Abstract

Spodoptera frugiperda is a species of the order Lepidoptera. It is one of the species of fall armyworm moths distinguished by its larval life stage, is found in different regions of Africa, and can cause incredible damage. This is the first report produced by the preparation of an indexed combinatorial library of novel chalcone derivatives 3a–kvia treatment of 4-formylphenyl4-methylbenzenesulfonate (1) with some acetyl compounds 2a–k in the presence of NaOH. The structures of the synthesized compounds were proven by different spectroscopic techniques such as infrared, 1H NMR, 13C NMR, and elemental analyses. In this work, we studied their toxicity effect against S. frugiperda, followed by a structure–reaction relationship. Moreover, newly prepared chalcone derivatives were tested as insecticides using S. frugiperda. It has been found that most compounds have good to excellent potential effectiveness. Among all of the compounds, 3b, 3g, and 3j exhibited excellent effectiveness. Furthermore, compound 3c showed the most activity, with LC50 = 9.453 ppm of the second instar larva and LC50 = 66.930 of the fourth instar larva .
1. Introduction
Spodoptera frugiperda is one of the most dangerous insects that have recently entered Egypt1−3 and has affected farmers, particularly those who currently grow maize. It was also responsible for the destruction of the corn crop in the year 2021. It can devour 80 other crops in a few days, including rice, cotton, sugar cane, sorghum, and other important crops.4,5 Recently, researchers introduced some newly prepared organic compounds, which are sustainable against this insect and harmless to people and the environment.6 The synthesized compounds that contain a chalcone moiety or a sulfonate group were very effective against S. frugiperda,7 and it is known that chalcones are one of the most important types of natural products belonging to the flavonoid family.8 Moreover, chalcones are important compounds in medicinal and biological activities such as antileishmanial, antiparasitic,9 anticancer, anti-inflammatory, cardiovascular, antitumor,10 and antitubercular activities.11 Moreover, they represent anti-agents in the agricultural field.12,13 Accordingly, these compounds have been used in engineered agrochemicals and to formulate manufactured mixtures possessing many pharmacological effects.14,15 Due to the presence of a conjugated enone system, chalcones are very reactive species, Figure 1.16 Therefore, chalcone compounds are considered widely applicable for the design and synthesis of novel biological and agricultural fields.17
Figure 1.
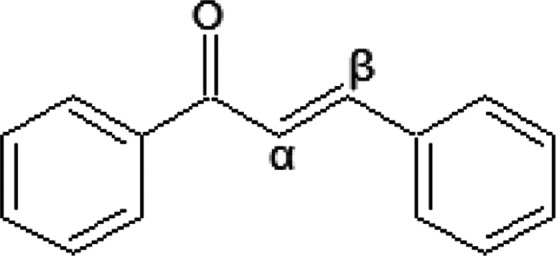
Molecule of chalone.
Therefore, in view of these observations and in conjunction with our previous interest in the synthesis of heterocyclic rings,18−25 we aimed here to find new insecticides for the synthesis of novel chalcone derivatives and the subsequent assessment of their toxicological and biological bio-efficacy against S. frugiperda.
2. Results and Discussion
2.1. Chemistry
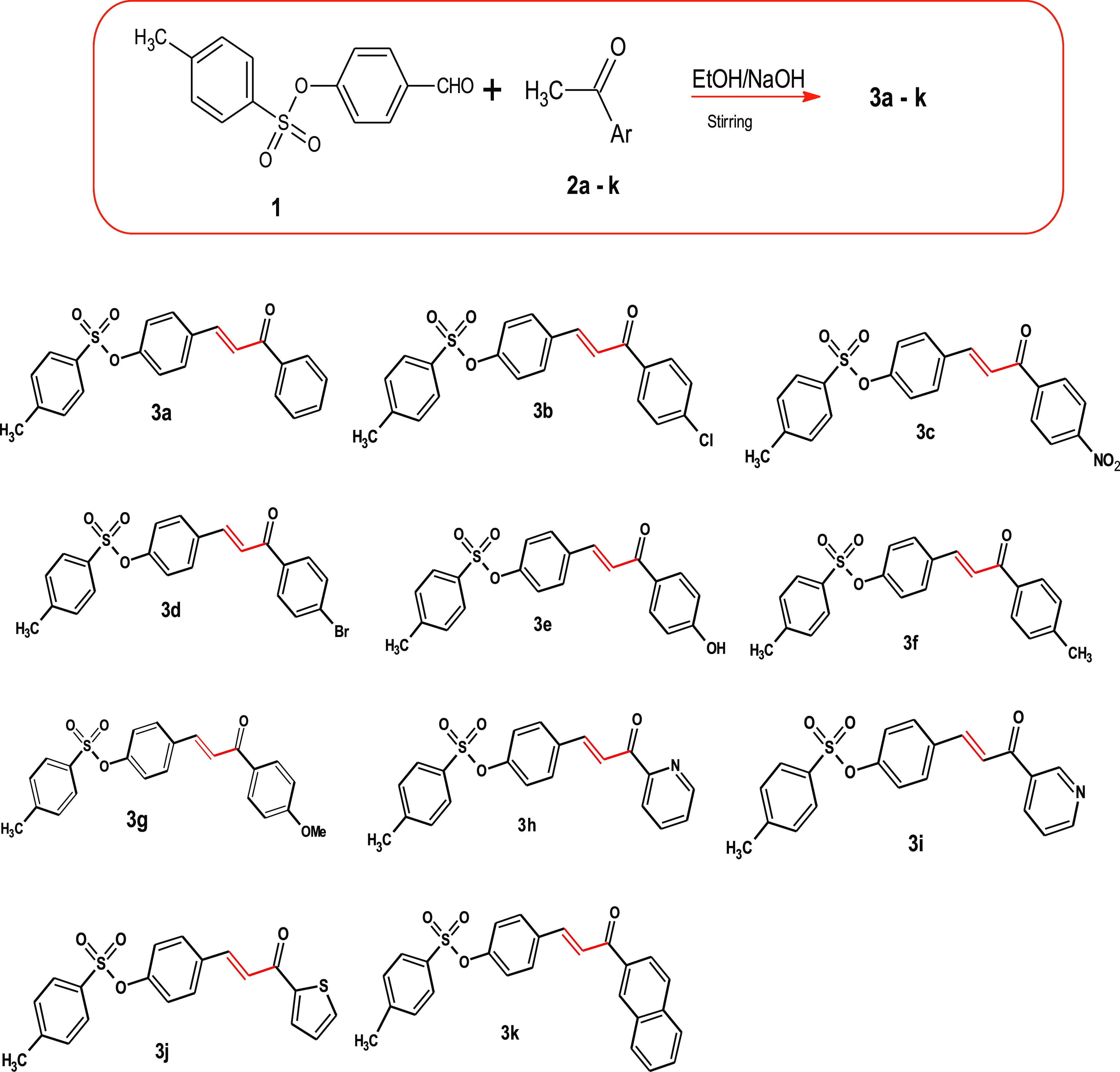
In this paper, it was presented that the reaction between 4-formylphenyl-4-methyl benzenesulfonate (1) and acetyl derivatives 2a–k, such as acetophenone (2a), 4-chloroacetophenone (2b), 4-bromoacetophenone (2c), 4-hydroxyacetophenone (2d), 4-methyl-acetophenone (2e), 4-methoxyacetophenone (2f), 2-acetylpyridine (2g), 4-acetylpyridine (2h), 2-acetylthiophene (2i), and 2-acetylnaphthalene (2k), in the presence of alcoholic sodium hydroxide afforded chalcone derivatives 3a–k in excellent yield (Scheme 1). Most of the advanced reactions are safe and easy, and pollution issues associated with toxic solvent use were avoided because we used a green solvent (mixed water and ethanol).
Scheme 1. Synthesis of Chalcone Derivatives.
The structures of the obtained chalcones 3a–k were determined by infrared (IR), 1H NMR, 13C NMR, and elemental analyses. The infrared spectra of these synthesized products 3a–k displayed the apparent bands in the region of δ 1678–1655 cm–1 due to C=O groups. The 1H NMR (DMSO-d6) spectra exhibited only aromatic proton signals located in the region δ 8.60–8.25 ppm, in addition to CH3 groups for all compounds at δ 2.44–2.41 ppm. Moreover, compounds 3e and 3f showed new signals at δ 2.40 ppm and at δ 3.88 ppm due to methyl and methoxy groups, respectively. Furthermore, their 13C NMR spectra indicated that new signals appeared at δ 189.62 and at δ 189.00 ppm due to C=O groups. Additionally, elemental analyses and mass spectra confirmed the correct structure.
2.2. Insecticidal Behavior
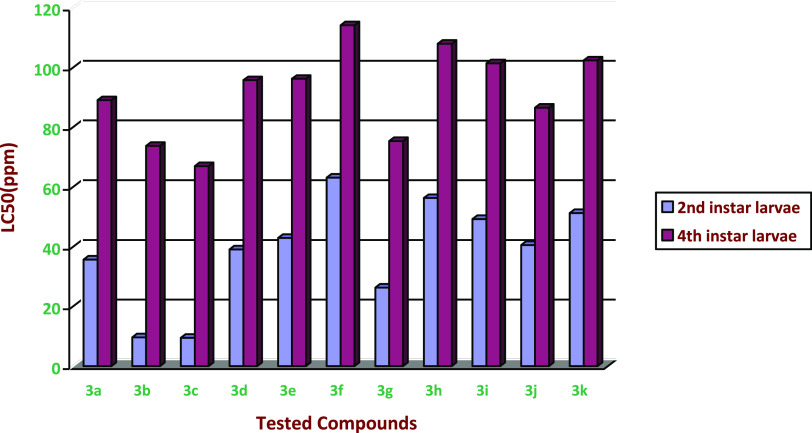
The laboratory bioassay analysis was conducted to estimate the insecticidal bioefficacy screening of 11 newly synthesized target compounds as toxicity agents against a sensitive strain of the second and fourth larval instar of the S. frugiperda pest. The synthesized and tested target compounds after 3 days of treatment gave high to moderate insecticidal activity, with LC50 values ranging from 63.100 to 9.543 ppm for second instar larvae and from 114.10 to 66.930 ppm for fourth instar larvae. Thus, the 11 tested target compounds showed insecticidal activity with the above-mentioned data after 3 days of treatment for S. frugiperda second and fourth instar larvae. To estimate the toxicological application of the newly synthesized compounds as insecticidal agents, their toxic activities against S. frugiperda were tested. Thus, the LC50 values were determined, as shown in Table 1 and Figures 2 and 3. The results show that compound 3c was more active than other chalcone-synthesized derivatives. The new synthesized compounds were screened for their insecticidal bioefficacy as shown below:
Table 1. Insecticidal Efficacy for Recently Synthesized Compounds after 3 Days of the Second and Fourth S. frugiperdsa Innstar Larvae.
| second
instar larvae |
fourth
instar larvae |
|||||||
|---|---|---|---|---|---|---|---|---|
| comp. | LC50 (ppm) | x2 | slope | toxic ratio | LC50 (ppm) | x2 | slope | toxic ratio |
| 3a | 35.646 | 0.887 | 1.113 ± 0.261 | 0.265 | 89.002 | 0.298 | 0.954 ± 0.267 | 0.752 |
| 3b | 9.616 | 0.596 | 0.930 ± 0.259 | 0.975 | 73.720 | 0.232 | 1.000 ± 0.255 | 0.907 |
| 3c | 9.453 | 0.969 | 0.711 ± 0.251 | 1 | 66.930 | 0.397 | 1.061 ± 0.268 | 1 |
| 3d | 39.036 | 0.699 | 1.040 ± 0.257 | 0.165 | 95.720 | 0.510 | 0.931 ± 0.288 | 0.699 |
| 3e | 42.921 | 0.377 | 0.956 ± 0.257 | 0.150 | 96.164 | 0.0958 | 0.888 ± 0.296 | 0.695 |
| 3f | 63.100 | 0.026 | 0.776 ± 0.266 | 0.149 | 114.10 | 0.261 | 0.853 ± 0.287 | 0.586 |
| 3g | 26.247 | 1.538 | 1.541 ± 0.288 | 0.360 | 75.308 | 1.197 | 1.003 ± 0.287 | 0.888 |
| 3h | 56.260 | 0.110 | 0.872 ± 0.255 | 0.168 | 107.93 | 0.186 | 0.851 ± 0.286 | 0.620 |
| 3i | 49.212 | 0.231 | 0.928 ± 0.256 | 0.192 | 101.36 | 0.235 | 0.891 ± 0.287 | 0.660 |
| 3j | 40.530 | 0.533 | 0.987 ± 0.257 | 0.232 | 86.511 | 0.346 | 0.968 ± 0.288 | 0.773 |
| 3k | 51.232 | 0.124 | 0.8625 ± 0.25 | 0.184 | 102.32 | 0.146 | 0.895 ± 0.277 | 0.648 |
Figure 2.
Insecticidal activity of selective chalcone derivatives 3a–k against the second and fourth S. frugiperda instar larvae after treatment.
Figure 3.

Insecticidal activities of selective compounds 3a–k for the second and fourth S. frugiperda instar larvae after treatment.
2.2.1. Insecticidal Bioefficacy Screening for the Second Instar Larvae of S. frugiperda after Treatment
As shown in Figures 1 and 2, the results of target compounds 3a–k tested against the instar larvae after 72 h are shown in Table 1. The results assured that all synthesized compounds have high to low insecticidal activity against the second larvae of S. frugiperda, with LC50 values ranging from 63.100 to 9.453 ppm, in which the results of compounds 3c, 3b, and 3g have promising insecticidal activity against the second instar larvae of S. frugiperda. Compounds 3c, 3b, 3g, and 3a possess high insecticidal bioefficacy and their LC50 values are 9.453, 9.616, 26.24, and 35.64 ppm, in which the toxic ratio is 1, 0.957, 0.360, and 0.265, respectively.
2.2.2. Insecticidal Bioefficacy Screening for the Fourth Instar Larvae of S. frugiperda after Treatment
As shown in Figures 1 and 2, the results of synthesized compounds 3a–k for the fourth instar larvae are confirmed, and the LC50 values are presented in Table 1. All the synthesized compounds exhibit strong to weak insecticidal activity, which showed varied values from 114.100 to 66.930 ppm. The median lethal concentration LC50 and slope values of the newly synthesized compounds were computerized by using a regression analysis program and reported as parts per million (ppm), Figure 3. The insecticidal activity of the target compounds 3a–k had an LC50 value as follows: 89.002, 73.720, 66.930, 95.720, 96.164, 114.10, 75.308, 107.93, 101.36, 86.511, and 102.32, respectively, in which second instar larvae are represented by black lines and fourth instar larvae are represented by red lines. As shown in the analysis data, chalcone derivative compound 3c is more active toward S. frugiperda than other synthesized compounds.
3. Structure–Activity Relationship
In this work, the insecticidal activity was estimated by the authors systematically against second and fourth instar larvae of S. frugiperda. In Table 1 and Figures 2 and 3, the high activity associated with compound 3c may be due to the presence of a nitrogen dioxide (NO2) moiety and a sulfonate group in the chemical structure, in addition to the conjugated enone system, whose presence in a compound structure makes it a very reactive species. Additionally, compound 3b gives high activity, which may be attributed to the presence of chloride atoms in their chemical structure group. The insecticidal activity of compounds 3a–k was compared to the effect of substituents; the obtained results showed that 3c > 3b > 3g > 3j > 3a > 3d > 3f > 3i > 3h > 3k > 3e.
4. Conclusions
By using an environmentally friendly method, we developed and synthesized a novel and ecologically sound series of some new bioactive chalcone derivatives that possess insecticidal toxicity. The prepared novel target compound structures were validated by using IR, 1H, and 13C NMR spectroscopic techniques and elemental analyses. Also, under laboratory conditions, the toxicological evaluation parameters of the newly synthesized products were investigated against S. frugiperda and LC50 values were estimated. Compounds 3c, 3b, 3g, and 3a show high insecticidal bioefficacy, with LC50 values of 9.453, 9.616, 26.247, and 35.646 ppm, respectively.
5. Materials and Methods
All the used chemical materials are from Aldrich, and they were used as received without any further purification. By using thin layer chromatography, all reactions were monitored. All melting points were recorded using the Kofler melting point device . IR spectroscopy was measured using a Bruker Alpha platinum-attenuated total reflection spectrophotometer. 1H NMR and 13C NMR data were detected using the Bruker BioSpin AG spectrometer at 400 MHz. The larvae of S. frugiperda insects were gathered from the cornfields of the Institute of Plant Protection at Shandweel, Sohag, Egypt.
6. Experimental Section
6.1. Chemistry
6.1.1. Synthesis of Chalcone Derivatives 3a–k
A sodium hydroxide solution of (1 mmol, 5 mL) was dissolved in water and added drop by drop to a mixture of (1 mmol) compound 1 and acetyl derivatives 2a–k, namely, acetophenone (2a), 4-chloroacetophenone (2b), 4-bromoacetophenone (2c), 4-hydroxyacetophenone (2d), 4-methyl-acetophenone (2e), 4-methoxyacetophenone (2f), 2-acetylpyridine (2g), 4-acetylpyridine (2h), 2-acetylthiophene (2i), and 2-acetylnaphthalene (2k). At room temperature, the mixture was stirred for 5 min in cold, neutralized with dilute HCl solution, washed with water several times, and filtered, and the formed precipitate was crystallized using ethanol.
6.1.2. 4-[3-Oxo-3-phenylprop-1-en-1-yl]phenyl-4-methylbenzenesulfonate (3a)
Yield: 87%; mp 146 °C. IR (ν) (KBr) cm–1: 3050 (C–H aromatic), 2951 (C–H aliphatic), 1661 (C=O), 1331, 1150 (SO2) cm–1; 1H NMR (DMSO-d6): δ 7.10–8.17 (m, 13H Ar–H and d, 2H of 2C–H), 2.43 (s, 3H, CH3). 13C NMR (DMSO-d6): δ 188.53, 150.75, 146.43, 145.75, 141.96, 136.20, 134.21, 134.22, 131.83, 130.97, 130.76, 129.40, 128.73, 123.54, 122.47, 21.46; Anal. Calcd for C22H18O4S (378); calcd C (69.82%), H (4.79%), S (8.47). Found: C (69.81%), H (4.76%), S (8.45%).
6.1.3. 4-[3-(4-Chlorophenyl)-3-oxoprop-1-en-1-yl]phenyl-4-methylbenzenesulfonate (3b)
Yield: 92%; mp 154 °C. IR (ν) (KBr) cm–1: 3069 (C–H aromatic), 2924 (C–H aliphatic), 1662 (C=O), 1369, 1147 (SO2) cm–1; 1H NMR (DMSO-d6): δ 7.13–8.29 (m, 12H Ar–H and d, 2H of 2C–H), 2.45 (s, 3H, CH3). 13C NMR (DMSO-d6): δ 188.53, 150.81, 146.43, 143.16, 138.70, 136.56, 134.29, 131.84, 131.05, 130.93, 130.75, 129.38, 128.73, 123.40, 122.97, 21.64; Anal. Calcd for C22H17ClO4S (412.5); calcd C (64.40%), H (4.15%), S (7.77%). Found: C (64.39%), H (4.16%), S (7.75%).
6.1.4. 4-[3-(4-Nitrophenyl)-3-oxoprop-1-en-1-yl]phenyl-4-methylbenzenesulfonate (3c)
Yield: 88%; mp 138 °C. IR (ν) (KBr) cm–1: 3096 (C–H aromatic), 2935 (C–H aliphatic), 1668 (C=O) 1358, 1141 (SO2) cm–1; 1H NMR (DMSO-d6): δ 6.72–8.38 (m, 12H Ar–H and d, 2H of 2C–H), 2.45 (s, 3H, CH3). 13C NMR (DMSO-d6): δ 189.87, 151.01, 150.41, 146.46, 144.21, 134.10, 131.91, 131.82, 130.76, 130.38, 128.73, 128.35, 124.32, 123.26, 123.02, 21.64. Anal. Calcd for C22H17NO6S (423) calcd C (62.40%), H (4.05%), N (3.31%). Found: C (62.38%), H (4.04%), N (3.29%).
6.1.5. 4-[3-(4-Bromophenyl)-3-oxoprop-1-en-1-yl]phenyl-4-methylbenzenesulfonate (3d)
Yield: 83%; mp 159 °C. IR (ν) (KBr) cm–1: 3068 (C–H aromatic), 2986 (C–H aliphatic), 1660 (C=O), 1366, 1140 (SO2) cm–1; 1H NMR (DMSO-d6): δ 6.72–8.38 (m, 12H Ar–H and d, 2H of 2C–H), 2.45 (s, 3H, CH3). 13C NMR (DMSO-d6): δ 189.62, 150.73, 146.34, 142.70, 137.41, 134.38, 133.70, 131.85, 131.27, 130.97, 129.27, 129.01, 128.73, 123.73, 122.96, 21.64; Anal. Calcd for C22H17BrO4S (337) calcd C (57.78%), H (3.75%), S (7.01%). Found: C (57.75%), H (3.73%), S (7.00%).
6.1.6. 4-[3-(4-Hydroxyphenyl)-3-oxoprop-1-en-1-yl]phenyl-4-methylbenzenesulfonate (3e)
Yield: 85%; mp 170 °C. IR (ν) (KBr) cm–1: 3424 (OH), 3057 (C–H aromatic), 2924 (C–H aliphatic), 1655 (C=O), 1369, 1151 (SO2) 1H NMR (DMSO-d6): δ 7.02–8.82 (m, 12H Ar–H and d, 2H of 2C–H), 4.72 (s, 1H, OH), 2.45 (s, 3H, CH3). 13C NMR (DMSO-d6): δ 189.62, 151.63, 148.34, 144.70, 138.40, 135.38, 133.70, 132.85, 132.27, 130.97, 128.27, 128.09, 128.63, 124.73, 121.96, 21.64; Anal. Calcd for C22H18O5S (394) calcd C (66.99%), H (4.60%), S (8.13%). Found: C (66.97%), H (4.58%), S (8.12%).
6.1.7. 4-[3-(4-Methylphenyl)-3-oxoprop-1-en-1-yl]phenyl 4-methylbenzenesulfonate (3f)
Yield: 94%; mp 182 °C. IR (ν) (KBr) cm–1: 3064 (C–H aromatic), 2925 (C–H aliphatic), 1668 (C=O), 1302, 1178 (SO2) cm–1; 1H NMR (DMSO-d6): δ 7.09–8.06 (m, 12H Ar–H and d, 2H of 2C–H), 2.43 (s, 3H, CH3), 2.40 (s, 3H, CH3). 13C NMR (DMSO-d6): δ 189.00, 150.67, 146.40, 144.16, 142.30, 135.41, 134.44, 134.38, 131.84, 130.88, 130.74, 129.82, 129.15, 128.72, 123.73, 122.93, 21.64; Anal. Calcd for C23H20O4S (392) calcd C (7.39%), H (5.14%), S (8.17%). Found: C (70.39%), H (5.13%), S (8.16%).
6.1.8. 4-[3-(4-Methoxyphenyl)-3-oxoprop-1-en-1-yl]phenyl-4-methylbenzenesulfonate (3g)
Yield: 92%; mp 144. 163 °C. IR (ν) (KBr) cm–1: 3081 (C–H aromatic), 2968 (C–H aliphatic), 1658 (C=O), 1362, 1151 (SO2) 1H NMR (DMSO-d6): δ 7.08–8.16 (m, 12H Ar–H and d, 2H of 2C–H), 3.88 (s, 3H, OCH3), 2.43 (s, 3H, CH3); 13C NMR (DMSO-d6): δ 189.76, 163.82, 150.58, 146.41, 141.92, 134.55, 13.86, 131.43, 130.93, 130.75, 130.03, 128.73, 123.74, 122.92, 114.54, 56.07, 21.64; Anal. Calcd for C23H20O5S (408) calcd C (67.63%), H (4.94%), S (7.85%). Found: C (67.60%), H (4.91%), S (7.82%).
6.1.9. 4-[3-Oxo-3-(pyridin-2-yl)prop-1-en-1-yl]phenyl-4-methylbenzenesulfonate(3h)
Yield: 70%; mp 142 °C. IR (ν) (KBr) cm–1: 3021 (C–H aromatic), 2918 (C–H aliphatic), 1669 (C=O), 1354, 1146 (SO2); 1H NMR (DMSO-d6): δ 6.86–8.68 (m, 12H Ar–H and d, 2H of 2C–H), 2.44 (s, 3H, CH3). 13C NMR (DMSO-d6): δ 194.53, 150.80, 146.44, 143.63, 136.43, 143.11, 132.43, 131.81, 130.94, 130.75, 129.03, 128.51, 128.13, 126.98, 125.37, 122.12, 21.63; Anal. Calcd for C21H17NO4S (379) calcd C (66.47%), H (4.52%), N (3.69%). Found: C (66.44%), H (4.51%), N (3.67%).
6.1.10. 4-[3-Oxo-3-(pyridin-2-yl)prop-1-en-1-yl]phenyl-4-methylbenzenesulfonate (3i)
Yield: 72%; mp 138 °C. IR (ν) (KBr) cm–1: 3068 (C–H aromatic), 2936 (C–H aliphatic), 1673 (C=O), 1336, 1129 (SO2); 1H NMR (DMSO-d6): δ 7.06–8.10 (m, 12H Ar–H and d, 2H of 2C–H), 2.45 (s, 3H, CH3). 13C NMR (DMSO-d6): δ 194.03, 151.80, 145.34, 141.63, 133.43, 133.11, 132.43, 131.81, 130.94, 129.80, 129.03, 128.51, 128.13, 126.98, 126.37, 124.12, 21.63; Anal. Calcd for C21H17NO4S (379) calcd C (66.47%), H (4.52%), N (3.69%). Found: C (66.44%), H (4.51%), N (3.67%).
6.1.11. 4-[3-Oxo-3-(thiophen-2-yl)prop-1-en-1-yl]phenyl-4-methylbenzenesulfonate (3j)
Yield: 74%; mp 167 °C; IR (ν) (KBr) cm–1: 3083 (C–H aromatic), 2952 (C–H aliphatic), 1667 (C=O), 1335, 1149 (SO2); 1H NMR (DMSO-d6): δ 7.12–8.31 (m, 11H Ar–H and d, 2H of 2C–H), 2.44 (s, 3H, CH3). 13C NMR (DMSO-d6): δ 182.97, 150.57, 146.45, 145.75, 133.43, 133.11, 131.81, 130.94, 129.80, 129.03, 128.51, 128.13, 126.98, 123.54, 122.97, 21.64; Anal. Calcd for C20H16O4S2 (384) calcd C (62.48%), H (4.19%), S (16.68%). Found: C (62.45%), H (4.16%), S (16.65%).
6.1.12. 4-[3-(Naphthalen-2-yl)-3-oxoprop-1-en-1-yl] Phenyl-4-methylbenzenesulfonate (3k)
Yield: 76%; mp 186 °C. IR (ν) (KBr) cm–1: 3075 (C–H aromatic), 2947 (C–H aliphatic), 1678 (C=O), 1323, 1117 (SO2); 1H NMR (DMSO-d6): δ 7.06–8.10 (m, 12H Ar–H and d, 2H of 2C–H), 2.45 (s, 3H, CH3). 13C NMR (DMSO-d6): δ 193.41, 150.81, 146.41, 143.60, 136.44, 134.13, 133.92, 132.42, 131.84, 130.89, 130.34, 129.02, 128.59, 128.12, 126.96, 125.66, 125.36, 122.99, 21.63; Anal. Calcd for C26H20NO4S (428) calcd C, (72.88%), H (4.70%), S (7.48%). Found: C, (72.85%), H (4.68%), S (7.45%).
6.2. Biology Assay
Via the leaf dipping bioassay method, the toxic activity of all newly prepared chalcone derivatives was established.12,26−30 For the most active synthesized derivatives, the screening results helped us find out the appropriate concentrations that kill 50% (LC50) of the second and fourth instar larvae of S. frugiperda. In this report, for every compound of synthesized chalcone derivatives, we took five concentrations plus 0.1% Tween 80 as the surfactant. We offered similar size to the second and fourth instar larvae and put them in disks (9 cm in diameter) of castor bean leaves. They were then dipped in the prepared concentration for 10 s and left to dry. In glass jars (5 pounds), the larvae were placed, and every treatment was repeated three times (10 larvae per each). The S. frugiperda instar larvae were provided by the Shandweel Agricultural Research Station, Sohag, Egypt. The control was prepared as a disk dipped in distilled water and Tween 80, where the untreated larvae were transferred and allowed to feed on castor bean for 48 h. After 72 h, the mortality percentage was recovered for all synthesized compounds. The mortality relapse line measurements were elucidated by probit analysis.31 Mortality was redressed by Abbott’s formula.32 The harmfulness index was detected by sun equations.33
Acknowledgments
The authors gratefully acknowledge Sohag University, Sohag 82524, Egypt; Jouf University, 2014 Sakaka, Saudi Arabia; Aswan University, 81528 Aswan, Egypt, and the Research Institute of Plant Protection, Agriculture Research Center, 12112 Giza, for the facility and support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c04814.
IR spectra, 1H NMR spectra, and 13C NMR spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Deshmukh S.; Pavithra H. B.; Kalleshwaraswamy C. M.; Shivanna B. K.; Maruthi M. S.; Mota-Sanchez D. Field Efficacy of Insecticides for Management of Invasive Fall Armyworm, Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) on Maize in India. Fla. Entomol. 2020, 103, 221–227. 10.1653/024.103.0211. [DOI] [Google Scholar]
- Maruthadurai R.; Ramesh R. Occurrence, damage pattern and biology of fall armyworm, Spodoptera frugiperda (J.E. smith) (Lepidoptera: Noctuidae) on fodder crops and green amaranth in Goa, India. Phytoparasitica 2020, 48, 15–23. 10.1007/s12600-019-00771-w. [DOI] [Google Scholar]
- Assefa F.; Ayalew D. Status and control measures of fall armyworm (Spodoptera frugiperda) infestations in maize fields in Ethiopia: A review. Cogent Food Agric. 2019, 5, 1641902–1641917. 10.1080/23311932.2019.1641902. [DOI] [Google Scholar]
- Storer N. P.; Babcock J. M.; Schlenz M.; Meade T.; Thompson G. D.; Bing J. W.; Huckaba R. M. Discovery and Characterization of Field Resistance to Bt Maize: Spodoptera frugiperda (Lepidoptera: Noctuidae) in Puerto Rico. J. Econ. Entomol. 2010, 103, 1031–1038. 10.1603/ec10040. [DOI] [PubMed] [Google Scholar]
- Romanelli G. P.; Virla E. G.; Duchowicz P. R.; Gaddi A. L.; Ruiz D. M.; Bennardi D. O.; del Valle Ortiz E. V.; Autino J. C. Sustainable synthesis of flavonoid derivatives, QSAR study and insecticidal activity against the fall armyworm, Spodoptera frugiperda (Lep.: Noctuidae). J. Agric. Food Chem. 2010, 58, 6290–6295. 10.1021/jf100073j. [DOI] [PubMed] [Google Scholar]
- Tavares W. S.; Cruz I.; Petacci F.; de Assis Júnior S. L.; de Sousa Freitas S. S.; Zanuncio J. C.; Serrão J. E. Potential use of Asteraceae extracts to control Spodoptera frugiperda (Lepidoptera: Noctuidae) and selectivity to their parasitoids Trichogramma pretiosum (Hymenoptera: Trichogrammatidae) and Telenomus remus (Hymenoptera: Scelionidae). Ind. Crops Prod. 2009, 30, 384–388. 10.1016/j.indcrop.2009.07.007. [DOI] [Google Scholar]
- Ameta K. L.; Kumar B.; Rathore N. S. Microwave Induced Improved Synthesis of Some Novel Substituted 1, 3-Diarylpropenones and their Antimicrobial Activity. E-J. Chem. 2011, 8, 665–670. 10.1155/2011/165047. [DOI] [Google Scholar]
- Nielsen S. F.; Christensen S. B.; Cruciani G.; Kharazmi A.; Liljefors T. Antileishmanial Chalcones: Statistical Design, Synthesis, and Three-Dimensional Quantitative Structure–Activity Relationship Analysis. J. Med. Chem. 1998, 41, 4819–4832. 10.1021/jm980410m. [DOI] [PubMed] [Google Scholar]
- Anto R. J.; Sukumaran K.; Kuttan G.; Rao M. N. A.; Subbaraju V.; Kuttan R. Anticancer and antioxidant activity of synthetic chalcones and related compounds. Cancer Lett. 1995, 97, 33–37. 10.1016/0304-3835(95)03945-s. [DOI] [PubMed] [Google Scholar]
- Konieczny M. T.; Horowska B.; Kunikowski A.; Konopa J.; Wierzba K.; Yamada Y.; Asao T. Synthesis of polyhydroxylated derivatives of phenyl vinyl sulfone as structural analogs of chalcones. Synthsis 2001, 2001, 1363–1367. 10.1055/s-2001-15223. [DOI] [Google Scholar]
- Lin Y. M.; Zhou Y.; Flavin M. T.; Zhou L. M.; Nie W.; Chen F. C. Chalcones and flavonoids as anti-tuberculosis agents. Bioorg. Med. Chem. 2002, 10, 2795–2802. 10.1016/s0968-0896(02)00094-9. [DOI] [PubMed] [Google Scholar]
- El-Gaby M. S. A.; Ammar Y. A.; Drar A. M.; Gad M. A. Insecticidal bioefficacy screening of some chalcone and acetophenone hydrazone derivatives on Spodopetra Frugiperda (Lepidoptera: Noctuidae). Curr. Chem. Lett. 2022, 11, 263–268. 10.5267/j.ccl.2022.4.003. [DOI] [Google Scholar]
- Böger M.; Dur D.; Gsell L.; Hall R. G.; Karrer F.; Kristiansen O.; Maienfisch P.; Pascual A.; Rindlisbacher A. Synthesis and structure activity relationships of benzophenone hydrazone derivatives with insecticidal activity. Pest Manage. Sci. 2001, 57, 191–202. . [DOI] [PubMed] [Google Scholar]
- Liu M.; Wang Y.; Wangyang W.-Z.; Liu F.; Cui Y.-L.; Duan Y.-S.; Wang M.; Liu S.-Z.; Rui C.-H. Design, synthesis and insecticidal activities of phthalamides containing a hydrazone substructure. J. Agric. Food Chem. 2010, 58, 6858–6863. 10.1021/jf1000919. [DOI] [PubMed] [Google Scholar]
- Wu J.; Song B. A.; Hu D. Y.; Yue M.; Yang S. Design, synthesis and insecticidal activities of novel pyrazole amides containing hydrazone substructures. Pest Manage. Sci. 2012, 68, 801–810. 10.1002/ps.2329. [DOI] [PubMed] [Google Scholar]
- Prasad Y. R.; Rao A. L.; Rambabu R.; Kumar P. R. Synthesis and biological evaluation of some novel chalcone derivatives. Orient. J. Chem. 2007, 23, 927–937. [Google Scholar]
- Singh P.; Anand A.; Kumar V. Recent developments in biological activities of chalcones: a mini review. Eur. J. Med. Chem. 2014, 85, 758–777. 10.1016/j.ejmech.2014.08.033. [DOI] [PubMed] [Google Scholar]
- Hussein B. R. M.; Ali A. M. Multicomponent Reaction for Synthesis of Novel 2-Tosyloxyphenylpyridines. J. Heterocycl. Chem. 2019, 56, 1420–1425. 10.1002/jhet.3521. [DOI] [Google Scholar]
- Khodairy A.; Ali M. A.; El-Wassimy M. T. 4-Toluenesulfonamide as a Building Block for Synthesis of Novel Triazepines, Pyrimidines, and Azoles. J. Heterocycl. Chem. 2016, 53, 1544–1553. 10.1002/jhet.2461. [DOI] [Google Scholar]
- Khodairy A.; Ali M. A.; Aboelez M. O.; El-Wassimy M. T. One-Pot Multicomponent Synthesis of Novel 2-Tosyloxyphenylpyrans under Green and Conventional Condition with Anti-inflammatory Activity. J. Heterocycl. Chem. 2017, 54, 1442–1449. 10.1002/jhet.2730. [DOI] [Google Scholar]
- Mourad A. F. E.; Amer A. A.; El-Shaieb K. M.; Ali M.; Aly A. A. 4-Hydroxy-1-phenylquinolin-2(1H)-one in One-pot Synthesis of Pyrimidoquinolines and Related Compounds under Microwave Irradiation and Conventional Conditions. J. Heterocycl. Chem. 2016, 53, 383–388. 10.1002/jhet.2286. [DOI] [Google Scholar]
- Khodairy A.; Shaaban K. M.; Ali M.; El-Wassimy M. T.; Nagwa S. A. Eco-friendly and efficiently synthesis, anti-inflammatory activity of 4-tosyloxyphenylpyrans via multi-component reaction under ultrasonic irradiation and room temperature conditions. J. Chem. Pharm. Res. 2015, 7, 332–340. [Google Scholar]
- Khodairy A.; Ali M.; El-Wassimy M. T. Synthesis of Novel Chromene, Pyridine, Pyrazole, Pyrimidine, and Imidazole Derivatives via One-pot Multicomponent Reaction. J. Heterocycl. Chem. 2017, 54, 3342–3349. 10.1002/jhet.2954. [DOI] [Google Scholar]
- Khodairy A.; Ali M.; El-Wassimy M. T. Synthesis and Reactions of New Thiazoles and Pyrimidines Containing Sulfonate Moiety. J. Heterocycl. Chem. 2018, 55, 964–970. 10.1002/jhet.3126. [DOI] [Google Scholar]
- Ahmed E. A.; Soliman A. M. M.; Ali Ali. M.; El-Remaily M. A. A. Boosting the catalytic performance of zinc linked amino acid complex as an eco-friendly for synthesis of novel pyrimidines in aqueous medium. Appl. Organomet. Chem. 2021, 35, e6197 10.1002/aoc.6197. [DOI] [Google Scholar]
- Abdelhamid A. A.; Elsaghiera A. M. M.; Aref S. A.; Gad M. A.; Ahmed N. A.; Abdel-Raheem Sh. A. A. Preparation and biological activity evaluation of some benzoylthiourea and benzoylurea compounds. Curr. Chem. Lett. 2021, 10, 371–376. 10.5267/j.ccl.2021.6.001. [DOI] [Google Scholar]
- Gad M. A.; Aref S. A.; Abdelhamid A. A.; Elwassimy M. M.; Abdel-Raheem Sh. A. A. Biologically active organic compounds as insect growth regulators (IGRs): introduction, mode of action, and some synthetic methods. Curr. Chem. Lett. 2021, 10, 393–412. 10.5267/j.ccl.2021.5.004. [DOI] [Google Scholar]
- Abdelhamid A. A.; Elwassimy M. M.; Aref S. A.; Gad M. A. Chemical design and bioefficacy screening of new insect growth regulators as potential insecticidal agents against Spodoptera littoralis (Boisd.). Biotechnol. Rep. 2019, 24, e00394 10.1016/j.btre.2019.e00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelhamid A. A.; Salama K. S. M.; Elsayed A. M.; Gad M. A.; Ali Ali El-Remaily M. A. A. A. Synthesis and Toxicological effect of some new pyrrole derivatives as prospective insecticidal agents against the cotton leafworm, spodoptera littoralis (Boisduval). ACS Omega 2022, 7, 3990–4000. 10.1021/acsomega.1c05049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhite E. A.; Marae I. S.; Gad M. A.; Mohamed Sh. K.; Mague J. T.; Abuelhassan S. Pyridine Derivatives as Insecticides. Part 3. Synthesis, Crystal Structure, and Toxicological Evaluation of Some New Partially Hydrogenated Isoquinolines against Aphis gossypii (Glover, 1887). J. Agric. Food Chem. 2022, 70, 9637–9644. 10.1021/acs.jafc.2c02776. [DOI] [PubMed] [Google Scholar]
- Finny D. J.Probit Analysis: A Statistical Treatment of the Sigmoid Response Curve, 2nd ed.; Cambridge University Press: Cambridge, U.K., 1952. [Google Scholar]
- Abbott W. S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. 10.1093/jee/18.2.265a. [DOI] [Google Scholar]
- Sun Y. P. Toxicity Index-An Improved Method of Comparing the Relative Toxicity of Insecticides1. J. Econ. Entomol. 1950, 43, 45–53. 10.1093/jee/43.1.45. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.