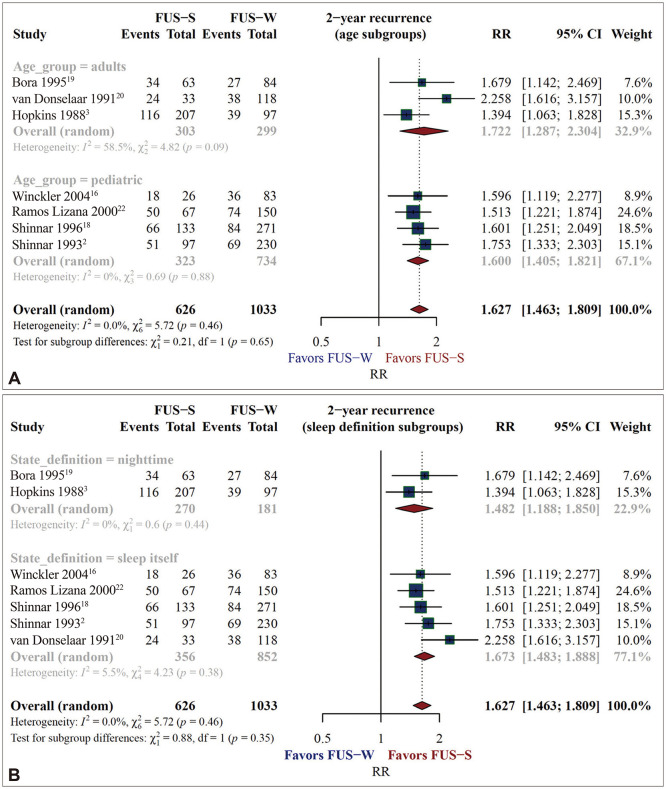
The seven studies finally enrolled comprised three involving adults and four involving children. Two studies defined sleep as nighttime, and five studies defined it as being asleep. The age groups and sleep-state definitions did not affect the pooled RR and SRR estimates in the subgroup analyses.
The forest plots in Fig. 4 present the results of the subgroup analyses for RRs. The RR was 1.722 (95% CI=1.287–2.304, I2=58.5%) among the adult studies and 1.600 (95% CI=1.405–1.821, I2=0.0%) among the pediatric studies. The Q value for the subgroup difference test in this analysis was 0.21, with p=0.65. The RR was 1.482 (95% CI=1.188–1.850, I2=0.0%) in the nighttime-definition studies, and 1.673 (95% CI=1.483–1.888, I2=5.5%) in the being-asleep-definition studies. The Q value for the subgroup difference test in this analysis was 0.88, with p=0.35.
Fig. 4. No significant changes of RRs of seizure recurrence after 2 years of follow-up in subgroup analyses by subject age or sleep definition. The RR was 1.722 in the three adult studies and 1.600 in the four pediatric studies (A). The RR was 1.482 in the two studies that defined sleep as nighttime and 1.673 in the five studies that defined it as actually being asleep (B). FUS, first unprovoked seizure; FUS-S, FUS while sleeping, FUS-W, FUS when waking; RR, relative risk.
The forest plots in Supplementary Fig. 1 (in the online-only Data Supplement) present the results of the subgroup analyses for SRRs. The SRR was 57.2% (95% CI=51.5–62.7, I2=43.0%) in the adult studies and 60.8% (95% CI=47.6–72.6, I2=77.4%) in the pediatric studies. The Q value for the subgroup difference test was 0.24, with p=0.62. The SRR was 55.6% (95% CI=49.6–61.4, I2=0.0%) in the nighttime-definition studies and 62.8% (95% CI=51.1–73.2, I2=75.6%) in the being-asleep-definition studies. The Q value for the subgroup difference test was 1.21, with p=0.27.
Sensitivity analyses
In the sensitivity analyses that revalidated the pooled estimates while removing potential biases, the RRs were consistent with the result of a primary meta-analysis. The results of the sensitivity analyses are also presented in Supplementary Fig. 2 (in the online-only Data Supplement). The analysis of the remaining five studies revealed an RR of 1.57 (95% CI=1.39–1.76, p<0.0001, I2=0.0%), eliminating two studies that completely excluded the cases with ASM use. In another analysis of the five remaining trials, the RR was 1.56 (95% CI=1.39–1.75, p<0.0001, I2=0.0%), removing two studies that did not include remote symptomatic cases.
The sensitivity analyses performed on the SRRs of people with FUS-S also yielded values similar to those in the initial meta-analysis. The forest plots of the sensitivity analyses are presented in Supplementary Fig. 3 (in the online-only Data Supplement). The SRR was 56.9% (95% CI=48.7–64.7, I2=65.5%) in the analysis of studies that controlled for cases that used ASM, while it was 59.3% (95% CI=49.5–68.3, I2=69.9%) in the analysis of studies that controlled for remote symptomatic patients.
Studies without follow-up time-point information
We could not obtain SRRs at follow-up time points for 5 of the 13 studies. Those studies provided the follow-up duration of the subjects as a mean or a median, or only descriptive information.5,6,14,15,17 As a result, we could not include these studies in the meta-analyses, which are instead summarized in Table 3. The SRRs ranged from 39.7% to 82.8% in the FUS-S group and from 28.1% to 52.8% in the FUS-W group.
Table 3. SRRs in people with FUS in studies with unclear follow-up time points.
| Study | Follow-up time point | SRR (%) | |
|---|---|---|---|
| FUS-S | FUS-W | ||
| Camfield 19856 | 31.4±16.0 months (mean±SD) | 46.5 | 52.8 |
| Sathirapanya 20205 | Median, 33.6 months | 39.7 | 28.1 |
| Martinovic´ 199717 | Mean, 4.1 years | 82.8 | 38.1 |
| Maia 201714 | 86 cases: 26 at >5 years, 35 at 2–5 years, 25 at <2 years | 63.6 | 29.7 |
| Scotoni 200415 | Mean 25.7 months among patients with no recurrence, 84% for >6 months, 70% for >1 year | 47.9 | 30.3 |
FUS, first unprovoked seizure; FUS-S, FUS while sleeping, FUS-W, FUS when waking; SRR, seizure recurrence rate
Certainty of evidence for the outcomes
The RR estimate was rated as having a low certainty in the certainty-of-evidence evaluation using GRADEpro GDT 2015 (Supplementary Table 2 in the online-only Data Supplement).11,12 This was attributed to the observational design of the studies included in the meta-analyses. However, given the subject matter of this review, it appears that we may be able to draw a different judgement. It is impossible to randomize whether FUS artificially occurs while sleeping or when awake. The studies in this review that included consecutive subjects with FUS suggest that the process was naturally randomized. Considering that, we may judge the certainty of the evidence of the RR estimate as moderate or high. The SRRs in FUS-S and FUS-W had high certainty evidence (Supplementary Table 3 in the online-only Data Supplement).
DISCUSSION
The pooled estimated RR of seizure recurrence between FUS-S and FUS-W was statistically significant in this review. The SRR was 62.7% higher in FUS-S than in FUS-W. There was no heterogeneity among the RRs of the primary studies when combining them into a pooled estimate. Furthermore, the subgroup analysis demonstrated that the RRs did not differ between age groups. This means that our review has clearly indicated that if FUS occurs while sleeping, seizure recurrence is more likely.
Previous research on the impact of sleep on SRR in people with FUS has not offered enough information, with reports of the SRR being 56% for adults with FUS-S and 53% for children with FUS-S.2,3 The pooled estimate SRR among people with FUS-S was higher in the present review, with the probability of seizure recurrence after 2 years of follow-up being 59.8% in FUS-S and 36.5% in FUS-W. This information will help determine how to manage people with FUS in clinical practice.
The ILAE released a practical clinical definition of epilepsy in 2014.1 According to one of the three criteria in the definition, epilepsy can be diagnosed if the risk of a seizure recurrence in the next 10 years is at least 60% in a person who has had one unprovoked or reflex seizure. After integrating primary studies in this review, the SRR in people with FUS-S was 59.8% after 2 years of follow-up, indicating that if they are followed up for 10 years, the SRR will be considerably higher than 60%. Based on the practical and clinical definitions from the ILAE, FUS-S is likely to be diagnosed as epilepsy.
Epilepsy is a tendency to develop seizures that persist in the brain. However, physicians find it challenging to diagnose epilepsy using this description alone in practice. This review has provided physicians with essential clinical information that they can refer to when deciding on ASM treatment for people with FUS. According to the results of this review, the SRR of FUS-S is sufficiently high, so it can be a meaningful basis when actively considering ASM treatment.
We concede that some confounding effects might have been present in the primary studies included in this review. Various circumstances can influence seizure recurrence in people with FUS. For example, the SRR is higher in remote symptomatic patients than in idiopathic cases.23,24 Among the primary studies included in this review, the confounding factor controls for seizure recurrence varied among the recruited people with FUS. Controls for ASM use, remote symptomatic cases, and seizure types differed among studies, with some not controlling them at all. The variability of the confounding factor controls might have influenced the heterogeneity of SRR among the primary studies. To improve study quality, researchers should account for confounding factors. Researchers will only be able to determine SRRs more precisely in the future if they can collect studies that have carefully adjusted for confounding factors.
We divided the primary studies into pediatric and adult studies based on the age of the subjects. Also, in some of the studies, the sleep state was defined as nighttime rather than being asleep. Such research could not consider FUS that happened at night but not when sleeping. We conducted subgroup analyses based on the target age groups and sleep definitions to account for these elements of the primary studies. Subject ages and sleep definitions did not affect the pooled estimates in our meta-analyses. Gathering more primary studies in the future might make it possible to find distinctions that we were unable to.
It was challenging to identify follow-up time-point information for SRRs in several of the selected studies, which meant that those studies could not be included in our meta-analyses. Because the follow-up period was presented a mean or median value or as descriptive information in those studies, the follow-up periods of subjects would be variable. Such studies might have included subjects with short follow-up periods to accurately assess seizure recurrence. More accurate data will be accessible in the future if researchers conduct more prospective studies with specific follow-up time points.
For the follow-up time point other than 2 years, only 2 to 4 of the 13 studies included in this review had information on SRR, thus the SRR outcomes at those time points were not acceptable for meta-analysis. It will be feasible to obtain a better understanding of the prognosis of people with FUS in the future if an appropriate number of studies are gathered with defined follow-up points, especially with long-term follow-up durations exceeding 2 years.
All of the studies suitable for inclusion in this systematic review were hospital-based. Finding studies conducted on the general population is challenging. To obtain more practical information, it would be better for future analyses to include studies that extracted information from large samples from the general population.
Lastly, there was another limitation from methodological aspects of this review. In this review, one author evaluated the risk of bias, extracted data, and assessed the certainty of the evidence, and another author checked their reliability. Since this process was performed separately but not independently by the two authors, the risk of error might have been present. Nevertheless, a methodological limitation of this type would not have greatly affected the overall conclusions of this review.
Footnotes
- Conceptualization: Tae-Won Yang, Young-Soo Kim, Do-Hyung Kim, Oh-Young Kwon.
- Data curation: all authors.
- Formal analysis: Oh-Young Kwon.
- Investigation: Hongmin Ha, Tae-Won Yang, Oh-Young Kwon.
- Methodology: Tae-Won Yang, Oh-Young Kwon.
- Project administration: Oh-Young Kwon.
- Software: Tae-Won Yang, Oh-Young Kwon.
- Supervision: Oh-Young Kwon.
- Validation: all authors.
- Visualization: Tae-Won Yang, Oh-Young Kwon.
- Writing—original draft: Oh-Young Kwon.
- Writing—review & editing: Tae-Won Yang, Oh-Young Kwon.
Conflicts of Interest: Oh-Young Kwon, a contributing editor of the Journal of Clinical Neurology, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Funding Statement: None
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2022.18.6.642.
Quality of the 13 selected studies assessed with the cohort version of the Newcastle-Ottawa Scale
GRADEpro GDT 2015 certainty-of-evidence evaluation for relative risk (RR) of seizure recurrence in people with a first unprovoked seizure (FUS) according to sleeping or waking state after 2 years of clinical follow-up
GRADEpro GDT 2015 certainty-of-evidence evaluation for seizure recurrence rate (SRR) after 2 years of clinical follow-up in people with FUS-S and people with FUS-W
No significant change of seizure recurrence rates (SRRs) after 2 years of follow-up in subgroup analyses by subject age or sleep definition. The SRR was 57.2% in adult studies and 60.8% in pediatric studies (A), and 55.6% in nighttime-definition studies and 62.8% in being-asleep-definition studies (B). CI, confidence interval; FUS-S, first unprovoked seizure while sleeping.
No significant change of relative risks (RRs) after 2 years of follow-up in sensitivity analyses. The RRs were consistent with the findings of the primary meta-analysis. The RR was 1.57 when excluding two studies that controlled antiseizure medication (ASM) use (A), and 1.56 when excluding two studies that controlled remote symptomatic cases (B).
No significant changes of seizure recurrence rate (SRR) after 2 years of follow-up in sensitivity analyses. Sensitivity analyses of SRRs performed by removing each potential bias factor also showed results consistent with those of the primary meta-analysis. The SSR was 56.9% when excluding two studies that controlled antiseizure medication (ASM) use (A), and 59.3% when excluding two studies that controlled remote symptomatic cases (B). CI, confidence interval; FUS, first unprovoked seizure; FUS-S, FUS while sleeping.
References
- 1.Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475–482. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- 2.Shinnar S, Berg AT, Ptachewich Y, Alemany M. Sleep state and the risk of seizure recurrence following a first unprovoked seizure in childhood. Neurology. 1993;43:701–706. doi: 10.1212/wnl.43.4.701. [DOI] [PubMed] [Google Scholar]
- 3.Hopkins A, Garman A, Clarke C. The first seizure in adult life. Value of clinical features, electroencephalography, and computerised tomographic scanning in prediction of seizure recurrence. Lancet. 1988;1:721–726. doi: 10.1016/s0140-6736(88)91535-8. [DOI] [PubMed] [Google Scholar]
- 4.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sathirapanya P, Praipanapong A, Kongkamol C, Chongphattararot P. Predictors of early recurrent seizure after first seizure presentation to an emergency service: a retrospective cohort study. Seizure. 2020;78:1–6. doi: 10.1016/j.seizure.2020.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Camfield PR, Camfield CS, Dooley JM, Tibbles JA, Fung T, Garner B. Epilepsy after a first unprovoked seizure in childhood. Neurology. 1985;35:1657–1660. doi: 10.1212/wnl.35.11.1657. [DOI] [PubMed] [Google Scholar]
- 7.R Core Team. R: a language and environment for statistical computing. R version 4.1.1 [software] Vienna: R Foundation for Statistical Computing; 2021. [updated 2021 Aug 10]. Available from: https://www.R-project.org/ [Google Scholar]
- 8.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet] Ottawa: Ottawa Hospital Research Institute; 2000. [updated 2000 Jan 1]. [cited 2021 Nov 3]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp . [Google Scholar]
- 10.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Hoboken, NJ: Wiley-Blackwell; 2019. [Google Scholar]
- 11.McMaster University. GRADEpro GDT: GRADEpro guideline development tool [software] Hamilton: Evidence Prime, Inc.; 2020. [Google Scholar]
- 12.Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. [place unknown]: The GRADE Working Group; 2013. [updated 2021 Oct 20]. Available from: https://gdt.gradepro.org/app/handbook/handbook.html . [Google Scholar]
- 13.The EndNote Team. EndNote 20 [software] Philadelphia, PA: Clarivate Analytics; 2020. [Google Scholar]
- 14.Maia C, Moreira AR, Lopes T, Martins C. Risk of recurrence after a first unprovoked seizure in children. J Pediatr (Rio J) 2017;93:281–286. doi: 10.1016/j.jped.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Scotoni AE, Manreza ML, Guerreiro MM. Recurrence after a first unprovoked cryptogenic/idiopathic seizure in children: a prospective study from São Paulo, Brazil. Epilepsia. 2004;45:166–170. doi: 10.1111/j.0013-9580.2004.16503.x. [DOI] [PubMed] [Google Scholar]
- 16.Winckler MI, Rotta NT. Clinical and electroencephalographic follow-up after a first unprovoked seizure. Pediatr Neurol. 2004;30:201–206. doi: 10.1016/j.pediatrneurol.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Martinović Z, Jović N. Seizure recurrence after a first generalized tonic-clonic seizure, in children, adolescents and young adults. Seizure. 1997;6:461–465. doi: 10.1016/s1059-1311(97)80021-0. [DOI] [PubMed] [Google Scholar]
- 18.Shinnar S, Berg AT, Moshe SL, O’Dell C, Alemany M, Newstein D, et al. The risk of seizure recurrence after a first unprovoked afebrile seizure in childhood: an extended follow-up. Pediatrics. 1996;98(2 Pt 1):216–225. [PubMed] [Google Scholar]
- 19.Bora I, Seçkin B, Zarifoglu M, Turan F, Sadikoglu S, Ogul E. Risk of recurrence after first unprovoked tonic-clonic seizure in adults. J Neurol. 1995;242:157–163. doi: 10.1007/BF00936889. [DOI] [PubMed] [Google Scholar]
- 20.van Donselaar CA, Geerts AT, Schimsheimer RJ. Idiopathic first seizure in adult life: who should be treated? BMJ. 1991;302:620–623. doi: 10.1136/bmj.302.6777.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, Huang Z, Tang J, Li Y. Risk factors following first spontaneous epileptic seizure in children below 3 years of age. Int J Neurosci. 2017;127:745–751. doi: 10.1080/00207454.2016.1243105. [DOI] [PubMed] [Google Scholar]
- 22.Ramos Lizana J, Cassinello Garciá E, Carrasco Marina LL, Vázquez López M, Martín González M, Muñoz Hoyos A. Seizure recurrence after a first unprovoked seizure in childhood: a prospective study. Epilepsia. 2000;41:1005–1013. doi: 10.1111/j.1528-1157.2000.tb00286.x. [DOI] [PubMed] [Google Scholar]
- 23.Berg AT, Shinnar S. The risk of seizure recurrence following a first unprovoked seizure: a quantitative review. Neurology. 1991;41:965–972. doi: 10.1212/wnl.41.7.965. [DOI] [PubMed] [Google Scholar]
- 24.Hauser WA, Rich SS, Lee JR, Annegers JF, Anderson VE. Risk of recurrent seizures after two unprovoked seizures. N Engl J Med. 1998;338:429–434. doi: 10.1056/NEJM199802123380704. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Quality of the 13 selected studies assessed with the cohort version of the Newcastle-Ottawa Scale
GRADEpro GDT 2015 certainty-of-evidence evaluation for relative risk (RR) of seizure recurrence in people with a first unprovoked seizure (FUS) according to sleeping or waking state after 2 years of clinical follow-up
GRADEpro GDT 2015 certainty-of-evidence evaluation for seizure recurrence rate (SRR) after 2 years of clinical follow-up in people with FUS-S and people with FUS-W
No significant change of seizure recurrence rates (SRRs) after 2 years of follow-up in subgroup analyses by subject age or sleep definition. The SRR was 57.2% in adult studies and 60.8% in pediatric studies (A), and 55.6% in nighttime-definition studies and 62.8% in being-asleep-definition studies (B). CI, confidence interval; FUS-S, first unprovoked seizure while sleeping.
No significant change of relative risks (RRs) after 2 years of follow-up in sensitivity analyses. The RRs were consistent with the findings of the primary meta-analysis. The RR was 1.57 when excluding two studies that controlled antiseizure medication (ASM) use (A), and 1.56 when excluding two studies that controlled remote symptomatic cases (B).
No significant changes of seizure recurrence rate (SRR) after 2 years of follow-up in sensitivity analyses. Sensitivity analyses of SRRs performed by removing each potential bias factor also showed results consistent with those of the primary meta-analysis. The SSR was 56.9% when excluding two studies that controlled antiseizure medication (ASM) use (A), and 59.3% when excluding two studies that controlled remote symptomatic cases (B). CI, confidence interval; FUS, first unprovoked seizure; FUS-S, FUS while sleeping.
Data Availability Statement
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.