Abstract
Introduction and Objective:
The lack of precision to identify patients with early-stage diabetic kidney disease (DKD) at near-term risk for progressive decline in kidney function results in poor disease management often leading to kidney failure requiring unplanned dialysis. The KidneyIntelX is a multiplex, bioprognostic, immunoassay consisting of 3 plasma biomarkers and clinical variables that uses machine learning to generate a risk score for progressive decline in kidney function over 5-year in adults with early-stage DKD. Our objective was to assess the impact of KidneyIntelX on management and outcomes in a Health System in the real-world evidence (RWE) study.
Methods:
KidneyIntelX was introduced into a large metropolitan Health System via a population health-defined approved care pathway for patients with stages 1 to 3 DKD between [November 2020 to March 2022]. Decision impact on visit frequency, medication management, specialist referral, and selected lab values was assessed. We performed an interim analysis in patients through 6-months post-test date to evaluate the impact of risk level with clinical decision-making and outcomes.
Results:
A total of 1686 patients were enrolled in the RWE study and underwent KidneyIntelX testing and subsequent care pathway management. The median age was 68 years, 52% were female, 26% self-identified as Black, and 94% had hypertension. The median baseline eGFR was 59 ml/minute/1.73 m2, urine albumin-creatinine ratio was 69 mg/g, and HbA1c was 7.7%. After testing, a clinical encounter in the first month occurred in 13%, 43%, and 53% of low-risk, intermediate-risk, and high-risk patients, respectively and 46%, 61%, and 71% had at least 1 action taken within the first 6 months. High-risk patients were more likely to be placed on SGLT2 inhibitors (OR = 4.56; 95% CI 3.00-6.91 vs low-risk), and more likely to be referred to a specialist such as a nephrologist, endocrinologist, or dietician (OR = 2.49; 95% CI 1.53-4.01) compared to low-risk patients.
Conclusions:
The combination of KidneyIntelX, clinical guidelines and educational support resulted in changes in clinical management by clinicians. After testing, there was an increase in visit frequency, referrals for disease management, and introduction to guideline-recommended medications. These differed by risk category, indicating an impact of KidneyIntelX risk stratification on clinical care.
Keywords: diabetic kidney disease, early-stage, KidneyIntelX, decision impact study, Real World Evidence
Introduction
Diabetic kidney disease (DKD) develops in approximately 1 out of 4 adults with type 2 diabetes (T2D) with nearly 50 000 individuals progressing to kidney failure, resulting in dialysis or a kidney transplant every year.1 The estimated glomerular filtration rate (eGFR) and urinary albumin creatinine ratio (UACR), used in the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines for risk stratification,2 lacks the precision to identify patients who will experience progressive decline in kidney function (PDKF), which is especially true for patients with early-stage DKD (Grade (G)1-G3).3 As a result, primary care physicians (PCP) and diabetologists are often not able to appropriately risk stratify and counsel patients on the progressive nature of their disease. Easily interpretable and accurate prognostic tools that integrate into clinical workflow are lacking, resulting in suboptimal treatment and referral delays to a nephrology or other specialist. This has led, in part, to an unacceptable number of PDKF and kidney failure cases in this population4-8 with a high proportion of patients starting unplanned dialysis.1,9,10
Prediction of near-term risk for kidney disease progression in the early stages is often not assessed. The Kidney Failure Risk Equation (KFRE) is an online tool used to predict risk of kidney failure using several clinical variables such as age, gender, eGFR, and UACR. Unfortunately, the KFRE has not been validated in individuals with relatively preserved kidney function, such as those with early-stage DKD. KFRE is similar to KDIGO in that it is limited by the physiologic variability of both eGFR and UACR,11,12 as well as confounded by hyperfiltration in early stages of DKD.11,13,14 As a result, PCPs and other specialists are not able to appropriately risk stratify and counsel patients on the progressive nature of DKD.7,8,15-18
Several plasma biomarkers have been investigated to aid in the prediction of kidney disease progression. Three of the most widely studied are soluble tumor necrosis factor receptors (TNFR) 1 and 2 as well as plasma kidney injury molecule-1 (KIM-1).19-24 Although these markers have uniformly shown independent associations with kidney function decline, they have only recently been combined into a single assay with clinical data (ie, a bioprognostic, biological risk assessment tool) to predict progression of kidney disease in the KidneyIntelX assay.25,26 Analytically and clinically validated, this assay is approved in the United States for clinical use in T2D patients with early-stage DKD to predict risk of a progressive decline of their kidney function.
Until recently, easily integrated and accurate prognostic models that combine clinical data from patients’ electronic health records (EHR) and blood-based biomarkers, as in the KidneyIntelX assay, have not been available. Machine learning tools can combine biomarkers and EHR data and identify those patients at high-risk of progressive decline in kidney function. An individualized, targeted approach such using the KidneyIntelX, has the potential to improve patient outcomes through prioritizing the use of cardiorenal protective medications such as angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARBs), sodium glucose transporter 2 inhibitors (SGLT2i), and non-steroidal mineralocorticoid antagonists27,28 as well as efficient resource allocation at the PCP level.
Through the RWE study and in collaboration with a large metropolitan Hospital Population Health Chronic Disease management program Mount Sinai (MS) Hospital, New York, NY, we introduced the KidneyIntelX bio-prognostic, immunoassay test into the T2D, early- stage DKD patient population. Here, the objective was to assess clinical decision-making and outcomes based on the KidneyIntelX risk score for early-stage DKD disease progression. We hypothesized that by introducing an awareness of risk for early-stage DKD progression in combination with educational materials, providers would be prompted to act according to established guidelines and introduce changes earlier in patient management to improve clinical outcomes.
Methods
Patients with T2D and early-stage DKD (with documented eGFR of 30-59 ml/minute/1.73 m2 [G3a, G3b] or an eGFR ≥ 60 with albuminuria [UACR] ≥30 mg/g [A2, A3]) were prospectively enrolled by MS Hospital population health care navigation team to have a KidneyIntelX test as part of the RWE study (NCT04802395) between November 2020 and March 2022 (Figure 1). The study was approved by the MS Institutional Review Board with individual patient consent waived due to patient volume and number of providers. All providers received a 1-time, in-service by the study principal investigator or population health staff member who reviewed the study design, clinical and analytic validation of the KidneyIntelX assay, and alignment of the risk score with a MS recommended KDIGO/American Diabetes Association (ADA) guideline2,29,30 approach to patient management. Educational reinforcement was provided by the Renalytix medical affairs group and the population health care navigation team to address any outstanding operational and clinical questions.
Figure 1.
RWE clinical study diagram.
This is a prospective collection of clinical data at baseline, 6-month posttest and a minimum 6 months pre-baseline serving as an internal patient control. Data collected included age, height, gender, race, weight, blood pressure, eGFR, UACR, HbA1c, and glucose levels, as well as a relevant medication history (record of statin use, changes in dose, type, frequency of anti-hypertensives, diuretics, and kidney protective medications etc.), clinical decisions (change in visit frequency), and associated outcomes extracted from medical records and curated by study clinicians. Participant clinical data, provider clinical decisions and actions/outcomes were captured within the designated 6-month post-test period.
The primary objective was the demonstration of risk-based clinical impact within 3- to 6-months of baseline KidneyIntelX test. Clinical impact was defined by any of the following measures: 20% increase in referrals by any provider to specialty services (eg, dietician, diabetologist, or nephrologist), 20% increase in use of statins or dose adjustment of ACEi/ARB, or 20% increase of patients treated with SGLT2 inhibitors or glucagon-like peptide 1 (GLP1) agonists.
Every provider received a KidneyIntelX test report that contains low-, intermediate-, and high-risk categories and a guideline-based clinical care pathway developed and approved by MS Population Health (Figure 2). Each report contained the recommended care pathway based on the patient’s level of risk and included visit frequency (1-3×/year), specialist referral, medication use and modifications (ie, maximal titration of ACEi/ARBs), or introduction of novel therapies (eg, SGLT2i). Determination of a new referral to a specialty consult service (ie, nephrology, endocrinology, nutrition), any new prescriptions, or modification to any existing prescription medication for ACEi/ARB, SLGT2i, or GLP-1 agonists was based on a 6-month pre-baseline to 6-month post-test assessment, which also included impact on risk level categorization. Patient compliance with filling the prescription was not available for this interim evaluation but is planned for the long-term study outcomes.
Figure 2.
KidneyIntelX contains a risk score and categorization linked to a guideline driven care path.
Statistical Analyses
This was a prospective data collection study to evaluate the impact of the KidneyIntelX test result on clinical decision-making and outcomes. The primary outcomes evaluated were the percent change of descriptive statistics between high- and low-risk patients, as well as non-adjusted and adjusted odds ratio (OR) by clinical covariates (ie, age, sex, race, eGFR, and UACR) with 95% confidence intervals. Only the adjusted OR will be reported unless statistical differences are noted.
Results
A total of 1686 patients were enrolled in the MS RWE study at 20 practice locations with 75 medical providers. The median age was 68 years, 54% female, 29% Black, and 94% with hypertension. Six-month prior baseline median systolic blood pressure was 141 mmHg, with eGFR at 59 ml/minute/1.73 m2, UACR of 64 mg/g, and 7.9% HbA1c (Table 1). All patients had a KidneyIntelX test result, with post-test follow-up at 6-months. The risk breakdown of RWE population was similar to the clinical validation cohort29 (high-risk 12% vs 17%, intermediate-risk 40% vs 37%, and low-risk 48% vs 46%). Race stratification identified 34% of the Black population (n = 70) to be high-risk, which was nearly double that of White patients (17%, n = 35). The majority (61%, n = 1028) of all enrolled patients were seen by their PCP, while others saw an endocrinologist (24%, n = 405), nephrologist (13%, n = 219), or other specialist (2%, n = 34).
Table 1.
RWE Interim Cohort Demographics.
| All patients | Low-risk | Intermediate-risk | High-risk | |
|---|---|---|---|---|
| N = 1686 | N = 804 | N = 678 | N = 204 | |
| Age, years median (IQR) | 68 (13) | 69 (13) | 67 (14.75) | 68 (12.25) |
| % Female | 53.9% | 56.1% | 52.2% | 50.5% |
| Race, n (%) | ||||
| Black | 496 (29.4%) | 236 (29.4%) | 190 (28.0%) | 70 (34.3%) |
| White | 406 (24.1%) | 214 (26.6%) | 157 (23.2%) | 35 (17.2%) |
| Other | 784 (46.5%) | 354 (44.0%) | 331 (48.8%) | 99 (48.5%) |
| Ethnicity, n (%) | ||||
| Hispanic/Latino | 22 (1.3%) | 9 (1.1%) | 9 (1.3%) | 4 (2.0%) |
| Not Hispanic/Latino | 143 (8.5%) | 66 (8.2%) | 60 (8.8%) | 17 (8.3%) |
| Not specified | 1521 (90.2%) | 729 (90.7%) | 609 (89.8%) | 183 (89.7%) |
| Comorbidities | ||||
| Hypertension, n (%) | 1588 (94.2%) | 742 (92.3%) | 647 (95.4%) | 199 (97.5%) |
| CAD, n (%) | 480 (28.5%) | 199 (24.8%) | 212 (31.3%) | 69 (33.8%) |
| Heart failure, n (%) | 103 (6.1%) | 34 (4.2%) | 52 (7.7%) | 17 (8.3%) |
Abbreviation: CAD, coronary artery disease.
Importantly, 53% of all KidneyIntelX high-risk patients had a follow-up visit within 1 month and 57% had action taken (medication change or referral) within 3 months compared to 13% and 35%, respectively, for low-risk individuals. Traditionally, the standard-of-care (SOC) for follow-up visit frequency is every 12 months. Thus, these results reflect a needed change in management for high-risk patients with regard to visit frequency and any action taken. It is worth noting that this increase of visit frequency occurred during the COVID-19 pandemic when triaging patients for in-person visits was based on their risk level, while directing lower risk individuals to telehealth.
Thirteen percent of low-risk, 43% of intermediate-risk, and 53% of high-risk patients were seen by their primary care physician in the first month, and 46%, 61%, and 71% had at least 1 action taken within the first 6 months. New referrals by risk group were 6%, 12%, and 15% (OR = 2.49, 95% CI: 1.53-4.01 for high- vs low-risk; Figure 3). The OR suggests that high-risk patients are almost 2.5× more likely to be referred to a specialty service compared to a low-risk patient. High-risk patients were referred predominately to nephrology (63%) and endocrinology (37%), while across all risk groups, PCPs referred patients first to endocrinology (53%), nephrology (34%) second, and a small percentage (13%) was referred to the nutrition service.
Figure 3.
Time to specialist referral. Time to specialist referral based on (a) KidneyIntelX and (b) Sankey Flow diagram demonstrating proportion of referrals based on provider. Greater than 20% increase in referrals for patients at high- versus low-risk for progression of their DKD ordered by their Primary Care Physician (high-risk vs low-risk, new referrals; OR = 2.49; 95% CI: 1.53-4.01).
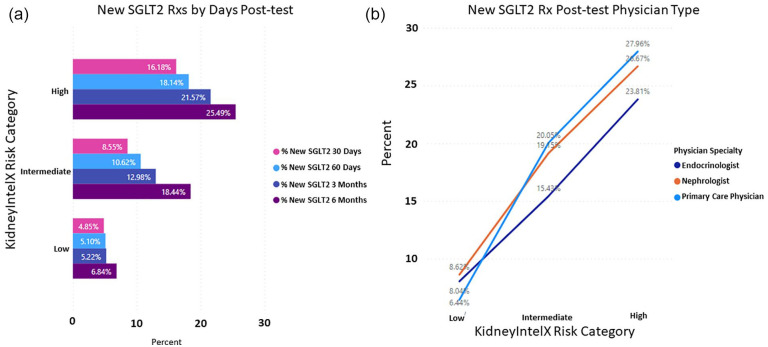
When evaluating new or modified prescriptions for anti-hypertensive at 6-months, both ACEi and ARBs achieved a greater than 20% change in the high-risk group (ACEi, OR = 1.36; 95% CI: 0.77-2.30; ARBs, OR = 1.65; 95% CI: 1.01-2.63). The ORs are mostly suggestive of trends in change of dose or type of antihypertensives between high and low risk patients. Most of these changes were directed by the PCP of the low-and intermediate-risk groups, with an overall increase in the use of ARBs in the intermediate-risk group, as per guideline recommendations on the KidneyIntelX test report. Even more pronounced were the changes associated with new prescriptions for SGLT2i at 6 months in the high- (25%) vs low-risk (7%) groups (OR = 4.56; 95% CI: 3.00-6.91; Figure 4), which is more than 4.5× the rate in high-risk patients compared to low-risk patients. Primary care physicians represented 32% of the physicians ordering SGLT2i in the high-risk group followed by nephrologists (30%) and endocrinologists (28%).
Figure 4.
Decision impact of KidneyIntelX risk level on new prescriptions for SGLT2 inhibitors. (a) Post-test result at 6 months in the high- (25%) vs low-risk (7%) groups (OR = 4.56; 95% CI: 3.00-6.91) and (b) the physician type most likely to order SGLT2 inhibitors.
Early evidence suggests that the introduction of the SGLT2i lowered HbA1c levels most notably in the high-risk category (median 8.2% HbA1c at 6 months pre KidneyIntelX vs 7.45% post-test, Table 2). Although eGFR and SBP remained unchanged, there were reductions in the median UACR levels within the low- and intermediate-risk groups, which is where the PCPs introduced modifications to the use of ACEi/ARBs (Table 2).
Table 2.
Pre- and Post-Test Variables.
| All patients | Low-risk | Intermediate-risk | High-risk | |
|---|---|---|---|---|
| N = 1686 | N = 804 | N = 678 | N = 204 | |
| Pre-test median | ||||
| SBP | 141 | 137 | 145 | 150.5 |
| DBP | 80 | 80 | 81 | 82 |
| eGFR | 59.5 | 61 | 64.5 | 46.5 |
| UACR | 64 | 33 | 104.5 | 720.5 |
| HbA1c | 7.95 | 7.4 | 8.25 | 8.2 |
| Post-test median | ||||
| SBP | 140 | 135 | 145 | 149 |
| DBP | 80 | 80 | 81.5 | 81.5 |
| eGFR | 60.5 | 61.5 | 63.5 | 46.5 |
| UACR | 59 | 26 | 88.5 | 726.5 |
| HbA1c | 7.6 | 7.1 | 8 | 7.45 |
Abbreviations: DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1C; SBP, systolic blood pressure; UACR, urine albumin-to-creatinine ratio.
Discussion
In the present study, we demonstrate that patients with early-stage DKD who were identified as high-risk via the KidneyIntelX score received earlier follow-up visits, necessary change in medications or specialist referral compared to those who were identified as low- or intermediate-risk patients. Specifically, high-risk patients were more likely to be referred to a nephrologist and by 6 months, these patients had a significant increase in anti-hypertension medications compared to those of intermediate- and low-risk who were more likely to receive SOC. Most pronounced was the increased frequency in new SGLT2i prescriptions among high-risk patients ordered by treating physicians, with favorable effects on HbA1c. Collectively, these interim data provide evidence that the KidneyIntelX individualized risk score positively influenced disease management decisions, which should ultimately lead to improved outcomes for patients with early-stage DKD.
Although DKD is present in one-third of adults with T2D, most people are unaware they have the condition.31 This lack of understanding is primarily due to the intra-individual variability of the markers used to diagnose and efficiently prognosticate DKD using eGFR and UACR. Moreover, UACR is often not performed at the frequency recommended by guidelines for this population due to the high within-person variability.12,32 The current diagnostic approach, especially when used to predict progression of “early-stage” DKD, is further flawed with the use of staging systems such as KDIGO that rely on small changes within these highly variable markers to stratify patients’ risk of disease progression at the individual level.33
The KidneyIntelX is a first-in-class bio-prognostic (ie, blood-based biomarkers combined with clinical data to predict outcomes) assay to predict the progressive decline of DKD function by quantifying TNFR1, TNFR2, and Kim-1 in blood and combine these values with specific clinical features to generate a unique patient risk score and category. This integrated risk score has near-term clinical implications, especially when linked to clinical decision support (CDS) and embedded care pathways. This is different from other clinically based tools like KFRE, which uses kidney replacement therapy as the endpoint, is based on eGFR and UACR variables, is not validated in early stage DKD and is not linked to care pathways. Thus, the wide range of risk at 2 and 5 years provided by this tool raises practical issues on how best to use at the patient care management level.33
The current standard for clinical risk stratification (KDIGO risk strata)2 has 3 risk strata that overlap with the population of DKD patients that we included in our study. The KidneyIntelX also uses a risk score with 3 risk strata (low, intermediate, and high) incorporating KDIGO classification components (eGFR and UACR), as well as the addition of other clinical variables, and 3 blood-based biomarkers. As a result, the ability to accurately risk-stratify DKD patients was increased, thereby enabling improved patient management. As demonstrated in the RWE study, the clinical impact with the KidneyIntelX assay was evident across all risk groups and in line with the report embedded ADA and KDIGO guidelines/MS care path. Several important observations were identified, specifically related to the level of risk for the individual patient. Fifty-two percent of high-risk patients were seen within 1 month (compared to yearly standard of care recommendation) and 57% had some action taken within 3 months. Similarly, 66% of referrals in this group occurred within the first 3 months and 63% of these referrals were to the nephrology service by their PCP. At 6 months, there were significant fold changes in anti-hypertensive (ACEi/ARBs; OR X), initiation of SGLT2i (OR 4.56) and increased referrals to nephrologists, endocrinologists, or dieticians (OR = 2.49) in high-risk vs low-risk patients.
The increase in referrals to nephrology,34 improved awareness of kidney health, referral to dieticians, medication review by pharmacists, reinforcement of usage of antagonists of the renin angiotensin aldosterone system, and increased use of recently approved novel medications (eg, SGLT2 inhibitors and GLP-1 receptor agonists) represents important changes made to help slow progression of DKD.35-38 Furthermore, UACR levels were lower in the low- and intermediate-risk groups where PCPs modified dosing and introduced additional medications (ie, ARBs) based on guideline-directed plans to address hypertension and ultimately improve kidney function.
The current study has limitations. First, the patient characterization in this study did not include information such as compliance with filling prescriptions, co-morbidities, or additional clinical laboratory values beyond what was measured in the clinic. Additional information such as health insurance coverage, which would potentially impact the ability for some patients to obtain recommended medications was also not recorded. It is worth noting that these attributes will be included as we reach important study milestones at year 1 and 2. Second, we were not able to capture frequency of emergency room visits and/or hospitalizations at this interim time-point. However, this also will be captured in the 2 to 5 years outcome and is included as an objective for that future study report. We will also continue to build upon our interrogation of patient and population level controls to re-affirm current observations.
Conclusion
In summary, DKD is an increasingly complex and systemic problem challenging modern healthcare systems. The ability to identify a patient’s level of risk for kidney function decline in early-stage DKD has the potential to change the trajectory of the disease through awareness and implementation of clinical solutions. As suggested in this RWE study, the KidneyIntelX individualized risk score was associated with management decisions of PCPs including visit frequency, specialist referrals, and implementation of cardiovascular and kidney medications, which should ultimately result in improved outcomes. It is anticipated that the continued enrollment and follow-up will further support and add to these interim observations.
Acknowledgments
JetPub Scientific Communications, LLC assisted the authors in the preparation of this manuscript, in accordance with Good Publication Practice (GPP3) guidelines.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AZ, FL, and MJD are employees of Renalytix; SC and GN are consultants for Renalytix.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Renalytix.
Trial Registration Number: Real World Evidence (RWE) study, NCT04802395.
Study Enrollment Dates: November 2020 to March 2022.
IRB Approval: Mount Sinai Institutional Review Board (HS#-21-00165, Approved: 3/3/2022)
Ethical Approval: All authors assert that all procedures contributing to this work comply with the ethical standards of the Mount Sinai Institutional Review Board.
Ethics and Consent to Participate: In accordance with the Declaration of Helsinki, this study was approved by the Mount Sinai Institutional Review Board (IRB). Individual patient consent waived due to patient volume and number of providers.
ORCID iD: Michael J. Donovan  https://orcid.org/0000-0003-0772-598X
https://orcid.org/0000-0003-0772-598X
References
- 1. USRDS. Annual data report. Accessed August 22, 2022. https://www.usrds.org/annual-data-report/
- 2. Official Journal of the international Society of nephrology KDIGO 2012. Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Accessed August 22, 2022. www.publicationethics.org [DOI] [PubMed]
- 3. Dunkler D, Gao P, Lee SF, et al. Risk prediction for early CKD in type 2 diabetes. Clin J Am Soc Nephrol. 2015;10(8):1371-1379. doi: 10.2215/CJN.10321014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sprangers B, Evenepoel P, Vanrenterghem Y. Late referral of patients with chronic kidney disease: no time to waste. Mayo Clin Proc. 2006;81(11):1487-1494. [DOI] [PubMed] [Google Scholar]
- 5. Kagoma YK, Weir MA, Iansavichus AV, et al. Impact of estimated GFR reporting on patients, clinicians, and health-care systems: a systematic review. Am J Kidney Dis. 2011;57(4):592-601. [DOI] [PubMed] [Google Scholar]
- 6. Hingwala J, Wojciechowski P, Hiebert B, et al. Risk-based triage for nephrology referrals using the kidney failure risk equation. Can J Kidney Heal Dis. 2017;4:1-13. doi: 10.1177/2054358117722782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boulware LE, Troll MU, Jaar BG, Myers DI, Powe NR. Identification and referral of patients with progressive CKD: a national study. Am J Kidney Dis. 2006;48(2):192-204. [DOI] [PubMed] [Google Scholar]
- 8. Agrawal V, Ghosh AK, Barnes MA, McCullough PA. Perception of indications for nephrology referral among internal medicine residents: a national online survey. Clin J Am Soc Nephrol. 2009;4(2):323-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gillespie BW, Morgenstern H, Hedgeman E, et al. Nephrology care prior to end-stage renal disease and outcomes among new ESRD patients in the USA. Clin Kidney J. 2015;8(6):772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Winkelmayer WC, Liu J, Chertow GM, Tamura MK. Predialysis nephrology care of older patients approaching end-stage renal disease. Arch Intern Med. 2011;171(15):1371-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oshima M, Shimizu M, Yamanouchi M, et al. Trajectories of kidney function in diabetes: a clinicopathological update. Nat Rev Nephrol. 2021;17(11):740-750. [DOI] [PubMed] [Google Scholar]
- 12. Waikar SS, Rebholz CM, Zheng Z, et al. Biological variability of estimated GFR and albuminuria in CKD. Am J Kidney Dis. 2018;72(4):538-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang Y, Xu G. Update on pathogenesis of glomerular hyperfiltration in early diabetic kidney disease. Front Endocrinol. 2022;13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Quinn GZ, Abedini A, Liu H, et al. Renal histologic analysis provides complementary information to kidney function measurement for patients with early diabetic or hypertensive disease. J Am Soc Nephrol. 2021;32(11):2863-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Duggal V, Montez-Rath ME, Thomas IC, Goldstein MK, Tamura MK. Nephrology referral based on laboratory values, kidney failure risk, or both: a study using veterans affairs health system data. Am J Kidney Dis. 2022;79(3):347-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lea JP, McClellan WM, Melcher C, Gladstone E, Hostetter T. CKD risk factors reported by primary care physicians: do guidelines make a difference? Am J Kidney Dis. 2006;47(1):72-77. [DOI] [PubMed] [Google Scholar]
- 17. Shahinian VB, Saran R. The role of primary care in the management of the chronic kidney disease population. Adv Chronic Kidney Dis. 2010;17(3):246-253. [DOI] [PubMed] [Google Scholar]
- 18. Datar M, Ramakrishnan S, Montgomery E, Coca SG, Vassalotti JA, Goss T. A qualitative study documenting unmet needs in the management of diabetic kidney disease (DKD) in the primary care setting. BMC Public Health. 2021;21(1):1-9. doi: 10.1186/S12889-021-10959-7/TABLES/3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coca SG, Nadkarni GN, Huang Y, et al. Plasma biomarkers and kidney function decline in early and established diabetic kidney disease. J Am Soc Nephrol. 2017;28(9):2786-2793. doi: 10.1681/ASN.2016101101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gutiérrez OM, Shlipak MG, Katz R, et al. Associations of plasma biomarkers of inflammation, fibrosis, and kidney tubular injury with progression of diabetic kidney disease: a cohort study. Am J Kidney Dis. 2022;79(6):849-857.e1. doi: 10.1053/J.AJKD.2021.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Niewczas MA, Gohda T, Skupien J, et al. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol. 2012;23(3):507-515. doi: 10.1681/ASN.2011060627/-/DCSUPPLEMENTAL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Niewczas MA, Pavkov ME, Skupien J, et al. A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat Med. 2019;25(5):805-813. doi: 10.1038/S41591-019-0415-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schrauben SJ, Shou H, Zhang X, et al. Association of multiple plasma biomarker concentrations with progression of prevalent diabetic kidney disease: findings from the chronic renal insufficiency cohort (CRIC) study. J Am Soc Nephrol. 2021;32(1):115-126. doi: 10.1681/ASN.2020040487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sen T, Li J, Neuen BL, et al. Effects of the SGLT2 inhibitor canagliflozin on plasma biomarkers TNFR-1, TNFR-2 and KIM-1 in the CANVAS trial. Diabetologia. 2021;64(10):2147-2158. doi: 10.1007/S00125-021-05512-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chan L, Nadkarni GN, Fleming F, et al. Derivation and validation of a machine learning risk score using biomarker and electronic patient data to predict progression of diabetic kidney disease. Diabetologia. 2021;64(7):1504-1515. doi: 10.1007/S00125-021-05444-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lam D, Nadkarni GN, Mosoyan G, et al. Clinical utility of KidneyIntelX in early stages of diabetic kidney disease in the CANVAS Trial. Am J Nephrol. 2022;53(1):21-31. doi: 10.1159/000519920 [DOI] [PubMed] [Google Scholar]
- 27. Mima A. A narrative review of diabetic kidney disease: previous and current evidence-based therapeutic approaches. Adv Ther. 2022;39(8):3488-3500. doi: 10.1007/S12325-022-02223-0 [DOI] [PubMed] [Google Scholar]
- 28. Cohen S, Sternlicht H, Bakris GL. Mineralocorticoid receptor antagonists in the treatment of diabetic kidney disease: their application in the era of SGLT2 inhibitors and GLP-1 receptor agonists. Curr Diab Rep. 2022;22(5):213-218. doi: 10.1007/S11892-022-01461-4 [DOI] [PubMed] [Google Scholar]
- 29. American Diabetes Association. Diabetes care. Volume 43 Issue Supplement_1. Accessed August 22, 2022. https://diabetesjournals.org/care/issue/43/Supplement_1
- 30. de Boer IH, Caramori ML, Chan JCN, et al. Executive summary of the 2020 KDIGO Diabetes Management in CKD Guideline: evidence-based advances in monitoring and treatment. Kidney Int. 2020;98(4):839-848. doi: 10.1016/J.KINT.2020.06.024 [DOI] [PubMed] [Google Scholar]
- 31. Chu CD, McCulloch CE, Banerjee T, et al. CKD awareness among US adults by future risk of kidney failure. Am J Kidney Dis. 2020;76(2):174-183. doi: 10.1053/J.AJKD.2020.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stempniewicz N, Vassalotti JA, Cuddeback JK, et al. Chronic kidney disease testing among primary care patients with type 2 diabetes across 24 U.S. health care organizations. Diabetes Care. 2021;44(9):2000-2009. doi: 10.2337/DC20-2715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Major RW, Cockwell P, Nitsch D, Tangri N. The next step in chronic kidney disease staging: individualized risk prediction. Kidney Int. 2022;102(3):456-459. doi: 10.1016/J.KINT.2022.06.012 [DOI] [PubMed] [Google Scholar]
- 34. Smart NA, Dieberg G, Ladhani M, Titus T. Early referral to specialist nephrology services for preventing the progression to end-stage kidney disease. Cochrane Database Syst Rev. 2014;2014(6):CD007333. doi: 10.1002/14651858.CD007333.PUB2 [DOI] [PubMed] [Google Scholar]
- 35. Sarafidis P, Ferro CJ, Morales E, et al. SGLT-2 inhibitors and GLP-1 receptor agonists for nephroprotection and cardioprotection in patients with diabetes mellitus and chronic kidney disease. A consensus statement by the EURECA-m and the DIABESITY working groups of the ERA-EDTA. Nephrol Dial Transplant. 2019;34(2):208-230. doi: 10.1093/NDT/GFY407 [DOI] [PubMed] [Google Scholar]
- 36. Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776-785. doi: 10.1016/S2213-8587(19)30249-9 [DOI] [PubMed] [Google Scholar]
- 37. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306. doi: 10.1056/NEJMOA1811744/SUPPL_FILE/NEJMOA1811744_DATA-SHARING.PDF [DOI] [PubMed] [Google Scholar]
- 38. Perkovic V, de Zeeuw D, Mahaffey KW, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6(9):691-704. doi: 10.1016/S2213-8587(18)30141-4 [DOI] [PubMed] [Google Scholar]