Abstract
Background
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the causative agent of the coronavirus disease 2019 (COVID-19), has recently posed a threat to global health by spreading at a high rate and taking millions of lives worldwide. Along with the respiratory symptoms, there are gastrointestinal manifestations and one of the most common gastrointestinal symptoms is diarrhea which is seen in a significant percentage of COVID-19 patients.
Literature review
Several studies have shown the plausible correlation between overexpressed angiotensin converting enzyme 2 (ACE2) in enterocytes and SARS-CoV-2, as ACE2 is the only known receptor for the virus entry. Along with the dysregulated ACE2, there are other contributing factors such as gut microbiome dysbiosis, adverse effects of antiviral and antibiotics for treating infections and inflammatory response to SARS-CoV-2 which bring about increased permeability of gut cells and subsequent occurrence of diarrhea. Few studies found that the SARS-CoV-2 is capable of damaging liver cells too. No single effective treatment option is available.
Limitations
Confirmed pathophysiology is still unavailable. Studies regarding global population are also insufficient.
Conclusion
In this review, based on the previous works and literature, we summarized the putative molecular pathophysiology of COVID-19 associated diarrhea, concomitant complications and the standard practices of management of diarrhea and hepatic manifestations in international setups.
Keywords: COVID-19, SARS-CoV-2, Diarrhea, Angiotensin-converting enzyme-2, Antibiotic-associated diarrhea, Diarrhea management
Introduction
Since December 2019, COVID-19 has become a global threat to public health and the economy. The main causative agent of this disease, SARS-CoV-2, first appeared in Wuhan city of China and the World Health Organization declared it a global pandemic on March 2020 (Huang et al., 2020). Along with a great toll on economic growth and interruption of the general lifestyle of people, COVID-19 has become one of the major public health crises infecting around 533 million people till June 12, 2022, taking more than 6.3 million lives reported from 226 countries (World Health Organization, 2022). However, there is a probability that the number of infected people and deaths is much higher because of underreporting and receiving support from the practice of telemedicine (Abdel-Naser, 2021).
The causing agent of COVID-19, SARS-CoV-2, is a member of the coronavirus group and Nidovirale family. The main characteristics of the members of this family are the spike (S)-like structures of the viruses, the ability to spread swiftly and be converted into a new variant of concern, and higher infectivity and mortality rates, which make this virus a novel one (Loo and Letchumanan, 2021). After the virus entering into the body via respiration, it affects the lungs, resulting in cough with sputum production, shortness of breath, and in severe cases, acute respiratory distress syndrome, respiratory failure, and sometimes, even death (Mohamed et al., 2021). There are several other organs that are affected by COVID-19, and those extrapulmonary manifestations include cardiovascular disorders such as myocarditis, pericarditis, arrhythmias, acute coronary syndrome, and heart failure; renal disorders, e.g., acute tubular necrosis. Along with these adverse effects on the organs themselves, hepatic dysfunctions are also seen in many patients, and this may lead to an elevated level of enzymes such as alanine transaminase (ALT), aspartate transaminase (AST), and serum bilirubin. In addition, some dermatological changes due to COVID-19 were also experienced by patients, which include vesicles, maculopapular rashes, petechiae, purpura, urticaria, pernio, distal limb ischemia, and livedo racemose (Gottlieb and Long, 2020). A significant number of people also experience neurological symptoms such as cephalgia, peripheral neuropathy, encephalopathy, cerebrovascular disorders, and vertigo (Johnson et al., 2020). In general, the practice of telemedicine may have contributed to the underestimation of the true magnitude of the pandemic (Abdel-Naser, 2021).
Although the key manifestations of patients with COVID-19 were fever and sometimes respiratory symptoms, many case reports showed different percentages of patients having gastrointestinal (GI) symptoms, and altogether the range varies from 2% to 79.1% (Guan et al., 2020; Jin et al., 2020; Lin et al., 2020; Wang D et al., 2020; Wang et al., 2021, Zhang et al., 2020a). These symptoms can emerge anytime during COVID-19 infection, in the beginning or after the onset of fever, or even later. The most common GI symptoms include diarrhea, lack of appetite, nausea, vomiting, and sometimes abdominal pain (Gu et al., 2020). In severe cases, patients experience bleeding in the GI tract (GIT) (Wang et al., 2021).
Although diarrhea is the most common GI manifestation, the underlying causes are still unclear. Because GIT manifestations are found in nearly one-fifth of patients with COVID-19 (Henry et al., 2020), this article is going to offer insights into GIT symptoms, associated mechanisms, and pathological changes caused by SARS-CoV-2 infection and provide an outline of current treatment options.
Pathophysiology and mechanism of gastrointestinal tract-related manifestations
Mode of entry into the cell and molecular pathophysiology of diarrhea
SARS-CoV-2 is the largest known RNA virus having a genome of 30kb in length, and the RNA is a single-stranded positive sense in nature. All known coronaviruses share a few common structural features. One of them is the structural proteins (SPs). There are four basic SPs: S, membrane (M), envelope (E), and nucleocapsid (N) proteins, and all of them are encoded by the viral genome (RNA) (Malik, 2020). These SPs play significant roles in viral invasion into the host cells. The S protein attaches to the host cell glycoprotein receptor, angiotensin-converting enzyme 2 (ACE2), initiating the whole process of infection. The shaping of virion particles and binding to N is the responsibility of the M protein. E protein helps in the assembly and release of viral particles, whereas N protein aids the genome to bind to the replication-transcription complex and to proceed with the whole replication process.
It was believed that the SARS-CoV-2 virus enters the host cell via two pathways. One is pH-dependent, and another is pH-independent. In a pH-independent pathway, the viral E gets fused with the M of the host cell, and the genetic material is then delivered into the host cell. In contrast, the pH-dependent pathway, which is now established as the main mechanism of entry, uses a clathrin-mediated endocytic pathway involving the binding to the extracellular domains of ACE2 receptors (Bayati et al., 2021; Hu et al., 2020). ACE2 is a transmembrane metallopeptidase enzyme found mostly in the plasma M of cells (Hamming et al., 2004). In the pH-dependent pathway, acidic pH stimulates the fusion of viral particles and endosomal M. After the formation of the endosome, the virion is activated and releases the genomic contents into the host cell cytoplasm for further replication process (Wang et al., 2008).
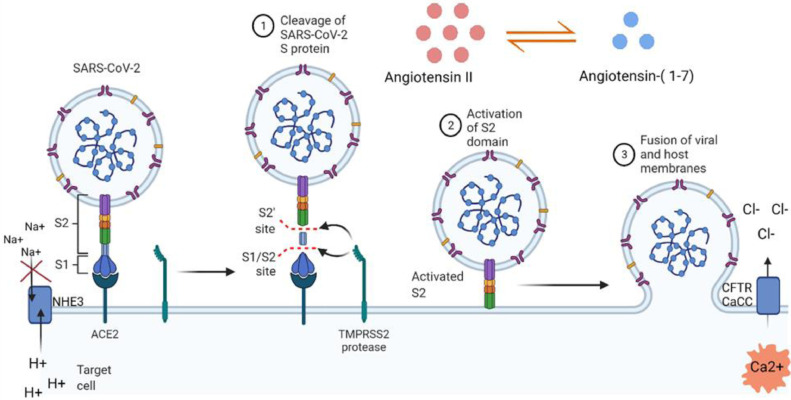
The S of SARS-CoV-2, which initiates the process of endosome formation, is a homotrimer composed of three similar copies of the same glycoprotein chain, which are attached together. An S has two structural subunits: S1 and S2. The S1 subunit contains a receptor-binding domain (RBD) having a residue 394 (glutamine) (Shang et al., 2020) that can be recognized by the critical lysine 31 on the human ACE2 receptor (Wu et al., 2012). The S2 subunit, in contrast, contains a transmembrane part of the S protein and is crucial for fusing the viral M with the Ms of the host cell (Hoffmann et al., 2020; Letko et al., 2020). These two subunits are generally fused together, but after attaching to host cell ACE2 via RBD of S protein, the S is cleaved into S1 and S2, which is mediated by human cell-derived proteases such as furin, type II transmembrane serine protease(TMPRSS2), trypsin-like proteases, plasmin, elastase, and factor Xa (Davidson et al., 2020; Hussain et al., 2020; Letko et al., 2020; Lippi et al., 2020; Walls et al., 2020; Wang et al., 2021; Wruck and Adjaye, 2020). Following segregation, the S1 subunit binds to ACE2, and the S2 subunit subsequently leads to the fusion of the viral M and the host cell M via a molecular mimic of soluble N-ethylmaleimide-sensitive factor protein receptor-mediated cellular M fusion, resulting in endocytosis (Hamming et al., 2004; Walls et al., 2020). When endocytosed, viral RNA enters the host cell and directs to the replication of the virus (Figure 1 ).
Figure 1.
Partial mechanisms of COVID-19-associated diarrhea. SARS-CoV-2 binds to ACE2 receptor and the S protein of viral envelop is cleaved into S1 and S2 (1). Activated S2 leads to the fusion of viral membrane and host cell membrane (2 & 3). Entry of the virus into the host cell causes ionic imbalance in the host contributing to a leaky gut. This figure was partially adapted from ‘Mechanism of SARS-CoV-2 Viral Entry’ by BioRender.com (2022) and retrieved from https://app.biorender.com/biorender-templates.
ACE2: Angiotensin-converting enzyme; CaCC: Ca2+ activated Cl− Channel; CFTR: CF transmembrane conductance regulator; NHE3: Na+-H+ exchanger 3; S, spike.
Several studies are showing that ACE2 is ubiquitous within the human body, expressed primarily on the luminal surface of epithelial cells, particularly overexpressed on the intestinal epithelial cells of the gut, smooth muscle cells, and endothelial cells of blood vessels, lung, heart, brain, testis, and renal tubular epithelial cell (Ortiz-Melo and Gurley, 2016; Patel et al., 2016; Turner et al., 2004; Zhang et al., 2020; Zhao et al., 2020), but underexpressed on the crypt of epithelial cells and in the colon (Hashimoto et al., 2012). Because COVID-19 symptoms are primarily respiratory tract oriented, and, in most cases, GIT related, and the presence of ACE2 receptors is high in there, it can be said that the virus infects and replicates within the GIT (Wong et al., 2020) resulting in COVID-19-associated diarrhea. One of the main basic body mechanisms that SARS-CoV-2 interrupts is the Renin-Angiotensin-Aldosterone System (RAAS). Principal functions of this system include the elimination of oxidative stress, vasoconstriction, and inflammation which are regulated by angiotensin II, one of the three components of RAAS. Two other components of this system are ACE and angiotensin receptor 1 (AT1R); angiotensin II exerts these effects after binding to AT1R (Obukhov et al., 2020). The ACE/angiotensin II/AT1R axis has proinflammatory and vasoconstrictive effects (Aksoy et al., 2020), and the ACE2/ angiotensin (1-7)/Mas pathway downregulates this axis resulting in anti-inflammatory effects (Jia et al., 2009). After entering into the cell, SARS-CoV-2 and ACE2 receptor both are internalized in the endolysosomal compartment, and the ACE2 receptor is degraded there following the disruption of RAAS (Hoffmann et al., 2020; Letko et al., 2020; Zang et al., 2020). There is another enzyme, named ‘A disintegrin and metalloprotease domain 17 (ADAM17)’, which acts as sheddase and participates in shedding of ACE2, Epidermal growth factor receptor (EFGR) ligands, and tumor necrosis factor-alpha (Xu et al., 2020). When ADAM17 mediates the cleavage of ACE2, the level of ACE2 in the cell M drops, and this phenomenon leads to proinflammatory effects by changing the balance of the ACE/angiotensin II/AT1R pathway (Megyeri et al., 2021).
Another mechanism involves two viroporins of SARS-CoV-2. One of them is the E protein which is an M protein forming a homopentameric ion channel. This ion channel shows selective permeability for monovalent ions like K+, Na+, and Cl− and a bivalent ion, Ca2+ (Cao et al., 2020). When the E proteins accumulate in the endoplasmic reticulum and Golgi Ms, they carry Ca2+ to the cytoplasm from these compartments. Elevated Ca2+ in cytoplasm can increase the rate of transportation of apical Cl− outside of the cell through the Cl− channel activated by Ca2+ (Barrett and Keely, 2000). Another protein is Orf3a, which is also an ion channel protein that carries K+ ions through viroporins, localized on plasma M and endomembrane (Figure 1) (Ren et al., 2020; Xu et al., 2020). The primary function of Orf3a in the cytoplasmic M might be the leaking of K+ ions from intestinal epithelial cells. This intracellular imbalance of multiple ions caused by these viroporins leads to activation of NRP3 inflammasome (NOD-, LRR-, and Pyrin domain-containing 3), resulting in secretion of interleukin (IL)-1β, following cell death (Lin et al., 2020; Theobald et al., 2021). This IL-1β then creates a local inflammatory environment by activating innate immune cells and, thus, a systemic cytokine storm. Together, the indirect cytokine storm and the direct viroporin activation trigger the ionic imbalance in enterocytes and finally leads to the occurrence of diarrhea (Megyeri et al., 2021).
The third possible mechanism of diarrhea involves an amino acid transporter named broad neutral amino acid transporter 1(B0AT1), which takes part in Na+- coupled transportation of tryptophan, glutamine, leucine, and phenylalanine. ACE2 binds to B0AT1, creating a heterodimer complex (Andring et al., 2020), and when SARS-CoV-2 attaches to ACE2:B0AT1 complex, it can impair the transportation of Na+ and neutral amino acid (Obukhov et al., 2020). As a consequence, amino acid starvation takes place, which may reduce Na+ uptake, leading to the development of diarrhea.
Altered gut microbiota
One of the most common characteristics of the human intestine is the habitat of a large number of commensal microbes, and the diversity is incredible, consisting of around 1000-1500 species. For instance, in the GI system, the colon has almost 33% of all the bacterial cells present in the human body (Lee et al., 2021). The main roles of these large populations of gut microbes are boosting the host metabolism, protecting the host against disease-causing organisms by habitat colonization and immunoregulatory responses, and developing and strengthening the host immune system and maintaining immune homeostasis (Iacob et al., 2018; Laville et al., 2019; Lee et al., 2021; Pan et al., 2020; Rodionov et al., 2019; Sharma et al., 2019; Shin et al., 2019)
When the normal population of gut microbiota is altered, it may affect the respiratory tract through the common mucosal immune system. An alternate situation can also take place, where the respiratory tract microbes are compromised, resulting in digestive tract-related disorders caused by immunoregulation. Several factors can negatively influence gut microbes. Such factors include age, any disease, concomitant infections, overuse or premature termination of antibiotic uptake, and viral-induced inflammation resulting in high or low plasma concentrations of proinflammatory mediators, e.g., cytokines, chemokines, and inflammation markers. Sometimes, probiotic strains (e.g., Bifidobacteria), which produce butyrate, a short-chain fatty acid playing important metabolic functions and maintains the integrity of intestinal epithelium, are lowered due to amino acid starvation. When these factors come together, individually or all together, they lead to alterations in infection susceptibility and disease severity through dysbiosis of community structure and function (Mangiola et al., 2018; Perisetti et al., 2020; Xiang et al., 2020).
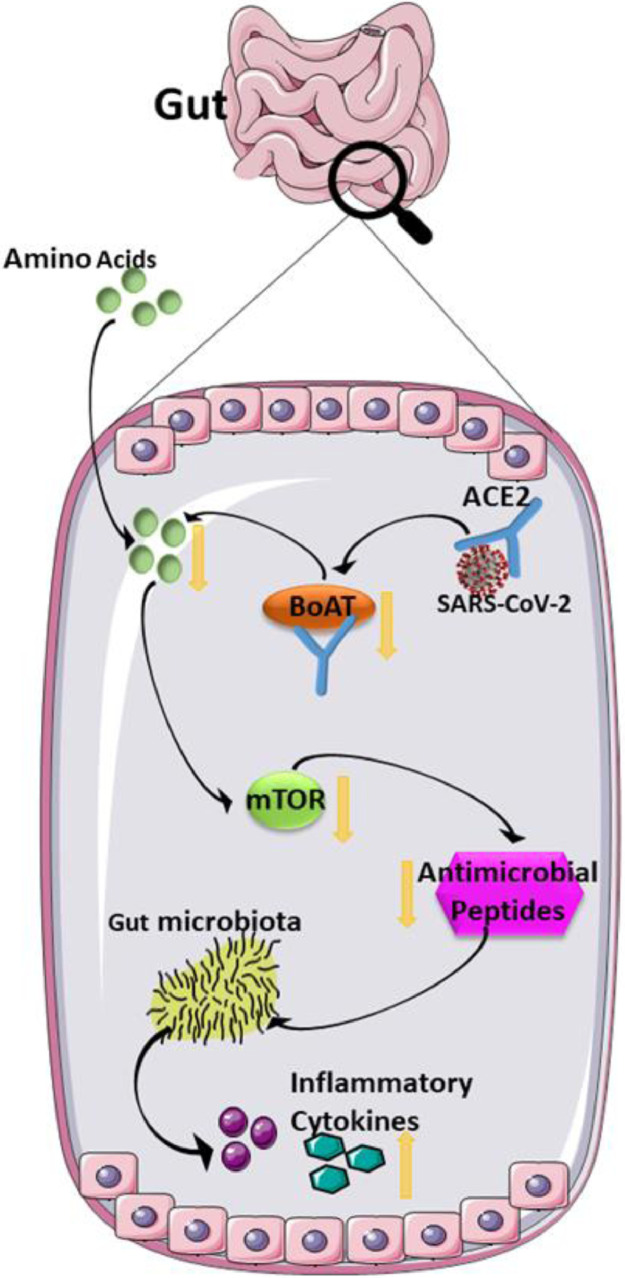
Another factor that affects the normal population of gut microbiota is reduced serum levels of neutral amino acids, especially tryptophan. ACE2 has functions other than initiation of viral entry into host cell, which regulate the amino acid homeostasis in the intestine by transporting it there. Intestinal SARS-CoV-2 may influence tryptophan absorption by influencing the ACE2:B0AT1 transportation complex. This influence further affects the mammalian target of rapamycin, which controls the presence of antimicrobial peptides. Reduced tryptophan level and the lowered expression of antimicrobial peptides, together, can negatively affect gut microbial ecology leading to transmissible susceptibility to colitis (Hashimoto et al., 2012; Xu et al., 2020), intestinal inflammation, and diarrhea (Figure 2 ). This leads to the speculation of an association between COVID-19 and gut microbiota (Gao et al., 2020). Two of the most common gut bacteria are Lactobacillus and Bifidobacterium, and they were found to decrease in some patients with COVID-19 (Xu et al., 2020).
Figure 2.
Mechanisms involved in COVID-19-associated diarrhea. SARS-CoV-2 enters into a host cell by ACE2 receptor, where it disrupts the B0AT/ACE2 absorption pathway and then interrupts the activation of mTOR resulting in the reduction of antimicrobial peptide production. Altogether, these affect the normal gut microbiome contributing to inflammatory cytokine production. This figure was partially generated by Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
ACE2: angiotensin-converting enzyme; B0AT, broad neutral amino acid transporter; mTOR, mammalian target of rapamycin.
Side effects of medications used to treat COVID-19
Antiviral drugs, antibiotics, and corticosteroids are used in the treatment of patients with COVID-19 (Megyeri et al., 2021), but there are possible side effects of these medicines. One of the major side effects is antibiotic-associated diarrhea which could also be mistaken as a COVID-19 symptom. Depending on the time of onset of diarrhea, it could be early COVID-19-associate diarrhea or late antibiotic-associated diarrhea (Maslennikov et al., 2021). In case of the first one, viral RNA is found in the stool sample depicting that it is caused by the virus itself, and it is usually benign, self-limiting, and the need for medical attention is limited. However, in the fecal sample of patients with late diarrhea, viral RNA was absent, leading to the idea of antibiotic-associated diarrhea, which could be fatal if untreated.
Antibiotics are not the treatment option for COVID-19 itself but are administered for treating the secondary bacterial infection caused by frequently detected Klebsiella pneumoniae, Staphylococcus aureus, Streptococcus pneumoniae, Escherichia coli, Acinetobacter spp., Neisseria meningitides, and Haemophilus influenzae (Handel et al., 2009; Sharifipour et al., 2020) and the infection rate is higher in critically ill patients who are in the intensive care unit (Pourajam et al., 2022). The use of antimicrobial drugs can alter the normal intestinal microbiota leading to antibiotic-associated diarrhea, as mentioned earlier. They also affect antibody production and immune system, which could prolong the clearance of SARS-CoV-2 from the gut (Perisetti et al., 2020). Another problem may arise if the patient is treated with broad-spectrum antibiotics, which leads to the possibility of Clostridiodes difficile infection (CDI), even long after recovery from COVID-19. When someone has both CDI and COVID-19, it damages the intestine more severely, resulting in more severe GIT-associated symptoms (Granata et al., 2020).
Antiviral drugs such as favipiravir and remdesivir, which are RNA polymerase inhibitors, lopinavir, and ritonavir, used to treat patients with COVID-19, also cause diarrhea in some cases (Mifsud et al., 2019; Perisetti et al., 2020; Ye et al., 2020).
There is another treatment option that may cause diarrhea, namely the use of monoclonal antibodies (mAbs) such as sarilumab, siltuximab, and tocilizumab. The main purpose of these mAbs is to offset the effects of proinflammatory cytokines such as IL-6 by inhibiting the IL-6 receptors (Hanna et al., 2021).
Inflammatory response to gastrointestinal tract and bile acid diarrhea
One of the mechanisms by which SARS-CoV-2 damages the intestinal tract is via inflammatory response leading to diarrhea, and the major causes of this ‘inflammatory storm’ are excessively released cytokines and immune dysregulation (Liu et al., 2020; Wu et al., 2020).
Intestinal inflammation may trigger COVID-19-associated diarrhea through some mechanisms, including reduced reabsorption of secreted bile acids in the terminal ileum. Bile acids are major organic substances of bile that are absorbed in the distal small intestine and then return to the liver, re-secreted from there, which is known as enteropathic circulation. During this process, only 5% of the total bile acids are lost during defecation, which means the process is precise (Ticho et al., 2019).
There is a bile acid absorption system on the apical epithelial surface of gut epithelia which is controlled by an apical sodium-dependent bile acid transporter. In the case of ileal inflammation, a lot of inflammatory cytokines are present, and if particularly, IL-6 is present, it decreases the expression of apical sodium-dependent bile acid transporter (ASBT) (Craddock et al., 1998; Ha et al., 2021; Lazaridis et al., 1997; Neimark et al., 2006; Shirohata et al., 2022), which leads to decreased bile acid absorption capacity at the distal ileum (Oelkers et al., 1997). As a result, bile acid in the colon increases, and this stimulates colonic water secretion and peristalsis, leading to bile acid diarrhea (Ticho et al., 2019).
Concomitant complications and underlined factors
In addition to respiratory and GIT symptoms, SARS-CoV-2 can damage hepatocytes with subsequent hepatic dysfunction (15-78% of the cases), which results in increased alkaline phosphatase (ALP) (4.6%), ALT (20.6%), AST (22.8%), and bilirubin (7.8%) levels and slightly reduced albumin (39.8%) level. (Zarifian et al., 2021).
It is hypothesized that the main reason behind COVID-19-associated liver injury is ACE2; in other words, where ACE2 receptors are present, COVID-19-associated pathogenesis will be present too. Upregulation of ACE2 is thought to be one mechanism of pathogenesis in liver tissues as a result of the hepatocyte regeneration in liver homeostasis (Pu and Zhou, 2022). The main function of ACE2 is to offset the vasoconstriction effect of angiotensin II, thus decreasing the liver damage through the renin-angiotensin system (Paizis et al., 2005). However, a recent study showed that ACE2 was highly expressed in hepatocytes and cholangiocytes, suggesting the possibility of COVID-19-associated liver injury (Chai et al., 2020; Pu and Zhou, 2022). Another hypothesis suggests that the liver injury in COVID-19 might be due to a secondary injury from hypoxia, which leads to a sharp increase of ACE2 in hepatocytes and bile duct cells (Paizis et al., 2005) and thus increased entry of the virus into the liver.
Management of COVID-19-associated diarrhea and liver injuries
COVID-19-associated diarrhea is mostly moderate or mild, so it is often self-limiting. Drug-induced diarrhea does not need medical attention and resolves spontaneously with either continued use or withdrawal of the offending drug (Abraham and Sellin, 2007). Two studies found that the most commonly used and offending drugs were azithromycin, ceftriaxone, hydroxychloroquine, glucocorticoids, and some antivirals (Maslennikov et al., 2021; Sultana et al., 2020). When there is more frequent diarrhea, or there is the possibility of drug intolerance, adjustment of doses or cessation of the culprit drug could be the best option.
If the diarrhea in patients with COVID-19 develops in the later course of illness, the risk of mortality could increase. In a study, it was found that the rate of mortality due to the late onset of diarrhea was 50%, compared to a lower rate (4.3%) if the onset of diarrhea was in the first 20 days of the infection (Maslennikov et al., 2021). Sometimes, severe dehydration might develop, and in that case, hospitalization, fluid management, and potassium monitoring (Desforges et al., 1990) are essential, but fluid administration should be supervised continuously in patients with severe sepsis and lung involvement (World Health Organization, 2020).
Because there is no homogeneity in the causes of development, COVID-19-associated diarrhea requires different management for different patients. Varied combinations of antibiotics and probiotics were used; however, none showed significant benefit over the others (Maslennikov et al., 2021). Proper use of antibiotics in the case of patients with infective diarrhea required knowledge of the locally circulating pathogen spectrum and their antibiotic sensitivity pattern. Medicines like metronidazole, vancomycin, sulfasalazine, and the probiotic Saccharomyces boulardii, were also used to treat diarrhea that occurred during the later course of the illness (Maslennikov et al., 2021).
Different probiotics were used for different types of diarrhea. However, the results are not always promising. Probiotics, especially Lactobacillus and dicotahedral montmorillonite might be another treatment option for COVID-19-associated diarrhea. Although the effectiveness of probiotics (especially Lactobacillus and dicotahedral montmorillonite) is still unknown for human-coronavirus-associated diarrhea, it worked for animal coronavirus-associated diarrhea (Kumar et al., 2010).
In critically ill patients, antibiotic-associated diarrhea or CDI may occur, and as a treatment option or preventive measure, Lactobacillus-containing probiotic preparation might be administered if CDI tests are found positive for patients with COVID-19 (Maslennikov et al., 2021).
Because COVID-19-associated liver injury is often caused by mostly antiviral drugs like lopinavir and ribavirin, antipyretic analgesics, antibiotics, and herbal products, controlled use or complete suspension of these drugs can be the only way of avoiding liver injury. Another preferable way can be a dynamic observation of the patients with elevated AST/ALT level.
Gaps in knowledge
Although diarrhea is one of the most prevalent symptoms of COVID-19, not enough studies have been conducted regarding this. Molecular pathophysiology is also not well established; most of them are hypothesized. Studies related to other GIT manifestations like anorexia, nausea, and vomiting are also insufficient, and the causes are less known. Geographical location-based studies to observe the relationship between these symptoms and ethnicity are less published. Moreover, potential cofounder of GIT symptoms- adverse effects of drugs, gut microbiota dysbiosis, and inflammatory responses can change the prevalence of these symptoms in patients with COVID-19. More studies are needed to evaluate the degree of damage and extent of the risk due to SARS-CoV-2 infection and GIT manifestations.
Conclusion
Diarrhea is the most frequent GI symptom in patients with COVID-19. ACE2 is the main receptor for entry into the gut epithelial cell, which is found abundantly in the intestine. The virus enters into enterocyte and replicates there, leading to an inflammatory response in the intestine, and production of various proinflammatory chemokines and cytokines. Some of them trigger the increase of permeability. Viroporins of SARS-CoV-2 also directly contribute to ionic imbalance in the intestine resulting in COVID-19-associated diarrhea. Another gut-specific mechanism of COVID-19 is the dysregulation of the ACE2:B0AT1 complex, and the modification of this complex reduces Na+ uptake and amino acid starvation, which is a contributing factor to diarrhea. Alteration of gut microbiota and side effects of medications are also involved in causing diarrhea.
Because medication choice to treat COVID-19-associated symptoms may lead to further complications like liver damage, the dynamic monitoring of liver function and cytokine production is a must to check for further complications and fatality. Extensive studies regarding ACE2 and immune responses should be conducted in the future to treat COVID-19, and ACE2 can be a good target for the COVID-19 vaccine.
Declaration of competing interest
The authors have no competing interests to declare.
Acknowledgments
Funding
No funding source was applicable for this manuscript.
Ethical approval
No ethical approval was required for this manuscript.
Acknowledgments
International Centre for Diarrhoeal Disease Research Bangladesh (icddr,b) acknowledges supports from the Governments of Bangladesh, Canada, Sweden, and the UK for providing core/unrestricted support.
Author contributions
RTJ and SAS designed the concept of the review manuscript. RTJ prepared the first draft. SAS reviewed and edited the review with appropriate key points and figures. MS assisted in writing the diarrhea management section. RH made further contribution to the draft version. MSA supervised and revised the manuscript. All authors have read and agreed to this version of the manuscript.
References
- Abdel-Naser MB. COVID-19 pandemic and teledermatology in low-income regions. Edorium J Dermatol. 2021;3 10000402MA2021. [Google Scholar]
- Abraham B, Sellin JH. Drug-induced diarrhea. Curr Gastroenterol Rep. 2007;9:365–372. doi: 10.1007/s11894-007-0044-x. [DOI] [PubMed] [Google Scholar]
- Aksoy H, Karadag AS, Wollina U. Angiotensin II receptors: impact for COVID-19 severity. Dermatol Ther. 2020;33:e13989. doi: 10.1111/dth.13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andring JT, McKenna R, Stevens BR. Amino acid transporter B0AT1 influence on ADAM17 interactions with SARS-CoV-2 receptor ACE2 putatively expressed in intestine, kidney, and cardiomyocytes. bioRxiv. 2020:361873. doi: 10.1101/2020.10.30.361873. [DOI] [Google Scholar]
- Barrett KE, Keely SJ. Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol. 2000;62:535–572. doi: 10.1146/annurev.physiol.62.1.535. [DOI] [PubMed] [Google Scholar]
- Bayati A, Kumar R, Francis V, McPherson PS. SARS-CoV-2 infects cells after viral entry via clathrin-mediated endocytosis. J Biol Chem. 2021;296:100306. doi: 10.1016/j.jbc.2021.100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Yang R, Wang W, Lee I, Zhang R, Zhang W, et al. Computational Study of the Ion and Water Permeation and Transport Mechanisms of the SARS-CoV-2 Pentameric E Protein Channel. Front Mol Biosci. 2020;7:565797. doi: 10.3389/fmolb.2020.565797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv. 2020:931766. doi: 10.1101/2020.02.03.931766. [DOI] [Google Scholar]
- Craddock AL, Love MW, Daniel RW, Kirby LC, Walters HC, Wong MH, et al. Expression and transport properties of the human ileal and renal sodium-dependent bile acid transporter. Am J Physiol. 1998;274:G157–GG69. doi: 10.1152/ajpgi.1998.274.1.G157. [DOI] [PubMed] [Google Scholar]
- Davidson AM, Wysocki J, Batlle D. Interaction of SARS-CoV-2 and other coronavirus with ACE (angiotensin-converting enzyme)-2 as their main receptor: therapeutic implications. Hypertension. 2020;76:1339–1349. doi: 10.1161/HYPERTENSIONAHA.120.15256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desforges JF, Avery ME, Snyder JD. Oral therapy for acute diarrhea. N Engl J Med. 1990;323:891–894. doi: 10.1056/NEJM199009273231307. [DOI] [PubMed] [Google Scholar]
- Gao QY, Chen YX, Fang JY. 2019 Novel coronavirus infection and gastrointestinal tract. J Dig Dis. 2020;21:125–126. doi: 10.1111/1751-2980.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb M, Long B. Dermatologic manifestations and complications of COVID-19. Am J Emerg Med. 2020;38:1715–1721. doi: 10.1016/j.ajem.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granata G, Bartoloni A, Codeluppi M, Contadini I, Cristini F, Fantoni M, et al. The burden of Clostridioides difficile infection during the COVID-19 pandemic: a retrospective case-control study in Italian hospitals (CloVid) J Clin Med. 2020;9:3855. doi: 10.3390/jcm9123855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Han B, Wang J. COVID-19: gastrointestinal manifestations and potential fecal–oral transmission. Gastroenterology. 2020;158:1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha S, Jin B, Clemmensen B, Park P, Mahboob S, Gladwill V, et al. Serotonin is elevated in COVID-19-associated diarrhoea. Gut. 2021;70:2015–2017. doi: 10.1136/gutjnl-2020-323542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, Van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handel A, Longini IM, Antia R. Intervention strategies for an influenza pandemic taking into account secondary bacterial infections. Epidemics. 2009;1:185–195. doi: 10.1016/j.epidem.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna R, Dalvi S, Sălăgean T, Pop ID, Bordea IR, Benedicenti S. Understanding COVID-19 pandemic: molecular mechanisms and potential therapeutic strategies. An evidence-based review. J Inflamm Res. 2021;14:13–56. doi: 10.2147/JIR.S282213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M, et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BM, De Oliveira MHS, Benoit J, Lippi G. Gastrointestinal symptoms associated with severity of coronavirus disease 2019 (COVID-19): a pooled analysis. Intern Emerg Med. 2020;15:857–859. doi: 10.1007/s11739-020-02329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and Is blocked by a clinically proven protease inhibitor. Cell. 2020;181 doi: 10.1016/j.cell.2020.02.052. 271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu TY, Frieman M, Wolfram J. Insights from nanomedicine into chloroquine efficacy against COVID-19. Nat Nanotechnol. 2020;15:247–249. doi: 10.1038/s41565-020-0674-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M, Jabeen N, Amanullah A, Baig AA, Aziz B, Shabbir S, et al. Molecular docking between human TMPRSS2 and SARS-CoV-2 spike protein: conformation and intermolecular interactions. AIMS Microbiol. 2020;6:350–360. doi: 10.3934/microbiol.2020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacob S, Iacob DG, Luminos LM. Intestinal microbiota as a host defense mechanism to infectious threats. Front Microbiol. 2018;9:3328. doi: 10.3389/fmicb.2018.03328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia HP, Look DC, Tan P, Shi L, Hickey M, Gakhar L, et al. Ectodomain shedding of angiotensin-converting enzyme 2 in human airway epithelia. Am J Physiol Lung Cell Mol Physiol. 2009;297:L84–L96. doi: 10.1152/ajplung.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Lian JS, Hu JH, Gao J, Zheng L, Zhang YM, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KD, Harris C, Cain JK, Hummer C, Goyal H, Perisetti A. Pulmonary and extra-pulmonary clinical manifestations of COVID-19. Front Med (Lausanne) 2020;7:526. doi: 10.3389/fmed.2020.00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laville E, Perrier J, Bejar N, Maresca M, Esque J, Tauzin AS, et al. Investigating host microbiota relationships through functional metagenomics. Front Microbiol. 2019;10:1286. doi: 10.3389/fmicb.2019.01286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaridis KN, Pham L, Tietz P, Marinelli RA, Degroen PC, Levine S, et al. Rat cholangiocytes absorb bile acids at their apical domain via the ileal sodium-dependent bile acid transporter. J Clin Invest. 1997;100:2714–2721. doi: 10.1172/JCI119816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LH, Wong SH, Chin SF, Singh V, Ab Mutalib NS. Editorial: Human microbiome: symbiosis to pathogenesis. Front Microbiol. 2021;12:605783. doi: 10.3389/fmicb.2021.605783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Jiang X, Zhang Z, Huang S, Zhang Z, Fang Z, et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69:997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- Lippi G, Lavie CJ, Henry BM. Sanchis-Gomar F. Do genetic polymorphisms in angiotensin-converting enzyme 2 (ACE2) gene play a role in coronavirus disease 2019 (COVID-19)? Clin Chem Lab Med. 2020;58:1415–1422. doi: 10.1515/cclm-2020-0727. [DOI] [PubMed] [Google Scholar]
- Liu J, Li S, Liu J, Liang B, Wang X, Wang H, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBiomedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo K-Y, Letchumanan V. COVID-19: Malaysia's fight against this deadly virus. Prog Micobes Mol Biol. 2021;4 [Google Scholar]
- Malik YA. Properties of coronavirus and SARS-CoV-2. Malays J Pathol. 2020;42:3–11. [PubMed] [Google Scholar]
- Mangiola F, Nicoletti A, Gasbarrini A, Ponziani FR. Gut microbiota and aging. Eur Rev Med Pharmacol Sci. 2018;22:7404–7413. doi: 10.26355/eurrev_201811_16280. [DOI] [PubMed] [Google Scholar]
- Maslennikov R, Svistunov A, Ivashkin V, Ufimtseva A, Poluektova E, Efremova I, et al. Early viral versus late antibiotic-associated diarrhea in novel coronavirus infection. Medicine. 2021;100:e27528. doi: 10.1097/MD.0000000000027528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megyeri K, Dernovics Á, Al-Luhaibi ZII, Rosztóczy A. COVID-19-associated diarrhea. World J Gastroenterol. 2021;27:3208–3222. doi: 10.3748/wjg.v27.i23.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mifsud EJ, Hayden FG, Hurt AC. Antivirals targeting the polymerase complex of influenza viruses. Antiviral Res. 2019;169:104545. doi: 10.1016/j.antiviral.2019.104545. [DOI] [PubMed] [Google Scholar]
- Mohamed DZ, Ghoneim ME-S, Abu-Risha SE-S, Abdelsalam RA, Farag MA. Gastrointestinal and hepatic diseases during the COVID-19 pandemic: manifestations, mechanism and management. World J Gastroenterol. 2021;27:4504–4535. doi: 10.3748/wjg.v27.i28.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neimark E, Chen F, Li X, Magid MS, Alasio TM, Frankenberg T, et al. c-Fos is a critical mediator of inflammatory-mediated repression of the apical sodium-dependent bile acid transporter. Gastroenterology. 2006;131:554–567. doi: 10.1053/j.gastro.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Obukhov AG, Stevens BR, Prasad R, Li Calzi S, Boulton ME, Raizada MK, et al. SARS-CoV-2 infections and ACE2: clinical outcomes linked with increased morbidity and mortality in individuals with diabetes. Diabetes. 2020;69:1875–1886. doi: 10.2337/dbi20-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelkers P, Kirby LC, Heubi JE, Dawson PA. Primary bile acid malabsorption caused by mutations in the ileal sodium-dependent bile acid transporter gene (SLC10A2) J Clin Invest. 1997;99:1880–1887. doi: 10.1172/JCI119355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Melo DI, Gurley SB. Angiotensin-converting enzyme 2 and the kidney. Curr Opin Nephrol Hypertens. 2016;25:59–66. doi: 10.1097/MNH.0000000000000182. [DOI] [PubMed] [Google Scholar]
- Paizis G, Tikellis C, Cooper ME, Schembri JM, Lew RA, Smith AI, Shaw T, Warner FJ, Zuilli A, Burrell LM, Angus PW. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin-converting enzyme 2. Gut. 2005;54:1790–1796. doi: 10.1136/gut.2004.062398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115:766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/angiotensin 1–7 Axis of the renin–angiotensin system in heart failure. Circ Res. 2016;118:1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perisetti A, Goyal H, Gajendran M, Boregowda U, Mann R, Sharma N. Prevalence, Mechanisms, and implications of gastrointestinal symptoms in COVID-19. Front Med (Lausanne) 2020;7:588711. doi: 10.3389/fmed.2020.588711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourajam S, Kalantari E, Talebzadeh H, Mellali H, Sami R, Soltaninejad F, et al. Secondary bacterial infection and clinical characteristics in patients with COVID-19 admitted to two intensive care units of an academic hospital in iran during the first wave of the pandemic. Front Cell Infect Microbiol. 2022;12:784130. doi: 10.3389/fcimb.2022.784130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu W, Zhou B. Hepatocyte generation in liver homeostasis, repair, and regeneration. Cell Regen. 2022;11:2. doi: 10.1186/s13619-021-00101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Shu T, Wu D, Mu J, Wang C, Huang M, et al. The ORF3a protein of SARS-CoV-2 induces apoptosis in cells. Cell Mol Immunol. 2020;17:881–883. doi: 10.1038/s41423-020-0485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov DA, Arzamasov AA, Khoroshkin MS, Iablokov SN, Leyn SA, Peterson SN, et al. Micronutrient requirements and sharing capabilities of the human gut microbiome. Front Microbiol. 2019;10:1316. doi: 10.3389/fmicb.2019.01316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifipour E, Shams S, Esmkhani M, Khodadadi J, Fotouhi-Ardakani R, Koohpaei A, et al. Evaluation of bacterial co-infections of the respiratory tract in COVID-19 patients admitted to ICU. BMC Infect Dis. 2020;20:646. doi: 10.1186/s12879-020-05374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V, Rodionov DA, Leyn SA, Tran D, Iablokov SN, Ding H, et al. B-vitamin sharing promotes stability of gut microbial communities. Front Microbiol. 2019;10:1485. doi: 10.3389/fmicb.2019.01485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Noh JR, Chang DH, Kim YH, Kim MH, Lee ES, et al. Elucidation of Akkermansia muciniphila probiotic traits driven by mucin depletion. Front Microbiol. 2019;10:1137. doi: 10.3389/fmicb.2019.01137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirohata A, Ariyoshi R, Fujigaki S, Tanaka K, Morikawa T, Sanuki T, et al. A case of COVID-19 diarrhea relieved by bile acid sequestrant administration. Clin J Gastroenterol. 2022;15:393–400. doi: 10.1007/s12328-022-01598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana J, Cutroneo PM, Crisafulli S, Puglisi G, Caramori G, Trifirò G. Azithromycin in COVID-19 patients: pharmacological mechanism, clinical evidence and prescribing guidelines. Drug Saf. 2020;43:691–698. doi: 10.1007/s40264-020-00976-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theobald SJ, Simonis A, Kreer C, Zehner M, Fischer J, Albert M-C, et al. The SARS-CoV-2 spike protein primes inflammasome-mediated interleukin-1- beta secretion in COVID-19 patient-derived macrophages. EMBO Mol Med. 2021;13:e14150. doi: 10.15252/emmm.202114150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ticho AL, Malhotra P, Dudeja PK, Gill RK, WA Alrefai. Intestinal absorption of bile acids in health and disease. Compr Physiol. 2019;10:21–56. doi: 10.1002/cphy.c190007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner AJ, Hiscox JA, Hooper NM. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol Sci. 2004;25:291–294. doi: 10.1016/j.tips.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls AC, Park Y-J, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181 doi: 10.1016/j.cell.2020.02.058. 281–292.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yang P, Liu K, Guo F, Zhang Y, Zhang G, et al. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008;18:290–301. doi: 10.1038/cr.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MK, Yue HY, Cai J, Zhai YJ, Peng JH, Hui JF, et al. COVID-19 and the digestive system: a comprehensive review. World J Clin Cases. 2021;9:3796–3813. doi: 10.12998/wjcc.v9.i16.3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SH, Lui RN, Sung JJ. Covid-19 and the digestive system. J Gastroenterol Hepatol. 2020;35:744–748. doi: 10.1111/jgh.15047. [DOI] [PubMed] [Google Scholar]
- World health Organization . World Health Organization; Geneva: 2020. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance. [Google Scholar]
- World Health Organization . World Health Organization; Geneva: 2022. Weekly epidemiological update on COVID-19 - 11 May 2022. 91 ed2022. [Google Scholar]
- Wruck W, Adjaye J. SARS-CoV-2 receptor ACE2 is co-expressed with genes related to transmembrane serine proteases, viral entry, immunity and cellular stress. Sci Rep. 2020;10:21415. doi: 10.1038/s41598-020-78402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, Peng G, Wilken M, Geraghty RJ, Li F. Mechanisms of host receptor adaptation by severe acute respiratory syndrome coronavirus. J Biol Chem. 2012;287:8904–8911. doi: 10.1074/jbc.M111.325803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Ho W, Huang Y, Jin DY, Li S, Liu SL, et al. SARS-CoV-2 is an appropriate name for the new coronavirus. Lancet. 2020;395:949–950. doi: 10.1016/S0140-6736(20)30557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z, Koo H, Chen Q, Zhou X, Liu Y, Simon-Soro A. Potential implications of SARS-CoV-2 oral infection in the host microbiota. J Oral Microbiol. 2020;13:1853451. doi: 10.1080/20002297.2020.1853451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Xu X, Jiang L, Dua K, Hansbro PM, Liu G. SARS-CoV-2 induces transcriptional signatures in human lung epithelial cells that promote lung fibrosis. Respir Res. 2020;21:182. doi: 10.1186/s12931-020-01445-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Wang B, Zhang T, Xu J, Shang S. The mechanism and treatment of gastrointestinal symptoms in patients with COVID-19. Am J Physiol Gastrointest Liver Physiol. 2020;319 doi: 10.1152/ajpgi.00148.2020. G245–52-g52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang R, Gomez Castro MF, McCune BT, Zeng Q, Rothlauf PW, Sonnek NM, et al. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci Immunol. 2020;5:eabc3582. doi: 10.1126/sciimmunol.abc3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarifian A, Zamiri Bidary M, Arekhi S, Rafiee M, Gholamalizadeh H, Amiriani A, et al. Gastrointestinal and hepatic abnormalities in patients with confirmed COVID-19: a systematic review and meta-analysis. J Med Virol. 2021;93:336–350. doi: 10.1002/jmv.26314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. Am J Respir Crit Care Med. 2020;202:756–759. doi: 10.1164/rccm.202001-0179LE. [DOI] [PMC free article] [PubMed] [Google Scholar]