Abstract
CO2 capture from industry sectors or directly from the atmosphere is drawing much attention on a global scale because of the drastic changes in the climate and ecosystem which pose a potential threat to human health and life on Earth. In the past decades, CO2 capture technology relied on classical liquid amine scrubbing. Due to its high energy consumption and corrosive property, CO2 capture using solid materials has recently come under the spotlight. A variety of porous solid materials were reported such as zeolites and metal–organic frameworks. However, amine-functionalized porous materials outperform all others in terms of CO2 adsorption capacity and regeneration efficiency. This review provides a brief overview of CO2 capture by various amines and mechanistic aspects for newcomers entering into this field. This review also covers a state-of-the-art regeneration method, visible/UV light-triggered CO2 desorption at room temperature. In the last section, the current issues and future perspectives are summarized.
1. Introduction
Since the onset of the Industrial Revolution in the 18th century, the atmospheric concentration of carbon dioxide increased from ca. 280 ppm1 to 417 ppm in 2022. The climate change and sea level rise of 1–3 mm per year arise from the record-high level of atmospheric CO2 concentration. Besides, CO2-induced ocean acidification is considered to have an impact on organisms and the ecosystem.2,3 Provided that no action is taken, the atmospheric CO2 concentration is estimated to rise up to 450 ppm by 2050.4 Because of surging public concerns about potential environmental damage, carbon capture and storage/sequestration (CCS)5 or utilization (CCU)6 became one of the key research topics in the 21st century. CCS aims at geological storage of CO2 in the deep underground. On the other hand, CCU is a concept in which captured CO2 is utilized as a carbon source for chemical feedstocks such as fuels and fine chemicals. The scientific community and industry are gradually coming to a conclusion that CCS has limitations, taking into account the economic feasibility. Hence, industries are shifting toward exploration of CCU technologies.7 In any case, CO2 capture is the very first step and thus the most critical technology to pave the way for facilitating the industrialization of CCS and CCU. In the industrial sector, pressure swing adsorption (PSA) and amine scrubbing have traditionally been applied for some decades.8 PSA, which has been the main technology in the last a few decades, separates hydrogen out of other gases, including CO2. Since PSA aims at purification of H2, the resulting concentration of CO2 collected from off-gas is too low to utilize CO2 for further chemical processes.8 On the other hand, amine scrubbing has been applied since 1930 and allows CO2 collection with high purity (>99%).9 CO2 is captured by using monoethanolamine solution (20–30 wt % in water) and then released at 100–120 °C. Amine scrubbing produces a huge amount of degraded solvent as waste. Therefore, the current scrubbing technologies are energy intensive and therefore not very economical. To improve CO2 capture technology, two major drawbacks of amine scrubbing need to be overcome: (1) energy-inefficient regeneration at high temperature and (2) waste management of the degraded solvent containing amines. In light of this, CO2 capture using solid materials has recently come under the spotlight.10 CO2 adsorption on solid materials occurs at solid–gas interfaces and thus requires no solvent, leading to the complete elimination of the solvent disposal process. Normally, CO2 adsorption on solid materials is carried out at ambient temperature, followed by regeneration of the materials at 80–120 °C to release CO2. Therefore, high energy input during the regeneration process is still inevitable. Solid adsorbent-based technology can be applied for two different capturing concepts. One is the direct air capture (DAC) which captures CO2 directly from the atmosphere. The other is CO2 capture from the off-gas of industrial sectors such as power plants and factories for fine chemical synthesis. DAC is considered to be a “negative emission technology” because already released CO2 in the air (417 ppm) has to be captured. However, DAC is much less cost-effective compared to CO2 capture from exhaust streams because of the low concentration of CO2 in the air and thus the associated thermodynamic barrier.4 On the other hand, CO2 capture from industrial off-gas is considered as “zero emission technology” in order not to increase the current CO2 level any further. Depending on emission sources, the CO2 concentration differs in a wide range of 3–100%.11 For example, the off-gas from coal-fired power plants contains 10–15% CO2, whereas fermentation plants for ethanol production emit 98–99% CO2.11 Hence, CO2 capture from these plants is thermodynamically favored and can be cost-effective. Public concerns about global warming and awareness of the importance of CO2 removal make both “negative emission technology” and “zero emission technology” a high priority. Such an urgent demand has lately been extending the horizon of solid adsorbent materials with new concepts, and in the past decade we have witnessed tremendous advance in this field.10 Porous materials have been extensively explored, targeting high CO2 adsorption capacity such as carbonaceous adsorbents, ionic liquids, zeolites, metal oxides, and metal–organic frameworks (MOFs). The overview of different adsorbents is summarized in a previously published review article.10
In this review, we outline properties, advantages, disadvantages, and future perspectives of amine-functionalized materials, foreseeing their potential for high CO2 adsorption capacity, relatively low regeneration temperature, cost-effectiveness, and industrial applications. This article not only summarizes different amine-based materials but also sums up the effects of impurities in off-gas streams and adsorption mechanisms and process engineering.
2. Amine-Functionalized Solid
Amine-functionalized solid adsorbents light a path for mitigating the issues encountered by liquid amine scrubbing, such as the loss of volatile amines by vaporization. The support materials with high surface area and porosity are functionalized with amines, such that the amines are well-dispersed as uniformly as possible. Hence, the functionalized materials possess the positive properties of both components (an amine and a support). The support materials are often functionalized in two different ways: wet impregnation or anchoring on the surface with covalent bonds. This chapter discusses what types of amines and solid support materials have already been explored. Table 1 lists adsorbent materials reported by different research groups in the last 15 years.
Table 1. Summary of CO2 Adsorption Performance of Different Adsorbents.
| Support | Absorbent | Amine loading [wt %] | Adsorption capacity [mmol/g] | Adsorption temperature [°C] | Desorption temperature [°C] | Adsorption conditions | Reference |
|---|---|---|---|---|---|---|---|
| SBA-15 | functionalized by different aminosilanes | - | 2.2 to 3.2 | 25 | 95 to 105 | pure CO2, static | Boukoussa et al.31 |
| SBA-15 and fumed silica | TEPA, impregnated | - | 3.25 to 3.97 | 75 | 115 | 15% CO2, no information about gas flow | Chao et al.32 |
| β-zeolite | TEPA, impregnated | 38.4 | 2.90 | 30 | 135 | 10% CO2, 30 mL/min | Fischer et al.12 |
| protonated titanate nanotubes (PTNTs) | TEPA, impregnated | 60 | 4.13 | 75 | 100 | 10% CO2, 10 mL/min | Guo et al.13 |
| nanoporous titanium oxyhydrate | TEPA, impregnated | 60 | 3.1 | 60 | 100 | 1% CO2 and 1% H2O, 300 mL/min | Irani et al.33 |
| carbon nanotubes (CNTs) | TEPA, impregnated | 75 | 5.0 | 60 | 90 | 10% CO2 and 1% H2O, 300 mL/min | Irani et al.14 |
| PMMA (HP2MG) | TEPA and modified TEPA, impregnated | - | 2.58 to 4.30 | 25 | 100 | pure CO2, 200 mL/min | Jo at al.15 |
| silica gel | TEPA and PEI, impregnated | 40 | 2.64 to 3.32 | 75 | 100 | pure CO2, 100 mL/min | Jung at al.34 |
| PAN carbon fibers | TEPA and TETA, impregnated | 50 | 4.30 to 5.44 | 25 | 90 | 10% CO2, 30 mL/min | Kuang at al.35 |
| mesoporous SiO2 (MCM-41) | EPA, DETA, TEPA, and PEHA, impregnated | 40 | 1.19 to 2.34 | 35 | 100 | 10% CO2, no information about gas flow | Liu at al.17 |
| TiO2 | monoethanolamine, impregnated | 40 | 1.09 | 45 | 90 | 1% CO2, 300 mL/min | Sun at al.36 |
| MR10 (LDPE) | BPEI, impregnated | 50 | 2.64 | 25 | 70 | different concentrations of CO2, 3 mL/min | Wang at al.22 |
| fumed silica | BPEI and IL, impregnated | 30 and 10 | - | 25 | 50 | 5000 ppm of CO2, 200 mL/min | Weisshar at al.23 |
| nanosilica | LPEI, impregnated | different loadings | 1.0 to 3.5 | different temperatures | 50–60 | different concentrations of CO2, 3 mL/min | Zhang at al.21 |
| - | IRMOF-74-III-(CH2NH2)2, alkylamine functionality | - | 1.2 | 25 | 120 | pure CO2, static | Flaig et al.29 |
| - | N-containing polymer NUT-4, imine linker | - | 6.9 | 25 | 25 | 10% CO2 | Geng et al.27 |
| - | N-doped copolymer | 3.1 | 0 | - | pure CO2, static | Qi et al. |
2.1. Type of Amines
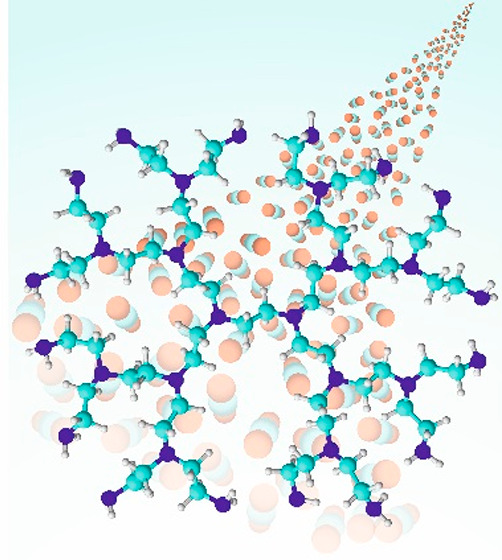
As described in the Introduction, the monoethanolamine solution has been used as a main sorption component for amine scrubbing. Since monoethanolamine is not suitable for the functionalization of solids due to its high volatility and the small adsorption capacity of CO2, longer-chain amines have often been employed in the past. A prime example in this respect is tetraethylenepentamine (TEPA), which contains five amine groups and thus has the theoretical capacity of chemisorption of up to five CO2 molecules per one TEPA molecule. Because the past research mainly focused on the adsorption capacity, the high adsorption capacity of TEPA attracted much attention.12−16 As a result, TEPA has become one of the most widely used amines for the functionalization of solid materials. Besides, other ethyleneamines, such as triethylenetetramine (TETA) and pentaethylenehexamine (PEHA), are also suitable for the functionalization as they have similar performance in CO2 adsorption.17 Recently, the amine-containing polymer, i.e., polyethylenimine (PEI), has also come to the forefront of research. PEI has two different structures: branched polyethylenimine (BPEI) and linear polyethylenimine (LPEI).18−21 Due to the significantly lower price and availability in the market, BPEI was studied more extensively compared to LPEI. Both PEI types have adsorption capacity similar to or slightly lower than TEPA. However, the regeneration temperature of PEI is much lower, especially LPEI, compared to shorter ethyleneamines.21,22 Another exciting aspect of the use of PEI is the influence of the polymer size. Smaller PEI molecules are better suited for the impregnation of porous materials because they can diffuse more uniformly into the pores than large PEI molecules. Larger PEI molecules, on the other hand, are less soluble in water, resulting in better regeneration stability under humid conditions. This is an important factor considering long-term operations. As shown in Table 1, different adsorption temperatures 25–75 °C were chosen for each of the reports. The concept of such adsorption conditions was built upon the exploitation of the off-gas heat from industrial sectors. The source of CO2 emission from the industrial sectors is normally higher than room temperature, e.g., coal and petroleum power plants (40–65 °C).11 Therefore, the adsorption performance in this temperature range is important for industrial applications. Due to the climate change and global concerns about energy and the environment, the research direction is shifting toward how to lower the regeneration temperature without compromising the high adsorption capacity. Weisshar et al. reported that a hybrid adsorbent of BPEI with ionic liquid released CO2 even at 50 °C.23 The zwitterions formed by the reaction of amines and CO2 interact with counterion pairs of the ionic liquid, leading to the weakening of the C–N bond as shown in Figure 1.
Figure 1.
Proposed interaction of BPEI and ionic liquid. Copyright 2016 American Chemical Society. Reprinted with permission from ref (23).
Yogo et al. reported a different approach to improving both adsorption and desorption properties by chemical medication of amino groups.24 TEPA substituted with bulky alkyl groups was found to enhance the adsorption capacity and to lower the regeneration temperature. They achieved 88% efficiency in the desorption at 60 °C by sweeping an inert gas.
2.2. Support Materials
The choice of an appropriate support material is driven by several factors. Two important properties for the support material are the thermal stability and chemical inertness. Accordingly, the support material should not be decomposed at relatively high temperature and should not react with amines during functionalization so that the amines remain available for the chemisorption of CO2. Table 1 shows the various support materials tested in recent years. It is worth noticing that all supports reported fulfill the two criteria mentioned above. However, additional properties must be considered to finely tune the adsorption and desorption performance. For example, additional decisive properties are specific surface area, porosity, and pore volume, whereby the adsorption performance is interrelated. The specific surface area plays an important role in impregnating amines onto the support material uniformly. Support materials with high surface area can impregnate a larger amount of amines, leading to higher adsorption capacity. Because BPEI is a highly viscous liquid, thick liquid BPEI layers are formed on support materials with low specific surface area. The thick liquid layers inhibit gas diffusion, resulting in the low efficiency for CO2 adsorption. Furthermore, the porosity and the pore volume are important for the diffusion of gaseous molecules through the support material. Materials with high porosity and large pore volume are favored due to better mass transport through the support material. Such a property can enhance the adsorption capacity as well as rapid desorption of CO2. Thus, the choice of support material drastically influences the performance of the adsorbents.25 Furthermore, the number of acid sites on the surface also has an influence on the sorption properties of the amine. The beneficial effects of acidic sites of the support material can be attributed to the interaction between the acid sites and the amine groups. The presence of strong surface acidity contributes to the formation of protonated amine species on which CO2 weakly adsorbs. This is beneficial to the repeated cycles of adsorption and desorption.26 On one hand, the higher thermostability of the entire system and better distribution of the amine on the surface of the support material result in more efficient adsorption.26
2.3. Functionalized Polymeric Adsorbents
There has been a new strategy to add the CO2-capturing property to polymeric compounds such as porous polymers27,28 and metal–organic frameworks (MOFs)29,30 with amine-functionalized linkers. MOFs are classified as coordination polymers with a metal cation center and organic linker. Yagi and co-workers reported that IRMOF-74-III was functionalized with primary or secondary amines.30 IRMOF-74-III-CH3 without amine functionalization also adsorbed CO2, but the adsorption capacity dropped by 80% under humid conditions. This behavior can be explained by the CO2 adsorption to the open Mn sites where H2O competitively coordinates. On the other hand, the moisture did not affect the performance of IRMOF-74-III-CH2NH2, indicating that the CO2 uptake occurs with the amine linkers and that the open Mn sites are not available. The regeneration of IRMOF-74-III-CH2NH2 required 90 °C to remove CO2. They extended the synthetic method toward functionalization by diamine and reported that IRMOF-74-III-(CH2NH2)2 adsorbed 2.33 times more CO2 than monoamine-functionalized IRMOF-74-III-CH2NH2.29 The complete regeneration was achieved at 120 °C in a vacuum.
Recently, Sun and co-workers have proposed nitrogen-doped porous carbons (NPCs) for CO2 capture.27,28 The NPCs were prepared through polymerization of 2,4,6-tris(chloromethyl)mesitylene (TCM) and p-phenylenediamine (PD) to a polymer of NUT-4 (NUT stands for Nanjing Tech University). The obtained NUT-4 was further carbonized at elevated temperatures (400–700 °C) to obtain NPCs. The CO2 adsorption capacity of 6.9 mmol/g was achieved with NPCs containing CO2-philic N sites. The complete regeneration of the adsorbent was possible under mild condition (25 °C, 30 mmHg, 60 min).
3. Moisture Effect
Amine-functionalized solid materials are advantageous over porous adsorbents because of their high tolerance against moisture. In most of the cases, the presence of moisture even enhances the CO2 adsorption capacity14,20,22,33,36−40 yet keeps the regeneration temperature in the same range.23 The atmosphere contains 18–60% humidity on average over the year. The exhaust gas from power plants also contains moisture. Therefore, both DAC and CO2 capture from industrial off-gas using amine-functionalized materials can greatly benefit from the enhancement effect of CO2 adsorption by moisture. Figure 2 shows typical examples of reaction schemes of amines with CO2 under dry and humid conditions.14 Under dry conditions, two primary amines react with one CO2 molecule to form an ammonium ion and carbamate ion. The secondary amines follow the same reaction path. Therefore, the maximum efficiency can only be 0.5 mol of CO2 per mole of amine. Under humid conditions, both primary and secondary amines react with CO2 and H2O to form ammonium ions and bicarbonate. Hence, the theoretical value of moles of CO2 per mole of primary and secondary amines doubles to 1.0 under humid conditions. Likewise, under humid conditions, CO2 and water react with tertiary amines by forming an ammonium ion and bicarbonate and by binding one mole of CO2 per mole of tertiary amine. These reactions schemes are examples out of all the potential reaction paths, e.g., the formation of carbamic acid. The actual reaction appears to be more complicated than the schemes described in Figure 2.
Figure 2.
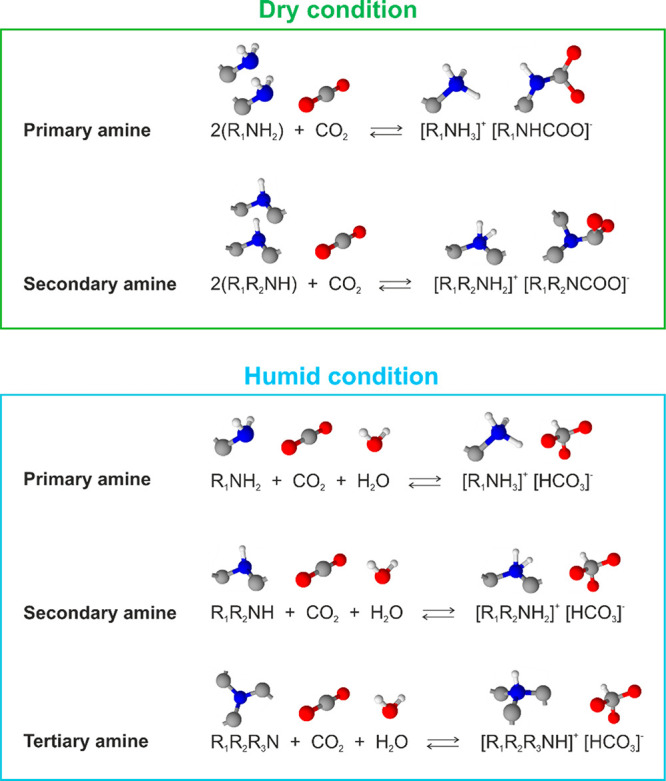
Examples of reaction schemes of CO2 adsorption under dry and humid conditions.
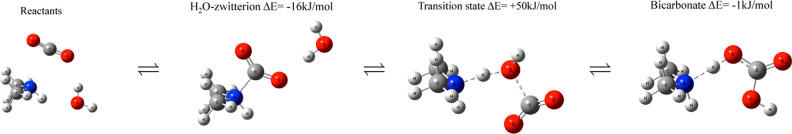
According to the theoretical estimation, the presence of moisture should double the CO2 adsorption capacity for primary and secondary amines. Thus, the enhancement effect of moisture depends on the type of amines used and relative humidity (RH). On PEI supported on resin22 or polymer,39 CO2 adsorption capacity increases proportionally with RH. With 100 RH % at a dew point of 25 °C, the CO2 breakthrough period was twice as long as that of dry conditions.23 The recyclability test was also conducted using a triamine-grafted mesoporous material.38 Under dry conditions, CO2 uptake dropped by 15% after 700 cycles because of the formation of a stable urea, whose reaction is not reversible. Under humid conditions, the same CO2 adsorption capacity was kept over 700 cycles. H2O was reported to react with urea to form carbamate, leading to the long-term durability of the adsorbent. Another striking feature of the moisture effect is that the desorption temperature was never influenced by the presence of moisture even though CO2 uptake was doubled.23 Normally, the desorption temperature increases for samples with higher loading amount of amine.41 A clear answer to this unique effect of the moisture was given by quantum chemical calculations.37 As in Figure 3, a H2O-stabilized zwitterion is formed during the CO2 adsorption process, which helps CO2 transport through PEI. Therefore, under humid conditions the diffusion barrier can be minimized.
Figure 3.
Water-assisted formation of bicarbonate via a zwitterion. Copyright 2016 American Chemical Society. Reprinted with permission from ref (37).
4. Tolerance for Impurities
In this section, the effects of SO2, NOx, CO, and O2 on CO2-capturing property are summarized. The performance of liquid amine scrubbing with monoethanolamine (MEA) is tremendously deteriorated by the presence of impurities such as SO2 and O2 in flue gas streams.42 Therefore, it is of vital importance to investigate the potential degradation of amine-functionalized materials under such conditions for industrial applications. Xu et al. investigated the effects of impurities in a flue gas on CO2-capturing property using 50 wt % of BPEI (Mn = 600) finely dispersed in the pores of MCM-41.43 They employed the actual off-gas from a natural gas-fired boiler containing 7.4–7.7% CO2, 14.6% H2O, ∼4.45% O2, 200–300 ppm of CO, 60–70 ppm of NOx, and 73–74% N2. N2, O2, and CO had little influence on CO2 adsorption, while NOx slightly decreased the amount of CO2 adsorbed. However, CO2 was captured 3000 times more than NOx, demonstrating that BPEI/MCM-41 can efficiently separate CO2 from the flue gas. Rezaei et al. reported single-component adsorption44 and multicomponent adsorption45 using SO2, NOx, and CO2 with different amine types. They demonstrated that SO2 greatly influenced CO2 adsorption at 35 °C but had much less influence at 75 °C.44 This phenomenon originates from the fact that SO2 adsorption capacity decreases with an increase in temperature, while CO2 adsorption capacity increases. The coadsorption experiments showed that the presence of SO2 considerably deteriorates CO2 adsorption performance.45 Among all the amine types, secondary amine was less influenced by SO2. Coadsorption with NO has no impact on CO2 adsorption, whereas NO2 slightly suppressed CO2 adsorption capacity.45
The degradation of amine-functionalized materials by oxygen was also extensively studied.46−51 Normally, the off-gas from power plants and some fine chemical factories contain a certain concentration of oxygen. Therefore, O2 tolerance of the amine-based materials should be taken into account for industrial applications with long-term operation. The reason for the degradation by O2 has never been clearly demonstrated. Heydari-Gorji et al. carried out infrared spectroscopic and NMR analysis of a triamine-grafted material (TRI).48,49 Upon the TRI deactivation, a new IR band emerged at 1665–1680 cm–1, but only a minor change was confirmed by 13C NMR except for a weak peak at 158 ppm. What these changes indicate remain uncertain. However, they assumed that the possible formation of imine, carbamic acid, and nitrone cannot be ruled out. Further research is required to draw a solid conclusion on the oxidative degradation mechanism of TRI. Bali at al. thoroughly investigated the oxidative degradation of PEI and poly(allylamine) (PAA) on mesoporous alumina support employing infrared, Raman, and 13C NMR spectroscopy.47 Their data clearly proved the formation of a C=O bond associated with deactivation of PEI. They concluded that undesirable formation of amides, acids, and imides leads to lower basicity of the nitrogen species in the polymer and thus low CO2 adsorption capacity. According to their findings, PAA may serve as a better adsorbent with high oxidative stability. Ahmadalinezhad et al. also came to a similar conclusion by using 1D and 2D NMR techniques.46 BPEI is the most unstable amine among the amines tested, and PAA and LPEI can be more resistant to the oxidative atmosphere.
5. Mechanistic Studies
The advance in spectroscopy, in situ techniques, and quantum mechanical modeling in the past decade promoted mechanistic investigations to unveil the underlying mechanism of CO2 adsorption and desorption. Commonly used techniques in this field are infrared spectroscopy,23,52−60 nuclear magnetic resonance (NMR),61−66 and density functional theory (DFT).37,67−70 This chapter briefly summarizes what information can be gained by each technique and major findings related to CO2 adsorption mechanisms.
NMR studies were mainly reported by the group of Jones and colleagues,61−66 exploiting 1H NMR,6613C NMR,64,6515N dynamic nuclear polarization (DNP) NMR,64 rotational-echo double-resonance 15N(13C) and 13C(15N) (REDOR) NMR,62 and two-dimensional 13C–1H heteronuclear correlation (HETCOR) NMR.61,6313C NMR is a powerful tool to identify reaction pathways and surface species formed, such as carbamic acid, carbamate, bicarbonate, and urea. In particular, its combination with IR spectroscopy offers a firm understanding of molecular structure because the frequency difference between asymmetric and symmetric stretching vibrations of carboxylate ions provides insight into the molecular interaction.64 REDOR NMR is useful to gain information about short- and long-range dipolar coupling between isolated pairs of heteronuclei. 15N(13C) REDOR NMR identified amide-like species such as carbamic acid and carbamate. Together with its counterpart, 13C(15N) REDOR NMR, REDOR NMR indicated that carbamate species are isolated from each other.62 2D NMR with HETCOR sequence detects the correlation of two different nuclei (c.f., 13C and 1H) via single-bond spin–spin coupling and thus unveils which proton is bonded to which carbon groups. Chen et al. reported that 13C and 1H HETCOR NMR detected two distinct bicarbonate species at 100 K, which are coupled to different protons, respectively.63 Their follow-up study evidenced that one bicarbonate species is coupled to H2O molecules present on the walls of the mesoporous material, while another bicarbonate species is coordinated to H2O molecules in the pores.61
DFT calculations contributed considerably to understanding what species is favorably formed and what reaction path is reasonable for the reaction of amines with CO2.37,67−70 Early stage research was reported by Mebane et al. that the formation of a zwitterion was unstable in a polar environment of anhydrous PEI.67 However, a dielectric medium under humid conditions stabilizes the zwitterion. They also proposed linear and ring topologies to be responsible for CO2 diffusion in the bulk PEI under humid conditions.37 DFT calculations in combination with adsorption isotherms demonstrated that CO2 uptake caused the volume expansion of the amine layers and thus the decrease in the pore volume.69 Therefore, sticky amines with high viscosity might increase the diffusion resistance and have a negative impact on CO2 adsorption property.
In situ infrared spectroscopy is the most widely utilized technique to follow the reaction path in this field because the setup is relatively cheap and can be operated easily.23,52−60,64,71 Since there is an extensive review on IR spectroscopic studies published in 2019,54 we only describe major findings briefly and update that what has been newly reported since then. A number of reports contributed to assign IR bands observed during CO2 adsorption such as carbamic acid, carbamate-physisorbed CO2, and ammonium ions. The detailed band assignment is given in a previous review article.54 The most frequently used amine, TEPA, was also investigated by in situ IR spectroscopy. By increasing the thickness of TEPA layers, the interaction between amines was enhanced, forming zwitterions with NH and NH2 groups.59 This phenomenon suppresses CO2 gas diffusion in a thicker layer of liquid TEPA and leads to high-temperature desorption of CO2 at 100 °C. The addition of polyethylene glycol (PEG) with low molecular weight TEPA was reported to increase the ratio of weakly adsorbed CO2, whose band emerged at 2627 cm–1 (NH2–O).57 PEI also possesses similar characteristics of IR bands.56 IR spectroscopy together with thermogravimetric analysis (TGA) provided important information about BPEI behavior at different temperatures. Due to the high viscosity of liquid BPEI, adsorption capacity increased with temperature because of the reduction of the viscosity.56 There are several reports on amine-grafted/-immobilized materials.52,53,58,60 The IR spectroscopic features and behavior were similar to that of TEPA and BPEI. Effects of NO2 and SO2 on CO2 adsorption were also investigated by in situ IR spectroscopy.55 Those acidic gases form NH–NO2 and NH–SO2 complexes assigned at 1650 cm–1, leading to the deterioration of CO2 adsorption performance. NO2 has less influence on the amount of CO2 adsorbed even at 200 ppm, but the presence of SO2 leads to the considerable reduction of CO2 adsorption above 50 ppm. Weisshar et al. have recently demonstrated that in situ IR spectroscopy could be a useful tool to monitor the weakening of the C–N bond of carbamic acid and carbamate.23 Such an attempt would be beneficial for designing an adsorbent material with low-temperature regeneration.
6. Reactor and Process Design
6.1. Vacuum Swing Desorption
Temperature vacuum temperature swing desorption (TVSD) is considered to be useful because a high-level vacuum is not required.72 The process optimization is critical to not waste time, energy, and CO2 gas during the desorption process so that the maximum efficiency for collecting pure CO2 can be achieved. In the TVSD process, CO2 is first chemisorbed on an absorbent material at atmospheric conditions (typically 25 °C and 1 bar). After saturation of the material with CO2, the chamber with the adsorbent is closed and evacuated by a vacuum pump, removing the excess gas at the begging and weakly adsorbed CO2. In order to desorb further CO2, the absorbent bed is heated to the desorption temperature.73Figure 4 displays the schematic diagram of a typical TVSD setup. There is also an attempt without any temperature swing, i.e., vacuum swing desorption (VSD), operated at 90 °C.74 VSD can achieve 95% CO2 purity and 90% recovery. Due to the high-temperature operation, the degradation of CO2 adsorption capacity is expected with repeated cycles. Hence, the fabrication of thermally stable adsorbents must be taken into account.74 Compared to VSD, TVSD is more efficient when higher CO2 concentration is required. In some cases, >99% CO2 can be achieved with 0.23 mmol/g/h of the desorption rate.72 Under humid conditions during the vacuum process, the desorption rate increased to 3.75 mmol/g/h, confirming the beneficial kinetics assisted by steam. However, additional energy is required for the desorption of water if H2O is coadsorbed with CO2. The recyclability test revealed that 8% loss in CO2 adsorption capacity was observed after 50 cycles (>1500 h).72 Gebald et al. also reported cyclic experiments using TVSD with amine-functionalized cellulose.75 100 cycles of adsorption and regeneration at 90 °C caused 2% loss of the N content. However, this report showed a high potential that TVSD can be well applied for DAC.
Figure 4.
Water-assisted formation of bicarbonate via a zwitterion. Copyright 2016 American Chemical Society. Reprinted with permission from ref (72).
6.2. Steam-Stripping Regeneration
Instead of TVSD, steam-stripping regeneration combined with temperature swing desorption (TSD) has been explored to collect pure CO2.76−82 In the regeneration process, a stream saturated with H2O carries a mixture of CO2 and H2O, and then a concentrated CO2 can be obtained by compression and condensation.83 There is a report on studying how to minimize the energy consumption, employing superheated steam from low-pressure steam turbine and a heat exchanger.76 This so-called “direct steam-stripping process” was found to lower the energy consumption by 23.2% compered to a conventional stripping method. The steam stripping can also be combined with vacuum swing adsorption, named steam-aided vacuum swing adsorption (SA-VSA).77 Yogo et al. demonstrated highly efficient CO2 capturing performance using a system with three columns: the first column for adsorption, the second column for rinsing, and the third column for desorption.77 The SA-VSA process with an amine-impregnated mesoporous MSU-F silica can achieve >98% of CO2 purity and >93% of recovery rate. They also estimated the required heat for the regeneration to be much lower than that of liquid amine scrubbing and other amine-based solid adsorbents.
A challenge for its industrial application is the H2O tolerance of amines at elevated temperature. After steam treatment, the adsorption capacity drops to a certain degree.83,84 For long-term operations, the fabrication of robust amine-based adsorbents is desired. Sayari et al. reported that amine-grafted SBA-15 showed high hydrothermal stability, and its exposure to steam for 48 h caused no change in the adsorptive and structural properties.85 A choice of support materials is also a key factor for the steam tolerance. Upon exposure to steam, alumina support is partially transformed into boehmite, but it does not alter the amine efficiency.80 Therefore, γ-Al2O3 is considered to be a promising support material to impregnate PEI.
6.3. Reactor Design
The design of the adsorption–desorption chamber is also a crucial factor to achieve high efficiency with amine-functionalized solid materials. There are two flow-reactor types considered: (1) fixed-bed reactor86 and (2) fluidized-bed reactor.87 In the fixed-bed reactor. The solid adsorbent is placed such that its position always remains the same when a gas stream flows through the fixed bed.86 On the contrary, in the fluidized bed reactor, the adsorbent powders are swirled up with a gas stream and fly around inside the chamber.87 The advantage of the fixed-bed reactor is that the process engineering and reactor design are much simpler and cheaper than the fluidized-bed reactor. Furthermore, a significantly larger quantity of adsorbent can be used per unit of space, which results in better process efficiency. However, the fluidized-bed reactor benefits from high adsorption rate and uniform heat distribution because the adsorption process is exothermic.87
6.4. Process Design and Cost Estimate
A techno-economic analysis (TEA) was considered to assess the applicability of reactor systems with amine-functionalized solids for large-scale CO2 capture from exhaust gases. This included four different reactor types (fixed bed, fluidized bed, moving bed, and rapid thermal swing) and was compared with the liquid amine scrubbing often used today. By integrating the adsorption/desorption and heating/cooling processes, TEA estimated a cost of the process by each reactor which falls into the range of 48.1–75.2 $/t-CO2 and a heating recovery of 45–58%. These results support the use and need for CO2 capture for flue gas purification.88 The lowest price of 48.1 $/t-CO2 can be achieved with the fixed-bed adsorption configuration. Considering the current cost of 62.8 $/t-CO2 with amine scrubbing using 30 wt % of MEA, CO2 capture with amine-functionalized solid materials has a high potential to compete in the market.
Zhao et al. designed and evaluated a 200 kWth pilot reactor for the energy balance of three different regeneration processes (thermal regeneration with CO2 stream, vacuum regeneration, steam-stripping regeneration).89 Amine-functionalized resin was selected to evaluate the efficiency and energy needs for each of the regeneration processes. The least energy is required for the thermal regeneration. Compared to the liquid amine scrubbing often used today, the energy consumption can be reduced by 30% or more. However, if the thermal regeneration is supported by an additional vacuum system, the energy consumption is higher than the thermal process itself but still much lower than the liquid amine scrubbing. In addition, the whole reactor is complicated and more expensive when the vacuum is applied. The steam-stripping regeneration requires the most energy, but the process can be greatly optimized when the steam condensation and heat recovery are taken into account.89 The total annual cost also depends on the plant size. Mazzotti et al. performed a techno-economic assessment and found that the cost of commercially available adsorbent considered gas had much less impact on the process cost, but the size and shape of the adsorption system critically affect the investment cost.90 The adsorption-based process can be competitive at the small scale (less than 100 tons of flue gas per day) and low recovery rate (less than 40%). However, the classical liquid amine scrubbing is still the most cost-effective at most of the plant sizes and recovery rates.
To perform a cost estimation for CO2 capture and concentration (CCC) technology, a model was created to calculate the cost per ton of CO2 captured for a 500 MW power plant. The absorbent used was zeolite 13X, which can also be replaced by an amine-functionalized material. Based on the calculations, a tremendous amount of energy can be saved when the concentration of CO2 in the flue gas is high enough. Besides, the adsorption efficiency of the reactor can be increased by shortening the reactor length with the larger diameter. At an off-gas CO2 concentration of 15%, the cost estimate was $32.8 to $34.4 per ton of CO2 captured.91
7. Light-Triggered CO2 Adsorption and Desorption
In this section, a state-of-the-art CO2 desorption method is described. In the past decade, photosensitive functional groups are applied for low-temperature regeneration of adsorbents by visible and/or UV light.92−94 This method allows CO2 adsorption–desorption cycles to be operated under mild conditions (1 bar and room temperature). Lyndon et al. reported that a zinc-based MOF with 4,4′-dicarboxylate (AzDC) and trans-1,2-bis(4-pyridyl)ethylene (4,4′-BPE) as a framework released CO2 under UV light irradiation at 30–31 °C.92 In situ FT-IR spectroscopy detected the change in C–C–C and C–C–N bending modes, originating from cis–trans isomerization of Zn(AzDC)(4,4′-BPE)0.5 under UV irradiation. The desorption capacity was 42% under static irradiation conditions and 64% under dynamic measurements.
Sun and co-workers reported photosensitive MOFs functionalized with azobenzene for CO2 adsorption–desorption by UV and visible lights.93 Tetraethylenepentamine (TEPA) was impregnated onto an UiO-type MOF functionalized with azobenzene (U-azo). Azobenzene changes its form between trans and cis under visible and UV lights, respectively. Exploiting this phenomenon, CO2 bound to TEPA is released by UV light irradiation. U-azo has the CO2 adsorption capacity of 43.4 cm3/g (ca. 1.94 mmol/g), among which 45.6% of adsorbed CO2 can be released. They also demonstrated that CO2 can be selectively separated using this principle out of gas mixtures of CO2/N2 and CO2/CH4. They further developed a novel adsorbent which works only with visible light.94 MCM-41 was functionalized with (3-aminopropyl)triethoxysilane (APTES) and Disperse Red 1 (DR1). Without irradiation, the photosensitive DR1 is in trans configuration, leading to CO2 capture on amines. Under visible-light irradiation, DR1 transforms into cis configuration, inducing CO2 desorption. The CO2 adsorption capacity was 32.1 cm3/g (ca. 1.43 mmol/g), 40% of which can be reversibly adsorbed and desorbed upon visible-light irradiation.
8. Summary
A great deal of research has been reported on CO2 capture by amine-based solid materials. In the last two decades, we witnessed a tremendous advance in material synthesis, optimization, and mechanistic aspects. However, practical use in the industrial sector still needs to overcome the following issues:
-
(1)
Long-term thermal stability during the regeneration process
-
(2)
Oxidative degradation
-
(3)
Material fabrication with lower regeneration temperatures
-
(4)
Reactor engineering and process optimization
Especially, point 3, “material fabrication with lower regeneration temperatures”, can mitigate points 1 and 2 because thermal stability and oxygen resistance would be greatly improved when the regeneration is operated in a low-temperature range of 40–60 °C. The cost estimate clearly proved that CO2 capture by amine-functionalized solid materials can reasonably be appointed to the position currently taken by the liquid amine scrubbing. The 2021 United Nations Climate Change Conference commonly referred to as the 26th United Nations Climate Change conference (COP26) set new goals for the increase in the global temperature level and CO2 emissions. The decision made in COP26 will dramatically change and refine research directions of CCS and CCU technologies. Amine-functionalized solid materials will be one of the main contributing technologies for achieving these goals.
The authors declare no competing financial interest.
References
- Zhao X. Y.; Qian J. L.; Wang J.; He Q. Y.; Wang Z. L.; Chen C. Z. Using a tree ring delta C-13 annual series to reconstruct atmospheric CO2 concentration over the past 300 years. Pedosphere 2006, 16 (3), 371–379. 10.1016/S1002-0160(06)60065-9. [DOI] [Google Scholar]
- Kawahata H.; Fujita K.; Iguchi A.; Inoue M.; Iwasaki S.; Kuroyanagi A.; Maeda A.; Manaka T.; Moriya K.; Takagi H.; Toyofuku T.; Yoshimura T.; Suzuki A. Perspective on the response of marine calcifiers to global warming and ocean acidification-Behavior of corals and foraminifera in a high CO2 world ″hot house″. Prog. Earth Planet. Sci. 2019, 10.1186/s40645-018-0239-9. [DOI] [Google Scholar]
- Tresguerres M.; Hamilton T. J. Acid base physiology, neurobiology and behaviour in relation to CO2-induced ocean acidification. J. Exp. Biol. 2017, 220 (12), 2136–2148. 10.1242/jeb.144113. [DOI] [PubMed] [Google Scholar]
- Keith D. W. Why Capture CO2 from the Atmosphere?. Science 2009, 325 (5948), 1654–1655. 10.1126/science.1175680. [DOI] [PubMed] [Google Scholar]
- Agaton C. B. Application of real options in carbon capture and storage literature: Valuation techniques and research hotspots. Sci. Total Environ. 2021, 795, 148683. 10.1016/j.scitotenv.2021.148683. [DOI] [PubMed] [Google Scholar]
- Sabri M. A.; Al Jitan S.; Bahamon D.; Vega L. F.; Palmisano G. Current and future perspectives on catalytic-based integrated carbon capture and utilization. Sci. Total Environ. 2021, 790, 148081. 10.1016/j.scitotenv.2021.148081. [DOI] [PubMed] [Google Scholar]
- Rafiee A.; Khalilpour K. R.; Milani D. Chapter 8 - CO2 Conversion and Utilization Pathways. In Polygeneration with Polystorage for Chemical and Energy Hubs; Khalilpour K. R., Ed.; Academic Press: 2019; pp 213–245. [Google Scholar]
- Robert A. M.Handbook of Petroleum Refining Processes, 3rd ed.; McGraw-Hill Education: New York, 2004. [Google Scholar]
- Rochelle G. T. Amine Scrubbing for CO2 Capture. Science 2009, 325 (5948), 1652. 10.1126/science.1176731. [DOI] [PubMed] [Google Scholar]
- D’Alessandro D. M.; Smit B.; Long J. R. Carbon Dioxide Capture: Prospects for New Materials. Angew. Chem., Int. Ed. 2010, 49 (35), 6058–6082. 10.1002/anie.201000431. [DOI] [PubMed] [Google Scholar]
- Bains P.; Psarras P.; Wilcox J. CO2 capture from the industry sector. Prog. Energy Combust. Sci. 2017, 63, 146–172. 10.1016/j.pecs.2017.07.001. [DOI] [Google Scholar]
- Fisher J. C.; Tanthana J.; Chuang S. S. C. Oxide-Supported Tetraethylenepentamine for CO2 Capture. Environ. Prog. Sustain. Energy 2009, 28 (4), 589–598. 10.1002/ep.10363. [DOI] [Google Scholar]
- Guo L. P.; Hu X.; Hu G. S.; Chen J.; Li Z. M.; Dai W.; Dacosta H. F. M.; Fan M. H. Tetraethylenepentamine modified protonated titanate nanotubes for CO2 capture. Fuel Process. Technol. 2015, 138, 663–669. 10.1016/j.fuproc.2015.07.007. [DOI] [Google Scholar]
- Irani M.; Jacobson A. T.; Gasem K. A. M.; Fan M. H. Modified carbon nanotubes/tetraethylenepentamine for CO2 capture. Fuel 2017, 206, 10–18. 10.1016/j.fuel.2017.05.087. [DOI] [Google Scholar]
- Jo D. H.; Jung H.; Shin D. K.; Lee C. H.; Kim S. H. Effect of amine structure on CO2 adsorption over tetraethylenepentamine impregnated poly methyl methacrylate supports. Sep. Purif. Technol. 2014, 125, 187–193. 10.1016/j.seppur.2014.01.048. [DOI] [Google Scholar]
- Xie W. L.; Ji X. Y.; Fan T. T.; Feng X.; Lu X. H. CO2 Uptake Behavior of Supported Tetraethylenepentamine Sorbents. Energy Fuels 2016, 30 (6), 5083–5091. 10.1021/acs.energyfuels.6b00558. [DOI] [Google Scholar]
- Liu Z. L.; Teng Y.; Zhang K.; Chen H. G.; Yang Y. P. CO2 adsorption performance of different amine-based siliceous MCM-41 materials. J. Energy Chem. 2015, 24 (3), 322–330. 10.1016/S2095-4956(15)60318-7. [DOI] [Google Scholar]
- Prud’homme A.; Nabki F. Comparison between Linear and Branched Polyethylenimine and Reduced Graphene Oxide Coatings as a Capture Layer for Micro Resonant CO2 Gas Concentration Sensors. Sensors 2020, 20 (7), 1824. 10.3390/s20071824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosu C.; Pang S. H.; Sujan A. R.; Sakwa-Novak M. A.; Ping E. W.; Jones C. W. Effect of Extended Aging and Oxidation on Linear Poly(propylenimine)-Mesoporous Silica Composites for CO2 Capture from Simulated Air and Flue Gas Streams. ACS Appl. Mater. Interfaces 2020, 12 (34), 38085–38097. 10.1021/acsami.0c09554. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Goeppert A.; Olah G. A.; Prakash G. K. S. Remarkable effect of moisture on the CO2 adsorption of nano-silica supported linear and branched polyethylenimine. J. CO2 Util. 2017, 19, 91–99. 10.1016/j.jcou.2017.03.008. [DOI] [Google Scholar]
- Zhang H.; Goeppert A.; Prakash G. K. S.; Olah G. Applicability of linear polyethylenimine supported on nano-silica for the adsorption of CO2 from various sources including dry air. RSC Adv. 2015, 5 (65), 52550–52562. 10.1039/C5RA05428A. [DOI] [Google Scholar]
- Wang W.; Liu F.; zhang Q.; Yu G.; Deng S. Efficient removal of CO2 from indoor air using a polyethyleneimine-impregnated resin and its low-temperature regeneration. Chem. Eng. J. 2020, 399, 125734. 10.1016/j.cej.2020.125734. [DOI] [Google Scholar]
- Weisshar F.; Gau A.; Hack J.; Maeda N.; Meier D. M. Toward Carbon Dioxide Capture from the Atmosphere: Lowering the Regeneration Temperature of Polyethylenimine-Based Adsorbents by Ionic Liquid. Energy Fuels 2021, 35 (10), 9059–9062. 10.1021/acs.energyfuels.1c00392. [DOI] [Google Scholar]
- Yamada H.; Chowdhury F. A.; Fujiki J.; Yogo K. Enhancement Mechanism of the CO2 Adsorption–Desorption Efficiency of Silica-Supported Tetraethylenepentamine by Chemical Modification of Amino Groups. ACS Sustain. Chem. Eng. 2019, 7 (10), 9574–9581. 10.1021/acssuschemeng.9b01064. [DOI] [Google Scholar]
- Cogswell C. F.; Xie Z.; Wolek A.; Wang Y.; Stavola A.; Finkenaur M.; Gilmore E.; Lanzillotti M.; Choi S. Pore structure-CO2 adsorption property relations of supported amine materials with multi-pore networks. J. Mater. Chem. A 2017, 5 (18), 8526–8536. 10.1039/C7TA01616F. [DOI] [Google Scholar]
- Liu Y.; Liu J.; Yao W. Y.; Cen W. L.; Wang H. Q.; Weng X. L.; Wu Z. B. The effects of surface acidity on CO2 adsorption over amine functionalized protonated titanate nanotubes. RSC Adv. 2013, 3 (41), 18803–18810. 10.1039/c3ra42597e. [DOI] [Google Scholar]
- Geng J. C.; Xue D. M.; Liu X. Q.; Shi Y. Q.; Sun L. B. N-Doped Porous Carbons for CO2 Capture: Rational Choice of N-Containing Polymer with High Phenyl Density as Precursor. AIChE J. 2017, 63 (5), 1648–1658. 10.1002/aic.15531. [DOI] [Google Scholar]
- Qi S. C.; Wu J. K.; Lu J.; Yu G. X.; Zhu R. R.; Liu Y.; Liu X. Q.; Sun L. B. Underlying mechanism of CO2 adsorption onto conjugated azacyclo-copolymers: N-doped adsorbents capture CO2 chiefly through acid-base interaction?. J. Mater. Chem. A 2019, 7 (30), 17842–17853. 10.1039/C9TA04785A. [DOI] [Google Scholar]
- Flaig R. W.; Osborn Popp T. M.; Fracaroli A. M.; Kapustin E. A.; Kalmutzki M. J.; Altamimi R. M.; Fathieh F.; Reimer J. A.; Yaghi O. M. The Chemistry of CO2 Capture in an Amine-Functionalized Metal–Organic Framework under Dry and Humid Conditions. J. Am. Chem. Soc. 2017, 139 (35), 12125–12128. 10.1021/jacs.7b06382. [DOI] [PubMed] [Google Scholar]
- Fracaroli A. M.; Furukawa H.; Suzuki M.; Dodd M.; Okajima S.; Gándara F.; Reimer J. A.; Yaghi O. M. Metal–Organic Frameworks with Precisely Designed Interior for Carbon Dioxide Capture in the Presence of Water. J. Am. Chem. Soc. 2014, 136 (25), 8863–8866. 10.1021/ja503296c. [DOI] [PubMed] [Google Scholar]
- Boukoussa B.; Hakiki A.; Bouazizi N.; Beltrao-Nunes A. P.; Launay F.; Pailleret A.; Pillier F.; Bengueddach A.; Hamacha R.; Azzouz A. Mesoporous silica supported amine and amine-copper complex for CO2 adsorption: Detailed reaction mechanism of hydrophilic character and CO2 retention. J. Mol. Struct. 2019, 1191, 175–182. 10.1016/j.molstruc.2019.04.035. [DOI] [Google Scholar]
- Chao K. J.; Klinthong W.; Tan C. S. CO2 Adsorption Ability and Thermal Stability of Amines Supported on Mesoporous Silica SBA-15 and Fumed Silica. J. Chin. Chem. Soc. 2013, 60 (7), 735–744. 10.1002/jccs.201200507. [DOI] [Google Scholar]
- Irani M.; Gasem K. A. M.; Dutcher B.; Fan M. H. CO2 capture using nanoporous TiO(OH)2/tetraethylenepentamine. Fuel 2016, 183, 601–608. 10.1016/j.fuel.2016.06.129. [DOI] [Google Scholar]
- Jung H.; Lee C. H.; Jeon S.; Jo D. H.; Huh J.; Kim S. H. Effect of amine double-functionalization on CO2 adsorption behaviors of silica gel-supported adsorbents. Adsorption 2016, 22 (8), 1137–1146. 10.1007/s10450-016-9837-2. [DOI] [Google Scholar]
- Kuang Y.; He H.; Chen S.; Wu J.; Liu F. Adsorption behavior of CO2 on amine-functionalized polyacrylonitrile fiber. Adsorption 2019, 25 (4), 693–701. 10.1007/s10450-019-00070-0. [DOI] [Google Scholar]
- Sun Z. Y.; Fan M. H.; Argyle M. Supported Monoethanolamine for CO2 Separation. Ind. Eng. Chem. Res. 2011, 50 (19), 11343–11349. 10.1021/ie2005115. [DOI] [Google Scholar]
- Li K. J.; Kress J. D.; Mebane D. S. The Mechanism of CO2 Adsorption under Dry and Humid Conditions in Mesoporous Silica-Supported Amine Sorbents. J. Phys. Chem. C 2016, 120 (41), 23683–23691. 10.1021/acs.jpcc.6b08808. [DOI] [Google Scholar]
- Sayari A.; Belmabkhout Y. Stabilization of Amine-Containing CO2 Adsorbents: Dramatic Effect of Water Vapor. J. Am. Chem. Soc. 2010, 132 (18), 6312–6314. 10.1021/ja1013773. [DOI] [PubMed] [Google Scholar]
- Veneman R.; Frigka N.; Zhao W. Y.; Li Z. S.; Kersten S.; Brilman W. Adsorption of H2O and CO2 on supported amine sorbents. Int. J. Greenh. Gas Control. 2015, 41, 268–275. 10.1016/j.ijggc.2015.07.014. [DOI] [Google Scholar]
- Zhou Z.; Balijepalli S. K.; Nguyen-Sorenson A. H. T.; Anderson C. M.; Park J. L.; Stowers K. J. Steam-Stable Covalently Bonded Polyethylenimine Modified Multiwall Carbon Nanotubes for Carbon Dioxide Capture. Energy Fuels 2018, 32 (11), 11701–11709. 10.1021/acs.energyfuels.8b02864. [DOI] [Google Scholar]
- Wang X.; Song C. Temperature-programmed desorption of CO2 from polyethylenimine-loaded SBA-15 as molecular basket sorbents. Catal. Today 2012, 194 (1), 44–52. 10.1016/j.cattod.2012.08.008. [DOI] [Google Scholar]
- Uyanga I. J.; Idem R. O. Studies of SO2- and O2-Induced Degradation of Aqueous MEA during CO2 Capture from Power Plant Flue Gas Streams. Ind. Eng. Chem. Res. 2007, 46 (8), 2558–2566. 10.1021/ie0614024. [DOI] [Google Scholar]
- Xu X.; Song C.; Miller B. G.; Scaroni A. W. Adsorption separation of carbon dioxide from flue gas of natural gas-fired boiler by a novel nanoporous “molecular basket” adsorbent. Fuel Process. Technol. 2005, 86 (14), 1457–1472. 10.1016/j.fuproc.2005.01.002. [DOI] [Google Scholar]
- Rezaei F.; Jones C. W. Stability of Supported Amine Adsorbents to SO2 and NOx in Postcombustion CO2 Capture. 1. Single-Component Adsorption. Ind. Eng. Chem. Res. 2013, 52 (34), 12192–12201. 10.1021/ie4019116. [DOI] [Google Scholar]
- Rezaei F.; Jones C. W. Stability of Supported Amine Adsorbents to SO2 and NOx in Postcombustion CO2 Capture. 2. Multicomponent Adsorption. Ind. Eng. Chem. Res. 2014, 53 (30), 12103–12110. 10.1021/ie502024z. [DOI] [Google Scholar]
- Ahmadalinezhad A.; Sayari A. Oxidative degradation of silica-supported polyethylenimine for CO2 adsorption: insights into the nature of deactivated species. Phys. Chem. Chem. Phys. 2014, 16 (4), 1529–1535. 10.1039/C3CP53928H. [DOI] [PubMed] [Google Scholar]
- Bali S.; Chen T. T.; Chaikittisilp W.; Jones C. W. Oxidative Stability of Amino Polymer-Alumina Hybrid Adsorbents for Carbon Dioxide Capture. Energy Fuels 2013, 27 (3), 1547–1554. 10.1021/ef4001067. [DOI] [Google Scholar]
- Heydari-Gorji A.; Belmabkhout Y.; Sayari A. Degradation of amine-supported CO2 adsorbents in the presence of oxygen-containing gases. Micropor. Mesopor. Mater. 2011, 145 (1–3), 146–149. 10.1016/j.micromeso.2011.05.010. [DOI] [Google Scholar]
- Heydari-Gorji A.; Sayari A. Thermal, Oxidative, and CO2-Induced Degradation of Supported Polyethylenimine Adsorbents. Ind. Eng. Chem. Res. 2012, 51 (19), 6887–6894. 10.1021/ie3003446. [DOI] [Google Scholar]
- Vu Q. T.; Yamada H.; Yogo K. Oxidative Degradation of Tetraethylenepentamine-Impregnated Silica Sorbents for CO2 Capture. Energy Fuels 2019, 33 (4), 3370–3379. 10.1021/acs.energyfuels.8b04307. [DOI] [Google Scholar]
- Wang M.; Yao L.; Wang J.; Zhang Z.; Qiao W.; Long D.; Ling L. Adsorption and regeneration study of polyethylenimine-impregnated millimeter-sized mesoporous carbon spheres for post-combustion CO2 capture. Appl. Ener. 2016, 168, 282–290. 10.1016/j.apenergy.2016.01.085. [DOI] [Google Scholar]
- Bacsik Z.; Hedin N. Effects of carbon dioxide captured from ambient air on the infrared spectra of supported amines. Vib. Spectrosc. 2016, 87, 215–221. 10.1016/j.vibspec.2016.10.006. [DOI] [Google Scholar]
- Danon A.; Stair P. C.; Weitz E. FTIR Study of CO2 Adsorption on Amine-Grafted SBA-15: Elucidation of Adsorbed Species. J. Phys. Chem. C 2011, 115 (23), 11540–11549. 10.1021/jp200914v. [DOI] [Google Scholar]
- Hedin N.; Bacsik Z. Perspectives on the adsorption of CO2 on amine-modified silica studied by infrared spectroscopy. Curr. Opin. Green Sustain. 2019, 16, 13–19. 10.1016/j.cogsc.2018.11.010. [DOI] [Google Scholar]
- Pyo S. W.; Manianglung C.; Ko Y. S. In-situ IR study on stability of amine-impregnated CO2 adsorbents to acidic gases. Catal. Today 2020, 352, 198–203. 10.1016/j.cattod.2020.01.036. [DOI] [Google Scholar]
- Chakravartula Srivatsa S.; Bhattacharya S. Amine-based CO2 capture sorbents: A potential CO2 hydrogenation catalyst. J. CO2 Util. 2018, 26, 397–407. 10.1016/j.jcou.2018.05.028. [DOI] [Google Scholar]
- Tanthana J.; Chuang S. S. C. In Situ Infrared Study of the Role of PEG in Stabilizing Silica-Supported Amines for CO2 Capture. ChemSusChem 2010, 3 (8), 957–964. 10.1002/cssc.201000090. [DOI] [PubMed] [Google Scholar]
- Tumuluri U.; Isenberg M.; Tan C. S.; Chuang S. S. C. In Situ Infrared Study of the Effect of Amine Density on the Nature of Adsorbed CO2 on Amine-Functionalized Solid Sorbents. Langmuir 2014, 30 (25), 7405–7413. 10.1021/la501284y. [DOI] [PubMed] [Google Scholar]
- Wilfong W. C.; Srikanth C. S.; Chuang S. S. C. In Situ ATR and DRIFTS Studies of the Nature of Adsorbed CO2 on Tetraethylenepentamine Films. ACS Appl. Mater. Interfaces 2014, 6 (16), 13617–13626. 10.1021/am5031006. [DOI] [PubMed] [Google Scholar]
- Yu J.; Chuang S. S. C. The Structure of Adsorbed Species on Immobilized Amines in CO2 Capture: An in Situ IR Study. Energy Fuels 2016, 30 (9), 7579–7587. 10.1021/acs.energyfuels.6b01423. [DOI] [Google Scholar]
- Chen C. H.; Sesti E. L.; Lee J. J.; Mentink-Vigier F.; Sievers C.; Jones C. W.; Hayes S. E. NMR Reveals Two Bicarbonate Environments in SBA15-Solid-Amine CO2 Sorbents. J. Phys. Chem. C 2021, 125 (30), 16759–16765. 10.1021/acs.jpcc.1c04145. [DOI] [Google Scholar]
- Chen C. H.; Shimon D.; Lee J. J.; Didas S. A.; Mehta A. K.; Sievers C.; Jones C. W.; Hayes S. E. Spectroscopic Characterization of Adsorbed 13CO2 on 3-Aminopropylsilyl-Modified SBA15 Mesoporous Silica. Environ. Sci. Technol. 2017, 51 (11), 6553–6559. 10.1021/acs.est.6b06605. [DOI] [PubMed] [Google Scholar]
- Chen C. H.; Shimon D.; Lee J. J.; Mentink-Vigier F.; Hung I.; Sievers C.; Jones C. W.; Hayes S. E. The ″Missing″ Bicarbonate in CO2 Chemisorption Reactions on Solid Amine Sorbents. J. Am. Chem. Soc. 2018, 140 (28), 8648–8651. 10.1021/jacs.8b04520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo G. S.; Lee J. J.; Chen C. H.; Hayes S. E.; Sievers C.; Jones C. W. Elucidation of Surface Species through in Situ FTIR Spectroscopy of Carbon Dioxide Adsorption on Amine-Grafted SBA-15. ChemSusChem 2017, 10 (1), 266–276. 10.1002/cssc.201600809. [DOI] [PubMed] [Google Scholar]
- Moore J. K.; Sakwa-Novak M. A.; Chaikittisilp W.; Mehta A. K.; Conradi M. S.; Jones C. W.; Hayes S. E. Characterization of a Mixture of CO2 Adsorption Products in Hyperbranched Aminosilica Adsorbents by 13C Solid-State NMR. Environ. Sci. Technol. 2015, 49 (22), 13684–13691. 10.1021/acs.est.5b02930. [DOI] [PubMed] [Google Scholar]
- Park S. J.; Lee J. J.; Hoyt C. B.; Kumar D. R.; Jones C. W. Silica supported poly(propylene guanidine) as a CO2 sorbent in simulated flue gas and direct air capture. Adsorption 2020, 26 (1), 89–101. 10.1007/s10450-019-00171-w. [DOI] [Google Scholar]
- Mebane D. S.; Kress J. D.; Storlie C. B.; Fauth D. J.; Gray M. L.; Li K. J. Transport, Zwitterions, and the Role of Water for CO2 Adsorption in Mesoporous Silica-Supported Amine Sorbents. J. Phys. Chem. C 2013, 117 (50), 26617–26627. 10.1021/jp4076417. [DOI] [Google Scholar]
- Muller K.; Lu D. Y.; Senanayake S. D.; Starr D. E. Monoethanolamine Adsorption on TiO2(110): Bonding, Structure, and Implications for Use as a Model Solid-Supported CO2 Capture Material. J. Phys. Chem. C 2014, 118 (3), 1576–1586. 10.1021/jp409098p. [DOI] [Google Scholar]
- Chen Y.; Yuan H. L.; Xia H.; Jiang W.; Yang C.; Hu G. S.; Lan Y. Z.; Fan M. H. The volume expansion effect of amine during CO2 adsorption process: An experimental study combined with theoretical calculations. J. Colloid Interface Sci. 2020, 572, 190–197. 10.1016/j.jcis.2020.03.088. [DOI] [PubMed] [Google Scholar]
- Lee Z. R.; Quinn L. D. J.; Jones C. W.; Hayes S. E.; Dixon D. A. Predicting the Mechanism and Products of CO2 Capture by Amines in the Presence of H2O. J. Phys. Chem. A 2021, 125 (45), 9802–9818. 10.1021/acs.jpca.1c05950. [DOI] [PubMed] [Google Scholar]
- Chen C.-H.; Sesti E. L.; Lee J. J.; Mentink-Vigier F.; Sievers C.; Jones C. W.; Hayes S. E. NMR Reveals Two Bicarbonate Environments in SBA15-Solid-Amine CO2 Sorbents. J. Phys. Chem. C 2021, 125 (30), 16759–16765. 10.1021/acs.jpcc.1c04145. [DOI] [Google Scholar]
- Wijesiri R. P.; Knowles G. P.; Yeasmin H.; Hoadley A. F. A.; Chaffee A. L. Desorption Process for Capturing CO2 from Air with Supported Amine Sorbent. Ind. Eng. Chem. Res. 2019, 58 (34), 15606–15618. 10.1021/acs.iecr.9b03140. [DOI] [Google Scholar]
- Wurzbacher J. A.; Gebald C.; Brunner S.; Steinfeld A. Heat and mass transfer of temperature-vacuum swing desorption for CO2 capture from air. Chem. Eng. J. 2016, 283, 1329–1338. 10.1016/j.cej.2015.08.035. [DOI] [Google Scholar]
- Krishnamurthy S.; Boon J.; Grande C.; Lind A.; Blom R.; Boer R.; Willemsen H.; Scheemaker G. Screening Supported Amine Sorbents in the Context of Post-combustion Carbon Capture by Vacuum Swing Adsorption. Chem. Ing. Technol. 2021, 93 (6), 929–940. 10.1002/cite.202000172. [DOI] [Google Scholar]
- Gebald C.; Wurzbacher J. A.; Tingaut P.; Steinfeld A. Stability of Amine-Functionalized Cellulose during Temperature-Vacuum-Swing Cycling for CO2 Capture from Air. Environ. Sci. Technol. 2013, 47 (17), 10063–10070. 10.1021/es401731p. [DOI] [PubMed] [Google Scholar]
- Fang M. X.; Xiang Q. Y.; Wang T.; Le Moullec Y.; Lu J. H.; Jiang W. M.; Zhou X. P.; Zhang J. B.; Chen G. F. Experimental Study on the Novel Direct Steam Stripping Process for Postcombustion CO2 Capture. Ind. Eng. Chem. Res. 2014, 53 (46), 18054–18062. 10.1021/ie503517y. [DOI] [Google Scholar]
- Fujiki J.; Chowdhury F. A.; Yamada H.; Yogo K. Highly efficient post-combustion CO2 capture by low-temperature steam-aided vacuum swing adsorption using a novel polyamine-based solid sorbent. Chem. Eng. J. 2017, 307, 273–282. 10.1016/j.cej.2016.08.071. [DOI] [Google Scholar]
- Li W.; Choi S.; Drese J. H.; Hornbostel M.; Krishnan G.; Eisenberger P. M.; Jones C. W. Steam-Stripping for Regeneration of Supported Amine-Based CO2 Adsorbents. ChemSusChem 2010, 3 (8), 899–903. 10.1002/cssc.201000131. [DOI] [PubMed] [Google Scholar]
- Plaza M. G.; Rubiera F.; Pevida C. Evaluating the Feasibility of a TSA Process Based on Steam Stripping in Combination with Structured Carbon Adsorbents To Capture CO2 from a Coal Power Plant. Energy Fuels 2017, 31 (9), 9760–9775. 10.1021/acs.energyfuels.7b01508. [DOI] [Google Scholar]
- Sakwa-Novak M. A.; Jones C. W. Steam Induced Structural Changes of a Poly(ethylenimine) Impregnated gamma-Alumina Sorbent for CO2 Extraction from Ambient Air. ACS Appl. Mater. Interfaces 2014, 6 (12), 9245–9255. 10.1021/am501500q. [DOI] [PubMed] [Google Scholar]
- Sandhu N. K.; Pudasainee D.; Sarkar P.; Gupta R. Steam Regeneration of Polyethylenimine-Impregnated Silica Sorbent for Postcombustion CO2 Capture: A Multicyclic Study. Ind. Eng. Chem. Res. 2016, 55 (7), 2210–2220. 10.1021/acs.iecr.5b04741. [DOI] [Google Scholar]
- Xiang Q. Y.; Le Moullec Y.; Fang M. X.; Valle-Marcos J. C.; Lu J. H.; Jiang W. M.; Zhou X. P.; Chen G. F.; Luo Z. Y. In Novel Solvent Regeneration Process through Direct Steam Stripping, 12th International Conference on Greenhouse Gas Control Technologies (GHGT), Austin, TX, Oct 05–09, 2014; pp 1392–1398.
- Chaikittisilp W.; Kim H. J.; Jones C. W. Mesoporous Alumina-Supported Amines as Potential Steam-Stable Adsorbents for Capturing CO2 from Simulated Flue Gas and Ambient Air. Energy Fuels 2011, 25 (11), 5528–5537. 10.1021/ef201224v. [DOI] [Google Scholar]
- Fayaz M.; Sayari A. Long-Term Effect of Steam Exposure on CO2 Capture Performance of Amine-Grafted Silica. ACS Appl. Mater. Interfaces 2017, 9 (50), 43747–43754. 10.1021/acsami.7b15463. [DOI] [PubMed] [Google Scholar]
- Jahandar Lashaki M.; Ziaei-Azad H.; Sayari A. Insights into the Hydrothermal Stability of Triamine-Functionalized SBA-15 Silica for CO2 Adsorption. ChemSusChem 2017, 10 (20), 4037–4045. 10.1002/cssc.201701439. [DOI] [PubMed] [Google Scholar]
- Yu Q.; Brilman D. W. F., Design strategy for CO2 adsorption from ambient air using a supported amine based sorbent in a fixed bed reactor. In 13th International Conference on Greenhouse Gas Control Technologies, Ghgt-13; Dixon T., Laloui L., Twinning S., Eds.; 2017; Vol. 114, pp 6102–6114.
- Esmaeili Rad F.; Abbasian J.; Arastoopour H. Numerical simulation of CO2 adsorption in a fluidized bed using solid-supported amine sorbent. Can. J. Chem. Eng. 2021, 99 (7), 1595–1606. 10.1002/cjce.24000. [DOI] [Google Scholar]
- Jung W. H.; Lee J. Economic evaluation for four different solid sorbent processes with heat integration for energy-efficient CO2 capture based on PEI-silica sorbent. Energy 2022, 238, 121864. 10.1016/j.energy.2021.121864. [DOI] [Google Scholar]
- Zhao W. Y.; Veneman R.; Chen D. G.; Li Z. S.; Cai N. S.; Brilmana D. W. F.. Post-Combustion CO2 Capture Demonstration Using Supported Amine Sorbents: Design and Evaluation of 200 kW(th) Pilot. In 12th International Conference on Greenhouse Gas Control Technologies, Ghgt-12; Dixon T., Herzog H., Twinning S., Eds.; 2014; Vol. 63, pp 2374–2383.
- Zanco S. E.; Pérez-Calvo J.-F.; Gasós A.; Cordiano B.; Becattini V.; Mazzotti M. Postcombustion CO2 Capture: A Comparative Techno-Economic Assessment of Three Technologies Using a Solvent, an Adsorbent, and a Membrane. ACS Engineering Au 2021, 1 (1), 50–72. 10.1021/acsengineeringau.1c00002. [DOI] [Google Scholar]
- Susarla N.; Haghpanah R.; Karimi I. A.; Farooq S.; Rajendran A.; Tan L. S. C.; Lim J. S. T. Energy and cost estimates for capturing CO2 from a dry flue gas using pressure/vacuum swing adsorption. Chem. Eng. Res. Des. 2015, 102, 354–367. 10.1016/j.cherd.2015.06.033. [DOI] [Google Scholar]
- Lyndon R.; Konstas K.; Ladewig B. P.; Southon P. D.; Kepert P. C. J.; Hill M. R. Dynamic Photo-Switching in Metal–Organic Frameworks as a Route to Low-Energy Carbon Dioxide Capture and Release. Angew. Chem., Int. Ed. 2013, 52 (13), 3695–3698. 10.1002/anie.201206359. [DOI] [PubMed] [Google Scholar]
- Jiang Y.; Tan P.; Qi S.-C.; Liu X.-Q.; Yan J.-H.; Fan F.; Sun L.-B. Metal–Organic Frameworks with Target-Specific Active Sites Switched by Photoresponsive Motifs: Efficient Adsorbents for Tailorable CO2 Capture. Angew. Chem., Int. Ed. 2019, 58 (20), 6600–6604. 10.1002/anie.201900141. [DOI] [PubMed] [Google Scholar]
- Wu Q.-R.; Tan P.; Gu C.; Zhou R.; Qi S.-C.; Liu X.-Q.; Jiang Y.; Sun L.-B. Smart adsorbents for CO2 capture: Making strong adsorption sites respond to visible light. Sci. China Mater. 2021, 64 (2), 383–392. 10.1007/s40843-020-1423-8. [DOI] [Google Scholar]