Abstract
Neurofeedback training (NFT) is a noninvasive neuromodulation method for children with attention-deficit/hyperactivity disorder (ADHD). Brain rhythms, the unique pattern in electroencephalogram (EEG), are widely used as the training target. Most of current studies used a fixed frequency division of brain rhythms, which ignores the individual developmental difference of each child. In this study, we validated the feasibility of NFT using individual beta rhythm. A total of 55 children with ADHD were divided into two groups using the relative power of individual or fixed beta rhythms as the training index. ADHD rating scale (ADHD-RS) was completed before and after NFT, and the EEG and behavioral features were extracted during the training process. After intervention, the attention ability of both groups was significantly improved, showing a significant increase in beta power, a decrease in scores of ADHD-RS and an improvement in behavioral and other EEG features. The training effect was significantly better with individualized beta training, showing more improvement in ADHD-RS scores. Furthermore, the distribution of brain rhythms moved towards high frequency after intervention. This study demonstrates the effectiveness of NFT based on individual beta rhythm for the intervention of children with ADHD. When designing a NFT protocol and the corresponding data analysis process, an individualized brain rhythm division should be applied to reflect the actual brain state and to accurately evaluate the effect of NFT.
Keywords: ADHD, Neurofeedback, EEG, Attention, Individual rhythm
Introduction
Neurofeedback training (NFT) is a type of biofeedback that enhances cognitive and physical performance by measuring neural activity in real time and giving feedback to participants in an intuitive way (auditory, visual, tactile, etc.) (Gruzelier 2014a; Simkin et al. 2014; Sitaram et al. 2017). Participants are guided to spontaneously modulate their neural activity by using certain mental strategies (Nan et al. 2012; Kober et al. 2013). As a noninvasive and endogenous noninvasive neuromodulation method, NFT is easy to perform and is suitable for a wide range of people. NFT has been widely used in the modulation of various mental and neurological diseases, such as epilepsy, depression, anxiety, and brain damage, and it is also used in the enhancement of cognitive abilities, such as attention, memory, emotion, movement, and creativity (Gruzelier 2014a).
Attention-deficit/hyperactivity disorder (ADHD) is one of the most prevalent developmental neuropsychiatric disorders, affecting approximately 5% of school-age children (Polanczyk et al. 2007; Konrad and Eickhoff 2010). Children with ADHD are characterized by inappropriate inattention, hyperactivity and impulsiveness (Del Barrio 2016). Pharmacotherapy is currently the most effective therapy for the core symptoms of ADHD (Sibley et al. 2014). However, the drawbacks of medication are a lack of long-lasting effects and the presence of side effects, such as effects on sleep and decreased appetite (Storebø et al. 2015), with an additional 13.2% to 64% of patients experiencing drug discontinuation and non-adherence problems (Adler and Nierenberg 2010). NFT is a promising alternative option for intervention in children with ADHD. Theta increases and/or beta decreases have been widely found in ADHD EEG studies, and increases in motor cortical beta rhythms can also be found in attention tasks (Arns et al. 2013; Chen et al. 2019a). Therefore, NFT based on beta and theta/beta ratio (TBR) are widely used for ADHD interventions. Recent meta-studies have shown that NFT is effective in the treatment of ADHD and that the use of NFT in children with ADHD has shown longer-lasting effects than control treatments (Arns and Kenemans 2014; Van Doren et al. 2019; Enriquez-Geppert et al. 2019; Orendáčová and Kvašňák 2021).
However, certain studies reported lower effects of NFT on ADHD treatment compared to medication (Ogrim et al. 2012; Geladé et al. 2017), and some experiments could not even replicate the results of NFT efficacy in the treatment of ADHD (Logemann et al. 2010; Cortese et al. 2016). This result may be due to the use of fixed frequency division of brain rhythms. The frequency range of brain rhythms is not constant. During childhood and adolescence, the alpha frequency range increases with age and subsequently becomes stable (Niedermeyer 1999; Stroganova et al. 1999; Bazanova 2008; Bazanova and Vernon 2014). The EEG abnormalities of each ADHD child differ in time, space, and frequency range (Chen et al. 2019b). The majority of existing rhythm-based NFT studies used fixed rhythm ranges that were defined using adult EEG data. Such NFT may cause training to be ineffective or even harmful to the participants (Arns et al. 2014). Additionally, given the complex relationship between attention and brain rhythms, there is a hypothesis that NFT may further influence the frequency ranges of brain rhythms, causing fixed rhythms to further deviate from the actual brain state. In addition, previous studies have reported significant results mainly at the group level (Arns et al. 2009; Enriquez-Geppert et al. 2019), and few studies have discussed the effects of NFT on the brain rhythms of individuals (Nan et al. 2012; Albert et al. 2017; Weber et al. 2020).
Therefore, we argue that NFT as an intervention for children with ADHD should be conducted using individual brain rhythms. In this study, individual beta NFTs were compared to fixed beta NFTs to validate their effectiveness. We further explored the relationship between changes in rhythms and the improvement in attention ability during training, providing the evidence for individualized and precise interventions for children with ADHD.
Methods
Participants
Sixty-one children with ADHD were recruited in the current study, and 55 participants successfully completed the training (16 girls, age range: 4–10 yr, mean age: 6.03 ± 1.69 yr). The inclusion criteria for children with ADHD were as follows: (1) met the structured and interviewer-administered scale based on DSM-IV; (2) right-handed; (3) no lifetime history of head trauma with loss of consciousness; (4) no history of neurological illness or other severe disease; (5) normal vision or corrected normal vision, no color blindness or color weakness; and (6) no history of taking stimulants or other medication for ADHD symptoms. The study was conducted in the laboratory and approved by the Ethics Committee of Beijing Normal University. Written consent was obtained from the parents or guardians of the participants after a complete description of the experimental procedures.
Before the first NFT session and 2–5 days after the last NFT session, the parents were asked to complete the ADHD rating scale (ADHD-RS) (DuPaul et al. 1998), which consists of 2 subscales: inattention (9 items) and hyperactivity-impulsivity (9 items). Each item is scored on a 1–4 scale, indicating that the behavior occurs ‘rarely or never’, ‘somewhat’, ‘often’ or ‘always or very often’, respectively. The scores of each subscale are summed as the inattention score and hyperactivity score, respectively, and the scores of all 18 items are summed as the total score. Higher scores on the ADHD-RS indicate more severe corresponding symptoms.
Neurofeedback training
EEG Acquisition
A single-channel EEG system with active electrodes was used to ensure signal quality (Jielian Co., Ltd., Jiangxi, China). Most of previous ADHD studies reported theta increases and/or beta decreases at location Cz (Arns et al. 2013; Lenartowicz and Loo 2014), in this study, EEG was recorded at Cz using a linked-ear reference (M1/M2) at a sampling rate of 1000 Hz, and the contact impedance was kept below 30 kΩ. The 55 children were divided into two groups: the first group (noted as the iBeta group, 28 participants, 8 girls) used the relative power of individual beta rhythms as the training index, and the second group (noted as the Beta group, 27 participants, 8 girls) used the relative power of a fixed beta rhythm as the training index. Both trainer and participants/parents were blinded for the type of training.
The power spectrum was obtained using Welch’s method, and the relative power was defined as the ratio between the sum of the power spectrum within the beta range and the sum of the power in the frequency range of 4–45 Hz. The fixed beta range is 13–30 Hz, but the individual beta was calculated before each training session. As an intermediate rhythm between high- and low-frequency rhythms, alpha rhythms is key to defining the individual division of brain rhythms. Previous studies have proposed a variety of methods for determining the alpha range (Corcoran et al. 2018). To facilitate the real-time calculation process, the center of gravity (CoG) was used to determine the individual alpha peak frequency (iAPF):
where f indicates the frequency points within 7–14 Hz and S(f) is the power spectrum of EEG (Klimesch et al. 1993; Klimesch 1999). The fixed divisions of EEG frequency band have been widely used in previous studies (Gruzelier 2014a, b; Marzbani et al. 2016). Since the iAPF is about 10 Hz across populations (Grandy et al. 2013a; Bazanova and Vernon 2014; Haegens et al. 2014; Christie et al. 2017), the individual rhythms were defined as proportional to iAPF to approximate the frequency bands that are widely used. The fixed and individual divisions of the frequency band are shown in Table 1. For convenience, the relative powers of rhythms using fixed division were noted as Theta, Alpha, Beta and Gamma, and the relative powers of individual rhythms were noted as iTheta, iAlpha, iBeta, and iGamma.
Table 1.
Frequency ranges of each rhythm using individual and fixed division
| Rhythm name | Fixed division | Individual division |
|---|---|---|
| Theta | 4–8 Hz | 4 Hz-0.8*iAPF |
| Alpha | 8–13 Hz | 0.8*iAPF-1.3*iAPF |
| Beta | 13–30 Hz | 1.3*iAPF-3.0*iAPF |
| Gamma | 30–45 Hz | 3*iAPF-45 Hz |
Neurofeedback training
In this study, visual feedback was delivered by the size of a video played on a screen, and the video materials consisted of cartoons or a natural documentary selected by the children to ensure their interest and participation. Figure 1 shows the graphical interface displayed to participants during the NFT process. The bar on the left side of the video shows the real-time value of the current training index, and the bar on the right side shows the level of artifact contamination in the current EEG data.
Fig. 1.

The graphical interface for visual feedback during NFT
One NFT session consists of two stages: a 2-min resting-state baseline and a 21-min training. During the resting-state baseline, the participant sat quietly in front of the 21-inch screen, and the trainer guided the participant to remain quiet with eyes open for 2 min. At this time, the bar graphs on both sides of the screen remained unchanged, and the video was randomly zoomed in or out.
In this study, we used 2-s segments with a 1-s sliding window for the real-time calculation of training index during NFT. For consistency, this 2-min baseline EEG was cut into 119 2-s segments with a 1-s overlap. Electromyogram (EMG) is the one of the main artifacts due to head movement or teeth clenching during NFT, and is characterized as wide frequency range (> 40 Hz). The relative power of EMG was calculated as follows:
where the S(f) is the power spectrum of each segment and the power of line noise (48–52 Hz) was removed from the power within each band. A total of 119 values were obtained and transformed into z-scores using the z-transform, and all segments with an absolute value of z-score greater than 2 were discarded to eliminate EMG contamination. Finally, the iAPF and iBeta (or Beta) of all remaining segments were calculated and averaged as the baseline for further training.
During the training process, the two groups used iBeta or Beta as the training index. For the iBeta group, the iBeta and EMG ratio were calculated in real time using a 2-s data segment with a 1-s overlap, such that the size of the video and the two bars were updated once per second. The initial size of the video is 50% of the size of the whole screen and zooms in and out during the training process. The size of the video has five levels from the initial to the maximum state (100%) and five levels from the initial to the minimum state (20%). The left bar corresponds to 80% to 120% of the baseline iBeta, and the current iBeta value is displayed in real time. When iBeta exceeds 120% of the baseline level, the video window zooms in by one level as a reward for visual feedback until it reaches the maximum, and when iBeta is lower than 80% of the baseline level, the video window zooms out by one level as a punishment until the minimum is reached. When iBeta is within the two thresholds, the video window remains unchanged. The right bar corresponds to 80% to 120% of the baseline EMG value. The video play is paused, and the size of the video window remains unchanged when the EMG level exceeds 120% of the baseline level. In the process of NFT, the trainer guided participants to enlarge the video as much as possible through “mental control” while reducing physical movement to avoid behaviors that may seriously contaminate the EEG.
Each participant was trained twice or three times a week, with at least one day between two training sessions. A total of 20 training sessions were conducted, and the participants were asked to complete all training sessions within two months. Each training session contained a 2-min resting-state baseline and 6 training epochs. Each epoch lasted for 3 min, with a 0.5-min break between epochs. The video was set to the initial size at the beginning of each epoch. We followed the CRED-nf checklist (Ros et al. 2020) to ensure the validity of this experiment (see Supplementary Material).
Data analysis
EEG and behavioral data analysis
For both the iBeta and Beta groups, the EEG data recorded at Cz under the baseline state were cut into 2 s segments with a 1 s overlap. The iAPF and the relative power of each brain rhythm were obtained and averaged for further statistical analysis. The NFT system recorded the rewards and punishments that the participants received during the training process. The “reward rate” and “maximum duration” of the participants in each training session were obtained as their behavioral features. The reward rate was defined as the ratio between the number of rewards and the total number of feedback events in each training session. The maximum duration was defined as the maximum duration in seconds that the participant kept the size of video to the maximum.
Statistical Analysis
For each of the three ADHD-RS scores, mixed analysis of variance (MANOVA) was conducted to test the training effect (pre- and post) and group effect (iBeta and Beta). For each of the EEG features and behavioral features obtained above, MANOVA was also applied to test the training effect (20 training sessions) and group effect (iBeta and Beta). For the statistical analysis stated above, the significance level was set to 0.05, and the Bonferroni method (Dunnett 1955) was applied to correct the significance threshold to avoid type I error.
Results
Training effect on features of both groups
The three ADHD-RS scores of the two groups before and after NFT are shown in Fig. 2. The MANOVA results showed that the main effects of training on the three scores were significant (Finattention(1,53) = 43.285, = 0.450; Fhyperactivity(1,53) = 49.170, = 0.481; Ftotal(1,53) = 69.690, = 0.568; p < 0.001), and the interaction effect between group and training were also significant (Finattention(1,53) = 7.742, p = 0.007, = 0.450; Fhyperactivity(1,53) = 7.948, p = 0.007, = 0.130; Ftotal(1,53) = 11.812, p = 0.001, = 0.182). The main effect of group on the hyperactivity subscale score and the total score were significant (Fhyperactivity(1,53) = 7.364, p = 0.009, = 0.122; Ftotal(1,53) = 9.645, p = 0.003, = 0.154), and the main effect of group on the inattention subscale score was not significant after correction, but it showed a trend (Finattention(1,53) = 5.264, p = 0.025, = 0.090). The simple effect analyses were significant for both groups on the hyperactivity and total scores (F(1,53) > 10, p < 0.001), but on the inattention subscale score, iBeta group showed significant simple effect (FiBeta(1,53) = 44.63, p = 0.000), Beta group did not after correction (FBeta(1,53) = 7.08, p = 0.010;). The simple effect analyses showed that there were no between-group differences before NFT (Finattention(1,53) = 0.02, p = 0.882; Fhyperactivity(1,53) = 0.11, p = 0.740; Ftotal(1,53) = 0.02, p = 0.885), and after NFT, there were significant between-group differences (Finattention (1,53) = 12.18, p = 0.001; Fhyperactivity(1,53) = 15.77, p = 0.000; Ftotal(1,53) = 20.22, p = 0.000), indicating that the decrease in the scores of the iBeta group was significantly higher than that of the Beta group.
Fig. 2.
ADHD-RS scores of the two groups before and after NFT. a Inattention scores; b hyperactivity scores; c total scores. The vertical line represents the standard deviation of the feature values
The changes in the behavioral features of the two groups with the 20 training sessions are shown in Fig. 3. The MANOVA results showed that the training effect on reward rate was significant (F(19, 1007) = 6.852, p = 0.000, = 0.114), the group effect was not significant (F(1, 53) = 0.271, p = 0.605, = 0.005), and there was no interaction (F(19, 1007) = 0.738, p = 0.781, = 0.014). Neither the training effect nor the group effect were significant for maximum duration (F(19, 1007) = 1.547, p = 0.063, = 0.028; F(1, 53) = 0.019, p = 0.891, = 0.000).
Fig. 3.
Changes in behavioral features of the two groups with 20 training sessions. a Reward rate of the iBeta group; b reward rate of the Beta group; c maximum duration of the iBeta group; d maximum duration of the Beta group. The diamond in each subfigure represents the mean value of the feature values; the shaded area represents the standard deviation. Both groups showed significant training effect and no significant group effect on reward rate, but showed no training and group effect on maximum duration
MANOVA was conducted on the relative power of brain rhythms and iAPF under the baseline condition. As shown in Table 2, there were significant main effect of training on most of the EEG features (F(19, 1007) > 3, p < 0.001, > 0.065), but none of EEG features showed a significant group effect (F(1,53) < 2, p > 0.15, < 0.035) and no interaction effect between training and group was found (F(19, 1007) < 1.6, p > 0.060, > 0.018) except for iTheta (F(19,1007) = 2.104, p = 0.004, = 0.038).
Table 2.
The MANOVA results of EEG features
| EEG feature | Main effect of training | Interaction effect | ||||
|---|---|---|---|---|---|---|
| F(19, 1007) | p-value | F(19, 1007) | p-value | |||
| iTheta | 14.167 | 0.000* | 0.211 | 2.104 | 0.004* | 0.038 |
| iAlpha | 1.649 | 0.686 | 0.030 | 1.437 | 0.101 | 0.026 |
| iBeta | 14.132 | 0.000* | 0.211 | 1.539 | 0.065 | 0.028 |
| iGamma | 23.582 | 0.000* | 0.308 | 0.968 | 0.497 | 0.018 |
| Theta | 26.821 | 0.000* | 0.336 | 1.296 | 0.176 | 0.024 |
| Alpha | 3.787 | 0.000* | 0.067 | 1.091 | 0.354 | 0.020 |
| Beta | 42.680 | 0.000* | 0.446 | 1.223 | 0.230 | 0.023 |
| Gamma | 17.933 | 0.000* | 0.253 | 1.125 | 0.319 | 0.021 |
| iAPF | 45.719 | 0.000* | 0.463 | 0.982 | 0.480 | 0.018 |
Bold values indicated the overestimation of Alpha and misdetection of the training effect
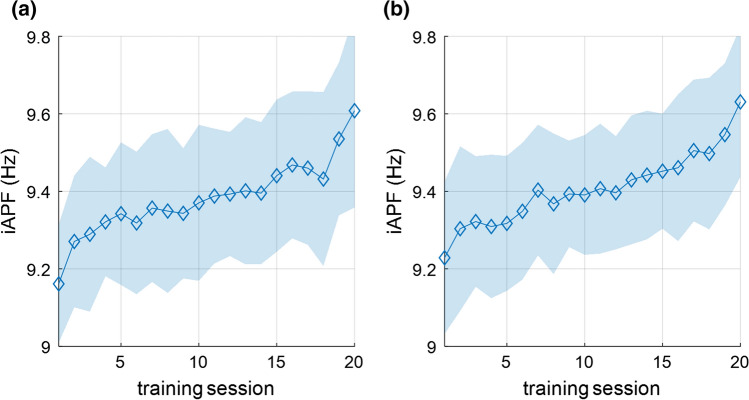
It is worth noting that there is significant training effect for Alpha (F(19,1007) = 3.787, p = 0.000, = 0.067), but not for iAlpha (F(19,1007) = 1.649, p = 0.686, = 0.030). To further explain this phenomenon, the changes in iAPF with training sessions are shown in Fig. 4. The iAPF of both groups gradually increased in 20 training sessions (F(19, 1007) = 45.719, p = 0.000, = 0.463), indicating that the distribution of brain rhythms of both groups moved towards high frequency, resulting in overestimation of Alpha and misdetection of the training effect. The fixed division of brain rhythms cannot reflect the actual brain state, especially when the distribution of brain rhythms is shifted during NFT.
Fig. 4.
Change in iAPF of the two groups in the 20 training sessions. a iBeta group; b Beta group. The iAPF of both groups gradually increased during NFT
Correlation between iBeta difference and the difference of other features
To further explore the relationship between the training effect on the training index and on other features, the two-tailed Pearson correlation between the iBeta difference and the difference of other features under the baseline condition was analyzed. Given that the main effects of group on the ADHD-RS scores were significant or showed a trend, the correlation analyses were conducted within the iBeta group, and the correlation about the features that showed no group effect were examined using data from both groups. As shown in Fig. 5, the increase in iBeta was not significantly correlated with the difference in inattention score of ADHD-RS (Fig. 5a, p = 0.176) but showed a significant correlation with the difference in hyperactivity score and the total score (Fig. 5b, p = 0.010; Fig. 5c, p = 0.011). In terms of behavioral features, the correlations between the iBeta difference and the difference in behavioral features of both groups are shown in Fig. 6. An increase in iBeta was significantly correlated with an improvement in the reward rate (Fig. 6a, p = 0.000) and the maximum duration (Fig. 6b, p = 0.003). The correlations between the iBeta difference and the difference in EEG features of both groups are shown in Fig. 7. The increase in iBeta also showed a significant correlation with the reduction in iTheta and the increase in iGamma (Fig. 7a, b, p < 0.005). However, as shown in Fig. 7c, there was no significant correlation between iBeta differences and the increase in iAPF (p = 0.576), indicating that the movement of rhythm distribution was not affected by the improvement of iBeta of both groups.
Fig. 5.
The two-tailed Pearson correlation between iBeta difference and difference of ADHD-RS scores of iBeta group. a Correlation with a decrease in inattention score; b correlation with a decrease in hyperactivity score; c correlation with a decrease in total score
Fig. 6.
The two-tailed Pearson correlation between iBeta differences and the differences in behavioral features of both groups. a Correlation with increase of reward rates; b correlation with increase of maximum duration
Fig. 7.
The two-tailed Pearson correlation between iBeta differences and EEG features of both groups. a Correlation with the decrease in iTheta; b correlation with the increase in iGamma; c correlation with the increase of iAPF
Discussion
In this study, a total of 55 children with ADHD were divided into two groups and underwent NFT using the relative power of individual or fixed beta rhythm as the training index. After NFT, both groups showed a decrease in ADHD-RS scores, a significant increase in beta relative power and an improvement in behavioral features, indicating that the attention ability of participants was improved significantly. The training effect of the iBeta group was significantly better than that of the Beta group, showing more improvement in the ADHD-RS scores, indicating that NFT based on individual divisions of brain rhythms is more effective than NFT based on fixed divisions.
The NFT process is a learning process (Hammer et al. 2012). The current research on neurofeedback learning includes three categories: within-session learning, across-session learning and baseline learning (Gruzelier 2014c). Within-session learning reflected the adaption to the NFT process and the dynamic process of spontaneous learning, while across-session learning and baseline learning indicated the cumulative process of learning. In this study, the reward rate and the maximum duration increased gradually as the training progressed (Fig. 3). The individual brain rhythm features showed significant baseline learning except for iAlpha (Fig. 4 and Table 2). Tracking and analyzing the changes in the index in the process of neurofeedback learning can help to elucidate the dynamic evolution of the brain state. In addition, previous studies have provided a variety of theoretical explanations for neurofeedback learning, such as operational learning theories (Skinner 1984) and cognitive learning theory (Yin et al. 2009). The current analysis of the learning process can further provide evidence to verify and improve these theories.
The development of each child with ADHD is different, and therefore, we applied an individualized division method for brain rhythm. An interesting result is that in Table 2, using fixed frequency division methods, Alpha showed significant training effect but not for iAlpha. And furtherly, we found that in the process of NFT, the brain rhythm distribution of participants gradually moved towards high frequency (Fig. 4), which makes it inappropriate to analyze EEG using a fixed rhythm division. A previous study also found that due to the decrease in the iAPF of children with ADHD, the TBR was overly estimated using fixed frequency division (Lansbergen et al. 2011). Discussions also exist on the accuracy and robustness of auxiliary diagnosis of children with ADHD using TBR (Arns et al. 2013; Lenartowicz and Loo 2014), suggesting that TBR may not be reliable in discriminating between individuals with or without ADHD.
A number of studies have shown the correlation between training effects and the degree of behavior improvement (Ros et al. 2009; Nan et al. 2012; Schabus et al. 2014). In this study, the training effects of iBeta of both groups were similar, and there were also significant correlations between the increase in iBeta and the improvement in ADHD-RS, behavioral and EEG features (Figs. 5, Fig. 6 and Fig. 7), showing that more changes in iBeta are related to more changes in brain state and thus have a stronger impact on attention ability. It can be speculated that NFT can cause changes in the brain state, which in turn lead to changes in attention ability. These findings showed the possible correlation among brain rhythm, brain state, and attention ability from the perspective of intervention and response.
NFT changes the brain state of children, and this change is also reflected in the distribution of individual brain rhythms of children, showing an increase in iAPF as the training progresses. Previous studies have shown that iAPF is related to a wide range of cognitive abilities, such as memory, exercise, and intelligence (Grandy et al. 2013b; Bazanova and Vernon 2014; Haegens et al. 2014; Christie et al. 2017), Therefore, it is speculated that the use of NFT based on individual beta rhythms improve attention ability and also affect more general cognitive ability by improving the frequency distribution of rhythms. Some previous studies directly use iAPF as the index for NFT, mainly reporting the effects of interventions on the elderly and participants with cognitive diseases, such as memory decline (Angelakis et al. 2007), mild cognitive impairment (Lavy et al. 2019) and learning disability (Pérez-Elvira et al. 2021). In the future study, the relationship among the distribution of brain rhythms, cognitive abilities and NFT will be verified in future research.
The current study contains major limitations. The unique evaluation scores (ADHD-RS) were completed only by the parents, resulting in restricted effectiveness and potential lack of objectivity. Secondly, due to the experimental settings and participants, this study lacks a sham group to control for placebo and social effect (Zoefel et al. 2011; Viviani and Vallesi 2021). Finally, there is no follow-up data, resulting in the lasting effect of NFT remains unknown (Van Doren et al. 2019). In future studies, the experimental design will be improved, and the evaluation methods will be refined to strictly control the influence of potential subjectivity and placebo effect, and to verify the lasting effect of NFT.
Conclusion
In this study, a total of 55 children with ADHD were divided into two groups and underwent NFT using the relative power of individual or fixed beta rhythm as the training index. NFT based on individual divisions of brain rhythms is more effective than NFT based on fixed divisions. The attention ability of participants was significantly improved and NFT based on individual divisions of brain rhythms is more effective than NFT based on fixed divisions. There were significant correlations between the increase in iBeta and the improvement in ADHD-RS scores, EEG and behavioral features. To the best of our knowledge, this is the first study to report the movement of the distribution of brain rhythms towards high frequency after beta NFT. Therefore, when designing a neurofeedback protocol and the corresponding data analysis process, an individualized method for brain rhythm division should be applied to reflect the actual brain state and to accurately evaluate the effect of NFT.
Acknowledgements
This research was supported by the National Defense Basic Scientific Research Program of China (JCKY2018110B011) and the National Natural Science Foundation of China (61827811).
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
None of the authors have potential conflicts of interest to be disclosed.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhang Hao and Chen He authors have contributed equally to this work.
References
- Adler LD, Nierenberg AA. Review of medication adherence in children and adults with ADHD. Postgrad Med. 2010;122:184–191. doi: 10.3810/pgm.2010.01.2112. [DOI] [PubMed] [Google Scholar]
- Albert J, Sánchez-Carmona AJ, Fernández-Jaén A, López-Martín S. Neurofeedback for ADHD: a critical review and suggested future directions. Curr Dev Disord Rep. 2017;4:86–93. doi: 10.1007/s40474-017-0117-y. [DOI] [Google Scholar]
- Angelakis E, Stathopoulou S, Frymiare JL, et al. EEG neurofeedback: A brief overview and an example of peak alpha frequency training for cognitive enhancement in the elderly. Clin Neuropsychol. 2007;21:110–129. doi: 10.1080/13854040600744839. [DOI] [PubMed] [Google Scholar]
- Arns M, Kenemans JL. Neurofeedback in ADHD and insomnia: vigilance stabilization through sleep spindles and circadian networks. Neurosci Biobehav Rev. 2014;44:183–194. doi: 10.1016/j.neubiorev.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Arns M, De Ridder S, Strehl U, et al. Efficacy of neurofeedback treatment in ADHD: The effects on inattention, impulsivity and hyperactivity: A meta-analysis. Clin EEG Neurosci. 2009;40:180–189. doi: 10.1177/155005940904000311. [DOI] [PubMed] [Google Scholar]
- Arns M, Conners CK, Kraemer HC. A decade of EEG theta/beta ratio research in ADHD: a meta-analysis. J Atten Disord. 2013;17:374–383. doi: 10.1177/1087054712460087. [DOI] [PubMed] [Google Scholar]
- Arns M, Heinrich H, Strehl U. Evaluation of neurofeedback in ADHD: The long and winding road. Biol Psychol. 2014;95:108–115. doi: 10.1016/j.biopsycho.2013.11.013. [DOI] [PubMed] [Google Scholar]
- Bazanova OM. Age related alpha activity change differs for males and females and for low and high alpha frequency EEG pattern. Revista Espanola De Neurop- Sicologia. 2008;10:82–83. [Google Scholar]
- Bazanova OM, Vernon D. Interpreting EEG alpha activity. Neurosci Biobehav Rev. 2014;44:94–110. doi: 10.1016/j.neubiorev.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Chen H, Chen W, Song Y, et al. EEG characteristics of children with attention-deficit/hyperactivity disorder. Neuroscience. 2019;406:444–456. doi: 10.1016/j.neuroscience.2019.03.048. [DOI] [PubMed] [Google Scholar]
- Chen H, Song Y, Li X. Use of deep learning to detect personalized spatial-frequency abnormalities in EEGs of children with ADHD. J Neural Eng. 2019 doi: 10.1088/1741-2552/ab3a0a. [DOI] [PubMed] [Google Scholar]
- Christie S, di Fronso S, Bertollo M, Werthner P. Individual alpha peak frequency in ice hockey shooting performance. Front Psychol. 2017;8:1–8. doi: 10.3389/fpsyg.2017.00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran AW, Alday PM, Schlesewsky M, Bornkessel-Schlesewsky I. Toward a reliable, automated method of individual alpha frequency (IAF) quantification. Psychophysiology. 2018;55:1–44. doi: 10.1111/psyp.13064. [DOI] [PubMed] [Google Scholar]
- Cortese S, Ferrin M, Brandeis D, et al. Neurofeedback for attention-deficit/hyperactivity disorder: meta-analysis of clinical and neuropsychological outcomes from randomized controlled trials. J Am Acad Child Adolesc Psychiatry. 2016;55:444–455. doi: 10.1016/j.jaac.2016.03.007. [DOI] [PubMed] [Google Scholar]
- Del Barrio V. Diagnostic and statistical manual of mental disorders. Washington, D.C.: American Psychiatric Pub; 2016. [Google Scholar]
- Dunnett CW. A multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc. 1955;50:1096–1121. doi: 10.1080/01621459.1955.10501294. [DOI] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R (1998) ADHD Rating Scale—IV: Checklists, norms, and clinical interpretation.
- Enriquez-Geppert S, Smit D, Pimenta MG, Arns M. Neurofeedback as a treatment intervention in ADHD: current evidence and practice. Curr Psychiatry Rep. 2019;21:46. doi: 10.1007/s11920-019-1021-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geladé K, Bink M, Janssen TWP, et al. An RCT into the effects of neurofeedback on neurocognitive functioning compared to stimulant medication and physical activity in children with ADHD. Eur Child Adolesc Psychiatry. 2017;26:457–468. doi: 10.1007/s00787-016-0902-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandy TH, Werkle-Bergner M, Chicherio C, et al. Peak individual alpha frequency qualifies as a stable neurophysiological trait marker in healthy younger and older adults. Psychophysiology. 2013;50:570–582. doi: 10.1111/psyp.12043. [DOI] [PubMed] [Google Scholar]
- Grandy TH, Werkle-Bergner M, Chicherio C, et al. Individual alpha peak frequency is related to latent factors of general cognitive abilities. Neuroimage. 2013;79:10–18. doi: 10.1016/j.neuroimage.2013.04.059. [DOI] [PubMed] [Google Scholar]
- Gruzelier JH. EEG-neurofeedback for optimising performance. I: A review of cognitive and affective outcome in healthy participants. Neurosci Biobehav Rev. 2014;44:124–141. doi: 10.1016/j.neubiorev.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Gruzelier JH. EEG-neurofeedback for optimising performance. II: Creativity, the performing arts and ecological validity. Neurosci Biobehav Rev. 2014;44:142–158. doi: 10.1016/j.neubiorev.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Gruzelier JH. EEG-neurofeedback for optimising performance. III: A review of methodological and theoretical considerations. Neurosci Biobehav Rev. 2014;44:159–182. doi: 10.1016/j.neubiorev.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Haegens S, Cousijn H, Wallis G, et al. Inter- and intra-individual variability in alpha peak frequency. Neuroimage. 2014;92:46–55. doi: 10.1016/j.neuroimage.2014.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer EM, Halder S, Blankertz B, et al. Psychological predictors of SMR-BCI performance. Biol Psychol. 2012;89:80–86. doi: 10.1016/j.biopsycho.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res Rev. 1999;29:169–195. doi: 10.1016/S0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Schimke H, Pfurtscheller G. Alpha frequency, cognitive load and memory performance. Brain Topogr. 1993;5:241–251. doi: 10.1007/BF01128991. [DOI] [PubMed] [Google Scholar]
- Kober SE, Witte M, Ninaus M, et al. Learning to modulate one’s own brain activity: the effect of spontaneous mental strategies. Front Hum Neurosci. 2013;7:695. doi: 10.3389/fnhum.2013.00695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad K, Eickhoff SB. Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Hum Brain Mapp. 2010;31:904–916. doi: 10.1002/hbm.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansbergen MM, Arns M, van Dongen-Boomsma MM, et al. The increase in theta/beta ratio on resting-state EEG in boys with attention-deficit/hyperactivity disorder is mediated by slow alpha peak frequency. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:47–52. doi: 10.1016/j.pnpbp.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Lavy Y, Dwolatzky T, Kaplan Z, et al. Neurofeedback improves memory and peak alpha frequency in individuals with mild cognitive impairment. Applied Psychophysiol Biofeedback. 2019;44:41–49. doi: 10.1007/s10484-018-9418-0. [DOI] [PubMed] [Google Scholar]
- Lenartowicz A, Loo SK. Use of EEG to diagnose ADHD. Curr Psychiatry Rep. 2014;16:498. doi: 10.1007/s11920-014-0498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logemann HNA, Lansbergen MM, Van Os TWDP, et al. The effectiveness of EEG-feedback on attention, impulsivity and EEG: A sham feedback controlled study. Neurosci Lett. 2010;479:49–53. doi: 10.1016/j.neulet.2010.05.026. [DOI] [PubMed] [Google Scholar]
- Marzbani H, Marateb HR, Mansourian M. Neurofeedback: a comprehensive review on system design, methodology and clinical applications. Basic Clin Neurosci. 2016;7:143–158. doi: 10.15412/J.BCN.03070208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan W, Rodrigues JP, Ma J, et al. Individual alpha neurofeedback training effect on short term memory. Int J Psychophysiol. 2012;86:83–87. doi: 10.1016/j.ijpsycho.2012.07.182. [DOI] [PubMed] [Google Scholar]
- Niedermeyer E. The normal EEG of the waking adult. Electroencephalography: Basic Principles. Clin Appl Relat Fields. 1999;167:149–173. [Google Scholar]
- Ogrim G, Kropotov J, Hestad K. The quantitative EEG theta/beta ratio in attention deficit/hyperactivity disorder and normal controls: Sensitivity, specificity, and behavioral correlates. Psychiatry Res. 2012;198:482–488. doi: 10.1016/j.psychres.2011.12.041. [DOI] [PubMed] [Google Scholar]
- Orendáčová M, Kvašňák E. Effects of transcranial alternating current stimulation and neurofeedback on alpha (EEG) dynamics: a review. Front Hum Neurosci. 2021;15:1–17. doi: 10.3389/fnhum.2021.628229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Elvira R, Oltra-Cucarella J, Carrobles JA, et al. Individual alpha peak frequency, an important biomarker for live z-score training neurofeedback in adolescents with learning disabilities. Brain Sci. 2021;11:1–22. doi: 10.3390/brainsci11020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk G, De Lima MS, Horta BL, et al. The worldwide prevalence of ADHD: A systematic review and metaregression analysis. Am J Psychiatry. 2007;164:942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- Ros T, Moseley MJ, Bloom PA, et al. Optimizing microsurgical skills with EEG neurofeedback. BMC Neurosci. 2009;10:87. doi: 10.1186/1471-2202-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros T, Enriquez-Geppert S, Zotev V, et al. Consensus on the reporting and experimental design of clinical and cognitive-behavioural neurofeedback studies (CRED-nf checklist) Brain. 2020;143:1674–1685. doi: 10.1093/brain/awaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabus M, Heib DPJ, Lechinger J, et al. Enhancing sleep quality and memory in insomnia using instrumental sensorimotor rhythm conditioning. Biol Psychol. 2014;95:126–134. doi: 10.1016/j.biopsycho.2013.02.020. [DOI] [PubMed] [Google Scholar]
- Sibley MH, Kuriyan AB, Evans SW, et al. Pharmacological and psychosocial treatments for adolescents with ADHD: an updated systematic review of the literature. Clin Psychol Rev. 2014;34:218–232. doi: 10.1016/j.cpr.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Simkin DR, Thatcher RW, Lubar J. Quantitative EEG and neurofeedback in children and adolescents. anxiety disorders, depressive disorders, comorbid addiction and attention-deficit/hyperactivity disorder, and brain injury. Child Adolesc Psychiatr Clin N Am. 2014;23:427–464. doi: 10.1016/j.chc.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Sitaram R, Ros T, Stoeckel L, et al. Closed-loop brain training: The science of neurofeedback. Nat Rev Neurosci. 2017;18:86–100. doi: 10.1038/nrn.2016.164. [DOI] [PubMed] [Google Scholar]
- Skinner BF. The operational analysis of psychological terms. Behav Brain Sci. 1984;7:547–553. doi: 10.1017/S0140525X00027187. [DOI] [Google Scholar]
- Storebø OJ, Ramstad E, Krogh HB, et al. Methylphenidate for children and adolescents with attention deficit hyperactivity disorder (ADHD) Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD009885.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroganova TA, Orekhova EV, Posikera IN. EEG alpha rhythm in infants. Clin Neurophysiol. 1999;110:997–1012. doi: 10.1016/S1388-2457(98)00009-1. [DOI] [PubMed] [Google Scholar]
- Van Doren J, Arns M, Heinrich H, et al. Sustained effects of neurofeedback in ADHD: a systematic review and meta-analysis. Eur Child Adolesc Psychiatry. 2019;28:293–305. doi: 10.1007/s00787-018-1121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviani G, Vallesi A. EEG-neurofeedback and executive function enhancement in healthy adults: A systematic review. Psychophysiology. 2021 doi: 10.1111/psyp.13874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber LA, Ethofer T, Ehlis AC. Predictors of neurofeedback training outcome: A systematic review. NeuroImage: Clin. 2020 doi: 10.1016/j.nicl.2020.102301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Mulcare SP, Hilário MRF, et al. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci. 2009;12:333–341. doi: 10.1038/nn.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoefel B, Huster RJ, Herrmann CS. Neurofeedback training of the upper alpha frequency band in EEG improves cognitive performance. Neuroimage. 2011;54:1427–1431. doi: 10.1016/j.neuroimage.2010.08.078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.