Abstract
There has been an important change in the clinical characteristics and immune profile of Coronavirus disease 2019 (COVID‐19) patients during the pandemic thanks to the extensive vaccination programs. Here, we highlight recent studies on COVID‐19, from the clinical and immunological characteristics to the protective and risk factors for severity and mortality of COVID‐19. The efficacy of the COVID‐19 vaccines and potential allergic reactions after administration are also discussed. The occurrence of new variants of concerns such as Omicron BA.2, BA.4, and BA.5 and the global administration of COVID‐19 vaccines have changed the clinical scenario of COVID‐19. Multisystem inflammatory syndrome in children (MIS‐C) may cause severe and heterogeneous disease but with a lower mortality rate. Perturbations in immunity of T cells, B cells, and mast cells, as well as autoantibodies and metabolic reprogramming may contribute to the long‐term symptoms of COVID‐19. There is conflicting evidence about whether atopic diseases, such as allergic asthma and rhinitis, are associated with a lower susceptibility and better outcomes of COVID‐19. At the beginning of pandemic, the European Academy of Allergy and Clinical Immunology (EAACI) developed guidelines that provided timely information for the management of allergic diseases and preventive measures to reduce transmission in the allergic clinics. The global distribution of COVID‐19 vaccines and emerging severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) variants with reduced pathogenic potential dramatically decreased the morbidity, severity, and mortality of COVID‐19. Nevertheless, breakthrough infection remains a challenge for disease control. Hypersensitivity reactions (HSR) to COVID‐19 vaccines are low compared to other vaccines, and these were addressed in EAACI statements that provided indications for the management of allergic reactions, including anaphylaxis to COVID‐19 vaccines. We have gained a depth knowledge and experience in the over 2 years since the start of the pandemic, and yet a full eradication of SARS‐CoV‐2 is not on the horizon. Novel strategies are warranted to prevent severe disease in high‐risk groups, the development of MIS‐C and long COVID‐19.
Keywords: allergy, angiotensin‐converting enzyme 2, COVID‐19, immunity, vaccine
1. INTRODUCTION
The COVID‐19 pandemic, caused by infection with SARS‐CoV‐2, has led to alarming numbers of infections and deaths worldwide since it was first reported in December 2019. 1 SARS‐CoV‐2 belongs to the beta‐coronavirus genus and is closely related to SARS‐CoV. 2 , 3 SARS‐CoV‐2 binds to angiotensin‐converting enzyme 2 (ACE2) via its spike protein to enter cells. 3 , 4 The host serine protease transmembrane protease serine 2 (TMPRSS2) cleaves the spike protein and thus enable cellular membrane fusion. 5 , 6 The host protease furin cleaves the full‐length spike precursor to S1 and S2 peptides. 7 S1 directly binds to neuropilin‐1 on the cell surface and may facilitate cell invasion and infectivity of SARS‐CoV‐2. 7 , 8 ACE2 and TMPRSS2 are expressed at the epithelial sites of the lung and skin, whereas other host molecules that may be involved in SARS‐CoV‐2 invasion such as CD147, cyclophilins, CD26, and related molecules are expressed in both epithelium and immune cells. 9
The global administration of COVID‐19 vaccines has dramatically decreased the infection rate, severity, and mortality of this disease. 10 , 11 However, new SARS‐CoV‐2 variants of concern (VOC) have emerged that will dampen the protective immunity induced by natural infection and current vaccines and lead to breakthrough infection. 12 , 13
In this review, we highlight recent studies on the clinical and immunological characteristics of COVID‐19 in the context of allergy and asthma. The impact of asthma on the susceptibility and severity of COVID‐19 is not fully understood and it is discussed here in further detail. Moreover, recent studies on the immune responses and protective effects of COVID‐19 vaccines are summarized. The possible culprit components of the vaccines that can induce an allergic reaction are elaborated, along with appropriate vaccination measures for reducing the risk of anaphylaxis.
2. CLINICAL AND LABORATORY CHARACTERISTICS OF COVID‐19
2.1. Clinical characteristics of COVID‐19
The clinical scenarios of COVID‐19 are diverse and range from asymptomatic to critical illness and even fatal outcomes. 6 The symptoms of COVID‐19 include dry cough, fatigue, fever, myalgia, headache, diarrhea, and respiratory failure. 1 Olfactory and gustatory dysfunctions have been also identified as distinct symptoms of SARS‐CoV‐2 infection, especially in the western countries. 14 Thus, the respiratory symptoms of COVID‐19 may be confused with those of allergic rhinitis (AR) and the common cold. 15 Skin manifestations of COVID‐19 include vesicular, urticarial, and maculopapular eruptions and livedo, necrosis, and other vasculitis forms 16 and are more common among European and North Americans than among Asians. 17 The heterogeneity of COVID‐19 warrants the elucidation of the phenotypes and endotypes of COVID‐19 that will benefit from precision medicine. 18 , 19 In addition, persistent symptoms such as fatigue, brain fog, body aches, and loss of smell may persist for months following acute infection and are referred as post‐acute COVID‐19 syndrome or long‐COVID. 20 , 21 After a 1‐year follow‐up, most COVID‐19‐recovered patients regain their physical and functional status, although it remains lower than individuals without infection. 22
Children younger than 15 years of age appear to be susceptible to SARS‐CoV‐2 infection, although most of them are asymptomatic or develop mild symptoms. 23 Multisystem inflammatory syndrome in children (MIS‐C) has been described in COVID‐19 patients with an overall 2% mortality. 24 MIS‐C predominantly affects children between 6 and 12 years. Most MIS‐C children were critically ill, mostly from shock and/or left ventricular dysfunction, with less severe or no respiratory involvement. 25 Regarding the treatment of MIS‐C, intravenous immune globulin (IVIG) plus glucocorticoids were associated with a lower risk of cardiovascular dysfunctions but not the recovery from disease when compared to IVIG treatment alone. 26 , 27
2.2. Laboratory findings of SARS‐CoV‐2 infection
The nucleic acid amplification test using quantitative reverse transcription PCR (RT‐qPCR) is now widely used for the detection of SARS‐CoV‐2 infection. Modifications on RT‐qPCR such as sample pooling test and using probes against replication intermediates and new variants, and novel methods such as next‐generation sequencing, microfluidic assays, and clustered regularly interspaced short palindromic repeats (CRISPR)‐associated–based diagnostic testing have been adopted to enhance the diagnostic efficacy and sensitivity of SARS‐CoV‐2 infection. 28 IgG antibody assay against the receptor binding domain (RBD) and S1 domain was developed as an alternative diagnostic test, albeit with varying accuracies depending on the assay used. 29 Overall, antibody tests for SARS‐CoV‐2 have high specificity but relatively lower sensitivity, which varied with different immunoassays and epitopes. 30 Antigen testing is inexpensive, can provide results in a few minutes, and be performed at home. Population‐wide antigen testing was demonstrated to reduce the transmission of SARS‐CoV‐2. 31 Asymptomatic SARS‐CoV‐2 infection is associated with a longer duration of viral shedding and needs to be promptly detected to stop viral transmission. 32 , 33 The concentrations of neutrophil extracellular traps increase in the plasma, trachea aspirate, and lung autopsies and contribute to the pathophysiology of COVID‐19. 34 , 35 Thus, blood neutrophil extracellular traps can be a potential biomarker for SARS‐CoV‐2 infection. In addition, asymptomatic patients have lower levels of SARS‐CoV‐2 specific IgG antibodies, which decay during the early convalescent phase. 32 Laboratory changes during COVID‐19 have been reviewed and are summarized in our previous studies. 36 , 37 White blood cells, lymphopenia, lactate dehydrogenase, procalcitonin, high‐sensitivity C‐reactive protein, proinflammatory cytokines interleukin (IL)‐6, IL‐1β, Krebs von den Lungen‐6, and ferritin were identified as potential biomarkers for monitoring the progression and predicting the outcomes of COVID‐19. 37 , 38 Recently, machine learning (ML) approaches have been developed to help select the most useful laboratory sets of laboratory findings to diagnose COVID‐19 more efficiently. 39 One ML model was constructed based on laboratory parameters and chest CT scan to differentiate between COVID‐19 and other viral pneumonia, bacterial pneumonia, and other pneumonia. 39 , 40 Another study trained several ML models using data on reported comorbidities, medications, symptoms, and laboratory parameters on hospital admission and over the disease course to rapidly identify SARS‐CoV‐2 positive patients, high‐risk patients, and recognize longitudinal warning signs of a possible fatal outcome. 40 ML models, and likely together with artificial intelligence, may be helpful in the construction of decision tree of COVID‐19 in the future.
3. RISK AND PROTECTIVE FACTORS FOR SUSCEPTIBILITY, SEVERITY, AND MORTALITY OF COVID‐19
3.1. Protective factors
Health systems resilience is critical for the control of the COVID‐19 pandemic. 41 A healthy diet has been identified as a protective factor against SARS‐CoV‐2 infection. 36 Both the incidence rate and mortality of COVID‐19 were lower in Bacillus Calmette‐Guerin (BCG)‐vaccinated countries than in those without vaccination program. 42 , 43 Moreover, BCG vaccination during early childhood seems to selectively protect against infection in the elderly. 44 BCG was suggested to enhance innate immune responses, leading to “trained immunity” and confer protection against viral infections. 45 Similarly, recent administration of the mumps‐measles‐rubella vaccine was observed to be associated with a reduction in SARS‐CoV‐2 infection in males 46 and severity of COVID‐19, 47 although the real correlation between this vaccine and COVID‐19 is still unclear. 48
Lower levels of nasal ACE2 49 and airway cathepsin L/CTSL1, 50 a protease that cleaves and primes the SARS‐CoV‐2 spike protein, may contribute to the mild disease of COVID‐19 in children. Atopy and type 2 inflammation have been shown to be associated with a decreased expression of ACE2 in airway epithelial cells and thus lower susceptibility to SARS‐CoV‐2. 51 Mechanistically, the type 2 cytokine IL‐13 reduced ACE2 expression, 51 intracellular viral load, and cell‐to‐cell transmission, while increasing the cilial keratan sulfate coating in airway epithelial cells, suggesting a role of IL‐13 in attenuating viral shedding and thus reducing the entry, replication, and spread of SARS‐CoV‐2. 52 IL‐13 in combination with mucus was shown to reduce SARS‐CoV‐2 RNA by 90%–97% after inoculation of this virus in cultured human bronchial epithelial cells. 53 Another recent study in preprint also demonstrated that IL‐13 decreased the expression of long ACE2 mRNA and reduced glycosylation of full‐length ACE2 protein, leading to reduction in the apical expression of ACE2 on the ciliated airway epithelial cells. 54 These data indicate that the inflammatory milieu may be an important factor affecting the expression of ACE2 and other SARS‐CoV‐2‐relevant receptors, and thus affect the susceptibility and infection. Genetic variation of allergic disease was associated with a lower risk of COVID‐19. 55
Most studies suggested that AR and chronic rhinosinusitis (CRS) are not associated with a higher risk of susceptibility and severity of COVID‐19, 36 , 56 , 57 although reduced ACE2 expression was observed in bronchial epithelial cells from patients with concomitant AR and allergic asthma. 51 The expression of ACE2 in nasal polyp tissues of patients with CRS was lower than that of healthy controls. 58 The expression of ACE2 and TMPRSS2 in the olfactory mucosa was also lower patients of CRS with nasal polyps (CRSwNP) compared to healthy controls, and the protein expression of ACE2 was negatively correlated with eosinophils numbers in olfactory mucosa. 59 Moreover, the expression of ACE2 was upregulated by interferon (IFN)‐γ and downregulated by type 2 cytokines in nasal epithelial cells. 51 , 60 , 61 On the other hand, another study showed that early Th2 inflammation and attenuated IFN‐γ production in the nose may indicate worse clinical outcomes. 62 Moreover, elevated IL‐13 was associated with severe COVID‐19, and this effect may be due to IL‐13‐induced hyaluronan deposition in the lung. Treatment with dupilumab, a monoclonal antibody against IL‐4 and IL‐13, was associated with less severe COVID‐19, while IL‐13 neutralization reduced hyaluronan deposition and disease severity and death in SARS‐CoV‐2–infected mice. 63 Based on the results of this study, a pilot phase IIA clinical trial observing the safety and efficacy of dupilumab on forty moderate or severe COVID‐19 patients found that dupilumab did not increase the ventilator‐free survival at day 28, but it did reduce the mortality at day 60 when compared to placebo. 64 All these data suggest a complex association among type 2 inflammation, ACE2 expression, IL‐13, SARS‐CoV‐2 infection, and COVID‐19 outcomes.
The corticosteroid dexamethasone can increase ventilator‐free days 65 and reduce the death rate 66 in severe and critically ill COVID‐19 patients. Mechanistically, dexamethasone treatment was shown to restrain neutrophil pathogenicity by reducing IFNactive‐neutrophils and expanding immunosuppressive immature neutrophils. 67 Treatment with inhaled corticosteroids (ICS) reduced the level of ACE2 in induced sputum 68 from asthma patients and in bronchial epithelia from patients with chronic obstructive pulmonary diseases. 69 Clinical studies reported that inhaled budesonide in COVID‐19 patients reduced time to recovery and resulted in less severe outcome. 70 , 71 Thus, proper systemic and ICS treatment may benefit to COVID‐19 patients. Inhalable SARS‐CoV‐2‐specific siRNA and human ACE2‐containing nanocatchers were shown to reduce SARS‐CoV‐2 infection 72 , 73 and lung inflammation, 72 as shown in SARS‐CoV‐2 infected mice.
3.2. Risk factors
The emergence of new variants of SARS‐CoV‐2, such as Omicron and its subvariants, present a challenge for the eradication of COVID‐19 as they have evolved immune escape from the neutralizing antibodies developed in previous infections and vaccinations. 74 , 75 COVID‐19 breakthrough infection associated with the new variants has become a major concern. 76 Whole genome sequencing revealed two distinct mechanisms that can predispose an individual to life‐threatening COVID‐19, namely failure to control viral replication or an enhanced tendency toward pulmonary inflammation and intravascular coagulation. 77 Older age, male sex, cardiovascular and metabolic comorbidities, racial/ethnic disparities, chronic kidney diseases, and cancer have been identified as risk factors for SARS‐CoV‐2 infection and worse outcomes of COVID‐19. 36 , 37 A recent meta‐analysis identified that obesity was associated with an increased risk of COVID‐19‐related hospitalization and death, especially for those with extreme obesity. 78 Healthcare workers are at higher risk of SARS‐CoV‐2 infection compared to non‐healthcare workers. 37 , 79 Blood levels of neutrophil elastase 80 and histone‐DNA 81 were associated with severe, systemic, and multi‐organ manifestations of COVID‐19. Higher levels of bacterial DNA in the system circulation were associated with severe and fatal COVID‐19. 82 COVID‐19 patients with inborn errors of immunity, except type I IFN immunity errors, exhibit an almost similar natural COVID‐19 course compared to the general population. 83 Interestingly, individuals with blood group A are at higher risk of SARS‐CoV‐2 infection and severe disease, whereas blood group O may be protective against COVID‐19. 84
The higher expression of entry receptors ACE2 and TMPRSS2 increase the susceptibility to SARS‐CoV‐2 infection. 36 Smoking was associated with higher expression of ACE2, TMPRSS2, FURIN, and BSG in bronchial brushes 9 and represents a risk factor for COVID‐19 mortality when not adjusted for chronic respiratory diseases. 85
Air pollution has been shown to be associated with SARS‐CoV‐2 infection and COVID‐19 mortality. 86 Mechanistically, air pollutants such as nitrogen dioxide, ozone, and particulate matters (PM) may disrupt the airway epithelial barrier and impair the defense against respiratory viruses. 86 , 87 The airway epithelial barrier interacts with the respiratory microbiome to shape the immune response in the lungs. 88 In addition, air pollution may contribute to chronic systemic inflammation and a higher prevalence of comorbidities such as cardiovascular and respiratory diseases, which have been demonstrated to be risk factors for severe COVID‐19. 87 , 89 , 90 Air pollution is also correlated with a higher expression of ACE2 receptor in the lung. 91 Furthermore, fine PMs such as PM2.5 and PM10 may act as carriers of SARS‐CoV‐2 and promote the transmission of this virus. 89 Lockdown during COVID‐19 was associated with a reduction in PM2.5 concentrations due to reduced traffic emission. 92 Interestingly, the concentrations of airborne pollen correlated with the infection rates of SARS‐CoV‐2 in thirty‐one countries across both hemispheres. 93 This may be attributed to the impairment of innate antiviral immunity of airway epithelia upon pollen exposure. 93 The severity and excess death rate of COVID‐19 in northern Italy were suggested to have a link with the increased air pollution, 87 in accordance with several other studies. 94 , 95 , 96
3.3. New variants of concerns
New variants of SARS‐CoV‐2 emerged sequentially, becoming the predominant strains during the pandemic. These variants have distinct ACE2 binding affinity, virulence, transmissibility, and host immune responses 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 (Figure 1). Currently, the most pronounced risk factor for SARS‐CoV‐2 infection is the emergence of new variants or subvariants that are resistant to neutralizing antibodies and with higher transmissibility. 110 The subvariants BA.4 and BA.5, most likely stem from Omicron lineage BA.2, 110 were first spotted in South Africa 108 , 110 and are now spreading to Europe, the United States, 111 and other regions worldwide. 112 SARS‐CoV‐2 variants Omicron BA.4 and BA.5 belong to the single‐strand RNA (ssRNA) virus, like rhinovirus (RV) and influenza virus. However, variants Delta and Omicron are distinct from RV and influenza in many aspects including classification, virion size, receptors, risk groups, and incubation period and mortality after infection. The biological and clinical characteristics of RV, influenza A, SARS‐CoV‐2 variants delta, Omicron BA.1 and BA.5 are compared in Table 1.
FIGURE 1.
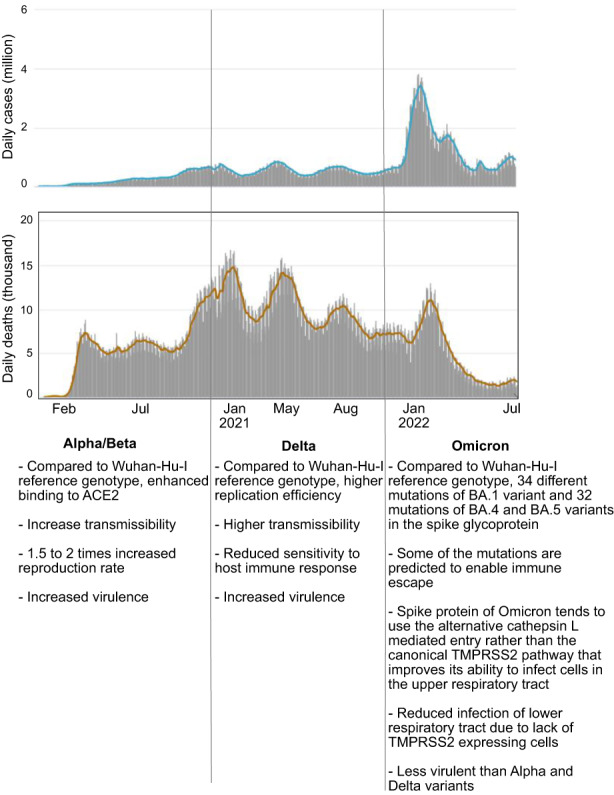
Daily new cases and deaths, and the characteristics of different mutations in the COVID‐19 coronavirus pandemic. The data were obtained from https://www.worldometers.info/coronavirus/.
TABLE 1.
Comparison of biological and clinical characteristics of SARS‐CoV‐2 variants, rhinovirus, and influenza virus
| Rhinovirus 116 , 117 | Influenza A 118 , 119 | SARS‐CoV‐2 Delta B.1.617.2 120 , 121 | SARS‐CoV‐2 Omicron B.1.1.529 120 , 122 | SARS‐CoV‐2 Omicron BA.5 123 , 124 , 125 | |
|---|---|---|---|---|---|
| Classification |
Picornaviridae Enterovirus Rhinovirus A/80 subtypes Rhinovirus B/32 subtypes Rhinovirus C/57 subtypes |
Orthomyxoviridae Alphainfluenzavirus Influenza A virus |
Coronaviridae Orthocoronavirinae Betacoronavirus Sarbecovirus SARS‐CoV‐2 |
Coronaviridae Orthocoronavirinae Betacoronavirus Sarbecovirus SARS‐CoV‐2 |
Coronaviridae Orthocoronavirinae Betacoronavirus Sarbecovirus SARS‐CoV‐2 |
| Genome | ssRNA positive‐strand linear | ssRNA negative‐strand segments | ssRNA positive‐strand linear | ssRNA positive‐strand linear | ssRNA positive‐strand linear |
| Size |
Rhinovirus A: 6.5–7.2 kb Rhinovirus B: 6.9–7.3 kb Rhinovirus C: 6.7–7.2 kb |
13.1–13.6 kb | 29.8 kb | 29.6 kb | 29.6 kb |
| Receptor |
Rhinovirus A: Immunoglobulin‐type cell adhesion molecule (ICAM‐1) B: low‐density lipoprotein receptor (LDLR) C: Cadherin‐related family 126 and member‐3 (CDHR3) |
Sialic acid (NeuAc alpha 2,3Gal and NeuAc alpha 2,6Gal) |
Angiotensin‐converting enzyme 2 (ACE2) | Angiotensin‐converting enzyme 2 (ACE2) | Angiotensin‐converting enzyme 2 (ACE2) |
| Incubation period | 2–3 days 129 | 2–5 days 129 | 4–7 days | 2–4 days | 2–4 days |
| Local diseases |
Rhinovirus A/B: Common cold, acute otitis media, rhinosinusitis Rhinovirus C: Lower respiratory tract infections, bronchiolitis and pneumonia, wheezing and asthma exacerbations, croup |
Pneumonia, headache, chills, dry cough, fever, myalgia, fatigue, anorexia, nasal congestion, rhinorrhea, sneezing, conjunctivitis | Fever and chills, cough, shortness of breath/dyspnea, burnout, myalgia, headache, loss of taste or smell, sore throat, nasal congestion, runny nose, nausea, vomiting, diarrhea | Runny nose, headache, fatigue, hoarseness, sneezing, sore throat, fever, cough, loss of smell or taste | Most infected individuals are asymptomatic. For those with symptoms, runny nose, cough, sore throat/pharyngitis, headache, were reported by more than 50% of patients. No pneumonia was reported 130 |
| System diseases | Systemic manifestation of RV infection is rare. It may cause fever, malaise, co‐infection with other pathogens, and exacerbate preexisting diseases such as asthma, chronic obstructive pulmonary disease | Viremia, myocarditis, myositis, central nervous system symptoms (irritability, lethargy, noise, confusion, encephalopathy, encephalitis, etc.), toxic shock syndrome, cytokine storm, kidney failure, liver failure | Viremia, myocarditis, myositis, central nervous system symptoms (irritability, lethargy, noise, confusion, encephalopathy, encephalitis, etc.), toxic shock syndrome, cytokine storm, kidney failure, liver failure | More focused to elderly and risk groups, same type of diseases as in delta. Relatively low prevalence compared to delta | Most infected individuals are asymptomatic. For those with symptoms, fever, severe feeling of sickness, muscle or body aches were reported by >40% patients 130 |
| Risk groups | Babies and toddlers, chronic disease, primary and secondary immunodeficiencies | Older age, chronic diseases (asthma, coronary artery disease, diabetes, cirrhosis, chronic kidney failure, Parkinson's disease, etc.) | Adults 70 years and older, chronic heart disease, diabetes, obesity, immunodeficiency transplantation, cancer chemotherapy, chronic kidney disease, pregnancy | Adults 70 years and older, chronic heart disease, diabetes, obesity, immunodeficiency transplantation, cancer chemotherapy, chronic kidney disease, pregnancy | Age group of 40–59 years had the highest infection rate (44.0%), followed by age group 20–39 years (30.2%) and age group 60–79 years (15.1%) 130 |
| Mortality | 0.001% | 0.1% | 2%–4% | 0.01% | Similar to the Omicron BA.1 wave |
BA.4 and BA.5 have become the dominant VOC in many European countries. The hospitalization and death rate of BA.4 and BA.5 were significantly lower compared to previous waves of infection in South Africa, 108 which may be due to the high population immunity. However, in Portugal, the hospitalization and mortality caused by BA.4/5 were similar to that in the first wave of Omicron infection, which may be due to the higher proportion of elderly individuals in this country. 110 BA.4 and BA.5 carry additional mutations in the spike proteins assisting the immune escape from protection induced by 3‐dose vaccinations and by post‐vaccination infection of BA.1. 113 , 114 The Omicron variant is continuously evolving to escape antibody neutralization resulting in breakthrough infection of SARS‐CoV‐2 in both vaccinated and in previously infected individuals. 115
4. COVID‐19 AND ASTHMA
Observational studies indicate a potential protective factor of asthma for the morbidity and mortality of COVID‐19, 1 , 56 although conflicting data from the United States and United Kingdom (UK) suggested a higher prevalence of asthma in COVID‐19 patients. 131 A UK study found that asthmatic patients were associated with a higher risk of COVID‐19. 132 For allergic asthma, the protective effects have been partly attributed to the antiviral effect of eosinophils, 36 whose beneficial effects on COVID‐19 outcomes depend on ICS. 133 However, whether COVID‐19 patients with asthma are at higher risk of long‐COVID symptoms is still unclear as there are contradictory research studies. 134 , 135
It remains unclear whether asthma is a risk factor for the severe and worse outcome of COVID‐19. However, it appears to be related to the asthma phenotypes, treatments, and severity. 136 , 137 Studies demonstrated that Th2‐high inflammation may be associated with reduced, while Th2‐low inflammation may be associated with increased risk of SARS‐CoV‐2 infection and severity of COVID‐19. 138 Asthma was shown to be associated with an increased hospitalization risk of COVID‐19 both in adults 56 and in children. 139 Another study observed an increased hospitalization rate only in asthmatic patients needing regular ICS or regular/intermittent ICS with add‐on therapy. 132 The hospitalization rate of allergic asthmatics was 50% lower compared to non‐allergic asthmatics. 135 A recent meta‐analysis identified preexisting asthma as a risk factor for intensive care unit (ICU) admission among COVID‐19 patients. 140 The heterogeneity of asthma endotypes (allergic vs. non‐allergic asthma) may underly the different disease course in these studies. 36 , 135 Eosinopenia was associated with worse outcomes of COVID‐19, including longer duration of hospitalization, higher severity, and mortality. 1 , 37 , 135 Dynamic monitoring of eosinophils counts in addition to other laboratory indices, such as neutrophil‐to‐lymphocytes ratio lymphocytopenia and D‐dimer, may be used as predictive biomarkers of the outcomes of COVID‐19. 36 , 37 Biologicals were associated with lower susceptibility in asthmatic patients. 141 Omalizumab‐augmented IFN‐α production from plasmacytoid dendritic cells, 142 which may also contribute to the protecting effects of asthma against COVID‐19.
A lower expression of ACE2 in bronchial epithelial or lung tissue was observed in allergic asthmatic patients. 131 , 143 , 144 In addition, ICS may decrease the expression of ACE2 and TMPRSS2 in bronchial epithelia of asthmatic patients 68 and thus contribute to lower susceptibility to infection. A recent study in preprint showed that prior infection with rhinovirus restricted SARS‐CoV‐2 replication, but co‐infection augmented retinoic acid‐inducible gene 1 (RIG‐1) inflammasome activation and epithelial inflammation in patients with asthma, especially in the presence of house dust mites. 145 In a summary, the relationship between asthma, SARS‐CoV‐2, and COVID‐19 is complex and may be associated with the inflammatory phenotypes, treatments, and comorbidities. 146
5. LONG COVID‐19
A large number of patients recovering from COVID‐19 may suffer from long‐term complications of COVID‐19 including a large variety of symptoms, defined as “post‐acute COVID‐19 syndrome” or “long COVID‐19”. 147 The symptoms of long COVID‐19 are summarized in Table 2. The current evidence does not indicate an increased risk of long COVID‐19 in asthmatic patients, although studies with more patients are warranted. 144 Mechanistically, long COVID‐19 patients were characterized with over‐activated innate immune cells, lacked naive T and B cells, and showed persistent elevated expression of IFN‐β and IFN‐λ1 at 8 months after infection. 148 Mast cell activation may also contribute to long‐term COVID‐19 as evidenced by persistent mediators release. 149 Activated inflammatory caspases can induce pyroptosis, which may partly contribute to lymphopenia in COVID‐19, and the caspases inhibitors have been suggested to be potential therapeutics for severe and long COVID‐19. 150 Preexisting and de novo autoantibodies were more frequently detected in hospitalized or severe COVID‐19 patients and may play a role in post‐acute sequelae of COVID‐19. 151 Dysregulated respiratory CD8+ T cell responses were associated with impaired lung function after acute COVID‐19. 152 The mechanisms underlying COVID‐19 are still not fully understood. Currently, it was suggested that poor viral clearance, persistent inflammation, and autoimmunity were proposed as the major mechanisms contributing to long COVID‐19. 153 , 154 , 155 , 156 Elderly or female COVID‐19 patients, or those previously with a severe COVID‐19 disease are prone to have long COVID‐19, which may be associated with persistent dendritic cell deficiency, lymphopenia, and dysbiosis (Figure 2).
TABLE 2.
Reported symptoms of post‐acute COVID‐19 syndrome or long COVID‐19
| Organs and systems | Symptoms |
|---|---|
| General status | Fatigue, general asthenia, low fever, dizziness, sweating 147 , 157 |
| Neuro‐psychiatric system | Headache, insomnia, depression, distress, anxiety, dysphoria, post‐traumatic stress disorder, memory loss, loss of attention, anorexia, other mental health conditions 147 , 158 , 159 |
| Cardiovascular systems | Chest pain, palpitation, elevated heart rate, diastolic dysfunction, stress cardiomyopathy, changes in cardiac MRI, ongoing elevation in high‐sensitivity troponin T 20 , 147 , 158 , 159 , 160 |
| Upper respiratory tract | Runny nose, anosmia, sore throat, blocked nose 147 , 158 |
| Pulmonary system | Cough, phlegm, hemoptysis, chest tightness, chest pain, shortness of breath, ventilator and oxygen dependence, chest imaging abnormality, fibrotic lung disease, impaired pulmonary function 147 , 158 , 159 |
| Gastrointestinal system | Abdominal discomfort, diarrhea, constipation, vomiting, loss of appetite 158 |
| Hepato‐biliary system | Nausea, jaundice, transaminase increase 158 |
| Endocrine and metabolic system | New‐onset hyperglycemia and diabetes, diabetic ketoacidosis, subacute thyroiditis 147 , 159 |
| Genito‐urinary system | Proteinuria, hematuria, development of kidney injury, uncontrolled bladder, pollakiuria 20 , 158 , 161 |
| Vascular complications | Thromboembolism, bleeding, disseminated intravascular coagulation 20 , 153 |
| Reproduction | Male infertility 159 |
| Musculoskeletal system | Arthralgia, myalgia, muscle weakness 158 , 159 |
| Dermatological complications | Maculopapular exanthem, papulovesicular rash, urticaria, painful red acral purple papules, hair loss 158 , 159 |
| Others | Conjunctivitis, neck pain 153 |
FIGURE 2.
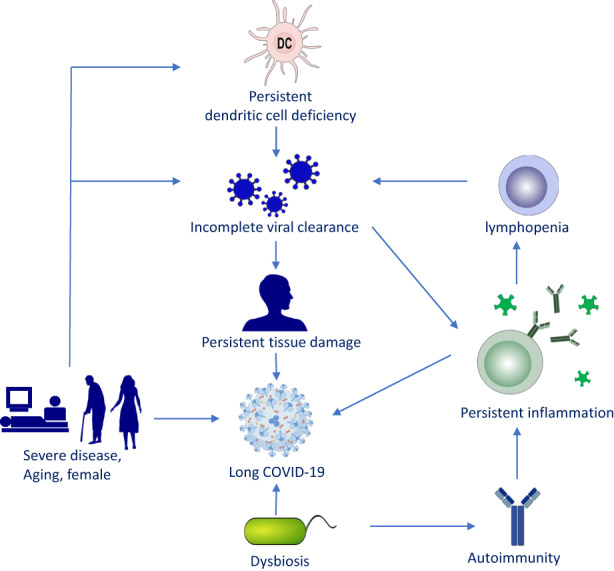
Proposed mechanisms of long COVID‐19. This schematic diagram shows the possible relevant factors contributing to long COVID‐19. Persistent tissue damage and inflammation might be the major mechanisms of persistent symptoms of COVID‐19. In addition, persistent dendritic cell deficiency, autoimmunity, and dysbiosis may also contribute to the long‐term symptoms. Severe disease, aging, and female are suggested as risk factors for the development of long COVID‐19.
6. IMMUNE RESPONSES AND PATHOGENESIS OF COVID‐19
The immunological pathogenicity of COVID‐19 is complex and may be associated with the virulence of SARS‐CoV‐2 and the lack of the temporal coordination between innate and adaptive immune responsces. 162 , 163 , 164 Other mechanisms such as pre‐existing immunity to SARS‐CoV‐2, superantigens, and autoimmunity have been also suggested to participate in the immune responses to this virus. 165 Adaptive responses to SARS‐CoV‐2 develop mainly to the spike protein. It is postulated that SARS‐CoV‐2 RNA is sensed by toll‐like receptor (TLR)‐3/7/8 and activates innate immune pathways. 154 , 166 SARS‐CoV‐2 can also be recognized by RIG‐I, the cytoplasmic sensor of RNA. 167 , 168 The interaction of RIG‐1 with viral genome directly abrogates viral replication in lung cells 167 ; on the contrary, the activation of RIG‐1 by SARS‐CoV‐2 triggers the epithelial cells to release inflammatory mediators, which then stimulate primary human macrophages to enhance cytokine production and drive cellular activation, especially in the presence of exogenous inflammatory stimuli. 168 SARS‐CoV‐2 replication induced a delayed type I IFN response in lung epithelial cells, which is regulated by melanoma differentiation‐associated gene 5 (MDA5). 169 Activation of NLRP3 inflammasomes participates in the pathophysiology of COVID‐19 and is associated with the severity of the disease. 170 Type I IFN‐mediated antiviral responses and activation of both CD4+Th1 and CD8+ cytotoxic T lymphocytes result in viral clearance in SARS‐CoV‐2‐infected subjects with mild symptoms. 171 Impairment in the number and function of dendritic cells may also lead to dysregulation in innate and adaptive immunity including antiviral response. 165 , 172 The insufficient antiviral response, 173 or autoantibodies against type I IFNs, 174 , 175 combined with the system inflammation induced by a large number of immune cells and resident tissue cells, may contribute to the cytokine storm in severe disease. 154
Virus‐specific IgM and IgA can be detected in the acute phase followed by an increase in virus‐specific IgG at a later stage of COVID‐19. 165 More severe COVID‐19 patients were associated with higher anti‐RBD IgA and IgG antibody responses when compared to those not hospitalized or asymptomatic. No deaths were reported in patients with higher an IgG antibody index (NT50/IgG >100). 176 Dupilumab (anti‐IL‐4/13 Rα) used in atopic dermatitis (AD) patients with COVID‐19 was associated with a lower IgG antibodies response. 177 Poor and delayed anti‐SARS‐CoV‐2 IgM and IgG antibodies responses correlated with poor outcomes of COVID‐19 in children. 178 A visible antibody cross‐reactivity was reported in infectious bronchitis virus, a non‐SARS‐CoV‐2 infection and chicken aerosol vaccines particularly in highly exposed veterinarians who administered the vaccines. However, this immune cross‐reactivity did not show a viral neutralizing activity and focused on non‐RBD antibodies, which substantially differ between SARS‐CoV‐2 and non‐SARS‐CoVs. 179
Mild COVID‐19 patients exhibited SARS‐CoV‐2‐specific memory B and T cells responses that display hallmarks of antiviral immunity. 180 The antigen‐driven activation of anti‐SARS‐CoV‐2 memory B cells persisted and matured up to 6 months after SARS‐CoV‐2 infection and may provide long‐term protection 181 Diverse autoantibody responses were identified in COVID‐19 patients and were correlated with disease severity and duration of hospitalization. 175
7. MANAGEMENT OF ALLERGIC DISEASES DURING THE COVID‐19 PANDEMIC
The COVID‐19 pandemic has shaped the ways medical services are conducted in order to accommodate for the imposed lockdown and social distancing measures. Accordingly, telemedicine was adopted by many physicians to guide the treatment and follow‐up of allergic patients. 182
Continuation of intranasal corticosteroids (INCS) was suggested for AR patients with COVID‐19 at the recommended doses. 183 Treatment with INCS before SARS‐CoV‐2 infection was associated with a lower risk of COVID‐19‐related hospitalization, ICU admission, or death. 184 A systematic review assessed the use of INCS on the olfactory dysfunction of COVID‐19 patients, but only identified a single study to include in the review. 185 The impact of INCS on the susceptibility and outcomes of COVID‐19 is still inconclusive. 57 Oral corticosteroids, biologicals, and surgical treatment should be avoided or suspended in CRS patients with SARS‐CoV‐2 infection. 57 Telemedicine was advocated to replace in‐person visits for the care of CRS patients during the COVID‐19 pandemic with high patient satisfaction. 186
Inhaled or oral corticosteroids should be continued to for asthmatic patients without SARS‐CoV‐2 infection to maintain asthma control, and oral corticosteroids and biologicals should be continued to treat severe asthma exacerbations. 187 In the case of asthmatic patients with confirmed SARS‐CoV‐2 infection, the use of inhalers should be indicated over nebulizers for the delivery of aerosolized medications to avoid viral transmission via aerosol. 188 Current evidence suggests that treatment with biologicals targeting type 2 inflammation does not increase the risk of SARS‐CoV‐2 infection and COVID‐19 severity 138 and may have beneficial effects. 141 Therefore, biologicals may be continued during COVID‐19 for asthmatic patients without SARS‐CoV‐2 infection.
Allergen immunotherapy (AIT) should be temporarily discontinued until recovery for SARS‐CoV‐2 positive asthmatic patients or in contact with confirmed cases of COVID‐19. 189 AIT can be continued in SARS‐CoV‐2 negative confirmed patients but with a prolonged injection interval. 189 Switching from subcutaneous to sublingual immunotherapy may be considered for AIT during COVID‐19. 190 Skin manifestations of COVID‐19 may be similar to other viral infections and drug hypersensitivity reactions (DHRs). 191 , 192 The diagnosis and management of DHRs induced by medications repurposed and off‐label for the treatment of COVID‐19 have been discussed in a few review papers. 191 , 192 , 193
Pulmonary function tests such as spirometry should be restricted to those patients with high clinical priority and using a high‐efficiency inline filter. The patients should be encouraged to perform peak expiratory flow measurement at home to reduce the possible transmission via small droplets and aerosol generated during the pulmonary function test. 194 , 195
Many studies have focused on the impact of the COVID‐19 pandemic on the status and control of allergic diseases. In asthmatic children, environmental changes, altered medical practice, and medication use, changes in transportation and travel patterns, school attendance, and physical activity impacted asthma control during the pandemic. 196 A survey revealed that the majority of the AD patients experienced AD flares with mild worsening of the disease during the COVID‐19 pandemic. 197 Dupilumab seems to be safe and crucial for a better outcome of COVID‐19 and should be continued in AD patients during the COVID‐19 pandemic. 198 During COVID‐19, AIT was initiated and continued by most physicians in patients without indications of SARS‐CoV‐2 infection. 199 In contrast, lockdown during COVID‐19 resulted in decreased numbers of patients initiating AIT but without significant impact on sublingual immunotherapy. 200
COVID‐19 was reported to increase the incidence of acute urticaria 201 and disease activity of chronic urticaria in males but not females, which may be associated with loss of omalizumab efficacy. 202 Single‐cell sequencing and proteomic analysis revealed that the cytokine storm associated with severe COVID‐19 may promote the activation of monocytes/macrophages and cytotoxic CD8+ T cells, which may be involved in the development of maculopapular drug rash associated with COVID‐19. 203
8. COVID‐19 VACCINES
Natural SARS‐CoV‐2 infection does not establish a strong antibody response that can prevent reinfection. 204 COVID‐19 vaccines offer promising protective immunity against SARS‐CoV‐2 infection. Two groups of vaccines are available for the global vaccination strategy for COVID‐19. The classic group includes subunit, inactivated, live‐attenuated, and virus‐like particle vaccines; novel approaches include RNA‐based vaccines, which deliver RNA coding target viral proteins into human cells. 205 Two mRNA‐based vaccines, BNT162b2 (Pfizer‐BioNTech) and Moderna mRNA vaccines were approved with emergency use or conditioned marked authorization by the USA, European Union, and other countries’ governmental agencies. Phase III clinical trials and real‐world data suggested that COVID‐19 vaccines have dramatically reduced severe COVID‐19 cases 206 and excess mortality due to COVID‐19 (Figure 3), although breakthrough infection in fully vaccinated individuals is not uncommon. 207
FIGURE 3.
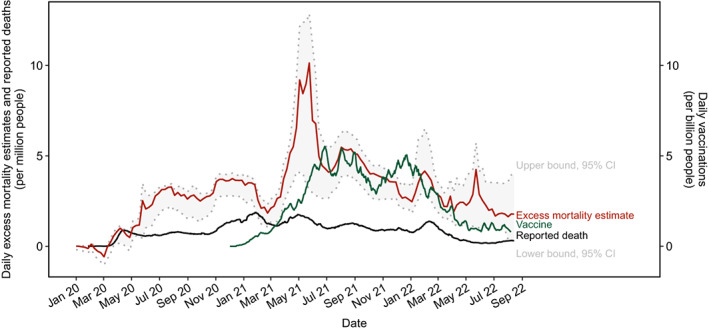
Time‐series visualization of global daily reported COVID‐19 deaths, excess mortality estimates, and vaccinations. Source data were downloaded from Our World in Data on August 19, 2022. Excess mortality estimates and the 95% confidence interval (CI) were modeled by the Economist. The lines indicate 7‐day rolling averages of daily reported deaths and excess mortality estimates per million people as well as all daily vaccination doses per billion people, including boosters that are counted individually.
8.1. Efficacy and immune responses of COVID‐19 vaccines
The RBD of the SARS‐CoV‐2 spike protein is the primary target for neutralizing antibodies induced by COVID‐19 vaccines. Specific IgG antibodies against conformational but not sequential RBD epitopes have the potential to block the binding of SARS‐CoV‐2 with ACE2 and confer protective immunity. 208 Mutations in RBD, as identified in several VOC, display increased binding affinity to ACE2 209 and/or reduced neutralization ability of sera from convalescent and BNT162B2 vaccinated individuals. 210 , 211 , 212 , 213 Noticeably, BNT162b2‐induced IgG antibodies had higher avidity to mutated RBD than those induced by natural infection. 74 The variant Omicron exhibited approximately fourfold greater immune escape relative to the Beta variant. 212 Prior COVID‐19 was associated with higher neutralization capacity for the ancestral virus after BNT162b2 vaccination. 212 More importantly, two doses of mRNA COVID‐19 vaccines provided almost non‐existent, whereas a booster dose yielded almost 75% protection against symptomatic infection of Omicron. 213 It is predicted that BNT162b2 boosted, or vaccination (two doses) combined with the previous infection can prevent 73% symptomatic infection by Omicron, which is significantly higher than in individuals with BNT162b2 vaccination only. 212
Other types of vaccines have also been administered around the world. CVnCoV is a vaccine based on unmodified RNA and induced only a 48% reduction in the incidence of symptomatic disease. 214 CV2CoV is the second‐generation unmodified mRNA vaccine but with optimized non‐coding regions and enhanced antigen expression. Compared to CVnCoV, CV2CoV induced higher titers of binding and neutralizing antibodies against SARS‐CoV‐2 variants, and memory B and T cell responses in non‐human primates. 215 The inactivated Sinopharm/BBIBP COVID‐19 vaccine is widely used in developing countries including China due to its low storage requirements. Seroconversion rates in unexposed individuals after first and second dose reached 40% and 100%, respectively, and younger individuals and women had the highest antibody concentrations. Previous SARS‐CoV‐2 infection was associated with a strong antibody response after a single dose of the BBIBP vaccine. A sharp increase in antibody concentrations was observed following SARS‐CoV‐2 infection after the first and second doses. 216 Virus‐like particle‐based COVID‐19 vaccines induced high levels of neutralizing antibodies and protection against infection with SARS‐CoV‐2 and its variants in mice and rhesus macaques. 217 , 218 PreS‐RBD vaccine was developed based on a recombinant fusion protein consisting of the human hepatitis B virus‐derived PreS antigen and two SARS‐CoV‐2 RBD domains. It induced a robust anti‐RBD IgG response in rabbits consisting of an early IgG1 and sustained IgG4, which can be detected in serum and mucosa secretions. Moreover, the vaccine‐induced antibodies potently inhibited the interaction of RBD with ACE2 and possessed the neutralizing ability of the omicron VOC. 219 The efficacy and immune responses elicited by different types of COVID‐19 vaccines are listed in Table 3.
TABLE 3.
Efficacy and immune response of different SARS‐CoV‐2 vaccines
| Vaccine name | Types of vaccines | Efficacy and immune responses |
|---|---|---|
| BNT162b2 | mRNA vaccines | |
| Moderna | mRNA vaccines | |
| AZD1222 | Viral vector |
|
| Sputnik V | Viral vector |
|
| Ad26.COV2.S | Viral vector |
|
| BBIBP COVID‐19 vaccine | Inactivated vaccines | |
| Covaxin (BBV152) | Viral vector |
|
| CoronaVac | Inactivated virus |
|
| AD5‐nCOV | Viral vector |
|
| CV2CoV | Unmodified RNA vaccines |
|
| PreS‐RBD vaccine | Recombinant fusion protein |
|
| NVX‐CoV2373 | Protein subunit |
Intramuscular injection is the major route for COVID‐19 vaccination. Recently, a diversity of animal experiments and clinical trials are ongoing to evaluate the immune responses and the safety and efficacy of intranasal administration of COVID‐19 vaccines, respectively. 220 It is hypothesized that intranasal vaccination may induce both mucosal and systemic immune responses, 221 and thus the local mucosal immunity may prevent the virus infection through the upper respiratory tract and block transmission to other people. 222 In mice, intranasal administration of virus‐based vaccine candidates expression SARS‐CoV‐2 spike protein elicited robust specific IgG and IgA antibodies and polyfunctional S‐specific Th1‐skewed CD4+ and cytotoxic CD8+ T‐cell immune responses. 221 Four COVID‐19 mucosal vaccines have been approved as booster for the prevention of COVID‐19 infection in China, Russia, India and Iran. 222 The exact efficacy of these mucosal vaccines is still unknown since no clinical trial data are released.
8.2. Allergic reactions to COVID‐19 vaccines
Local and systemic reactions were reported in phase III clinical trial of the ChAdOx1 nCoV‐19 vaccine, which were mostly mild and moderate in intensity. 241 Cutaneous adverse effects of the available COVID‐19 vaccines include injection site reactions, urticaria, angioedema, exacerbation of atopic eczema, and systemic allergic reactions including anaphylaxis. 242 Anaphylaxis to the BNT162b2 vaccine was reported several days after the initiation of public vaccinations. 243 Overall, the incidence of COVID‐19 mRNA vaccine‐associated anaphylaxis is very low, 244 with only was 4.8 per million doses for BNT162b2 and 5.1 per million doses for mRNA‐1273 according to data from Vaccine Safety Datalink. 245 Moreover, the second dose of the BNT162b2 vaccine elicited a higher rate of systemic events. 223 Nevertheless, the incidence of anaphylaxis‐associated with COVID‐19 vaccines is comparable to that of other vaccines 246 and the benefits may outweigh the potential risks of COVID‐19 vaccinations. 247 , 248 Unfortunately, the fear of an allergic reaction has caused vaccine hesitancy in the general public, haltering global vaccination efforts. 249
Polyethylene glycol (PEG) is an additive present in the Pfizer‐BioNtech and Moderna mRNA vaccines used to prevent premature degradation of the nanoparticless, 250 and it has been suggested to be the major culprit for anaphylaxis to COVID‐19 vaccines. 251 PEG and its derivatives are also widely used in household products including toothpaste, cosmetics, pharmaceuticals, and foods. 252 Many types of vaccines, therapeutic medications, and diagnostic media contain PEG with different molecular weights and their risks of anaphylaxis have been previously reported. 252 , 253 Exposure to products containing PEG via intravenous and intramuscular injection is the major route causing HSR to PEG. There is a study showing that lipid‐conjugated PEG or PEGylated liposomes have a stronger immunogenicity than PEG alone and may contribute to the anaphylaxis elicited by COVID‐19 mRNA vaccines. 254 , 255 Anti‐PEG IgE‐mediated HSR, complement activation‐related pseudoallergy induced by anti‐PEG IgM and IgG antibodies and potential interaction of PEG with mast cells and viral RNA have been suggested to underly COVID‐19 vaccine anaphylaxis. 254 , 256 IgG and IgM antibodies against PEG were found in up to 25% of the population without known prior exposure to PEGylated products and in up to 89% of patients with known prior exposure to PEGylated products. 257 Other excipients than PEG present in authorized COVID‐19 vaccines might also cause severe allergic reactions to COVID‐19 vaccines and need appropriate allergological assessment. 258
EAACI suggested performing in vivo tests (skin prick test and intradermal test) and in vitro tests (basophil activation test, BAT) on the vaccines or their components in individuals with severe reactions to the first dose of COVID‐19 vaccines. 259 The positive rate of skin test with PEG or mRNA vaccine in patients with reactions to COVID‐19 mRNA vaccine or with previous PEG or polysorbate allergies was very low. 260 , 261 Thus, the clinical significance of skin testing with mRNA vaccine in those with negative skin testing results with PEG is still unknown. BAT performed in patients with PEG allergy demonstrated that BNT162b2 and AZD1222 vaccines and PEGylated lipids, but not unmodified PEG, can activate mast cells. 255 Thus, positive BAT results in mRNA vaccines may be a potential diagnostic tool for confirming HSR to PEG excipient.
Studies have shown that most individuals with allergic reactions to the first dose tolerated well the second dose irrespective of the skin test results. 262 , 263 Thus, the second dose vaccine may be administered after careful evaluation and under careful monitoring in an allergy clinic. An EAACI position paper recommended that allergic patients without prior allergic reaction to any of the vaccine components and patients with mild and moderate allergies should not be excluded from COVID‐19 vaccinations. 259 On the contrary, it would put population immunity with vaccination at risk due to the high prevalence of allergic diseases. 259 However, anaphylaxis may occur after vaccination in the absence of a history of allergic disease. Therefore, strategies for the prevention, diagnosis, and treatment of severe allergic reactions and a list of recommended medications and equipment for vaccine centers were provided in the EAACI statement to minimize the risk of allergic reactions to COVID‐19 vaccines. 259 Anaphylaxis induced by COVID‐19 vaccines is rare but may be more severe in older people due to comorbidities and polypharmacy. 264 Intramuscular injection of adrenaline remains the first‐line therapy for anaphylaxis in older people. 264 Modified dosing or alternative vaccines were recommended by EAACI for those with confirmed reactions to COVID‐19 vaccines. 259 Herein, we provide a flow chart for the treatment of allergic reactions to COVID‐19 vaccines. This flow chart is modified based on EAACI recommendation and other consensus 259 , 265 , 266 (Figure 4).
FIGURE 4.
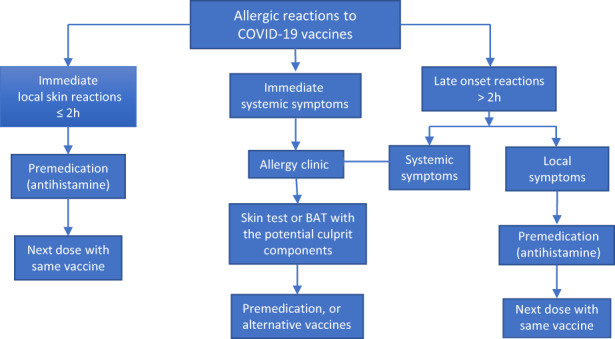
Flow chart for the management of individuals with allergic reactions to COVID‐19 vaccines. Allergic reactions to COVID‐19 vaccine can be divided into immediate and late‐onset reactions according to the symptom onset time after injection. Premedication such as antihistamines can be used for those with immediate or late onset local reactions before the next dose of vaccine. Those with systemic symptoms are recommended to be assessed with skin test or basophil activation test (BAT) in an allergy clinic and then treated with premedication or alternative vaccines. 259 , 265 , 266
9. PERSPECTIVES AND CONCLUSIONS
Anthropogenic activities, climate change, and global population movement set a perfect environment for new outbreaks of zoonotic pathogens. Using the knowledge and experience gained from the COVID‐19 pandemic, there is an urgent need to develop novel strategies to predict and prevent the emergence and transmission of novel pathogens. 267 Effective surveillance of new SARS‐CoV‐2 variants and reporting their transmissibility and rate of breakthrough infection is warranted to assist international policymaking. 268 The trajectory of COVID‐19 needs to be closely monitored to further our understanding of the immunity waning, antigen drifting, and re‐entries from zoonotic reservoirs. 269 As we deepen our knowledge of COVID‐19, vaccination strategies should be updated and revised, such as frequent administration of booster doses. The continuously mutating virus warrants the development of novel vaccines targeting current variant sequences. 270 The impacts of COVID‐19 on allergen sensitization and the incidence of allergic disease are still unknown and need to be investigated by the society of allergy and clinical immunology.
In conclusion, similarly to other respiratory viruses, full eradication of COVID‐19 is not on the horizon. Novel strategies should be developed for the prevention and management of this disease, particularly for patients at high risk of severe disease and to prevent MIS‐C and long COVID. The emergence of new VOC and global vaccinations efforts have substantially changed the clinical and immunological profiles of COVID‐19.
ACKNOWLEDGEMENTS
This work was supported by Scientific Research Initiative Funds of Shanxi Bethune Hosital.
CONFLICT OF INTEREST
C. A. Akdis has received research grants from the Swiss National Science Foundation, European Union (EU CURE, EU Syn‐Air‐G), European Union, Novartis Research Institutes, (Basel, Switzerland), Stanford University (Redwood City, Calif), and SciBase (Stockholm, Sweden); is the Co‐Chair for EAACI Guidelines on Environmental Science in Allergic diseases and Asthma; is on the Advisory Boards of Sanofi/Regeneron, Stanford University Sean Parker Asthma Allergy Center, Novartis, GlaxoSmithKline, Bristol‐Myers Squibb (London) and SciBase; and is the Editor‐in‐Chief of Allergy. M. Akdis has received research grants from Swiss National science Foundation, Bern; research grant from the Stanford University; Leading House for the Latin American Region, Seed Money Grant. She is in the Scientific Advisory Board member of Stanford University‐Sean Parker Asthma Allergy Center, CA; Advisory Board member of LEO Foundation Skin Immunology Research Center, Copenhagen; and Scientific Co‐Chair of World allergy Congress (WAC) Istanbul, 2022. The other authors have none to declare.
Zhang H‐p, Sun Y‐l, Wang Y‐f, et al. Recent developments in the immunopathology of COVID‐19. Allergy. 2023;78:369‐388. doi: 10.1111/all.15593
REFERENCES
- 1. Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020;75(7):1730‐1741. [DOI] [PubMed] [Google Scholar]
- 2. Riggioni C, Comberiati P, Giovannini M, et al. A compendium answering 150 questions on COVID‐19 and SARS‐CoV‐2. Allergy. 2020;75(10):2503‐2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wrapp D, Wang N, Corbett KS, et al. Cryo‐EM structure of the 2019‐nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260‐1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280 e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS‐CoV‐2 and COVID‐19. Nat Rev Microbiol. 2021;19(3):141‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Daly JL, Simonetti B, Klein K, et al. Neuropilin‐1 is a host factor for SARS‐CoV‐2 infection. Science. 2020;370(6518):861‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johnson BA, Xie X, Bailey AL, et al. Loss of furin cleavage site attenuates SARS‐CoV‐2 pathogenesis. Nature. 2021;591(7849):293‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Radzikowska U, Ding M, Tan G, et al. Distribution of ACE2, CD147, CD26, and other SARS‐CoV‐2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID‐19 risk factors. Allergy. 2020;75(11):2829‐2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lin DY, Gu Y, Wheeler B, et al. Effectiveness of Covid‐19 vaccines over a 9‐month period in North Carolina. N Engl J Med. 2022;386(10):933‐941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thompson MG, Stenehjem E, Grannis S, et al. Effectiveness of Covid‐19 vaccines in ambulatory and inpatient care settings. N Engl J Med. 2021;385(15):1355‐1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hacisuleyman E, Hale C, Saito Y, et al. Vaccine breakthrough infections with SARS‐CoV‐2 variants. N Engl J Med. 2021;384(23):2212‐2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Servellita V, Syed AM, Morris MK, et al. Neutralizing immunity in vaccine breakthrough infections from the SARS‐CoV‐2 Omicron and Delta variants. Cell. 2022;185(9):1539‐1548. e1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qiu C, Cui C, Hautefort C, et al. Olfactory and gustatory dysfunction as an early identifier of COVID‐19 in adults and children: an international multicenter study. Otolaryngol Head Neck Surg. 2020;163(4):714‐721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hagemann J, Onorato GL, Jutel M, et al. Differentiation of COVID‐19 signs and symptoms from allergic rhinitis and common cold: an ARIA‐EAACI‐GA(2) LEN consensus. Allergy. 2021;76(8):2354‐2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marzano AV, Cassano N, Genovese G, Moltrasio C, Vena GA. Cutaneous manifestations in patients with COVID‐19: a preliminary review of an emerging issue. Br J Dermatol. 2020;183(3):431‐442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tan SW, Tam YC, Oh CC. Skin manifestations of COVID‐19: a worldwide review. JAAD Int. 2021;2:119‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ranard BL, Megjhani M, Terilli K, et al. Identification of endotypes of hospitalized COVID‐19 patients. Front Med (Lausanne). 2021;8:770343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gutierrez‐Gutierrez B, Del Toro MD, Borobia AM, et al. Identification and validation of clinical phenotypes with prognostic implications in patients admitted to hospital with COVID‐19: a multicentre cohort study. Lancet Infect Dis. 2021;21(6):783‐792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nalbandian A, Sehgal K, Gupta A, et al. Post‐acute COVID‐19 syndrome. Nat Med. 2021;27(4):601‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nabavi N. Long covid: how to define it and how to manage it. BMJ. 2020;370:m3489. [DOI] [PubMed] [Google Scholar]
- 22. Huang L, Yao Q, Gu X, et al. 1‐year outcomes in hospital survivors with COVID‐19: a longitudinal cohort study. Lancet. 2021;398(10302):747‐758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lu X, Zhang L, Du H, et al. SARS‐CoV‐2 infection in children. N Engl J Med. 2020;382(17):1663‐1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383(4):334‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chou J, Thomas PG, Randolph AG. Immunology of SARS‐CoV‐2 infection in children. Nat Immunol. 2022;23(2):177‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McArdle AJ, Vito O, Patel H, et al. Treatment of multisystem inflammatory syndrome in children. N Engl J Med. 2021;385(1):11‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Son MBF, Murray N, Friedman K, et al. Multisystem inflammatory syndrome in children – initial therapy and outcomes. N Engl J Med. 2021;385(1):23‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rodino KG, Smith KP, Pettengill MA. Novel assays for molecular detection of severe acute respiratory syndrome coronavirus 2. Clin Lab Med. 2022;42(2):299‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mekonnen D, Mengist HM, Derbie A, et al. Diagnostic accuracy of serological tests and kinetics of severe acute respiratory syndrome coronavirus 2 antibody: a systematic review and meta‐analysis. Rev Med Virol. 2021;31(3):e2181. [DOI] [PubMed] [Google Scholar]
- 30. Chiereghin A, Zagari RM, Galli S, et al. Recent advances in the evaluation of serological assays for the diagnosis of SARS‐CoV‐2 infection and COVID‐19. Front Public Health. 2020;8:620222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Garcia‐Finana M, Buchan IE. Rapid antigen testing in COVID‐19 responses. Science. 2021;372(6542):571‐572. [DOI] [PubMed] [Google Scholar]
- 32. Long QX, Tang XJ, Shi QL, et al. Clinical and immunological assessment of asymptomatic SARS‐CoV‐2 infections. Nat Med. 2020;26(8):1200‐1204. [DOI] [PubMed] [Google Scholar]
- 33. Han MS, Choi EH, Chang SH, et al. Clinical characteristics and viral RNA detection in children with coronavirus disease 2019 in the Republic of Korea. JAMA Pediatr. 2021;175(1):73‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Radermecker C, Detrembleur N, Guiot J, et al. Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID‐19. J Exp Med. 2020;217(12), e20201012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Veras FP, Pontelli MC, Silva CM, et al. SARS‐CoV‐2‐triggered neutrophil extracellular traps mediate COVID‐19 pathology. J Exp Med. 2020;217(12), e20201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang JJ, Dong X, Liu GH, Gao YD. Risk and protective factors for COVID‐19 morbidity, severity, and mortality. Clin Rev Allergy Immunol. 2022. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gao YD, Ding M, Dong X, et al. Risk factors for severe and critically ill COVID‐19 patients: a review. Allergy. 2021;76(2):428‐455. [DOI] [PubMed] [Google Scholar]
- 38. Katzenschlager S, Zimmer AJ, Gottschalk C, et al. Can we predict the severe course of COVID‐19 – a systematic review and meta‐analysis of indicators of clinical outcome? PLoS One. 2021;16(7):e0255154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Du R, Tsougenis ED, Ho JWK, et al. Machine learning application for the prediction of SARS‐CoV‐2 infection using blood tests and chest radiograph. Sci Rep. 2021;11(1):14250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Styrzynski F, Zhakparov D, Schmid M, et al. Machine learning successfully detects COVID‐19 patients prior to PCR results and predicts their survival based on standard laboratory parameters. SSRN. 2022;Preprint. [DOI] [PMC free article] [PubMed]
- 41. Haldane V, De Foo C, Abdalla SM, et al. Health systems resilience in managing the COVID‐19 pandemic: lessons from 28 countries. Nat Med. 2021;27(6):964‐980. [DOI] [PubMed] [Google Scholar]
- 42. Escobar LE, Molina‐Cruz A, Barillas‐Mury C. BCG vaccine protection from severe coronavirus disease 2019 (COVID‐19). Proc Natl Acad Sci USA. 2020;117(30):17720‐17726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Miyasaka M. Is BCG vaccination causally related to reduced COVID‐19 mortality? EMBO Mol Med. 2020;12(6):e12661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Giamarellos‐Bourboulis EJ, Tsilika M, Moorlag S, et al. Activate: randomized clinical trial of BCG vaccination against infection in the elderly. Cell. 2020;183(2):315‐323 e319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Netea MG, Giamarellos‐Bourboulis EJ, Dominguez‐Andres J, et al. Trained immunity: a tool for reducing susceptibility to and the severity of SARS‐CoV‐2 infection. Cell. 2020;181(5):969‐977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lundberg L, Bygdell M, Stukat von Feilitzen G, et al. Recent MMR vaccination in health care workers and Covid‐19: a test negative case‐control study. Vaccine. 2021;39(32):4414‐4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gold JE, Baumgartl WH, Okyay RA, et al. Analysis of measles‐mumps‐rubella (MMR) titers of recovered COVID‐19 patients. mBio. 2020;11(6):e02628‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ozdemir O. Measles‐mumps‐rubella vaccine and COVID‐19 relationship. mBio. 2020;11(5):e01832‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Patel AB, Verma A. Nasal ACE2 levels and COVID‐19 in children. JAMA. 2020;323(23):2386‐2387. [DOI] [PubMed] [Google Scholar]
- 50. Muus C, Luecken MD, Eraslan G, et al. Single‐cell meta‐analysis of SARS‐CoV‐2 entry genes across tissues and demographics. Nat Med. 2021;27(3):546‐559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kimura H, Francisco D, Conway M, et al. Type 2 inflammation modulates ACE2 and TMPRSS2 in airway epithelial cells. J Allergy Clin Immunol. 2020;146(1):80‐88. e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Morrison CB, Edwards CE, Shaffer KM, et al. SARS‐CoV‐2 infection of airway cells causes intense viral and cell shedding, two spreading mechanisms affected by IL‐13. Proc Natl Acad Sci USA. 2022;119(16):e2119680119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bonser LR, Eckalbar WL, Rodriguez L, et al. The type 2 asthma mediator IL‐13 inhibits severe acute respiratory syndrome coronavirus 2 infection of bronchial epithelium. Am J Respir Cell Mol Biol. 2022;66(4):391‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stocker N, Radzikowska U, Wawrzyniak P, et al. Regulation of mRNA transcripts, protein isoforms, glycosylation and spatial localization of ACE2 and other SARS‐CoV‐2‐associated molecules in human airway epithelium upon viral infection and type 2 inflammation. bioRxiv. 2022; preprint.
- 55. Xu Y, Gao R, Zhu G, et al. Genetic variation of allergic disease is associated with the susceptibility to COVID‐19. J Infect. 2022;84(5):e92‐e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ren J, Pang W, Luo Y, et al. Impact of allergic rhinitis and asthma on COVID‐19 infection, hospitalization, and mortality. J Allergy Clin Immunol Pract. 2022;10(1):124‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Marin C, Hummel T, Liu Z, Mullol J. Chronic rhinosinusitis and COVID‐19. J Allergy Clin Immunol Pract. 2022;10(6):1423‐1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Takabayashi T, Yoshida K, Imoto Y, Schleimer RP, Fujieda S. Regulation of the expression of SARS‐CoV‐2 receptor angiotensin‐converting enzyme 2 in nasal mucosa. Am J Rhinol Allergy. 2022;36(1):115‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Marin C, Tubita V, Langdon C, et al. ACE2 downregulation in olfactory mucosa: eosinophilic rhinosinusitis as COVID‐19 protective factor? Allergy. 2021;76(9):2904‐2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ziegler CGK, Allon SJ, Nyquist SK, et al. SARS‐CoV‐2 receptor ACE2 is an interferon‐stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181(5):1016‐1035 e1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Blume C, Jackson CL, Spalluto CM, et al. A novel ACE2 isoform is expressed in human respiratory epithelia and is upregulated in response to interferons and RNA respiratory virus infection. Nat Genet. 2021;53(2):205‐214. [DOI] [PubMed] [Google Scholar]
- 62. Baker JR, Mahdi M, Nicolau DV Jr, et al. Early Th2 inflammation in the upper respiratory mucosa as a predictor of severe COVID‐19 and modulation by early treatment with inhaled corticosteroids: a mechanistic analysis. Lancet Respir Med. 2022;10(6):545‐556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Donlan AN, Sutherland TE, Marie C, et al. IL‐13 is a driver of COVID‐19 severity. JCI Insight. 2021;6(15):e150107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sasson J, Donlan AN, Ma JZ, et al. Safety and efficacy of dupilumab for the treatment of hospitalized patients with moderate to severe coronavirus disease 2019: a phase 2a trial. Open Forum Infect Dis. 2022;9(8):ofac343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator‐free in patients with moderate or severe acute respiratory distress syndrome and COVID‐19: the CoDEX randomized clinical trial. JAMA. 2020;324(13):1307‐1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid‐19. N Engl J Med. 2021;384(8):693‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sinha S, Rosin NL, Arora R, et al. Dexamethasone modulates immature neutrophils and interferon programming in severe COVID‐19. Nat Med. 2022;28(1):201‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Peters MC, Sajuthi S, Deford P, et al. COVID‐19‐related genes in sputum cells in asthma. Relationship to demographic features and corticosteroids. Am J Respir Crit Care Med. 2020;202(1):83‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Milne S, Li X, Yang CX, et al. Inhaled corticosteroids downregulate SARS‐CoV‐2‐related genes in COPD: results from a randomised controlled trial. Eur Respir J. 2021;58(1):2100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ramakrishnan S, Nicolau DV Jr, Langford B, et al. Inhaled budesonide in the treatment of early COVID‐19 (STOIC): a phase 2, open‐label, randomised controlled trial. Lancet Respir Med. 2021;9(7):763‐772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yu LM, Bafadhel M, Dorward J, et al. Inhaled budesonide for COVID‐19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open‐label, adaptive platform trial. Lancet. 2021;398(10303):843‐855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chang YC, Yang CF, Chen YF, et al. A siRNA targets and inhibits a broad range of SARS‐CoV‐2 infections including Delta variant. EMBO Mol Med. 2022;14(4):e15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhang H, Zhu W, Jin Q, et al. Inhalable nanocatchers for SARS‐CoV‐2 inhibition. Proc Natl Acad Sci USA. 2021;118(29):e2102957118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Planas D, Saunders N, Maes P, et al. Considerable escape of SARS‐CoV‐2 Omicron to antibody neutralization. Nature. 2022;602(7898):671‐675. [DOI] [PubMed] [Google Scholar]
- 75. Karim SSA, Karim QA. Omicron SARS‐CoV‐2 variant: a new chapter in the COVID‐19 pandemic. Lancet. 2021;398(10317):2126‐2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bergwerk M, Gonen T, Lustig Y, et al. Covid‐19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385(16):1474‐1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kousathanas A, Pairo‐Castineira E, Rawlik K, et al. Whole genome sequencing reveals host factors underlying critical Covid‐19. Nature. 2022:97‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sawadogo W, Tsegaye M, Gizaw A, Adera T. Overweight and obesity as risk factors for COVID‐19‐associated hospitalisations and death: systematic review and meta‐analysis. BMJ Nutr Prev Health. 2022;5(1):10‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Buonafine CP, Paiatto BNM, Leal FB, et al. High prevalence of SARS‐CoV‐2 infection among symptomatic healthcare workers in a large university tertiary hospital in Sao Paulo, Brazil. BMC Infect Dis. 2020;20(1):917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Karampoor S, Hesamizadeh K, Maleki F, et al. A possible pathogenic correlation between neutrophil elastase (NE) enzyme and inflammation in the pathogenesis of coronavirus disease 2019 (COVID‐19). Int Immunopharmacol. 2021;100:108137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bouchard BA, Colovos C, Lawson MA, et al. Increased histone‐DNA complexes and endothelial‐dependent thrombin generation in severe COVID‐19. Vascul Pharmacol. 2022;142:106950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Arunachalam PS, Wimmers F, Mok CKP, et al. Systems biological assessment of immunity to mild versus severe COVID‐19 infection in humans. Science. 2020;369(6508):1210‐1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhang Q, Bastard P, Effort CHG, Cobat A, Casanova JL. Human genetic and immunological determinants of critical COVID‐19 pneumonia. Nature. 2022;603(7902):587‐598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Liu N, Zhang T, Ma L, et al. The impact of ABO blood group on COVID‐19 infection risk and mortality: a systematic review and meta‐analysis. Blood Rev. 2021;48:100785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature. 2020;584(7821):430‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Woodby B, Arnold MM, Valacchi G. SARS‐CoV‐2 infection, COVID‐19 pathogenesis, and exposure to air pollution: what is the connection? Ann N Y Acad Sci. 2021;1486(1):15‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Fiorito S, Soligo M, Gao Y, Ogulur I, Akdis CA, Bonini S. Is epithelial barrier hypothesis the key to understanding the higher incidence and excess mortality during COVID‐19 pandemic? The case of Northern Italy. Allergy. 2022;77(5):1408‐1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Invernizzi R, Lloyd CM, Molyneaux PL. Respiratory microbiome and epithelial interactions shape immunity in the lungs. Immunology. 2020;160(2):171‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Annesi‐Maesano I, Maesano CN, D'Amato M, D'Amato G. Pros and cons for the role of air pollution on COVID‐19 development. Allergy. 2021;76(8):2647‐2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Aghapour M, Ubags ND, Bruder D, et al. Role of air pollutants in airway epithelial barrier dysfunction in asthma and COPD. Eur Respir Rev. 2022;31(163):210112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wang B, Chen H, Chan YL, Oliver BG. Is there an association between the level of ambient air pollution and COVID‐19? Am J Physiol Lung Cell Mol Physiol. 2020;319(3):L416‐L421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hammer MS, van Donkelaar A, Martin RV, et al. Effects of COVID‐19 lockdowns on fine particulate matter concentrations. Sci Adv. 2021;7(26):eabg7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Damialis A, Gilles S, Sofiev M, et al. Higher airborne pollen concentrations correlated with increased SARS‐CoV‐2 infection rates, as evidenced from 31 countries across the globe. Proc Natl Acad Sci USA. 2021;118(12):e2019034118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Travaglio M, Yu Y, Popovic R, Selley L, Leal NS, Martins LM. Links between air pollution and COVID‐19 in England. Environ Pollut. 2021;268(Pt A):115859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bozack A, Pierre S, DeFelice N, et al. Long‐term air pollution exposure and COVID‐19 mortality: a patient‐level analysis from New York City. Am J Respir Crit Care Med. 2022;205(6):651‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Dales R, Blanco‐Vidal C, Romero‐Meza R, Schoen S, Lukina A, Cakmak S. The association between air pollution and COVID‐19 related mortality in Santiago, Chile: a daily time series analysis. Environ Res. 2021;198:111284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Koelle K, Martin MA, Antia R, Lopman B, Dean NE. The changing epidemiology of SARS‐CoV‐2. Science. 2022;375(6585):1116‐1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Starr TN, Greaney AJ, Hilton SK, et al. Deep mutational scanning of SARS‐CoV‐2 receptor binding domain reveals constraints on folding and ACE2 binding. Cell. 2020;182(5):1295‐1310. e1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Davies NG, Abbott S, Barnard RC, et al. Estimated transmissibility and impact of SARS‐CoV‐2 lineage B.1.1.7 in England. Science. 2021;372(6538):eabg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Volz E, Mishra S, Chand M, et al. Assessing transmissibility of SARS‐CoV‐2 lineage B.1.1.7 in England. Nature. 2021;593(7858):266‐269. [DOI] [PubMed] [Google Scholar]
- 101. Dhar MS, Marwal R, Vs R, et al. Genomic characterization and epidemiology of an emerging SARS‐CoV‐2 variant in Delhi, India. Science. 2021;374(6570):995‐999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Mlcochova P, Kemp SA, Dhar MS, et al. SARS‐CoV‐2 B.1.617.2 Delta variant replication and immune evasion. Nature. 2021;599(7883):114‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Viana R, Moyo S, Amoako DG, et al. Rapid epidemic expansion of the SARS‐CoV‐2 Omicron variant in southern Africa. Nature. 2022;603(7902):679‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Balloux F, Tan C, Swadling L, et al. The past, current and future epidemiological dynamic of SARS‐CoV‐2. Oxf Open Immunol. 2022;3(1):iqac003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS‐CoV‐2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399(10332):1303‐1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Meng B, Abdullahi A, Ferreira I, et al. Altered TMPRSS2 usage by SARS‐CoV‐2 Omicron impacts infectivity and fusogenicity. Nature. 2022;603(7902):706‐714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Hui KPY, Ho JCW, Cheung MC, et al. SARS‐CoV‐2 Omicron variant replication in human bronchus and lung ex vivo. Nature. 2022;603(7902):715‐720. [DOI] [PubMed] [Google Scholar]
- 108. Tegally H, Moir M, Everatt J, et al. Emergence of SARS‐CoV‐2 Omicron lineages BA.4 and BA.5 in South Africa. Nat Med. 2022;28(9):1785‐1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wang Y, Chen R, Hu F, et al. Transmission, viral kinetics and clinical characteristics of the emergent SARS‐CoV‐2 Delta VOC in Guangzhou, China. EClinicalMedicine. 2021;40:101129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Callaway E. What Omicron's BA.4 and BA.5 variants mean for the pandemic. Nature. 2022;606(7916):848‐849. [DOI] [PubMed] [Google Scholar]
- 111. Phan T, Boes S, McCullough M, et al. First detection of SARS‐CoV‐2 Omicron BA.4 variant in Western Pennsylvania, United States. J Med Virol. 2022;94(9):4053‐4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Jian F, Yu Y, Song W, et al. Further humoral immunity evasion of emerging SARS‐CoV‐2 BA.4 and BA.5 subvariants. Lancet Infect Dis. 2022;22(11):1535‐1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Cao Y, Yisimayi A, Jian F, et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature. 2022;608(7923):593‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Tuekprakhon A, Nutalai R, Dijokaite‐Guraliuc A, et al. Antibody escape of SARS‐CoV‐2 Omicron BA.4 and BA.5 from vaccine and BA.1 serum. Cell. 2022;185(14):2422‐2433 e2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hachmann NP, Miller J, Collier AY, et al. Neutralization escape by SARS‐CoV‐2 Omicron subvariants BA.2.12.1, BA.4, and BA.5. N Engl J Med. 2022;387(1):86‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Jacobs SE, Lamson DM, St George K, Walsh TJ. Human rhinoviruses. Clin Microbiol Rev. 2013;26(1):135‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Neugebauer F, Bergs S, Liebert UG, Honemann M. Human rhinoviruses in pediatric patients in a Tertiary Care Hospital in Germany: molecular epidemiology and clinical significance. Viruses. 2022;14(8):1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Peteranderl C, Herold S, Schmoldt C. Human influenza virus infections. Semin Respir Crit Care Med. 2016;37(4):487‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Petrova VN, Russell CA. The evolution of seasonal influenza viruses. Nat Rev Microbiol. 2018;16(1):47‐60. [DOI] [PubMed] [Google Scholar]
- 120. Hirabara SM, Serdan TDA, Gorjao R, et al. SARS‐COV‐2 variants: differences and potential of immune evasion. Front Cell Infect Microbiol. 2021;11:781429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zhan Y, Yin H, Yin JY. B.1.617.2 (Delta) variant of SARS‐CoV‐2: features, transmission and potential strategies. Int J Biol Sci. 2022;18(5):1844‐1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Le TTB, Vasanthakumaran T, Thi Hien HN, et al. SARS‐CoV‐2 Omicron and its current known unknowns: a narrative review. Rev Med Virol. 2022;e2398. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Dhawan M, Saied AA, Emran TB, Choudhary OP. Emergence of Omicron variant's sublineages BA.4 and BA.5: risks assessment and possible countermeasures. New Microbes New Infect. 2022;48:100997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Tallei TE, Alhumaid S, AlMusa Z, et al. Update on the Omicron sub‐variants BA.4 and BA.5. Rev Med Virol. 2022;e2391. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Morris CP, Eldesouki RE, Sachithanandham J, et al. Omicron subvariants: clinical, laboratory, and cell culture characterization. medRxiv. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Basnet S, Palmenberg AC, Gern JE. Rhinoviruses and their receptors. Chest. 2019;155(5):1018‐1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Gillespie L, Roosendahl P, Ng WC, Brooks AG, Reading PC, Londrigan SL. Endocytic function is critical for influenza A virus infection via DC‐SIGN and L‐SIGN. Sci Rep. 2016;6:19428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Suzuki Y, Nagao Y, Kato H, et al. Human influenza A virus hemagglutinin distinguishes sialyloligosaccharides in membrane‐associated gangliosides as its receptor which mediates the adsorption and fusion processes of virus infection. Specificity for oligosaccharides and sialic acids and the sequence to which sialic acid is attached. J Biol Chem. 1986;261(36):17057‐17061. [PubMed] [Google Scholar]
- 129. Lessler J, Reich NG, Brookmeyer R, Perl TM, Nelson KE, Cummings DA. Incubation periods of acute respiratory viral infections: a systematic review. Lancet Infect Dis. 2009;9(5):291‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Goller KV, Moritz J, Ziemann J, et al. Differences in clinical presentations of Omicron infections with the lineages BA.2 and BA.5 in Mecklenburg‐Western Pomerania, Germany, between April and July 2022. Viruses. 2022;14(9):2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Gao YD, Agache I, Akdis M, et al. The effect of allergy and asthma as a comorbidity on the susceptibility and outcomes of COVID‐19. Int Immunol. 2022;34(4):177‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Bloom CI, Cullinan P, Wedzicha JA. Asthma phenotypes and COVID‐19 risk: a population‐based observational study. Am J Respir Crit Care Med. 2022;205(1):36‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Zein JG, Strauss R, Attaway AH, et al. Eosinophilia is associated with improved COVID‐19 outcomes in inhaled corticosteroid‐treated patients. J Allergy Clin Immunol Pract. 2022;10(3):742‐750 e714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Warner JO, Warner JA, Munblit D. Hypotheses to explain the associations between asthma and the consequences of COVID‐19 infection. Clin Exp Allergy. 2022;52(1):7‐9. [DOI] [PubMed] [Google Scholar]
- 135. Eggert LE, He Z, Collins W, et al. Asthma phenotypes, associated comorbidities, and long‐term symptoms in COVID‐19. Allergy. 2022;77(1):173‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Conway FM, Bloom CI, Shah PL. Susceptibility of patients with airways disease to SARS‐CoV‐2 infection. Am J Respir Crit Care Med. 2022;206(6):696‐703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Carr TF, Kraft M. Asthma and atopy in COVID‐19: 2021 updates. J Allergy Clin Immunol. 2022;149(2):562‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Adir Y, Saliba W, Beurnier A, Humbert M. Asthma and COVID‐19: an update. Eur Respir Rev. 2021;30(162):210152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Gaietto K, Freeman MC, DiCicco LA, et al. Asthma as a risk factor for hospitalization in children with COVID‐19: a nested case‐control study. Pediatr Allergy Immunol. 2022;33(1):e13696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Han X, Xu J, Hou H, Yang H, Wang Y. Significant association of pre‐existing asthma with an increased risk for ICU admission among COVID‐19 patients: evidence based on a meta‐analysis. J Infect. 2022;84(3):418‐467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Izquierdo JL, Soriano JB. Biologics may have a beneficial effect in asthma patients with COVID‐19. Eur Respir J. 2021;58(2):2101076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Gill MA, Liu AH, Calatroni A, et al. Enhanced plasmacytoid dendritic cell antiviral responses after omalizumab. J Allergy Clin Immunol. 2018;141(5):1735‐1743 e1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Jackson DJ, Busse WW, Bacharier LB, et al. Association of respiratory allergy, asthma, and expression of the SARS‐CoV‐2 receptor ACE2. J Allergy Clin Immunol. 2020;146(1):203‐206 e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Palmon PA, Jackson DJ, Denlinger LC. COVID‐19 infections and asthma. J Allergy Clin Immunol Pract. 2022;10(3):658‐663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Radzikowska U, Eljaszewicz A, Tan G, et al. Rhinovirus‐induced epithelial RIG‐I inflammasome activation suppresses antiviral immunity and promotes inflammatory responses in virus‐induced asthma exacerbations and COVID‐19. MedRxiv. 2021;Preprint.
- 146. Skevaki C, Karsonova A, Karaulov A, et al. SARS‐CoV‐2 infection and COVID‐19 in asthmatics: a complex relationship. Nat Rev Immunol. 2021;21(4):202‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Montani D, Savale L, Noel N, et al. Post‐acute COVID‐19 syndrome. Eur Respir Rev. 2022;31(163):210185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Phetsouphanh C, Darley DR, Wilson DB, et al. Immunological dysfunction persists for 8 months following initial mild‐to‐moderate SARS‐CoV‐2 infection. Nat Immunol. 2022;23(2):210‐216. [DOI] [PubMed] [Google Scholar]
- 149. Weinstock LB, Brook JB, Walters AS, Goris A, Afrin LB, Molderings GJ. Mast cell activation symptoms are prevalent in long‐COVID. Int J Infect Dis. 2021;112:217‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Plassmeyer M, Alpan O, Corley MJ, et al. Caspases and therapeutic potential of caspase inhibitors in moderate‐severe SARS‐CoV‐2 infection and long COVID. Allergy. 2022;77(1):118‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Knight JS, Caricchio R, Casanova JL, et al. The intersection of COVID‐19 and autoimmunity. J Clin Invest. 2021;131(24):e154886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Cheon IS, Li C, Son YM, et al. Immune signatures underlying post‐acute COVID‐19 lung sequelae. Sci Immunol. 2021;6(65):eabk1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Mehandru S, Merad M. Pathological sequelae of long‐haul COVID. Nat Immunol. 2022;23(2):194‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Merad M, Blish CA, Sallusto F, Iwasaki A. The immunology and immunopathology of COVID‐19. Science. 2022;375(6585):1122‐1127. [DOI] [PubMed] [Google Scholar]
- 155. Sumi T, Harada K. Immune response to SARS‐CoV‐2 in severe disease and long COVID‐19. iScience. 2022;25(8):104723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Yong SJ. Long COVID or post‐COVID‐19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis (Lond). 2021;53(10):737‐754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Raveendran AV, Jayadevan R, Sashidharan S. Long COVID: an overview. Diabetes Metab Syndr. 2021;15(3):869‐875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Hayes LD, Ingram J, Sculthorpe NF. More than 100 persistent symptoms of SARS‐CoV‐2 (long COVID): a scoping review. Front Med (Lausanne). 2021;8:750378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Desai AD, Lavelle M, Boursiquot BC, Wan EY. Long‐term complications of COVID‐19. Am J Physiol Cell Physiol. 2022;322(1):C1‐C11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Raman B, Bluemke DA, Luscher TF, Neubauer S. Long COVID: post‐acute sequelae of COVID‐19 with a cardiovascular focus. Eur Heart J. 2022;43(11):1157‐1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Kayaaslan B, Eser F, Kalem AK, et al. Post‐COVID syndrome: a single‐center questionnaire study on 1007 participants recovered from COVID‐19. J Med Virol. 2021;93(12):6566‐6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Azkur AK, Akdis M, Azkur D, et al. Immune response to SARS‐CoV‐2 and mechanisms of immunopathological changes in COVID‐19. Allergy. 2020;75(7):1564‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Sokolowska M, Lukasik ZM, Agache I, et al. Immunology of COVID‐19: mechanisms, clinical outcome, diagnostics, and perspectives – a report of the European Academy of Allergy and Clinical Immunology (EAACI). Allergy. 2020;75(10):2445‐2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Blanco‐Melo D, Nilsson‐Payant BE, Liu WC, et al. Imbalanced host response to SARS‐CoV‐2 drives development of COVID‐19. Cell. 2020;181(5):1036‐1045 e1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Maggi E, Azzarone BG, Canonica GW, Moretta L. What we know and still ignore on COVID‐19 immune pathogenesis and a proposal based on the experience of allergic disorders. Allergy. 2022;77(4):1114‐1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Khanmohammadi S, Rezaei N. Role of Toll‐like receptors in the pathogenesis of COVID‐19. J Med Virol. 2021;93(5):2735‐2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Yamada T, Sato S, Sotoyama Y, et al. RIG‐I triggers a signaling‐abortive anti‐SARS‐CoV‐2 defense in human lung cells. Nat Immunol. 2021;22(7):820‐828. [DOI] [PubMed] [Google Scholar]
- 168. Thorne LG, Reuschl AK, Zuliani‐Alvarez L, et al. SARS‐CoV‐2 sensing by RIG‐I and MDA5 links epithelial infection to macrophage inflammation. EMBO J. 2021;40(15):e107826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Yin X, Riva L, Pu Y, et al. MDA5 governs the innate immune response to SARS‐CoV‐2 in lung epithelial cells. Cell Rep. 2021;34(2):108628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Rodrigues TS, de Sa KSG, Ishimoto AY, et al. Inflammasomes are activated in response to SARS‐CoV‐2 infection and are associated with COVID‐19 severity in patients. J Exp Med. 2021;218(3):e20201707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Rydyznski Moderbacher C, Ramirez SI, Dan JM, et al. Antigen‐specific adaptive immunity to SARS‐CoV‐2 in acute COVID‐19 and associations with age and disease severity. Cell. 2020;183(4):996‐1012. e1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Galati D, Zanotta S, Capitelli L, Bocchino M. A bird's eye view on the role of dendritic cells in SARS‐CoV‐2 infection: perspectives for immune‐based vaccines. Allergy. 2022;77(1):100‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID‐19 patients. Science. 2020;369(6504):718‐724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Bastard P, Rosen LB, Zhang Q, et al. Auto‐antibodies against type I IFNs in patients with life‐threatening COVID‐19. Science. 2020;370(65):eabd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Wang EY, Mao T, Klein J, et al. Diverse functional autoantibodies in patients with COVID‐19. Nature. 2021;595(7866):283‐288. [DOI] [PubMed] [Google Scholar]
- 176. Garcia‐Beltran WF, Lam EC, Astudillo MG, et al. COVID‐19‐neutralizing antibodies predict disease severity and survival. Cell. 2021;184(2):476‐488. e411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Ungar B, Lavin L, Golant AK, et al. The impact of dupilumab treatment on severe acute respiratory syndrome coronavirus 2‐coronavirus disease 2019 antibody responses in patients with atopic dermatitis. Ann Allergy Asthma Immunol. 2022;128(6):734‐736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Sananez I, Raiden SC, Algieri SC, et al. A poor and delayed anti‐SARS‐CoV2 IgG response is associated to severe COVID‐19 in children. EBioMedicine. 2021;72:103615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Ardicli O, Carli T, Satitsuksanoa P, et al. Exposure to avian coronavirus vaccines is associated with increased levels of SARS‐CoV‐2‐cross‐reactive antibodies. Allergy. 2022;7(12):3648‐3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Rodda LB, Netland J, Shehata L, et al. Functional SARS‐CoV‐2‐specific immune memory persists after mild COVID‐19. Cell. 2021;184(1):169‐183. e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Sokal A, Chappert P, Barba‐Spaeth G, et al. Maturation and persistence of the anti‐SARS‐CoV‐2 memory B cell response. Cell. 2021;184(5):1201‐1213. e1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Pattini S, Malizia V, Travaglini A, et al. Telemedicine for allergic patients during COVID‐19. Pediatr Allergy Immunol. 2020;31(Suppl 26):102‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Bousquet J, Akdis CA, Jutel M, et al. Intranasal corticosteroids in allergic rhinitis in COVID‐19 infected patients: an ARIA‐EAACI statement. Allergy. 2020;75(10):2440‐2444. [DOI] [PubMed] [Google Scholar]
- 184. Strauss R, Jawhari N, Attaway AH, et al. Intranasal corticosteroids are associated with better outcomes in coronavirus disease 2019. J Allergy Clin Immunol Pract. 2021;9(11):3934‐3940 e3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Webster KE, O'Byrne L, MacKeith S, Philpott C, Hopkins C, Burton MJ. Interventions for the prevention of persistent post‐COVID‐19 olfactory dysfunction. Cochrane Database Syst Rev. 2021;7(7):CD013877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Morisada MV, Hwang J, Gill AS, Wilson MD, Strong EB, Steele TO. Telemedicine, patient satisfaction, and chronic rhinosinusitis care in the era of COVID‐19. Am J Rhinol Allergy. 2021;35(4):494‐499. [DOI] [PubMed] [Google Scholar]
- 187. Beaney T, Salman D, Samee T, Mak V. Assessment and management of adults with asthma during the covid‐19 pandemic. BMJ. 2020;369:m2092. [DOI] [PubMed] [Google Scholar]
- 188. Ari A. Use of aerosolised medications at home for COVID‐19. Lancet Respir Med. 2020;8(8):754‐756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Klimek L, Pfaar O, Worm M, et al. Allergen immunotherapy in the current COVID‐19 pandemic: a position paper of AeDA, ARIA, EAACI, DGAKI and GPA: position paper of the German ARIA Group (A) in cooperation with the Austrian ARIA Group (B), the Swiss ARIA Group (C), German Society for Applied Allergology (AEDA) (D), German Society for Allergology and Clinical Immunology (DGAKI) (E), Society for Pediatric Allergology (GPA) (F) in cooperation with AG Clinical Immunology, Allergology and Environmental Medicine of the DGHNO‐KHC (G) and the European Academy of Allergy and Clinical Immunology (EAACI) (H). Allergol Select. 2020;4:44‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Compalati E, Erlewyn‐Lajeunesse M, Runa Ali F, et al. Allergen immunotherapy in the era of SARS‐CoV‐2. J Investig Allergol Clin Immunol. 2020;30(6):459‐461. [DOI] [PubMed] [Google Scholar]
- 191. Martinez‐Lopez A, Cuenca‐Barrales C, Montero‐Vilchez T, Molina‐Leyva A, Arias‐Santiago S. Review of adverse cutaneous reactions of pharmacologic interventions for COVID‐19: a guide for the dermatologist. J Am Acad Dermatol. 2020;83(6):1738‐1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Dordal Culla MT, Herrera‐Lasso Regas V, Marti‐Garrido J, Rodriguez Cumplido D, Vazquez‐Revuelta P, Lleonart BR. Treating COVID‐19: review of drug hypersensitivity reactions. J Investig Allergol Clin Immunol. 2020;30(6):385‐399. [DOI] [PubMed] [Google Scholar]
- 193. Manjaly Thomas ZR, Leuppi‐Taegtmeyer A, Jamiolkowski D, et al. Emerging treatments in COVID‐19: adverse drug reactions including drug hypersensitivities. J Allergy Clin Immunol. 2020;146(4):786‐789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Virant FS, Randolph C, Nanda A, et al. Pulmonary procedures during the COVD‐19 pandemic: a workgroup report of the American Academy of Allergy, Asthma, and Immunology (AAAAI) asthma diagnosis and treatment (ADT) interest section. J Allergy Clin Immunol Pract. 2022;10(6):1474‐1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. McGowan A, Laveneziana P, Bayat S, et al. International consensus on lung function testing during the COVID‐19 pandemic and beyond. ERJ Open Res. 2022;8(1):00602‐2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Oreskovic NM, Kinane TB, Aryee E, Kuhlthau KA, Perrin JM. The unexpected risks of COVID‐19 on asthma control in children. J Allergy Clin Immunol Pract. 2020;8(8):2489‐2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Hernandez N, Sanclemente G, Tamayo L, Lopez A, Seidel A, Colombian Atopic Dermatitis Research Group M . Atopic dermatitis in the COVID‐19 era: results from a web‐based survey. World Allergy Organ J. 2021;14(8):100571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. El‐Qushayri AE, Mahmoud MA, Salman S, Sarsik S, Nardone B. Dupilumab therapy in atopic dermatitis is safe during COVID‐19 infection era: a systematic review and meta‐analysis of 1611 patients. Dermatol Ther. 2022;35(6):e15476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Pfaar O, Hamelmann E, Klimek L, et al. Allergen immunotherapy during the COVID‐19 pandemic‐A survey of the German Society for Allergy and Clinical Immunology. Clin Transl Allergy. 2022;12(3):e12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Rodriguez Del Rio P, Caimmi D, Rico P, et al. Real‐life report of allergen immunotherapy management during the COVID‐19 outbreak in France and Spain. Clin Exp Allergy. 2022;52(1):167‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Akca HM, Tuncer KK. Evaluation of urticaria patients before and during the period of the COVID‐19 pandemic: a retrospective study. Dermatol Ther. 2021;34(2):e14800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Kulu H, Atasoy M, Ozyurt K, et al. The COVID‐19 pandemic affects male patients with chronic spontaneous urticaria more than female patients. Front Immunol. 2021;12:722406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Mitamura Y, Schulz D, Oro S, et al. Cutaneous and systemic hyperinflammation drives maculopapular drug exanthema in severely ill COVID‐19 patients. Allergy. 2022;77(2):595‐608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Boyton RJ, Altmann DM. Risk of SARS‐CoV‐2 reinfection after natural infection. Lancet. 2021;397(10280):1161‐1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Excler JL, Saville M, Berkley S, Kim JH. Vaccine development for emerging infectious diseases. Nat Med. 2021;27(4):591‐600. [DOI] [PubMed] [Google Scholar]
- 206. Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF. Progress of the COVID‐19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol. 2021;21(10):626‐636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Lipsitch M, Krammer F, Regev‐Yochay G, Lustig Y, Balicer RD. SARS‐CoV‐2 breakthrough infections in vaccinated individuals: measurement, causes and impact. Nat Rev Immunol. 2022;22(1):57‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Gattinger P, Niespodziana K, Stiasny K, et al. Neutralization of SARS‐CoV‐2 requires antibodies against conformational receptor‐binding domain epitopes. Allergy. 2022;77(1):230‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Barton MI, MacGowan SA, Kutuzov MA, Dushek O, Barton GJ, van der Merwe PA. Effects of common mutations in the SARS‐CoV‐2 Spike RBD and its ligand, the human ACE2 receptor on binding affinity and kinetics. Elife. 2021;10:e70658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Vogel M, Augusto G, Chang X, et al. Molecular definition of severe acute respiratory syndrome coronavirus 2 receptor‐binding domain mutations: receptor affinity versus neutralization of receptor interaction. Allergy. 2022;77(1):143‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Weisblum Y, Schmidt F, Zhang F, et al. Escape from neutralizing antibodies by SARS‐CoV‐2 spike protein variants. Elife. 2020;9:e61312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Cele S, Jackson L, Khoury DS, et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. 2022;602(7898):654‐656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Carreno JM, Alshammary H, Tcheou J, et al. Activity of convalescent and vaccine serum against SARS‐CoV‐2 Omicron. Nature. 2022;602(7898):682‐688. [DOI] [PubMed] [Google Scholar]
- 214. Kremsner PG, Ahuad Guerrero RA, Arana‐Arri E, et al. Efficacy and safety of the CVnCoV SARS‐CoV‐2 mRNA vaccine candidate in ten countries in Europe and Latin America (HERALD): a randomised, observer‐blinded, placebo‐controlled, phase 2b/3 trial. Lancet Infect Dis. 2022;22(3):329‐340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Gebre MS, Rauch S, Roth N, et al. Optimization of non‐coding regions for a non‐modified mRNA COVID‐19 vaccine. Nature. 2022;601(7893):410‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Badano MN, Sabbione F, Keitelman I, et al. Humoral response to the BBIBP‐CorV vaccine over time in healthcare workers with or without exposure to SARS‐CoV‐2. Mol Immunol. 2022;143:94‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Kaewborisuth C, Wanitchang A, Koonpaew S, et al. Chimeric virus‐like particle‐based COVID‐19 vaccine confers strong protection against SARS‐CoV‐2 viremia in K18‐hACE2 mice. Vaccines (Basel). 2022;10(5):786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Volkmann A, Koopman G, Mooij P, et al. A capsid virus‐like particle‐based SARS‐CoV‐2 vaccine induces high levels of antibodies and protects rhesus macaques. Front Immunol. 2022;13:857440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Gattinger P, Kratzer B, Tulaeva I, et al. Vaccine based on folded RBD‐PreS fusion protein with potential to induce sterilizing immunity to SARS‐CoV‐2 variants. Allergy. 2022;77(8):2431‐2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Alu A, Chen L, Lei H, Wei Y, Tian X, Wei X. Intranasal COVID‐19 vaccines: From bench to bed. EBioMedicine. 2022;76:103841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Perez P, Astorgano D, Albericio G, et al. Intranasal administration of a single dose of MVA‐based vaccine candidates against COVID‐19 induced local and systemic immune responses and protects mice from a lethal SARS‐CoV‐2 infection. Front Immunol. 2022;13:995235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Waltz E. China and India approve nasal COVID vaccines – are they a game changer? Nature. 2022;609(7927):450. [DOI] [PubMed] [Google Scholar]
- 223. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383(27):2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2021;384(5):403‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Corbett KS, Flynn B, Foulds KE, et al. Evaluation of the mRNA‐1273 vaccine against SARS‐CoV‐2 in nonhuman primates. N Engl J Med. 2020;383(16):1544‐1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Guebre‐Xabier M, Patel N, Tian JH, et al. NVX‐CoV2373 vaccine protects cynomolgus macaque upper and lower airways against SARS‐CoV‐2 challenge. Vaccine. 2020;38(50):7892‐7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Voysey M, Costa Clemens SA, Madhi SA, et al. Single‐dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV‐19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881‐891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector‐based heterologous prime‐boost COVID‐19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671‐681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single‐dose Ad26.COV2.S vaccine against Covid‐19. N Engl J Med. 2021;384(23):2187‐2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Bos R, Rutten L, van der Lubbe JEM, et al. Ad26 vector‐based COVID‐19 vaccine encoding a prefusion‐stabilized SARS‐CoV‐2 Spike immunogen induces potent humoral and cellular immune responses. NPJ Vaccines. 2020;5:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Mercado NB, Zahn R, Wegmann F, et al. Single‐shot Ad26 vaccine protects against SARS‐CoV‐2 in rhesus macaques. Nature. 2020;586(7830):583‐588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1–2a trial of Ad26.COV2.S Covid‐19 vaccine. N Engl J Med. 2021;384(19):1824‐1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Kim JH, Marks F, Clemens JD. Looking beyond COVID‐19 vaccine phase 3 trials. Nat Med. 2021;27(2):205‐211. [DOI] [PubMed] [Google Scholar]
- 234. Ella R, Reddy S, Jogdand H, et al. Safety and immunogenicity of an inactivated SARS‐CoV‐2 vaccine, BBV152: interim results from a double‐blind, randomised, multicentre, phase 2 trial, and 3‐month follow‐up of a double‐blind, randomised phase 1 trial. Lancet Infect Dis. 2021;21(7):950‐961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS‐CoV‐2 vaccine in healthy adults aged 18‐59 years: a randomised, double‐blind, placebo‐controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Wu Z, Hu Y, Xu M, et al. Safety, tolerability, and immunogenicity of an inactivated SARS‐CoV‐2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double‐blind, placebo‐controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(6):803‐812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Zhu FC, Li YH, Guan XH, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type‐5 vectored COVID‐19 vaccine: a dose‐escalation, open‐label, non‐randomised, first‐in‐human trial. Lancet. 2020;395(10240):1845‐1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Wu S, Huang J, Zhang Z, et al. Safety, tolerability, and immunogenicity of an aerosolised adenovirus type‐5 vector‐based COVID‐19 vaccine (Ad5‐nCoV) in adults: preliminary report of an open‐label and randomised phase 1 clinical trial. Lancet Infect Dis. 2021;21(12):1654‐1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Heath PT, Galiza EP, Baxter DN, et al. Safety and efficacy of NVX‐CoV2373 Covid‐19 vaccine. N Engl J Med. 2021;385(13):1172‐1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Keech C, Albert G, Cho I, et al. Phase 1–2 trial of a SARS‐CoV‐2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383(24):2320‐2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Falsey AR, Sobieszczyk ME, Hirsch I, et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV‐19) Covid‐19 vaccine. N Engl J Med. 2021;385(25):2348‐2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Bogdanov G, Bogdanov I, Kazandjieva J, Tsankov N. Cutaneous adverse effects of the available COVID‐19 vaccines. Clin Dermatol. 2021;39(3):523‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. de Vrieze J. Pfizer's vaccine raises allergy concerns. Science. 2021;371(6524):10‐11. [DOI] [PubMed] [Google Scholar]
- 244. Alhumaid S, Al Mutair A, Al Alawi Z, et al. Anaphylactic and nonanaphylactic reactions to SARS‐CoV‐2 vaccines: a systematic review and meta‐analysis. Allergy Asthma Clin Immunol. 2021;17(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Klein NP, Lewis N, Goddard K, et al. Surveillance for adverse events after COVID‐19 mRNA vaccination. JAMA. 2021;326(14):1390‐1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Maltezou HC, Anastassopoulou C, Hatziantoniou S, Poland GA, Tsakris A. Anaphylaxis rates associated with COVID‐19 vaccines are comparable to those of other vaccines. Vaccine. 2022;40(2):183‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Haaf P, Kuster GM, Mueller C, et al. The very low risk of myocarditis and pericarditis after mRNA COVID‐19 vaccination should not discourage vaccination. Swiss Med Wkly. 2021;151:w30087. [DOI] [PubMed] [Google Scholar]
- 248. Lau CL, Galea I. Risk‐benefit analysis of COVID‐19 vaccines – a neurological perspective. Nat Rev Neurol. 2022;18(2):69‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Bellanti JA. COVID‐19 vaccines and vaccine hesitancy: role of the allergist/immunologist in promotion of vaccine acceptance. Allergy Asthma Proc. 2021;42(5):386‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Barbaud A, Garvey LH, Arcolaci A, et al. Allergies and COVID‐19 vaccines: an ENDA/EAACI position paper. Allergy. 2022;77(8):2292‐2312. [DOI] [PubMed] [Google Scholar]
- 251. Garvey LH, Nasser S. Anaphylaxis to the first COVID‐19 vaccine: is polyethylene glycol (PEG) the culprit? Br J Anaesth. 2021;126(3):e106‐e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Turner PJ, Ansotegui IJ, Campbell DE, et al. COVID‐19 vaccine‐associated anaphylaxis: a statement of the World Allergy Organization Anaphylaxis Committee. World Allergy Organ J. 2021;14(2):100517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Stone CA Jr, Liu Y, Relling MV, et al. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J Allergy Clin Immunol Pract. 2019;7(5):1533‐1540. e1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Bigini P, Gobbi M, Bonati M, et al. The role and impact of polyethylene glycol on anaphylactic reactions to COVID‐19 nano‐vaccines. Nat Nanotechnol. 2021;16(11):1169‐1171. [DOI] [PubMed] [Google Scholar]
- 255. Troelnikov A, Perkins G, Yuson C, et al. Basophil reactivity to BNT162b2 is mediated by PEGylated lipid nanoparticles in patients with PEG allergy. J Allergy Clin Immunol. 2021;148(1):91‐95. [DOI] [PubMed] [Google Scholar]
- 256. Zhou ZH, Stone CA Jr, Jakubovic B, et al. Anti‐PEG IgE in anaphylaxis associated with polyethylene glycol. J Allergy Clin Immunol Pract. 2021;9(4):1731‐1733 e1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Turk VE. Anaphylaxis associated with the mRNA COVID‐19 vaccines: approach to allergy investigation. Clin Immunol. 2021;227:108748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Caballero ML, Quirce S. Excipients as potential agents of anaphylaxis in vaccines: analyzing the formulations of currently authorized COVID‐19 vaccines. J Investig Allergol Clin Immunol. 2021;31(1):92‐93. [DOI] [PubMed] [Google Scholar]
- 259. Sokolowska M, Eiwegger T, Ollert M, et al. EAACI statement on the diagnosis, management and prevention of severe allergic reactions to COVID‐19 vaccines. Allergy. 2021;76(6):1629‐1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Pitlick MM, Sitek AN, Kinate SA, Joshi AY, Park MA. Polyethylene glycol and polysorbate skin testing in the evaluation of coronavirus disease 2019 vaccine reactions: early report. Ann Allergy Asthma Immunol. 2021;126(6):735‐738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Warren CM, Snow TT, Lee AS, et al. Assessment of allergic and anaphylactic reactions to mRNA COVID‐19 vaccines with confirmatory testing in a US regional health system. JAMA Netw Open. 2021;4(9):e2125524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Wolfson AR, Robinson LB, Li L, et al. First‐dose mRNA COVID‐19 vaccine allergic reactions: limited role for excipient skin testing. J Allergy Clin Immunol Pract. 2021;9(9):3308‐3320. e3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Kohli‐Pamnani A, Zapata K, Gibson T, Kwittken PL. Coronavirus disease 2019 messenger RNA vaccine skin tests and serum histamine levels in allergic reactions. Ann Allergy Asthma Immunol. 2022;128(3):339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Aurich S, Dolle‐Bierke S, Francuzik W, et al. Anaphylaxis in elderly patients‐data from the European Anaphylaxis Registry. Front Immunol. 2019;10:750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Chiang V, Leung ASY, Au EYL, et al. Updated consensus statements on COVID‐19 Vaccine Allergy Safety in Hong Kong. Asia Pac Allergy. 2022;12(1):e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Greenhawt M, Abrams EM, Shaker M, et al. The risk of allergic reaction to SARS‐CoV‐2 vaccines and recommended evaluation and management: a systematic review, meta‐analysis, GRADE assessment, and international consensus approach. J Allergy Clin Immunol Pract. 2021;9(10):3546‐3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Albery GF, Becker DJ, Brierley L, et al. The science of the host‐virus network. Nat Microbiol. 2021;6(12):1483‐1492. [DOI] [PubMed] [Google Scholar]
- 268. Kucharski AJ, Cohen C. Effective surveillance of variants. Science. 2022;375(6587):1349‐1350. [DOI] [PubMed] [Google Scholar]
- 269. Telenti A, Arvin A, Corey L, et al. After the pandemic: perspectives on the future trajectory of COVID‐19. Nature. 2021;596(7873):495‐504. [DOI] [PubMed] [Google Scholar]
- 270. Altmann DM, Boyton RJ. COVID‐19 vaccination: the road ahead. Science. 2022;375(6585):1127‐1132. [DOI] [PubMed] [Google Scholar]


