Abstract
Centimeter-scale tissue with angiogenesis has become more and more significant in organ regeneration and drug screening. However, traditional bioink has obvious limitations such as balance of nutrient supporting, printability, and vascularization. Here, with “secondary bioprinting” of printed microspheres, an innovative bioink system was proposed, in which the thermo-crosslinked sacrificial gelatin microspheres encapsulating human umbilical vein endothelial cells (HUVECs) printed by electrospraying serve as auxiliary component while gelatin methacryloyl precursor solution mixed with subject cells serve as subject component. Benefiting from the reversible thermo-crosslinking feature, gelatin microspheres would experience solid-liquid conversion during 37°C culturing and form controllable porous nutrient network for promoting the nutrient/oxygen delivery in large-scale tissue and accelerate the functionalization of the encapsulated cells. Meanwhile, the encapsulated HUVECs would be released and attach to the pore boundary, which would further form three-dimensional vessel network inside the tissue with suitable inducing conditions. As an example, vascularized breast tumor tissue over 1 cm was successfully built and the HUVECs showed obvious sprout inside, which indicate the great potential of this bioink system in various biomedical applications.
Keywords: Bioprinting, Angiogenesis, Microsphere, Large-scale tissue, Gelatin methacryloyl, Thermo-sensitive material
1. Introduction
In addition to various published microsphere functional units (micro tissues), in practical studies, centimeter-scale tissues based on hydrogel materials constructed by three-dimensional (3D) bioprinting technology[1,2] are also of great application value, such as drug screening based on large-scale tissues, and repair of large defects at the centimeter level[3]. However, the development of bioprinting of biologically active centimeter-scale large tissues has been impeded, mainly due to two reasons:
1.1. Limitation of the substances exchange inside and outside centimeter-scale structure
The spontaneously formed pores by hydrogel materials are always in micro scale. As a result, the distribution of nutrients, oxygen, and cell metabolites in centimeter-scale tissues often has gradients. It is difficult for cells inside large tissues to survive and cell metabolites cannot be smoothly discharged. Therefore, for the normal growth and functionalization of cells inside large centimeter-scale tissues, a bigger and richer network of nutrient channels needs to be built. Traditionally, researchers tend to print network scaffold or microfluidic constructure with designed routine program and directly form specific nutrient channels network. However, the added routine to perform nutrient channels would largely increase the printing duration and difficulty, which can bring more uncertainty to the printing feasibility and effectiveness. At present, some effective methods have been developed. Zhang et al. have published corresponding approach to form pores inside large-scale structures with phase separation principle[4]. Furthermore, in our previous work, irregular gelatin fragment has also been applied to form denser pores inside the large-scale tissues in vitro[5].
1.2. Difficulties of 3D angiogenesis inside large-scale structure
In actual organism, rich vessel network in different scales distributes in tissue to provide enough necessary blood supply for the surrounding cells. Thus, to be closer to the tissue in organism, in addition to the tissue subject cells that should be loaded in centimeter-scale tissues, complex network of 3D blood vessels should be also formed inside[6-9]. At present, a large number of biofabrication methods have been proposed and constructed the tissue structure of various kinds of loaded living cells. However, there are only few studies of angiogenesis within bioprinted structures, and building complex 3D vessel networks within centimeter-scale tissues remains challenging.
Microsphere has become one of the most significant bioprinting structures due to its promising properties, such as tiny size, high biocompatibility, and special rheological profiles[10,11]. Various effective fabrication methods have been proposed by introducing external energy to hydrogel precursor solution or solid bulk, namely, auxiliary dripping[12], diphase emulsion[13,14], lithography technology[15], and bulk crushing[16]. It has been widely used in the field of cell therapy[17], tissue models[18], and drug releasing[19]. In recent years, in addition to a kind of functional unit, microsphere has also been applied as bioink component or bioprinting aided tool, which has been widely known as “secondary printing.” For example, Burdick et al. published a bioprinting method to extrude microsphere-based bioink to build specific 3D structures[20]. Wang et al. successfully printed structures with alginate-microspheres-based bioink fabricated by microfluidic device and realized the tissue regeneration in vivo[21]. In the research of Hinton et al., sacrificial gelatin microspheres were applied to supporting the extruded hydrogel filament, as widely known as suspending bioprinting[22]. Furthermore, Jeon et al. also designed a biodegradable and photocrosslinkable microsphere supporting bath which could simultaneously permit smooth movement of the printing nozzle and maintain the outline of the printed structure[23].
As one of the most popular hydrogel materials applied in the field of biofabrication, gelatin also has its own unique thermo-sensitive properties in addition to the special characteristics obtained by modification (e.g., gelatin methacryloyl [GelMA] has irreversible photocrosslinking characteristics)[24-28]. The response of non-modified gelatin precursor solution to temperature has been verified to be obvious. Unlike GelMA precursor solution which would form an irreversible and stable covalent bond after photocrosslinking, the thermo-crosslinking process of non-modified gelatin precursor solution at low temperature would be reversible and mainly depends on hydrogen bond and triple-helix structure[29,30]. When the temperature rises above a certain temperature, the non-modified gelatin precursor solution would convert to solation state. Therefore, by combining the special features of non-modified gelatin and GelMA and specific microsphere fabrication method, it is expected to fabricate the thermo-sensitive microspheres, based on which an innovative bioink system could be designed to form richer nutrient channels within the centimeter-scale large tissue, followed by angiogenesis and in a suitable inducing way.
To address the aforementioned challenge in bioprinting of effective centimeter-scale tissue with angiogenesis, this paper will design an innovative bioink system for the extruding bioprinting scene of vascularized centimeter-scale tissue, namely, thermo-sensitive sacrificial microsphere-based bioink (TSM-B). As shown in Figure 1, the human umbilical vein endothelial cells (HUVECs)-laden gelatin microspheres with on-demand diameters were electrosprayed with high voltage electric field and thermo-crosslinked at low temperature as the auxiliary component (volume ≤50%). GelMA precursor solution was prepared as the subject component to further form the tissue structure by irradiation of 405 nm blue light. In the preparation of bioink, the electrospraying process of thermo-sensitive sacrificial gelatin microsphere (TSM) was analyzed. Based on this, TSM-B containing TSMs with different volume-to-volume ratios and diameters were prepared and the rheological properties and printability were tested. In the aspect of extrusion bioprinting of centimeter-scale 3D structure, the sol-gel transformation process of sacrificial microspheres inside the printed structure was observed to explore the formation of internal nutrient channels. Complex porous 3D structures were successfully printed with commercial 3D bioprinter. Finally, the ability of the bioink to build large vascularized tissue at the centimeter level was verified by in vitro printing human breast tumor tissue with angiogenesis. The encapsulated HUVECs showed obvious 3D sprout and the vascularization protein (VE-cadherin) was tested to be positive. We believe that this bioink system would become a powerful tool in corresponding biomedical applications in future.
Figure 1.

Sketch of the preparation of TSM-B and formation of porous centimeter-scale tissue with angiogenesis.
2. Materials and methods
2.1. Rheological testing of electrospraying ink
The non-modified gelatin was mixed in the phosphate buffered saline (PBS) at the concentration of 15% w/v and fully dissolved for 1 h with a water bath with magnetic mixer at 37°C. It was then filtered through a 0.22 mm filter for sterilization and kept at the temperature of 4°C. In the flow step measurement of gelatin electrospraying ink, a parallel plate rotor with a diameter of 50 mm was selected and the testing clearance was set to 0.2 mm. The shear rate was selected as 10 s-1, with a sampling period of 6 s and a total duration of 1200 s. The electrospraying ink was preheated in a 37°C water bath for 30 min, then the rheometer temperature was set to 30°C and 37°C respectively and the preheated electrospraying ink was warmed up again on the rheometer for 5 min. In the flow sweep, the rheometer temperature was maintained at 37°C and the shear rate range was set to 1 s-1~100 s-1. In the low amplification oscillation time sweep of thermo-crosslinking process, a parallel plate rotor with a diameter of 25 mm was chosen, and the testing clearance was set to 1 mm. The electrospraying ink was preheated in a 37°C water bath for 30 min and added to rheometer platform. Then, the testing temperature was set to 4°C and the testing was started at once. The oscillation angular frequency was set to 1 rad/s and the oscillation amplification was set to 0.01%. The sampling period was set to 12 s, and the total duration was set to 720 s. In the low amplification oscillation time sweep of extruding bioprinting process, the testing sample in the one of thermo-crosslinking process with stable G’ and G’’ was directly tested. The oscillation angular frequency was set to 10 rad/s and the other parameters remained unchanged.
2.2. Electrospraying of TSMs
The TSMs were prepared with nozzle-ring electric field. The environment temperature was set at 37°C and the humidity was 50%. The nozzle was selected as 27G (outer diameter 0.4 mm and inner diameter 0.2 mm), 28G (outer diameter 0.35 mm, and inner diameter 0.19 mm), 30G (outer diameter 0.3 mm and inner diameter 0.15 mm), and initially hydrophobically treated with nano waterproof sprays in order to ensure the surface hydrophobicity. The flow rate of the electrospraying ink was set to 50 mL/min and 100 mL/min. The applied voltage was selected according to the desired TSM diameter. When the gelatin microdroplets were finally received in silicon oil that was precooling to 4°C, the receiving Petri dish was transferred to refrigerator at 4°C with a thermo-crosslinking time of 400 s. Finally, the totally crosslinked TSMs were transferred to centrifugal tubes and centrifugated for 3 times at 1000 rpm to remove the silicon oil.
2.3. Rheological testing of GelMA precursor solution
Frozen-dried GelMA and photoinitiator LAP were mixed in the PBS at the concentration of 5% w/v and 0.5 w/v, respectively, and fully dissolved for 1 h in a water bath at 37°C. It was then filtered through a 0.22 mm filter for sterilization and kept at the temperature of 4°C. A parallel plate rotor with a diameter of 25 mm was selected and the testing clearance was set to 1 mm. In flow step measurement, GelMA precursor solution was preheated in a 37°C water bath for 30 min and transferred to rheometer at initial temperature of 37°C and kept for another 5 min. The cooling process was set at 4°C, 5 min followed by stabilization process at 24°C, 10 min. Sampling period was set to 10 s and the shear rate was 10 s-1. In flow sweep, GelMA precursor solution was scanned at a temperature of 24°C and the shear rate range was set to 0.1 s-1~100 s-1. In low amplification oscillation frequency sweep, the amplification was set to 1% and the oscillation angular frequency was set to 0.1 rad/s~1000 rad/s.
2.4. Preparation and rheological properties of TSM-B with different recipes
After TSMs were generated, GelMA precursor solution was loaded in 10 mL syringe and preheated at 37°C and was then transferred to 4°C refrigerator for 5 min. TSMs were added to the GelMA precursor solution at 120 s during cooling process at certain volume proportion according to the syringe scale and the syringe was constantly flipped. Finally, the syringe was transferred to bioprinter at 24°C and rest for more than 560 s. In the rheological testing of TSM-B with different recipes, the applied voltage in electrospraying was set as 0 kV, 2 kV, 3 kV, and 3.5 kV (35%) and the volume proportion was set as 50%, 35%, and 20% (3.5 kV), respectively. The testing temperature was set as 24°C. A parallel plate rotor with a diameter of 25 mm was selected. It is worth to be mentioned that because TSM-B was a kind of two-phase bioink with both solid and liquid phase, the testing clearance was set to 5 times the diameter of the selected TSMs. In flow sweep, GelMA precursor solution was scanned at a temperature of 24°C and the shear rate range was set to 0.1 s-1~100 s-1. In low amplification oscillation frequency sweep, the amplification was set to 1% and the oscillation angular frequency was set to 0.1 rad/s~1000 rad/s.
2.5. Observation of solation transferring process of TSMs
As an example, TSM-B was prepared with the TSMs electrosprayed under 3.5 kV voltage and the volume proportion was set as 35%. A little GFP-labeled GelMA (gelatin from the same batch with roughly the same molecular weight) was added to the electrospraying ink as tracer molecules (The gelatin/GFP-GelMA mass ratio was 100:1). The prepared TSM-B was poured into a cylindrical mold (∅9 mm × 6.3 mm) and photocrosslinked for 30 s with a 405 nm blue flashlight. The structures were then soaked in the PBS buffer and incubated in a 37°C incubator, which was shot with confocal fluorescence microscope after 0 h, 5 h, 10 h, and 24 h, respectively.
2.6. Observation of on-demand pores (nutrient channels) distribution
To initially observe the distribution of nutrient channels formed by TSM-B, the applied voltage in electrospraying was set as 0 kV, 2 kV, 3 kV, and 3.5 kV (35%) and the volume proportion was set as 50%, 35%, and 20% (3.5 kV), respectively. To facilitate the observation of nutrient channel patterns under confocal fluorescence microscope, a little GFP-GelMA was added to the GelMA precursor solution (The GelMA/GFP-GelMA mass ratio was 100:1). The prepared TSM-B was poured into the same cylindrical mold (∅9 mm × 6.3 mm), respectively, and photocrosslinked with 405 nm blue flashlight for 30 s. The structures were captured with the confocal fluorescence microscope with the functions of two-dimensional (2D) stitching and Z-stack. In terms of scanning electron microscope (SEM) morphology, the casted centimeter-scale 3D structures were soaked into PBS and incubated in a 37°C incubator. After 24 h, the samples were rapidly frozen with liquid nitrogen and transferred to a vacuum freeze dryer in a vacuum environment at −80°C for 24 h. Then, the freeze-dried samples were tear with scalpel along the cross sections, where were treated with a sputtering coating machine. Finally, the cross sections of the samples were observed and captured with SEM.
2.7. Printability of TSM-B and centimeter-scale structural establishment
The orthogonal experiments of printability were carried out with TSM-B and GelMA precursor solution. The tapered plastic nozzle types were set as 11G, 13G, 16G, 18G, and 20G, respectively. The movement speeds of printing nozzle were set as 40 mm/min, 60 mm/min, 80 mm/min, 100 mm/min, 120 mm/min, 140 mm/min, 160 mm/min, and 180 mm/min, respectively. The bioink flow rate was 150 mL/min. The TSMs were prepared at 3.5 kV voltage and their volume proportion was set as 35%. The print environment temperature was set to 24°C and the temperature of receiving platform was set to 10°C. In terms of 2D or 3D patterns printing, as above, a little GFP-GelMA or RFP-GelMA was added to the GelMA precursor solution. The extruding printing nozzle was 18G and the nozzle movement speed was set to 120 mm/min. Other printing parameters were the same as above. 3D models of complex centimeter-scale 3D structures were built with SolidWorks software and sliced with Repetier software to generate extruding printing path G-code. The layer height was set to 1 mm. The structures were captured with the confocal fluorescence microscope with the functions of 2D stitching and Z-stack.
2.8. Bioprinting of cell-laden centimeter-scale structures
The TSMs with the diameter of 800 mm and 1500 mm were prepared with 3.5 kV and 3.0 kV voltage, respectively. The cell density of GFP-HUVECs (purchased from Zhongqiaoxinzhou Co, Ltd, Shanghai, China) in the gelatin electrospraying ink and MDA-MB-231s (purchased from Zhongqiaoxinzhou Co, Ltd, Shanghai, China) in the GelMA precursor solution was both set as 5 × 106 cells/mL. The volume proportion of TSMs in TSM-B was set as 35%. The concentration of vascular endothelial growth factor (VEGF) in the GelMA precursor solution was set as 200 ng/mL. The bioprinting parameters were all the same with the ones in the printing of complex structures above. The tissue model was set as cube (1 cm × 1 cm × 1 cm). The photocrosslinked tissues were further cultured with complete Dulbecco’s modified eagle medium supplemented with 10% v/v fetal bovine serum and 1% v/v penicillin-streptomycin. In the experiments of growing state testing of MDA-MB-231s, the TSMs with 800 mm were applied. The ones with 1500 mm were applied in the bioprinting of breast tumor tissue with angiogenesis.
3. Results and discussion
3.1. Preparation and diameter regulation of TSMs
The received gelatin microdroplets in silicon oil were thermo-crosslinked at 4°C, followed by centrifugation to separate the TSMs from silicon oil, as shown in Figure 2A. Because the microdroplets at the nozzle tip were affected by gravity, electric field force and surface tension as shown in Figure 2D, the diameters of TSMs could be modified by nozzle size and applied voltage. To get uniform nutrient channels in the printed centimeter-scale structures, according to the previous research of our lab[31], the electrospraying state was selected as micro dripping state, which would occur in relatively lower voltage. The electrospraying process was observed with the high-speed camera, as shown in Figure 2B. To study the change in diameter under different electrospraying parameters, the nozzle size was selected as 27G, 28G, 30G and the voltages were set as 0.0 kV, 1.0 kV, 1.5 kV, 2.0 kV, 2.5 kV, 3.0 kV, and 3.5 kV, respectively. The flow rate of electrospraying ink was set as 50 µL/min and 100 µL/min. The generation frequency of microdroplets and the diameter measurement results are shown in Figure 2C and F, respectively. It could be found that the diameters of TSMs decreased with the reduction of the applied voltages could reach a minimum of about 800 mm. The generation frequency would be higher when smaller nozzle and higher voltage applied. The flow rate of the electrospraying ink did not show obvious effect on the frequency and diameter of microdroplets. From the optical images shown in Figure 2E, the prepared TSMs showed standard spheroid shape due to the existence of surface tension between gelatin microdroplet with aqueous phase and silicon oil with oil phase. Therefore, researchers could choose the TSMs with the required diameters to further form on-demand nutrient channels and angiogenesis distribution.
Figure 2.

Electrospraying process analysis of TSMs. (A) Post-treatment of the received gelatin microdroplets. (B) Images captured by high-speed camera. (C) Frequency of the droplet generation. (D) Force equilibrium of the gelatin microdroplets in the high voltage electric field. (E) Optical images of TSMs. (F) Diameters of TSMs. (G) Viscosity stabilization duration of electrospraying ink. (H) Shear-thinning profile of the electrospraying ink. (I) Thermo-crosslinking duration of TSMs at low temperature. (J) The stability of the crosslinked TSMs in further bioprinting temperature.
3.2. Rheological properties of gelatin electrospraying ink
The reversible crosslinking process of TSMs was achieved by low temperature. Since TSMs would undergo many processes such as centrifugation for separating from silicon oil, mixing with preheated GelMA precursor solution and extruding bioprinting of centimeter-scale 3D structure, for TSMs to finally play their role in constructing nutrient channels and angiogenesis, it is necessary to maintain its original gelation state until GelMA precursor solution is fully photocrosslinked. Throughout these processes, there are two main potential factors probably leading to the instability of the gelation state of TSMs. In terms of the loaded cells, before the electrospraying of TSMs, HUVECs should be initially mixed in gelatin precursor solution to form 3D vascular network in the final centimeter-scale tissue. During the thermo-crosslinking process, gelatin molecules have to bypass these cells and form hydrogel network, which causes the network inside TSMs to be interrupted to some extent. In addition, the movement and metabolic heat of HUVECs can also damage the in the process of preparing TSM-B, TSMs will be mixed with preheated GelMA precursor solution to guarantee the uniformity of the bioink. Besides, during the extruding process, to ensure the printability and the formation of stable and uniform filament, GelMA precursor solution in TSM-B should be at semi-gelation state. In the published study of extrusion printing based on GelMA based bioink, the printing temperature was often set to 20 – 24°C, which would cause TSMs to gradually undergo a solation transferring due to high temperature, losing its original 3D shape[32-36]. Therefore, it is necessary to increase the concentration of the gelatin precursor solution appropriately to increase the quantity and strength of the hydrogel network in TSMs, so that they would maintain a stable gelation state in subsequent “secondary printing.” Here, the concentration of gelatin precursor solution was set as 15% w/v.
In terms of the electrospraying process, the flow step measurement of gelatin electrospraying ink was carried out to explore the stabilization time of the viscosity in the electrospraying environment. In our previous research on the low-concentration GelMA microsphere electrospraying, the environment temperature was set as 30°C to get enough fluidity and shorter viscosity stabilization duration[31]. However, because the concentration of the applied gelatin electrospraying ink was much higher, its viscosity stabilization time may be greatly extended in this temperature. Here, the preheated electrospraying ink (37°C) was added to rheometer and the testing temperature was set to 30°C and 37°C, respectively. The testing results are shown in Figure 2. The viscosity of the preheated electrospraying ink in 30°C testing temperature continuously increased within the 1200 s testing process and failed to get the stable state. However, in the 37°C testing temperature, the viscosity maintained stable and was at the lower level, as shown in Figure 2G. Based on this, the flow sweep was carried out and the result showed that the electrospraying ink owned obvious shear-thinning property as shown in Figure 2H and would own lower viscosity when flowed from the nozzle tip, which was suitable for microdroplet electrospraying.
In terms of the low-temperature crosslinking process of TSMs, the received gelatin microdroplet in the silicon oil would be crosslinked at 4°C. Therefore, the thermo-crosslinking duration needs to be determined to get microdroplets totally crosslinked from 37°C to 4°C. Here, low amplification oscillation time sweep was carried out. The results as shown in Figure 2I demonstrated that, at the beginning 60 s, the electrospraying ink maintained fluid state (G’<G’’). At 60 s, gelatin electrospraying ink was at semi-gelation state. During 60 s~400 s, G’’ further increased and G’ decreased, demonstrating the gelation state was further enhanced. After 400 s, G’ and G’’ tended to be stable and the microdroplets was totally crosslinked to be TSMs. Therefore, the thermo-crosslinking treatment duration should be above 400 s to get solid TSMs for further extruding bioprinting.
In terms of the extruding bioprinting process, the TSMs should be mixed with GelMA precursor solution. According to the discussion above, this process would be carried out at the range of 20°C~24°C. Thus, the sol-gel state of the crosslinked microspheres in this temperature range should be tested. Here, low amplification oscillation time testing was carried out and the testing temperature was set as 24°C, as shown in Figure 2J. It could be found that even though G’ decreases and G’’ increased gradually, G’’ was greater than G’, indicating that the electrospraying ink maintain gelation state, proving that the prepared TSMs could be further used as an auxiliary component in TSM-B to participate in subsequent extruding printing.
3.3. Rheological properties of TSM-B with different recipes
To form stable and uniform filament during the extruding bioprinting, the temperature control method of TSM-B needs to be properly arranged. In the preparation process of TSM-B, to ensure that the original shapes of the added TSMs, it is necessary to initially reduce the temperature of the preheated GelMA precursor solution to weaken the solation transferring level. Meanwhile, in the cooling process, TSMs should be added when the GelMA precursor solution is still with a low viscosity so that the added TSMs can be evenly dispersed in the GelMA precursor solution. Moreover, in the extruding bioprinting process, GelMA precursor solution TSM-B should be stabilized at the printing temperature for a certain period of time, so that it will eventually be at semi-gelation state which is suitable for extruding bioprinting.
The rheological properties of 5% w/v GelMA precursor solution containing 0.5% w/v photoinitiator lithium phenyl-2, 4, 6-trimethylbenzoylphosphinate (LAP) were first tested. In terms of the preparation process of TSM-B, the viscosity of 37°C GelMA precursor solution in rapid cooling process at 4°C (5 min) and stabilizing process at 24°C was tested. As shown in Figure 3A, during the first 5 min cooling process, the temperature of GelMA precursor solution gradually dropped to 4°C, which would ensure the initial shape of TSMs. At 120 s, the temperature of GelMA precursor solution was around 15°C while the viscosity was still at a low level though, so that its fluidity was better and was conducive to the uniform dispersion of the TSMs in TSM-B. Subsequently, during the stabilization process in 24°C, the viscosity gradually decreased until the viscosity stabilized at 560 s. These results provide a guide of the time periods of temperature controlling during the preparation of TSM-B.
Figure 3.

Rheological properties of TSM-B with different recipes. (A) Viscosity stabilization duration of GelMA precursor solution during rapid cooling and recovery process. (B) Shear-thinning profile of the GelMA precursor solution. (C) Results of low amplification oscillation frequency sweep. (D) Shear-thinning profile of TSM-B composed of TSMs with different diameters. (E) Results of low amplification oscillation frequency sweep of TSM-B composed of TSMs with different diameters. (F) Shear-thinning profile of TSM-B composed of TSMs with different volume proportions. (G) Results of low amplification oscillation frequency sweep of TSM-B composed of TSMs with different volume proportions.
In terms of the extruding bioprinting process, the flow step measurement and low amplification oscillation frequency sweep of GelMA precursor solution were carried out. The testing temperature was set at the printing temperature (24°C) to explore the extruding printability in the stable viscosity state. As shown in Figure 3B, GelMA precursor solution owned obvious shear-thinning property at 24°C. At the same time, as shown in Figure 3C, GelMA precursor solution exhibited significant elastic feature in the low frequencies and viscous feature in the high ones, indicating that it could be successfully extruded (high frequency) with good fluidity while maintain the 3D shape after extrusion and deposition on the printing platform (low frequency). These results verified the promising extruding printability of GelMA precursor solution at 24°C.
TSM-B could be prepared according to different requirements and was mixed with TSMs with different diameters and volume proportions. Here, TSM-B with different TSM diameters or volume proportions were prepared. The flow sweep and low amplification oscillation frequency sweep at 24°C were carried out, respectively. As shown in Figure 3D-G, the addition of TSMs with different diameters and volume proportions had no obvious effect on the shear-thinning property and the sol-gel transferring feature of TSM-B in that GelMA precursor solution accounted larger proportion.
3.4. Morphology of on-demand nutrient channels in centimeter-scale structure
To explore the process of solation transferring process and diffusion process of TSMs, the casted centimeter-scale 3D structure based on TSM-B was soaked in PBS and transferred to a 37°C culture environment. As shown in Figure 4A, the area of green fluorescence concentrated in the location of original TSMs before incubation. At the 5th h, the green fluorescence intensity decreased, indicating that the TSMs has begun to transfer to solation state and diffuse outwards. At the 10th h, the green fluorescence intensity further decreased and spherical pores were formed. There was almost no green fluorescence signal in the 3D structure at the 24th h, indicating that the internal TSMs have fully finished the mission of forming nutrient channels and diffused outside the 3D structure.
Figure 4.
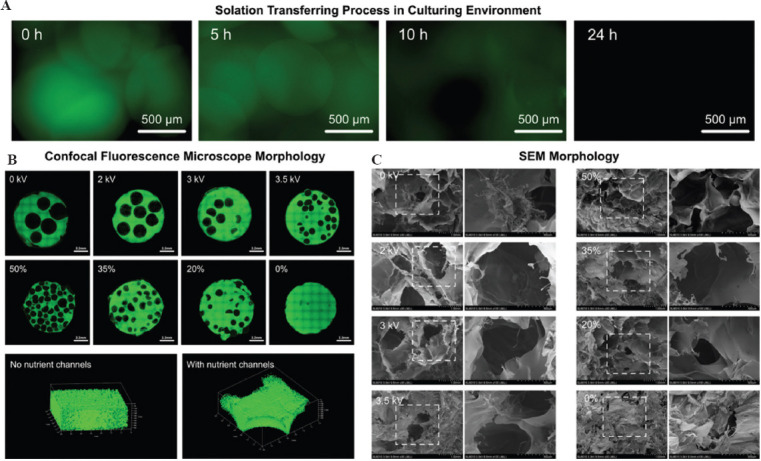
Morphology of on-demand nutrient channels in centimeter-scale structure. (A) Solation transferring process of the TSMs in the culturing environment. (B) Confocal fluorescence microscope morphology of the on-demand nutrient channels distribution. (C) SEM morphology of the on-demand nutrient channels distribution.
To get centimeter-scale structures with different nutrient channels distribution, the TSM-B was prepared with TSMs electrosprayed with different voltages at different volume proportions with GelMA precursor solution. To facilitate the observation of nutrient channel patterns under confocal fluorescence microscope, a little GFP-GelMA was added to GelMA precursor solution. As shown in Figure 4B, with the increasing of the volume proportions of TSMs, the quantity of nutrient channels increased and the distribution became more even. Moreover, by mixing TSMs with different diameters, pores (nutrient channels) with different sizes could be formed. With the decreasing of the TSMs diameters, the distribution of nutrient channels became more uniform.
In terms of microscale morphology, the incubated centimeter-scale structures after 24 h were treated and observed with SEM. The morphologies of the on-demand nutrient channels in the structures are shown in Figure 4C. In addition to the pores with microscale formed by GelMA precursor solution itself, the sol-gel transferring process of TSMs has successfully form more and larger pores in millimeter scale in the centimeter-scale 3D structures. A higher volume proportion diameters of TSMs could establish more and larger nutrient channels.
3.5. Printability of TSM-B in extruding bioprinting
A basic extruding printability indicator is the continuity of the extruded filament, as shown in Figure 5A. From the results of printability testing, as shown in Figure 5B and Figure S5 (637KB, pdf) , the addition of the electrosprayed TSMs did not restrict the printability of GelMA precursor solution, though the printable ranges of nozzle size and printing speed were a little different. Furthermore, the extruded filament diameters under different printing parameters were measured, as shown in Figure 5C. The filament diameters increased with the decreasing of the nozzle movement speed. Noticeably, the filament diameters were generally lower in TSM-B groups than the ones in GelMA groups. It was probably because when the extruded filament attached to the cooling platform of the bioprinter, the deposited filament would be fixed by friction and viscous force. Thus, with the continuous movement of the bioprinting nozzle, the pure GelMA segments between two gelatin microspheres inside the filament, which is being extruded, would be further stretched, so that the diameters of this part decreased.
Figure 5.

Printability of TSM-B in extruding bioprinting. (A) Sketch of printability of TSM-B. (B) Printability of TSM-B and GelMA precursor solution. (C) Filament diameter generated by TSM-B. (D) 2D patterns printed by TSM-B. (E) 3D structures printed by TSM-B.
Based on the detailed printability analysis above, a series of structural printings with the proposed TSM-B in practice was carried out. 2D patterns printing, including right-angle printing, curve printing, as well as multi-materials printing, were tested here (Figure 5D). In the close-up images, the pores inside the filaments could be clearly seen. In terms of 3D structures, we tried to establish centimeter-scale structures around 2.5 cm at maximum dimension. Three significant constructions in 3D bioprinting field, namely, scaffold, complex structures, and multi-materials structures, were established, as shown in Figure 5E. The formed spherical pores inside the structures could be seen, which confirmed the potential of the bioink in further building centimeter-scale tissue with rich nutrient channels and angiogenesis.
3.6. Growth of porous centimeter-scale breast tumor tissue printed by TSM-B
During the gradual deterioration process of tumors in organisms, their size will gradually increase to the centimeter scale. Meanwhile, in such a large tissue, rich 3D blood vessels will spontaneously form under appropriate induction condition such as VEGF[37,38] to provide adequate nutrients and oxygen for the growth of tumor cells. Therefore, in the related research of tumor tissue, the large scale tumor tissue with 3D vascular networks constructed in vitro owns much application value. The validity of the established centimeter-scale tumor tissue with angiogenesis is mainly reflected by two factors: the normal growth of the subject tumor cells and the vascularization of vessel cells. Firstly, GelMA precursor solution (containing VEGF) mixed with human breast tumor cells (MDA-MB-231s) was applied to prepare TSM-B, with which cubic tumor tissue at centimeter scale (1 cm × 1 cm × 1 cm) was printed to examine the effect of nutrient channels on the growth of tumor cells. As a control, the GelMA precursor solution (containing VEGF) without TSMs was printed in the same way as the centimeter-scale tumor tissue without nutrient channel.
On the 1st, 3rd, and 5th days of culture, the survival of MDA-MB-231s in the centimeter-scale 3D structures were tested with Live/Dead cell staining kits. It is worth emphasizing that because nutrient/oxygen supply at the center cross section of the centimeter-scale 3D structure would be the least adequate, the cell viability in the region can directly reflect the effectiveness of the printed tissue. Thus, the stained cells there were observed and captured with confocal fluorescence microscope and the viability was analyzed with ImageJ software, as shown in Figure 6A. The results showed that MDA-MB-231s in the centimeter-scale 3D structure without nutrient channels died from lack of nutrient and oxygen after 1-day culture, while the ones in the structures printed with TSM-B maintained the viability of more than 95% during 5-day culture benefiting from the rich nutrient channels, as shown in Figure 6B and D. Furthermore, the cell counting kit-8 (CCK-8) results of the loaded MDA-MB-231s showed that TSM-B could ensure the proliferation of the encapsulated cell, as shown in Figure 6E. In addition, the loaded F-actin and nucleus of MDA-MB-231s were stained by phalloidin and DAPI (4d,6-diamidino-2-phenylindole) to observe the cell morphology. The results showed that during the 5-day culture, most of the MDA-MB-231s in the centimeter-scale 3D structures with nutrient channels were spreading and migrating, while the ones in the structures without nutrient channels showed to be spherical shape or beginning to crack, as shown in Figure 6C. The results above verified the capability the TSM-B to provide promising growing environment and sufficient nutrient channels for the encapsulated subject cells.
Figure 6.

Growing of porous centimeter-scale breast tumor tissue printed by TSM-B. (A) Sketch of the sample observation. (B) Live/Dead testing of MDA-MB-231s. (C) F-actin and nucleus staining of MDA-MB-231s. (D) Viability of the loaded MDA-MB-231s. (E) Proliferation of the loaded MDA-MB-231s tested by CCK-8 kits.
3.7. Vascularization of endothelial cells in a centimeter-scale 3D structure
To demonstrate the ability of TSM-B to form 3D vessels spontaneously, GFP-HUVECs-loaded TSMs were electrosprayed under the voltage of 3.5 kV and 3 kV, respectively, with diameters of approximately 800 µm and 1500 µm. With the prepared TSMs, two kinds of TSM-B were prepared, respectively, with GelMA precursor solution (containing VEGF), with which cubic tissue at centimeter scale (1 cm × 1 cm × 1 cm) was printed to examine the effect of angiogenesis. From the images captured by fluorescence microscope, at the start of culture, GFP-HUVECs were initially distributed in the area of TSMs, as shown in Figure 7A. After culture, from the images shown in Figure 7C, GFP-HUVECs gradually settled and adhered to the inner surfaces of the nutrient channels with the solation transferring of TSMs. On the 2nd day of culture, the attached GFP-HUVECs in the structures with 1500 mm nutrient channels began to show the sign of 3D sprout under the induction of VEGF. On the 3rd day of culture, it could be found that GFP-HUVECs in porous structures with the two nutrient channel sizes began to form 3D sprout into the GelMA hydrogel. On the 5th day of culture, the fluorescence microscope images showed that GFP-HUVECs further formed longer 3D sprout structures into the GelMA hydrogel and began to exhibit angiogenesis in the 3D environment, as shown in Figure 7B.
Figure 7.

Angiogenesis in the centimeter-scale structures printed with TSM-B. (A) Distribution of GFP-HUVECs at the start of culture. (B) Fluorescence microscope images of 3D sprout of GFP-HUVECs on the 5th day. (C) Optical microscope images of attachment and 3D sprout of GFP-HUVECs on the 2nd and 3rd day.
Besides, β-tubulin proteins of the loaded GFP-HUVECs were stained and observed by confocal fluorescence microscope. As shown in Figure 8C, GFP-HUVECs showed obvious 3D sprout in the centimeter-scale 3D structures with both nutrient channel sizes. It is worth noting that, since the GFP-HUVECs density in the electrospraying ink was the same, the cell attaching density on the inner surface would be different. The attaching density obtained by 800 µm and 1500 µm TSMs was found to be 1333.33 cells/mm2 and 2500 cells/mm2 respectively (refers to the additional discussion in Supplementary File). Thus, more immense angiogenesis would happen at higher attaching density of endothelial cells. What is more, CD31, which is a kind of marker protein of HUVECs, could normally express, as shown in Figure 8A. The connection between GelMA hydrogel and cell membrane was established, which was verified by the staining results of vinculin[39-41], which is a common marker protein showing that cells are connected to the extracellular matrix with signal transmission, as shown in Figure 8B. In addition, VE-cadherin is a kind of specific protein showing cell-to-cell connection among endothelial cells[42-44], which can verify angiogenesis. From the staining result, it could be found that the encapsulated GFP-HUVECs in the centimeter-scale structures with two nutrient channel sizes normally expressed, as shown in Figure 8C which further confirmed the successful formation of vascular tissue in the constructed porous structures.
Figure 8.

Confocal fluorescence microscope images of corresponding proteins. (A) CD31, (B) Vinculin, (C) β-tubulin, (D) VE-cadherin.
3.8. Bioprinting of centimeter-scale breast tumor tissue with angiogenesis
Based on the above verification of normal growth of MDA-MB-231s and the 3D vascularization of GFP-HUVECs in the centimeter-scale 3D structures constructed with TSM-B, TSM-B composed of GelMA precursor solution mixed with MDA-MB-231s and TSM loading GFP-HUVECs were prepared and applied to print centimeter-scale breast tumor tissue with angiogenesis. As shown in Figure 9, after 7-day culture, longer sprout of GFP-HUVECs toward to the MDA-MB-231s could be found and more vessel branches formed in the tumor tissue. After 12-day culture, 3D sprouts of GFP-HUVECs from different nutrient channels began to converge with each other. Due to the increasing of VEGF in the 3D environment excreted by the encapsulated MDA-MB-231s, the 3D angiogenesis phenomenon was more obvious and the 3D vessel network became more complex. The results showed that in vitro bioprinting of centimeter-scale breast tumor tissue with angiogenesis was successfully achieved based on the proposed TSM-B, demonstrating its capability in more corresponding biomedical applications in future.
Figure 9.

Bioprinting of centimeter-scale breast tumor tissue with angiogenesis.
Centimeter-scale tissues based on hydrogel materials constructed by 3D bioprinting technology are of great application value. However, the question of the substances exchanging inside and outside centimeter-scale structure and 3D angiogenesis inside large-scale structure has restricted the development of centimeter-scale tissues. Combining to the hot spot of “secondary printing” of microspheres currently and the bran-new requirements of bioprinting to researchers, an innovative microsphere-based bioink system was invented in this paper, which was expected to become an important model in the research of tumor developing and anti-cancer drugs corresponding to angiogenesis.
4. Conclusion
Benefiting from the reversible thermo-sensitive crosslinking feature of non-modified gelatin, sacrificial microspheres that can respond to temperature changes were prepared by the electrohydrodynamics principle. Based on this, on-demand nutrient channels with different sizes and distribution could be formed in the centimeter-scale GelMA structures by sol-gel transferring, providing sufficient nutrient and oxygen for the encapsulated cells. To examine the feasibility of the proposed bioink system, the sol-gel transferring behavior, printability, and capability of nutrient channel formation were analyzed and verified in detail. More importantly, the sacrificial microspheres loaded endothelial cells could be further vascularized and form complex 3D vessel network in the printed breast tumor tissue, which indicated the great potential of this bioink system in various biomedical applications.
Funding
This study was sponsored by the National Key Research and Development Program of China (2018YFA0703000, Yong He), the National Natural Science Foundation of China (No. U1909218, Yong He), the Science Fund for Creative Research Groups of the National Natural Science Foundation of China (No. T2121004, Yong He).
Acknowledgments
This study was sponsored by the National Key Research and Development Program of China (2018YFA0703000, Yong He), the National Natural Science Foundation of China (No. U1909218, Yong He), the Science Fund for Creative Research Groups of the National Natural Science Foundation of China (No. T2121004, Yong He).
Conflict of interest
All authors declare no financial/commercial conflicts of interest.
Author contributions
Conceptualization: Yong He, Jianzhong Fu, Zichen Chen
Investigation: Mingjun Xie, Yuan Sun, Zhenliang Fu
Methodology: Mingjun Xie
Formal analysis: Mingjun Xie, Ji Wang, Lei Pan
Writing - original draft: Mingjun Xie
Writing - review & editing: Yong He, Ji Wang
References
- 1.He Y, Gu Z, Xie M, et al. Why Choose 3D Bioprinting?Part II:Methods and Bioprinters. BioDesign Manuf. 2020;3:1–4. https://doi.org/10.1007/s42242-020-00064-w. [Google Scholar]
- 2.Thakor J, Ahadian S, Niakan A, et al. Engineered Hydrogels for Brain Tumor Culture and Therapy. BioDesign Manuf. 2020;3:203–26. doi: 10.1007/s42242-020-00084-6. https://doi.org/10.1007/s42242-020-00084-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee M, Bae K, Guillon P, et al. Exploitation of Cationic Silica Nanoparticles for Bioprinting of Large-Scale Constructs with High Printing Fidelity. ACS Appl Mater Interfaces. 2018;10:37820–8. doi: 10.1021/acsami.8b13166. https://doi.org/10.1021/acsami.8b13166. [DOI] [PubMed] [Google Scholar]
- 4.Ying GL, Jiang N, Maharjan S, et al. Aqueous Two-Phase Emulsion Bioink-Enabled 3D Bioprinting of Porous Hydrogels. Adv Mater. 2018;30:1805460. doi: 10.1002/adma.201805460. https://doi.org/10.1002/adma.201805460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shao L, Gao Q, Xie C, et al. Sacrificial Microgel-laden Bioink-enabled 3D Bioprinting of Mesoscale Pore Networks. BioDesign Manuf. 2020;3:30–9. https://doi.org/10.1007/s42242-020-00062-y. [Google Scholar]
- 6.Eilken HM, Adams RH. Dynamics of Endothelial Cell Behavior in Sprouting Angiogenesis. Curr Opin Cell Biol. 2010;22:617–25. doi: 10.1016/j.ceb.2010.08.010. https://doi.org/10.1016/j.ceb.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Eng G, Lee BW, Parsa H, et al. Assembly of Complex Cell Microenvironments using Geometrically Docked Hydrogel Shapes. Proc Natl Acad Sci. 2013;110:4551–6. doi: 10.1073/pnas.1300569110. https://doi.org/10.1073/pnas.1300569110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee VK, Kim DY, Ngo H, et al. Creating Perfused Functional Vascular Channels using 3D Bio-printing Technology. Biomaterials. 2014;35:8092–102. doi: 10.1016/j.biomaterials.2014.05.083. https://doi.org/10.1016/j.biomaterials.2014.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakatsu MN, Sainson RC, Aoto JN, et al. Angiogenic Sprouting and Capillary Lumen Formation Modeled by Human Umbilical Vein Endothelial Cells (HUVEC) in Fibrin Gels:The Role of Fibroblasts and Angiopoietin-1. Microvasc Res. 2003;66:102–12. doi: 10.1016/s0026-2862(03)00045-1. https://doi.org/10.1016/s0026-2862(03)00045-1. [DOI] [PubMed] [Google Scholar]
- 10.Kratochvil MJ, Seymour AJ, Li TL, et al. Engineered Materials for Organoid Systems. Nat Rev Mater. 2019;4:606–22. doi: 10.1038/s41578-019-0129-9. https://doi.org/10.1038/s41578-019-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daly AC, Riley L, Segura T, et al. Hydrogel Microparticles for Biomedical Applications. Nat Rev Mater. 2019;5:20–43. doi: 10.1038/s41578-019-0148-6. https://doi.org/10.1038/s41578-019-0148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parsa S, Gupta M, Loizeau F, et al. Effects of Surfactant and Gentle Agitation on Inkjet Dispensing of Living Cells. Biofabrication. 2010;2:025003. doi: 10.1088/1758-5082/2/2/025003. https://doi.org/10.1088/1758-5082/2/2/025003. [DOI] [PubMed] [Google Scholar]
- 13.Caldwell AS, Campbell GT, Shekiro KM, et al. Clickable Microgel Scaffolds as Platforms for 3D Cell Encapsulation. Adv Healthc Mater. 2017;6:254. doi: 10.1002/adhm.201700254. https://doi.org/10.1002/adhm.201770080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leong W, Lau TT, Wang DA. A Temperature-cured Dissolvable Gelatin Microsphere-based Cell Carrier for Chondrocyte Delivery in a Hydrogel Scaffolding System. Acta Biomater. 2013;9:6459–67. doi: 10.1016/j.actbio.2012.10.047. https://doi.org/10.1016/j.actbio.2012.10.047. [DOI] [PubMed] [Google Scholar]
- 15.Chen X, Bai S, Li B, et al. Fabrication of Gelatin Methacrylate/Nanohydroxyapatite Microgel Arrays for Periodontal Tissue Regeneration. Int J Nanomed. 2016;11:4707–18. doi: 10.2147/IJN.S111701. https://doi.org/10.2147/ijn.s111701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinclair A, O'Kelly MB, Bai T, et al. Self-healing Zwitterionic Microgels as a Versatile Platform for Malleable Cell Constructs and Injectable Therapies. Adv Mater. 2018;30:e1803087. doi: 10.1002/adma.201803087. https://doi.org/10.1002/adma.201870291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seeto WJ, Tian Y, Winter RL, et al. Encapsulation of Equine Endothelial Colony Forming Cells in Highly Uniform, Injectable Hydrogel Microspheres for Local Cell Delivery. Tissue Eng Part C Methods. 2017;23:815–25. doi: 10.1089/ten.TEC.2017.0233. https://doi.org/10.1089/ten.tec.2017.0233. [DOI] [PubMed] [Google Scholar]
- 18.Xie M, Gao Q, Fu J, et al. Bioprinting of Novel 3D Tumor Array Chip for Drug Screening. BioDesign Manuf. 2020;3:175–88. https://doi.org/10.1007/s42242-020-00078-4. [Google Scholar]
- 19.Cai S, Shi H, Li G, et al. 3D-printed Concentration-controlled Microfluidic Chip with Diffusion Mixing Pattern for the Synthesis of Alginate Drug Delivery Microgels. Nanomaterials (Basel) 2019;9:1451. doi: 10.3390/nano9101451. https://doi.org/10.3390/nano9101451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Highley CB, Song KH, Daly AC, et al. Jammed Microgel Inks for 3D Printing Applications. Adv Sci (Weinh) 2019;6:1801076. doi: 10.1002/advs.201801076. https://doi.org/10.1002/advs.201801076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.An C, Liu W, Zhang Y, et al. Continuous Microfluidic Encapsulation of Single Mesenchymal Stem Cells using Alginate Microgels as Injectable Fillers for Bone Regeneration. Acta Biomater. 2020;111:181–96. doi: 10.1016/j.actbio.2020.05.024. https://doi.org/10.1016/j.actbio.2020.05.024. [DOI] [PubMed] [Google Scholar]
- 22.Hinton TJ, Jallerat Q, Palchesko RN, et al. Three-dimensional Printing of Complex Biological Structures by Freeform Reversible Embedding of Suspended Hydrogels. Sci Adv. 2015;1:e1500758. doi: 10.1126/sciadv.1500758. https://doi.org/10.1126/sciadv.1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeon O, Lee YB, Jeong H, et al. Individual Cell-only Bioink and Photocurable Supporting Medium for 3D Printing and Generation of Engineered Tissues with Complex Geometries. Mater Horiz. 2019;6:1625–31. doi: 10.1039/c9mh00375d. https://doi.org/10.1039/c9mh00375d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan MR, Sadiq MB. Importance of Gelatin, Nanoparticles and their Interactions in the Formulation of Biodegradable Composite Films:A Review. Polym Bull. 2020;78:4047–73. https://doi.org/10.1007/s00289-020-03283-4. [Google Scholar]
- 25.Ranasinghe RA, Wijesekara WL, Perera PR, et al. Functional and Bioactive Properties of Gelatin Extracted from Aquatic Bioresources-a Review. Food Rev Int. 2022;38(4):812–55. https://doi.org/10.1080/87559129.2020.1747486. [Google Scholar]
- 26.Rigueto CV, Nazari MT, Massuda LÁ, et al. Production and Environmental Applications of Gelatin-based Composite Adsorbents for Contaminants Removal:A Review. Environ Chem Lett. 2021;18:3. https://doi.org/10.1007/s10311-021-01184-0. [Google Scholar]
- 27.Rodríguez-Rodríguez R, Espinosa-Andrews H, Velasquillo-Martínez C, et al. Composite Hydrogels Based on Gelatin, Chitosan and Polyvinyl Alcohol to Biomedical Applications:A Review. Int J Polym Mater Polym Biomater. 2020;69:1–20. https://doi.org/10.1080/00914037.2019.1581780. [Google Scholar]
- 28.Yang Z, Chaieb S, Hemar Y. Gelatin-based Nanocomposites:A Review. Polym Rev. 2021;61:1–49. https://doi.org/10.1080/15583724.2021.1897995. [Google Scholar]
- 29.Shoulders MD, Raines RT. Collagen Structure and Stability. Ann Rev Biochem. 2009;78:929–58. doi: 10.1146/annurev.biochem.77.032207.120833. https://doi.org/10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marini JC, Forlino A, Cabral WA, et al. Consortium for Osteogenesis Imperfecta Mutations in the Helical Domain of Type I Collagen:Regions Rich in Lethal Mutations Align with Collagen Binding Sites for Integrins and Proteoglycans. Hum Mutation. 2007;28:209–21. doi: 10.1002/humu.20429. https://doi.org/10.1002/humu.20429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie M, Gao Q, Zhao H, et al. Electro-assisted Bioprinting of Low-concentration GelMA Microdroplets. Small. 2019;15:1804216. doi: 10.1002/smll.201804216. https://doi.org/10.1002/smll.201804216. [DOI] [PubMed] [Google Scholar]
- 32.Janmaleki M, Liu J, Kamkar M, et al. Role of Temperature on Bio-printability of Gelatin Methacryloyl Bioink in Two-step Cross-linking Strategy for Tissue Engineering Applications. Biomed Mater. 2020;16:015021. doi: 10.1088/1748-605X/abbcc9. https://doi.org/10.1088/1748-605x/abbcc9. [DOI] [PubMed] [Google Scholar]
- 33.Lee JY, Kim GH. A Cryopreservable Cell-laden GelMa-based Scaffold Fabricated using a 3D Printing Process Supplemented with an In Situ Photo-crosslinking. J Indust Eng Chem. 2020;85:249–57. https://doi.org/10.1016/j.jiec.2020.02.007. [Google Scholar]
- 34.Li H, Tan YJ, Kiran R, et al. Submerged and Non-submerged 3D Bioprinting Approaches for the Fabrication of Complex Structures with the Hydrogel Pair GelMA and Alginate/Methylcellulose. Addit Manuf. 2021;37:101640. https://doi.org/10.1016/j.addma.2020.101640. [Google Scholar]
- 35.Rastin H, Ormsby RT, Atkins GJ, et al. 3D Bioprinting of Methylcellulose/Gelatin-methacryloyl (MC/GelMA) Bioink with High Shape Integrity. ACS Appl Bio Mater. 2020;3:1815–26. doi: 10.1021/acsabm.0c00169. https://doi.org/10.1021/acsabm.0c00169. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Kankala RK, Zhu K, et al. Coaxial Extrusion of Tubular Tissue Constructs using a Gelatin/GelMA Blend Bioink. ACS Biomater Sci Eng. 2019;5:5514–24. doi: 10.1021/acsbiomaterials.9b00926. https://doi.org/10.1021/acsbiomaterials.9b00926. [DOI] [PubMed] [Google Scholar]
- 37.Albig AR, Schiemann WP. Fibulin-5 Antagonizes Vascular Endothelial Growth Factor (VEGF) Signaling and Angiogenic Sprouting by Endothelial Cells. DNA Cell Biol. 2004;23:367–79. doi: 10.1089/104454904323145254. https://doi.org/10.1089/104454904323145254. [DOI] [PubMed] [Google Scholar]
- 38.Gerhardt H, Golding M, Fruttiger M, et al. VEGF Guides Angiogenic Sprouting Utilizing Endothelial Tip Cell Filopodia. J Cell Biol. 2003;161:1163–77. doi: 10.1083/jcb.200302047. https://doi.org/10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grashoff C, Hoffman BD, Brenner MD, et al. Measuring Mechanical Tension Across Vinculin Reveals Regulation of Focal Adhesion Dynamics. Nature. 2010;466:263–6. doi: 10.1038/nature09198. https://doi.org/10.1038/nature09198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Humphries JD, Wang P, Streuli C, et al. Vinculin Controls Focal Adhesion Formation by Direct Interactions with Talin and Actin. J Cell Biol. 2007;179:1043–57. doi: 10.1083/jcb.200703036. https://doi.org/10.1083/jcb.200703036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ziegler WH, Liddington RC, Critchley DR. The Structure and Regulation of Vinculin. Trends Cell Biol. 2006;16:453–60. doi: 10.1016/j.tcb.2006.07.004. https://doi.org/10.1016/j.tcb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Dejana E, Bazzoni G, Lampugnani MG. Vascular Endothelial (VE)-cadherin:Only an Intercellular Glue? Exp Cell Res. 1999;252:13–9. doi: 10.1006/excr.1999.4601. https://doi.org/10.1006/excr.1999.4601. [DOI] [PubMed] [Google Scholar]
- 43.Dejana E, Orsenigo F, Lampugnani MG. The Role of Adherens Junctions and VE-cadherin in the Control of Vascular Permeability. J Cell Sci. 2008;121:2115–22. doi: 10.1242/jcs.017897. https://doi.org/10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- 44.Vestweber D. VE-cadherin:The Major Endothelial Adhesion Molecule Controlling Cellular Junctions and Blood Vessel Formation. Arterioscler Thromb Vasc Biol. 2008;28:223–32. doi: 10.1161/ATVBAHA.107.158014. https://doi.org/10.1161/atvbaha.107.158014. [DOI] [PubMed] [Google Scholar]


