Significance
In the current study, we show that prolonged neutropenia after SCT or chemotherapy is associated with alteration of gut microbiota composition. Our results reveal a cross talk between the intestinal microbiota and granulopoiesis during prolonged neutropenia, providing a new prospect in reactive granulopoiesis. Our finding is potentially clinically relevant, because rapid recovery of neutrophils after SCT and chemotherapy ensures safety of these treatment modalities by reducing risk of infections. Our finding may pave the way for the future clinical studies in which benefits of novel strategies of antibiotic usage sparing granulopoiesis-supporting bacteria or FMT or probiotics supporting granulopoiesis after SCT will be tested.
Keywords: hematopoietic stem cell transplantation, microbiota, granulopoiesis, neutropenia, Ruminococcaceae
Abstract
Granulopoiesis in the bone marrow adjusts cellular output as demand for neutrophils changes. Reactive granulopoiesis is induced by profound neutropenia, but its mechanism remains to be clarified. We herein explored its mechanisms using mouse models of syngeneic hematopoietic stem cell transplantation (SCT) and 5-fluorouracil-induced neutropenia. After SCT, T cell production of IL-17A was up-regulated. Neutrophil recovery was significantly delayed in IL-17A-deficient or T cell-deficient RAG1−/− mice, and adoptive transfer of wild-type (WT) T cells facilitated neutrophil engraftment. Gut decontamination with oral antibiotics suppressed T cell production of IL-17A and impaired neutrophil recovery. Transplantation of fecal microbiota collected from neutropenic, not naive, mice promoted neutrophil recovery in these mice, suggesting that neutropenia-associated microbiota had a potential to stimulate reactive granulopoiesis. Our study uncovered a cross talk between gut microbiota and neutropenia after SCT and chemotherapy.
Granulopoiesis in the bone marrow (BM) adjusts cellular output as demand for neutrophils changes. “Emergency granulopoiesis” is mediated by bacterial infection, whereas “reactive granulopoiesis” refers to enhanced granulopoiesis in the absence of active microbial infection that could be induced by inflammatory stimuli or neutropenia after hematopoietic stem cell transplantation (SCT) or cancer chemotherapy (1). While emergency granulopoiesis depends on granulocytecolony stimulating factor (G-CSF) production from endothelial cells promoted by pathogen-associated molecular patterns (2), the mechanism by which neutropenia induces granulopoiesis remains to be clarified. Several recent studies suggest a cross talk between the intestinal microbiota and hematopoiesis. Granulopoiesis in neonates depends on microbiota inherited from their mother (3). Gut microbiota plays a critical role in neutrophil senescence and granulopoiesis in the steady state of adults (4). It also plays a critical role in T cell reconstitution after SCT by promoting energy intake from the gut (5). In the current study, we took advantage of the mouse SCT model, in which lethally irradiated recipients were transplanted with purified lineagenegSca-1+c-kit+ (LSK) cells plus granulocyte macrophage progenitors (GMPs) from congenic donors and the mouse model of chemotherapy with 5-fluorouracil (5-FU) to study the precise mechanism by which gut microbiota promote reactive granulopoiesis.
Results
IL-17A Plays a Critical Role in Neutrophil Recovery after Prolonged Neutropenia.
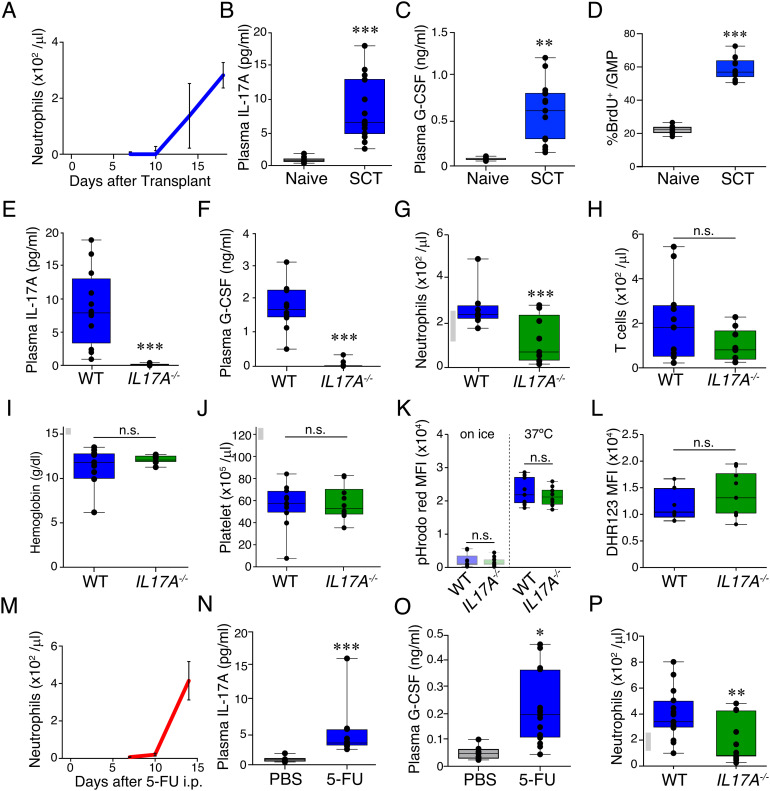
To induce prolonged neutropenia, lethally irradiated B6 mice were i.v. injected with 7,500 LSK cells plus 20,000 GMPs sorted from the BM of B6-CD45.1 donors on day 0. Flow cytometric analyses demonstrated that neutrophils were depleted in the peripheral blood for at least 10 d after SCT and reconstituted by day +18 after SCT (Fig. 1A). Neutrophils, monocytes, and B cells were completely replaced by donor-derived cells, while most of T cells remained to be recipient-derived (SI Appendix, Fig. S1 C and D). Cytometric bead array (CBA) targeting granulopoietic cytokines, such as G-CSF and IL-17A, demonstrated that plasma concentrations of IL-17A and G-CSF were significantly elevated after SCT compared with naive mice in association with enhanced proliferation of GMPs in the BM (Fig. 1 B–D).
Fig. 1.
IL-17A plays a critical role in reactive granulopoiesis in prolonged neutropenia. (A–C) Lethally irradiated B6 mice (CD45.2+) were transplanted with 7.5 × 103 LSKs plus 2 × 104 GMPs from B6-CD45.1+ donors. Absolute numbers of neutrophils in the peripheral blood (A) and plasma concentrations of IL-17A (B) and G-CSF (C) on day +18 after SCT (n = 15) and those in naive B6 mice (n = 6). (D) Naive or wild-type (WT) recipients were injected with BrdU (1 mg) on day + 18 after SCT and BM cells were harvested 2 h later (n: naive = 8, WT = 13). (E–L) Lethally irradiated IL-17A-deficient B6 mice (IL17A−/−) and control B6-WT mice were transplanted from WT B6-Ly5a donors as Fig. 1A. Plasma concentrations of IL-17A (E) and G-CSF (F) on day +18 (n = 18/group). Absolute numbers of neutrophils (G) and T cells (H), hemoglobin concentrations (I), and absolute numbers of platelets (J) in the peripheral blood on day +18 after SCT (n = 10/group). (K) Phagocytic capacity of neutrophils was assessed by incubating peripheral blood neutrophils with pHrodo red-labeled Escherichia coli for 15 min at 37°C or on ice (n = 9–10/group). (L) ROS production was evaluated after DHR123 labeling followed by stimulation with PMA (n = 9–10/group). B6-WT (M-O) or B6-IL17A−/− (P) mice were i.p. injected with 5-FU (200 mg/kg) or PBS on day 0. Absolute numbers of neutrophils in the peripheral blood (M, P) and plasma concentrations of IL-17A (N) and G-CSF (O) on day 14 (n = 12/group). Data from two (G–P), three (A–D), or four (E, F) similar experiments were combined and shown as mean ± SE. (G, I, J, and P) Gray bars on the left side of the figures represent reference ranges determined using data from naive mice (mean ± 2SD, n = 5) *P < 0.05, **P < 0.01, ***P < 0.005.
Given the critical role of the IL-17A/G-CSF axis in neonatal granulopoiesis (3), we next explored the role of IL-17A using B6-IL17A−/− mice as SCT recipients. After SCT, the elevation of both IL-17A and G-CSF was dramatically reduced in IL-17A-deficient recipients (Fig. 1 E and F). Neutrophil counts in the peripheral blood on day +18 after SCT were significantly lower in IL17A−/− recipients than those in WT recipients, while T cell counts, platelet counts, and hemoglobin concentrations were comparable between the two groups (Fig. 1 G–J), indicating that IL-17A plays a critical role in G-CSF production and neutrophil recovery after SCT. When WT and IL17A−/− recipients were cohoused for 4 wk before SCT, IL17A−/− mice again demonstrated delayed engraftment after SCT (SI Appendix, Fig. S2), indicating that the intrinsic difference of gut microbiota between WT and IL17A−/−mice did not contribute to the delayed neutrophil recovery (6). Next, we assessed whether phagocytic and bactericidal capacities of neutrophils could be affected by IL-17A production after SCT. We found that neutrophils in the WT and IL17A−/−recipients demonstrated comparable phagocytic activity against pHrodo red-labeled E. coli on day +18 after SCT (Fig. 1K). Furthermore, neutrophils in the WT and IL17A−/− recipients up-regulated reactive oxygen species (ROS) in response to PMA at a comparable level, suggesting that bactericidal activity of neutrophils was not affected by IL-17A after SCT (Fig. 1L). These data suggested that IL-17A plays a critical role in numerical recovery of neutrophils, whereas the functions of neutrophils were not affected by IL-17A production after SCT.
Next, the role of IL-17A in reactive granulopoiesis was evaluated in a chemotherapy-induced neutropenia model. When B6 mice were i.p. injected with 5-FU at a dose of 200 mg/kg, neutrophils were depleted for at least 10 d (Fig. 1M). Plasma concentrations of IL-17A and G-CSF were significantly elevated in 5-FU-treated mice (Fig. 1 N and O). Neutrophil recovery was significantly delayed in IL17A−/− mice compared with WT controls after 5-FU-treatment (Fig. 1P). Altogether, we concluded that neutropenia promotes production of IL-17A and G-CSF, which plays a critical role in neutrophil recovery.
IL-17A Plays a Critical Role in Granulopoiesis and BM Egress of Neutrophils after SCT.
Next, we explored the mechanisms by which the IL-17A/G-CSF axis promoted neutrophil recovery in the peripheral blood. To assess granulopoiesis in the BM, hematopoietic progenitor populations in the BM were enumerated on day +18 after SCT. Numbers of both the common myeloid progenitors (CMPs) and GMPs were significantly less in IL17A−/− recipients compared with WT controls, while those of whole nucleated cells (NCC), LSK cells, and megakaryocyte erythroid progenitors (MEPs) were comparable between the two groups (Fig. 2 A–E). BrdU+ proliferating GMPs were significantly reduced in IL17A−/− mice, indicating that IL-17A promotes reactive granulopoiesis in the BM after SCT (Fig. 2F). Next, we assessed the recently reported mouse granulopoietic subpopulations in the BM defined by c-kit and Ly6G expressions (SI Appendix, Fig. S1E) (7). Frequencies of immature fractions 1–4 were significantly reduced in IL17A−/− recipients compared with WT controls, resulting in the reduction of absolute numbers of fractions 2–4 (Fig. 2 G–I). In contrast, the proportion of fraction 5 which represents mature neutrophils was significantly increased in IL17A−/− compared with WT controls, and absolute numbers of fraction 5 were comparable between the two groups, suggesting that IL-17A plays an important role not only in reactive granulopoiesis but also in the BM egress of neutrophils. Thus, we evaluated the BM egress of neutrophils by observing the emergence of BrdU+ neutrophils in the peripheral blood after BrdU labeling of neutrophil-progenitors in the BM on day +18 after SCT (8, 9). Proportion of BrdU+ neutrophils in IL17A−/− recipients peaked 12 h later than that in WT recipients did (Fig. 2J), indicating that IL-17A promotes the BM egress of neutrophils after SCT. Although BrdU could also label myeloid progenitors in the extramedullary hematopoiesis which could be induced by myeloablation, we concluded that IL-17A plays a critical role in the BM egress of neutrophils based on the kinetics of the emergence of BrdU+ neutrophils after BrdU pulse together with the data indicating the retention of mature neutrophils (fraction 5) in the BM of IL17A−/−recipients (10).
Fig. 2.
IL-17A plays a critical role in BM granulopoiesis and BM egress of neutrophils after SCT. Lethally irradiated B6-IL17A−/− and B6-WT mice were transplanted as Fig. 1A. NCC (A), LSK (B), CMP (C), GMP (D), and MEP (E) in the BM of the bilateral femurs were enumerated on day +18 after SCT. Data from five similar experiments were combined and shown as mean ± SE (n = 22/group). (F) WT or IL17A−/− recipients were injected with BrdU (1 mg) on day +18 after SCT and BM cells were harvested 2 h later. Data from three similar experiments were combined and shown as mean ± SE (n = 13–14/group). (G–I) Fractions 1–5 within granulopoietic precursors gated as SI Appendix, Fig. S1E were enumerated in the femur 18 d after SCT. Representative dot plots (G), proportion (H), and absolute numbers (I) of each fraction. Data from four similar experiments were combined and shown as mean ± SE (n = 17/group). (J) WT (n = 5) or IL17A−/− (n = 3) recipient mice were i.p. injected with 1-mg BrdU on day +18 after SCT. Proportion of BrdU+ neutrophils in the peripheral blood is shown as mean ± SE. Data from one of two experiments are shown. *P < 0.05, *** P < 0.005.
T Cell Production of IL-17A Plays a Critical Role in Reactive Granulopoiesis.
It has been reported that T cells are the major producers of IL-17A in the steady states and after SCT (11). IL-17A expression in the purified T cells harvested on day +18 after SCT was significantly up-regulated compared with that in the naive controls (Fig. 3A). When T cell-deficient B6-RAG1−/− mice were used as recipients, plasma concentrations of IL-17A and G-CSF were significantly lower than those in the WT recipients on day +18 after SCT (Fig. 3 B and C). Neutrophil counts on day +18 were also lower in RAG1−/− recipients compared with WT recipients (Fig. 3D). To test the role of T cells in the reactive granulopoiesis after SCT, RAG1−/−recipients were i.v. injected with purified T cells from naive B6-WT or B6-IL17A−/− mice. While adoptive transfer of purified T cells from B6-WT or B6-IL17A−/− mice successfully repopulated T cells in RAG1−/− recipients (Fig. 3E), transfer of WT T cells, but not IL17A−/− T cells, significantly increased plasma concentrations of IL-17A and G-CSF (Fig. 3 F and G). Furthermore, transfer of WT T cells, but not IL17A−/−T cells, enhanced granulopoiesis in the BM and promoted the BM egress of neutrophils, resulting in earlier neutrophil recovery in the peripheral blood (Fig. 3 H–K). Furthermore, the adoptive transfer of WT T cells to IL17A−/− SCT recipients on day 0 significantly increased GMPs in the BM and neutrophils in the peripheral blood on day +18 after SCT (Fig. 3 L and M). Altogether, these data indicate that IL-17A production of T cells plays a critical role in reactive granulopoiesis after SCT.
Fig. 3.
T cell production of IL-17A plays a critical role in reactive granulopoiesis after SCT. SCT was performed as in Fig. 1A. (A) Splenic T cells were purified from SCT recipients on day +18 after SCT (n = 7–9/group). mRNA extracted from T cells was subjected to Q-PCR targeting IL-17A. (B–D) B6-WT (n = 9) or B6-RAG1−/− (n = 6) were transplanted as in Fig. 1A. Plasma concentration of IL-17A and G-CSF, and numbers of neutrophils in the peripheral blood on day +18. (E–I) RAG1−/− recipients were injected with 6 × 106 T cells from WT (n = 10–17) or IL17A−/− (n = 9–12) mice on day 0 of SCT or left untreated (n = 3–11). Numbers of T cells in the blood (E), plasma concentrations of IL-17A (F) and G-CSF (G), numbers of and GMPs in the bilateral femoral BM (H), proportion of fraction #1 to #4 (I) and fraction #5 (J) in the BM, numbers of neutrophils in the blood (K) on day +18. (L, M) IL17A−/− recipients were injected with 6 × 106 T cells from WT B6 mice on day 0 of SCT or left untreated (n = 7/group). Numbers of and GMPs in the bilateral femoral BM (J), and neutrophils in the blood (K) on day +18. Data from two (A–D,J, and K) and three (E–I) experiments were combined and shown as mean ± SE. *P < 0.05, **P < 0.01, and ***P < 0.005.
Gut Microbiota is Critical in Reactive Granulopoiesis after SCT and Chemotherapy.
To clarify the relationship between gut microbiota and granulopoiesis after SCT, a group of mice underwent gut decontamination by administrating a combination of three oral antibiotics (3ABx) including ampicillin (ABPC), streptomycin (SM), and vancomycin (VCM) from day −7 of SCT, resulting in 4-log reduction of bacterial load on day 0 (Fig. 4A). It has been reported that gut decontamination delayed hematopoietic recovery after syngeneic bone marrow transplantation (BMT) by suppressing absorption of nutritional elements (5). We confirmed that gut decontamination significantly delayed neutrophil recovery in association with reduced T cells in the peripheral blood and CMPs and GMPs in the BM (Fig. 4 B–F). Furthermore, we found that gut decontamination suppressed T cell transcription of IL-17A and abrogated the elevation of plasma concentrations of IL-17A and G-CSF after SCT, suggesting that gut microbiota promotes T cell production of IL-17A and plays a critical role in reactive granulopoiesis after SCT (Fig. 4 G–I). In the chemotherapy model with 5-FU injection, gut decontamination again abrogated the elevation of plasma concentrations of IL-17A and G-CSF accompanied by delayed recovery of neutrophils, that is consistent with the SCT model (Fig. 4 J–L).
Fig. 4.
The microbiota is critical in reactive granulopoiesis after SCT and chemotherapy. (A–I) Recipient mice were orally treated with three antibiotics (3ABx) including ABPC, SM, and VCM, or control diluent (Ctrl) from day −7 and transplanted as in Fig. 1A. (A) Fecal bacterial load on day 0 was measured using Q-PCR targeting 16S rRNA gene (n = 4/group). Numbers of neutrophils (B, C) and T cells (D) in the blood, and CMP (E) and GMP (F) in the right femoral BM on day +18 after SCT (n = 13/group). (G) Il17a expression in splenic T cells purified from naive B6, or Ctrl- or 3Abx-treated SCT recipients on day +18 (n = 5–7/group). Plasma concentrations of IL-17 (H) and G-CSF (I) from naive B6 (n = 6), or Ctrl- (n = 25) or 3Abx-treated (n = 19) recipients on day +18. (J–L) B6 mice treated with 3ABx or Ctrl for 7 d were i.p. injected with 5-FU (200 mg/kg) on day 0. Plasma concentrations of IL-17A (J) and G-CSF (K), and numbers of neutrophils in the blood (L) on days +7, +10, and +14 after 5-FU treatment (n = 12/group). Data from two (B–G, J–L) or three (H, I) similar experiments were combined and shown as mean ± SE. *P < 0.05, **P < 0.01, and ***P < 0.005.
Prolonged Neutropenia after SCT and 5-FU Injection Alters the Composition of Intestinal Microbiota.
Next, we tested whether prolonged neutropenia could alter the bacterial composition of gut microbiota by performing 16S rRNA sequencing on day +10 after SCT and 5-FU injection. Principal coordinate analysis and PERMANOVA test showed that bacterial composition of gut microbiota was significantly altered after SCT with significant change in the relative abundance of several genera (Fig. 5 A–C). In contrast, the bacterial composition of gut microbiota did not significantly alter after unmanipulated BMT. Importantly, neutrophils recovered by day +7 after BMT, which was much earlier than SCT possibly due to the presence of CMPs in the BM graft that, not like GMPs, can transiently form hematopoietic colonies and support hematopoiesis early after transplantation (Fig. 5D) (12). α-Diversity of gut microbiota represented as Simpson index and Shannon index did not significantly alter after SCT or 5-FU injection (SI Appendix, Fig. S3). To find bacterial genera, which increased or decreased during neutropenia, we searched the significantly and commonly increased or decreased bacterial genera after SCT and 5-FU, but not after unmanipulated BMT. We found that only 1 genus, uncultured genus-level group from the Ruminococcaceae (Ruminococcaceae UCG-014) significantly increased both after SCT and 5-FU-treatment, while unchanged after unmanipulated BMT, suggesting that prolonged neutropenia increased this bacterial genus (Fig. 5 C and F–I). At family level, Ruminococcaceae, containing genus Ruminococcaceae UCG-014, also significantly increased after SCT and 5-FU-treatment (SI Appendix, Fig. S4).
Fig. 5.
Prolonged neutropenia after SCT and 5-FU alters composition of intestinal microbiota. (A–C) SCT was performed as in Fig. 1A. 16S rRNA sequencing of fecal samples was performed on day +10. (A) Principal coordinate analysis of genus compositions of intestinal microbiota from one of three similar experiments (n = 4–6/group). Bacterial composition at phylum level (B, n = 15/group), and the genera significantly increased or decreased in SCT recipients compared with naive controls with relative abundance ≥ 0.1% in any groups (C, n = 15/group). (D,E) Lethally irradiated B6 mice were transplanted with 5 × 106 BM cells from syngeneic donors. Numbers of neutrophils in the blood on day +7 (n = 3–5/group) (D) and principal coordinate analysis of genus compositions of intestinal microbiota (E) on day +10 after BMT. (F, G) B6 mice were i.p. injected with 5-FU (200 mg/kg) on day 0, and 16S rRNA sequencing were performed on day +10 (n = 4–6/group). Venn diagrams of bacterial genus, the relative abundance of which was significantly increased (F) or decreased (G) in SCT recipients (blue), 5-FU-treated mice (yellow), or BMT recipients (green) compared with naive mice. Fold change of relative abundance of Ruminococcaceae UCG-014 in 5-FU-treated mice (H) and BMT recipients (I) compared with naive controls. *P < 0.05, **P < 0.01, ***P < 0.005, and ****P < 0.001.
Intestinal Microbiota Associated with Prolonged Neutropenia Promotes Reactive Granulopoiesis after SCT.
Finally, we tested if alteration of gut microbiota associated with neutropenia could promote reactive granulopoiesis using a fecal microbiota transplantation (FMT) model, in which gut-decontaminated recipients underwent FMT on day +14 after SCT using fecal suspension harvested from SCT recipients who were not given antibiotics on day +10 (Fig. 6A). 16S rRNA sequencing performed 5 d after FMT confirmed that the gut bacterial composition in the FMT recipients transplanted with fecal suspension from SCT recipients was significantly different from that in the recipients performed FMT from the naive mice (Fig. 6B). As expected, the relative abundance of Ruminococcaceae was significantly more in the FMT recipients receiving the fecal suspension from SCT mice compared with those receiving FMT from the naive mice (Fig. 6C). FMT from SCT recipients increased plasma concentrations of IL-17A significantly more than FMT from naive control mice (Fig. 6D). When gut-decontaminated B6-IL17A−/− mice were used as the recipients, FMT from SCT recipients did not induce IL17A production, indicating that neutropenia-associated gut microbiota stimulated host cells to produce IL-17A (plasma IL-17A concentration: 0.43 ± 0.12 pg/ml). Elevation of IL-17A was associated with increased numbers of GMPs in the BM, and enhanced neutrophil recovery in the peripheral blood, indicating that neutropenia-associated microbiota stimulates reactive granulopoiesis in the BM and promotes neutrophil recovery in the peripheral blood (Fig. 6 E and F).
Fig. 6.
Intestinal microbiota induced by prolonged neutropenia promotes reactive granulopoiesis after SCT. Lethally irradiated B6 mice were transplanted as in Fig. 1A. Fecal suspension was prepared using feces obtained from SCT recipients on day +10 after SCT. Another group of B6 mice were treated with 3ABx and transplanted as Fig. 4A. 3ABx was lifted on day +13 and FMT was performed on day +14 using fecal suspension from SCT recipients or naive mice. (A) Experimental scheme of FMT. (B, C) 16S rRNA sequencing of fecal samples was performed on day +20 after SCT (n = 3–5/group). Principal coordinate analysis of genus compositions of intestinal microbiota (B) and fold change of relative abundance of Ruminococcaceae (C). (D) Plasma concentrations of IL-17A measured on 4 d after FMT from naive mice or SCT recipients (n = 8/group). (E) GMPs in the femur enumerated 4 d after FMT (n = 15/group). (F) Absolute numbers of neutrophils in the peripheral blood enumerated 1–6 d after FMT (n = 8–9/group). Data from two (D, F) or three (E) experiments were combined and shown as mean ± SE *P < 0.05, **P < 0.01, and ***P < 0.005.
Discussion
In the current study, we show that T cell production of IL-17A plays a critical role in reactive granulopoiesis in prolonged neutropenia after SCT or chemotherapy. In the steady state, IL-23/IL-17A/G-CS Fcytokine-controlled loop acts as a neutrophil rheostat, which regulates BM granulopoiesis and maintains neutrophil homeostasis (13–15). It is likely that this rheostat function also induces reactive granulopoiesis after neutrophil depletion; it has been reported that brief neutropenia (2–3 d) induced by anti-Ly6G mAb promoted IL-17A and G-CSF production in TLR4-dependent and T cell-independent manner (16, 17). A recent study demonstrated that B cells and group 2 innate lymphoid cells (ILC2s) in the BM play a major role in reactive granulopoiesis 2 d after 5-FU injection (18). In our study of prolonged neutropenia for more than 10 d, T cells played a major role in IL-17A production and reactive granulopoiesis in prolonged neutropenia; adoptive transfer of WT T cells, not IL17A−/− T cells, into RAG−/− and IL17A−/− recipients enhanced IL-17A and G-CSF production, leading to neutrophil recovery. Although reactive granulopoiesis in regenerative hematopoietic response may recapitulate the process of neutrophil development in the neonatal period which is also dependent on microbiota and IL-17A/G-CSF axis, granulopoiesis in the early life is uniquely dependent on the IL-17A production from ILC3s, possibly due to the immature status of neonatal T cells (3). Thus, mechanisms of reactive granulopoiesis may be context- and time course dependent. It has been reported that IL-17A enhances granulopoiesis via production of G-CSF (3). Furthermore, IL-17A may directly induce granulopoiesis by acting on hematopoietic progenitor cells; IL-17A induces the expression of transcriptional factors involved in granulopoiesis such as C/EBPβ and promotes differentiation of granulocyte progenitors in clonogenic assay of BM cells (19–21). IL-17A also induces the expression of various chemokines including neutrophil chemoattractants, which could affect the BM egress of neutrophils (20). It remains to be clarified whether serum concentrations of these neutrophil-attracting chemokines could be affected by IL-17A during neutropenia. It is also intriguing to test whether there could be a correlation between IL-17A concentrations or polymorphisms and neutrophil counts in the human peripheral blood in the future studies.
Gut decontamination suppressed T cell production of IL-17A and delayed neutrophil recovery after SCT and chemotherapy, indicating that intestinal microbiota plays a critical role in the reactive granulopoiesis during neutropenia in a T cell-dependent manner. It has been reported that gut decontamination resulted in impaired neutrophil recovery and T cell reconstitution after syngeneic BMT, and caloric supplementation through sucrose in drinking water rescued hematopoietic recovery in these mice, suggesting that microbiota-dependent energy absorption plays a major role in reactive granulopoiesis after transplantation (5). Although it remains to be clarified if caloric supplementation rescued granulopoiesis in a T cell-dependent manner or directly acted on granulopoiesis, our data indicate that microbiota plays a critical role in IL-17A production and T cell reconstitution after SCT, suggesting that microbiota supports reactive granulopoiesis in a T cell dependent manner. In vivo or ex vivo T cell depletion is frequently performed to reduce chronic graft-versus-host disease after allogeneic SCT. Kinetics of neutrophil engraftment after infusion of T cell-depleted graft is comparable with conventional T cell-replete transplantation (22). Consistently, the current study showed that recipient T cells persisted after total body irradiation (TBI) could support neutrophil recovery after SCT in which T cell-depleted graft were used. Interestingly, in vivo T cell depletion using antithymocyte globulin (ATG) which deplete both donor and recipient T cells significantly delayed neutrophil engraftment after allo-SCT (22, 23). We previously reported that low-dose ATG did not affect the engraftment after allogeneic peripheral blood transplantation (24). Thus, the impact of T cell-depletion on the engraftment could be dependent on the thoroughness of T cell eradication.
Microbiota also plays a major role in steady-state granulopoiesis; germfree mice or antibiotic-treated mice demonstrated impaired BM granulopoiesis and reduction in the blood neutrophil count (4, 25–29). Bacterial components such as TLR-ligands and NOD-ligands as well as bacterial metabolites such as short chain fatty acids (SCFAs) maintain steady-state granulocytes (4, 13, 27, 29–35). Our data indicated that the gut microbiota plays a critical role in the enhanced production of IL-17A in T cells after prolonged neutropenia. Various microbiota-dependent factors such as IL-1β, IL-23, and IL-25, and commensal-derived adenosine tri-phosphate (ATP) promote the development of Th17 (36–38). Segmented filamentous bacteria promote Th17 differentiation via serum amyloid A production in epithelial cells (39–41). It remains to be clarified whether these mechanisms could be also involved in T cell production of IL-17A during prolonged neutropenia.
In the current study, prolonged neutropenia after SCT or chemotherapy led to significant alteration of the gut microbiota. In contrast, TBI-conditioned unmanipulated BMT, which caused short period neutropenia, did not significantly alter the composition of gut microbiota, suggesting that duration of neutropenia was critical to alter the gut microbiota. We and others have shown that administration of antibiotics reduces the production of antimicrobial peptides and induces intestinal dysbiosis both in human and mouse allogeneic SCT (42–46). Autologous SCT also leads to intestinal dysbiosis, which was associated with survival in patients with multiple myeloma (47). Although antibiotic use is the most likely cause of dysbiosis after autologous SCT, we showed that syngeneic SCT induced gut dysbiosis without administration of antibiotics, in association with prolonged neutropenia. Although mechanisms by which neutropenia modifies gut microbiota remains to be clarified, gut microbial ecology is maintained by neutrophils in the intestine (13, 48, 49). Neutrophils migrate into the intestinal lumen and produce high amounts of reactive oxygen species and antimicrobial peptides, leading to the suppression of bacterial translocation and overgrowth of pathobionts (50, 51). Neutrophils also enhance the expression of antimicrobial peptides in the gut epithelial cells, which potentially modifies gut microbiota (52).
When the gut-decontaminated SCT recipients underwent FMT from SCT recipients not receiving antibiotics, plasma concentrations of IL-17A and numbers of BM GMPs and peripheral blood neutrophils were increased. The only genus increased both in SCT-induced and chemotherapy-induced neutropenia was Ruminococcaceae UCG-014 that was not significantly increased after short-term neutropenia after BMT. Although this genus is not culturable and the detailed biological functions remain largely unknown, bacteria belonging to Ruminococcaceae are major components of a healthy gut microbiota and major producers of SCFAs (53, 54). Multiple species belonging to Ruminococcaceae along with species in Clostridium, Bacteroides, and Bifidobacterium were included in the 20-strain mixture which was isolated from human stool samples as potent IL-17A inducers (39). Ruminococcus also contains species that play a critical role in releasing nutrients from complex dietary starch (55, 56). High abundance of Ruminococcus 2, Faecalibacterium, and Akkermansia in the stool collected from patients who underwent allogeneic SCT predicts rapid neutrophil engraftment (56). High abundance of Ruminococcaceae is also associated with a favorable outcome after CAR T cell therapy (57). All these indicate the importance of Ruminococcaceae as stimulators of T cell functions, which play a critical role in reactive granulopoiesis during prolonged neutropenia.
In conclusion, we found that depletion of neutrophils stimulated T cell production of IL-17A in a microbiota-dependent manner, that enhanced reactive granulopoiesis. Furthermore, prolonged neutropenia-associated alteration of gut microbiota with the expansion of Ruminococcaceae stimulated IL-17A production and reactive granulopoiesis more potently than naive gut microbiota did. Our results revealed a cross talk between the intestinal microbiota and granulopoiesis during prolonged neutropenia, further providing a new prospect in reactive granulopoiesis. Rapid recovery of neutrophils after SCT and chemotherapy ensures safety of these treatment modalities by reducing risk of infections. It may be beneficial to revisit the strategies to use prophylactic and empiric administration of antibiotics so that we can leave granulopoiesis-supporting bacteria intact. FMT or probiotics that support granulopoiesis could be useful to enhance the donor engraftment after SCT (56). Fibers and SCFAs could also support reactive granulopoiesis during prolonged neutropenia. These novel strategies are promising and need to be tested in the clinical studies.
Methods
Mice.
Female C57BL/6 (CD45.2+) mice were purchased from Charles River Japan (Yokohama, Japan). B6-IL17A−/− mice were generated as previously reported (58). B6-RAG1−/− mice (Stock# 002216) and B6-CD45.1 mice (Stock# 002014) were purchased from the Jackson Laboratory (Bar Harbor, ME). The age of the mice ranged between 8 and 10 wk. All animal experiments were performed under the auspices of the institutional animal care and use committee of Hokkaido University (approval number; 17-0026, 22-0013).
SCT.
LSKs were sorted as cells negative for a set of lineage markers including CD3ε, CD11b, B220, Ly-76, and Ly-6G/Ly-6C while positive for Sca-1 and c-kit cells, and GMPs were sorted as lineage negative, CD16/CD32+, and CD34+ cells, using FACS Aria III (BD Biosciences, Franklin Lakes, NJ). Gating strategy for sorting is shown in SI Appendix, Fig. S1A. C57BL/6 (B6)-WT, B6-IL17A−/−, or B6-RAG1−/− recipients (CD45.2+) were i.v. injected with 7.5 × 103 LSK cells plus 2 × 104 GMPs from B6-CD45.1 donors after lethal X-ray TBI (11 Gy) delivered in two doses with 4-h intervals on day -1. In unmanipulated BMT, lethally irradiated B6 recipients were transplanted with 1 × 106 unmanipulated BM cells from syngeneic donors. In indicated experiments, B6-RAG1−/− SCT recipients were i.v. injected with 6 × 106 purified T cells from B6-WT or B6-IL17A−/− mice on day 0.
Reagent.
Naive B6 mice were i.p. injected with 200 mg/kg 5-FU (Sigma-Aldrich, St. Louis, MO) on day 0. In indicated experiments, recipient mice were orally treated with a combination of three antibiotics, ABPC (FUJIFILM Wako Pure Chemical Corp., Osaka, Japan), SM (FUJIFILM Wako Pure Chemical Corp), and VCM (Chem-Impex International Inc., Wood Dale, IL) at 1g/L each in drinking water from day −7 or left untreated.
Complete Blood Count.
Peripheral blood was collected from the retro-orbital plexus using heparin-coated capillary tubes, and the complete blood count was performed using pocH-100iV analyzer (Sysmex, Kobe, Japan).
CBA.
Plasma concentrations of IL-17A and G-CSF were determined using BD Cytometric Bead Array Flex Sets (BD Biosciences) and FACSCanto II (BD Biosciences), according to the manufacturer’s instruction.
Flow Cytometry.
The cell suspension was stained with antibodies listed in SI Appendix, Table S1. Dead cells were removed from the analyses as 4′,6-Diamidino-2-phenylindole (DAPI, Dojindo Laboratories, Kumamoto, Japan)-positive cells. Peripheral blood cells were analyzed with a FACS Canto II or FACS Aria III (BD Biosciences) using a gating strategy shown in SI Appendix, Fig. S1B.
Neutrophil Phagocytosis Assay.
Neutrophil phagocytic activity was evaluated using pHrodo Red E. coli BioParticles Phagocytosis Kits for Flow Cytometry (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Briefly, peripheral blood from WT or IL17A−/− SCT recipients was incubated with pHrodo red-labeled E. coli at 37°C or on ice for 15 min. Cells were washed and stained with APC-conjugated anti-Ly6G mAbs. Uptake of pHrodo red-labeled particles at 37°C in Ly6G+ cells was evaluated using a flow cytometer. Cells incubated with pHrodo red-labeled particles on ice were analyzed as negative controls.
ROS Production in Neutrophils.
To evaluate bactericidal activity of neutrophils, ROS production in response to PMA was evaluated using the Neutrophil/Monocyte Respiratory Burst Assay Kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s protocol. Peripheral blood was incubated with PE-conjugated anti-Ly6G mAbs for 15 min on ice and then labeled with dihydrorhodamine 123 (DHR 123) for 15 min at 37°C. Next, cells were stimulated with 0.2 μM PMA for 45 min at 37°C. DHR123 signals in the neutrophils were evaluated using a flow cytometer.
BrdU Labeling.
To label proliferating neutrophil progenitors in the BM, mice were injected i.p. with 1-mg BrdU (Sigma-Aldrich) on day +18 after SCT, and emergence of BrdU+ neutrophils in the peripheral blood was tracked sequentially after BrdU injection. To stain BrdU in the cells, cells were fixed and permeabilized with the transcription factor staining buffer set (eBiosciences, San Diego, CA), treated with 0.3 mg/ml DNaseI (Sigma-Aldrich), and stained with anti-BrdU mAbs. Dead cells were excluded from analyses using Live/Dead Fixable Viability Dye eFluor 450 (eBioscience).
Quantitative PCR (Q-PCR).
Total RNA was extracted from sorted T cells, using ISOGEN II (Nippon Gene, Tokyo, Japan). Reverse transcription was conducted to generate cDNA templates using ReverTra Ace qPCR RT Master Mix with gDNA Remover (Toyobo life science, Osaka, Japan). Q-PCR was performed using specific primers and probe sets listed in SI Appendix, Table S2, TaqMan Universal PCR master mix, and Applied Biosystems StepOnePlus (Thermo Fisher Scientific, Waltham, MA).
FMT.
Fresh feces have been collected from naive B6 mice or SCT recipients on day 10 after SCT and diluted in PBS at a concentration of 40-mg feces/ml and filtered with a cell strainer (70 μm). Gut decontaminated SCT recipients were orally administrated with fecal suspension prepared as above 14 d after SCT.
Fecal DNA Extraction and 16S rRNA Gene Sequencing.
Fecal DNA was obtained from 200-mg fresh feces using the QIAamp Fast DNA Stool Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instruction. V3-V4 variable region of 16S rRNA gene in each fecal DNA sample was amplified by PCR using the universal primer set of Bakt 341F (5′-CCTACGGGNGGCWGCAG-3′) and Bakt 805R (5′- GACTACHVGGGTATCTAATCC-3′) (59). Twenty-five μL reaction mixtures containing 12.5-ng fecal DNA, 200 nM each primer, and 1x KAPA HiFi Hot Start Ready Mix (Kapa Biosystems, Wilmington, MA) were prepared and first PCR was conducted under the following conditions: 95°C for 3 min, 25 cycles of 95°C for 30 s, 55°C for 30 s and 72°C for 30 s, followed by 72°C for 5 min. First PCR products were purified by AMPure XP beads (Beckman Coulter, Brea, CA), then subjected to a second PCR for adding indexing adapters containing sample-specific 8-bp barcodes by Nextera XT Index Kit v2 Set B (Illumina, San Diego, CA). Fifty μL of reaction mixtures containing 5 μL of first PCR amplicons, 5 μL of each indexing primer, and 1x KAPA HiFi Hot Start Ready Mix were subjected to a second PCR under the following conditions: 95°C for 3 min, 8 cycles of 95°C for 30 s, 55°C for 30 s and 72°C for 30 s, followed by 72°C for 5 min. To prepare amplicon library, each second PCR product was adjusted to 4 nM after the purification by AMPure XP beads, pooled 4 μL each, and diluted to 4 nM after the quantification by KAPA Library Quantification Kit Lightcycler 480 qPCR Mix (Kapa Biosystems). The amplicon library was mixed with 5% equimolar PhiX Control v3 (Illumina), and sequenced on a MiSeq instrument using the MiSeq 600-cycle v3 kit (Illumina) by pair-end sequencing mode.
16S rRNA-Gene-based Taxonomic Analysis.
Demultiplexed pair-end fastq files obtained from Miseq were analyzed by QIIME2 pipeline ver.2019.7 (60). Sequences were quality-filtered, denoised, and chimera removed by DADA2 plugin (61) with following parameters: --p-trim-left-f 17; --p-trim-left-r 21; --p-trunc-len-f 280; --p-trunc-len-r 200; --p-max-ee-f 2; and --p-max-ee-r 2. Then, the phylogenic tree was created by FastTree (62) after alignment with MAFFT (63). Taxonomy of each feature was assigned based on 99% sequence similarities to the Silva database (v132). Using QIIME2 workflow, principal coordinate analysis based on weighted UniFrac distance, an index of β-diversity was conducted.
Statistics.
Mann–Whitney U tests were used to compare data between two groups. Ordinary one-way ANOVA tests were used to compare data between three groups. The kinetic between groups were compared using the 2-way ANOVA tests. Analyses were performed using Prism (version 7.0; GraphPad Software, San Diego, CA). P < 0.05 was considered statistically significant. Statistical significance in β-diversity of microbiota was determined by the permutational multivariate analysis of variance (PERMANOVA) test in QIIME2 pipeline.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
This study was supported by Japan Society for the Promotion of Science KAKENHI (25293217 and 20K21610 to T.T., 21K08409 to D.H., 21K16259 to TA, 20K17366 to HO), the Center of Innovation Program of JST (T.T.), COI-NEXT of JST (JPMJPF2108 to K.N.), Promotion and Standardization of the Tenure-Track System (D.H.), and Suhara Memorial Foundation (D.H.).
Author contributions
D.H. and T.T. designed research; X.C., D.H., K.E., S.T., Y.S., R.S., Y.H., R.K., H.S., K.Y., Z.Z., S.H., E.H., T. Ara, and T.T. performed research; X.C., D.H., K.E., S.T., Y.S., R.S., Y.H., R.K., H.S., K.Y., Z.Z., S.H., E.H., T. Ara, H.O., Y.I., K.N., T. Ayabe, and T.T. analyzed data; and X.C., D.H., K.E., and T.T. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission. H.O. is a guest editor invited by the Editorial Board.
Contributor Information
Daigo Hashimoto, Email: daihashi00@gmail.com.
Takanori Teshima, Email: teshima@med.hokudai.ac.jp.
Data, Materials, and Software Availability
16S rRNA sequence data have been deposited in NCBI SRA (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA894100).
Supporting Information
References
- 1.Manz M. G., Boettcher S., Emergency granulopoiesis. Nat. Rev. Immunol. 14, 302–314 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Boettcher S., et al. , Endothelial cells translate pathogen signals into G-CSF-driven emergency granulopoiesis. Blood 124, 1393–1403 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deshmukh H. S., et al. , The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nat. Med. 20, 524–530 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balmer M. L., et al. , Microbiota-derived compounds drive steady-state granulopoiesis via MyD88/TICAM signaling. J. Immunol. 193, 5273–5283 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Staffas A., et al. , Nutritional support from the intestinal microbiota improves hematopoietic reconstitution after bone marrow transplantation in mice. Cell Host Microbe 23, 447–457.e444 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bi Y. J., et al. , IL-17A-dependent gut microbiota is essential for regulating diet-induced disorders in mice. Sci. Bull. 62, 1052–1063 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Satake S., et al. , C/EBPbeta is involved in the amplification of early granulocyte precursors during candidemia-induced "emergency" granulopoiesis. J. Immunol. 189, 4546–4555 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Casanova-Acebes M., et al. , Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell 153, 1025–1035 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiao J., et al. , Central role of conventional dendritic cells in regulation of bone marrow release and survival of neutrophils. J. Immunol. 192, 3374–3382 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inra C. N., et al. , A perisinusoidal niche for extramedullary haematopoiesis in the spleen. Nature 527, 466–471 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varelias A., et al. , Lung parenchyma-derived IL-6 promotes IL-17A-dependent acute lung injury after allogeneic stem cell transplantation. Blood 125, 2435–2444 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Na Nakorn T., Traver D., Weissman I. L., Akashi K., Myeloerythroid-restricted progenitors are sufficient to confer radioprotection and provide the majority of day 8 CFU-S. J. Clin. Invest. 109, 1579–1585 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mei J., et al. , Cxcr2 and Cxcl5 regulate the IL-17/G-CSF axis and neutrophil homeostasis in mice. J. Clin. Invest. 122, 974–986 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwarzenberger P., et al. , Requirement of endogenous stem cell factor and granulocyte-colony-stimulating factor for IL-17-mediated granulopoiesis. J. Immunol. 164, 4783–4789 (2000). [DOI] [PubMed] [Google Scholar]
- 15.Veldhoen M., Interleukin 17 is a chief orchestrator of immunity. Nat. Immunol. 18, 612–621 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Bugl S., et al. , Steady-state neutrophil homeostasis is dependent on TLR4/TRIF signaling. Blood 121, 723–733 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Cain D. W., Snowden P. B., Sempowski G. D., Kelsoe G., Inflammation triggers emergency granulopoiesis through a density-dependent feedback mechanism. PLoS One 6, e19957 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sudo T., et al. , Group 2 innate lymphoid cells support hematopoietic recovery under stress conditions. J. Exp. Med. 218, e20200817 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruddy M. J., et al. , Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J. Biol. Chem. 279, 2559–2567 (2004). [DOI] [PubMed] [Google Scholar]
- 20.Onishi R. M., Gaffen S. L., Interleukin-17 and its target genes: Mechanisms of interleukin-17 function in disease. Immunology 129, 311–321 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan W., et al. , Requirement of TPO/c-mpl for IL-17A-induced granulopoiesis and megakaryopoiesis. J. Leukoc. Biol. 94, 1303–1308 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malard F., et al. , Ex vivo and in vivo T cell-depleted allogeneic stem cell transplantation in patients with acute myeloid leukemia in first complete remission resulted in similar overall survival: On behalf of the ALWP of the EBMT and the MSKCC. J. Hematol. Oncol. 11, 127 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soiffer R. J., et al. , Prospective, randomized, double-blind, phase III clinical trial of anti-T-lymphocyte globulin to assess impact on chronic graft-versus-host disease-free survival in patients undergoing HLA-matched unrelated myeloablative hematopoietic cell transplantation. J. Clin. Oncol. 35, 4003–4011 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shiratori S., et al. , Low-dose anti-thymocyte globulin for GVHD prophylaxis in HLA-matched allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplant. 56, 129–136 (2021). [DOI] [PubMed] [Google Scholar]
- 25.Goris H., de Boer F., van der Waaij D., Myelopoiesis in experimentally contaminated specific-pathogen-free and germfree mice during oral administration of polymyxin. Infect. Immun. 50, 437–441 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tada T., Yamamura S., Kuwano Y., Abo T., Level of myelopoiesis in the bone marrow is influenced by intestinal flora. Cell Immunol. 173, 155–161 (1996). [DOI] [PubMed] [Google Scholar]
- 27.Clarke T. B., et al. , Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat. Med. 16, 228–231 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khosravi A., et al. , Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe 15, 374–381 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Josefsdottir K. S., Baldridge M. T., Kadmon C. S., King K. Y., Antibiotics impair murine hematopoiesis by depleting the intestinal microbiota. Blood 129, 729–739 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee S., et al. , Bone marrow CX3CR1+ mononuclear cells relay a systemic microbiota signal to control hematopoietic progenitors in mice. Blood 134, 1312–1322 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kovtonyuk L. V., et al. , IL-1 mediates microbiome-induced inflammaging of hematopoietic stem cells in mice. Blood 139, 44–58 (2022). [DOI] [PubMed] [Google Scholar]
- 32.Hergott C. B., et al. , Peptidoglycan from the gut microbiota governs the lifespan of circulating phagocytes at homeostasis. Blood 127, 2460–2471 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwamura C., Bouladoux N., Belkaid Y., Sher A., Jankovic D., Sensing of the microbiota by NOD1 in mesenchymal stromal cells regulates murine hematopoiesis. Blood 129, 171–176 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan H., et al. , The bacterial microbiota regulates normal hematopoiesis via metabolite-induced type 1 interferon signaling. Blood Adv. 6, 1754–1765 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang D., et al. , The microbiota regulates hematopoietic stem cell fate decisions by controlling iron availability in bone marrow. Cell Stem Cell 29, 232–247 e237 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaw M. H., Kamada N., Kim Y. G., Nunez G., Microbiota-induced IL-1beta, but not IL-6, is critical for the development of steady-state TH17 cells in the intestine. J. Exp. Med. 209, 251–258 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zaph C., et al. , Commensal-dependent expression of IL-25 regulates the IL-23-IL-17 axis in the intestine. J. Exp. Med. 205, 2191–2198 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atarashi K., et al. , ATP drives lamina propria T(H)17 cell differentiation. Nature 455, 808–812 (2008). [DOI] [PubMed] [Google Scholar]
- 39.Atarashi K., et al. , Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell 163, 367–380 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sano T., et al. , An IL-23R/IL-22 circuit regulates epithelial serum amyloid a to promote local effector Th17 responses. Cell 163, 381–393 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ladinsky M. S., et al. , Endocytosis of commensal antigens by intestinal epithelial cells regulates mucosal T cell homeostasis. Science 363, eaat4042 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jenq R. R., et al. , Intestinal blautia is associated with reduced death from graft-versus-host disease. Biol. Blood Marrow Transplant. 21, 1373–1383 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jenq R. R., et al. , Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J. Exp. Med. 209, 903–911 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eriguchi Y., et al. , Graft-versus-host disease disrupts intestinal microbial ecology by inhibiting paneth cell production of alpha-defensins. Blood 120, 223–231 (2012). [DOI] [PubMed] [Google Scholar]
- 45.Stein-Thoeringer C. K., et al. , Lactose drives enterococcus expansion to promote graft-versus-host disease. Science 366, 1143–1149 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peled J. U., et al. , Microbiota as predictor of mortality in allogeneic hematopoietic-cell transplantation. N. Engl. J. Med. 382, 822–834 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khan N., et al. , Fecal microbiota diversity disruption and clinical outcomes after auto-HCT: A multicenter observational study. Blood 137, 1527–1537 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flannigan K. L., et al. , IL-17A-mediated neutrophil recruitment limits expansion of segmented filamentous bacteria. Mucosal Immunol. 10, 673–684 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jee J., et al. , Cxcr2 signaling and the microbiome suppress inflammation, bile duct injury, and the phenotype of experimental biliary atresia. PLoS One 12, e0182089 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Molloy M. J., et al. , Intraluminal containment of commensal outgrowth in the gut during infection-induced dysbiosis. Cell Host Microbe 14, 318–328 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang D., Frenette P. S., Cross talk between neutrophils and the microbiota. Blood 133, 2168–2177 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zindl C. L., et al. , IL-22-producing neutrophils contribute to antimicrobial defense and restitution of colonic epithelial integrity during colitis. Proc. Nat. Acad. Sci. U.S.A. 110, 12768–12773 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gu Y., et al. , Influence of the densities and nutritional components of bacterial colonies on the culture-enriched gut bacterial community structure. AMB Express 11, 78 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koh A., De Vadder F., Kovatcheva-Datchary P., Backhed F., From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 165, 1332–1345 (2016). [DOI] [PubMed] [Google Scholar]
- 55.Ze X., Duncan S. H., Louis P., Flint H. J., Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J. 6, 1535–1543 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schluter J., et al. , The gut microbiota is associated with immune cell dynamics in humans. Nature 588, 303–307 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith M., et al. , Gut microbiome correlates of response and toxicity following anti-CD19 CAR T cell therapy. Nat. Med. 28, 713–723 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakae S., et al. , Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 17, 375–387 (2002). [DOI] [PubMed] [Google Scholar]
- 59.Herlemann D. P., et al. , Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J. 5, 1571–1579 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bolyen E., et al. , Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Callahan B. J., et al. , DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Price M. N., Dehal P. S., Arkin A. P., FastTree 2–approximately maximum-likelihood trees for large alignments. PLoS One 5, e9490 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Katoh K., Standley D. M., MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
16S rRNA sequence data have been deposited in NCBI SRA (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA894100).