Abstract
Joint attention (JA), infants' ability to engage in triadic attention with another person and a separate object or event, emerges in infancy. Responding to joint attention (RJA) develops earlier than initiating joint attention (IJA) and may benefit from a reconceptualization from a competence to a skill that varies in performance. Investigating associations between RJA performance and important skills of toddlerhood such as language, social responsiveness, and executive function (EF) in typically developing samples can better elucidate how RJA may serve as a developmental precursor to later dimensional skills, with implications for both typical and atypical development. Here, 210 (82% White) infants completed the Dimensional Joint Attention Assessment (DJAA), a naturalistic play‐based assessment of RJA, at 8–15 months. At 16–38 months social responsiveness, verbal ability, and EF were assessed. Multilevel models showed that DJAA scores were associated with later verbal abilities and parent‐reported social responsiveness. Exploratory analyses showed trend‐level associations between RJA and EF. Results establish the content validity of the DJAA as a measure of RJA, and longitudinal associations with later verbal ability and social responsiveness. Future work should examine EF emergence and consolidation, and RJA and later EF associations.
1. INTRODUCTION
Joint attention, and responding to joint attention (RJA) specifically, represent social‐cognitive and social‐communicative skills that emerge in infancy in typically developing populations. Previous work demonstrates associations between RJA and important outcomes in toddlerhood, such as language (e.g., Brooks & Meltzoff, 2008; Carpenter et al., 1998; Tomasello, 2003) and Theory of Mind (e.g., Brooks & Meltzoff, 2015; Charman et al., 2000). Atypical or delayed RJA is observed in infants/toddlers diagnosed with autism spectrum disorder (ASD; e.g., Stallworthy, Lasch, et al., 2022), a disorder also associated with delays or differences in social responsiveness (engaging in emotionally, socially appropriate behaviors) and executive function. However, differences in executive functions are associated with childhood psychopathology broadly (e.g., Martel et al., 2017; White et al., 2017), and social responsiveness impairment is also not specific to only ASD (Cholemkery et al., 2014; Grzadzinski et al., 2011; Jalbrzikowski et al., 2013; Pine et al., 2008; Reiersen et al., 2008). Better understanding of the associations between RJA and later skills of toddlerhood in typically developing populations may further elucidate patterns of normative development and may also inform how early perturbations in RJA may be linked with later atypical development.
In the latter half of the first year of life, infants begin to exhibit joint attention: the ability to coordinate one's gaze and attention with another person to an object or event external to the social dyad. Triadic joint attention has been observed and studied as early as 6–12 months (Brune & Woodward, 2007; Striano & Rochat, 1999; Vaughan Van Hecke et al., 2012) and is often cited as an important precursor to the development of understanding others' intentions and engaging in joint goals and experiences later in toddlerhood and beyond (e.g., Brooks & Meltzoff, 2015; Tomasello et al., 2005). Often, joint attention is discussed in terms of both responding to joint attention (RJA; following the direction of attention of another person to an object or event of interest) and initiating joint attention (IJA; successfully and intentionally directing another person's attention to something of interest).
An extensive body of work supports the distinction between RJA and IJA, but few microgenetic studies have been conducted to elucidate dynamic interplay of the two constructs. The emergence of RJA typically precedes IJA. But that said, RJA does not abruptly manifest. Infants demonstrate social interest and engagement very early in life. At 6 weeks old, infants look more to a social partner when that partner alternates attention between an object and the infant rather than directing attention only to the object (Striano et al., 2007). Longitudinal work suggests that infants' gaze following to both parents and strangers increases between 2 and 4 months of age with accuracy stabilizing by 6–8 months (Gredebäck et al., 2010). While this early gaze following may imply attentional engagement from young infants, this is necessary but not sufficient for triadic joint attention or engagement. Younger infants (4‐ and 5‐month‐olds) do not show enhanced object processing following joint visual interactions with adults, whereas older infants, 7‐ and 9‐month‐olds, do (Cleveland et al., 2007; Cleveland & Striano, 2007). This suggests some degree of a qualitative shift in how infants process or use information from these interactions to be full participants in a joint, triadic experience. Therefore, RJA represents not only the end of a cascade of early gaze following skills, but also the beginning or “catalyst” of a qualitatively new flavor of social interaction that infants may undertake with others—a triadic engagement.
As reviewed more thoroughly elsewhere (e.g., Gabouer & Bortfeld, 2021), joint attention is often viewed as an important milestone of social cognition, demonstrating early perspective taking and mentalizing abilities that are associated with later social phenomena unique to humans such as joint intentionality (Brooks & Meltzoff, 2015; Tomasello, 2018; Tomasello et al., 2005). This perspective also emphasizes that joint attention is not passively achieved, but rather necessitates active processing of another's perspective and intentions by an infant. However, a “leaner” interpretation of joint attention instead emphasizes the visual mechanisms of gaze following (e.g., Butterworth, 1991) and deemphasizes the role of social cognition. This interpretation argues that early gaze following and RJA are dependent on an infant's ability to infer the presence of some interesting or salient stimuli from the “geometric orientation” of another's gaze or head turn. Indeed, 6‐ to 12‐month‐old infants' tendency to stop at the first possible stimulus of interest in their scan path (Butterworth, 1991; Butterworth & Jarrett, 1991) suggests that visual salience may sometimes take precedence when infants know less about their social partner's intentions or interests or demonstrate less efficient endogenous control over their visual attention earlier in life. While 12‐ to 15‐month‐old's show improved ability to locate a target by following adults' gaze (Butterworth & Jarrett, 1991), it is unclear if this should be attributed to spatial orienting precision, attentional control, or maturing social cognition.
Examination of the neural underpinnings of the visual attention system and reorienting response operable during RJA suggests that spatial geometry alone is insufficient to explain the phenomenon (Redcay et al., 2010). Prior work suggests that both the dorsal and ventral attention systems (broadly associated with endogenous and exogenous attention, respectively) are engaged in visual reorienting (Corbetta et al., 2008), and that right posterior superior temporal sulcus (rpSTS) and right temporo‐parietal junction (rTPJ) are specifically involved with RJA in adults. Previous studies (e.g., Corbetta et al., 2000; Downar et al., 2001) also suggest that, in adults, the ventral system's role in RJA is related specifically to reorienting to stimuli that are task‐relevant—not just visually salient.
1.1. Joint attention and language
Joint attention plays a key role in language development. Having one's attention directed to objects by another (often a caregiver) allows for early label mapping and word learning (Baldwin, 1991; Mundy, 2018; Striano et al., 2006). Infants who successfully engaged in RJA at 10–11 months showed faster vocabulary growth over the following 10 months than those who did not (Brooks & Meltzoff, 2008), suggesting that joint attention facilitates language development even when infants are not initiating the engagement themselves. Later in development, infant‐initiated triadic engagement provides an opportunity for infants to inquire about new objects and experiences in their environments via proto‐communicative skills such as pointing. Mundy et al. (2007) also found that RJA at 12 months was associated with receptive language skill at 24 months, even after controlling of infants' general cognitive development. Previous work (e.g., Mundy et al., 1986; Sigman et al., 1999) has also found associations between joint attention and expressive language both predictively and concurrently, and a recent meta‐analysis suggests that RJA is associated with both concurrent and later expressive and receptive language abilities (Bottema‐Beutel, 2016). Based on these and similar studies, there is a reason to believe that more complex or advanced joint attention in infancy could lead to greater language competencies in toddlerhood.
1.2. Joint attention and social responsiveness
If we are to view joint attention as an early milestone of social cognition, then it is possible that social ability or motivation either contributes to joint attention development (e.g., Stallworthy, Berry, et al., 2022; Tomasello et al., 2005) or may be increased by early or frequent engagement with social partners via joint attention. However, little work has examined whether RJA in a typically developing sample relates to broader domains of social responsiveness, the ability to engage in socially contingent and emotionally appropriate behavior with others (Marrus et al., 2015). Also referred to as a reciprocal social behavior, this trait is heritable (Marrus et al., 2020), continuously distributed in the population (Constantino & Todd, 2003; Marrus et al., 2020), and is associated with a range/variety of atypical outcomes (Cholemkery et al., 2014; Grzadzinski et al., 2011; Jalbrzikowski et al., 2013; Pine et al., 2008; Reiersen et al., 2008; Wagner et al., 2019). Previous work in a typically developing sample has found associations between IJA behaviors at 12 months and social responsiveness and competence at 30 months (Vaughan Van Hecke et al., 2007). Relatedly, RJA behaviors in a sample of infants with prenatal substance exposure were associated with increased compliance, expression, and positive play behaviors at 36 months (Sheinkopf et al., 2004). Whether RJA shows that similar predictive utility in a typically developing community sample is currently unknown.
In typically developing individuals, sharing attention with others through joint attention is thought to underlie social understanding, as both are part of a “social‐communicative representational system” from infancy (Charman et al., 2000). Infant response to joint attention involves an understanding that their attention is separate from that of another individual's, and in the case of IJA, that the attention of others can be manipulated or influenced by the infant (Mundy, 2018). This is thought to be more than just a circumstance of shared attention, but rather an experience shared with a social partner (Carpenter et al., 1998; Tomasello et al., 2005). Joint attention also serves as additional practice with early perspective taking and allows for infants to build their social knowledge and experience (Mundy, 2018). Indeed, as noted earlier, many of the neural regions underlying joint attention, such as the dorsal medial prefrontal cortex and rTPJ, are isomorphic with those most necessary and common for social cognition and mentalizing later in life (Mundy, 2003; Redcay et al., 2010). If joint attention is interpreted as inherently focused on sharing attention and an experience with another person (Mundy et al., 2009), then whatever early social motivation or responsiveness drives an infant to engage in joint attention may also be present later in toddlerhood and reflected in a young child's social behaviors. Additionally, investigating the relationship between RJA in infancy and later social responsiveness could help identify an early indicator of social communication or social deficits in populations with and without increased likelihood of atypical development.
1.3. Joint attention and executive function
On a basic level, joint attention involves flexibly shifting (or reorienting) attention between a social partner and an object or event of interest (Mundy & Newell, 2007). RJA specifically requires the integration of both the dorsal and ventral attention systems (Eggebrecht et al., 2017). While the ventral system is more broadly implicated in spatial relations between the self and others and the environment (which are certainly relevant to RJA), the involvement of the dorsal system also implies a level of attentional control over reorienting of visual attention in service of the goal of engagement with the social partner. If RJA involves a degree of flexible regulation of attentional in the service of a goal, it may be conceptualized as an early manifestation of attentional flexibility requisite to later‐developing facets of executive function (EF), the neurocognitive skills involved in goal‐directed problem solving (Carlson et al., 2013).
There has been a relative lack of focus on the associations between RJA and later executive function performance (Gago Galvagno et al., 2019), especially given established associations between RJA and theory of mind, the latter of which has been correlated with executive functions by toddlerhood (Carlson et al., 2004). Mundy (2003) has also previously described joint attention as “the social application of executive attention skills,” which implies the mastery of some level of cognitive control by late infancy or toddlerhood. Previous research provides mixed evidence of associations between early joint attention and later executive function abilities. Vaughan Van Hecke et al. (2012) found a relation between infants' RJA abilities at 12 months and more mature distraction behaviors in a delay of gratification task at 36 months. However, others (such as Miller & Marcovitch, 2015) failed to find associations between executive function and joint attention at earlier ages such as 14 or 18 months, potentially due to emerging, and thus difficult to measure, executive function abilities at those ages.
These mixed results are also intriguing considering prior work examining associations between RJA and theory of mind, the ability to attribute intent, knowledge, and other mental states to others to make predictions about others' behavior. Theory of mind may represent a social application of executive function, requiring cognitive flexibility and updating information about the perspectives of others (Frye et al., 1995). Theory of mind and executive function task performance are concurrently associated by 24 months of age (Carlson et al., 2004) with more recent work replicating this relationship as early as 18–24 months (Gago Galvagno et al., 2019). Although associations between executive function and joint attention are less clear, previous studies have found associations between RJA and theory of mind over longer periods of development, such that RJA at 9–12 months has been associated with theory of mind task performance at 4–4.5 years (Brooks & Meltzoff, 2015; Engel de Abreu et al., 2014; Sodian & Kristen‐Antonow, 2015). These results suggest that more precise measurement may elucidate associations between joint attention and later EF, akin to those associations observed between joint attention and theory of mind.
1.4. Measurement of RJA
Previous work has typically characterized variations in RJA as a variability in the timing of the observed competence (Carpenter et al., 1998) or variability in the rate or count of the observed behavior within a given sampling frame (Mundy et al., 2007). While many measures of joint attention and RJA in the first 2 years of life exist, theoretical and procedural differences mean that not every measure is equally suited for dimensional characterization of the latent RJA construct (see Tasker & Schmidt, 2008). For example, the Early Social Communication Scale (Mundy et al., 1996) includes tasks to quantify IJA, RJA, and infants' request behaviors among others. However, it offers only one variation of an RJA bid to infants, limiting its ability to capture the dimensional variation in RJA skill performance. Other gaze‐following protocols (e.g., Brooks & Meltzoff, 2008; Wetherby & Prizant, 2002) have similar limitations in that RJA is conceptualized as a competence to be acquired or demonstrated. Operationalizing RJA as a competence typically yields either dichotomous pass/fail values or percentage correct/accurate responses during a given assessment period. The question remains as to whether RJA is best conceptualized as a competence to be demonstrated or as a skill that can vary in performance or developmental sophistication. Other measures, such as the Communication Complexity Scale (Brady et al., 2012), were developed primarily for use in individuals with intellectual disabilities or developmental delays and thus make comparisons of associations between typical and atypical populations difficult.
An alternative measure, the Dimensional Joint Attention Assessment (DJAA; Elison et al., 2013), reconceptualized variability in RJA as the degree of developmental sophistication by which a child responds to examiner's cues that vary in redundancy or overtness. The task is administered as a naturalistic play‐based assessment. Additionally, administration of four series of progressively more redundant attentional bids is thought to elicit a broader developmental variation in RJA abilities, putatively capturing an underlying RJA construct, and provides more opportunities for infants to display RJA skills that vary in developmental sophistication (e.g., preliminary gaze following to a social partner's pointing hand rather than an intended object of interest). A focal characterization of RJA behaviors, rather than broader early communication behaviors (e.g., Brady et al., 2012; Salley et al., 2020), is intentional to allow for the potential to quantify dimensional variability in the RJA skill level. The DJAA was designed for 9‐ to 15‐month‐olds but has been used in infants from 8 to 18 months. Recent evidence suggests that the DJAA can be administered in clinic settings (Reilly et al., 2021; Stallworthy, Sifre, et al., 2022). DJAA performance is modulated by intrauterine growth during fetal development (Stallworthy, Sifre, et al., 2022), reflects variations in early socioeconomic environments (Reilly et al., 2021), and is attenuated in infants at an increased familial risk for autism spectrum disorder who later receive a diagnosis (Stallworthy, Lasch, et al., 2022).
RJA is an early emerging skill with theoretical and empirical associations with language and theory of mind. Mixed evidence exists regarding associations between RJA and later executive function, and no work (to our knowledge) has examined associations between RJA and later social responsiveness, despite the important implications that work may have for our understanding of both typical and atypical development.
1.5. The current study
The current study represents an ongoing effort to establish the construct validity of RJA, conceptualized and operationalized as a skill that can vary in performance and developmental sophistication (see also Elison et al., 2013; Reilly et al., 2021; Stallworthy, Berry, et al., 2022, 2022b, 2022c), as opposed to a competence to be categorically demonstrated (e.g., Carpenter et al., 1998) or tallied within a given sampling period (e.g., Mundy et al., 2007). Utilizing the DJAA, we aimed to demonstrate that previous findings in the literature linking RJA in the first years of life to later language abilities in toddlerhood generalized to our alternative conceptualization and operationalization of RJA in a typically developing population, eventually allowing for application of similar analyses in samples at higher likelihood of ASD or other atypical development. We hypothesized that RJA behaviors at 8–15 months would be positively associated with later language abilities in toddlerhood (17–38 months), even after accounting for early verbal ability and other variables historically associated with a verbal level, such as participant sex and maternal education. Additionally, we aimed to characterize an association between joint attention and later social responsiveness as reported by parents of typically developing toddlers. Based on established delays and deficits in both joint attention and social responsiveness in ASD (e.g., Dawson et al., 2004), both constructs of which should vary across the typical‐to‐atypical continuum, we hypothesized that RJA behaviors at 8–15 months would be associated with parent‐reported social responsiveness at 16–31 months of age. Finally, we examined associations between RJA performance at 8–15 months and later executive function abilities from 24 to 38 months. We also examined family income as a possible moderator of these associations. Due to mixed findings regarding the association between RJA measures and executive function tasks in previous literature, we did not have a directional hypothesis and considered this analysis exploratory.
2. METHOD
2.1. Participants and procedures
Participants represented a convenience sample recruited via the Institute of Child Development's Participant Pool, a state‐wide research registry that draws primarily from the Twin Cities metropolitan area. Families can enter the participant registry in three ways. First, families can be contacted via publicly available birth records early in their child's life (i.e., before their first birthday) and then they can return a postcard indicating their interest in being included in the participant registry and hearing about various studies for which their child may be eligible. Second, children of various ages can be enrolled by their caregivers at large public events where participation in the participant registry is advertised (e.g., children's museums, street fairs). Finally, some families hear about the participant registry through friends, family, or participation in other developmental research studies at the University and choose to add their contact information via an online interest form.
A portion of participants were recruited as part of the Baby Connectome Project (n = 155; Howell et al., 2019), an accelerated longitudinal design, and remaining participants (n = 55) were recruited as part of a more traditional longitudinal sampling framework. Participants did not vary on any measures or demographic variables based on their recruitment origin. The following inclusion criteria were stipulated for each study: 1) > 35 weeks gestation at birth; 2) birthweight of at least 2000 g; 3) no major pregnancy and/or delivery complications; and 4) no significant genetic, medical, neurological, or neurodevelopmental condition that affects growth, development, or cognition.
All participants (N = 210, 52% female, 83% White) completed the DJAA and Mullen Scales of Early Learning (MSEL) in the lab between 8 and 15 months (M = 11.5 months, SD = 1.9). Most participants came in for more than one visit during that age period (range = 1–3 visits, M visits = 1.9, SD visits = .77) for a total of 424 valid DJAA visits. Because of the sampling structure of the Baby Connectome Project, many (but not all) participants contributed data at more than one “outcome” data point from 16 to 38 months of age.
Between ages 16 and 31 months (M = 21.3 months, SD = 3.3), parents of 102 children completed the Video‐Reference Rating of Reciprocal Social Behavior (vrRSB; Marrus et al., 2015, 2020) online. Most parents completed this measure on more than one occasion (range = 1–3 data points, M datapoints = 1.7) for a total of 170 valid responses recorded. On average, there was a gap of 7.6 months between participants' first DJAA assessment and their first recorded vrRSB (SD = 2.8, range = 2.3–16.9).
At 17–38 months (M age = 26.1, SD age = 5.2), 123 participants (50% female) again completed the MSEL, with participants completing 1–4 visits each (M visits = 1.6), yielding 200 valid “outcome” MSEL assessments overall. On average, there was a gap of 11.9 months between participants' first DJAA assessment and toddler‐aged MSEL assessment (SD = 3.3, range = 5.5–22.6).
Finally, at 24–38 months (M age = 28.4, SD age = 4.65), 87 participants (57% female) completed the Minnesota Executive Function Scales (MEFS; Carlson & Zelazo, 2014) in the lab often on the same day as the MSEL. Participants completed the MEFS 1–2 times each (M datapoints = 1.3), resulting in 114 different visits with MEFS data. There was an average of 15.2 months between participants' first DJAA assessment and first MEFS assessment (SD = 3.9, range = 8.2–25.5). Distributions of all participant ages for all measures and timing of all measures/visits can be found in Figure 1, and descriptive statistics for all measures can be found in Table 1. Demographic information for all participants can be found in Table 2. The present study was conducted according to guidelines laid down in the Declaration of Helsinki with written informed consent obtained from a parent or guardian for each child before any assessment or data collection. All procedures involving human subjects in this study were approved by the institutional review board at the University of Minnesota, Twin Cities.
FIGURE 1.
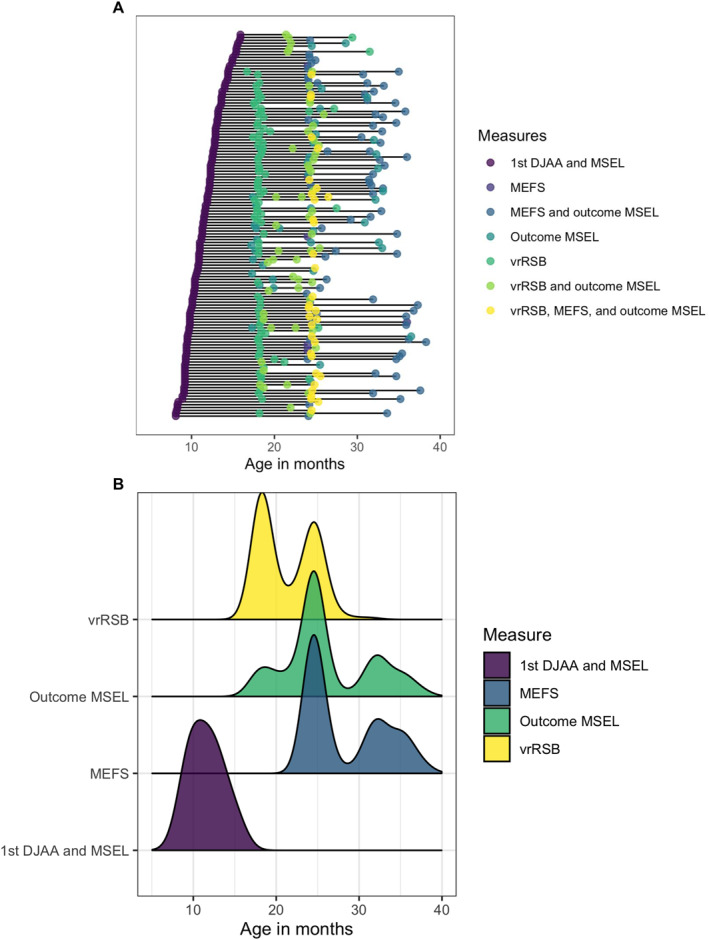
Ages at which participants completed various measures. (a) Each row represents a participant. Each data point is a visit, which may include multiple assessments (e.g., vrRSB and outcome MSEL collected at the same visit/age). (b) Age distributions for each measure. DJAA, Dimensional Joint Attention Assessment; MSEL, Mullen Scales of Early Learning; MEFS, Minnesota Executive Function Scale; vrRSB, Video‐Reference Rating of Reciprocal Social Behavior
TABLE 1.
Descriptive statistics for all measures by time point
| Early measures (8–15m) | Outcome measures (16–38 m) | |||||
|---|---|---|---|---|---|---|
| Measures and scales | N (%F) | Mean (SD) | Range | N (%F) | Mean (SD) | Range |
| DJAA |
|
|
||||
| Number of visits | ||||||
| Age at 1st visit |
|
8.1–15.9 | ||||
| Raw scores | ||||||
| Mean score |
|
0.125–4 | ||||
| Age‐conditioned mean score at 1st visit |
|
−1.98 to 1.98 | ||||
| MSEL |
|
|
||||
| Number of visits | 200 |
|
1–4 | |||
| Age at visit |
|
8.1–15.9 |
|
17.2–38.3 | ||
| Expressive Lang |
|
21–76 |
|
28–80 | ||
| Receptive Lang |
|
24–80 |
|
24–80 | ||
| Fine motor |
|
20–80 |
|
20–80 | ||
| Visual Reception |
|
25–80 |
|
28–80 | ||
| Verbal |
|
58–160 |
|
58–165 | ||
| Nonverbal |
|
66–162 |
|
66–163 | ||
| ELC |
|
63–138 |
|
64–154 | ||
| vrRSB |
|
|||||
| Number of visits | 170 |
|
1–3 | |||
| Age at visit |
|
16.7–31.5 | ||||
| RSB total score |
|
4–41 | ||||
| MEFS |
|
|||||
| Number of visits | 114 |
|
1–2 | |||
| Age at visit |
|
24–38.3 | ||||
| Total score |
|
0–55 | ||||
| Standard score |
|
72–118 | ||||
| National %ile for age |
|
3–88 | ||||
Abbreviations: DJAA, Dimensional Joint Attention Assessment; ELC, Early Learning Composite; F, female; MEFS, Minnesota Executive Function Scale; MSEL, Mullen Scales of Early Learning; N, number sampled; SD, standard deviation; vrRSB, the Video‐Reference Rating of Reciprocal Social Behavior.
TABLE 2.
Demographic information for sample
| Completed DJAA (n = 210) | In Outcome Models (n = 123) | |||
|---|---|---|---|---|
| Demographic measure | N | Percentage | N | Percentage |
| Maternal education | ||||
| Some high school | 1 | 0.5 | 1 | 0.8 |
| High school | 2 | 1.0 | 1 | 0.8 |
| Some college/2‐year degree | 16 | 7.6 | 12 | 9.8 |
| College degree | 80 | 38.1 | 41 | 33.3 |
| Some graduate school | 16 | 7.6 | 9 | 7.3 |
| Graduate degree | 93 | 44.3 | 57 | 46.3 |
| Not reported | 2 | 1.0 | 2 | 1.6 |
| Family income | ||||
| <25k | 1 | 0.5 | 1 | 0.8 |
| 25–35K | 5 | 2.4 | 3 | 2.4 |
| 35–50K | 10 | 4.8 | 4 | 3.3 |
| 50–75K | 34 | 16.2 | 24 | 19.5 |
| 75–100K | 52 | 24.8 | 31 | 25.2 |
| 100–150K | 59 | 28.1 | 31 | 25.2 |
| 150–200K | 23 | 11.0 | 15 | 12.2 |
| Over 200K | 25 | 11.9 | 13 | 10.6 |
| Not reported | 1 | 0.5 | 1 | 0.8 |
| Ethnicity | ||||
| Hispanic | 13 | 6.2 | 11 | 8.9 |
| Non‐Hispanic | 194 | 92.4 | 111 | 90.2 |
| Not reported | 3 | 1.4 | 1 | 0.8 |
| Race | ||||
| Asian | 4 | 1.9 | 0 | ‐ |
| Black/African American | 1 | 0.5 | 1 | 0.8 |
| Multi‐racial | 31 | 14.8 | 21 | 17.1 |
| White | 174 | 82.9 | 101 | 82.1 |
2.2. Measures
The Dimensional Joint Attention Assessment (DJAA; Elison et al., 2013) is a naturalistic, play‐based interaction to assess the developmental sophistication of an infant's responding to joint attention (RJA) performance. The assessment takes place between an administrator and an infant participant. It involves four series of four presses, which involve progressively more redundant experimenter bids for joint attention with the infant (see Figure 2). The DJAA does not require specific toys or stimuli to administer, and any kinds of visual stimuli (e.g., a picture of a dog or house) or toys (e.g., blocks, a ball, and a toy car) may be used. Additionally, toys or other stimuli can be reused within and between the four series of presses. While infant IJA can be observed during a DJAA administration, it is not the focal target of the assessment. In this study, the DJAA was administered as further described in Elison et al. (2013) without modifications.
FIGURE 2.

A typical sequence of DJAA bids, all preceded by gaining an infant's visual attention. (a) First bid includes gaze and head turn. (b) Second bid includes gaze, head turn, and verbal cue. (c) Third cue includes gaze, head turn, and point. (d) Fourth bid includes gaze, head turn, point, and verbal cue. Sequence in administered from (a) to (d)
Scores from the four series are averaged to obtain a mean DJAA score, ranging from 0 (indicated that an infant did not demonstrate any RJA behavior across any of the 4 series of examiner‐initiated interaction series) to 4 (indicating that an infant responded to the most sophisticated, least obvious examiner‐initiated interaction in each of the four series of RJA interactions). Administrators may score “in‐vivo” during an administration (i.e., noting scores to each series as they are obtained) or post‐administration using recordings of the administration. This strategy is only recommended if recordings have adequate capture of infant and administrator movements and looking (e.g., can see infants turn their head or otherwise shift gaze). The mean DJAA score has previously been found to have an inter‐rater reliability ICC of 0.98 (Stallworthy, Lasch, et al., 2022), including in‐vivo versus post‐administration scoring. While administered in a lab setting at all visits in this study, the DJAA has also been successfully administered in medical clinics at well‐child visits (Reilly et al., 2021), highlighting its adaptability and naturalistic format. Additionally, the DJAA is appropriate for both typically developing populations and those who may show delayed or atypical RJA, such as infants at elevated familial likelihood for ASD due to older siblings with the diagnosis (Stallworthy, Berry, et al., 2022, Stallworthy, Lasch, et al., 2022), and infants born premature (Stallworthy, Sifre, et al., 2022). DJAA can be administered as young as 8 months and as late at 18 months.
The Mullen Scales of Early Learning (MSEL; Mullen, 1995) is a standardized developmental assessment validated for children from birth to 68 months of age. Prior to 25 months, it provides standardized T‐scores in five subscales: Gross Motor, Fine Motor, Visual Reception, Receptive Language, and Expressive Language. The latter four scales can be summed into an Early Learning Composite (ELC), which reflects the overall developmental level. A Nonverbal score is composed of Fine Motor and Visual Reception scores, and a Verbal score is composed of Expressive and Receptive Language scores. Descriptive statistics of summary scale standard scores and subscale t‐scores in both the “early” period (concurrent w/DJAA assessments) and “outcome” period are presented in Table 1.
The Video‐Reference Rating of Reciprocal Social Behavior (vrRSB; Marrus et al., 2015, 2020) is a 48‐item parent‐report questionnaire for assessing social responsiveness and has been validated in 18‐ to 30‐month‐olds (Doyle et al., 2021; Hawks et al., 2018; Lasch et al., 2020; Marrus et al., 2015, 2020). It includes 13 items that ask parents to directly compare their child's behavior to that of a 19‐month‐old exemplar in a short video. All other questions are designed to capture typical variability in social behaviors and social responsiveness in early toddlerhood. A summary score is derived from all 48 items with a higher score indicating greater impairment in social responsiveness. The questionnaire takes about 15 min for parents to complete.
The vrRSB was designed as a downward extension of the Social Responsiveness Scale, 2nd edition (SRS‐2; Constantino & Todd, 2003), a widely used measure of social responsiveness in ASD research that also shows dimensional variations in the general population. Previous work has demonstrated a significant correlation between vrRSB's total score at 18 months and Social Responsiveness Scale's total score at 36 months (Marrus et al., 2015, 2020). To support the goal of establishing associations between RJA and developmental outcomes in typically developing samples and lay the groundwork for future efforts focused on the risk in early development, the vrRSB was selected due to its compatibility with the SRS.
The Minnesota Executive Function Scale (MEFS; Carlson & Zelazo, 2014) is a tablet‐based version of the Dimensional Change Card Sort (DCCS; Zelazo, 2006) with graduated levels of difficulty to extend its developmental sensitivity. Children must flexibly use rules to guide their decision‐making on a trial‐by‐trial basis, following increasingly complex rules. The MEFS engages working memory, inhibition, and cognitive flexibility (Perone, et al., 2018), three major facets theorized to make up the executive function (EF). It is standardized from 2 years to adulthood (Carlson, 2021) with initial difficulty based on age and adjustments based on performance. It takes about four minutes to complete on average. The MEFS has been normed in the United States on over 51,000 children with a test‐retest reliability of 0.86 (Carlson, 2021). MEFS administration was attempted in 108 participants at 156 total visits. Participants failed to complete the measure due to behavioral issues at 19 visits. Technology issues led to failure at 23 visits, leaving useable data from 87 participants and 114 visits. Descriptive scores are presented in Table 1.
Demographic variables. Maternal educational attainment and family income bracket were self‐reported at the time of enrollment. Descriptive statistics for all reporting participants can be found in Table 2. Because of their low representation within the sample, income groups from “<$25k” to “$50–75k” were collapsed into one “<$75k” group when examining the effect of family income. For the purposes of analysis, maternal education was recoded into two binary variables, “College” and “Graduate,” indicating a mother who had earned a college degree (91% of sample) or graduate degree (45% of sample), respectively. When the effects of maternal education were included in a model, both dichotomous variables were included as predictors. However, in situations where only one variable contributed significantly to the model, the other was removed. Thus, models presented in results may include the effects of only College or Graduate and still include the effects of maternal education.
2.3. Analytic strategy
As a preliminary step, we assessed the direction and strength of zero‐order correlations between our outcomes of interest, DJAA performance, and demographic variables of maternal education and family income.
Conditioning DJAA mean scores on age. As presented in Supplementary Information, and consistent with previous reports (Elison et al., 2013; Reilly et al., 2021; Stallworthy, Lasch, et al., 2022), age was significantly correlated with DJAA mean score, r(422) = .57, p < .001. To assess associations between the developmental sophistication of RJA performance and later developmental abilities more precisely, we conditioned DJAA scores on participants' age at the time of DJAA assessment. That is to say, when considering the DJAA score as a predictor in later regressions, we used individual participants' residual values from regressions with age at DJAA predicting DJAA mean score as the outcome, rather than the raw DJAA mean score. Using residuals as age‐conditioned scores in place of raw scores allows for a consideration of the DJAA scores in the context of a participant's age at the time of that DJAA assessment. This better accounts for the fact that, for example, a 9‐month‐old earning a DJAA mean score of 1 is far more “typical” or “average” for that age and developmental stage than a 14‐month‐old earning that same mean DJAA score. Additionally, it is a more parsimonious method of accounting for participant age at DJAA assessment in later regressions predicting outcomes (compared to the alternative of a main effect of DJAA scores, a main effect of age at DJAA assessment, and an interaction between the two variables).
While previous studies with the DJAA have suggested a quadratic effect of age on DJAA mean scores (Stallworthy, Lasch, et al., 2022), a single linear effect of age fit our data best and was thus used to calculate the visit‐level age‐conditioned DJAA scores. These age‐conditioned DJAA scores were used in all regressions predicting later developmental abilities. Additionally, while many participants completed the DJAA at multiple visits, and no practice effect was found, we chose to use only participants' age‐conditioned DJAA scores at their earliest visit as predictors, in hopes of capturing the increased variance in RJA abilities at a developmental point when RJA is emerging, and the most interindividual variation is to be expected. Prior work has also suggested that RJA measured earlier in development is more strongly associated with various outcomes, such as language (Morales et al., 2000). Additionally, we sought to demonstrate whether a single “snapshot” DJAA score would be associated with later outcomes to better approximate the role DJAA, or similar assessments could play in screening for RJA delays in clinical settings in the future (e.g., Reilly et al., 2021). Analyses completed by averaging age conditioned scores across all DJAA visits for each participant are presented in the Supplementary Information and show strikingly similar effect sizes and directions as analyses with scores from each participant's first visit presented here. Although participant sex was also associated with the DJAA mean score (see Supplementary Information), we did not condition DJAA scores on sex in the same way as we wished to examine the main effect of sex on later developmental outcomes as well as interactions.
Associations between RJA and developmental outcomes. Regressions were carried out to examine associations between RJA scores at 8–15 months (conditioned on age at assessment) and later developmental outcomes of language ability, social responsiveness, and executive function at ages ranging from 16 to 38 months. Because many participants had multiple data points for most outcome measures, multilevel regressions were computed using the lme4 R package (Bates et al., 2015) to account for interindividual variance and intra‐individual continuity in these outcome variables. Multiple models were fit for each outcome variable with Akaike Information Criterion (AIC; Akaike, 1973) and AIC with correction for finite sample sizes (AICc) used to assess comparative fit of models and account for different sample sizes across models (Burnham & Anderson, 2002). AICc is a numerical value that estimates the quality of a given statistical model (relative to other models) and is used to assist in model selection.
RJA and verbal development. We aimed to demonstrate generalizability of previous findings reporting associations between RJA in the first 2 years of life and later performance on verbal tasks (e.g., Brooks & Meltzoff, 2008; Mundy et al., 2007). Verbal ability at time of DJAA assessment and participant sex were both examined as possible variables to help account for variance in verbal ability at 18–38 months and to better isolate the association between DJAA performance and later verbal outcomes. Additionally, regressions also examined nonverbal development (i.e., MSEL Nonverbal standard scores) as the dependent variable to examine RJA abilities' unique association with verbal and language development rather than simply cognitive development in general. These analyses are presented in Supplementary Information.
RJA and social responsiveness . Associations between DJAA performance at 8–15 months and vrRSB scores at 16–32 months were examined via multilevel regression. Preliminary analyses suggested that a linear effect of age, rather than a quadratic or exponential effect, best fit the data. As Marrus et al. (2015) previously found significant associations between vrRSB scores and both verbal development and sex, both were examined as possible covariates.
RJA and executive function. Considering mixed results of previous studies investigating possible associations between early RJA skills and later‐emerging executive function abilities, exploratory regressions were carried out with MEFS total scores from 24 to 38 months as the dependent variable and DJAA performance and participant sex among other possible predictors. The total score was used as an outcome variable rather than the standard score to examine developmental trends in executive function development within this sample. Analyses with standard score as an outcome are presented in Supplementary information.
3. RESULTS
3.1. Preliminary analyses
Zero‐order correlations between outcome measures at various time points were examined to assess the basic statistical associations between all measures. These correlations are presented in Supplemental Tables 2 and 3. Contrary to our hypotheses, DJAA scores were correlated with MSEL Nonverbal scores at 17–38 months, r(198) = 0.25, p < .001. Correlations between DJAA scores and the two subscales of the Nonverbal Standard Score (Visual Reception and Fine Motor) were examined to better elucidate the findings of this regression. Full analyses are presented in Supplementary Information (Tables S2 and S3).
3.2. Associations between joint attention and developmental outcomes
Multilevel regressions were carried out to examine associations between age‐conditioned DJAA mean scores at 8–15 months and later developmental abilities. Demographic variables of maternal education and family income were also included as covariates where appropriate (i.e., when they showed statistically significant initial correlations with outcome variables).
Joint attention and verbal development. Regressions revealed associations between age‐conditioned DJAA scores and later verbal development (as measured by the MSEL Verbal scale) between 17 and 38 months. Various models assessed also included main and interaction effects of participant sex and MSEL Verbal level concurrent with DJAA assessment. Participants' verbal abilities in toddlerhood were associated with maternal college degree (r(206) = 0.178, p = .010) and graduate degree attainment (r(206) = 0.219, p = .001), so both were assessed as possible covariates. Full models are presented in Table 3.
TABLE 3.
Models examining associations between age‐conditioned Dimensional Joint Attention (DJAA) scores at 8–15 m and later verbal development
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 | Model 8 | Model 9 | |
|---|---|---|---|---|---|---|---|---|---|
| (Intercept) |
|
|
|
|
|
|
|
|
|
| Age |
|
|
|
|
|
|
|
|
|
| Early MSEL verbal score |
|
|
|
|
|
|
|
|
|
| Age‐conditioned DJAA score |
|
|
|
|
|
||||
| Sex |
|
|
|
|
|
|
|||
| Age x sex |
|
|
|
|
|||||
| Maternal graduate degree |
|
|
|||||||
| Early MSEL verbal x maternal graduate degree |
|
||||||||
| AICc | 1706.220 | 1688.040 | 1679.120 | 1689.570 | 1679.980 | 1680.900 | 1671.340 | 1649.88 | 1650.27 |
| R 2 | 0.654 | 0.647 | 0.646 | 0.646 | 0.656 | 0.645 | 0.657 | 0.659 | 0.665 |
| Log likelihood | −849.01 | −838.87 | −833.34 | −838.57 | −832.70 | −833.16 | −827.29 | −815.46 | −814.55 |
| Num. obs. | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 198 | 198 |
| Num. groups: Participant | 123 | 123 | 123 | 123 | 123 | 123 | 123 | 121 | 121 |
| Var: Participant (intercept) | 233.34 | 182.14 | 158.21 | 180.55 | 166.93 | 157.03 | 145.73 | 130.96 | 130.02 |
| Var: Residual | 123.480 | 124.850 | 125.3400 | 124.9900 | 119.5300 | 125.5900 | 119.680 | 120.98 | 119.72 |
Abbreviation: MSEL, Mullen Scales of Early Learning.
†p < .10, * = p < 05; ** = p < .01; *** = p < .001;
In the best‐fitting model, Model 8, age‐conditioned DJAA mean scores were associated with later MSEL Verbal scores, t(118) = 3.58, b = 5.59, = 0.251, p < .001. Age at outcome MSEL, participant sex, maternal graduate education, and early verbal ability also helped explain variance. Figure 3 illustrates the association between DJAA mean scores and MSEL Verbal scores, after controlling for these other variables. As seen in Table 3, this model accounted for 65.9% of variance in MSEL Verbal scores.
FIGURE 3.
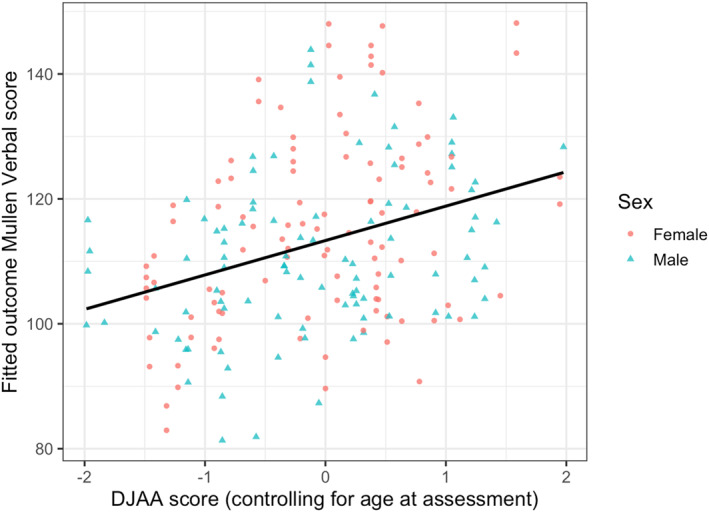
Dimensional Joint Attention Assessment (DJAA) score at 8–15 months is associated with later verbal abilities as measured by the MSEL (Mullen Scales of Early Learning). Y‐axis values are derived from fitted MSEL scores in a multilevel model controlling for sex, age at outcome assessment, maternal graduate education, and verbal ability at DJAA assessment and are utilized to better illustrate the DJAA‐MSEL association
Joint attention and social responsiveness. A series of regressions revealed the associations between vrRSB scores at 16–31 months and participant sex, age, and age‐conditioned DJAA scores and MSEL verbal performance at 8–15 months. vrRSB scores were also associated with both family income (r(166) = −0.186, p = .016) and maternal graduate degree (r(167) = −0.156, p = .043), so both were explored as possible predictors. As seen in Table 4, a model with the main effect of DJAA mean score, age at vrRSB, family income, and maternal graduate degree (Model 8) had the best fit based on Akaike Information Criteria‐corrected (AICc). In this model, the DJAA score was associated with social responsiveness, b = −1.98, = −0.183, p = .002 as shown in Figure 4. Intra‐class correlation (ICC) for vrRSB scores ranged from 0.338 to 0.447, suggesting that a considerable portion of variance in social responsiveness was explained at the participant level across this developmental period.
TABLE 4.
Models examining associations between age‐conditioned Dimensional Joint Attention (DJAA) scores at 8–15 m and later social responsiveness at 16–31m
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 | Model 8 | |
|---|---|---|---|---|---|---|---|---|
| (Intercept) |
|
|
|
|
|
|
|
|
| Age at vrRSB |
|
|
|
|
|
|
|
|
| Age‐conditioned DJAA score |
|
|
|
|
|
|
||
| Early MSEL verbal score |
|
|
|
|||||
| Age at vrRSB x early MSEL verbal |
|
|||||||
| Age at vrRSB x DJAA score |
|
|||||||
| Family income |
|
|
||||||
| Maternal college degree |
|
|||||||
| Maternal graduate degree |
|
|
||||||
| AICc | 1125.11 0 | 1115.29 0 | 1123.18 0 | 1115.09 0 | 1114.260 | 1101.10 | 1109.50 | 1094.29 |
| R 2 | 0.479 | 0.482 | 0.472 | 0.474 | 0.504 | 0.486 | 0.493 | 0.493 |
| Log likelihood | −558.43 | −552.46 | −556.41 | −551.29 | −548.68 | −544.29 | −547.40 | −539.79 |
| Num. obs. | 170 | 170 | 170 | 170 | 170 | 168 | 169 | 167 |
| Num. groups: Participant | 102 | 102 | 102 | 102 | 102 | 101 | 101 | 100 |
| Var: Participant (intercept) | 21.27 | 17.35 | 19.43 | 16.22 | 16.69 | 16.41 | 16.73 | 15.93 |
| Var: Residual | 24.80 0 | 24.68 0 | 25.05 0 | 24.93 0 | 23.55 0 | 24.59 | 24.34 | 24.39 |
Abbreviations: MSEL, Mullen Scales of Early Learning; vrRSB, Video‐Reference Rating of Reciprocal Social Behavior.
†p < .10, *p < 05; **p < .01; ***p < .001.
FIGURE 4.
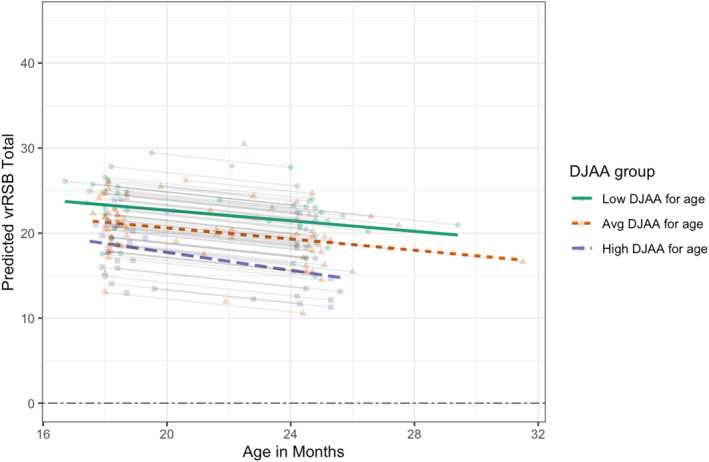
Age‐conditioned Dimensional Joint Attention Assessment (DJAA) mean scores are associated with score on the Video‐Reference Rating of Reciprocal Social Behavior (vrRSB) at 16–31 months, after accounting for participant age, family income, and maternal graduate degree attainment
Joint attention and executive function. As illustrated in Table S3, a raw DJAA mean score was not associated with the MEFS total score (r(112) = −0.105, p = .27). This association was still not significant after conditioning the DJAA score on age at visit (r(112) = 0.13, p = .16). However, family income was significantly associated with the MEFS total score (r(111) = 0.192, p = .042). Exploratory regressions were carried out to further examine relationships between DJAA scores, family income, MEFS performance, and possible covariates of these relationships. Models are presented in Table 5. As seen in Model 6, DJAA score, age at MEFS, and family income all independently helped explain variations in MEFS total scores. Model 7, the best fitting model based on AICc values, included an interaction between age at MEFS assessment and family income, b = 0.256, = 1.27, p = .013, such that children from higher income families saw faster increases in executive function ability across the age period sampled (seen in Figure 5). When this interaction effect was included in Model 7, the DJAA score was no longer a significant predictor in the model.
TABLE 5.
Models predicting Minnesota Executive Function Scales (MEFS) total score at 24–38 months of age
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 | |
|---|---|---|---|---|---|---|---|
| (Intercept) |
|
|
|
|
|
|
|
| Age at MEFS |
|
|
|
|
|
|
|
| Early MSEL verbal score |
|
|
|||||
| Age‐conditioned DJAA score |
|
|
|
|
|
||
| Age at MEFS x DJAA score |
|
||||||
| Family income |
|
|
|||||
| Age at MEFS x Family income |
|
||||||
| AICc | 783.7500 | 784.3200 | 781.830 | 782.0900 | 782.8200 | 772.04 | 767.97 |
| R 2 | 0.591 | 0.593 | 0.591 | 0.579 | 0.593 | 0.567 | 0.608 |
| Log likelihood | −387.69 | −386.88 | −385.64 | −384.65 | −385.02 | −379.62 | −376.45 |
| Num. obs. | 114 | 114 | 114 | 114 | 114 | 113 | 113 |
| Num. groups: Participant | 87 | 87 | 87 | 87 | 87 | 86 | 86 |
| Var: Participant (intercept) | 19.20 | 18.54 | 17.16 | 15.11 | 16.71 | 13.53 | 14.78 |
| Var: Residual | 35.140 | 34.970 | 35.020 | 35.910 | 34.870 | 35.86 | 32.21 |
Abbreviations: DJAA, Dimensional Joint Attention Assessment; MSEL, Mullen Scales of Early Learning.
†p < .10, *p < 05; **p < .01; ***p < .001.
FIGURE 5.
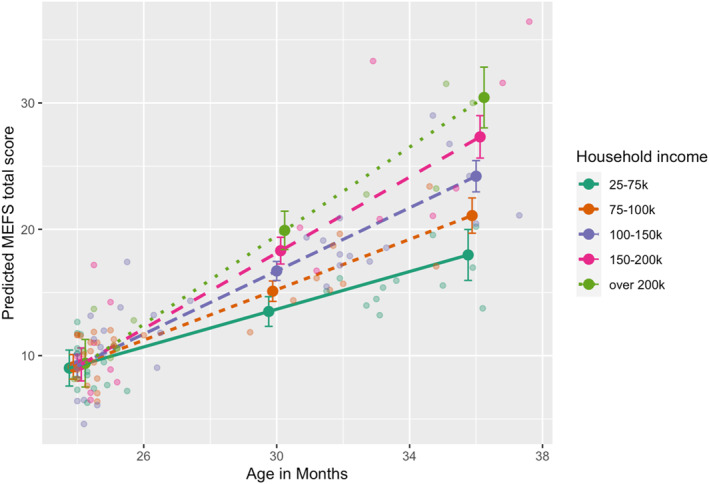
Family income is increasingly associated with Minnesota Executive Function Scales (MEFS) total score from 24 to 38 months
4. DISCUSSION
The current study aimed to characterize associations between RJA at 8–15 months and critical developmental abilities at 16–38 months and thus expand a body of evidence supporting the construct validity of RJA conceptualized and operationalized as a skill that can vary in developmental sophistication. Due to the wide age range at which RJA behaviors were assessed, RJA abilities were considered based on age at assessment with scores conditioned accordingly. Age‐conditioned DJAA scores were then a measure of how advanced or developmentally sophisticated a child's RJA skills were in comparison to other same‐aged infants.
4.1. Joint attention and later verbal ability
RJA abilities were strongly associated with verbal ability (as measured by the MSEL) from 17 to 38 months, even after controlling for participant sex, verbal ability at RJA measurement, and maternal graduate degree attainment. This aligns with previous findings of associations between joint attention in infancy and language measures in toddlerhood (e.g., Bottema‐Beutel, 2016). They also support the theory that joint attention is an important opportunity for infants to increase their vocabulary and language skills through label mapping and proto conversations related to objects of interest in a triadic engagement. Additionally, these associations between DJAA performance and later language abilities serve as additional content validation of the DJAA as a dimensional measure of RJA ability.
It is important to also consider the other covariates that accounted for variance in verbal ability in toddlerhood. Verbal abilities showed continuity from infancy (assessment at RJA time point) and toddlerhood. Consistent with previous literature, females showed higher levels of verbal ability in toddlerhood, though this effect also decreased with increasing age, consistent with a pattern of males achieving equal language abilities by preschool age. It is unclear how sex and its interaction of age may have changed in this model if we had measured language abilities into a higher age range (e.g., 4 or 5 years old). Higher maternal education (attainment of a graduate degree) was also predictive of greater verbal ability in toddlerhood.
Contrary to our expectations, DJAA scores were associated with later nonverbal abilities as measured by the MSEL. While this association is explored further in Supplementary Information, it is worth examining possible reasons for this finding. Theoretically, this association may implicate the high level of gaze following and triadic engagement required for more advanced items in the MSEL Visual Reception subscale (e.g., following examiner instructions to point to a single matching shape on a page with multiple stimuli). This would not only support the idea that joint attention is strongly associated with language development, but also that skills beyond language, such as visual orienting precision, are further fostered and developed by infant's continued engagement in joint attention with others even after the first year of life. Additionally, as early MSEL nonverbal scores at the time of DJAA assessment were associated with MSEL nonverbal scores at follow‐up (r(196) = 0.190, p = .007), it may also suggest that infants with more developed nonverbal skills show more advanced RJA and gaze following for their age and continue to have more advanced nonverbal skills into toddlerhood.
4.2. RJA and later social responsiveness
The best‐fitting model predicting vrRSB based on AICc included an association between DJAA and later social responsiveness, after accounting for age at vrRSB assessment, family income, and maternal graduate degree attainment. Associations between early social responsiveness and maternal education are intriguing, as previous work (e.g., Moody et al., 2017) also identified associations between measures of social responsiveness (such as the SRS) and demographic variables, such as maternal education. Further work is required to determine if the effect of maternal education in this sample is suggestive of earlier emergence or more rapid development of social responsiveness in children of more highly educated mothers, or if perhaps this factor is only influential in a relatively early period of development, such as toddlerhood, with children of less educated mothers “catching up” at some point between early childhood and adulthood.
While social responsiveness is generally considered a trait‐like feature in children and adults (Wagner et al., 2019), participant age was a significant predictor of vrRSB scores in our analyses. It is unclear if this is related to “emergence” or “stabilization” of social responsiveness before age three or some other cause. Longitudinal work assessing social responsiveness over a broader developmental period (e.g., early toddlerhood into early school age) may help to further clarify these patterns.
Overall, these results suggest that a trait‐like sociality or social motivation may be both early emerging (i.e., identifiable in the first year of life) and influence or indicate an infant's likelihood of success in engaging in joint attention with others, leading to comparatively better joint attention abilities for an infant's given age. Likewise, it may suggest that infants who are more successful at engaging in joint attention may find such interactions socially rewarding, and be more likely to seek similar interactions throughout infancy and toddlerhood, augmenting the acquisition of social skills, and thus respond more appropriately to social cues and situations in toddlerhood.
Collectively, our findings continue to establish the construct validity of the DJAA by relating DJAA performance to verbal abilities and social responsiveness in toddlerhood. These findings also build upon previous work establishing associations between early brain development and RJA behaviors in the first year of life as measured by the DJAA (Elison et al., 2013). It will be important to establish that these associations between RJA performance and both verbal and social development in this community‐ascertained sample also replicate in other populations with which the DJAA has been successfully used such as premature infants (Stallworthy, Sifre, et al., 2022) and infants at increased familial likelihood of ASD diagnosis (Stallworthy, Berry, et al., 2022; Stallworthy, Lasch, et al., 2022).
4.3. Joint attention and executive function
RJA performance was not correlated with executive function ability at 24–38 months. Based on previous literature, various models examined suggested associations between the MEFS total score and early verbal abilities, RJA scores, and family income with various interactions with age (which is important when using a raw, rather than age‐standardized score). As seen in Model 6, the main effects of both DJAA and family income were both significantly associated with executive function performance. However, Model 7 (the best‐fitting model based on AICc) suggests that age at MEFS moderates the effect of family income, such that participants from higher income brackets showed faster growth in executive function ability from 24 to 38 months. It should also be noted that when the interaction between age at MEFS and family income was included in Model 7, the main effect of DJAA was no longer significant.
The findings within this family of regressions highlight the exploratory nature of these analyses as well as the continued need to better understand associations between early‐developing RJA and later executive function skills, which have been linked to both social and cognitive abilities throughout early childhood and beyond (e.g., Carlson, et al., 2013; Morgan et al., 2019). While we also replicated previous findings of associations between SES (here measured only with family income) and early executive functions (see Lawson et al., 2018 for a meta‐analysis) in a relatively medium‐to‐high‐income sample (median income bracket $75–100k), there is a sufficient theory to suggest great caution when inferring causality between early SES and children's developmental abilities or trajectories. As Nketia et al. (2021) recently reviewed, generalizing the statistical effects of demographics variables such as SES without considering the nature of a sample and the multiple alternative possible interpretations can lead to deficit‐focused conclusions that further marginalize children already more likely to experience various types of adversity in Western societies.
More specifically, our finding that family income is increasingly associated with better performance on a tablet‐based assessment of flexible rule following as children age should not be interpreted as a deficit of executive functions in children from lower income families. With only observational data and no experimental manipulation of income or other SES factors, we also cannot confidently interpret these data to mean that children in especially wealthy early environments develop executive functions at an especially fast rate in early childhood compared to their peers at more typical income levels. As Nketia et al. (2021) specifically note, we cannot clearly compare children across these income groups without employing a normativity bias. If we do so, we fail to account for the varying unique developmental challenges that children in diverse childrearing environments must adapt to, and that we will inevitably fail to capture all forms of adaptation properly with tasks and tools designed without this diversity of experiences in mind.
These exploratory analyses are not offered as a statement on the nature or timing of executive function development, but instead elicit questions about the nature of both joint attention and executive functions early in life, and how timing and precision of measurement influence the associations between these two important abilities. As stated previously, earlier literature has found mixed results regarding relationships between early joint attention and later executive function abilities. In this smaller sample, we failed to provide conclusive evidence to clarify or support previous work. There are many possible contributing reasons for this. We chose to assess executive functions relatively early in toddlerhood. It is possible that had we assessed executive functions starting at 36 or 48 months and into early school age, associations with earlier RJA abilities would have been more conclusive.
However, timing an assessment of executive functions to find a significant association with earlier joint attention is complicated by a question of when exactly we expect executive functions to emerge, consolidate, or be validly measured by state‐of‐the‐art instruments. The skills we consider primary facets of executive functions in adulthood (inhibitory control, working memory, and cognitive flexibility) are not fully developed at 24–38 months, although different facets or predeterminants such as “executive efficiency” may be measured as early as 12 months (e.g., Vaughan Van Hecke et al., 2012).
An additional aspect of our mixed findings was our relatively novel measure of executive function abilities. While the measure of executive function we used (the MEFS) was presented on a tablet and took only a short time to complete, it still required a certain level of experience with tablets, attentional focus, and fine motor skills that were not present in all ages and participants with whom the assessment was attempted. The associations between joint attention (both RJA and IJA) and later executive function are certainly areas for continued study and investigation going forward.
These analyses also did not consider all possible mediators of the association between early joint attention and later executive function. For example, Kuhn et al. (2014) found that language at 2 and 3 years old mediated the association between early production of communicative gestures (such as pointing, a form of IJA) and executive functions across a variety of measures at 4 years of age. In this large, prospective sample, these associations were consistent across families classified as low‐income and not. Both our sample size and the timing of our measures limit our ability to approximate a replication of Kuhn et al. (2014), given we used both a different measure of early communication (RJA vs. forms of IJA) and a novel measure of executive function (the MEFS vs. a battery of tasks).
4.4. Strengths
Overall, a strength of our study was the time span of development covered. Assessing outcomes at multiple time points across more than a year of toddlerhood allowed for an examination of the longitudinal emergence (e.g., executive function) and stability (e.g., social responsiveness) of important developmental abilities of toddlerhood. It also provided the opportunity to examine how age moderated some associations between DJAA and other variables associated with outcomes of interest. Further, our use of a dimensional measure of RJA skill performance offers an opportunity to characterize a broader range of RJA skills in infancy.
Conceptualizing RJA as a skill that can vary in developmental sophistication, as opposed to simply a competence to be acquired, may yield new information about the construct itself, and thus its nomological network of associations. This approach also acknowledges the nonlinear and dynamic nature of RJA development, rather than scoring the behavior in a binary manner. We demonstrated generalizability of one of the most consistent associations with RJA, namely language abilities, and showed new evidence of a developmental association between RJA and reciprocal social behavior, both features of which are implicated in autism spectrum disorder.
4.5. Limitations
Although our data and analyses spanned 2 years of development, some analyses (e.g., those examining executive function outcomes) were limited by smaller sample sizes. However, even if those analyses are taken as preliminary or exploratory, we demonstrated associations between a naturalistic play‐based measure of RJA in infancy and both later developing language and social responsiveness. Continuing investigation of associations between joint attention and later executive function may benefit from increased sample sizes and a broader range of sample ages, aiming to better characterize EF's emergence and “consolidation” into a unitary construct measured by assessments such as the MEFS.
While we have chosen to focus on these three main abilities in early childhood, we also acknowledge that many other factors likely influence their development. For example, we did not include any measures of temperament that may be related either to outcome variables examined here or to joint attention itself. This area of investigation can serve as a future area of study. It may also help to clarify the developmental origins of joint attention abilities and the mechanisms through which they are associated with later developmental skills.
Overall, we are limited by the observational nature of this data, as we cannot make casual claims about the nature of the DJAA's relationships with any of the outcome variables assessed in this study. Further, this sample is skewed toward a higher family income level and is very well‐educated (91% of mothers with college degrees, compared to 43% of millennial women; Fry & Bialik, 2019). This sample is also overly representative of White families (83% of our sample compared to about 50% of children in the United States born in the same time frame (The Annie E. Casey Foundation, 2020)). Further work is needed to assess the validity of the DJAA and its associations with developmental outcomes in a more representative sample.
5. CONCLUSION
This study revealed significant associations between the DJAA, a play‐based assessment of RJA, in 8‐ to 15‐month‐olds and later verbal abilities. This finding serves as a form of additional content and criterion validation for the DJAA. Additionally, we established associations between RJA and later social responsiveness, an important indicator of a child's social development and social understanding in toddlerhood. We failed to find conclusive evidence of associations between DJAA scores measured at 8–15 months and executive function abilities at 24–38 months. Overall, this study provides evidence that RJA skills are an important social‐cognitive skill being practiced throughout infancy rather than simply “accomplished” and are associated with later developmental abilities critical to children's social‐communicative development.
CONFLICT OF INTEREST
CML and JTE has no conflicts to declare. SMC is a Co‐founder and holds equity in Reflection Sciences, Inc., which has licensed the Minnesota Executive Function Scale (MEFS) from the University of Minnesota. These interests have been reviewed and managed by the University of Minnesota in accordance with its Conflict of Interest policies.
Supporting information
Supporting Information S1
ACKNOWLEDGMENTS
This study was supported by a NIMH sponsored Biobehavioral Award for Innovative New Scientists (R01 MH104324) to J. Elison, and in part by the UNC/UMN Baby Connectome Project (U01MH110274). C. Lasch was funded by the National Science Foundation Graduate Research Fellowships Program (NSF‐GRFP) and NIMH T32 predoctoral fellowship (NIMH T32 MH015755). The funders had no role in study design, analysis, data interpretation, or the writing of the report. We thank all the children and families who participated in our studies. Lastly, we are indebted to the ELAB members for their dedication to the broader project, specifically Elayne Vollman, Rachel Roisum, Sharlotte Irwin, and Kristen Gault.
Lasch, C. , Carlson, S. M. , & Elison, J. T. (2023). Responding to joint attention as a developmental catalyst: Longitudinal associations with language and social responsiveness. Infancy, 28(2), 339–366. 10.1111/infa.12515
DATA AVAILABILITY STATEMENT
The majority of the sample utilized in this manuscript (74%) is available as part of the Baby Connectome data via the NDA. The remaining portion of raw deidentified data and analysis scripts are available upon request to the senior author. Authors are in the process of transferring scripts and remaining data to a publicly available repository.
REFERENCES
- Akaike, H. (1973). Information theory and an extension of the maximum likelihood principle. In Petrov B. N. & Csaki F. (Eds.), Second international symposium on information theory (pp. 267–281).
- Baldwin, D. A. (1991). Infants’ contribution to the achievement of joint reference. Child Development, 62(5), 875–890. 10.1111/j.1467-8624.1991.tb01577.x [DOI] [PubMed] [Google Scholar]
- Bates, D. , Mächler, M. , Bolker, B. , & Walker, S. (2015). Fitting linear mixed‐effects models using lme4. Journal of Statistical Software, 67(1), 1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Bottema‐Beutel, K. (2016). Associations between joint attention and language in autism spectrum disorder and typical development: A systematic review and meta‐regression analysis. Autism Research, 9(10), 1021–1035. 10.1002/aur.1624 [DOI] [PubMed] [Google Scholar]
- Brady, N. C. , Fleming, K. , Thiemann‐Bourque, K. , Olswang, L. , Dowden, P. , Saunders, M. D. , & Marquis, J. (2012). Development of the communication complexity scale. American Journal of Speech‐Language Pathology, 21(1), 16–28. 10.1044/1058-0360(2011/10-0099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks, R. , & Meltzoff, A. N. (2008). Infant gaze following and pointing predict accelerated vocabulary growth through two years of age: A longitudinal, growth curve modeling study. Journal of Child Language, 35(1), 207–220. 10.1017/S030500090700829X [DOI] [PubMed] [Google Scholar]
- Brooks, R. , & Meltzoff, A. N. (2015). Connecting the dots from infancy to childhood: A longitudinal study connecting gaze following, language, and explicit theory of mind. Journal of Experimental Child Psychology, 130, 67–78. 10.1016/j.jecp.2014.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune, C. W. , & Woodward, A. L. (2007). Social cognition and social responsiveness in 10‐month‐old infants. Journal of Cognition and Development, 8(2), 133–158. 10.1080/15248370701202331 [DOI] [Google Scholar]
- Burnham, K. P. , & Anderson, D. R. (2002). Model selection and multimodel inference: A practical information‐theoretic approach (2nd ed.). Springer‐Verlag. [Google Scholar]
- Butterworth, G. (1991). The ontogeny and phylogeny of joint visual attention. In Whiten A. (Ed.), Natural theories of mind: Evolution, development and simulation of everyday mindreading (pp. 223–232). Basil Blackwell. [Google Scholar]
- Butterworth, G. , & Jarrett, N. (1991). What minds have in common is space: Spatial mechanisms serving joint visual attention in infancy. British Journal of Developmental Psychology, 9(1), 55–72. 10.1111/j.2044-835x.1991.tb00862.x [DOI] [Google Scholar]
- Carlson, S. M. (2021). Minnesota executive function scale: Technical report. Reflection Sciences, Inc. www.reflectionsciences.com/wp‐content/uploads/MEFS‐Technical‐Report‐July‐2021.pdf [Google Scholar]
- Carlson, S. M. , Mandell, D. J. , & Williams, L. (2004). Executive function and theory of mind: Stability and prediction from ages 2 to 3. Developmental Psychology, 40(6), 1105–1122. 10.1037/0012-1649.40.6.1105 [DOI] [PubMed] [Google Scholar]
- Carlson, S. M. , & Zelazo, P. D. (2014). Minnesota executive function scale – test manual. Reflection Sciences. [Google Scholar]
- Carlson, S. M. , Zelazo, P. D. , & Faja, S. (2013). Executive function. In The Oxford handbook of developmental psychology. In Body and mind (Vol. 1, pp. 706–743). [Google Scholar]
- Carpenter, M. , Nagell, K. , Tomasello, M. , Butterworth, G. , & Moore, C. (1998). Social cognition, joint attention, and communicative competence from 9 to 15 months of age. Monographs of the Society for Research in Child Development, 63(255). 10.4135/9781452286143.n496 [DOI] [PubMed] [Google Scholar]
- Charman, T. , Baron‐Cohen, S. , Swettenham, J. , Baird, G. , Cox, A. , & Drew, A. (2000). Testing joint attention, imitation, and play infancy precursors to language and theory of mind. Cognitive Development, 15(4), 481–498. 10.1016/s0885-2014(01)00037-5 [DOI] [Google Scholar]
- Cholemkery, H. , Mojica, L. , Rohrmann, S. , Gensthaler, A. , & Freitag, C. M. (2014). Can autism spectrum disorders and social anxiety disorders be differentiated by the social responsiveness scale in children and adolescents? Journal of Autism and Developmental Disorders, 44(5), 1168–1182. 10.1007/s10803-013-1979-4 [DOI] [PubMed] [Google Scholar]
- Cleveland, A. , Schug, M. , & Striano, T. (2007). Joint attention and object learning in 5‐and 7‐month‐old infants. Infant and Child Development: An International Journal of Research and Practice, 16(3), 295–306. 10.1002/icd.508 [DOI] [Google Scholar]
- Cleveland, A. , & Striano, T. (2007). The effects of joint attention on object processing in 4‐ and 9‐month‐old infants. Infant Behavior and Development, 30(3), 499–504. 10.1016/j.infbeh.2006.10.009 [DOI] [PubMed] [Google Scholar]
- Constantino, J. N. , & Todd, R. D. (2003). Autistic traits in the general population: A twin study. Archives of General Psychiatry, 60(5), 524–530. 10.1001/archpsyc.60.5.524 [DOI] [PubMed] [Google Scholar]
- Corbetta, M. , Kincade, J. M. , Ollinger, J. M. , McAvoy, M. P. , & Shulman, G. L. (2000). Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature Neuroscience, 3(3), 292–297. 10.1038/73009 [DOI] [PubMed] [Google Scholar]
- Corbetta, M. , Patel, G. , & Shulman, G. L. (2008). The reorienting system of the human brain: From environment to theory of mind. Neuron, 58(3), 306–324. 10.1016/j.neuron.2008.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson, G. , Toth, K. , Abbott, R. , Osterling, J. , Munson, J. , Estes, A. , & Liaw, J. (2004). Early social attention impairments in autism: Social orienting, joint attention, and attention to distress. Developmental Psychology, 40(2), 271–283. 10.1037/0012-1649.40.2.271 [DOI] [PubMed] [Google Scholar]
- Downar, J. , Crawley, A. P. , Mikulis, D. J. , & Davis, K. D. (2001). The effect of task relevance on the cortical response to changes in visual and auditory stimuli: An event‐related fMRI study. NeuroImage, 14(6), 1256–1267. 10.1006/nimg.2001.0946 [DOI] [PubMed] [Google Scholar]
- Doyle, C. M. , Lasch, C. , Vollman, E. P. , Desjardins, C. D. , Helwig, N. E. , Jacob, S. , Wolff, J. J. , & Elison, J. T. (2021). Phenoscreening: A developmental approach to research domain criteria‐motivated sampling. The Journal of Child Psychology and Psychiatry and Allied Disciplines, 62(7), 884–894. 10.1111/jcpp.13341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggebrecht, A. T. , Elison, J. T. , Feczko, E. , Todorove, A. , Wolff, J. J. , Kandala, S. , Adams, C. M. , Snyder, A. Z. , Lewis, J. D. , Estes, A. M. , Zwaigenbaum, L. , Botteron, K. N. , McKinstry, R. C. , Constantino, J. N. , Evans, A. , Hazlett, H. C. , Dager, S. , Paterson, S. J. , Schultz, R. T. , & Pruett, J. R., Jr. (2017). Joint attention and brain functional connectivity in infants and toddlers. Cerebral Cortex, 27(3), 1709–1720. 10.1093/cercor/bhw403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elison, J. T. , Wolff, J. J. , Heimer, D. C. , Paterson, S. J. , Gu, H. , Hazlett, H. C. , Styner, M. , Gerig, G. , & Piven, J. , & for the IBIS Network . (2013). Frontolimbic neural circuitry at 6 months predicts individual differences in joint attention at 9 months. Developmental Science, 16(2), 186–197. 10.1111/desc.12015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel de Abreu, P. M. , Abreau, N. , Nikaedo, C. C. , Puglisi, M. L. , Tourinho, C. J. , Miranda, M. C. , Befi‐Lopes, D. M. , Bueno, O. F. A. , & Martin, R. (2014). Executive functioning and reading achievement in school: A study of Brazilian children assessed by their teachers as ‘poor readers. Frontiers in Psychology, 5, 550. 10.3389/fpsyg.2014.00550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry, R. , & Bialik, K. (2019). Millennial life: How young adulthood today compares with prior generations. Pew Research. https://www.pewresearch.org/social‐trends/2019/02/14/millennial‐life‐how‐young‐adulthood‐today‐compares‐with‐prior‐generations‐2/ [Google Scholar]
- Frye, D. , Zelazo, P. D. , & Palfai, T. (1995). Theory of mind and rule‐based reasoning. Cognitive Development, 10(4), 483–527. 10.1016/0885-2014(95)90024-1 [DOI] [Google Scholar]
- Gabouer, A. , & Bortfeld, H. (2021). Revisiting how we operationalize joint attention. Infant Behavior and Development, 63(April), 101566. 10.1016/j.infbeh.2021.101566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gago Galvagno, L. G. , De Grandis, M. C. , Clerici, G. D. , Mustaca, A. E. , Miller, S. E. , & Elgier, A. M. (2019). Regulation during the second year: Executive function and emotion regulation links to joint attention, temperament, and social vulnerability in a Latin American sample. Frontiers in Psychology, 10(JULY), 1–13. 10.3389/fpsyg.2019.01473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gredebäck, G. , Fikke, L. , & Melinder, A. (2010). The development of joint visual attention: A longitudinal study of gaze following during interactions with mothers and strangers. Developmental Science, 13(6), 839–848. 10.1111/j.1467-7687.2009.00945.x [DOI] [PubMed] [Google Scholar]
- Grzadzinski, R. , Di Martino, A. , Brady, E. , Mairena, M. A. , O’Neale, M. , Petkova, E. , Lord, C. , & Castellanos, F. X. (2011). Examining autistic traits in children with ADHD: Does the autism spectrum extend to ADHD? Journal of Autism and Developmental Disorders, 41(9), 1178–1191. 10.1007/s10803-010-1135-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawks, Z. W. , Marrus, N. , Glowinski, A. L. , & Constantino, J. N. (2018). Early origins of autism comorbidity: Neuropsychiatric traits correlated in childhood are independent in infancy. Journal of Abnormal Child Psychology, 1(2), 369–379. 10.1007/s10802-018-0410-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell, B. R. , Styner, M. A. , Gao, W. , Yap, P. T. , Wang, L. , Baluyot, K. , Yacoub, E. , Chen, G. , Potts, T. , Salzwedel, A. , Li, G. , Gilmore, J. H. , Piven, J. , Smith, J. K. , Shen, D. , Ugurbil, K. , Zhu, H. , Lin, W. , & Elison, J. T. (2019). The UNC/UMN Baby Connectome Project (BCP): An overview of the study design and protocol development. NeuroImage, 185, 891–905. 10.1016/j.neuroimage.2018.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalbrzikowski, M. , Krasileva, K. E. , Marvin, S. , Zinberg, J. , Andaya, A. , Bachman, P. , Cannon, T. D. , & Bearden, C. E. (2013). Reciprocal social behavior in youths with psychotic illness and those at clinical high risk. Development and Psychopathology, 25(4pt1), 1187–1197. 10.1017/s095457941300045x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn, L. J. , Willoughby, M. T. , Wilbourn, M. P. , Vernon‐Feagans, L. , & Blair, C. B. , & Family Life Project Key Investigators . (2014). Early communicative gestures prospectively predict language development and executive function in early childhood. Child Development, 85(5), 1898–1914. https://doi‐org.ezp3.lib.umn.edu/10.1111/cdev.12249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasch, C. , Wolff, J. J. , & Elison, J. T. (2020). Examining criterion‐oriented validity of the repetitive behavior scales for early childhood (RBS‐EC) and the video‐referenced rating of reciprocal social behavior (vrRSB). Development and Psychopathology, 32(3), 779–789. 10.1017/s0954579419001159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson, G. M. , Hook, C. J. , & Farah, M. J. (2018). A meta‐analysis of the relationship between socioeconomic status and executive function performance among children. Developmental Science, 21(2), e12529. 10.1111/desc.12529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrus, N. , Glowinski, A. L. , Jacob, T. , Klin, A. , Jones, W. , Drain, C. E. , Holzhauer, K. E. , Hariprasad, V. , Fitzgerald, R. T. , Mortenson, E. L. , Sant, S. M. , Cole, L. , Siegel, S. A. , Zhang, Y. , Agrawal, A. , Heath, A. C. , & Constantino, J. N. (2015). Rapid video‐referenced ratings of reciprocal social behavior in toddlers: A twin study. The Journal of Child Psychology and Psychiatry and Allied Disciplines, 56(12), 1338–1346. 10.1111/jcpp.12391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrus, N. , Grant, J. D. , Harris‐Olenak, B. , Albright, J. , Bolster, D. , Haber, J. R. , Jacob, T. , Zhang, Y. , Heath, A. C. , Agrawal, A. , Constantino, J. N. , Elison, J. T. , & Glowinski, A. L. (2020). Genetic architecture of reciprocal social behavior in toddlers: Implications for heterogeneity in the early origins of autism spectrum disorder. Development and Psychopathology, 32(4), 1190–1205. 10.1017/S0954579420000723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel, M. M. , Pan, P. M. , Hoffmann, M. S. , Gadelha, A. , do Rosário, M. C. , Mari, J. J. , Manfro, G. G. , Miguel, E. C. , Paus, T. , Bressan, R. A. , Rohde, L. A. , & Salum, G. A. (2017). A general psychopathology factor (P factor) in children: Structural model analysis and external validation through familial risk and child global executive function. Journal of Abnormal Psychology, 126(1), 137–148. 10.1037/abn0000205 [DOI] [PubMed] [Google Scholar]
- Miller, S. E. , & Marcovitch, S. (2015). Examining executive function in the second year of life: Coherence, stability, and relations to joint attention and language. Developmental Psychology, 51(1), 101–114. 10.1037/a0038359 [DOI] [PubMed] [Google Scholar]
- Moody, E. J. , Reyes, N. , Ledbetter, C. , Wiggins, L. , DiGuiseppi, C. , Alexander, A. , Jackson, S. , Lee, L. C. , Levy, S. E. , & Rosenberg, S. A. (2017). Screening for autism with the SRS and SCQ: Variations across demographic, developmental and behavioral factors in preschool children. Journal of Autism and Developmental Disorders, 47(11), 3550–3561. 10.1007/s10803-017-3255-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales, M. , Mundy, P. , Delgado, C. E. , Yale, M. , Messinger, D. , Neal, R. , & Schwartz, H. K. (2000). Responding to joint attention across the 6‐through 24‐month age period and early language acquisition. Journal of Applied Developmental Psychology, 21(3), 283–298. 10.1016/s0193-3973(99)00040-4 [DOI] [Google Scholar]
- Morgan, P. L. , Farkas, G. , Wang, Y. , Hillemeier, M. M. , Oh, Y. , & Maczuga, S. (2019). Executive function deficits in kindergarten predict repeated academic difficulties across elementary school. Early Childhood Research Quarterly, 46, 20–32. 10.1016/j.ecresq.2018.06.009 [DOI] [Google Scholar]
- Mullen, E. M. (1995). Mullen scales of early learning. AGS Publishing. [Google Scholar]
- Mundy, P. (2003). Annotation: The neural basis of social impairments in autism: The role of the dorsal medial‐frontal cortex and anterior cingulate system. The Journal of Child Psychology and Psychiatry and Allied Disciplines, 44(6), 793–809. 10.1111/1469-7610.00165 [DOI] [PubMed] [Google Scholar]
- Mundy, P. (2018). A review of joint attention and social‐cognitive brain systems in typical development and autism spectrum disorder. European Journal of Neuroscience, 47(September), 1–18. 10.1111/ejn.13720 [DOI] [PubMed] [Google Scholar]
- Mundy, P. , Block, J. , Delgado, C. , Pomares, Y. , Vaughan Van Hecke, A. V. , & Parlade, M. V. (2007). Individual differences and the development of joint attention in infancy. Child Development, 78(3), 938–954. 10.1111/j.1467-8624.2007.01042.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy, P. , Hogan, A. , & Doehring, P. (1996). A preliminary manual for the abridged early social communication scale (ESCS).
- Mundy, P. , & Newell, L. (2007). Attention, joint attention, and social cognition. Current Directions in Psychological Science, 16(5), 269–274. 10.1111/j.1467-8721.2007.00518.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy, P. , Sigman, M. , Ungerer, J. , & Sherman, T. (1986). Defining the social deficits of autism: The contribution of nonverbal communication measures. Journal of Child Psychology and Psychiatry, 27(5), 657–669. 10.1111/j.1469-7610.1986.tb00190.x [DOI] [PubMed] [Google Scholar]
- Mundy, P. , Sullivan, L. , & Mastergeorge, A. M. (2009). A parallel and distributed‐processing model of joint attention, social cognition and autism. Autism Research: Official Journal of the International Society for Autism Research, 2(1), 2–21. 10.1002/aur.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nketia, J. , Amso, D. , & Brito, N. H. (2021). Towards a more inclusive and equitable developmental cognitive neuroscience. Developmental cognitive neuroscience, 52, 101014. Advance online publication. 10.1016/j.dcn.2021.101014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perone, S. , Palanismay, J. , & Carlson, S. M. (2018). Age‐related change in brain rhythms from early to middle childhood: Links to executive function. Developmental Science, 21(6), e12691. 10.1111/desc.12691 [DOI] [PubMed] [Google Scholar]
- Pine, D. S. , Guyer, A. E. , Goldwin, M. , Towbin, K. A. , & Leibenluft, E. (2008). Autism spectrum disorder scale scores in pediatric mood and anxiety disorders. Journal of the American Academy of Child & Adolescent Psychiatry, 47(6), 652–661. 10.1097/chi.0b013e31816bffa5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay, E. , Dodell‐Feder, D. , Pearrow, M. J. , Mavros, P. L. , Kleiner, M. , Gabrieli, J. D. E. , & Saxe, R. (2010). Live face‐to‐face interaction during fMRI: A new tool for social cognitive neuroscience. NeuroImage, 50(4), 1639–1647. 10.1016/j.neuroimage.2010.01.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiersen, A. M. , Constantino, J. N. , & Todd, R. D. (2008). Co‐occurrence of motor problems and autistic symptoms in attention‐deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 47(6), 662–672. 10.1097/chi.0b013e31816bff88 [DOI] [PubMed] [Google Scholar]
- Reilly, E. B. , Stallworthy, I. C. , Mliner, S. B. , Troy, M. F. , Elison, J. T. , & Gunnar, M. R. (2021). Infants' abilities to respond to cues for joint attention vary by family socioeconomic status. Infancy: The Official Journal of the International Society on Infant Studies, 26(2), 204–222. 10.1111/infa.12380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salley, B. , Brady, N. C. , Hoffman, L. , & Fleming, K. (2020). Preverbal communication complexity in infants. Infancy, 25(1), 4–21. 10.1111/infa.12318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinkopf, S. J. , Mundy, P. , Claussen, A. H. , & Willoughby, J. (2004). Infant joint attention skill and preschool behavioral outcomes in at‐risk children. Development and Psychopathology, 16(02), 273–291. 10.1017/S0954579404044517 [DOI] [PubMed] [Google Scholar]
- Sigman, M. , Ruskin, E. , Arbeile, S. , Coronoa, R. , Dissanayake, C. , Espinosa, M. , Kim, N. , López, A. , & Zierhut, C. (1999). Continuity and change in the social competence of children with autism, Down syndrome, and developmental delays. Monographs of the Society for Research in Child Development, 64(1), 1–10. Serial No. 256). 10.1111/1540-5834.00002 [DOI] [PubMed] [Google Scholar]
- Sodian, B. , & Kristen‐Antonow, S. (2015). Declarative joint attention as a foundation of theory of mind. Developmental Psychology, 51(9), 1190–1200. 10.1037/dev0000039 [DOI] [PubMed] [Google Scholar]
- Stallworthy, I. C. , Berry, D. , Davis, S. , Wolff, J. J. , Burrows, C. A. , Swanson, M. R. , Grzadzinski, R. L. , Botteron, K. N. , Dager, S. R. , Estes, A. M. , Hazlett, H. C. , Zwaigenbaum, L. , Schultz, R. T. , Piven, J. , Elison, J. T. , Pruett, J. R. , & Marrus, N. , & IBIS Network . (2022). Quantifying latent social motivation and its associations with joint attention and language in infants at high and low risk for autism spectrum disorder. Developmental Science. 10.1111/desc.13336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallworthy, I. C. , Lasch, C. , Berry, D. , Wolff, J. J. , Pruett, J. R., Jr. , Marrus, N. , Swanson, M. R. , Botteron, K. N. , Dager, S. R. , Estes, A. M. , Hazlett, H. C. , Schultz, R. T. , Zwaigenbaum, L. , Piven, J. , & Elison, J. T. , & IBIS Network . (2022). Variability in responding to joint attention cues in the first year is associated with autism outcome. Journal of the American Academy of Child & Adolescent Psychiatry, 61(3), 413–422. 10.1016/j.jaac.2021.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallworthy, I. C. , Sifre, R. , Fenoglio, A. , Dahl, C. , Georgieff, M. K. , & Elison, J. T. (2022). Birthweight moderates the association between chronological age and infants’ abilities to respond to cues for joint attention. Developmental Psychiobiology, 64(2), e22239. 10.1002/dev.22239 [DOI] [PubMed] [Google Scholar]
- Striano, T. , Reid, V. M. , & Hoehl, S. (2006). Neural mechanisms of joint attention in infancy. European Journal of Neuroscience, 23(10), 2819–2823. 10.1111/j.1460-9568.2006.04822.x [DOI] [PubMed] [Google Scholar]
- Striano, T. , & Rochat, P. (1999). Developmental link between dyadic and triadic social competence in infancy. British Journal of Developmental Psychology, 17(4), 551–562. 10.1348/026151099165474 [DOI] [Google Scholar]
- Striano, T. , Stahl, D. , Cleveland, A. , & Hoehl, S. (2007). Sensitivity to triadic attention between 6 weeks and 3 months of age. Infant Behavior and Development, 30(3), 529–534. 10.1016/j.infbeh.2006.12.010 [DOI] [PubMed] [Google Scholar]
- Tasker, S. L. , & Schmidt, L. A. (2008). The “dual usage problem” in the explanations of “joint attention” and children’s socioemotional development: A reconceptualization. Developmental Review, 28(3), 263–288. 10.1016/j.dr.2007.07.001 [DOI] [Google Scholar]
- The Annie E. Casey Foundation . (2020, November 4). What is generation alpha? The Annie E. Casey Foundation. https://www.aecf.org/blog/what‐is‐generation‐alpha [Google Scholar]
- Tomasello, M. (2003). Constructing a language: A usage‐based theory of language acquisition. Harvard University Press. [Google Scholar]
- Tomasello, M. (2018). How children come to understand false beliefs: A shared intentionality account. Proceedings of the National Academy of Sciences, 115(34), 8491–8498. 10.1073/pnas.1804761115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello, M. , Carpenter, M. , Call, J. , Behne, T. , & Moll, H. (2005). Understanding and sharing intentions: The origins of cultural cognition. Behavioral and Brain Sciences, 28(5), 675–691. 10.1017/s0140525x05000129 [DOI] [PubMed] [Google Scholar]
- Vaughan Van Hecke, A. , Mundy, P. C. , Acra, C. F. , Block, J. J. , Delgado, C. E. , Parlade, M. V. , Meyer, J. A. , Neal, A. R. , & Pomares, Y. B. (2007). Infant joint attention, temperament, and social competence in preschool children. Child Development, 78(1), 53–69. 10.1111/j.1467-8624.2007.00985.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan Van Hecke, A. V. , Mundy, P. , Block, J. J. , Delgado, C. E. F. , Parlade, M. V. , Pomares, Y. B. , & Hobson, J. A. (2012). Infant responding to joint attention, executive processes, and self‐regulation in preschool children. Infant Behavior and Development, 35(2), 303–311. 10.1016/j.infbeh.2011.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, R. E. , Zhang, Y. , Gray, T. , Abbacchi, A. , Cormier, D. , Todorov, A. , & Constantino, J. N. (2019). Autism‐related variation in reciprocal social behavior: A longitudinal study. Child Development, 90(2), 441–451. 10.1111/cdev.13170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherby, A. M. , & Prizant, B. M. (2002). Communication and symbolic behavior scales: Developmental profile. Paul H. Brookes. [Google Scholar]
- White, L. K. , Moore, T. M. , Calkins, M. E. , Wolf, D. H. , Satterthwaite, T. D. , Leibenluft, E. , Pine, D. S. , Gur, R. C. , & Gur, R. E. (2017). An evaluation of the specificity of executive function impairment in developmental psychopathology. Journal of the American Academy of Child & Adolescent Psychiatry, 56(11), 975–982. e3. 10.1016/j.jaac.2017.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo, P. D. (2006). The dimensional change card sort (DCCS): A method of assessing executive function in children. Nature Protocols, 1, 297–301. 10.1038/nprot.2006.46 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
The majority of the sample utilized in this manuscript (74%) is available as part of the Baby Connectome data via the NDA. The remaining portion of raw deidentified data and analysis scripts are available upon request to the senior author. Authors are in the process of transferring scripts and remaining data to a publicly available repository.


