Abstract
The rising global incidence of cancer and high attrition rates of anticancer drugs make it imperative to design novel screening platforms to increase the success rate of chemotherapeutic agents. Advances in cell culture models from two-dimensional to three-dimensional platforms, along with microfluidics, have resulted in the creation of tumor-on-a-chip technology, which enables high-throughput molecular screening and helps to simulate the dynamic tumor microenvironment. Furthermore, advancements in bioprinting have allowed the structural and physiological aspects of the tumor to be recreated accurately and help to mimic cell-cell interactions and cell-extracellular matrix. This paper provides a comprehensive review of three-dimensional bioprinting to fabricate a tumor-on-a-chip platform to advance the discovery and screening of anticancer agents and provides a perspective on the challenges and future directions associated with the adoption of this technology to advance cancer research.
Keywords: 3D bioprinting, Tumor-on-a-chip platform, Anticancer drug screening
1. Introduction
Cancer is the second leading cause of mortality worldwide, accounting for 9.6 million fatalities in 2018, or one in every six deaths[1]. Global Cancer Statistics (GLOBACAN) estimated that 19.3 million new cancer cases and over 10 million cancer deaths would occur in 2020, indicating an upward trend in cancer incidence worldwide[2,3]. The high burden of cancer and cancer-related mortality has led to huge investment in time and money by pharmaceutical companies in drug development of oncologic treatments. Even after extensive research has been conducted, patients’ response to cancer therapies still remains unpredictable, and cancer is linked to a significant financial burden, particularly in developing nations[4,5]. The success rate of anticancer medications entering clinical trials and receiving U.S. Food and Drug Administration marketing approval is between 5% and 10%, which is significantly lower than that of drugs for other diseases[6-8]. Candidates that show promise in the preclinical stage often fail during clinical development, suggesting that current in vitro preclinical models used for drug screening are not reliable in predicting in vivo efficacy and toxicity of anticancer drugs in humans[8]. The standard treatment for cancer relies on a “one-size-fits-all” treatment approach using chemotherapeutic agents that may not always show positive responses in all patients with cancer, thereby necessitating the development of personalized or precision treatment options for certain patient populations. High attrition rates for anticancer drug candidates suggest the development of more effective preclinical platforms for screening anticancer compounds will potentially increase their success rate in the clinical phase pipeline[9].
Conventionally, cancer drug discovery and preclinical screening have relied on animal models and monolayer cell cultures (two-dimensional or 2D models) which most often cannot recapitulate the physiological properties and dynamics of the human tumor microenvironment (TME)[10]. Due to a lack of biological and mechanical stimuli that cancer cells would ordinarily encounter in vivo, planar 2D cell cultures have some constraints. The TME is complicated, with malignant cells interacting with one another and various types of cells entrenched in a three-dimensional (3D) extracellular matrix (ECM)[11-13]. In addition to the ethical considerations and time-consuming nature of animal trials, the success rate of translating animal models to cancer clinical trials is about 8%. This indicates the inability of animal trials to replicate human responses accurately in complex processes, such as human carcinogenesis and progression, and capture interspecies differences[14,15].
Three-dimensional models allow for the reconstruction of the complex TME and are therefore significant in the advancement of anticancer treatments. Cell-cell and cell-ECM interactions, carcinogenesis, drug discovery, gene expression, metabolic profiling, and protein profiling of cells may all be studied using 3D cell culture[16,17]. The interaction of cells and biomaterials is mainly based on the biomaterials’ structural and physical properties, such as pore size feature, material size feature, mechanical, and surface properties. For example, porous constructs of biomaterials promote the cell migration, viability, morphology, and alignment[18].
Tumors can be effectively cultivated in a 3D microenvironment or ECM, allowing cells to be exposed to oxygen and nutritional gradients, resulting in disparities in cell proliferation. These features of tumors cannot be recreated in 2D models, making 3D models better equipped for drug screening[19,20]. For 3D modeling of the TME, several techniques have been developed, including spheroid culture, organoid culture, biopolymer scaffolds, and tumor-on-a-chip platforms[21]. Three-dimensional cancer models can potentially reduce the costs associated with the drug development phase by decreasing the number of animals needed for preclinical studies and allowing for more accurate predictions of drug candidates in the clinical study phase.
The advancement of printing from 2D to 3D approach has resulted in the creation of 3D tumor tissue constructs that may be utilized to study cancer biology and evaluate prospective drug candidates. By permitting the construction of numerous distinct types of cells and biomaterials with great precision and repeatability, 3D bioprinting can capture the entire complexity of the TME[12]. Integration of the vascular network with cancer cells can be performed by techniques, such as sacrificial bioprinting, microfluidics, and stereolithography bioprinting[22-24]. Bioprinting may be used to develop tumor-on-a-chip systems that combine additive manufacturing, tissue engineering, and biomaterials to mimic the physiological dynamic properties of a tumor. These systems hold a lot of potential for low-cost, high-throughput anticancer drug screening[25-27].
Among additive manufacturing, melt electrowriting (MEW) is an emerging method that can increases the resolution of fabrication and hence, enhance the function. MEW applies a potential difference between the nozzle and the collector when the jet is direct-written, to maintain a molten fluid column at low flow rates. In this case, MEW has its advantage in adapting to various manufacturing requirements, with well-defined fibers that range from 820 nm to 130 µm in length[28].
This paper comprehensively reviews the tumor-on-a-chip technology fabricated by bioprinting for its application in anticancer drug screening. The TME, evolution of cell cultures in cancer research, advantages and techniques of bioprinting, and development of tumor-on-a-chip platforms by bioprinting are discussed.
2. Tumor microenvironment
The experimental gap that exists between in vitro models for screening anticancer drugs and in the efficacy of treatments largely contributes to their limited success. Understanding the cellular and molecular composition of tumors is important to developing models that recapitulate the TME. These models will then most accurately predict the success rate of potential drug candidates.
In addition to understanding malignant tumor cells, a thorough exploration of the TME and complicated interactions that occur between cells is necessary[12]. Cells within the tumor, stromal TME, and ECM, which provide structural support for cells in the extracellular space, affect the tumor’s behaviour[12,29-31]. Proteins, extracellular vesicles, cytokines, growth factors, and hormones are all found in the ECM, which is fed through a vascular network. Endothelial cells, fibroblasts, and mesenchymal stem/stromal cells (MSCs) are all examples of stromal cells[32,33]. Immune cells (T-lymphocytes, B-lymphocytes, natural killer [NK] cells, and macrophages), adipocytes, and pericytes are among the other biological components found in the TME. Communication between cells, cell-ECM, and the network of cytokines, proteins, and chemokines affects tumor behavior, such as tumorigenesis, angiogenesis, metastasis, and resistance to drugs[34]. Another important feature of the TME is leaky vasculature caused by altered endothelial cell junctions that compromise the vascular barrier function. Further, necrosis of endothelial cells and leaky, disorganized vasculature is responsible for tumor cell extravasation and metastasis[35,36].
2.1. Role of cells in the TME
(1) Stromal cells
Endothelial cells provide nutritional support for tumor growth and development as well as immune system protection. Antiangiogenic treatment targets endothelial cells, which are one of the primary targets[37,38]. Both normal fibroblasts and cancer-associated fibroblasts (CAFs) are found in the TME. The normal fibroblasts are able to transform into a CAF-like phase or completely into CAFs under certain mechanisms, including contacting with signals (Notch signaling or Eph-ephrins signalling), undergoing DNA damage (chemotherapy or radiotherapy), physiological stress (disrupted metabolism), and accepting inflammatory signals (interleukin [IL]-1, IL-6 and tumor necrosis factor [TNF])[39]. The CAFs release growth factors and chemokines that help malignant cells develop and survive while also encouraging other cells to migrate to the tumor[40].
MSCs interact with tumor cells through secreting growth hormones or cytokines and transferring mitochondria. They contribute to vascular network extension by differentiating into vascular pericytes and smooth muscle cells. They play a role in every step of tumor growth, including evading immune surveillance, promoting tumor angiogenesis, establishing therapeutic resistance, invasion and metastasis, and generation of stem tumor cells[41,42].
(2) Immune cells
The most prominent immune cells found in the TME are macrophages, which can account for up to 50% of the tumor mass in certain situations[37]. There are two subsets of macrophages in general, which are classically activated macrophages (M1 macrophages) and alternatively activated macrophages (M2 macrophages). M1 macrophages encourage the inflammation response against tumor cells, while M2 macrophages suppress the immune function and favor the tumor progression. Hence, most tumor-associated macrophages residing in the tumor cells are M2 macrophages, which reduce the phagocytizing ability of tumor cells and promote the spread of tumor cells to other tissues and organs.
NK cells recognize and kill tumor cells via the activation of cell surface receptors and alert the immune system in cases of infection or malignancy. This function makes them highly explored for cancer immunotherapy[43,44]. Other immune cells such as T-cells and mastocytes interact with malignant cells that have been altered, thereby promoting oncogenesis[45-47].
2.1.3. Other cells
Adipocytes secrete a number of chemicals that allow them to act as paracrine factor in the treatment of breast and ovarian cancer[48]. Increase in the number of adipocytes that correlates with obesity leads to instability and hypoxia in the TME, which causes tumor invasion and metastasis. The release of factors, such as IL-6 and extracellular vesicles, by adipose tissue cause cancer progression by enhancing the tumor cell oncogenic phenotype or stimulating the adjacent normal cells to adopt a pro-cancer phenotype.
Pericytes are multipotent perivascular cells that play an important part in the formation of the vasculature. In addition to having a favorable or negative influence on tumor development, pericytes can preserve blood vascular permeability, prevent tumor cell dissemination, and contribute to metastasis and cancer progression by altering pericyte/endothelial cell interactions[49-52].
2.2. ECM in TME
The ECM is an important component of the TME and contains a diversity of proteins, such as collagen, fibronectin, laminin, vitronectin, tenascin, glycoproteins, proteoglycans, and polysaccharides[52,53]. The chemokines and angiogenic factors connected to the ECM-tumor cell interaction can change the tensile and compressive strength of tumor tissue[54-56]. Upregulation of biological factors, such as transglutaminase and lysyl oxidase, and secretion of proteases (matrix metalloproteases and cysteine proteases) within the TME result in remodeling of the ECM, inflammation, angiogenesis, and tumor progression[57,58]. Based on the ECM modification in the TME, therapeutic treatments against cancer are focused on targeting the ECM. However, there are several factors that could interfere with therapeutic treatment, such as the complex nature of ECM proteins and their isoforms, barrier caused by ECM proteins hindering drug delivery, and modulation of immune response[59].
The TME is a dynamic environment, and its composition, morphological, physical, and mechanical characteristics influence tumor growth, metastasis, and anticancer medication efficacy. As a result, a detailed understanding of the interactions between cancer cell and ECM may make it easier to create realistic tumor models for anticancer drug screening and identification of promising therapeutic candidates.
3. Models for anticancer drug screening
This section describes the features and advantages of various cell cultures and animal models used for anticancer drug screening. Figure 1 represents the progression in terms of increasing complexity to recapitulate the TME from 2D cell cultures to bioprinted tumor constructs[19].
Figure 1.
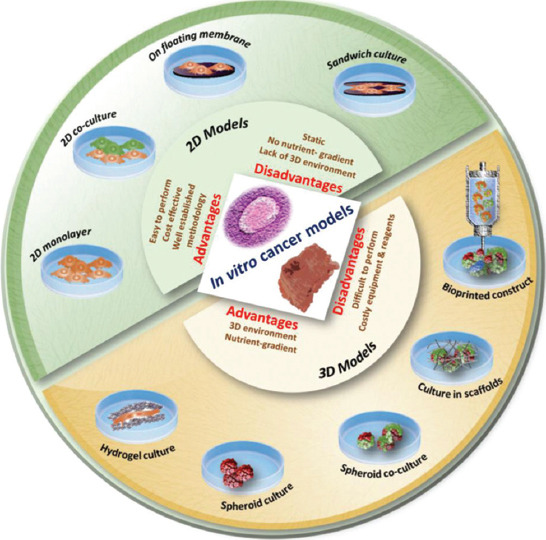
Various in vitro cancer models are used in anticancer drug screening (from reference[19] licensed under Creative Commons Attribution 4.0 license)
3.1. 2D cell cultures
Two-dimensional models have been typically used for anticancer drug screening due to their ease of use, low cost, and availability of functional assays[60]. Traditional 2D monolayer culture, monolayer co-cultures, cells grown on floating membranes, and cell monolayer sandwiched between membranes are the most often used 2D models in cancer research and screening. Despite their advantages related to usability and cost, 2D model cell cultures lack the ability to accurately identify potential anticancer compounds aimed at treating solid tumors. In cancer patients, drugs that have demonstrated substantial performance in 2D in vitro models have not always worked as well[61]. The fact that 2D cell cultures are frequently produced as monolayers, which differ greatly from the 3D architecture of tumors, explain this phenomenon. Drug, nutrients, and oxygen exposure are often uniform across all cells in a monolayer whereas inner tumor cells have limited drug exposure[62]. Furthermore, 2D models cannot capture cell-cell and cell-ECM interactions, and they suffer from damage to tissue-specific construction, and biochemical and mechanical cues caused by the removal of secreted biomolecules by media replacement[63-65]. Two-dimensional cell cultures are made up of immortalized cell lines, and recurrent passages of cell lines can result in genotypic and phenotypic changes, obstructing proper growth and responsiveness to stimuli. Cell supports in Petri dishes or flasks are stiffer than soft tissues of tumors, which affects gene expression profiles and drug sensitivity[66].
Several limitations associated with 2D cell culture models have led to the development and use of in vivo animal models and 3D models that can closely mimic the physical, geometrical, biochemical, and genetic profiles of the TME.
3.2. In vivo animal models
In vivo investigations in animal models are most often the next logical step following successful in vitro determination of the efficacy of potential drug candidates. Although in vivo models provide essential information on tumor growth and progression, they suffer from several limitations, such as high cost, large variations observed between the animals used, inter-species differences, and ethical considerations relating to animal use. Because of the variations in gene expression, protein expression, and soluble molecules (cytokines, growth factors, etc.) that are crucial for the investigation of cancer progression, animal models such as rat models utilized in cancer research are not indicative of human responses to anticancer treatments[66-68]. Despite their limitations, animal models continue to remain the gold standard for anticancer compounds due to their physiological similarity to humans. The large gap between 2D cell cultures and animal models necessitates the development of suitable 3D models that eliminate species differences in drug response observed during animal testing and enables the recreation of the complex TME.
3.3. Three-dimensional cell culture models
The advances in microfluidics, cell biology, and tissue engineering have paved the way for the creation of 3D cell culture models that can mimic the pathophysiology of tumor cells and their responses to anticancer treatments. Correct cell polarization, original form, genetic profile, and TME heterogeneity may all be obtained using 3D models[12]. Tumors are 3D formations made up of a heterogeneous distribution of various cells buried in stromal tissue; therefore, 3D models can better depict the TME and cellular behavior[19].
To create realistic human models for chemotherapeutic drug testing, primary cancer cells obtained from patient tumor biopsies or cell lines can be employed. The different 3D cell cultures include spheroids, organoids, scaffolds/hydrogels, and 3D-bioprinted constructs.
Spheroids are micro-sized, single-cell, or multicell aggregates that represent the 3D architecture of solid tumors and they can demonstrate cell-cell and cell-ECM interactions, unlike 2D cell culture systems[69,70]. Spheroids can form intrinsic metabolic gradients (oxygen, nutrients, by-products, and metabolites) that lead to the establishment of heterogenous structures composed of a mixture of acidic, hypoxic, proliferative, and quiescent cells mimicking the tumor structure[71]. They can be engineered to contain different cell types (usually seen in TME), so that realistic cell-cell physical and signaling interactions can be replicated. Spheroid cultures can be made by various methods, such as low-adhesion plates, hanging drop plates, bioreactors, and micropatterned or nanopatterned surfaces[72,73]. Practical challenges associated with spheroid culture include the production of uniform size spheroids, the lack of precise control overpopulation of different cell types in spheroids, and the lack of standardized high-throughput screening assays using spheroids[74] Furthermore, the tumor vasculature cannot be recreated using spheroids[60].
Organoids are in vitro aggregates produced from stem cells that can self-organize and recapitulate tissue/organ functionality[75]. Embryonic stem cells, induced pluripotent stem cells, and adult stem cells are all examples of stem cell organoids[76]. Organoids can accurately replicate in vivo tumor architecture and genetic expressions, but they lack some cell types found in vivo and the necessary vasculature for nutrient and waste transport; therefore, they may only replicate the early stages of organ development, making them unsuitable for screening platform replication[73].
Scaffolds/hydrogels are synthetic 3D structures constructed of materials with varying porosities, permeabilities, surface chemistries, and mechanical properties that are meant to simulate the TME[73]. Scaffolds are constructed of biological or synthetic polymers (collagen, Matrigel, gellan gum, hyaluronic acid, polystyrene, and polycaprolactone). Although, natural materials allow for a physiologically relevant microenvironment for cell attachment and reorganization, they suffer from batch-to-batch variability and complex composition[65,73]. Polymeric scaffolds use hydrogels to generate supports for 3D cultures which can be hydrolytically or enzymatically biodegraded. Because of their water content, synthetic polymeric scaffolds offer better repeatability as well as changeable biochemical and mechanical characteristics, and allow for the movement of nutrients, oxygen, waste, and soluble components[77,78]. Scaffolds are routinely made using processes, such as 3D printing, particle leaching, and electrospinning[70]. Perfusing the animals and properly dispersing cells on the scaffold are, however, challenging[60].
To generate tissue-like structures, 3D bioprinting refers to the additive layer-by-layer construction of diverse materials containing various cells (cancer and non-cancer cells in the case of anticancer medication research) and biological components[79]. It allows for the reproduction of the complex 3D architecture and allows the model complex cell-ECM interactions with the added advantage of high throughput capability.
The accuracy of bioprinting could be significantly improved via the incorporation of a microfluidics print head into one of the additive manufacturing techniques (one of the additive manufacturing techniques). This microfluidic strategy enables to control the spinning of desirable hollow microfibers continuously on the chip and hence, displays morphological and structural complexity. These hollow structures ease the fabrication of structure that mimics native tissues as they possess important features such as natural vascular shape, large surface area, high permeability, and high mechanical flexibility[80]. Besides, a low-viscosity cell-laden bioink is used as it enhances the migration and alignment of the cells within each microfiber[81]. With a mixture of multiple biomaterials, a heterogeneous composition microfiber is created. The creation of distinctive multicompartmental structures or complex multi-compositional architecture microfibers has great potential in the application of various biomedical fields, such as in cancer drug research[82].
In addition to 3D bioprinting, other additive manufacturing technologies, such as MEW, allow for the recreation of highly tailored architectures and scaffold constructs using computer-written programs for biomedical applications. MEW microfibrous polycaprolactone filter was prepared to allow for size- and immunoaffinity-based capture and on-site culture of EpCAM-positive cancer cells. The following sections describe 3D bioprinting in detail with reference to cancer research[73,74].
4. Bioprinting in cancer research
Three-dimensional bioprinting is the process of printing cells, biocompatible materials, and supporting components into complex 3D living tissues with the required cell/organoid architecture, topology, and functioning using computer-aided design[83]. Unlike other 3D cell culture models discussed in section 3.3 such as spheroids and organoids that follow a non-guided spontaneous self-assembly development of tissues and organs, bioprinting allows for spatial control of matrix properties and cells in order to more accurately depict the TME and also enables designs that simulate tumor vascularization[16,21]. Three approaches are used for bioprinting: biomimicry that uses bioengineering to replicate the intracellular and extracellular components of organs or tissues, autonomous self-assembly that relies on cells to drive the desired microarchitecture and functional tissues, and fabrication and assembly of mini tissue blocks into a large construct via rational design, self-assembly, or both[84-86].
Figure 2 shows the trend in the number of publications related to bioprinting and cancer from 2013 to 2021. These results were obtained from the PubMed database using search terms such as “bioprinting,” “3D printing,” “cancer,” “drug screening,” “tumor-on-a-chip,” and appropriate MesH terms. A clear upward trend in research related to 3D bioprinting technology is evident. Several review papers on the evolution of cell cultures for cancer drug screening, organ-on-chip platforms, and bioprinting have also been published.
Figure 2.

Search results for cancer and bioprinting from PubMed database (2013 – 2021).
4.1. Bioinks
Bioinks are important tools for the fabrication of artificial living tissue constructs that can mimic all properties of native tissues through 3D printing technologies. Bioinks are the building blocks of bioprinted constructions, consisting of a biocompatible hydrogel in which the cells of interest as well as nutrition and growth factors are embedded[81]. Biocompatibility, predictable gelation, capacity to imitate structural, physicochemical, rheological and biological features of the ECM, amenability to scale-up, production under good manufacturing principles, and minimal batch-to-batch variability are all desired qualities of the hydrogels[18,87]. They can be composed of only cells, but usually, a carrier substance such as a natural or synthetic polymer, or both are used to act as a scaffold on which cells can adhere, proliferate, and grow, allowing for protection of cells during the printing process, and serving as a template that can be chemically modified or cross-linked to allow for the formation of a 3D construct[87]. Bioinks are classified into six categories: peptide- or protein-based bioinks (hyaluronic acid, heparin, and chitosan), polysaccharide-based bioinks, ECM-based bioinks, synthetic polymer-based bioinks (gelatin methacrylate [GelMA], Pluronic, and polycaprolactone), cell-aggregate or pellet-based bioinks (fibrin-based bioinks), and tissue-derived decellularized matrix (carbon-based, clay-based, ceramic nanoparticles, or nanofibers, and nanocrystals)[87]. Alginate, collagen, and agarose are often utilized as biopolymers because of their low cytotoxicity, high water content, and biocompatibility[88]. The merging of cancer and stromal cells is done independently to generate viable tumor models.
Cancer bioinks consist of patient-derived cancer cells (primary cancer cells, cancer stem cells, circulating tumor cells, and CAFs) mixed with a biopolymer (GelMA, a methacrylate form of gelatin, alginate, hyaluronic acid, or collagen), growth agents, and nutrition. Matrigel, a gelatinous ECM protein generated by mouse sarcoma cells, is commonly utilized to provide the ideal environment for cancer cell proliferation and carcinogenesis, as well as to imitate the morphological properties of in vivo tumors[18,89].
Stromal bioinks contain healthy stromal cells such as endothelial cells, mesenchymal/hematopoietic stem cells, fibroblasts, and other tissue cells. Patient-derived primary cells or BioBank primary cell lines should be used to mimic the microenvironment along with natural and synthetic polymers. Furthermore, endothelial cells or endothelial progenitor cells should also be incorporated with angiogenic factors to recapitulate tumor blood vessel features, such as extensive branching and leaky vasculature[18,90,91].
4.2. Techniques and steps of bioprinting
Inkjet bioprinting, microextrusion bioprinting, laser-assisted bioprinting (LAB), and stereolithography are the most common techniques for bioprinting cancer tumor structures (Figure 3).
Figure 3.

3D printing techniques for cancer tumor modeling. (A) Inkjet bioprinting. (B) Microextrusion bioprinting. (C) Laser-assisted bioprinting. (D) Stereolithography bioprinting (from ref.[92] licensed under Creative Commons Attribution 4.0 license).
Inkjet bioprinting uses a variety of energy sources, including sound, temperature, and electricity, to generate droplets of bioink in a precise pattern and at a rapid rate. Inkjet bioprinting is economical and allows for fast and high-resolution printing[12,19]. However, thermal and mechanical stresses can damage cells and this technique cannot work with bioinks of high viscosity, thus posing a limitation to the printing of hydrogels with high cell densities. This method has been used in the regeneration of skin and cartilage. Human ear and sheep meniscus have been inkjet-bioprinted using nanocellulose bioink[92,93].
The deposition of material extruded through a nozzle in a well-defined continuous stream is the basis for extrusion-based bioprinting. It permits deposition of materials in higher viscosity compared to inkjet bioprinting, making it suited for rebuilding highly cellular tissues. However, the shear stresses created can lead to cellular damage. To maintain cell stability and survival, strict control of the quantity of biomaterial, pressure, and nozzle diameter is required[18,82]. Aortic valve conduits, vascular grafts, and cartilage structures have all been printed using this technology[94-96].
LAB is a relatively new technology in which a laser pulse is focused on the donor ribbon and converted into a shockwave to activate the bioink underneath[81]. The high resolution of LAB (~10 µm) allows it to be used to make structures of native tissues at or near the scale of a single cell and its contactless and nozzle-free nature precludes problems associated with clogging of nozzles[92]. Laser direct-write permits numerous cell types to be encapsulated in microbeads and has been used to develop and construct multicellular tumor spheroids of uniform size and shape[97].
Stereolithography bioprinting is based on the use of bioinks made of light-sensitive polymers which are deposited in a layer-by-layer fashion and then exposed to a patterned light source for curing and formation of 3D constructs. This method is associated with good cell viability and lack of shear forces avoids cell injury. However, the lack of compatible materials, high costs, low cell density for avoiding light scattering, and a long processing time are some of the drawbacks that limit its use[12,87]. Figure 4 outlines the steps for bioprinting after selecting a particular bioprinting technique.
Figure 4.

Steps to fabricate the tumor microenvironment by 3D bioprinting (from ref.[16] licensed under Creative Commons Attribution 4.0 license).
The first step in bioprinting involves the choice of a suitable bioprinting technique for the fabrication of the tumor (in case of cancer) or specific tissue. The choice of the technique depends on the cellular density of the tissue being recreated and other factors such as resolution required, and the ability of the cells to resist thermal or mechanical damage or injury by shear stress application. Then, computer-assisted design or images of the tissue can be used to recreate the structural architecture of the tumor which will guide the 3D development. To reproduce the TME, bioinks containing malignant and healthy cells from patients (cancer and stromal bioinks) are combined with other biopolymers, medium, and growth factors in a precise ratio. To print the build, bioinks are applied according to the computer-assisted design. Following layer-by-layer printing of the model, the construct is crosslinked (photocrosslinking or ionic crosslinking, depending on the hydrogel employed) and matured in a culture medium[18,98,99]. Tumorigenesis is aided by cell division, proliferation, and differentiation during the post-printing stage of tumor creation. The maturation of constructs is time-sensitive and necessary to develop the most effective personalized anticancer regimen. Accelerated tissue maturation is a difficult problem to solve, and it is still being studied. To accomplish tissue maturation, static culture systems can be utilized, such as the incubation of tissue spheroids, which produces tissue cohesion and maturation as well as the accumulation of ECM molecules[100-102]. Physicochemical measurements and biological assays are used to characterize bioprinted cancer constructs. Various methods used include atomic force microscopy to measure the stiffness of constructs by nanoindentation, and scanning electron microscopy to characterize the topological features of the construct. Cell viability is another important characteristic that may be determined using a calcein-AM staining approach that distinguishes between living and dead cells. Protein expression associated with the maintenance and development of cancer as well as the creation of ECM components and membrane proteins are determined using immunohistochemistry and immunofluorescence techniques[18,103,104].
4.3. Advantages and challenges of bioprinting
Unlike other 3D cell culture models described in section 3.3, bioprinting offers several advantages as it allows for the reproduction of the complex TME and ECM by the accurate spatial distribution of different cell types which is essential for realistic tumor modeling. It allows for cell-cell and cell-ECM interactions and modeling of leaky tumor vasculature, which is vital for understanding drug delivery to tumors and developing suitable chemotherapeutic agents. Bioprinting technologies are amenable to automation and high-throughput testing abilities, which are important for screening assays. Despite these clear advantages of 3D bioprinting, this technology is still in its initial stages and facing problems, such as scalability, high cost of equipment and labor-intensive nature, and difficulty in developing well-established vascular networks in tumors. Further problems associated with techniques, such as nozzle clogging, cell viability issues, and the use of hydrogels that are not suitable for luminescence or fluorescence assays by HTS due to their vicious nature, necessitate further research to explore the full potential of bioprinting in cancer applications[16,19,87].
5. Tumor-on-a-chip platforms
5.1. Concept
Tumor-on-a-chip technology, which is based on the integration of manufactured tissues into microfluidic devices, has emerged as a unique tool for cancer research. This method integrates microfluidics, microfabrication, tissue engineering, and biomaterials research, and it has the potential to revolutionize cancer biology[60]. Three-dimensional cell culture models described in section 3.3 and bioprinting can help generate cancer tumor models that mimic tumor heterogeneity and vasculature in a high-throughput and reliable manner. Spheroids, organoids, scaffolds, and bioprinted constructions are static models that do not recreate aspects of live tissues that are crucial for their function, such as tissue-tissue interfaces, spatio-temporal gradients of chemicals and oxygen, and mechanically dynamic milieu. The microfluidic approach is based on the exposure of the constructed tumor tissue to a continuous fluid flow to integrate dynamic mechanical cues such as shear stress into these systems[105,106]. Interstitial fluid flow in and around the tissues is particularly important in tumor models as it affects cell cycle arrest in tumor cell lines, and the migration of cancer cells in the direction of fluid modulates gene expression and cell proliferation, and helps generate gradients of chemicals and biomolecules, which play a role in cancer metastasis[105-107]. Oxygen gradients may be created using microfluidic chips, simulating the physiological effects of oxygen on tumor development and metastasis[62].
The tumor-on-a-chip device is a microfluidic device that can develop tumors by providing tissue culture, nutrition and small molecule supply, and means of waste disposal[60]. A complex tissue structure comprising tumor cells, stromal cells, and blood arteries is developed on the chip, either self-organized or spatially arranged by design (through bioprinting). The tumor-on-a-chip technology can be automated to run many drug screening assays at the same time and identify the response and mechanism in real-time[108]. Cell migration in the tumor environment, metastasis events, anticancer drug screening and therapy response, or study of the transport of anticancer medicines in tumorous tissue can be achieved by the microfluidic platform[25]. Thus, 3D tumor-on-a-chip platforms enable the recreation of tumor-stroma interactions in relevant ECM mimetic matrices, as well as dynamic manipulation of biochemical and biophysical parameters, such as pH, oxygen, nutrients, metabolites, and cells (e.g., immune cells, mesenchymal, and stromal cells).
5.2. Techniques to produce tumor-on-a-chip
The following variables must be considered while fabricating tumor-on-a-chip systems: Microfluidic system, 2D/3D cell culture models that comprise various cell types and the physicochemical environment, stimulus-loading components, and sensors for monitoring and reading the results[109,110]. The TME and its functional parts, including cancer cells, cancer stem cells, and stromal cells, as well as the supportive ECM and blood/lymphatic-like conduit, require a basic understanding. Suitable tumor-ECM biomimetic biomaterials should be chosen to fabricate the 3D architecture and these biomaterials should have suitable rheological properties to allow for construct maintenance and resolution.
Manufacturing techniques, such as photolithography, soft lithography self-assembly, replica molding, microcontact printing, and bioprinting, have been used to produce organ-on-a-chip platforms. Photolithography involves the use of masks, photoresists, ultraviolet light, and etching technology. This approach involves creating a mask based on the desired structure and then coating a layer of photoresist on a substrate such as a silicon wafer, glass, or quartz. Ultraviolet light is used to remove portions of the photoresist material from the substrate surface and create a mold. After that, the design is transferred to a substrate, resulting in a microfluid chip with microflow channels[111,112]. The soft lithography technique is based on the use of a microchannel mold prepared by photolithography. To make an elastomeric stamp with patterned microstructures, a liquid polymer such as polydimethylsiloxane (PDMS) is poured into the mold. By transferring the pattern from the stamp to other polymer structures, complex 3D microchannels may be formed. A closed-circuit channel is created and is then sealed with a glass slide[113-115]. Replica molding uses a photolithographically patterned silicon mold, PDMS pouring, and heat curing to build a device that is affixed to a flat, smooth surface, such as glass, to create a microfluidic chip with microchannels. Microcontact printing is an extension of the replica molding process in which the PDMS stamp is printed on a substrate along with biofunctional molecules, such as proteins[116]. The majority of the technologies discussed above using photolithography, which is costly and time-consuming, can only create microfluidic chips without microtissues, stimulus-loading components, or readout sensors, all of which require additional procedures to create[114].
Due to features such as optical transparency, breathability, biocompatibility, and flexibility, PDMS is the most popular material utilized to create microfluidic chip devices, allowing for continuous viewing of tumor constructions for real-time monitoring of cell behavior and therapy response. PDMS substrates offer a higher porosity and flexibility compared to glass or plastic mimicking the soft tissues. However, PDMS is hydrophobic and can bind or adsorb hydrophobic molecules that are problematic during drug screening. Poly(methyl methacrylate) (PMMA) substrates bound to etched polyethylene terephthalate membrane are impermeable to lipophilic molecules[117,118]. Other materials used to prepare microfluidic devices include gelatin, photocrosslinked GelMA, bacterial cellulose paper, and basement membrane extract (BME/Matrigel)[62].
5.3. Bioprinting to fabricate tumor-on-a-chip constructs
Recently, bioprinting has emerged as a preferred choice for tumor-on-a-chip fabrication. Bioprinting allows for the 3D simultaneous printing of multiple cell types and biofunctional materials directly onto a cell-compatible substrate with high reproducibility and spatial resolution. This is essential because it allows bioinks containing numerous cell types, such as CAFs, immunological cells, and endothelial cells. that may form vascular networks to replicate the heterogeneous tumor environment. Bioprinting also aids in the heterogeneous distribution of physiologically relevant proteins and growth factors that are involved in tumor signaling, proliferation, and migration[119-121]. Bioprinting allows for the effect of non-malignant cells on tumor evolution to be evaluated through tumor-stroma interactions. Another advantage of bioprinting that is particularly important in manufacturing is that it can directly print or pattern cells in microfluidic devices, modeling vasculature, and biological barriers. Furthermore, 3D bioprinting technique enables the engineering of vessel-like tubular constructs for assembling 3D vascular components during the fabrication process, allowing for real-time personalization as opposed to pre-programmed channel architectures used in standard microfluidic chip manufacturing[122]. This feature of bioprinting is particularly critical owing to the deregulated tumor vasculature that differs in terms of heterogeneity, permeability, and multi-directional flow from the supplying healthy tissue[122]. Drug distribution is considerably affected by the leaky and poorly structured blood arteries feeding tumors, and these changes are difficult to mimic in other tissue models. Bioprinting enables in vitro replication of altered vascular structures to better assess the effects of drug treatments and delivery[99]. Bioprinting minimizes the unpredictability imposed by standard cell seeding methods in microfluidic chips by allowing for spatially controlled deposition of cell-laden ECM-biomimetic hydrogel bioinks within a microfluidic device[121]. The combination of on-chip biosensors with the capabilities of microfluidic systems to screen many anticancer drugs concurrently enables high-throughput screening in real time, speeding up the drug screening process. Thus, bioprinting is an emerging technology that can create tumor-on-a-chip platforms through its ability to mimic physical, chemical, and mechanical cues and perform high-throughput studies.
The next section focuses on examples in literature that have used bioprinting technology to fabricate tumor-on-a-chip systems to evaluate drug effects and for drug screening.
5.4. Examples of bioprinted tumor-on-a-chip in anticancer drug screening
Biomimetic 3D in vitro tumor models or personalized bioprinted constructs, such as tumor-on-a-chip models, are emerging tools that can be used to test an array of chemotherapeutic agents. Bioprinted tumor-on-a-chip systems can recapitulate the TME, recreate tumor-stroma interactions in ECM-mimetic matrices, and allow for the manipulation of factors such as pH, oxygen, nutrients, and cells.
The PubMed database (https://pubmed.ncbi.nlm.nih.gov) was searched using identifying terms such as “tumor-on-a-chip,” “tumor-on-a-chip,” “tumor-on-chip,” “tumor-on-chip,” “3D bioprinting”, “cancer,” and associated MesH terms with the objective of identifying articles that report works using tumor-on-a-chip or microfluidic platforms fabricated by bioprinting for drug screening. Figure 5 shows a flowchart for the selection of appropriate publications for this review.
Figure 5.

Flowchart for identifying publications related to “tumor-on-a-chip” and “bioprinting” to be included in the review (2013 – 2021).
Table 1 shows the characteristics of the studies related to the use of bioprinting technology to make tumor-on-a-chip platforms. Hamid et al. developed a microfluidic system to assess drug metabolism. A tissue platform was constructed using photolithography with a PDMS enclosure. SU-8, an epoxy-based resin material was used to create microfluidic chips with channels of different porosities (300, 500, and 700 µm). MDA-MB-231 cell lines (human breast adenocarcinoma cells) were bioprinted into the channels using an extrusion-based method. Fluorescent staining revealed similar cell growth in all three chips. Cell proliferation studies showed an increasing trend of proliferation in all three chips; however, the number of cells on the chips was in the order of 300 µm > 500 µm > 700 µm. The high surface-area-to-volume ratio and shear stresses were credited for high cell growth in the chips with narrower channels. Drug metabolism was investigated by seeding 7-ethoxy-4-trifluoromethyl coumarin (EFC) solution into the cell-laden chips. Metabolism of EFC to its metabolite, HFC, was observed in all three chips after 12 h as only trace amounts of EFC were quantified on the chips. This study demonstrated that bioprinting could be used to fabricate a microfluidic system to assess drug metabolism especially in cases where drugs are expensive (particularly during the early stages of drug discovery and development) as only small volumes of fluids are necessary to investigate metabolism[123].
Table 1.
Studies utilizing bioprinting for fabrication of tumor-on-a-chip platforms.
| Reference | Bioprinting technique | Cells | Bioink | Substrate | Purpose |
|---|---|---|---|---|---|
| Hamid (2014)[117] | Extrusion | MDA-MB-231 cell line (human breast adenocarcinoma) | NA | PDMS | Investigation of drug metabolism |
| Hamid (2015)[118] | Extrusion | MDA-MB-231 cell line (human breast adenocarcinoma) and HepG2 cell line (liver cancer) | NA | PDMS | Co-culture of cancer cells |
| Zhang (2016)[119] | Inkjet | HepG2 cells (liver cancer) and U251 cells (glioblastoma) | Alginate sodium | PDMS | Drug metabolism and diffusion |
| Cao (2019)[13] | Extrusion | MCF-7 breast tumor cells | GelMA, alginate, photoinitiator | PDMS and PMMA | In vitro drug screening |
| Cheng (2019)[120] | Extrusion | MCF-7 breast tumor cells | Hydrophobic petroleum jelly-liquid paraffin | In vitro drug screening | |
| Li (2019)[121] | Extrusion | SMMC-7721 cells (human hepatocellular carcinoma) | Hydroxypropyl chitin and Matrigel | PDMS | Drug screening |
| Mi (2019)[122] | Inkjet | MDA-MB-231 cell line (human breast adenocarcinoma) | NA | PDMS | Anticancer drug effect |
| Yi (2019)[123] | Extrusion | Human U-87 glioblastoma cell line | Brain decellularized ECM (BdECM) | Glass | Chemoradiation and drug screening |
| Xie (2020)[124] | Inkjet | MDA-MB-231 cell line (human breast adenocarcinoma) | GelMA | Conductive membrane | Drug screening |
In another study, Hamid et al. used a mask less fabrication technique to develop a microfluidic chip eliminating the need for the use of conventional photolithography. This novel approach involved the integration of biologically compatible materials and plasma chemistry to enhance surface functionalization and direct cell deposition. Extrusion-based bioprinting was used to co-culture MDA-MB-231 cells (human breast adenocarcinoma cells) and HepG2 (liver cancer cells). Fluorescent-based tracking of the cells indicated that they integrated together and there was even cell distribution and proliferation in the chip. This platform has the potential to be used to produce advanced microfluidic arrays[124].
A microfluidic chip was used as a platform for inkjet printing of HepG2 and U251 (glioblastoma cells) using alginate sodium as the printing matrix by Zhang et al.[119] This study was the first to integrate inkjet printing and microfluidic chip. SU-8 2050, a negative photoresist, was used to create the microchip and then PDMS was used to form the substrate. The viscosity of alginate sodium and the voltage of the printer were adjusted and optimized to allow for the reliable printing of the alginate hydrogel droplets. Cell suspension with HepG2 and U251 cells was prepared in alginate sodium and co-patterned into the channels of the microfluidic chip. A metabolism and diffusion study of the model drug, Tegafur (prodrug of 5-fluoro uracil) was performed following inkjet printing of the cells. Staining and confocal microscopy were used to determine cell viability. Tegafur was metabolized in the co-culture cell system by HepG2 cells to the parent drug, 5-FU which exhibited anti-cancer effects on U251 cells. This approach involved spatially controlled patterning of cells in a microfluidic chip, which can be used for cell culture, simulation, and analysis[125].
Cao et al. created a tumor model consisting of a bioprinted hollow blood vessel and a lymphatic vessel pair to imitate genuine perfusion and draining microcirculation systems and enable the investigation of anticancer medication transport kinetics (TOC-BBL)[13]. A blood and lymphatic vascular pair with tumor cells implanted within a hydrogel niche was bioprinted using extrusion-based bioprinting with adjustable bioinks (alginate, GelMA, PEG combinations). The bioprinted vascular arteries were housed in PDMS and PMMA layers, which also served as a reservoir for the tumor cell culture. The bioinks’ mechanical properties, such as printability, elastic modulus, and rheological behavior, were optimized by experimenting with different PEG concentrations. Fluorescein isothiocyanate diffusion was used to determine the permeability of the vessels and the bioink composition was chosen to mimic the permeability values of native blood and lymphatic vessel pairs in vivo. The TOC-BBL system’s performance was assessed using doxorubicin diffusion. Comparative evaluation of doxorubicin delivery in one channel (blood vessel only) or two channels (blood and lymphatic vessel pair) was performed. Cell viability differed between these two configurations emphasizing the importance of inclusion of a draining lymphatic vessel in the TOC-BBL platform. The surface of the bioprinted vessel pair was seeded with endothelial cells (human umbilical vein endothelial cells [HUVECs] and HLECs). Due to the expression of junction biomarker CD31, slower diffusion rates were seen when these cells were used. Through replicating delivery and drainage microcirculation channels, the TOC-BBL platform is intended to increase knowledge of the kinetics of drug and biomolecule transport by diffusion[13].
Cheng et al. used matrix-assisted sacrificial 3D printing to create a membrane with perfusable microchannels using a hydrophobic fugitive ink (petroleum jelly-liquid paraffin) placed within a bacterial cellulose hydrogel matrix[126]. Bacterial cellulose offers various advantages, including a long shelf life that allows it to be rehydrated to create realistic tissue models, as well as a simple and low-cost cell growth substrate, high porosity, high water-holding capacity, and good biocompatibility. To construct a vascularized breast tumor model, MCF-7 breast cancer cells were seeded onto the device’s paper matrix and HUVECs were employed to fill the surface of the microchannels. The cells were found to be alive by fluorescence microscopy, and both endothelium and tumor cells multiplied over the 14-day culture period. Tamoxifen was administered into the endothelialized microchannels and the paper devices were cultured for 48 h to assess drug response. The cytotoxicity generated by pharmacological therapy was demonstrated by confocal pictures. This research might lead to a new method for creating simple and low-cost in vitro tissue models, which could be useful in drug screening and customized treatment[126].
Li et al.[121] established an in vitro hepatoma model with extremely homogenous 3D tumor clusters based on 3D cell printing, co-culture, and microfluidics. The human hepatocellular carcinoma SMC-7721 cell line was used to create three models: a 2D model, a 3D printed model (3DP), and a 3D printed + microfluidic model (3DPF). The cell clusters were dissolved in a bioink composed of hydroxypropyl chitin and Matrigel and injected into the microfluidic chips. In comparison to the 3DP model, the 3DPF model provides a microenvironment with a greater degree of bionics for the cells in the chip, allowing for better credibility of pharmacodynamic test findings. The effect of Metuzumab, a monoclonal antibody drug, was tested in these models. Cell proliferation, size characterization, and antibody-dependent cellular cytotoxicity (ADCC tests) were used to compare the models. The 2D model was more responsive to medication dosage, which might be attributed to the distribution of hepatoma cells in the 2D model versus the 3D model, where they are aggregated and less likely to interact with drugs. The 3D model had better mimicking effect in in vivo tests. The proliferation efficiencies of the cells were higher in the 3DPF model compared to the 3DP model because of microfluidic perfusion in the former. Under the same dose of drug treatment, the ADCC test performed better in the 3DPF model than in the 3DP model, indicating that Metuzumab-mediated ADVV effects were also successfully implemented in the 3DPF model. Because of biomimetic transport and a 3D cell environment, the 3DPF model’s pharmacodynamic results highly resemble those of animal trials. The 3DPF model may also be used to investigate the toxicity and metabolism of different antibody-based medicines. The importance of including fluid flow dynamics in 3D printed models is shown by these findings[127].
Mi et al. developed a breast tumor-on-a-chip device by using inkjet bioprinting on a microfluidic chip[128]. MDA-MB-231 cells (human breast adenocarcinoma cells) and endothelial cells (HUVECs) were printed on a PDMS chip made by soft-photolithography. The cells showed good viability post-printing and this system was then used to test the responses of the cancer cells to paclitaxel, a microtubule-stabilizing drug which is expected to interfere with the migration capacity of cells. It was observed that paclitaxel caused a dose-dependent decrease in cell migration ability. This platform has the potential to be used for cell analysis, cancer development, and drug screening and metabolism[128].
Yi et al.[129] fabricated a patient-derived glioblastoma-on-chip. In this study, patient-derived tumor cells, vascular endothelial cells, and decellularized ECM from brain tissue were bioprinted in a compartmentalized cancer-stroma concentric ring structure that maintains a radial oxygen gradient to mimic the structural, biochemical, and biophysical features of the tumor and represent the heterogeneous ecology of glioblastoma tumors using extrusion-based printing. The bioink was made up of brain decellularized ECM (BdECM), which solidified after deposition and served as a cell-supporting matrix. The capacity of GBM-on-chip to replicate therapy effects in patients after chemoradiation and temozolomide (TMS, an anticancer medication) treatment was investigated. The resistance to chemoradiation and TMS for GBM cells produced on GBM-on-chip and clinical patient responses were shown to be related, demonstrating that this microfluidic technology is viable and precisely reproduces the patient’s treatment resistance. The GBM-on-chip can be utilized to find the best medication combination for treating GBM patients, paving the way for more individualized cancer therapies[129].
Xie et al. demonstrated the formation of a 3D tumor array chip for anticancer drug screening[124]. Electrohydrodynamic 3D printing was used to deposit a bioink (gelatin methacryloyl hydrogel, GelMA) containing MDA-MB-231 breast cancer cells on a conductive membrane. In a 3D environment, the cells displayed critical tumor features, such as spreading, survival, and metastasis, as well as distinct cell cycles, suggesting their potential to spread and metastasize even after being exposed to high voltage. On this 3D tumor array chip, the effects of epirubicin (a cell-permeable anthracycline antitumor medication) and paclitaxel were assessed by assessing MDA-MB-231 cell death, vascular permeability factor (VEGF) expression, and NAD+ expression for cell proliferation. This platform was feasible to screen antitumor drugs and can be integrated with conventional screening procedures[130].
6. Challenges and future directions
The highly complex and dynamic nature of tumors necessitates the development of a suitable biomimetic platform to screen anticancer drugs that recapitulates the TME. Thre-dimensional bioprinting has emerged as a suitable fabrication method that can be integrated with microfluidics to create tumor-on-a-chip platforms that can be used via a high-throughput method for anticancer drug screening. Bioprinting offers special benefits since it enables the simulation of ECM, cells, and other biomaterials’ spatial dispersion and layer-by-layer assembly. The development of organs, or specifically tumor cells in this case, can also contribute to personalized medicine due to their ability to recreate patient-specific TMEs. Despite these advantages, bioprinting suffers from some challenges that need to be overcome before it can be used widely. Resolutions to problems with printing efficiency, printing resolution, and repeatability as well as standardization of cell sources and biomaterials are necessary to ensure the adoption of bioprinting technology. Polymer choices for bioprinting are limited and there is always a risk of drug degradation upon heating. Although bioprinting can be performed in an aseptic environment, sterilization of the finished product is usually performed which can cause heat and light-induced degradation of polymers. Regulatory concerns exist regarding the approval of bioprinted constructs as these are usually tailored for individual patients and as a result do not meet the participant number criteria for approval that is usually required by health authorities. Additionally, most imaging techniques, optical analysis, and chemical evaluation methods have been developed for 2D assays, which may not be suitable for analyzing 3D constructs. Furthermore, processes for the preparation of tumors need to be optimized and standardized.
Coupling bioprinted tumors with microfluidics (tumor-on-a-chip) enables the study of specific microenvironmental components on cancer cells, tumor-stromal and ECM interactions, and allows for obtaining data in real time on a more realistic platform. Advancements in printing technology that enable joint printing of tissues and cells can help realize tumor-on-a-chip technology as a viable method to prepare screening platforms for chemotherapeutics. Although this technology is theoretically sound, practical challenges limit its use. Obstacles in technology creation, design, optimization, analysis, and validation are among them. Material properties such as mechanical properties, biocompatibility, and ease of handling limit the choice of substrates for PDMS. Although PDMS fulfills most of the criteria for studying biological mechanisms, it is not suitable for hydrophobic drugs including anticancer compounds, making it necessary to explore other materials that can be molded and printed, such as epoxy resins, off-stoichiometry thiol-enes and perfluorinated polymers. For widespread use, the methods that manufacture tumor-on-a-chip platforms should be amenable to scale-up activities to facilitate large-scale manufacture, which requires investigation of materials and bioinks and a focus on user training. To fully achieve the potential of tumor-on-a-chip devices, various manufacturing, operability, and regulatory challenges must be solved.
Future work that can facilitate the adoption of tumor-on-a-chip for high-throughput screening of potential drug molecules should involve correlation of the results obtained with tumor-on-a-chip system with xenograft models or clinical tumor tissues. Validation of tumor-on-a-chip models is an important step to facilitate the widespread use of on-chip technology, improve drug discovery and personalized medicine, and help reduce unethical animal testing. Cancer xenograft models that mimic the complexity and variability of human tumors can be used to validate tumor-on-a-chip models. Correlation between human cancer xenograft in mouse and tumor-on-a-chip wherein the tumor-on-a-chip technology can mimic many of the relevant characteristics of cancer cells and the TME, and host response indicates a progress toward improved drug discovery[131]. The emergence of cancer immunotherapy that relies on the patients’ immune system activation has led to research in developing immunocompetent tumor-on-a-chip models to understand tumor-immune system interactions and screen potential anticancer immunotherapies[132]. The combined effect of TME with tumor-associated immune cells under dynamic conditions will help in providing realistic data outputs for anticancer screening. Based on the immunosuppressive effect of myeloid-derived suppressor cells and regulatory T-cells (Tregs), they should be incorporated into the tissue or tumor cells[133]. Since tumors have markedly different molecular and biological signatures from one another which can affect drug efficacy, incorporation of patient-derived cancer cells in the fabrication of tumor models can be used to recapitulate this heterogeneity. Few studies have investigated the use of patient cells with the bioprinting platform, prompting the need for further research in this area[134,135]. Patient-derived samples have been used for chemotherapy drug testing by Mazzochi et al. and Lim et al. wherein similarity was observed between drug responses in vitro and in the patients[136,137].
The optimization of the bioprinting technique can use of stimulus-sensitive hydrogels as bioinks which consider the dynamic nature of the TME allowing for the controlled release of growth factors, and incorporation of ECM-mimetic biomaterials that mimic native tissues are challenging and need to be explored in detail[122,138]. In addition, the mechanical properties of the tissue being modeled, such as stiffness and adhesion sites, should also be considered when developing bioinks. To enable the use of tumor-on-a-chip technology, it needs to be coupled with effective tools to enable on-chip analysis. However, information on tools and techniques for on-chip analysis is often lacking. Further research on physical sensors on-chip for the evaluation of gas exchange, pH levels, and metabolic markers is necessary. Standardization of biosensors to allow for use in high-throughput assays and to optimize therapeutic performance is another area that needs to be explored further. On-chip imaging methods to characterize tumors in terms of size, morphology, and viability can be achieved by techniques, such as confocal laser scanning microscopy, fluorescence hyperspectral imaging, and integration of optical elements into the microchip, which represent other avenues for analysis[139]. Another important area that use of bioprinting and microfluidics technology requires work on the part of regulatory agencies in further and create standardized guidelines for the requirements of processes for the approval and clinical translation of bioprinted models[19].
7. Conclusion
The fabrication of tumor-on-a-chip has the potential to reduce the dependence on animal models for cancer research by providing a platform for the screening of chemotherapeutic compounds and personalized medicine. Although studies have shown the ability of these platforms to capture the dynamic environment of tumor-stromal interactions and ECM, challenges related to manufacturing (bioinks, vasculature fabrications, process optimization, and standardization) and regulatory approval still exist. Advancements in bioprinting processes such as the development of hybrid printers for the fabrication of tissue and chip and the integration of biosensors and read-out displays are expected to allow for the widespread adoption of tumor-on-a-chip for cancer modeling and drug development.
Funding
This work was supported by institutional grants from the National Key R&D Program (2020YFF0305101, 2019YFC0119301, 2019YFC0119303).
Conflict of interest
The authors declare that they do not have any competing interests.
Author contributions
Supervision: Weiqing Wan
Conceptualization: Lingling Fang, Weiqing Wan
Investigation: Lingling Fang, Yu Liu, Junfeng Qiu
Methodology: Lingling Fang, Yu Liu
Formal analysis: Junfeng Qiu
Visualization: Junfeng Qiu
Writing – original draft: Lingling Fang, Yu Liu
Writing – review and editing: Lingling Fang, Weiqing Wan
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global Cancer Statistics 2018:GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. https://doi.org/10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020:GLOBACAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. https://doi.org/10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Colombet M, Soerjomataram I, et al. Cancer Incidence and Mortality Patterns in Europe:Estimates for 40 Countries and 25 Major Cancers in 2018. Eur J Cancer. 2018;103:356–87. doi: 10.1016/j.ejca.2018.07.005. https://doi.org/10.1016/j.ejca.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Stewart B, Wild CP. World Cancer Report 2014. IARC Publication 2014 [Google Scholar]
- 5.Knowlton S, Onal S, Yu CH, et al. Bioprinting for Cancer Research. Trends Biote. 2015;33:504–13. doi: 10.1016/j.tibtech.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Hay M, Thomas DW, Craighead JL, et al. Clinical Development Success Rates for Investigational Drugs. Nat Biotechnol. 2014;32:40–51. doi: 10.1038/nbt.2786. https://doi.org/10.1038/nbt.2786. [DOI] [PubMed] [Google Scholar]
- 7.Smietana K, Siatkowski M, Møller M. Trends in Clinical Success Rates. Nat Rev Drug Discov. 2016;15:379. doi: 10.1038/nrd.2016.85. https://doi.org/10.1038/nrd.2016.85. [DOI] [PubMed] [Google Scholar]
- 8.Heylman C, Sobrino A, Shirure VS, et al. A strategy for integrating essential three-dimensional microphysiological systems of human organs for realistic anticancer drug screening. Exp Biol Med (Maywood) 2014;239:1240–54. doi: 10.1177/1535370214525295. https://doi.org/10.1177/1535370214525295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imamura Y, Mukohara T, Shimono Y, et al. Comparison of 2D- and 3D-Culture Models as Drug-Testing Platforms in Breast Cancer. Oncol Rep. 2015;33:1837–43. doi: 10.3892/or.2015.3767. https://doi.org/10.3892/or.2015.3767. [DOI] [PubMed] [Google Scholar]
- 10.Chi CW, Lao YH, Ahmed R, et al. High-Throughput Tumor-on-a-chip Platform to Study Tumor-Stroma Interactions and Drug Pharmacokinetics. Adv Healthc Mater. 2020;9:2000880. doi: 10.1002/adhm.202000880. https://doi.org/10.1002/adhm.202000880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He Y, Nie J, Xie M, et al. Why Choose 3D Printing?Part III:Printing in 3D Models for Drug Screening. Bio Des Manufac. 2020;3:160–3. https://doi.org/10.1007/s42242-020-00067-7. [Google Scholar]
- 12.Samadian H, Jafari S, Sepand MR, et al. 3D Bioprinting Technology to Mimic the Tumor Microenvironment:Tumor-on-a-chip Concept. Mat Today Adv. 2021;12:100160. https://doi.org/10.1016/j.mtadv.2021.100160. [Google Scholar]
- 13.Cao X, Ashfaq R, Cheng F, et al. A Tumor-on-a-chip System with Bioprinted Blood AND Lymphatic Vessel Pair. Adv Funct Mater. 2019;29:1807173. doi: 10.1002/adfm.201807173. https://doi.org/10.1002/adfm.201807173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radhakrishnan J, Varadaraj S, Dash SK, et al. Organotypic Cancer Tissue Models for Drug Screening:3D Constructs, Bioprinting and Microfluidic Chips. Drug Discov Today. 2020;25:879–90. doi: 10.1016/j.drudis.2020.03.002. https://doi.org/10.1016/j.drudis.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Mak IW, Evaniew N, Ghert M. Lost in Translation:Animal Models and Clinical Trials in Cancer Treatment. Am J Transl Res. 2014;6:114–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Kronemberger GS, Miranda GA, Tavares RS, et al. Recapitulating Tumorigenesis In Vitro:Opportunities and Challenges of 3D Bioprinting. Front Bioeng Biotechnol. 2021;9:682498. doi: 10.3389/fbioe.2021.682498. https://doi.org/10.3389/fbioe.2021.682498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nath S, Devi GR. Three-Dimensional Culture Systems in Cancer Research:Focus on Tumor Spheroid Model. Pharmacol Therap. 2016;163:94–108. doi: 10.1016/j.pharmthera.2016.03.013. https://doi.org/10.1016/j.pharmthera.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaeri A, Cao K, Zhang F, et al. A Review of the Structural and Physical Properties that Govern Cell Interactions with Structured Biomaterials Enabled by Additive Manufacturing. Bioprinting. 2022;26:e00201. https://doi.org/10.1016/j.bprint.2022.e00201. [Google Scholar]
- 19.Augustine R, Kalva SN, Ahmad R, et al. 3D Bioprinted Cancer Models:Revolutionizing Personalized Cancer Therapy. Trans Oncol. 2021;14:101015. doi: 10.1016/j.tranon.2021.101015. https://doi.org/10.1016/j.tranon.2021.101015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopatina T, Gai C, Deregibus MC, et al. Cross Talk Between Cancer and Mesenchymal Stem Cells Through Extracellular Vesicles Carrying Nucleic Acids. Front Oncol. 2016;6:239. doi: 10.3389/fonc.2016.00125. https://doi.org/10.3389/fonc.2016.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Datta P, Dey M, Ataie Z, et al. 3D Bioprinting for Reconstituting the Cancer Microenvironment. Precision Oncol. 2020;4:18. doi: 10.1038/s41698-020-0121-2. https://doi.org/10.1038/s41698-020-0121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joyce JA, Pollard JW. Microenvironmental Regulation of Metastasis. Nat Rev Cancer. 2009;9:239. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turley SJ, Cremasco V, Astarita JL. Immunological Hallmarks of Stromal Cells in the Tumour Microenvironment. Nat Rev Immunol. 2015;15:669. doi: 10.1038/nri3902. https://doi.org/10.1038/nri3902. [DOI] [PubMed] [Google Scholar]
- 24.Cao Y. Tumor Angiogenesis and Therapy. Biomed Pharmacother. 2005;59:S340–3. doi: 10.1016/s0753-3322(05)80070-8. [DOI] [PubMed] [Google Scholar]
- 25.Trujillo-de Santiago G, Flores-Garza BG, Tavares-Negrete JA, et al. The Tumor-on-chip:Recent Advances in the Development of Microfluidic Systems to Recapitulate the Physiology of Solid Tumors. Materials. 2019;12:2945. doi: 10.3390/ma12182945. https://doi.org/10.3390/ma12182945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van den Berg A, Mummery CL, Passier R, et al. Personalised Organs-on-chips:Functional Testing for Precision Medicine. Lab Chip. 2019;19:198–205. doi: 10.1039/c8lc00827b. https://doi.org/10.1039/c8lc00827b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han B, Qu C, Park K, et al. Recapitulation of Complex Transport and Action of Drugs at the Tumor Microenvironment Using Tumor-Microenvironment-on-chip. Cancer Lett. 2016;380:319–29. doi: 10.1016/j.canlet.2015.12.003. https://doi.org/10.1016/j.canlet.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMaster R, Hoefner C, Hrynevich A, et al. Tailored Melt Electrowritten Scaffolds for the Generation of Sheet-like Tissue Constructs from Multicellular Spheroids. Adv Healthc Mater. 2019;8:e1801326. doi: 10.1002/adhm.201801326. https://doi.org/10.1002/adhm.201801326. [DOI] [PubMed] [Google Scholar]
- 29.Chiantore MV, Mangino G, Zangrillo MS, et al. Role of the Microenvironment in Tumourigenesis:Focus on Virus-induced Tumors. Curr Med Chem. 2015;22:958–74. doi: 10.2174/0929867322666141212121751. https://doi.org/10.2174/0929867322666141212121751. [DOI] [PubMed] [Google Scholar]
- 30.Peppicelli S, Andreucci E, Ruzzolini J, et al. The Acidic Microenvironment as a Possible Niche of Dormant Tumor Cells. Cell Mol Life Sci. 2017;74:2761–71. doi: 10.1007/s00018-017-2496-y. https://doi.org/10.1007/s00018-017-2496-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butturini E, de Prati AC, Boriero D, et al. Tumor Dormancy and Interplay with Hypoxic Tumor Microenvironment. Int J Mol Sci. 2019;20:4305. doi: 10.3390/ijms20174305. https://doi.org/10.3390/ijms20174305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu T, Dai Y. Tumor Microenvironment and Therapeutic Response. Cancer Lett. 2017;387:61–8. doi: 10.1016/j.canlet.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 33.O'Loghlen A. Role for Extracellular Vesicles in the Tumour Microenvironment. Philos Trans R Soc B Biol Sci. 2018;373:20160488. doi: 10.1098/rstb.2016.0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erdogan B, Ao M, White LM, et al. Cancer-Associated Fibroblasts Promote Directional Cancer Cell Migration by Aligning Fibronectin. J Cell Biol. 2017;216:3799–816. doi: 10.1083/jcb.201704053. https://doi.org/10.1083/jcb.201704053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naumov GN, Folkman J, Straume O, et al. Tumor-Vascular Interactions and Tumor Dormancy. APMIS. 2008;116:569–85. doi: 10.1111/j.1600-0463.2008.01213.x. https://doi.org/10.1111/j.1600-0463.2008.01213.x. [DOI] [PubMed] [Google Scholar]
- 36.Quail DF, Joyce JA. Microenvironmental Regulation of Tumor Progression and Metastasis. Nat Med. 2013;19:1423–37. doi: 10.1038/nm.3394. https://doi.org/10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arneth B. Tumor Microenvironment. Medicina. 2019;56:15. doi: 10.3390/medicina56010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hida K, Akiyama K, Ohga N, et al. Tumour Endothelial Cells Acquire Drug Resistance in a Tumour Microenvironment. J Biochem. 2013;153:243–9. doi: 10.1093/jb/mvs152. https://doi.org/10.1093/jb/mvs152. [DOI] [PubMed] [Google Scholar]
- 39.Sahai E, Astsaturov I, Cukierman E, et al. A Framework of Advancing Our Understanding of Cancer-associated Fibroblasts. Nat Rev Cancer. 2020;20:174–86. doi: 10.1038/s41568-019-0238-1. https://doi.org/10.1002/adma.201601504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanahan D, Coussens LM. Accessories to the Crime:Functions of Cells Recruited to the Tumor Microenvironment. Cancer Cell. 2012;21:309–22. doi: 10.1016/j.ccr.2012.02.022. https://doi.org/10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 41.Sun Z, Wang S, Zhao RC. The Roles of Mesenchymal Stem Cells in Tumor Inflammatory Microenvironment. J Hematol Oncol. 2014;7:14. doi: 10.1186/1756-8722-7-14. https://doi.org/10.1186/1756-8722-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanahan D, Coussens LM. Accessories to the Crime:Functions of Cells Recruited to the Tumor Microenvironment. Cancer Cell. 2012;21:309–22. doi: 10.1016/j.ccr.2012.02.022. https://doi.org/10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 43.Bi J, Tian Z. NK Cell Exhaustion. Front Immunol. 2017;8:760. doi: 10.3389/fimmu.2017.00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang C, Hu Y, Shi C. Targeting Natural Killer Cells for Tumor Immunotherapy. Front Immunol. 2020;11:60. doi: 10.3389/fimmu.2020.00060. https://doi.org/10.3389/fimmu.2020.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Payne KK, Bear HD, Manjili MH. Adoptive Cellular Therapy of Cancer:Exploring Innate and Adaptive Cellular Crosstalk to Improve Anti-tumor Efficacy. Future Oncol. 2014;10:1779–94. doi: 10.2217/fon.14.97. https://doi.org/10.2217/fon.14.97. [DOI] [PubMed] [Google Scholar]
- 46.Nassar D, Blanpain C. Cancer Stem Cells:Basic Concepts and Therapeutic Implications. Annu Rev Pathol. 2016;11:47–76. doi: 10.1146/annurev-pathol-012615-044438. https://doi.org/10.1146/annurev-pathol-012615-044438. [DOI] [PubMed] [Google Scholar]
- 47.Albini A, Bruno A, Gallo C, et al. Cancer Stem Cells and the Tumor Microenvironment:Interplay in Tumor Heterogeneity. Connect Tissue Res. 2015;56:414–25. doi: 10.3109/03008207.2015.1066780. https://doi.org/10.3109/03008207.2015.1066780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Lope LR, Alcíbar OL, López AA, et al. Tumour-adipose Tissue Crosstalk:Fuelling Tumour Metastasis by Extracellular Vesicles. Philos Trans R Soc London Ser B Biol Sci. 2018;373:20160485. doi: 10.1098/rstb.2016.0485. https://doi.org/10.1098/rstb.2016.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ribeiro AL, Okamoto OK. Combined Effects of Pericytes in the Tumor Microenvironment. Stem Cells Int. 20152015:868475. doi: 10.1155/2015/868475. https://doi.org/10.1155/2015/868475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barrow AD, Colonna M. Exploiting NK Cell Surveillance Pathways for Cancer Therapy. Cancers. 2019;11:55. doi: 10.3390/cancers11010055. https://doi.org/10.3390/cancers11010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mravic M, Asatrian G, Soo C, et al. From Pericytes to Perivascular Tumours: Correlation Between Pathology, Stem Cell Biology, and Tissue Engineering. Int Orthop. 2014;38:1819–24. doi: 10.1007/s00264-014-2295-0. https://doi.org/10.1007/s00264-014-2295-0. [DOI] [PubMed] [Google Scholar]
- 52.Insua-Rodriguez J, Oskarsson T. The Extracellular Matrix in Breast Cancer. Adv Drug Deliv Rev. 2016;97:41–55. doi: 10.1016/j.addr.2015.12.017. https://doi.org/10.1016/j.addr.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 53.Cheng YQ, Wang SB, Liu JH, et al. Modifying the Tumour Microenvironment and Reverting Tumour Cells:New Strategies for Treating Malignant Tumours. Cell Prolif. 2020;53:e12865. doi: 10.1111/cpr.12865. https://doi.org/10.1111/cpr.12865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trivanovic D, Krstic J, Djordjevic IO, et al. The Roles of Mesenchymal Stromal/Stem Cells in Tumor Microenvironment Associated with Inflammation. Mediators Inflamm. 20162016:7314016. doi: 10.1155/2016/7314016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sangaletti S, Chiodoni C, Tripodo C, et al. Common Extracellular Matrix Regulation of Myeloid Cell Activity in the Bone Marrow and Tumor Microenvironments. Cancer Immunol Immunother. 2017;66:1059–67. doi: 10.1007/s00262-017-2014-y. https://doi.org/10.1007/s00262-017-2014-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mohammadi H, Sahai E. Mechanisms and Impact of Altered Tumour Mechanics. Nat Cell Bio. 2018;20:766–74. doi: 10.1038/s41556-018-0131-2. https://doi.org/10.1038/s41556-018-0131-2. [DOI] [PubMed] [Google Scholar]
- 57.Levental KR, Yu H, Kass L, et al. Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. https://doi.org/10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Masola V, Bellin G, Gambaro G, et al. Heparanase:A Multitasking Protein Involved in Extracellular Matrix (ECM) Remodeling and Intracellular Events. Cell. 2018;7:236. doi: 10.3390/cells7120236. https://doi.org/10.3390/cells7120236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nallanthighal S, Heiserman JP, Cheon DJ. The Role of the Extracellular Matrix in Cancer Stemness. Front Cell Dev Biol. 2019;7:86. doi: 10.3389/fcell.2019.00086. https://doi.org/10.3389/fcell.2019.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsai HF, Trubelja A, Shen AQ. Tumour-On-A-Chip:Microfluidic Models of Tumour Morphology, Growth and Microenvironment. J R Soc Interface. 2017;14:20170137. doi: 10.1098/rsif.2017.0137. https://doi.org/10.1098/rsif.2017.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shoemaker RH. The NCI60 Human Tumour Cell Line Anticancer Drug Screen. Nat Rev Cancer. 2016;6:813–23. doi: 10.1038/nrc1951. https://doi.org/10.1038/nrc1951. [DOI] [PubMed] [Google Scholar]
- 62.Liu X, Fang J, Huang S, et al. Tumor-on-a-chip:From Bioinspired Design to Biomedical Application. Microsyst Nanoeng. 2021;7:50. doi: 10.1038/s41378-021-00277-8. https://doi.org/10.1038/s41378-021-00277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kapalczynska M, Kolenda T, Przybyla W, et al. 2D and 3D Cell Cultures-A Comparison of Different Types of Cancer Cell Cultures. Arch Med Sci. 2018;14:910. doi: 10.5114/aoms.2016.63743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ryan SL, Baird AM, Vaz G, et al. Drug Discovery Approaches Utilizing Three-Dimensional Cell Culture. Assay Drug Dev Technol. 2016;14:19–28. doi: 10.1089/adt.2015.670. https://doi.org/10.1089/adt.2015.670. [DOI] [PubMed] [Google Scholar]
- 65.Brancato V, Oliviera JM, Correlo VM, et al. Could 3D Models of Cancer Enhance Drug Screening? Biomaterials. 2020;232:119744. doi: 10.1016/j.biomaterials.2019.119744. https://doi.org/10.1016/j.biomaterials.2019.119744. [DOI] [PubMed] [Google Scholar]
- 66.Skardal A, Devarasetty M, Forsythe S, et al. A Reductionist Metastasis-on-a-chip Platform for In Vitro Tumor Progression Modeling and Drug Screening. Biotechnol Bioeng. 2016;113:2020–32. doi: 10.1002/bit.25950. https://doi.org/10.1002/bit.25950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saglam-Metiner P, Gulce-Iz S, Biray-Avci C. BioengineeringnInspired Threegdimensional Culture Systems:Organoids to Create Tumor Microenvironment. Gene. 2019;686:203–12. doi: 10.1016/j.gene.2018.11.058. https://doi.org/10.1016/j.gene.2018.11.058. [DOI] [PubMed] [Google Scholar]
- 68.Carvalho V, Maia I, Souza A, et al. In Vitro Stenotic Arteries to Perform Blood Analogues Flow Visualizations and Measurements:A Review. Open Biomed Eng J. 2020;14:87–102. https://doi.org/10.2174/1874120702014010087. [Google Scholar]
- 69.Friedrich J, Seidel C, Ebner R, et al. Spheroid-Based Drug Screen:Considerations and Practical Approach. Nat Protoc. 2009;4:309–24. doi: 10.1038/nprot.2008.226. https://doi.org/10.1038/nprot.2008.226. [DOI] [PubMed] [Google Scholar]
- 70.Costa EC, Moreira AF, de Melo-Diogo D, et al. 3D Tumor Spheroids:An Overview on the Tools and Techniques Used for Their Analysis. Biotechnol Adv. 2016;34:1427–41. doi: 10.1016/j.biotechadv.2016.11.002. https://doi.org/10.1016/j.biotechadv.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 71.Hoffmann OI, Ilmberger C, Magosch S, et al. Impact of the Spheroid Model Complexity on Drug Response. J Biotechnol. 2015;205:14–23. doi: 10.1016/j.jbiotec.2015.02.029. https://doi.org/10.1016/j.jbiotec.2015.02.029. [DOI] [PubMed] [Google Scholar]
- 72.Nunes AS, Barros AS, Costa EC, et al. 3D Tumor Spheroids as In Vitro Models to Mimic In Vivo Human Solid Tumors Resistance to Therapeutic Drugs. Biotechnol Bioeng. 2019;116:206–26. doi: 10.1002/bit.26845. https://doi.org/10.1002/bit.26845. [DOI] [PubMed] [Google Scholar]
- 73.Fang Y, Eglen RM. Three-Dimensional Cell Cultures in Drug Discovery and Development. SLAS Discov. 2017;22:456–72. doi: 10.1177/1087057117696795. https://doi.org/10.1177/1087057117696795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kitaeva KV, Rutland CS, Rizvanov AA, et al. Cell Culture Based In Vitro Test Systems for Anticancer Drug Screening. Front Bioeng Biotechnol. 2020;8:322. doi: 10.3389/fbioe.2020.00322. https://doi.org/10.3389/fbioe.2020.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clevers H. Modeling Development and Disease with Organoids. Cell. 2016;165:1586–97. doi: 10.1016/j.cell.2016.05.082. [DOI] [PubMed] [Google Scholar]
- 76.Velasco V, Shariati SA, Esfandyarpour R. Microtechnology-Based Methods for Organoid Models. Microsyst Nanoeng. 2020;6:76. doi: 10.1038/s41378-020-00185-3. https://doi.org/10.1038/s41378-020-00185-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lutolf MP, Lauer-Fields JL, Schmoekel HG, et al. Synthetic Matrix Metalloproteinase-Sensitive Hydrogels for the Conduction of Tissue Regeneration:Engineering Cell Invasion Characteristics. Proc Natl Acad Sci U S A. 2003;100:5413–8. doi: 10.1073/pnas.0737381100. https://doi.org/10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nguyen KT, West JL. Photopolymerizable Hydrogels for Tissue Engineering Applications. Biomaterials. 2002;23:4307–14. doi: 10.1016/s0142-9612(02)00175-8. https://doi.org/10.1016/s0142-9612(02)00175-8. [DOI] [PubMed] [Google Scholar]
- 79.Charbe N, McCarron PA, Tambuwala MM. Three-Dimensional Bio-Printing:A New Frontier in Oncology Research. World J Clin Oncol. 2017;8:21. doi: 10.5306/wjco.v8.i1.21. https://doi.org/10.5306/wjco.v8.i1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zaeri A, Zgeib R, Cao K, et al. Numerical Analysis on the Effects of Microfluidic-based Bioprinting Parameters on the Microfiber Geometrical Outcomes. Sci Rep. 2022;12:3364. doi: 10.1038/s41598-022-07392-0. https://doi.org/10.1038/s41598-022-07392-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Colosi C, Shin SR, Manoharan V, et al. Microfluidic Bioprinting of Heterogenous 3D Tissue Constructs Using Low-viscosity Bioink. Adv Mater. 2016;28:677–84. doi: 10.1002/adma.201503310. https://doi.org/10.1002/adma.201503310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu Y, Wei W, Wang Y, et al. Simple Spinning of Heterogeneous Hollow Microfibers on Chip. Adv Mater. 2016;28:6649–55. doi: 10.1002/adma.201601504. [DOI] [PubMed] [Google Scholar]
- 83.Murphy SV, Atala A. 3D Bioprinting of Tissues and Organs. Nat Biotechnol. 2014;32:773–85. doi: 10.1038/nbt.2958. https://doi.org/10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 84.Ingber DE, Mow VC, Butler D, et al. Tissue Engineering and Developmental Biology:Going Biomimetic. Tissue Eng. 2006;12:3265–83. doi: 10.1089/ten.2006.12.3265. https://doi.org/10.1089/ten.2006.12.3265. [DOI] [PubMed] [Google Scholar]
- 85.Mironov V, Visconti RP, Kasyanov V, et al. Organ Printing:Tissue Spheroids as Building Blocks. Biomaterials. 2009;30:2164–74. doi: 10.1016/j.biomaterials.2008.12.084. https://doi.org/10.1089/ten.2006.12.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kelm JM, Lorber V, Snedeker JG, et al. A Novel Concept for Scaffold-Free Vessel Tissue Engineering:Self-assembly of Microtissue Building Blocks. J Biotechnol. 2010;148:46–55. doi: 10.1016/j.jbiotec.2010.03.002. https://doi.org/10.1016/j.jbiotec.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 87.Tiwari AP, Thorat ND, Pricl S, et al. Bioink:A 3D-Bioprinting Tool for Anticancer Drug Discovery and Cancer Management. Drug Discov Today. 2021;26:1574–90. doi: 10.1016/j.drudis.2021.03.010. https://doi.org/10.1016/j.drudis.2021.03.010. [DOI] [PubMed] [Google Scholar]
- 88.Hospodiuk M, Dey M, Sosnoski D, et al. The Bioink:A Comprehensive Review on Bioprintable Materials. Biotechnol Adv. 2017;35:217–39. doi: 10.1016/j.biotechadv.2016.12.006. https://doi.org/10.1016/j.biotechadv.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 89.Kleinman HK, Martin GR. Matrigel:Basement Membrane Matrix with Biological Activity. Semin Cancer Biol. 2005;15:378–86. doi: 10.1016/j.semcancer.2005.05.004. https://doi.org/10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 90.Yin Z, Dong C, Jiang K, et al. Heterogeneity of Cancer-Associated Fibroblasts and Roles in the Progression, Prognosis, and Therapy of Hepatocellular Carcinoma. J Hematol Oncol. 2019;12:101. doi: 10.1186/s13045-019-0782-x. https://doi.org/10.1186/s13045-019-0782-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Belgodere JA, King CT, Bursavich JB, et al. Engineering Breast Cancer Microenvironments and 3D Bioprinting. Front Bioeng Biotechnol. 2018;6:66. doi: 10.3389/fbioe.2018.00066. https://doi.org/10.3389/fbioe.2018.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Foyt DA, Norma MA, Yu TT, et al. Exploiting Advanced Hydrogel Technologies to Address Key Challenges in Regenerative Medicine. Adv Healthcare Mat. 2018;7:1700939. doi: 10.1002/adhm.201700939. https://doi.org/10.1002/adhm.201700939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Markstedt K, Mantas A, Tournier I, et al. 3D Bioprinting Human Chondrocytes with Nanocellulose-Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromol. 2015;16:1489–96. doi: 10.1021/acs.biomac.5b00188. https://doi.org/10.1021/acs.biomac.5b00188. [DOI] [PubMed] [Google Scholar]
- 94.Duan B, Hockaday LA, Hang KH, et al. 3D Bioprinting of Heterogeneous Aortic Valve Conduits with Alginate/Gelatin Hydrogels. J Biomed Mater Res A. 2013;101:1255–64. doi: 10.1002/jbm.a.34420. https://doi.org/10.1002/jbm.a.34420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Norotte C, Marga FS, Niklason LE, et al. Scaffold-Free Vascular Tissue Engineering Using Bioprinting. Biomaterials. 2009;30:5910–7. doi: 10.1016/j.biomaterials.2009.06.034. https://doi.org/10.1016/j.biomaterials.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rhee S, Puetzer JL, Mason BN, et al. 3D Bioprinting of Spatially Heterogeneous Collagen Constructs for Cartilage Tissue Engineering. ACS Biomater Sci Eng. 2016;2:1800–5. doi: 10.1021/acsbiomaterials.6b00288. https://doi.org/10.1021/acsbiomaterials.6b00288. [DOI] [PubMed] [Google Scholar]
- 97.Kingsley DM, Roberge CL, Rudkouskaya A, et al. Laser-Based 3D Bioprinting for Spatial and Size Control of Tumor Spheroids and Embryoid Bodies. Acta Biomater. 2019;95:357–70. doi: 10.1016/j.actbio.2019.02.014. https://doi.org/10.1016/j.actbio.2019.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lim KS, Galarraga JH, Cui X, et al. Fundamentals and Applications of Photo-Cross-Linking in Bioprinting. Chem Rev. 2020;120:10662–94. doi: 10.1021/acs.chemrev.9b00812. https://doi.org/10.1021/acs.chemrev.9b00812. [DOI] [PubMed] [Google Scholar]
- 99.Shin JY, Yeo YH, Jeong JE, et al. Dual-Crosslinked Methylcellulose Hydrogels for 3D Bioprinting Applications. Carbohydr Polym. 2020;238:116192. doi: 10.1016/j.carbpol.2020.116192. https://doi.org/10.1016/j.carbpol.2020.116192. [DOI] [PubMed] [Google Scholar]
- 100.Konig G, McAllister TN, Dusserre N, et al. Mechanical Properties of Completely Autologous Human Tissue Engineered Blood Vessels Compared to Human Saphenous Vein and Mammary Artery. Biomaterials. 2009;30:1542–50. doi: 10.1016/j.biomaterials.2008.11.011. https://doi.org/10.1016/j.biomaterials.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rago AP, Dean DM, Morgan JP. Controlling Cell Position in Complex Heterotypic 3D Microtissues by Tissue Fusion. Biotechnol Bioeng. 2009;102:1231–41. doi: 10.1002/bit.22162. https://doi.org/10.1002/bit.22162. [DOI] [PubMed] [Google Scholar]
- 102.Xu Q, Norma JT, Shrivastav J, et al. In Vitro Models of TGF-b-induced Fibrosis Suitable for High-throughput Screening of Antifibrotic Agents. Am J Physiol. 2007;293:F631–40. doi: 10.1152/ajprenal.00379.2006. https://doi.org/10.1152/ajprenal.00379.2006. [DOI] [PubMed] [Google Scholar]
- 103.Wang Y, Shi W, Kuss M, et al. 3D Bioprinting of Breast Cancer Models for Drug Resistance Study. ACS Biomater Sci Eng. 2018;4:4401–11. doi: 10.1021/acsbiomaterials.8b01277. https://doi.org/10.1021/acsbiomaterials.8b01277. [DOI] [PubMed] [Google Scholar]
- 104.Murphy SV, Atala A. 3D Bioprinting of Tissues and Organs. Nat Biotechnol. 2014;32:773–85. doi: 10.1038/nbt.2958. https://doi.org/10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 105.Knowlton S, Joshi A, Yenilmez B, et al. Advancing Cancer Research Using Bioprinting for Tumour-on-chip Platforms. Int J Bioprint. 2016;2:3–8. https://doi.org/10.18063/ijb.2016.02.003. [Google Scholar]
- 106.Huh D, Hamilton GA, Ingber DE. From 3D Cell Culture to Organs-on-chips. Trends Cell Biol. 2011;21:745–54. doi: 10.1016/j.tcb.2011.09.005. https://doi.org/10.1016/j.tcb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chang SF, Chang CA, Lee DY, et al. Tumour Cell Cycle Arrest Induced by Shear Stress:Roles of Integrins and Smad. Proc Nat Acad Sci. 2008;105:3927–32. doi: 10.1073/pnas.0712353105. https://doi.org/10.1073/pnas.0712353105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Skardal A, Aleman J, Forsythe S, et al. Drug Compound Screening in Single and Integrated Multi-Organoid Body-on-a-chip Systems. Biofabrication. 2020;12:025017. doi: 10.1088/1758-5090/ab6d36. https://doi.org/10.1088/1758-5090/ab6d36. [DOI] [PubMed] [Google Scholar]
- 109.Bhatia SN, Ingber DE. Microfluidic Organs-on-chips. Nat Biotechnol. 2014;32:760–72. doi: 10.1038/nbt.2989. https://doi.org/10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 110.Yang Q, Lian Q, Xu F. Perspective:Fabrication of Integrated Organ-On-Achip Via Bioprinting. Biomicrofluidics. 2017;11:031301. doi: 10.1063/1.4982945. https://doi.org/10.1063/1.4982945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Asano H, Shiraishi Y. Development of Paper-Based Microfluidic Analytical Device for Iron Assay Using Photomask Printed with 3D Printer for Fabrication of Hydrophilic and Hydrophobic Zones on Paper by Photolithography. Anal Chim Acta. 2015;883:55–60. doi: 10.1016/j.aca.2015.04.014. https://doi.org/10.1016/j.aca.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 112.Carvalho V, Gonçalves I, Lage T, et al. 3D Printing Techniques and Their Applications to Organ-on-a-chip Platforms:A Systematic Review. Sensors. 2021;21:33014. doi: 10.3390/s21093304. https://doi.org/10.3390/s21093304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Qin D, Xia Y, Whitesides GM. Soft Lithography for Micro-and Nanoscale Patterning. Nat Protoc. 2010;5:491–502. doi: 10.1038/nprot.2009.234. https://doi.org/10.1038/nprot.2009.234. [DOI] [PubMed] [Google Scholar]
- 114.McDonald JC, Whitesides GM. Poly (Dimethylsiloxane) as a Material for Fabricating Microfluidic Devices. Acc Chem Res. 2002;35:491–9. doi: 10.1021/ar010110q. https://doi.org/10.1021/ar010110q. [DOI] [PubMed] [Google Scholar]
- 115.Thakare K, Jerpseth L, Pei Z, et al. Bioprinting of Organ-on-chip Systems:A Literature Review from a Manufacturing Perspective. J Manuf Mater Process. 2021;5:91. https://doi.org/10.3390/jmmp5030091. [Google Scholar]
- 116.Bernard A, Renault JP, Michel B, et al. Microcontact Printing of Proteins. Adv Mater. 2000;12:1067–70. https://doi.org/10.1002/1521-4095(200007)12:14<1067:aid-adma1067>3.0.co;2-m. [Google Scholar]
- 117.Auner AW, Tasneem KM, Markov DA, et al. Chemical-PDMS Binding Kinetics and Implications for Bioavailability in Microfluidic Devices. Lab Chip. 2019;19:864–74. doi: 10.1039/c8lc00796a. https://doi.org/10.1039/c8lc00796a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nguyen T, et al. Robust chemical bonding of PMMA microfluidic devices to porous PETE membranes for reliable cytotoxicity testing of drugs. Lab Chip. 2019;19:3706–13. doi: 10.1039/c9lc00338j. https://doi.org/10.1039/c9lc00338j. [DOI] [PubMed] [Google Scholar]
- 119.Dababneh AB, Ozbolat IT. Bioprinting Technology:A Current State-of-the-art Review. J Manuf Sci Eng. 2014;136:061016. [Google Scholar]
- 120.Xu F, Celli J, Rizvi I, et al. A Three-Dimensional In Vitro Ovarian Cancer Coculture Model Using a High-Throughput Cell Patterning Platform. Biotechnol J. 2011;6:204–12. doi: 10.1002/biot.201000340. https://doi.org/10.1002/biot.201000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Keenan TM, Folch A. Biomolecular Gradients in Cell Culture Systems. Lab Chip. 2008;8:34–57. doi: 10.1039/b711887b. https://doi.org/10.1039/b711887b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Monteiro MV, Zhang YS, Gaspar VM, et al. 3D-Bioprinted Cancer-on-a-chip:Level-Up Organotypic In Vitro Models. Trends Biotechnol. 2021;40:432–47. doi: 10.1016/j.tibtech.2021.08.007. https://doi.org/10.1016/j.tibtech.2021.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hamid Q, Wang C, Zhao Y, et al. A Three-dimensional Cell-laden Microfluidic Chip for In Vitro Drug Metabolism Detection. Biofabrication. 2014;6:025008. doi: 10.1088/1758-5082/6/2/025008. https://doi.org/10.1088/1758-5082/6/2/025008. [DOI] [PubMed] [Google Scholar]
- 124.Hamid Q, Wang C, Snyder J, et al. Maskless Fabrication of Cell-laden Microfluidic Chips with Localized Surface Functionalization for the Co-culture of Cancer Cells. Biofabrication. 2015;7:015012. doi: 10.1088/1758-5090/7/1/015012. https://doi.org/10.1088/1758-5090/7/1/015012. [DOI] [PubMed] [Google Scholar]
- 125.Zhang J, Chen F, He Z, et al. A Novel Approach for Precisely Controlled Multiple Cell Patterning in Microfluidic Chip by Inkjet Printing and Detection of Drug Metabolism and Diffusion. Analyst. 2016;141:395. doi: 10.1039/c6an00395h. https://doi.org/10.1088/1758-5090/7/1/015012. [DOI] [PubMed] [Google Scholar]
- 126.Cheng F, Cao X, Li H, et al. Generation of Cost-effective Paper-based Tissue Models Through Matrix-assisted Sacrificial 3D Printing. Nano Lett. 2019;19:3603–11. doi: 10.1021/acs.nanolett.9b00583. https://doi.org/10.1021/acs.nanolett.9b00583.s001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li Y, Zhang T, Pang Y, et al. 3D Bioprinting of Hepatoma Cells and Application with Microfluidics for Pharmacodynamic Test of Metuzumab. Biofabrication. 2019;11:034102. doi: 10.1088/1758-5090/ab256c. https://doi.org/10.1088/1758-5090/ab256c. [DOI] [PubMed] [Google Scholar]
- 128.Mi S, Yang S, Liu T, et al. A Novel Controllable Cell Array Printing Technique on Microfluidic Chips. Trans Biomed Eng. 2019;66:2512–20. doi: 10.1109/TBME.2019.2891016. https://doi.org/10.1109/tbme.2019.2891016. [DOI] [PubMed] [Google Scholar]
- 129.Yi HG, Jeong YH, Kim Y, et al. A Bioprinted Glioblastoma-on-a-chip for the Identification of Patient-Specific Responses to Chemoradiotherapy. Nat Biomed Eng. 2019;3:509–519. doi: 10.1038/s41551-019-0363-x. https://doi.org/10.1038/s41551-019-0363-x. [DOI] [PubMed] [Google Scholar]
- 130.Xie M, Gao Q, Fu J, et al. Bioprinting of Novel 3D Tumor Array Chip for Drug Screening. Biodes Manuf. 2020;3:175–88. https://doi.org/10.1007/s42242-020-00078-4. [Google Scholar]
- 131.Komen J, van Neerven SM, van den Berg A, et al. Mimicking and Surpassing the Xenograft Model with Cancer-on-chip Technology. EBioMedicine. 2021;66:103303. doi: 10.1016/j.ebiom.2021.103303. https://doi.org/10.1016/j.ebiom.2021.103303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Maulana TI, Kromidas E, Wallstabe L, et al. Immunocompetent Cancer-on-chip Models to Assess Immuno-oncology Therapy. Adv Drug Del Rev. 2021;173:281–305. doi: 10.1016/j.addr.2021.03.015. https://doi.org/10.1016/j.addr.2021.03.015. [DOI] [PubMed] [Google Scholar]
- 133.Krishnamoorthy M, Gerhardt L, Vareki SM. Immunosuppressive Effects of Myeloid-Derived Suppressor Cells in Cancer and Immunotherapy. Cells. 2021;10:1170. doi: 10.3390/cells10051170. https://doi.org/10.3390/cells10051170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kumar SA, Delgado M, Mendez VE, et al. Applications of Stem Cells and Bioprinting for Potential Treatment of Diabetes. World J Stem Cells. 2019;11:13–32. doi: 10.4252/wjsc.v11.i1.13. https://doi.org/10.4252/wjsc.v11.i1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jovic TH, Combellack EJ, Jessop ZM, et al. 3D Bioprinting and the Future of Surgery. Front Surg. 2020;7:609836. doi: 10.3389/fsurg.2020.609836. https://doi.org/10.3389/fsurg.2020.609836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Izumchenko E, Paz K, Ciznadija D, et al. Patient-derived xenografts effectively capture responses to oncology therapy in a heterogeneous cohort of patients with solid tumors. Ann Oncol. 2017;28:2595–605. doi: 10.1093/annonc/mdx416. https://doi.org/10.1093/annonc/mdx416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang N, Yin Y, Xu SJ, et al. 5-Fluorouracil:Mechanisms of resistance and reversal strategies. Molecules. 2008;13:1551–69. doi: 10.3390/molecules13081551. https://doi.org/10.3390/molecules13081551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Boso D, Maghin E, Carraro E, et al. Extracellular matrix-derived hydrogels as biomaterial for different skeletal muscle tissue replacements. Materials (Basel) 2020;13:2483. doi: 10.3390/ma13112483. https://doi.org/10.3390/ma13112483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Weissleder R, Pittet MJ. Imaging in the Era of Molecular Oncology. Nature. 2008;452:580–9. doi: 10.1038/nature06917. https://doi.org/10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]


