Abstract
Ox-/thiazoline groups in nonribosomal peptides are formed by a variant of peptide-forming condensation domains called heterocyclization (Cy) domains and appear in a range of pharmaceutically important natural products and virulence factors. Recent cryo-EM, crystallographic, and NMR studies of Cy domains make it opportune to revisit outstanding questions regarding their molecular mechanisms. This review covers structural and dynamical findings about Cy domains that will inform future bioengineering efforts and our understanding of natural product synthesis.
Keywords: heterocycle, pantetheine, cyclodehydration, bioengineering, protein-protein interactions, allostery
Introduction
Nonribosomal peptide synthetase (NRPS) heterocyclization (Cy) domains (Fig. 1) afford ox-/thiazoline groups in a variety of natural products [1–3]. Found in three oxidation states (Fig. 1A, bond-line structures), these heterocycles can increase potency for biological targets [4,5]. Nature has developed alternative strategies for incorporating ox-/thiazoline groups within peptides depending on whether they are synthesized ribosomally or nonribosomally [6–9]. Ribosomal systems use in trans interactions after polymerization [10], whereas nonribosomal systems install ox-/thiazoline groups as the peptide is produced [11].
Figure 1. Structures of NRPS Cy domains.
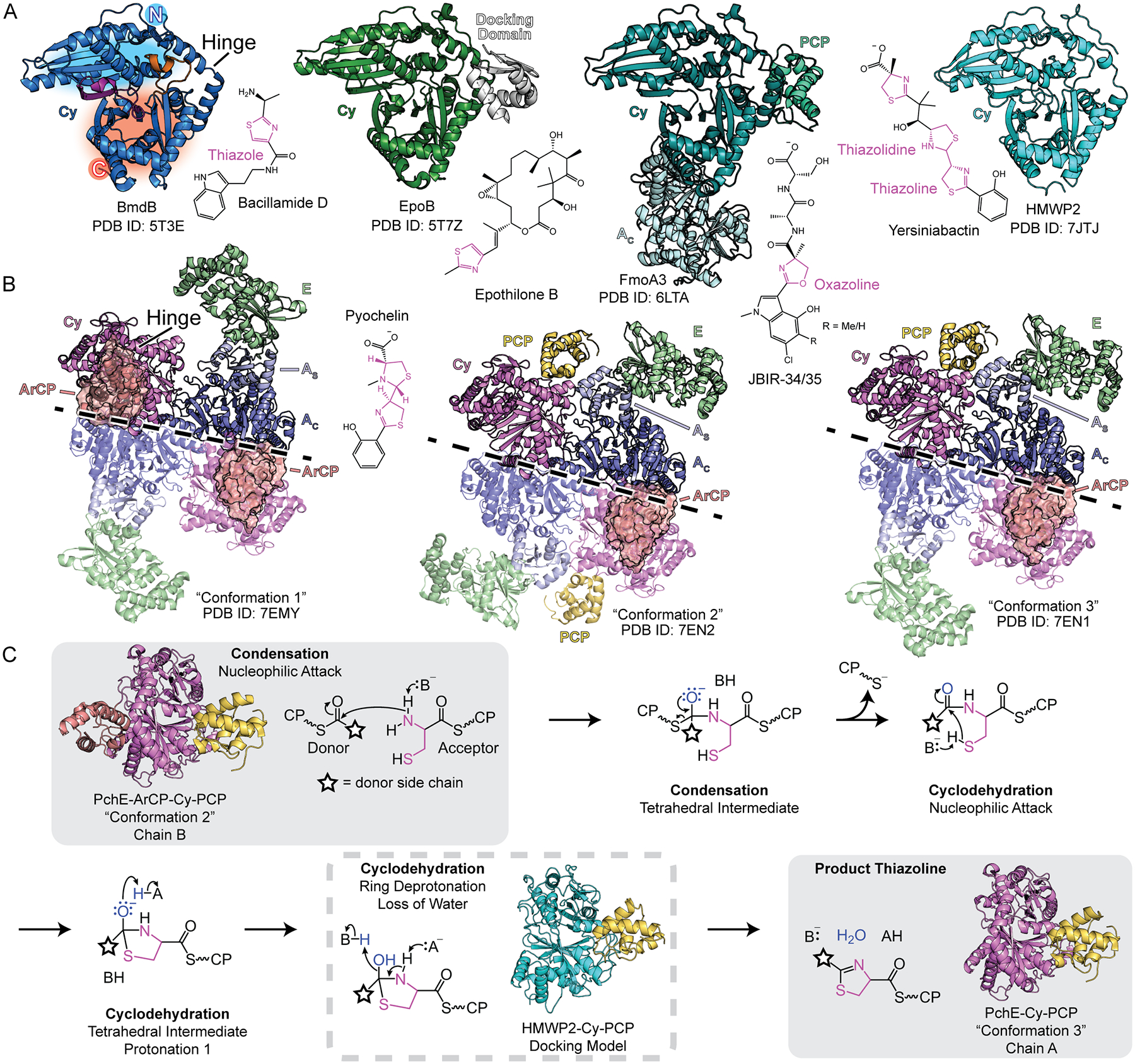
A) Crystal structures of Cy domains [12,13,16,17] alongside their products. Heterocycles are colored pink. In BmdB, the N- and C-terminal subdomains are shaded blue and red, respectively. The latch and floor loop are purple and orange, respectively. B) Three conformations of homodimeric PchE modules determined by cryo-EM [15], with the dimer interface marked by a dashed line. ArCPs are represented in surface. C) Catalytic scheme for Cy domains, including currently available structures resembling proposed catalytic states. Atoms for the heterocycle are colored magenta, and atoms of the water leaving group are blue.
Recent studies of JBIR-34/35 [12], yersiniabactin [13,14], pyochelin [15], epothilone [16], and bacillamide [17,18] synthetases have expanded our knowledge of Cy domain structure and function (Fig. 1A, B), complementing previous biochemical studies of NRPS Cy systems ([19] and Table S1 from [17]). These structures are of interest for bioengineering [20–24] as type 1 NRPSs follow a modular, assembly-line logic involving phosphopantetheine (Ppant)-loaded carrier proteins (CPs) that tether amino acid substrates installed by adenylation (A) domains [25,26], and modifying substrate incorporation affords new products [27,28]. Peptide bond formation requires two CPs and intervening condensation (C) domains (Fig. 1C, first step) or Cy domains. Thiazoline-producing Cy domains catalyze both condensation and cyclodehydration steps (Fig. 1C) [29], whereas oxazoline-producing Cy domains are typically found in tandem, with each domain catalyzing condensation or cyclodehydration [12,30].
C and Cy domains share little-to-no sequence similarity [16,17,31], enabling exaptation of the parent C-domain fold to additionally catalyze cyclodehydration. Both domains adopt a fold comprising two chloramphenicol acetyltransferase (CAT)-like subdomains (N- and C-terminal) connected by a hinge linker with two additional points of subdomain crossover, known as the floor loop and latch (Fig. 1A). The Cy-domain active site lies between the β-sheets of the N-terminal and C-terminal subdomains and is connected by tunnels to acceptor and donor CP binding sites. Cy domains contain a DxxxxDxxS motif [29], with DxxS in helix α4, identified with a structural role, an aspartate-threonine catalytic dyad required for cyclization, and a gating phenylalanine blocking the active site from bulk solvent, presumably to facilitate dehydration [16,17]. Here, we highlight observations in the current body of literature that link global conformational changes to local remodeling of CP and substrate binding sites and propose a remodeling of the active site architecture for condensation and cyclodehydration within a dynamic protein scaffold.
Cy structures provide insight into global conformational change
The first Cy-domain crystal structures revealed a closed donor site entrance, blocked by a gating phenylalanine [16,32] which we refer to as a “closed-for-cyclodehydration” state. Recently, cryo-EM structures of PchE [15] captured a Cy domain bound to a substrate-loaded donor (Fig. 1C). Here, an “open-for-condensation” state displays a bound donor Ppant, with a C-terminal subdomain mobile lobe (containing strand β10) splayed away from strands β8 and β9 flanking the floor loop (Fig. 2A). Aligning all existing models [12–18] using β8 and β9 (Fig. 2A) displays rotation of the N-terminal subdomain, a twisting of the floor loop, and displacement of the mobile lobe (Fig. 2) [13,14], motions observed by normal mode analysis of HMWP2-Cy2 [13] and in C domains [32,33]. In some normal modes, these motions accompany rearrangement of α1, α10, and the β-turn (BT) at the acceptor site entrance (ASE, Fig. 2B) as well as opening of the donor site entrance (DSE) and rearrangement of C-terminal subdomain helices (e.g. α9/α11, Fig. 2) [13,14,17]. Thus, global rearrangements can remodel entrances to the active-site tunnel.
Figure 2. Motions common to Cy domains.
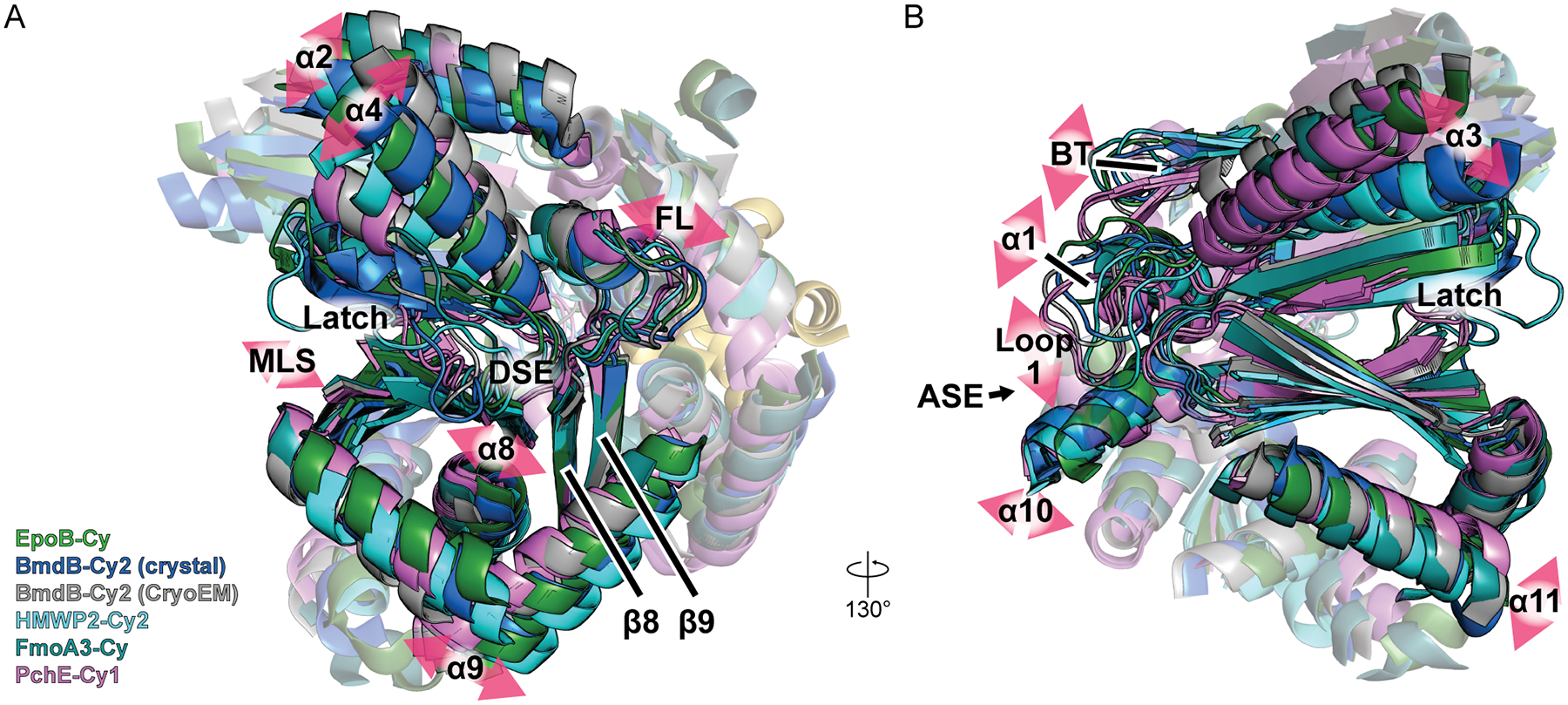
A,B) Structures [12,13,15–18] aligned by strands flanking the floor loop, viewed from the donor site entrance (A) and acceptor site entrance (B). Pink arrows highlight variations between models. MLS = mobile lobe strands, DSE = donor site entrance, ASE = acceptor site entrance.
The Cy-specific DxxxxDxxS motif, which when mutated led to decreased structural integrity or activity [12,15–18,30,34–37], appears to be part of the open-for-condensation to closed-for-cyclodehydration transition. In absence of CP, the second aspartate of the DxxxxDxxS motif usually engages the serine within the motif and an arginine in the floor loop (FL Arg) (Fig. 3A). Structures of the donor-bound and acceptor-bound PchE-Cy1 models (Fig. 3B) reveal conformational changes in an open-to-closed transition, where the second motif aspartate interacts with the Ppant arm. The serine then interacts with the backbone at the end of the floor loop, substituting for the lost interaction between the FL Arg and the aspartate, leading to a conformation that could indirectly affect the gating phenylalanine and open the DSE. The first aspartate of the DxxxxDxxS motif appears to transiently interact with a conserved arginine in α1 near the ASE, suggesting a possible role in sensing acceptor CP binding (Fig. 3D). Thus, DxxxxDxxS conformational changes accompany remodeling of the DSE and ASE upon CP and tethered Ppant binding, perhaps participating in communication between both sites.
Figure 3. Cy-domain motions may participate in substrate binding and allostery.
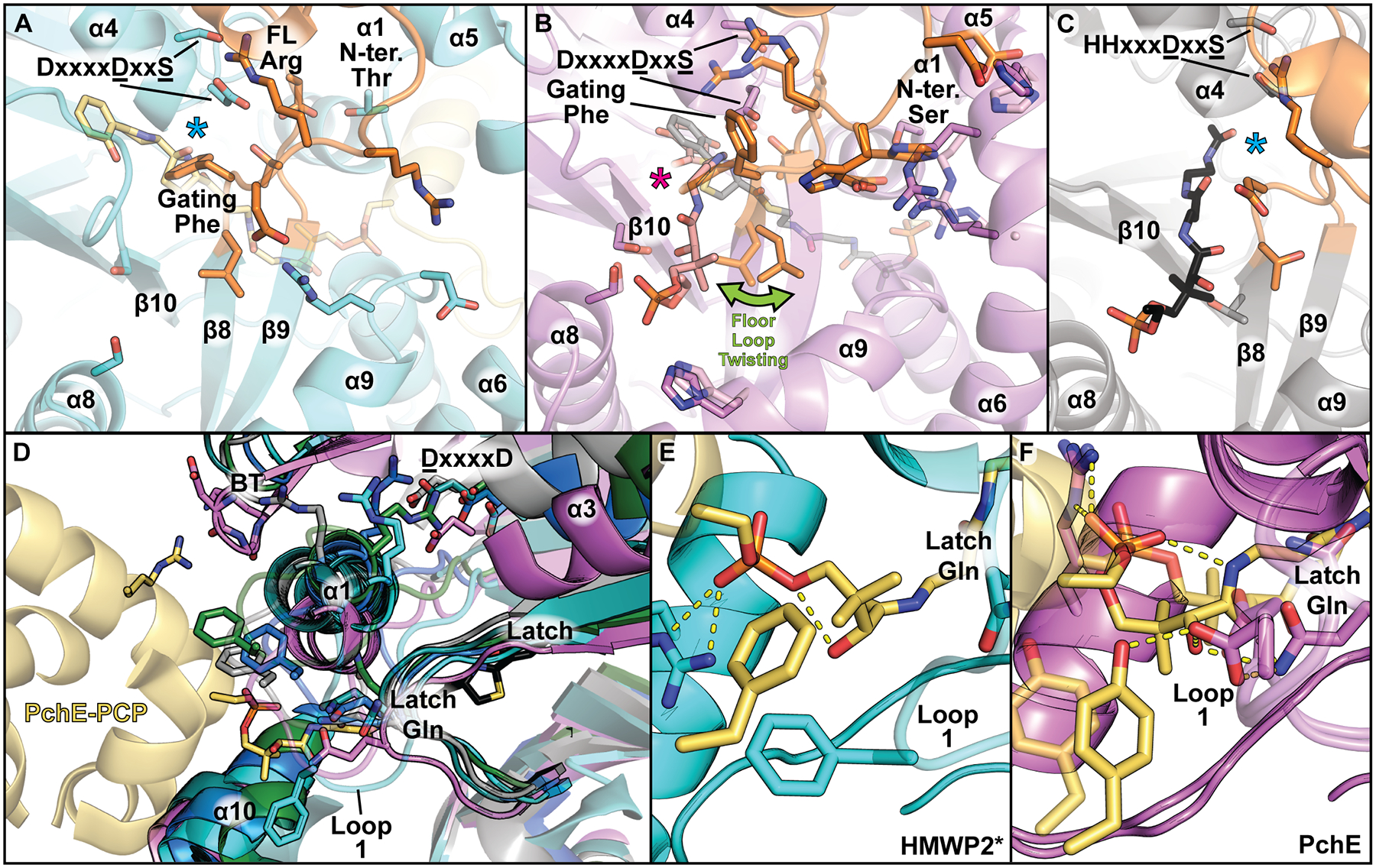
Comparison of donor site entrances of A) HMWP2-Cy2, B) PchE-Cy1 and C) LgrA-C [13,15,38] highlights twisting of the floor loop, with different floor loop arginine and motif aspartate conformations, as well as different Ppant and thioester positions (C). Electrophile positions are marked by asterisks and floor loops are colored orange. D) Acceptor site entrances of PchE-Cy1, HMWP2-Cy2, EpoB-Cy, FmoA3-Cy2 and BmdB-Cy2 [12,13,15–17]. A semi-conserved phenylalanine in loop 1 is displayed to help emphasize variation in loop 1 structure. E) Docking model of a cyclodehydration intermediate-bound state for HMWP2-Cy2 (acceptor PCP residues and Ppant are in yellow, cartoon for PCP α3 containing the displayed phenylalanine is omitted for clarity) F) Loaded and unloaded holo acceptor CP complexes in PchE highlight differences in pantetheine placement. Acceptor CP and Ppant are displayed as in panel E, and the pantetheine-only model is displayed with transparency.
Features of condensation and cyclodehydration
The open-for-condensation state of PchE [15] contrasts a prior CP-C donor-mimic LgrA complex with respect to donor electrophile positioning (Fig. 3B,C) [38]. Whereas the LgrA complex positions the electrophile near the N-terminus of α4, the PchE model instead places its thioester oxygen near the putative catalytic aspartate, inconsistently positioned for nucleophilic attack. In the closed-for-cyclodehydration state in HMWP2-Cy2 (Fig. 3A), the leaving group oxygen of a modelled cyclodehydration intermediate sits at the N-terminus of α4 [13]. The dipole of ⍺4 could then stabilize negative charge accumulation on the oxygen in the tetrahedral intermediates of both condensation and cyclodehydration steps [13,38]. These observations support an amine-first condensation near α4 (mirroring C-domain activity), followed by cyclization via the Cys acceptor’s nucleophilic thiol, with deprotonation by the putative catalytic dyad [13]. Future investigations should consider these alternative electrophile orientations before interpreting mechanisms.
Importantly, modeling of cysteinyl-PCP from structural comparison to the C domain of LgrA places the acceptor cysteine past the Cy-domain catalytic dyad [13,38], indicating rearrangements are necessary for cyclodehydration. At the acceptor site entrance, loop 1 (Fig. 3D) forms an extended surface where PCP and Ppant interactions may occur in the cyclodehydration complex. In PchE, a conserved glutamine within the latch is found to interact with the acceptor Ppant. In HMWP2-Cy2, this glutamine instead interacts with loop1, which forms part of a basin of attraction in which the Ppant can reside in a conformation specific for cyclodehydration [13]. Notably, the residues of this basin differ in PchE (Fig. 3E,F) compared to other systems, which may reflect different classes of Cy domains utilizing alternate interactions. Nonetheless, these observations suggest that Cy domains have two separate binding states for the acceptor Ppant: a condensation state that positions the loaded Ppant further into the active site (as in the C domain) in proximity to the donor substrate, and a cyclodehydration state that places the reactive functional groups of Cys/Thr/Ser sidechains near the catalytic dyad.
In comparison, tandem Cy domain architectures within (methyl)oxazoline systems may contain one Cy domain that lacks features that are important for facilitating cyclodehydration and a second Cy domain that lacks the appropriate interface for binding an acceptor CP [12,17]. The utility of tandem Cy domains was proposed to increase efficiency for cyclodehydration by serine/threonine in comparison to cysteine [33,39]; however, thiazoline systems containing single Cy domains have demonstrated the ability to generate oxazoline [40], and there are oxazoline-forming systems that contain Cy domains functional for both condensation and cyclodehydration (e.g. mycobactin) [30,37]. It seems likely that the Cy domains containing all of the prerequisite structural motifs and conserved amino acids can accept and cyclize Ser, Thr, or Cys, albeit with some selectivity for a particular donor substrate [41], and that overall specificity in turn comes from the coupled A domain. Future comparative studies will be necessary to better understand the evolutionary and molecular differences that favor tandem Cy domain architectures.
Global dynamics within Cy domains
Expanding upon static global conformations detailed above, recent solution studies captured Cy-domain global dynamics that appear necessary for domain communication. Here, dynamics is defined as structural fluctuations granting access to diverse local or global conformations, which can play a role in enzyme catalysis [42,43] or allosteric communication [44,45]. Global dynamics were observed in the Cy1 domain of HMWP2-Cy1 [14], with a footprint suggesting that the conformational changes of Figs. 2 and 3 reflect fluctuations in solution (Fig. 4A). Cy1 only engages with its substrate-loaded donor and not its holo form, thus avoiding unproductive interactions, as also reported for condensation domains [46,47]. Surprisingly, domain engagement generated an allosteric response reaching the acceptor site 40 Å away, suggesting communication between acceptor and donor sites (Fig. 4). To demonstrate a tie between structural dynamics and recognition of substrate-loaded CPs, the conserved dyad aspartate was mutated, which yielded a global response that impeded both the domain dynamics and carrier protein recognition. The results highlighted the importance of structural fluctuations in domain communication, in line with the interpretation of conformational changes discussed above. They also cautioned that, amidst dynamics, mutations may impart global responses confounding traditional interpretations using a single structure.
Figure 4. Cy structural dynamics modulate transient contacts for function.
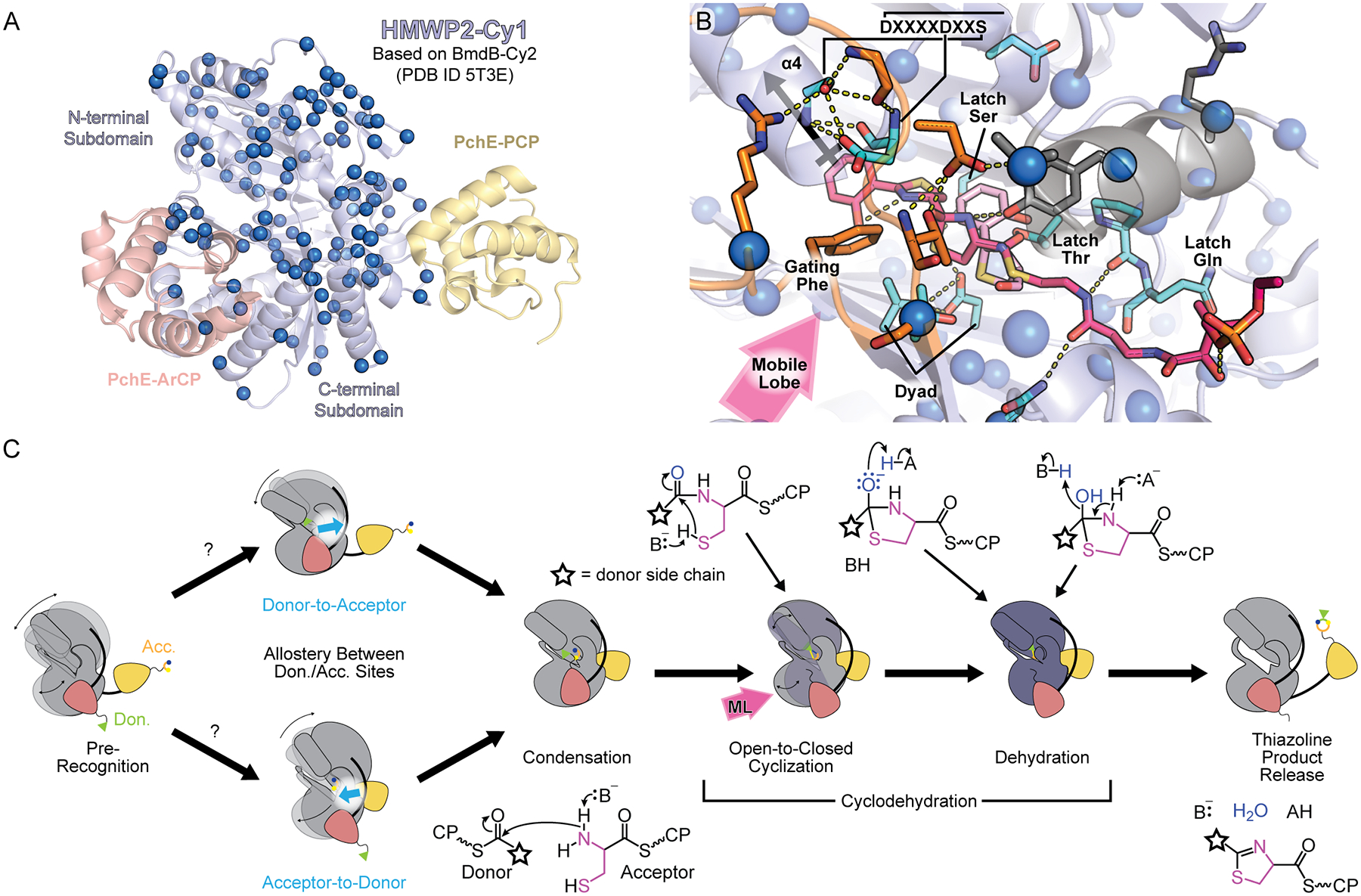
A) The dynamic footprint of HMWP2-Cy1 [11]. Carrier proteins from PchE [15] are superimposed to mark donor and acceptor sites. Cy residues exhibiting relaxation dispersion in solution NMR experiments are shown as spheres. B) Substrate funneling at the donor site may be mediated by a network of dynamic residues. The pink arrow labeled “Mobile Lobe” indicates the direction the mobile lobe would shift to open the donor site entrance. The cyclodehydration intermediate (pink sticks) and the PchE product-bound state (light pink sticks) are shown. Yellow dotted lines are polar contacts, including interactions linking the acceptor site (α1, in gray) to the floor loop (orange) and subsequently α4, which may mediate allosteric effects. C) Proposed role of conformational fluctuations in the catalytic cycle. Gray icons resemble observed states shared by C and Cy domains, whereas the purple icon represents an anticipated Cy domain-specific state. Carrier proteins are salmon and yellow orange.
Examining structures of Cy domains in light of dynamics provides alternative interpretations of mutagenesis. The dyad aspartate is remote from both donor and acceptor CP binding sites [13,16,17]. How then can mutating this residue lead to a dramatic global response and affect donor recognition? Mishra et al. noted that, in some Cy conformations, the aspartate can interact with a semi-conserved serine/threonine (Fig. 4B, Latch Ser), as confirmed by the structures of PchE Cy [15] (Fig. 4B, Latch Thr). These residues immediately precede the latch glutamine, which was found to interact with the acceptor Ppant in PchE (Fig. 4B, Latch Gln) but displays different conformations in unliganded Cy domains. Within a dynamic scaffold begetting an ensemble of conformations, mutating a residue destabilizes specific conformations. Mutating the dyad aspartate would remove contacts with the latch serine and threonine residues, destabilizing conformers; the global dynamics are then altered and apparently impede access to conformations required for donor recognition. Because fluctuations sample a variety of conformers, these mechanisms necessarily supplement our understanding of Cy function. Here, the dyad aspartate can act both as a general base and in mediating allosteric networks.
Dynamics may help form fully engaged complexes featuring a substrate-tethered Ppant in the active-site tunnel. Brief encounter complexes are increasingly thought to precede and promote fully bound forms [48], and such mechanisms appear to be involved in Cy-donor recognition [14]. Dynamics of HMWP2-Cy1 in solution (Fig. 4A) reveals a network of residues that could couple the donor site entrance with tunnel residues known to interact with the Ppant arm (Fig. 4B). That the entire region surrounding the donor site entrance is dynamic suggests that fluctuations in the region can couple the conformational changes we described at the donor site (Fig. 3A,B), such that contacts between substrates and the gating phenylalanine redistribute conformations at the second aspartate of the DxxxxDxxS motif to help funnel the substrate into the tunnel. Highly conserved residues in α1 that interact with the DxxxxDxxS motif, substrate and the floor loop (as described in Fig. 3D) also display relaxation dispersion profiles.
Importantly, global dynamics are also inferred for the C domain from static structures and normal mode analysis demonstrating variable orientations of the N- and C-terminal subdomains [32], which similarly present an opportunity for CP binding to affect the state of the donor and acceptor entrances [14]. Only open donor entrances with the mobile lobe splayed away from the floor loop strands have been experimentally observed for C domains [33], and it would not be necessary for the C domain to reach a closed-for-cyclodehydration state. However, details of C-domain catalysis also remain unresolved, so global conformational changes—perhaps including mobile lobe rearrangement—could be involved in yet unknown ways such as product release trajectories.
Overall, consideration of structural dynamics may resolve open questions around Cy domains (Fig. 4C), and future research should account for dynamics when determining molecular mechanisms. Similarly, future bioengineering efforts should consider aspects of the Cy-domain conformational landscape, which appear intimately connected to domain communication and catalysis of peptide bond formation and cyclodehydration. Indeed, the interactions between A and C domains within a module have been shown by Townsend and colleagues to have dramatic effects on reactivity, with different A domain specificity observed in the presence and absence of the neighboring C domain [49]. These findings echo the global conformational responses of Cy domains towards carrier proteins binding and speak to how allostery is likely involved in regulating NRPS productivity.
Final thoughts
An understanding of the molecular determinants of Cy reaction mechanisms is beginning to take shape, although many questions remain. Excitingly, we are now at the point of building an understanding of allosteric communication between CP binding events and remodeling of the Cy-domain active site. Further studies will be needed to detail these features for improved bioengineering of Cy domain-containing systems.
Highlights.
Heterocyclization domains catalyze both peptide bond formation and cyclodehydration in nonribosomal peptide synthetases
Heterocyclization domains appear to change conformations for distinct catalytic steps
Structural dynamics likely couple molecular communication with fold remodeling
Conformational changes and structural dynamics should be considered in future bioengineering of heterocyclization domains
Acknowledgements
Support for this research was provided by the National Institute of General Medical Sciences (NIGMS) (1R15GM123425-01) to D.P.D., and A.D.G. was supported by a Sanofi Genzyme Doctoral Research Fellowship. D.P.F. acknowledges support from NIGMS R01 GM104257.
Footnotes
Conflict of Interest
The authors declare that they have no conflicts of interest with the contents of this article.
References and recommended reading
papers of special interest (*) or outstanding interest (**)
- [1].Shen Q, Zhou H, Dai G, Zhong G, Huo L, Li A, Liu Y, Yang M, Ravichandran V, Zheng Z, Tang Y-J, Jiao N, Zhang Y, Bian X Characterization of a cryptic NRPS gene cluster in Bacillus velezensis FZB42 reveals a discrete oxidase involved in multithiazole biosynthesis, ACS Catalysis 12 (2022) 3371–3381. doi: 10.1021/acscatal.1c05131. [DOI] [Google Scholar]
- [2].Agrawal S, Acharya D, Adholeya A, Barrow CJ, Deshmukh SK Nonribosomal peptides from marine microbes and their antimicrobial and anticancer potential, Front. Pharmacol 8 (2017) 828. doi: 10.3389/fphar.2017.00828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Walsh CT, Nolan EM Morphing peptide backbones into heterocycles, Proc. Natl. Acad. Sci. U. S. A 105 (2008) 5655–5656. doi: 10.1073/pnas.0802300105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cole KE, Dowling DP, Boone MA, Phillips AJ, Christianson DW Structural basis of the antiproliferative activity of largazole, a depsipeptide inhibitor of the histone deacetylases, J. Am. Chem. Soc 133 (2011) 12474–12477. doi: 10.1021/ja205972n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Soor HS, Appavoo SD, Yudin AK Heterocycles: Versatile control elements in bioactive macrocycles, Bioorg. Med. Chem 26 (2018) 2774–2779. doi: 10.1016/j.bmc.2017.10.022. [DOI] [PubMed] [Google Scholar]
- [6].Walsh CT, Malcolmson SJ, Young TS Three ring posttranslational circuses: Insertion of oxazoles, thiazoles, and pyridines into protein-derived frameworks, ACS Chem. Biol 7 (2012) 429–442. doi: 10.1021/cb200518n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wenski SL, Thiengmag S, Helfrich EJN Complex peptide natural products: Biosynthetic principles, challenges and opportunities for pathway engineering, Synth. Syst. Biotechnol 7 (2022) 631–647. doi: 10.1016/j.synbio.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hubrich F, Bösch NM, Chepkirui C, Morinaka BI, Rust M, Gugger M, Robinson SL, Vagstad AL, Piel J Ribosomally derived lipopeptides containing distinct fatty acyl moieties, Proc. Natl. Acad. Sci. U. S. A 119 (2022) e2113120119. doi: 10.1073/pnas.2113120119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Funk MA, van der Donk WA Ribosomal natural products, tailored to fit, Acc. Chem. Res 50 (2017) 1577–1586. doi: 10.1021/acs.accounts.7b00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cox CL, Doroghazi JR, Mitchell DA The genomic landscape of ribosomal peptides containing thiazole and oxazole heterocycles, BMC Genomics 16 (2015) 778. doi: 10.1186/s12864-015-2008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sundaram S, Hertweck C On-line enzymatic tailoring of polyketides and peptides in thiotemplate systems, Curr. Opin. Chem. Biol 31 (2016) 82–94. doi: 10.1016/j.cbpa.2016.01.012. [DOI] [PubMed] [Google Scholar]
- **[12].Katsuyama Y, Sone K, Harada A, Kawai S, Urano N, Adachi N, Moriya T, Kawasaki M, Shin-ya K, Senda T, Ohnishi Y Structural and functional analyses of the tridomain-nonribosomal peptide synthetase FmoA3 for 4-methyloxazoline ring formation, Angew. Chem. Int. Ed. Engl 60 (2021) 14554–14562. doi: 10.1002/anie.202102760. [DOI] [PubMed] [Google Scholar]; This work reports cryo-EM and crystal structures of an oxazoline-producing system, providing an important comparison to the thiazol(in)e Cy domains.
- **[13].Gnann AD, Xia Y, Soule J, Barthélemy C, Mawani JS, Nzikoba Musoke S, Castellano BM, Brignole EJ, Frueh DP, Dowling DP High-resolution structures of a siderophore-producing cyclization domain from Yersinia pestis offer a refined proposal of substrate binding, J. Biol. Chem 298 (2022). doi: 10.1016/j.jbc.2022.102454. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper reports the currently highest resolution crystal structure for a Cy domain, that of HMWP2-Cy2 from yersiniabactin synthetase. Notably, the structure enabled carrier protein and cyclodehydration intermediate docking that informs on Cy domain mechanistic possibilities as well as normal mode calculations that identify movements within Cy domains.
- **[14].Mishra SH, Kancherla AK, Marincin KA, Bouvignies G, Nerli S, Sgourakis N, Dowling DP, Frueh DP Global protein dynamics as communication sensors in peptide synthetase domains, Sci. Adv 8 (2022) eabn6549. doi: 10.1126/sciadv.abn6549. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study presents the first NMR structure of a Cy domain, representing one of the few examples of NMR studies on protein domains over 50 kDa in size. This work captures relaxation dispersion in solution NMR experiments to probe dynamics with the Cy domain, as well as for active site mutants that globally alter Cy domain dynamics.
- **[15].Wang J, Li D, Chen L, Cao W, Kong L, Zhang W, Croll T, Deng Z, Liang J, Wang Z Catalytic trajectory of a dimeric nonribosomal peptide synthetase subunit with an inserted epimerase domain, Nat. Commun 13 (2022) 592. doi: 10.1038/s41467-022-28284-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work presents cryo-EM structures of the first Cy domain within pyochelin biosynthesis. The structures capture states of the Cy domain with loaded Ppants at either the acceptor or donor entrances, providing the first structural information for CP and Ppant binding to Cy domains.
- *[16].Dowling DP, Kung Y, Croft AK, Taghizadeh K, Kelly WL, Walsh CT, Drennan CL Structural elements of an NRPS cyclization domain and its intermodule docking domain, Proc. Natl. Acad. Sci. U. S. A 113 (2016) 12432–12437. doi: 10.1073/pnas.1608615113. [DOI] [PMC free article] [PubMed] [Google Scholar]; Here the authors report the first available crystal structure of an NRPS Cy domain and mutagenesis studies that interrogate the identified acive site, identifying a conserved asparate is required for heterocycle production. The structure is of the Cy domain from epothilone synthetase with its N-terminal docking domain, providing information on the location of the docking domain for binding CPs at the donor entrance site.
- *[17].Bloudoff K, Fage CD, Marahiel MA, Schmeing TM Structural and mutational analysis of the nonribosomal peptide synthetase heterocyclization domain provides insight into catalysis, Proc. Natl. Acad. Sci. U. S. A 114 (2017) 95–100. doi: 10.1073/pnas.1614191114. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work represents the second reported crystal structure of an NRPS Cy domain and mutagenesis that probed formation of both the condensation and cyclodehydration products. The authors’ work identifies a conserved catalyic aspartate and threonine dyad.
- **[18].Fortinez CM, Bloudoff K, Harrigan C, Sharon I, Strauss M, Schmeing TM Structures and function of a tailoring oxidase in complex with a nonribosomal peptide synthetase module, Nat. Commun 13 (2022) 548. doi: 10.1038/s41467-022-28221-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work reports crystal and cryo-EM structures of the bacillamide system and focuses on the tailoring oxidase BmdC. Structures include the Cy domain within BmdB in a modular context where the oxidase stabilizes dimerization of the NRPS module.
- [19].Dekimpe S, Masschelein J Beyond peptide bond formation: the versatile role of condensation domains in natural product biosynthesis, Nat. Prod. Rep 38 (2021) 1910–1937. doi: 10.1039/d0np00098a. [DOI] [PubMed] [Google Scholar]
- [20].Beck C, Garzón JFG, Weber T Recent advances in re-engineering modular PKS and NRPS assembly lines, Biotechnol. Bioprocess Eng 25 (2020) 886–894. doi: 10.1007/s12257-020-0265-5. [DOI] [Google Scholar]
- [21].Abbood N, Duy Vo T, Watzel J, Bozhüyük KAJ, Bode HB Type S non-ribosomal peptide synthetases for the rapid generation of tailormade peptide libraries, Chemistry (Easton) 28 (2022) e202103963. doi: 10.1002/chem.202103963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cai X, Zhao L, Bode HB Reprogramming promiscuous nonribosomal peptide synthetases for production of specific peptides, Org. Lett 21 (2019) 2116–2120. doi: 10.1021/acs.orglett.9b00395. [DOI] [PubMed] [Google Scholar]
- [23].Bozhüyük KAJ, Linck A, Tietze A, Kranz J, Wesche F, Nowak S, Fleischhacker F, Shi YN, Grün P, Bode HB Modification and de novo design of non-ribosomal peptide synthetases using specific assembly points within condensation domains, Nat. Chem 11 (2019) 653–661. doi: 10.1038/s41557-019-0276-z. [DOI] [PubMed] [Google Scholar]
- [24].Bozhüyük KAJ, Fleischhacker F, Linck A, Wesche F, Tietze A, Niesert CP, Bode HB De novo design and engineering of non-ribosomal peptide synthetases, Nat. Chem 10 (2018) 275–281. doi: 10.1038/nchem.2890. [DOI] [PubMed] [Google Scholar]
- [25].Reimer JM, Haque AS, Tarry MJ, Schmeing TM Piecing together nonribosomal peptide synthesis, Curr. Opin. Struct. Biol 49 (2018) 104–113. doi: 10.1016/j.sbi.2018.01.011. [DOI] [PubMed] [Google Scholar]
- [26].Süssmuth RD, Mainz A Nonribosomal Peptide Synthesis-Principles and Prospects, Angew. Chem. Int. Ed. Engl 56 (2017) 3770–3821. doi: 10.1002/anie.201609079. [DOI] [PubMed] [Google Scholar]
- [27].Calcott MJ, Owen JG, Ackerley DF Efficient rational modification of non-ribosomal peptides by adenylation domain substitution, Nat. Commun 11 (2020) 4554. doi: 10.1038/s41467-020-18365-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Stachelhaus T, Mootz HD, Marahiel MA The specificity-conferring code of adenylation domains in nonribosomal peptide synthetases, Chem. Biol 6 (1999) 493–505. doi:Doi 10.1016/S1074-5521(99)80082-9. [DOI] [PubMed] [Google Scholar]
- *[29].Konz D, Klens A, Schörgendorfer K, Marahiel MA The bacitracin biosynthesis operon of Bacillus licheniformis ATCC 10716: molecular characterization of three multi-modular peptide synthetases, Chem. Biol 4 (1997) 927–937 [DOI] [PubMed] [Google Scholar]; This study presents the early identification and characterization of the Cy domain within bacitracin biosynthesis and identifies sequence differences in comparison to the characteristic C domain motif.
- *[30].Di Lorenzo M, Stork M, Naka H, Tolmasky ME, Crosa JH Tandem heterocyclization domains in a nonribosomal peptide synthetase essential for siderophore biosynthesis in Vibrio anguillarum, Biometals 21 (2008) 635–648. doi: 10.1007/s10534-008-9149-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work provides biochemical studies of a tandem Cy domain system in anguibactin biosynthesis, revealing the necessity for both Cy domains for anguibactin synthesis. Inactivating mutations also report decreased virulence of V. anguillarum 775, supporting siderophore biosynthesis as a target for drug development.
- [31].Keating TA, Marshall CG, Walsh CT, Keating AE The structure of VibH represents nonribosomal peptide synthetase condensation, cyclization and epimerization domains, Nat. Struct. Biol 9 (2002) 522–526. doi: 10.1038/nsb810. [DOI] [PubMed] [Google Scholar]
- *[32].Bloudoff K, Rodionov D, Schmeing TM Crystal structures of the first condensation domain of CDA synthetase suggest conformational changes during the synthetic cycle of nonribosomal peptide synthetases, J. Mol. Biol 425 (2013) 3137–3150. doi: 10.1016/j.jmb.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work reports crystal structures of a C domain that adopt a different conformation than prior C domain structures, permitting evaluation of flexibility in the C domain. Normal mode analysis, targetted MD, and energy-minimized linear interpolation were used to plot trajectories between observed C domain states.
- [33].Bloudoff K, Schmeing TM Structural and functional aspects of the nonribosomal peptide synthetase condensation domain superfamily: discovery, dissection and diversity, Biochim. Biophys. Acta Proteins Proteom 1865 (2017) 1587–1604. doi: 10.1016/j.bbapap.2017.05.010. [DOI] [PubMed] [Google Scholar]
- [34].Keating TA, Miller DA, Walsh CT Expression, purification, and characterization of HMWP2, a 229 kDa, six domain protein subunit of Yersiniabactin synthetase, Biochemistry 39 (2000) 4729–4739 [DOI] [PubMed] [Google Scholar]
- [35].Kelly WL, Hillson NJ, Walsh CT Excision of the epothilone synthetase B cyclization domain and demonstration of in trans condensation/cyclodehydration activity, Biochemistry 44 (2005) 13385–13393. doi: 10.1021/bi051124x. [DOI] [PubMed] [Google Scholar]
- [36].Marshall CG, Hillson NJ, Walsh CT Catalytic mapping of the vibriobactin biosynthetic enzyme VibF, Biochemistry 41 (2002) 244–250 [DOI] [PubMed] [Google Scholar]
- [37].Duerfahrt T, Eppelmann K, Muller R, Marahiel MA Rational design of a bimodular model system for the investigation of heterocyclization in nonribosomal peptide biosynthesis, Chem. Biol 11 (2004) 261–271. doi: 10.1016/j.chembiol.2004.01.013. [DOI] [PubMed] [Google Scholar]
- *[38].Reimer JM, Eivaskhani M, Harb I, Guarné A, Weigt M, Schmeing TM Structures of a dimodular nonribosomal peptide synthetase reveal conformational flexibility, Science 366 (2019) eaaw4388. doi: 10.1126/science.aaw4388. [DOI] [PubMed] [Google Scholar]; This work reports crystal structures of large, dimodular complexes from linear gramicidin in various conformational states thought to correspond to steps of the reaction cycle of C domains. A condensation donor mimic-bound state suggests how the donor electrophile may be positioned for condensation.
- [39].Belshaw PJ, Roy RS, Kelleher NL, Walsh CT Kinetics and regioselectivity of peptide-to-heterocycle conversions by microcin B17 synthetase, Chem. Biol 5 (1998) 373–384. doi: 10.1016/s1074-5521(98)90071-0. [DOI] [PubMed] [Google Scholar]
- [40].Schneider TL, Walsh CT, O’Connor SE Utilization of alternate substrates by the first three modules of the epothilone synthetase assembly line, J. Am. Chem. Soc 124 (2002) 11272–11273. doi: 10.1021/ja0274498. [DOI] [PubMed] [Google Scholar]
- [41].Marshall CG, Burkart MD, Keating TA, Walsh CT Heterocycle formation in vibriobactin biosynthesis: Alternative substrate utilization and identification of a condensed intermediate, Biochemistry 40 (2001) 10655–10663. doi: 10.1021/bi010937s. [DOI] [PubMed] [Google Scholar]
- [42].Whittier SK, Hengge AC, Loria JP Conformational motions regulate phosphoryl transfer in related protein tyrosine phosphatases, Science 341 (2013) 899–903. doi: 10.1126/science.1241735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rozovsky S, McDermott AE Substrate product equilibrium on a reversible enzyme, triosephosphate isomerase, Proc. Natl. Acad. Sci. U. S. A 104 (2007) 2080–2085. doi: 10.1073/Pnas.0608876104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wodak SJ, Paci E, Dokholyan NV, Berezovsky IN, Horovitz A, Li J, Hilser VJ, Behar I, Karanicolas J, Stock G, Hamm P, Stote RH, Eberhardt J, Chebaro Y, Dejaegere A, Cecchini M, Changeux JP, Bolhuis PG, Vreede J, Faccioli P, Orioli S, Ravasio R, Yan L, Brito C, Wyart M, Gkeka P, Rivalta I, Palermo G, Mccammon JA, Panecka-Hofman J, Wade RC, Di Pizio A, Niv MY, Nussinov R, Tsai CJ, Jang H, Padhorny D, Kozakov D, Mcleish T Allostery in its many disguises: From theory to applications, Structure 27 (2019) 566–578. doi: 10.1016/j.str.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Saavedra HG, Wrabl JO, Anderson JA, Li J, Hilser VJ Dynamic allostery can drive cold adaptation in enzymes, Nature 558 (2018) 324–328. doi: 10.1038/s41586-018-0183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[46].Izoré T, Candace Ho YT, Kaczmarski JA, Gavriilidou A, Chow KH, Steer DL, Goode RJA, Schittenhelm RB, Tailhades J, Tosin M, Challis GL, Krenske EH, Ziemert N, Jackson CJ, Cryle MJ Structures of a non-ribosomal peptide synthetase condensation domain suggest the basis of substrate selectivity, Nat. Commun 12 (2021) 2511. doi: 10.1038/s41467-021-22623-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work reports structures of an acceptor PCP-bound C domain from fusachelin biosynthesis as well as simulation to investigate PCP and Ppant/substrate binding. Mutagenesis was performed to evaluate the role of a tunnel-lining arginine involved in positioning glycyl-Ppant in that C domain.
- [47].Shi C, Miller BR, Alexander EM, Gulick AM, Aldrich CC Design, synthesis, and biophysical evaluation of mechanism-based probes for condensation domains of nonribosomal peptide synthetases, ACS Chem. Biol 15 (2020) 1813–1819. doi: 10.1021/acschembio.0c00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Schilder J, Ubbink M Formation of transient protein complexes, Curr. Opin. Struct. Biol 23 (2013) 911–918. doi: 10.1016/j.sbi.2013.07.009. [DOI] [PubMed] [Google Scholar]
- [49].Li R, Oliver RA, Townsend CA Identification and characterization of the sulfazecin monobactam biosynthetic gene cluster, Cell Chem. Biol 24 (2017) 24–34. doi: 10.1016/j.chembiol.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


