Abstract
Mycotoxins pose significant risks to humans and livestock. In addition, contaminated food- and feedstuffs can only be discarded, leading to increased economic losses and potential ecological pollution. Mycotoxin removal and real-time toxin level monitoring are effective approaches to solve this problem. As a hot research hotspot, small peptides derived from phage display peptide libraries, combinatorial peptide libraries, and rational design approaches can act as coating antigens, competitive antigens, and anti-immune complexes in immunoassays for the detection of mycotoxins. Furthermore, as a potential approach to mycotoxin degradation, small peptides can mimic the natural enzyme catalytic site to construct artificial enzymes containing oxidoreductases, hydrolase, and lyase activities. In summary, with the advantages of mature synthesis protocols, diverse structures, and excellent biocompatibility, also sharing their chemical structure with natural proteins, small peptides are widely used for mycotoxin detection and artificial enzyme construction, which have promising applications in mycotoxin degradation. This paper mainly reviews the advances of small peptides in the detection of mycotoxins, the construction of peptide-based artificial enzymes, and their potential applications in mycotoxin control.
Keywords: small peptides, artificial enzymes, mycotoxin detection, mycotoxin removal, mycotoxin control
1. Introduction
Mycotoxins are the secondary metabolites produced by fungi during growth and have been a global concern for a long period. Many fungal species usually produce them as a defense against the changing environment, which mainly including Penicillium, Aspergillus, and Fusarium [1]. The most frequently encountered mycotoxins in agricultural products and foods include aflatoxins (AFs), ochratoxin A (OTA), deoxynivalenol (DON), zearalenone (ZEN or ZEA), fumonisins (FBs), T-2 toxin (T-2), HT-2 toxin (HT-2), ergot alkaloids (EAs), citrinin (CIT or CTN), and patulin (PAT) [2,3]. Because of their highly resistive characteristics, mycotoxins tend to remain in the food chain and affect a broad range of agricultural products and foods, including wheat, rice, maize, fruit, and other related processed foodstuffs [4]. Mycotoxins can impose acute or chronic effects on both humans and animals, leading to impaired immunity, endocrine abnormalities, and carcinogenic effects [5]. Many tend to co-occur with other sometimes structurally unrelated mycotoxins, which may bring multiplicative and synergistic effects to human and animal health [6,7]. Therefore, maximum levels (MLs) for mycotoxins have been set in agricultural products and foods by various countries and organizations (Table 1).
Table 1.
Maximum levels of mycotoxins in agricultural products and foods.
| Mycotoxin | Country * | Maximum Level (ML) (ug/kg) | ML in Infant and Young Children’s Food (µg/kg) |
|---|---|---|---|
| AFB1 | China | 5–20 | 0.5 |
| EU | AFB1: 2–12 AFB1 + AFB2 + AFG1 + AFG2: 4–15 |
0.1 | |
| U.S. | AFB1 + AFB2 + AFG1 + AFG2: 20 | - | |
| CAC | AFB1 + AFB2 + AFG1 + AFG2: 10–15 | - | |
| AFM1 | China | 0.5 | 0.5 |
| EU | 0.05 | 0.025 | |
| U.S. | 0.5 | - | |
| CAC | 0.5 | - | |
| OTA | China | 2–10 | - |
| EU | 2–80 | 0.5 | |
| CAC | 5 | - | |
| ZEN | China | 60 | - |
| EU | 50–400 | 20 | |
| DON | China | 1000 | - |
| EU | 500–1750 | 200 | |
| U.S. | 1000 | - | |
| CAC | 1000–2000 | 200 | |
| PAT | China | 50 | - |
| EU | 25–50 | 10 | |
| U.S. | 50 | - | |
| CAC | 50 | - | |
| FMs | EU | FB1 + FB2: 800–4000 | FB1 + FB2: 200 |
| U.S. | FB1 + FB2 + FB3: 2000–4000 | - | |
| CAC | FB1 + FB2: 2000–4000 | - | |
| CIT | EU | 2000 | - |
* Data obtained from: (1) The People’s Republic of China National Standard GB2761-2017; (2) European Union (EU): Regulations (EC) Nos. 2002/32/EC, 1881/2006, 2021/1399); (3) The United States (U.S.) Food and Drug Administration: Mycotoxins: toxins found in food infected by certain molds or fungi (Search date 2022.9.28); (4) Codex Alimentarius Commission (CAC): General standard for contaminants and toxins in food and feed cxs 193-1995 (Amended in 2019). AFB1: Aflatoxin B1; AFB2: Aflatoxin B2; AFG1: Aflatoxin G1; AFG2: Aflatoxin G2; FB1: Fumonisin B1; FB2: Fumonisin B2; FB3: Fumonisin B3.
Traditionally, analytical methods for mycotoxin detection can be divided into two categories: sophisticated instruments-based techniques for quantitative analysis and immunological techniques for the rapid real-time detection of bulk samples [8]. The instrumental analyses include high-performance liquid chromatography (HPLC), ultra-high liquid chromatography (UHPLC), and gas chromatography (GC) coupled with ultraviolet (UV), fluorescence (FLD), or mass spectrometric (MS) detection, allowing the largest range of mycotoxins to be determined with the highest sensitivity. However, these methods are only suitable for the analysis of mycotoxins in the laboratory not in real-time monitoring because of their expensive price, complicated sample preparation, time-consuming process, susceptible matrix interference, and the requirements of highly skilled analysts, which limit their wide application in resource-constrained regions. So, rapid methods that require only minimal sample preparation have been developed [9]. Most methods for rapid detection of mycotoxins are based on immunoassays [10]. Despite the possibility of expressing false-positive results or poor repeatability, the immunological method has the advantages of high specificity, high sensitivity, simple operation, and real-time detection, which make up for the above-mentioned shortcomings.
The enzyme-linked immunosorbent assay (ELISA) is the most frequently used technology for the rapid detection of mycotoxins [11]. The basic principle of the immunological assay is the reaction of antigens and antibodies. Therefore, it is important to prepare monoclonal antibodies that can specifically bind to small-molecule toxins in the ELISA method. As mycotoxins are small molecular haptens, mycotoxin-specific antigen preparation processes are complex, time-consuming, and labor-intensive, as well as having poor stability and high cost, which limits its wider application. Hence, chemically synthesized mycotoxin conjugates are inevitably introduced as coating or competitive antigens during the detection process. However, there are various drawbacks in the synthesis of mycotoxin conjugates, such as complex synthesis procedures, easy production of by-products, and the need to use poisonous mycotoxin standards that pose a threat to the safety of the detector and the environment. Thus, replacing mycotoxin conjugates with safe and renewable alternatives is essential for the development of eco-friendly immunoassays.
Small peptides can serve as competitors, inhibitors, mimotopes, drugs, reagents for affinity purification systems, and active elements in biosensors [12]. Recently, researchers found small peptides obtained from phages display peptide libraries can be used as biosensors for the detection of mycotoxins, and some of them are even more effective than antibodies [11]. Moreover, with the development of rational design technology, small peptides capable of having affinity to specific mycotoxins can be easily obtained by computer-aided design, screening, and solid-phase peptide synthesis [13]. Meanwhile, molecular modeling and simulation (MMS) methodologies capable of evaluating the performance of rationally designed peptide biosensors, with the potential to minimize biosensor development costs, improve product lifetime, and promote the multi-analyte detection of mycotoxins, are being developed [14]. According to the current reports, small peptides can act as coating antigens, competitive antigens, and anti-immune complexes in the detection of mycotoxins using immunoassays.
Although there are various methods to detect and even monitor mycotoxins in real time, contamination is still inevitable and remains a global problem [15]. Enzymes with high specificity, regioselectivity, and stereoselectivity can efficiently and specifically degrade specific mycotoxins such as AFB1, OTA, DON, and ZEN, and are becoming the most promising approach to address mycotoxin contamination after physical adsorption, chemical decomposition, and microbial detoxification [1,16]. However, few mycotoxin-degrading enzymes are applied commercially, and most enzymes are still being researched in the laboratory, because enzyme catalysis requires suitable conditions, usually effective under mild physiological conditions, but poorly tolerant to temperature, pH, and organic solvents, and easily losing catalytic activity. Therefore, it remains a challenging and meaningful mission to design a catalyst that can adapt to various complex and extreme industrial production environments, and also meet the requirements of catalyzing specific reactions. In recent years, with the research on the structure and catalytic mechanism of natural enzymes, artificial enzymes based on small peptides have become a research hot topic because of their promising break through the poor stability drawbacks of natural enzymes [17,18,19,20]. Despite the few relevant reports that are currently available [21,22], peptide-based artificial enzymes may provide a potential new approach for the removal of mycotoxins in the future.
Mycotoxin contamination not only harms human and animal health, but contaminated food and foodstuffs can only be discarded, which causes great economic loss and the risk of environmental pollution [23,24]. Accordingly, real-time on-site monitoring and post-contamination detoxification are the most effective solutions to mycotoxin contamination. Small peptides have various advantages, such as mature synthesis protocols, diverse structures, and excellent biocompatibility, and share the same chemical structure as proteins, which are widely used in biological, pharmaceutical, chemical, and other fields [25,26]. Meanwhile, with the application of small peptide-based immunological assays in mycotoxin detection and the development of peptide-based artificial enzymes with mycotoxin degradation potential, small peptides have promising applications in mycotoxin control [27,28]. This review mainly summarizes the feasible applications of small peptides in mycotoxin detection, peptide-based artificial enzymes, and their potential applications in mycotoxin removal, to provide new ideas for the detection and control of mycotoxins (Figure 1).
Figure 1.
Small peptides in mycotoxin detection and potential applications in mycotoxin degradation.
2. Design and Screening of Small Peptides
Peptide-based sensors and artificial enzymes have unique advantages and broad application prospects in mycotoxin control. To quickly obtain ideal small peptide sequences, it is crucial to develop convenient small peptide construction methods that can be applied to various scenarios. The main approaches currently used to design and screen small peptides with affinity to mycotoxins are phage display peptide libraries, artificial chemical synthesis, and rational design [29].
2.1. Phage Display Peptides
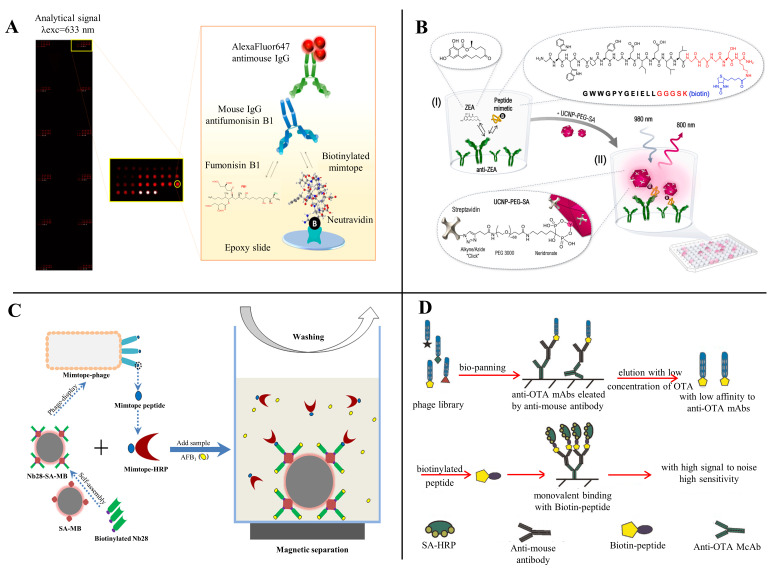
In 1985, Smith used genetic engineering to insert a foreign gene into the genome of filamentous bacteriophage to display the peptide encoded by the target gene as a fusion protein [30]. Subsequently, he cloned the synthesized oligonucleotide fragment with random sequence into filamentous bacteriophage to display one peptide on the surface of each bacteriophage particle after expression [31]. All these phages displaying different peptides constitute a library of phage display peptides, from which binding peptides to specific proteins were obtained by screening. Since it directly related the phage expression peptide to the coding gene, the DNA sequence can be easily obtained after amplification and cloning, thus establishing a random peptide library for phage surface display [32,33]. Therefore, phage display technology can display small peptides on the surface of phage rods, either on the backbone or on both ends, which can be achieved by the targeted insertion of a DNA sequence encoding foreign proteins or peptides [34]. Furthermore, the small peptides displayed on the phage surface can still recognize molecularly targeted binding sites.
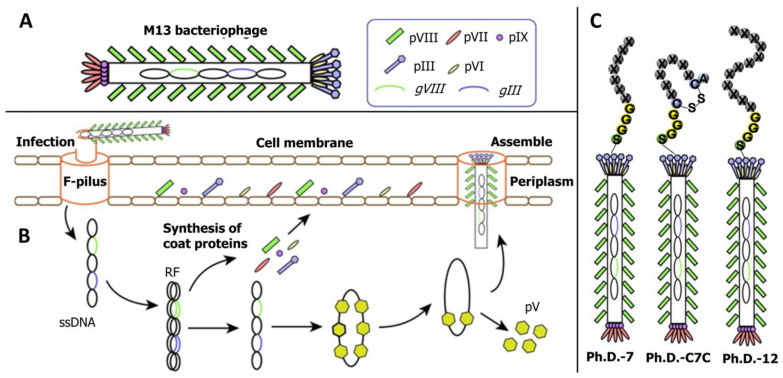
The phage display technology won the 2018 Nobel Prize in Chemistry, and is widely used for antigen–antibody library building, drug designing, vaccine research, pathogen detection, gene therapy, antigen epitope research, and cell signaling research. Although several phage display techniques have been developed, M13 filamentous phage is the most commonly used in screening small peptides corresponding to mycotoxins. M13 phage is an Escherichia coli-specific filamentous phage with a circular single-stranded DNA genome containing 11 genes, 5 of which are used to encode coat proteins (pIII, pVI, pVII, pVIII, and pIX), and its structure is illustrated in Figure 2A [34]. According to the location of the exogenous peptide chain insertion, the M13 phage display system could divide into pIII-mediated display and pVIII-mediated display. The process of filamentous phage adsorption, invasion, proliferation, and release is illustrated in Figure 2B [34]. The M13 phage can produce massive progenies in a short period without killing the host, which varies between the lytic and lysogenic phages. Currently, the main commercial phage display peptide library kits commonly used are loop-constrained heptapeptide library (Ph.D.-C7C), linear heptapeptide library (Ph.D.-7), and linear dodecapeptide library (Ph.D.-12) (Figure 2C). A random peptide library may display tens of millions of peptide epitopes, which can quickly make these phages bind to specific target molecules by bio-panning steps, and then the sequence of phage display peptide can be determined by gene sequence analysis; finally, the sequence of small peptides that has affinity with the target molecule can be obtained [35].
Figure 2.
The basic structure and life cycle of the M13 bacteriophage. (A) Structure of the M13 bacteriophage; (B) the life cycle of the M13 bacteriophage; (C) commercial M13 bacteriophage display peptide library. RF: Replicate form; pV: Phage protein.
2.2. Combinatorial Peptides
Combinatorial chemistry is a set of synthetic strategies and screening protocols that link building blocks, such as amino acids, nucleotides, and organic small molecules, in a combination to synthesize a chemical library containing a large number of compounds and screen them for compounds with some physical, chemical, or pharmacological activity [36,37]. The combinatorial peptide library technology originated from combinatorial chemistry, which has been widely used to screen novel affinity ligands that can be used as materials for affinity chromatography or as new receptors for sensors and biosensors [29,38]. In the combinatorial peptide library, 20 natural amino acids can be used as building blocks, and the size of the library is determined by the number of amino acids (n) that constitute a peptide of a specific length, which can be simply calculated as 20n. If a dipeptide library is constructed, there are 400 (202) combinations, a tripeptide library has 8000 (203) combinations, …, and an octapeptide will have 25,600,000,000 (208) combinations.
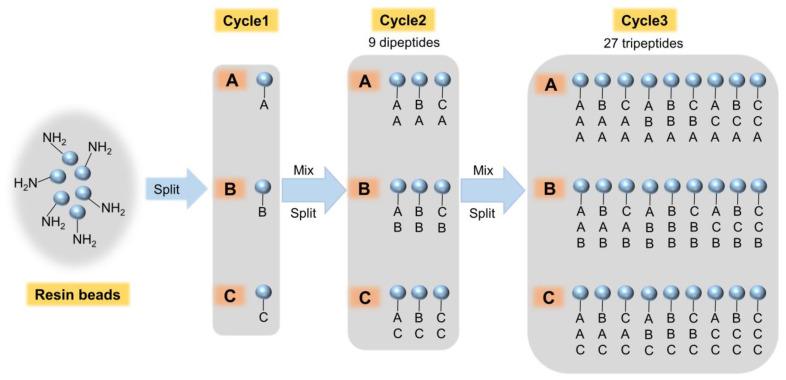
The phage display peptide library method is simple and economical, but this biological method is limited to peptides containing only L-amino acids. However, synthetic combinatorial libraries can accommodate D-amino acids, non-natural amino acids, and even organic molecules, which makes these methods highly general synthetic peptide libraries. The most commonly used method for constructing combinatorial peptide libraries is the one-bead one-compound library (OBOC) [38,39] (Figure 3). Lam et al. [40] first reported a pentapeptide library synthesized by resin beads (100–200 μm) as a carrier, in which only one pentapeptide is coupled to each resin bead. These pentapeptides can bind to the corresponding proteins, using specific proteins such as antibodies, ligands, and enzymes labeled with fluorescein or isotopes as probes. The specific pentapeptide can finally be screened and isolated from the peptide library based on these markers [40]. Giraudi et al. constructed a hexapeptide library by OBOC combinatorial synthesis and screened out a hexapeptide (SNLHPK), showing good affinity (Keq = 3.4 × 104 M−1) towards ochratoxin A (OTA). This hexapeptide can be used for the solid-phase extraction of OTA from wine and quantification of OTA in wine samples at concentration levels down to 0.10 μg/L [41]. The application of combinatorial chemistry in small-molecule drug screening is widespread [37,42]. Recently, research on DEL technology that combines DNA coding and combinatorial chemistry has shown explosive growth and has revolutionized small-molecule drug development, which may have broader use in mycotoxin detection [43].
Figure 3.
The one-bead one-compound combinatorial peptide library. A, B, and C represent any of the amino acids.
2.3. Rational Design Peptides
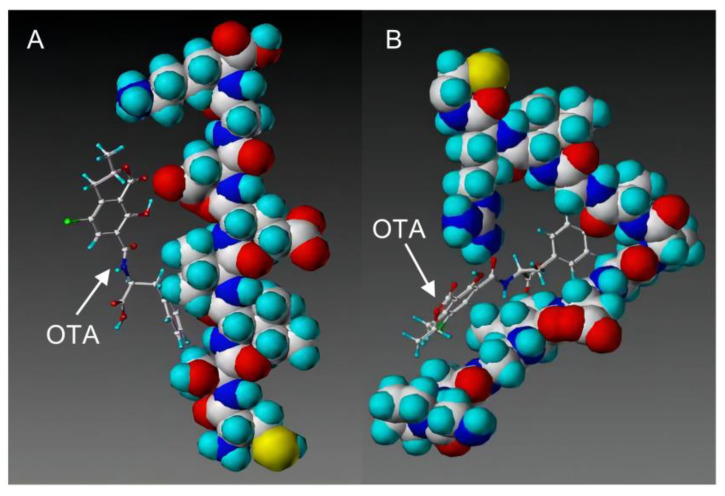
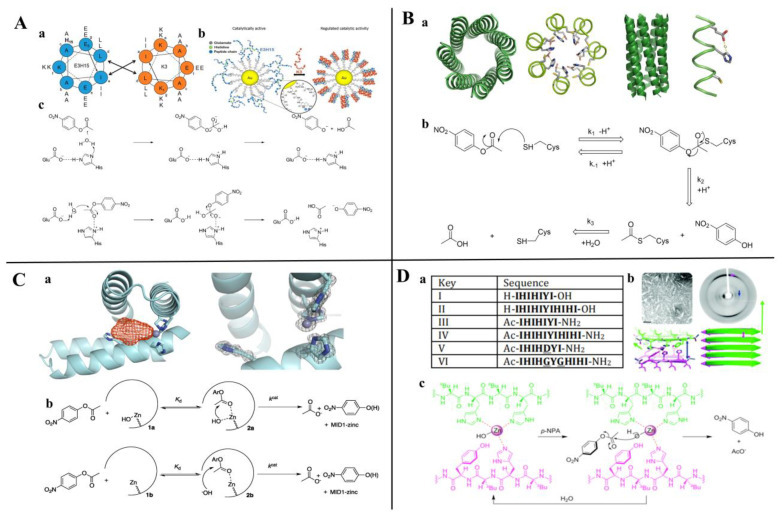
With the development of high-performance computing (HPC) and further knowledge of the structure and function of peptides, rational design strategies have achieved remarkable progress, and at present, we can accurately evaluate the three-dimensional structure of proteins based on the primary peptide sequences [44,45]. Therefore, there are increasingly small peptides synthesized based on rational design and generally divided into two categories. The first one is based on molecular modeling and docking techniques, and the small peptides are designed and screened by rational analysis. By evaluating the affinity of common amino acids with target molecules, the amino acids with higher affinity among them were selected to design potential small peptides with the help of software, and finally the best small peptide sequence was determined by artificial synthesis and experimental verification. Using this strategy, Parker and Heurich designed and screened small peptide sequences with specific affinity to AFB1 and OTA, respectively, and demonstrated through synthesis and analysis that these small peptides can be used in the rapid detection of AFB1 [46] and OTA [13,47] (Figure 4).
Figure 4.
Rationally designed peptide receptor binding with OTA ((A): GPAGIDGPAGIRC; (B): CSIVEDGL) [13]. Peptide sequences are represented as space-filled, and OTA and AFB1 as stick and ball structures.
As increasingly functional small peptides are mined and designed, there are various databases of small peptide sequences with different functions that can be easily retrieved. Therefore, another frequently rational design method for small peptides is to retrofit the already discovered small peptides. Although the affinity of the peptide synthesized by rational design is slightly imperfect, the peptide sequence can be optimized to improve its properties based on conformational relationships [48]. Currently, rationally designed small peptides are widely applied in the monitoring of disease biomarkers [49], pathogenic microbes [50], public hazardous [51], etc. Meanwhile, rationally designed peptides coupled with gold nanomaterials have gained` increasing interest in biosensors, cell imaging, drug delivery, and therapy, which have promising applications in medical and on-site detection [52].
3. Small Peptides in Mycotoxin Detection
Peptides have emerged as a promising approach to synthetic biomimetics [53]. The excellent properties of synthetic peptides in small-molecule contaminant (SMCs) recognition make them a potential alternative to antibodies and natural receptors in mycotoxin biosensor applications [54]. Small peptides show high-affinity binding to these small analytes and have stability, playing an increasingly important role in the rapid detection of mycotoxins [55]. Among immunologically based mycotoxin detection methods, peptides can serve as competing antigens, coating antigens, and anti-immune complexes (Table 2).
Table 2.
Small peptides in mycotoxin detection.
| Peptide Sequence | Peptide Screening Approach | Detection Principle | Peptide Function | Analyte | IC50/SC50 | Detection Limit | Linear Range | Reference |
|---|---|---|---|---|---|---|---|---|
| VYMNRKYYKCCK | Rational design | Peptide-based competitive ELISA | Coating antigen | OTA | 3.2 μg/L | 1.25 μg/L | 1.25–10 μg/L | [48] |
| IRPMVDP | M13 Ph.D.-7 | ELISA | Competing antigen | OTA | - | 150 pg/mL | 200–8000 pg/mL | [56] |
| AETYGFQLHAMK | M13 Ph.D.-12 | Phage Chemiluminescent ELISA | Competing antigen | OTA | 0.04 ng/mL | 0.005 ng/mL | 0.006–0.245 ng/mL | [57] |
| AEDRPFQLHLPV | M13 Ph.D.-12 | CLEIA | Coating antigen | OTA | 0.82 ng/mL | - | 0.31–2.17 ng/mL | [58] |
| AEDRPFQLHLPV | M13 Ph.D.-12 | Dot immunoassay | Coating antigen | OTA | - | 5.0 ng/mL | - | [58] |
| Biotin-KSGGGSNLHPK | Rational design | Dot-blot assay | Recognition element | OTA | - | 0.49 μg/kg | - | [59] |
| GMVQTIFGGGSK-Biotin | M13 Ph.D.-7 | Phage-free peptide ELISA | Competing antigen | OTA | 0.024 ng/mL | 0.001 ng/mL | 0.005–0.2 ng/mL | [60] |
| DLLWVPST | Phage display random linear 8-mer peptide library | Nc-MCLEIA | Anti-immune complex peptide | AFB1 | 0.089 ng/mL | 0.006 ng/mL | 0.019–0.407 ng/mL | [61] |
| YSWHEWYIPQLS | M13 Ph.D.-12 | MB-dcELISA | Competing antigen | AFB1 | 0.75 ng/mL | 0.13 ng/mL | 0.24–2.21 ng/mL | [62] |
| CVPSKPGLC | M13 Ph.D.-C7C | Bp-ELISA | Competing antigen | AFB1 | 0.92 ng/mL | 0.09 ng/mL | 0.23–3.36 ng/mL | [63] |
| CNVLPFDSIFRF | Rational design | Electrochemical immunosensor | Recognition element | AFB1 | - | 9.4 × 10−4 mg/L | 0.01–20 μg/L | [64] |
| ACPYPNHPYC | M13 Ph.D.-12 | Electrochemical immunosensor | Competing antigen | DON | 58.26 ng/mL | 0.07 g/mL | 0.1–10,000 pg/mL | [65] |
| AIRMIRIRTS | M13 Ph.D.-12 | D8-MBP ELISA | Coating antigen | DON | 57.98 ng/mL | 9.83 ng/mL | 11.32–286.77 ng/mL | [66] |
| ESYWATVPWTRH | M13 Ph.D.-12 | Phage-based dot-immunoassay | Coating antigen | ZEN | 1.8 ng/mL | 50 mg/kg | - | [67] |
| DAVILLM | M13 Ph.D.-7 | ELISA | Competing antigen | ZEN | - | 100 pg/mL | 100–10,000 pg/mL | [68] |
| DAVILLM | M13 Ph.D.-7 | PEC-ELISA | Competing antigen | ZEN | - | 10−6 ng/mL | 10−6–1 ng/mL | [69] |
| DAVILLM | M13 Ph.D.-7 | MSR-system | Competing antigen | ZEN | - | 1.06 × 10−7 ng/mL | 10−7–10−1 ng/mL | [70] |
| CMTTLFGEDC | Phage display random linear 8-mer peptide library | P-MCLEIA | Competing antigen | ZEN | 31.4 pg/mL | 4.3 pg/mL | 0.0086–0.1475 ng/mL | [71] |
| GWWGPYGEIELL | M13 Ph.D.-12 | Bioluminescent ELISA | Competing antigen | ZEN | 11.0 ng/mL | 4.2 ng/mL | 6.2–19.6 ng/mL | [72] |
| GWWGPYGEELLGGGSK-Biotin | M13 Ph.D.-12 | ULISA | Competing antigen | ZEN | 0.16 ng/mL | 0.02 ng/mL | 0.05–0.50 ng/mL | [73] |
| -- | Phage display random linear 12-mer peptide library | P-dcFLISA | Coating antigen | ZEN | 0.301 ng/ml | 0.023 ng/mL | 0.060–1.531 ng/ml | [74] |
| ACWELPTLACGGGS | M13 Ph.D.-C7C | ELISA | Coating antigen | FB1 | 6.06 ng/mL | 1.18 ng/mL | 1.77–20.73 ng/mL | [75] |
| NNAAMYSEMATD | M13 Ph.D.-12 | ELISA | Coating antigen | FB1 | 2.15 ng/mL | 0.32 ng/mL | - | [76] |
| TTLQMRSEMADD | M13 Ph.D.-12 | ELISA | Coating antigen | FB1 | 1.26 ng/mL | 0.21 ng/mL | - | [76] |
| VTPNDDTFDPFR | M13 Ph.D.-12 | ELISA | Competing antigen | FB1 | 37.1 ng/mL | 11.1 ng/mL | 0.13–25.6 ng/mL | [77] |
| VTPNDDTFDPFRGGGSK-Biotin | M13 Ph.D.-12 | MB-ELISA | Competing antigen | FB1 | 1.85 ng/mL | 0.029 ng/mL | 0.13–25.6 ng/mL | [78] |
| WLTPVGELV | Phage display random cyclic 8-, 9-, 10-mer peptide libraries | Competitive P-ELISA | Competing antigen | Clothianidin | 3.83 ng/mL | 1.11 ng/mL | 1.11–13.20 ng/mL | [79] |
| AVFTDQWWTG | Phage display random cyclic 8-, 9-, 10-mer peptide libraries | Noncompetitive P-ELISA | Anti-immune complex peptide | Clothianidin | 0.45 ng/mL | 0.26 ng/mL | 0.26–0.76 ng/mL | [79] |
| CTMHLSVYC | M13 Ph.D.-C7C | ELISA | Anti-immune complex peptide | Ethyl carbamate | 1.66 ng/mL | 0.54 ng/mL | 0.87–3.20 ng/mL | [80] |
| ((CSGLAEFMSC)2K)2KK-FITC | Rational design | Competitive FPIAs | Competing antigens | Benzothiostrobin | 19.71 ng/mL | 4.27 ng/mL | 4.27–129.42 ng/mL | [81] |
| ((CPDIWPTAWC)2K)2KK-FITC | Rational design | Noncompetitive FPIAs | Anti-immune complex peptide | Benzothiostrobin | 40.43 ng/mL | 9.27 ng/mL | 9.27–210.62 ng/mL | [81] |
| CFNGKDWLYC | Phage display random cyclic 8-mer peptide libraries | PHAIA | Coating antigen | PBA | 0.31 ng/mL | 0.05 ng/mL | 0.05–2.0 ng/mL | [82] |
OTA: Ochratoxin A; AFB1: Aflatoxin B1; DON: Deoxynivalenol; ZEN: Zearalenone; FB1: Fumonisin B1; PBA: Phenoxybenzoic acid.
3.1. Peptides as Competing Antigens
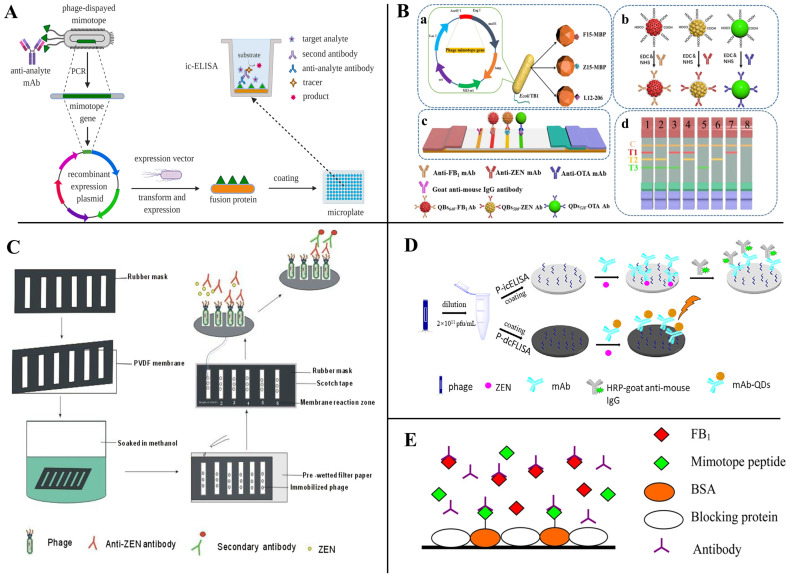
As stated earlier, competitive antigens are essential in competitive immunoassays, but the obtaining of specificity antigens always involves the use of mycotoxins. Peptides can serve as competing antigens and competitively bind with specific monoclonal antibody (mAb), which can avoid the use of poisonous mycotoxins [62,65]. In 1999, Yuan et al. [83] identified two phage-displayed mimotopes (SWGPFPF and SWGPLPF) and used them to detect DON firstly. Subsequently, mimotopes as competitive antigens for the detection of mycotoxins have been increasingly reported, involving OTA, AFB1, ZEN, FB1, and other mycotoxins [84]. Peltomaa et al. [77] reported the selection of a novel dodecapeptide (VTPNDDTFDPFR) from a 12-mer peptide library; then, a biotinylated synthetic derivative of this mimotope (VTPNDDTFDPFRGGGSK-Biotin) was used for the detection of FB1 by a competitive binding inhibition assay. Its 50% inhibitory concentration (IC50) was 37.1 ng/mL, with a detection limit (LOD) of 11.1 ng/mL, and a dynamic range from 17.3 to 79.6 ng/mL (Figure 5A). Recently, the authors used the same method to obtain a ZEN mimetic epitope peptide (GWWGPYGEIELL); the peptide was used to create fusion proteins with the bioluminescent Gaussia luciferase (GLuc) that were directly used as tracers for mycotoxin detection in a competitive immunoassay [72]. Meanwhile, using the same small peptide sequence developed a competitive upconversion-linked immunosorbent assay (ULISA) for ZEN monitoring with a LOD of 20 pg mL−1 [73] (Figure 5B).
Figure 5.
Peptides as competing antigens for mycotoxins detection. (A) FB1 detection; Reprinted with permission from Ref. [77]. 2017, Anal. Chem. (B) ZEN detection; Reprinted with permission from Ref. [73]. 2020, Biosens. Bioelectron. (C) AFB1 detection; Reprinted with permission from Ref. [62]. 2019, Talanta. (D) OTA detection; Reprinted with permission from Ref. [60]. 2016, Talanta.
Chen et al. [69] constructed a peptide@Tyr-RMC probe by selecting a mimotope peptide from a phage display library and labeling it a Tyr-RMC composite, which was used to develop a competitive ELISA for the ultrasensitive detection of ZEN. It has a linear range of 10−6–1.0 ng/mL, and a low detection limit of 10−6 ng/mL. Zhao et al. [62], using AFB1 as a model system, selected a mimotope (YSWHEWYIPQLS) from Ph.D-12 phage display peptide library, and the rapid magnetic-beads-based directed competitive ELISA (MB-dcELISA) was developed by mimotope ME17. The IC50 and LOD of the MB-dcELISA were 0.75 and 0.13 ng/mL, respectively, with a linear range of 0.24–2.21 ng/mL (Figure 5C). Zou et al. [60] obtained a mimotope from the commercial Ph.D.-7 phage display peptide library and connected it with biotin (GMVQTIF-GGGSK-biotin). The biotinylated 12-mer peptide was used as a competing antigen to develop a competitive peptide ELISA for OTA detection and showed a wide linear range of 0.005–0.2 ng/mL with the detection limit of 0.001 ng/mL. The IC50 was 0.024 ng/mL, which is approximately five times more sensitive as a competing antigen than the OTA-HRP conjugates used in the conventional ELISA (Figure 5D).
3.2. Peptides as Coating Antigens
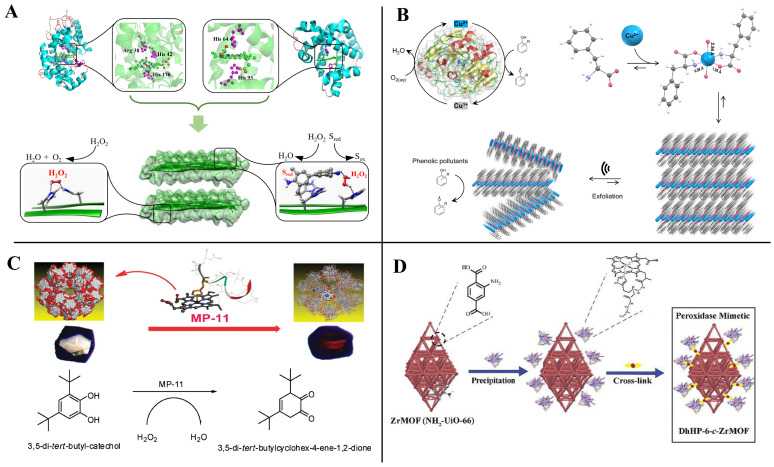
Peptides also can serve as competing antigens in immunological-based mycotoxin detection. He et al. [67] selected a phage display dodecapeptide (ESYWATVPWTRH) as a substitute for coating antigens and applied it for the rapid detection of ZEN by dot-immunoassay (Figure 6C). The cut-off level for detecting ZEN in cereal samples was 50 mg/kg and the results can be accomplished within 10 min. With the phage display peptide library technology, Zhou et al. [74] reported a phage mimotope-based direct competitive fluorescence immunosorbent assay (P-dcFLISA); the IC50 of P-dcFLISA was 0.301 ng/mL, which was lower than the phage-based indirect competitive enzyme-linked immunosorbent assay (P-icELISA) under the same conditions (Figure 6D). The LOD and detection range of P-dcFLISA was 0.023 ng/mL and 0.060–1.531 ng/mL, respectively. However, there is no corresponding amino acid sequence information in the article. Liu et al. [75], utilizing mimotope peptide-bovine serum albumin conjugate as a coating antigen, developed a peptide ELISA for detecting FB1, in which the IC50 and LOD were 6.06 ng/mL and 1.18 ng/mL, respectively (Figure 6E). Except for phage display peptides, small synthetic peptides by rational design are also used as coating antigens for mycotoxin detection. Bazin et al. [85] designed an OTA-binding peptide (VYMNRKYYKCCK) derived from an oxidoreductase and developed a peptide-based competitive enzyme-linked immunosorbent assay (peptide-based competitive ELISA) in which the peptide was the coating antigen.
Figure 6.
(A) Schematic of mimotope fusion protein as coating antigens for mycotoxin detection; Reprinted with permission from Ref. [11]. 2021, Food Chem. (B,C) Mimotope fusion protein as coating antigens for mycotoxin detection; (a) Biological expression strategy of peptide-MBP fusion protein, (b) Fabrication process of the prepared QDs/QBs-mAb probes, (c) schematic illustration of the tricolor mICA, (d) schematic illustration for the interpretation of test results; Reprinted with permission from Ref. [67], 2014, Food Control, and Ref. [86], 2020, J. Agric. Food. Chem. (D,E) Peptides as coating antigens for mycotoxin detection; Reprinted with permission from [74], 2022, J. Food Saf., and Ref. [75], 2013, J. Agric. Food. Chem.
Mimotope-based fusion proteins can also be used as synthetic coating antigens for the detection of mycotoxins [11] (Figure 6A). Xu et al. [76], using FB1 as a model hapten, screened two mimotopes (F1: NNAAMYSEMATD, F15: TTLQMRSEMADD) that have affinity to the anti-FB1 antibody from a 12-mer peptide library and developed a new method for the development of a sensitive and environmentally friendly immunoassay for FB1 based on the peptide–MBP (maltose-binding protein) fusion protein. Quantitative immunoassay for FB1 using F1-MBP and F15-MBP showed the LOD was 0.32 and 0.21 ng/mL, respectively, and the IC50 of the assay was 2.15 and 1.26 ng/mL, which was 10 times more sensitive than the conventional FB1-BSA conjugate-based ELISA. Meanwhile, using the same strategy, they also constructed a small peptide–MBP fusion protein-coated antigen that can be used to detect OTA and DON with good sensitivity and stability [58,66]. Recently, to monitor the co-contamination of mycotoxins in agricultural products and foods, Yan et al. [86] developed a mimotope–MBP fusion protein-based multiplex immunochromatographic assay (mICA) that can quickly and simultaneously detect FB1, ZEN, and OTA without the building-up process of mycotoxin conjugates. The LOD of peptide–MBP-based mICA for FB1, ZEN, and OTA were 0.25, 3.0, and 0.5 ng/mL, respectively (Figure 6B).
3.3. Anti-Immune Complex Peptides
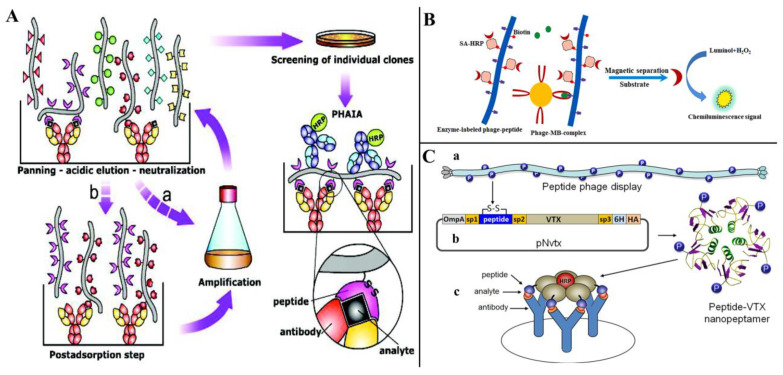
As small molecule contaminants (SMCs), mycotoxins usually only have one immunological binding site and are not suitable for detection by conventional sandwich non-competitive immunoassays [87]. Alternatively, the development of non-competitive immunoassays using specific recognizers for the immune complex of anti-SMC antibodies is a feasible strategy. Anti-immune complex peptide (AIcP) is a peptide that specifically binds to immune complexes, neither antibodies nor antigen monomers [88]. Biopanning the phage display random peptides library using antigen–antibody conjugates allows the screening of peptides that can bind specifically to immune complexes and can be used to establish non-competitive immunoassays [89]. Unlike mimotopes, anti-immune complex peptides recognize only complexes of antigens and antibodies and do not bind to antibodies or antigens alone, and because of this dual site recognition pattern, anti-immune complex peptides are often thought to improve the specificity of assays.
González-Sapienza’s team achieved promising results in the detection of pesticide contaminants using anti-immune complex peptides, demonstrating the advantages of the method in the detection of SMCs [82,89,90,91], and the process of panning anti-immune complex phages and establishing the phage anti-immune complex assay (PHAIA) is depicted in Figure 7A [90]. Zou et al. [61], using AFB1 and anti-AFB1 nanobody conjugates as the immune complex, screened anti-immune complex peptides from a phage display random linear 8-mer peptide library; the best binding peptide was biotinylated and coupled with horseradish peroxidase-labeled streptavidin (SA-HRP) for developing the magnetic-phage anti-immune complex immunoassay (MPHAIA) detection of AFB1 (Figure 7B). Phage-peptide p13 (DLLWVPST)-based MPHAIA, showing the lowest SC50 (50% signal saturation concentration) value (0.12 ng/mL) and the highest ODmax/SC50 ratio (12.75), was selected for further study. Under the ultimate condition, the LOD was 0.006 ng/mL, with a linear range of 0.019–0.407 ng/mL. Lassabe et al. [92] used a verotoxin (VTX) of Escherichia coli as a scaffold for multivalent display anti-immunocomplex peptides, and, among these peptides, ICX09m (CLEAPNVEAC) showed 10-fold increased sensitivity and excellent recovery than the competitive ELISA for clomazone detection (Figure 7C).
Figure 7.
Peptides as anti-immune complexes for small-molecule contaminant detection (SMC). (A) The process of panning anti-immune complex phages and establishing phage anti-immune complex assay (PHAIA). Reprinted with permission from Ref. [90]. 2007, Anal. Chem. (B) Noncompetitive magnetic-phage anti-immune complex immunoassay (Nc-MCLEIA) for AFB1 detection. Reprinted with permission from Ref. [61]. 2022, Food Chem. (C) Multivalent display anti-immunocomplex peptides on verotoxin for clomazone detection [92]; (a). The anti-immuncomplex peptide selected from phage libraries, (b). Peptide coding sequence cloned into the pNvtx vector and fused to the VTX gene, (c). Recombinant nanopeptamer conjugated to peroxidase to detect the formation of the immunocomplex.
Other studies use this method for residual pesticide and veterinary drug detection, but with few applications in mycotoxin detection. Although the non-competitive assay has advantages, the technique is still in the early stages of development, and the biggest challenge in developing this assay is the screening of anti-immune complex peptides. Therefore, improving the success rate of peptide screening for anti-immune complexes will significantly enhance the development of non-competitive immunoassays for small-molecule compounds, such as mycotoxins and pesticides. The anti-immune complex peptides of mycotoxins, pesticides, and other small molecules are summarized in Table 2.
4. Small Peptides in the Construction of Artificial Enzymes
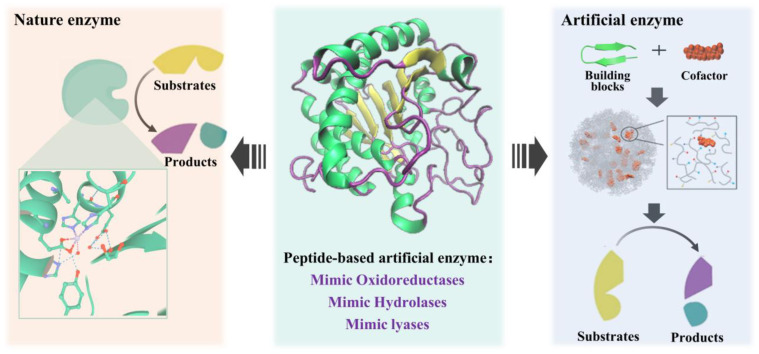
Small peptides are the ideal material to construct artificial enzymes because of the advantages of high similarity to natural enzymes and controllable structure [93,94]. The high efficiency of the catalytic activity of natural enzymes comes from two main aspects: (1) the active cavity that can just accommodate the substrate molecule; and (2) the attack of the catalytically active amino acid on the substrate and the stabilization of the transition state. Peptide-based artificial enzyme construction is mainly inspired by the structure and catalytic mechanism of the natural enzyme active site, and supramolecular catalysts with enzyme-like active sites can be generated by self-assembly of small-peptide molecules or supplemented with other supporting structures to mimic the natural enzyme active center (Figure 8).
Figure 8.
Artificial enzyme construction process by mimicking nature enzymes.
Peptide-based artificial enzymes have been proven efficient in the degradation of plasticizers [95], phenolic contaminants [96,97], Azo dyes [98], cellulose [99], and the treatment of Alzheimer’s disease [100]. Despite the few relevant reports on mycotoxin removal by peptide-based artificial enzymes at present, it may provide a potential new approach for mycotoxin control in the future. The peptide-based artificial enzymes that have been reported mainly mimic the catalytic activities of oxidoreductases, hydrolases, and lyases (Table 3).
Table 3.
Types and catalytic activities of some peptide-based artificial enzymes.
| Peptide-Based Artificial Enzyme | Structure | Substrate | Reaction Condition | kcat (×10−3 s−1) | KM (mM) | Kcat/KM (M−1 s−1) | References |
|---|---|---|---|---|---|---|---|
| Peptide-based oxidoreductase mimics | |||||||
| F-Cu(II) | Nanosheets | 2,4-DP | PBS buffer (pH 7.4), 22 °C | 11.90 × 103 | 0.19 | 62.65 | [97] |
| H15 | Nanosheets or Ribbons | TMB | 50 mM MES (pH 7.0), 25 °C | 0.13 | - | 0.70 | [101] |
| H2O2 | 0.11 | - | 3.13 × 10−2 | ||||
| Hemin(Phe+His) | Nanofibers | Pyrogallol | Toluene | 17.42 × 103 | - | - | [102] |
| Phosphate buffer (10 mM, pH 7.4) | 0.82 × 103 | ||||||
| Gel-6 | Nanofibers | Pyrogallol | Toluene | 25.95 × 103 | - | - | [103] |
| Phosphate buffer (10 mM, pH 7.4) | 1.51 × 103 | ||||||
| His-C7-Mn(II) | Nanospheres | OPD | pH 7.1, 25 °C, 10 mM OPD | 0.13 × 103 | 8.45 | 15.38 | [104] |
| H2O2 | pH 7.1, 25 °C, 280 mM H2O2 | 0.48 × 103 | 53.63 | 8.94 | |||
| NapFFH | Nanotubes | pNPA | PBS buffer (10 mM, pH 7.4), 25 °C | 1.96 | 0.93 | 2.11 | [105] |
| NapFFHCu | Shorter nanotubes | TMB | PBS buffer (10 mM, pH 7.4), 25 °C | 0.34 | 0.77 | 0.44 | [105] |
| H2O2 | 0.26 | 7.77 | 0.03 | ||||
| NapFFHH | Nanotubes | pNPA | PBS buffer (10 mM, pH 7.4), 25 °C | 0.37 | 2.56 | 0.14 | [105] |
| NapFFHHCu | Shorter nanotubes | TMB | PBS buffer (10 mM, pH 7.4), 25 °C | 0.11 | 4.32 | 0.26 | [105] |
| H2O2 | 0.39 | 4.84 | 0.08 | ||||
| NapFFRH | Nanotubes | pNPA | PBS buffer (10 mM, pH 7.4), 25 °C | 1.37 | 5.68 | 0.24 | [105] |
| NapFFRHCu | Thinner nanotubes | TMB | PBS buffer (10 mM, pH 7.4), 25 °C | 0.54 | 0.40 | 1.35 | [105] |
| H2O2 | 0.95 | 2.32 | 0.41 | ||||
| NapFFKH | Nanotubes | pNPA | PBS buffer (10 mM, pH 7.4), 25 °C | 1.13 | 4.19 | 0.27 | [105] |
| NapFFKHCu | Thinner nanotubes | TMB | PBS buffer (10 mM, pH 7.4), 25 °C | 2.67 | 1.28 | 2.08 | [105] |
| H2O2 | 1.74 | 1.11 | 1.57 | ||||
| NapFFDH | Nanotubes | pNPA | PBS buffer (10 mM, pH 7.4), 25 °C | 1.24 | 5.32 | 0.23 | [105] |
| NapFFDHCu | Nanospheres | TMB | PBS buffer (10 mM, pH 7.4), 25 °C | 0.28 | 1.90 | 0.15 | [105] |
| H2O2 | 0.20 | 7.61 | 0.03 | ||||
| NapFFSH | Nanotubes | pNPA | PBS buffer (10 mM, pH 7.4), 25 °C | 1.01 | 8.80 | 0.12 | [105] |
| NapFFSHCu | Nanospheres | TMB | PBS buffer (10 mM, pH 7.4), 25 °C | 0.51 | 0.79 | 0.64 | [105] |
| H2O2 | 1.12 | 16.22 | 0.07 | ||||
| LMLHLFL-hemin | Nanofibers | TMB | Phosphate buffer (100 mM, pH 6) | 0.47 × 103 | 9.9 × 10−3 | 0.47 × 105 | [106] |
| ABTS | 2.38 × 103 | 7.8 × 10−3 | 3.06 × 105 | ||||
| DhHP-6 | - | TMB | PBS buffer (10 mM, pH 7.4), 30 °C | - | 0.55 | Vm = 20.68 × 10−8 M s−1 | [96] |
| H2O2 | 0.47 | Vm = 3.89 × 10−8 M s−1 | |||||
| DhHP-6-c-ZrMOF | Nanoparticles | TMB | PBS buffer (10 mM, pH 7.4), 30 °C | - | 0.37 | Vm = 35.24 × 10−8 M s−1 | [96] |
| H2O2 | 0.35 | Vm = 5.484 × 10−8 M s−1 | |||||
| Peptide-based hydrolase mimics | |||||||
| CAII | Carbonic anhydrase (PDB:2CBA) | pNPA | pH 7.0, 25 °C | 37 | 22.1 | 1670 | [107] |
| pH 7.5, 25 °C | - | 30.53 | 2600 | ||||
| pH 8.0, 25 °C | 53 | 20.7 | 2550 | ||||
| pH 9.0, 25 °C | 56 | 23.9 | 2320 | ||||
| E3H15 | α-helix | pNPA | Tris-HCl (50 mM, pH 7.3), 25 °C | 0.19 | 0.98 | 0.20 | [108] |
| E3H15/K3 | 0.23 | 1.49 | 0.15 | ||||
| Au@E3H15 | Gold nanoparticles | 0.72 | 0.31 | 2.18 | |||
| Azo-GFGH | Nanofibers | pNPA | PBS (50 mM, pH 7.4), 25 °C (before UV) | 3.67 | 15.56 | 0.23 | [109] |
| PBS (50 mM, pH 7.4), 25 °C (after UV) | 3.00 | 15.97 | 0.18 | ||||
| 1NF | Nanofibers | pNPA | HEPES (10 mM, pH 7.4), 25 °C | 1.28 | 1.22 | 1.00 | [110] |
| 1TB | Twisted bundles | 1.07 | 0.63 | 1.71 | |||
| 2NF | Nanofibers | 1.15 | 2.01 | 0.575 | |||
| (di-2⊂γ-CD)NS | Nanosheets | 0.54 | 3.14 | 0.172 | |||
| Im-KL | Nanotubes | p-nitrophenyl 4-oxopentanoate
|
HEPES (25 mM, pH 8) | 1.50 | 0.74 | 2.1 | [111] |
| 1.17 | 2.4 | 0.48 | |||||
| Im-RL | p-nitrophenyl pentanoate
|
1.17 | 0.32 | 3.6 | |||
| 0.50 | 0.3 | 1.8 | |||||
| MAX1-H2H5 | β-structured fibrils | pNPA | Tris (15 mM, pH 7.4), 25 °C | 2.10 | 2.99 | 0.71 | [112] |
| Tris (15 mM, pH 9.0), 25 °C | 37.2 | 3.07 | 12.1 | ||||
| CAPS (15 mM, pH 10.0), 25 °C | 9.98 | 0.41 | 24.3 | ||||
| pNPMA | Tris (15 mM, pH 9.0), 25 °C | 69.8 | 0.15 | 452 | |||
| pNPP | Tris (15 mM, pH 9.0), 25 °C | 7.59 | 0.13 | 58.1 | |||
| MAX1-H2S5 | β-structured fibrils | pNPA | Tris (15 mM, pH 7.4), 25 °C | 0.78 | 0.75 | 1.04 | [112] |
| Tris (15 mM, pH 9.0), 25 °C | 17.4 | 1.31 | 13.3 | ||||
| CAPS (15 mM, pH 10.0), 25 °C | 47.4 | 0.48 | 99.4 | ||||
| pNPMA | Tris (15 mM, pH 9.0), 25 °C | 38.9 | 0.19 | 203 | |||
| pNPP | Tris (15 mM, pH 9.0), 25 °C | 7.27 | 0.05 | 145 | |||
| CoA-HSDmax | Nanofibers | pNPA | HEPES (10 mM, pH7.5), 35 °C | 3.00 | 16.29 | 0.19 | [113] |
| SA-H | 2.00 | 20.03 | 0.096 | ||||
| Q11H | Nanofibers | pNPA | PBS (10 mM, pH 7.4) | 1.95 | 21.68 | 0.09 | [114] |
| Q11HRmax | 2.64 | 17.63 | 0.15 | ||||
| SA-H | Nanofibers | pNPA | PBS (10 mM, pH 8.0), 25 °C | 6.73 × 10−2 | 7.79 | 0.088 | [93] |
| NIP-H | 5.93 × 10−2 | 4.28 | 0.14 | ||||
| AMIP-H | 6.87 × 10−2 | 2.66 | 0.258 | ||||
| IHQ-NP | β-sheets | pNPA | Tris-HCl (50 mM, pH8.0), 25 °C | 4.49 | 1.61 | 2.79 | [115] |
| Zn(II)-IHQ-NP | 10.53 | 1.21 | 8.69 | ||||
| Au@IHQ-NP | Nanoparticles | 2.41 | 0.49 | 4.95 | |||
| Zn(II)-Au@IHQ-NP | 7.97 | 0.50 | 16.06 | ||||
| F–Zn(II) | Amyloid-like structure | pNPA | Deionized water (pH 7.0), 22 °C | - | - | 76.54 | [116] |
| Tris-HCl (25 mM, pH 7.0), 22 °C | - | - | 10.62 | ||||
| VK2H | Nanofibers | pNPA | Tris-HCl (15 mM, pH 9.0) | 70 | 3.65 | 19.18 | [117] |
| Ac-IHIHIYI-NH2 | Nanofibers | pNPA | Tris-HCl (25 mM, pH 8.0), 37 °C | 8.26 × 103 | 0.02 | 355 | [118] |
| HKH-LLLAAA(K)-palmitoyl | Nanofibers | DNPA | HEPES(50 mM, pH 7.4), 25 °C | 16.7 | 0.85 | 19.76 | [119] |
| PepNTs-His-Argmax | Nanotubes | pNPA | HEPES(10 mM, pH 7.5), 25 °C | 1.38 | 0.76 | 1.82 | [120] |
| MID1-zinc | Artificial metalloprotease (PDB:3V1C) |
pNPA | HEPES (40 mM, pH 7.0), 25 °C | 42 | 1.18 | 35 | [121] |
| HEPES (40 mM, pH 7.5), 25 °C | 81 | 0.90 | 90 | ||||
| HEPES (40 mM, pH 8.0), 25 °C | 1.5 × 102 | 0.82 | 190 | ||||
| HEPES (40 mM, pH 8.5), 25 °C | 2.2 × 102 | 0.47 | 470 | ||||
| HEPES (40 mM, pH 9.0), 25 °C | 2.8 × 102 | 0.42 | 660 | ||||
| pNPP | HEPES (40 mM, pH 8.5), 37 °C | 0.2 | 1.2 × 10−2 | 14 | |||
| AuNPs@POMD-8pep | Nanoparticles | BAPNA | Tris-HCl 7.4, 37 °C | 2.18 × 106 | 0.16 g·L–1 | 8.26 × 105 L·g−1·min−1 | [122] |
| JAL-AK22 | Small peptide | MMP18-33 | PBS (pH 7.4), 37 ◦C | - | 0.17 | - | [123] |
| MMP18-40 | 0.15 | ||||||
| JAL-TA9 | Small peptide | Aβ1-20 | PBS (pH 7.4), 37 ◦C | - | 1.27 | - | [123,124,125] |
| Aβ11-29 | 0.56 | ||||||
| MMP18-33 | 0.17 | [126] | |||||
| MMP18-40 | 0.15 | ||||||
| ANA-TA9 | Small peptide | Aβ11-29 | PBS (pH 7.4), 37 ◦C | 0.02 | 0.32 | - | [100,127,128] |
| ANA-SA5 | Small peptide | Aβ11-29 | PBS (pH 7.4), 37 ◦C | 0.79 × 10−2 | 0.13 | - | [127] |
| ANA-YA4 | 0.01 | 0.15 | |||||
| Peptide-based lyase mimics | |||||||
| F-Zn(II) | Needle-like architectures | pNPA | Deionized water (pH 7.0), 22 °C | - | 0.17 | 76.54 | [116] |
| CO2 + H2O | Tris buffer (20 mM, pH 8) | 7.80×103 | 8.10 | 962.00 | |||
| YLK-18-opt | - |

|
5% CH3CN, 42.5 mM Na phosphate (pH 7.5), 25 °C | 3.50 × 10−3 | 1.80 | kcat/kuncat = 540 | [129] |

|
5% DMSO, 42.5 mM Na phosphate (pH 7.5), 25 °C | 6.83 × 10−3 | 0.90 | kcat/kuncat = 170 | |||
| FT-YLK-3 |

|
5% CH3CN, 42.5 mM Na phosphate (pH 7.5), 25 °C | 9.33 × 10−3 | 1.80 | kcat/kuncat = 1400 | ||

|
5% DMSO, 42.5 mM Na phosphate (pH 7.5), 25 °C | 2.00×10−3 | 1.1 | kcat/kuncat = 500 | |||
| FluS303-FTYLK3 | - |

|
10% CH3CN, 42.5 mM Na phosphate (pH 7.5), 25 °C | 3.83 × 10−3 | 8.00 × 10−3 | 4.83 × 10−1 | [130] |

|
5% CH3CN, 42.5 mM Na phosphate (pH 7.5), 25 °C | 12.30 × 10−3 | 1.10 | 1.12 × 10−2 | |||
| FT-YLK-3 |

|
10% CH3CN, 42.5 mM Na phosphate (pH 7.5), 25 °C | 3.33 × 10−3 | 0.13 | 2.50 × 10−2 | ||

|
5% CH3CN, 42.5 mM Na phosphate (pH 7.5), 25 °C | 9.33 × 10−3 | 1.80 | 5.17 × 10−3 | |||
| Ac-IHIHIQI-NH2 | Nanofibers | pNPA | Tris (25 mM, pH 8), 22 °C | 26 | 0.4 | 62 | [131] |
| [Hg(II)]S[Zn(II)(H2O/OH-)]N(TRIL9CL23H)3n+ | Artificial metalloprotease (PDB:3PBJ) |
pNPA | HEPES(50 mM, pH 7.5), 25 °C | 2.2 | 1.6 | 1.38 | [107] |
| HEPES(50 mM, pH 8.0), 25 °C | 5.4 | 1.7 | 3.1 | ||||
| CHES (50 mM, pH 8.5), 25 °C | 12 | 1.9 | 6.0 | ||||
| CHES (50 mM, pH 8.75), 25 °C | 21 | 2.0 | 10.8 | ||||
| CHES (50 mM, pH 9.0), 25 °C | 38 | 2.1 | 17.6 | ||||
| CHES (50 mM, pH 9.5), 25 °C | 40 | 1.7 | 23.3 | ||||
4.1. Peptide-Based Oxidoreductase Mimics
Small peptides can mimic oxidoreductase activities, such as laccase [97], peroxidase [101], and superoxide dismutase [132], alone or in combination with cofactors. Begum et al. [133], first using copper (II)-peptides mimicking the active site of laccase from Trametes versicolor (PDB: 1KYA), designed artificial enzymes that can catalyze oxidation and polymerization to degrade aromatic and cyclic pharmaceutically active compounds (PhACs). Tp-CuS 12 was demonstrated to be the best catalyst in degrading the cresols, 2,4-dichlorophenol, and ibuprofen, and revealed potential applications in environmental remediation. More recently, similarly inspired by the laccase catalytic site, Makam et al. [97] designed a simple, efficient, and robust phenylalanine-based single amino acid artificial enzyme (Figure 9B). It can catalyze the rapid oxidation of phenolic pollutants, which were approximately 5400-fold more cost-effective, 36-fold more sensitive, and 4-fold more efficient than a natural laccase enzyme. In addition to the rational design of copper-binding peptides with oxidoreductase activity, Xin et al. [134] found that the natural copper-binding peptide (Methanobactin, Mb) from Methylosinus trichosporium OB3b exhibited peroxidase activity; further studies showed that the catalytic efficiency of Mb-Cu was significantly enhanced by immobilizing it on gold nanoparticles (AuNPs).
Figure 9.
Peptide-based oxidoreductase mimics with natural heme-containing enzyme activity. (A) Laccase activity. Adapted from Ref. [101]. (B) Peroxidase activity [97]. (C,D) Immobilized into a mesoporous metal-organic framework. Reprinted with permission from Ref. [96], 2020, Catal. Commun., and Ref. [136], 2011, J. Am. Chem. Soc.
Microperoxidase-11 (MP-11) is a heme-containing peptide from Cytochrome c by proteolytic digestion [135]. The molecular weight of MP-11 is much smaller than that of natural peroxidase, so it has better solubility in organic solvents. Accordingly, Wariishi et al. [98] found that MP-11 exhibited effective decolorization activity against both azo and anthraquinone dyes in 90% methanol. Subsequently, Lykourinou et al. [136] and Ding et al. [96] immobilized MP-11 and MP-11 mimic (DhHP-6) in a metal-organic framework to improve their catalytic rates, respectively, and found that the catalytic properties of the newly constructed artificial enzymes were both improved, among which DhHP-6-c-ZrMOF could be a promising catalyst for the efficient degradation of phenol pollutants (Figure 9C,D).
Natural redox enzymes (e.g., peroxidase, laccase, and glucose oxidase) mainly rely on the precise arrangement of amino acid residues near the active site and cofactor to catalyze the redox reaction of the substrate efficiently, among which the cofactor plays a key role in electron transfer. However, when the temperature increases or pH fluctuates, the spatial arrangement of amino acid residues in the active site changes, leading to the movement, shedding, or aggregation of cofactors and resulting in the irreversible denaturation and inactivation of the enzyme. Based on the catalytic properties of histidine side chain imidazole, Liu et al. [101] designed polyhistidine peptides of different lengths (number of amino acids from 2 to 20), which were assembled to form large two-dimensional nanosheets or ribbons through hydrogen bonding, charge, or π–π stacking interactions, and the sizes reached up to millimeters. The catalytic activity H15 exhibited significant activity in catalyzing the oxidation of 3,3′,5,5′-tetramethylbenzidine (TMB), homovanillic acid (HVA), and nicotinamide adenine dinucleotide (NADH) by H2O2 in the absence of a heme cofactor and metal conditions (Figure 9A).
4.2. Peptide-Based Hydrolase Mimics
According to the degradation of the different chemical bonds, peptide-based artificial hydrolases mainly be used to catalyze ester bonds, peptide bonds, and glycosidic bond hydrolysis. Among them, ester bond degrading artificial enzymes have been reported more frequently. Esterases are enzymes that catalyze the rapid cleavage of ester bonds and the hydrolysis of esters to the corresponding alcohols and acids. It is commonly found in living organisms and widely used in food, medicine, chemistry, biology, and other fields. Meanwhile, the serine protease catalytic center amino acids histidine (His), aspartate (Asp), and serine (Ser), also known as the catalytic triad, are often introduced into peptide sequences to construct esterase mimics [137]. In addition, numerous studies have reported that histidine can act as an artificial enzyme active site to catalyze the hydrolysis of esters [101,138]. The main ester substrates that have been reported to be able to be catalyzed by peptide-based ester-bond-degradation artificial enzymes are p-nitrophenol acetate (pNPA) [121], p-nitrophenol propionate (pNPP) [112,121], p-nitrophenol butyrate (pNPB) [113], p-nitrophenyl acetate methyl ester (p-NPMA) [112], dioctyl phthalate (DEHP) [139], uridine 3’-(2,2,2-Trichloroethylphosphate) [140], acetyltyrosine ethyl ester (ATEE) [141], benzoyltyrosine ethyl ester (BTEE) [141], Cbz-Phe-ONP [142], BAPNA [122], etc.
Mikolajczak et al. [108] reported a peptide-gold nanoparticle coupling Au@E3H15 (E3H15, H2N-CGGYE5IAALEKEIAH15LEKEIAALEK-CO2H) with esterase properties by rational design, in which the catalytic activity is regulated by peptide conformational changes and can be significantly inhibited by the addition of small peptides with complementary α-helical structures (Figure 10A). Burton et al. [143] completed a de novo design of a peptide-based artificial hydrolase using the thermostable α-helical barrel, which can catalyze the hydrolysis of p-nitrophenyl acetate. Eventually, CC-Hept-Cys-His-Glu was heptameric in a solution, confirmed by the X-ray crystal structure (PDB: 5EZC) and showing the best activity with KM and kcat/KM at 134 μM and 3.7 M−1 s−1, respectively (Figure 10B). Similarly, Der et al. [121] de novo designed an artificial enzyme MID1-zinc (PDB: 3V1C) with the ability to catalyze pNPA and pNPP hydrolysis (Figure 10C).
Figure 10.
Peptide-based hydrolase mimics with esterase activity. (A) immobilized onto gold nanoparticles; (a) Peptide sequences of E3H15 and K3, (b) The structure of Au@E3H15 and mechanism for regulated catalytic activity, (c) Proposed mechanism for the hydrolysis of pNPA catalyzed by E3H15 and Au@E3H15 monolayer. Reprinted with permission from Ref. [108]. 2017, Biomacromolecules. (B) having catalytically active Cys-His-Glu triads by a de novo design; (a) The structure of CC-Hept-Ile-Cys-Ile, (b) Proposed mechanism for the reaction of CC-Hept-Cys-His-Glu with pNPA via a thioester intermediate. Adapted from Ref. [143]. (C) having a small cleft and open zinc coordination site; (a) The structure of MID1-zinc, (b) Proposed mechanism for the reaction of MID1-zinc with pNPA. Reprinted with permission from Ref. [121]. 2012, Biochemistry. (D) capable of self-assembling into amyloid structures; (a) Peptide designs, (b) The structure of peptide III, (c) The proposed mechanism of hydrolysis for the substrate pNPA by fibrils of peptide III. Reprinted with permission from Ref. [118]. 2017, Nanoscale.
Garawi et al. [118] designed a series of small peptide sequences capable of self-assembling into amyloid fibrils through the rational design approach (Figure 10D). Catalytic activity studies showed that III, IV, and VI were able to catalyze the degradation of pNPA, sequence III (Ac-IHIHIYI-NH2), showing the best catalytic activity with a kcat/Km of 355 M−1s−1. The catalytic mechanism may be that the water molecule activates the Zn2+–imidazole group complex, forming a tetrahedral structure that acts directly with the carboxyl group of pNPA to form an acyl-Zn-imidazole complex; the tyrosine in the sixth position assists the protonation process of the zinc ion–imidazole group complex. The successful construction of these artificial hydrolases provides evidence for understanding the evolution of natural enzymes and an effective strategy for the rational design of enzymes in the future.
In addition to mimicking esterase activity, peptide-based artificial enzymes can also be used to catalyze the degradation of peptide bonds and glycosidic bonds. Gao et al. [122] used the synergistic interaction of POMD (polyoxometalate with Wells-Dawson structure) and 7 peptides (N-His-Sar-His-Sar-His-Sar-His, Sar = sarcosine) to mimic natural trypsin activity. AuNPs were used to facilitate electron transfer between heptapeptides and POMD, and could also serve as a backbone for the artificial enzyme. POMD can be linked to the surface of AuNPs; the 7 peptides can also be immobilized on the surface of AuNPs by adding cysteine at the N-terminal, which finally constitutes a multifunctional artificial enzyme (AuNPs@POMD-8pep). The artificial enzyme has both protease activity and superoxide dismutase activity, and the specific activity was 8.80 × 105 U/mg using BAPNA as substrate, which was much higher than that of natural trypsin (5.14 × 10 U/mg) under the same conditions. He et al. [144] designed a series of β-folded heptapeptides containing multiple glutamic or aspartic acids, based on the self-assembly properties of small molecule peptides. All these small peptides were repetitive patterns of polar and nonpolar amino acids, consistent with the structural periodicity required for amyloid structure formation. After self-assembly, most of them were found to have measurable hydrolytic activity of cellobiose, with PC5 (Ac-FEFEAEA-CONH2) having the best catalytic activity. The factors affecting the catalytic activity are the amount of glutamate, the conformation of the small peptide backbone, the distribution of intramolecular hydrogen bonds, the spatial resistance to substrate, and the crystal state of the peptide nanofibers.
4.3. Peptide-Based Lyase Mimics
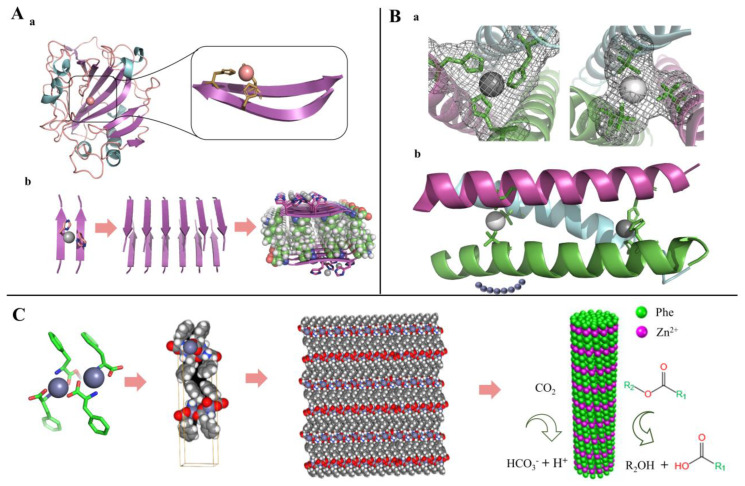
Lyases are enzymes that cleave C–C, C–O, C–N and other bonds by elimination to form multiple bonds or rings. Small peptides can mimic the reaction of the corresponding substrate catalyzed by aldolase and carbonic anhydrase [116,130]. Rufo et al. [131] designed a series of heptapeptides capable of self-assembling into amyloid structures and investigated whether they have enzyme-like catalytic effects. The results showed that, with the assistance of zinc ions, these self-assembled short peptides possess esterase activity and can catalyze the hydrolysis of pNPA. Among them, Ac-IHIHIQI-CONH2 showed the best catalytic activity; the KM, kcat, and kcat/KM were 0.4 mM, 2.6 × 10−2 s−1, and 62 M−1 s−1, respectively (Figure 11A). With the TRI family peptide (Ac-G(LKALEEK)4G-NH2) as a scaffold, Zastrow et al. [107] developed a true de novo metalloenzymes based on a designed catalytic metal site and a defined three-dimensional structure. [Hg(II)]S[Zn(II)(H2O/OH-)]N(TRIL9CL23H)3n+ catalyzes pNPA hydrolysis only ∼100-fold less than human carbonic anhydrase (CA)II, and the CO2 hydration occurs with efficiency within approximately 500-fold of CAII (Figure 11B).
Figure 11.
Peptide-based lyase mimics inspired by natural human carbonic anhydrase. (A) Peptide-based artificial enzyme capable of self-assembling into nanofibers; (a) Structure of human carbonic anhydrase showing a typical metal-binding motif, (b) Structure and assembly process of artificial enzyme. Adapted from Ref. [131]. (B) Artificial protein containing two separate metal sites by a de novo design; (a) Top-down view of the structural trigonal thiolate site (right) and side view of the tetrahedral catalytic site (left), (b) One of two trimers found in the asymmetric unit of the crystal structure. Adapted from Ref. [107]. (C) single phenylalanine self-assembling into needle-like architectures with carbonic anhydrase activity. Reprinted with permission from Ref. [93]. 2020, ACS Catal.
Phenylalanine has both positively as well as negatively charged groups and aromatic groups, and these properties allow phenylalanine to self-assemble to form specific nanostructures through hydrogen bonding, π–π stacking, and hydrophobic interactions. Abramovich et al. [145] found that phenylalanine can spontaneously self-assemble into supramolecular crossed β-sheet secondary structures with amyloid characteristics. Later, Makam et al. [116] constructed an artificial enzyme for the first time by self-assembling single phenylalanine with zinc ions to form a nanoneedle structure. The crystal structure diffraction analysis showed that one zinc ion was bound to two phenylalanines via amine and carboxylate groups; the layers were stabilized by π–π interactions. Activity validation showed that the artificial enzyme has both esterase and carbonic anhydrase activities, which is the smallest artificial hydrolase with the lowest molecular weight found to date, and the catalytic activity (kcat/KM = 46 × 10−2 (gl−1)−1 s−1) was 8-fold higher than that of natural carbonic anhydrase (kcat/KM = 5.7 × 10−2 (gl−1)−1 s−1) based on mass (Figure 11C).
Aldolase is a lyase that catalyzes the reversible conversion of fructose-1,6-bisphosphate to glyceraldehyde, 3-phosphate, and dihydroxyacetone phosphate. Tanaka and Carlos [129] tried to select aldolase peptides using an 18-residues peptide (YLK-18-opt) that can catalyze oxaloacetate decarboxylation. To study the catalytic activity of this peptide, a six-peptide library was designed at its C-terminus and displayed on the phage. The small peptides were obtained by panning phage display peptide library (YKLLKELLAKLKWLLRKLXXXXXX, X = natural 20 amino acids) that bound to substrate. Among the peptides, FT-YLK3 (YKLLKELLAKLKWLLRKLLGPTCLNH2) can catalyze the retro-aldol reaction of the substrate with a kcat/kuncat of 1900 and a Km of 1.8 mM. Subsequently, to increase substrate specificity, the authors added the small peptide FluS303 (YPNEFDWWDYYY) at the C-terminal of FT-YLK3 that has affinity to the substrate. The designed FluS303-FTYLK3 exhibits enhanced catalytic properties than FT-YLK3 [130]. More recently, Peme et al. [146] designed a peptide-based artificial enzyme that mimics the catalytic active site of the nature fructose-1,6-bisphosphate aldolase. The peptides exhibited asymmetric aldol reaction catalytic activity for aliphatic and aromatic ketone/aldehydes, and the aldol product yields can reach 44% with excellent enantioselectivity (93%).
4.4. Small Peptides in Mycotoxin Removal
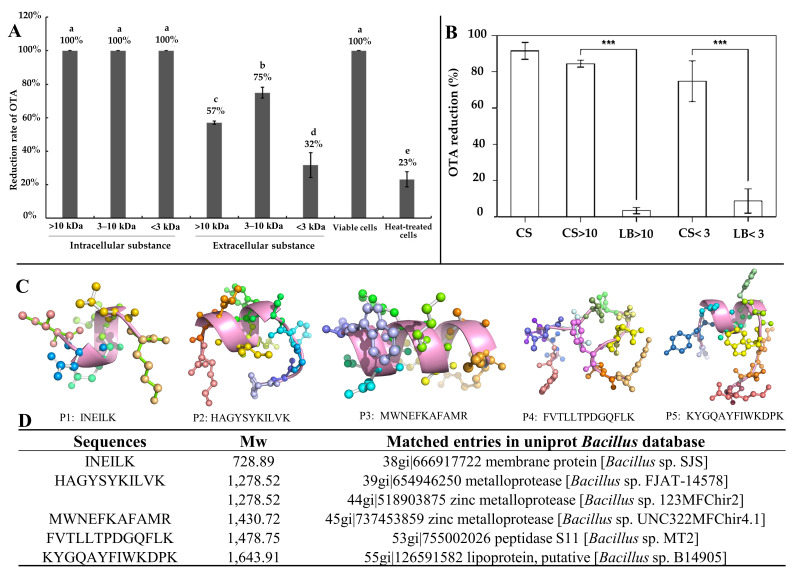
Our team previously isolated a Bacillus subtilis CW14 from fresh elk droppings with OTA-degrading ability, and the cell-free supernatant can also degrade OTA efficiently. Interestingly, when the strain culture supernatant was separated by ultrafiltration, both the fractions collected at > 10 kDa (84.9%) and < 3 kDa (74.8%) demonstrated a remarkable ability to remove OTA (Figure 12B) [21]. Then, a carboxypeptidase gene was likely responsible for the OTA degradation by the > 10 kDa fraction was cloned from the B. subtilis CW14 genome and has OTA-degrading ability, which was confirmed by expression of the gene in Escherichia coli. As for the <3 kDa fraction (CS < 3), the author found the further purified small peptides (0.7 kDa < Mw < 1.7 kDa), showing OTA-degrading activity (45.0%). We speculated that the OTA may be adsorption and removed by some small peptides. Finally, by comparison with the B. subtilis protein database, we found five small peptides with molecular weights ranging from 0.73 kDa to 1.64 kDa, which were fragments derived from membrane protein, metalloprotease, zinc metalloprotease, peptidase, and lipoprotein, respectively, and may be present in the CS < 3 fractions as shown in Figure 12C [21]. More recently, we obtained another Brevundimonas naejangsanensis ML17 strain from soil, which degraded OTA into OTα and OTB into OTβ with a degrading rate of 100% within 24 h. Similarly, when the ML17 strain intracellular fractions and extracellular components were separated by ultrafiltration. The <3 kDa fractions both degraded OTA within 24 h at a rate of 100% and 31.64% [22] (Figure 12A).
Figure 12.
Removal of OTA by microbial-source small molecular substances; (A) Brevundimonas naejangsanensis ML17 source < 3 kDa ultrafiltration fraction for OTA degradation; Data with different lowercase letters were considered to be significantly different (p < 0.05) by Duncan’s multiple comparison test; Reprinted with permission from Ref. [22]. 2022, Food Control. (B) Bacillus subtilis CW14 source < 3 kDa ultrafiltration fraction for OTA degradation; Bars with *** were significantly different based on ANOVA test (p < 0.001); Reprinted with permission from Ref. [21]. 2018, World Mycotoxin J. (C) Structure of the small peptide in Figure 12D predicted with PEP-FOLD 3.5 and displayed by pyMOL; (D) list of peptides in the Bacillus subtilis CW14 < 3 kDa ultrafiltration fraction, identified by LC-ESI-MS/MS using Mascot serve. Reprinted with permission from Ref. [21]. 2018, World Mycotoxin J.
Small peptides derived from microorganisms have diversity functions [147], based on the following evidence: (1) small peptides derived from Methylosinus trichosporium (Methanobactin, Mb) possess peroxidase activity [134]; (2) small peptide fragments derived from natural Tob1 protein (JAL-TA9 and ANA-TA9) can catalyze peptide bond degradation [124,126,127]; (3) heme-containing small peptides derived from horse heart cytochrome c (Microperoxidase-11, MP-11) possess peroxidase activity and can catalyze the degradation of azo dyes [98]; and (4) artificial enzymes constructed based on small peptides can mimic a variety of natural enzyme activities and catalyze the degradation of plasticizers [95], phenolic contaminants [96,97], cellulose [99], etc. Thus, we speculated that the small peptides derived from the ML17 strain may have OTA degradation activity. We are currently performing further purification and characterization of these small molecules. Since small peptides have various advantages over natural large molecule proteins, such as simple structure, being easily controlled, having thermal stability, and biocompatibility, this discovery may provide new perspectives for the green and safe removal of mycotoxins in the future.
5. Conclusions and Outlook
Small peptides can be constructed and screened in various ways, such as phage display peptide libraries, combinatorial chemistry, and rational design. With the advantages of mature synthesis protocols, diverse structures, excellent biocompatibility, and sharing the same chemical structure as proteins, small peptides are widely used for mycotoxin detection and artificial enzyme construction with mycotoxin degradation potentiality. With further research on structure–activity relationship, synthesis, and screening methods, small peptides are playing an increasingly important role in mycotoxin control.
In mycotoxin detection, small peptides can serve as mycotoxin extraction and clean-up materials to improve the sensitivity and reproducibility of traditional instrument detection. Since traditional methods conduct the real-time detection of mycotoxins in food and foodstuffs with difficulty, immunoassays based on antigen–antibody reactions can achieve rapid and highly sensitive detection. However, these approaches also have problems, such as the need to use mycotoxin standards and the difficulty and complexity of anti-toxin antibody preparation, while small peptides can be used as coating antigens, competitive antigens, anti-immune complex peptides avoiding the use of toxin standards, or simplifying antibody preparation, which have an important role in the detection of mycotoxins. Meanwhile, the application of peptide-based biosensors is becoming increasingly widespread in SMC detection areas and will play an invaluable role in the future.
In mycotoxin removal, compared with the traditional physical adsorption and chemical degradation methods, mycotoxin degradation enzymes have the advantages of higher catalytic efficiency, better specificity, and greater safety, which are the most promising degradation methods. However, the application of natural enzymes in the removal of mycotoxins is limited by their large molecular weight, complex structure, the difficulty of modification, and sensitivity to the environment. With further understanding of the structure and catalytic mechanism of natural enzymes, researchers found that small peptides, nucleic acid, cyclodextrins, metal nanoparticles, and other materials can mimic the catalysis center of natural enzymes to build artificial enzymes with similar activity. Notably, artificial enzymes constructed based on small peptides can mimic the catalytic activities of oxidoreductases, hydrolases, lyases, etc., and have been applied to the degradation of plasticizers, phenolic pollutants, azo dyes, cellulose, amyloid, etc., which provides a new perspective on the removal of mycotoxins.
Given that small peptides have so many potential possibilities and advantages in mycotoxin removal, we hope more researchers in related fields can participate in the discovery of more small peptides with a mycotoxin degradation function and investigate the principle and conformational relationship of the peptide-based mycotoxin removal of artificial enzymes to open up a new way for mycotoxin degradation. Meanwhile, owing to the excellent advantages of small peptides in constructing immunobiosensors, many researchers are already focusing on research into the applications of small peptides in mycotoxin detection, and we should pay more attention to promoting its practical application.
Currently, the major challenge is obtaining small peptide sequences with specific functions quickly and precisely; this requires a deep understanding of the chemical properties, folding patterns, and structures of small peptides. Notably, with the help of peptide structure prediction software such as PEP-FOLD, Alphafold2, and RoseTTAFold, the structure of a specific small-peptide sequence can be more easily and accurately predicted, which facilitates the rational design of small-peptide sequences. The construction of more small peptides with specific functions by rational design will be a future trend. Overall, it will provide new solutions for the detection and removal of mycotoxins or other small-molecule contaminants in the future in a greener, safer, and more efficient way.
Author Contributions
Conceptualization, Z.Z. (Zitong Zhao) and Z.L.; writing—original draft preparation, Z.Z. (Zitong Zhao) and Z.Z. (Zhenzhen Zhang); writing—review and editing, Z.Z. (Zitong Zhao), Z.L., Z.Z. (Zhenzhen Zhang) and H.Z.; supervision, Z.L.; funding acquisition, Z.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
In this work, we mainly summarize the feasible application of small peptides in mycotoxin detection, peptide-based artificial enzymes, and their potential applications in mycotoxin removal, to provide new ideas for the control of mycotoxins.
Funding Statement
National Natural Science Foundation of China (No. 32172170) and Shandong Natural Science Foundation (No. ZR202102260301).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Haque M.A., Wang Y., Shen Z., Li X., Saleemi M.K., He C. Mycotoxin contamination and control strategy in human, domestic animal and poultry: A review. Microb. Pathogen. 2020;142:104095. doi: 10.1016/j.micpath.2020.104095. [DOI] [PubMed] [Google Scholar]
- 2.Iqbal S.Z. Mycotoxins in food, recent development in food analysis and future challenges; A review. Curr. Opin. Food Sci. 2021;42:237–247. doi: 10.1016/j.cofs.2021.07.003. [DOI] [Google Scholar]
- 3.Khodaei D., Javanmardi F., Khaneghah A.M. The global overview of the occurrence of mycotoxins in cereals: A three-year survey. Curr. Opin. Food Sci. 2021;39:36–42. doi: 10.1016/j.cofs.2020.12.012. [DOI] [Google Scholar]
- 4.Awuchi C.G., Ondari E.N., Ogbonna C.U., Upadhyay A.K., Baran K., Okpala C.O.R., Korzeniowska M., Guiné R.P.F. Mycotoxins affecting animals, foods, humans, and plants: Types, occurrence, toxicities, action mechanisms, prevention, and detoxification strategies-A revisit. Foods. 2021;10:1279. doi: 10.3390/foods10061279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dey D.K., Kang J.I., Bajpai V.K., Kim K., Lee H., Sonwal S., Simal-Gandara J., Xiao J., Ali S., Huh Y.S., et al. Mycotoxins in food and feed: Toxicity, preventive challenges, and advanced detection techniques for associated diseases. Crit. Rev. Food Sci. Nutr. 2022;62:1–22. doi: 10.1080/10408398.2022.2059650. [DOI] [PubMed] [Google Scholar]
- 6.Fusilier K., Chilvers M.I., Limay-Rios V., Singh M.P. Mycotoxin co-occurrence in michigan harvested maize grain. Toxins. 2022;14:431. doi: 10.3390/toxins14070431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weaver A.C., Weaver D.M., Adams N., Yiannikouris A. Co-occurrence of 35 mycotoxins: A Seven-Year survey of corn grain and corn Silage in the United States. Toxins. 2021;13:516. doi: 10.3390/toxins13080516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anfossi L., Giovannoli C., Baggiani C. Mycotoxin detection. Curr. Opin. Biotechnol. 2016;37:120–126. doi: 10.1016/j.copbio.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Adunphatcharaphon S., Elliott C.T., Sooksimuang T., Charlermroj R., Petchkongkaew A., Karoonuthaisiri N. The evolution of multiplex detection of mycotoxins using immunoassay platform technologies. J. Hazard. Mater. 2022;432:128706. doi: 10.1016/j.jhazmat.2022.128706. [DOI] [PubMed] [Google Scholar]
- 10.Li R., Wen Y., Wang F., He P. Recent advances in immunoassays and biosensors for mycotoxins detection in feedstuffs and foods. J. Anim. Sci. Biotechnol. 2021;12:108. doi: 10.1186/s40104-021-00629-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li P., Deng S., Zech Xu Z. Toxicant substitutes in immunological assays for mycotoxins detection: A mini review. Food Chem. 2021;344:128589. doi: 10.1016/j.foodchem.2020.128589. [DOI] [PubMed] [Google Scholar]
- 12.Pavan S., Berti F. Short peptides as biosensor transducers. Anal. Bioanal. Chem. 2012;402:3055–3070. doi: 10.1007/s00216-011-5589-8. [DOI] [PubMed] [Google Scholar]
- 13.Heurich M., Altintas Z., Tothill I.E. Computational design of peptide ligands for ochratoxin A. Toxins. 2013;5:1202–1218. doi: 10.3390/toxins5061202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thyparambil A., Bazin I., Guiseppi-Elie A. Molecular modeling and simulation tools in the development of peptide-based biosensors for mycotoxin detection: Example of ochratoxin. Toxins. 2017;9:395. doi: 10.3390/toxins9120395. [DOI] [Google Scholar]
- 15.Misihairabgwi J., Ezekiel C., Sulyok M., Shephard G., Krska R. Mycotoxin contamination of foods in Southern Africa: A 10-year review (2007–2016) Crit. Rev. Food Sci. Nutr. 2019;59:43–58. doi: 10.1080/10408398.2017.1357003. [DOI] [PubMed] [Google Scholar]
- 16.Chen W., Li C., Zhang B., Zhou Z., Shen Y., Liao X., Yang J., Wang Y., Li X., Li Y. Advances in biodetoxification of ochratoxin A: A review of the past five decades. Front. Microbiol. 2018;9:1386. doi: 10.3389/fmicb.2018.01386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zozulia O., Dolan M.A., Korendovych I.V. Catalytic peptide assemblies. Chem. Soc. Rev. 2018;47:3621–3639. doi: 10.1039/C8CS00080H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall L.R., Korendovych I.V. Catalytic amyloids: Is misfolding folding? Curr. Opin. Chem. Biol. 2021;64:145–153. doi: 10.1016/j.cbpa.2021.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang M., Qi W. Assembled peptides for biomimetic catalysis. Artif. Protein Pept. Nanofibers. 2020;16:383–413. doi: 10.1016/B978-0-08-102850-6.00016-4. [DOI] [Google Scholar]
- 20.Duncan K.L., Ulijn R.V. Short peptides in minimalistic biocatalyst design. Biocatalysis. 2015;1:67–81. doi: 10.1515/boca-2015-0005. [DOI] [Google Scholar]
- 21.Hu H.N., Jia X., Wang Y.P., Liang Z.H. Removal of ochratoxin A by a carboxypeptidase and peptides present in liquid cultures of Bacillus subtilis CW14. World Mycotoxin J. 2018;11:559–570. doi: 10.3920/WMJ2017.2296. [DOI] [Google Scholar]
- 22.Peng M., Zhao Z., Liang Z. Biodegradation of ochratoxin A and ochratoxin B by Brevundimonas naejangsanensis isolated from soil. Food Control. 2022;133:108611. doi: 10.1016/j.foodcont.2021.108611. [DOI] [Google Scholar]
- 23.Cimbalo A., Alonso-Garrido M., Font G., Manyes L. Toxicity of mycotoxins in vivo on vertebrate organisms: A review. Food Chem. Toxicol. 2020;137:111161. doi: 10.1016/j.fct.2020.111161. [DOI] [PubMed] [Google Scholar]
- 24.Eskola M., Kos G., Elliott C.T., Hajšlová J., Mayar S. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25% Crit. Rev. Food Sci. Nutr. 2019;60:2773–2789. doi: 10.1080/10408398.2019.1658570. [DOI] [PubMed] [Google Scholar]
- 25.Liu Q., Wang J., Boyd B.J. Peptide-based biosensors. Talanta. 2015;136:114–127. doi: 10.1016/j.talanta.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 26.Dasgupta A., Das D. Designer peptide amphiphiles: Self-assembly to applications. Langmuir. 2019;35:10704–10724. doi: 10.1021/acs.langmuir.9b01837. [DOI] [PubMed] [Google Scholar]
- 27.Duran-Meza E., Diaz-Espinoza R. Catalytic amyloids as novel synthetic hydrolases. Int. J. Mol. Sci. 2021;22:9166. doi: 10.3390/ijms22179166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akagawa K. 19-Catalysis by peptides. In: Koutsopoulos S., editor. Peptide Applications in Biomedicine, Biotechnology and Bioengineering. Woodhead Publishing; Sawston, UK: 2018. pp. 513–564. [DOI] [Google Scholar]
- 29.Tothill I.E. Peptides as molecular receptors. In: Zourob M., editor. Recognition Receptors in Biosensors. Springer; New York, NY, USA: 2010. pp. 249–274. [DOI] [Google Scholar]
- 30.Smith G.P. Filamentous Fusion Phage: Novel Expression Vectors That Display Cloned Antigens on the Virion Surface. Science. 1985;228:1315–1317. doi: 10.1126/science.4001944. [DOI] [PubMed] [Google Scholar]
- 31.Kay B.K., Adey N.B., Yun-Sheng H., Manfredi J.P., Mataragnon A.H., Fowlkes D.M. An M13 phage library displaying random 38-amino-acid peptides as a source of novel sequences with affinity to selected targets. Gene. 1993;128:59–65. doi: 10.1016/0378-1119(93)90153-T. [DOI] [PubMed] [Google Scholar]
- 32.Smith G.P. Phage display: Simple evolution in a petri dish (nobel lecture) Angew. Chem. Int. Ed. 2019;58:14428–14437. doi: 10.1002/anie.201908308. [DOI] [PubMed] [Google Scholar]
- 33.Devlin J.J., Panganiban L.C., Devlin P.E. Random peptide libraries: A source of specific protein binding molecules. Science. 1990;249:404–406. doi: 10.1126/science.2143033. [DOI] [PubMed] [Google Scholar]
- 34.Sun W., Zhang Y., Ju Z. Mimotopes for mycotoxins diagnosis based on random peptides or recombinant antibodies from phage library. Molecules. 2021;26:7652. doi: 10.3390/molecules26247652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu P., Ghosh S., Gul A.R., Bhamore J.R., Park J.P., Park T.J. Screening of specific binding peptides using phage-display techniques and their biosensing applications. TrAC Trends Anal. Chem. 2021;137:116229. doi: 10.1016/j.trac.2021.116229. [DOI] [Google Scholar]
- 36.Liu R., Li X., Lam K.S. Combinatorial chemistry in drug discovery. Curr. Opin. Chem. Biol. 2017;38:117–126. doi: 10.1016/j.cbpa.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furka Á. Forty years of combinatorial technology. Drug Discov. Today. 2022;27:103308. doi: 10.1016/j.drudis.2022.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Bozovičar K., Bratkovič T. Evolving a peptide: Library platforms and diversification strategies. Int. J. Mol. Sci. 2019;21:215. doi: 10.3390/ijms21010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gray B.P., Brown K.C. Combinatorial peptide libraries: Mining for cell-binding peptides. Chem. Rev. 2014;114:1020–1081. doi: 10.1021/cr400166n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lam K.S., Salmon S.E., Hersh E.M., Hruby V.J., Kazmierski W.M., Knapp R.J. A new type of synthetic peptide library for identifying ligand-binding activity. Nature. 1991;354:82–84. doi: 10.1038/354082a0. [DOI] [PubMed] [Google Scholar]
- 41.Giraudi G., Ferrero V.E., Anfossi L., Baggiani C., Giovannoli C., Tozzi C. Solid-phase extraction of ochratoxin A from wine based on a binding hexapeptide prepared by combinatorial synthesis. J. Chromatogr. A. 2007;1175:174–180. doi: 10.1016/j.chroma.2007.10.057. [DOI] [PubMed] [Google Scholar]
- 42.Onda Y., Bassi G., Elsayed A., Ulrich F., Oehler S., Plais L., Scheuermann J., Neri D. A DNA-Encoded Chemical Library Based on Peptide Macrocycles. Chem. Eur. J. 2021;27:7160–7167. doi: 10.1002/chem.202005423. [DOI] [PubMed] [Google Scholar]
- 43.Boschetti E., Zilberstein G., Righetti P.G. Combinatorial peptides: A library that continuously probes low-abundance proteins. Electrophoresis. 2022;43:355–369. doi: 10.1002/elps.202100131. [DOI] [PubMed] [Google Scholar]
- 44.Baek M., DiMaio F., Anishchenko I., Dauparas J., Ovchinnikov S., Lee G.R., Wang J., Cong Q., Kinch L.N., Schaeffer R.D., et al. Accurate prediction of protein structures and interactions using a three-track neural network. Science. 2021;373:871–876. doi: 10.1126/science.abj8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jumper J., Evans R., Pritzel A., Green T., Figurnov M., Ronneberger O., Tunyasuvunakool K., Bates R., Žídek A., Potapenko A. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parker C. Ph.D. Thesis. Cranfield University; Cranfield, UK: 2008. Development of an Affinity Sensor for the Detection of Aflatoxin m1 in Milk; pp. 192–194. [Google Scholar]
- 47.Heurich M. Ph.D. Thesis. Cranfield University; Cranfield, UK: 2008. Development of an Affinity Sensor for Ochratoxin A; pp. 227–229. [Google Scholar]
- 48.Bazin I., Tria S.A., Hayat A., Marty J.L. New biorecognition molecules in biosensors for the detection of toxins. Biosensors & Bioelectronics. 2017;87:285–298. doi: 10.1016/j.bios.2016.06.083. [DOI] [PubMed] [Google Scholar]
- 49.Wu Y., Nizam M.N., Ding X., Xu F.-J. Rational design of peptide-functionalized poly (methacrylic acid) brushes for on-chip detection of protease biomarkers. ACS Biomater. Sci. Eng. 2018;4:2018–2025. doi: 10.1021/acsbiomaterials.7b00584. [DOI] [PubMed] [Google Scholar]
- 50.Mendive-Tapia L., Mendive-Tapia D., Zhao C., Gordon D., Benson S., Bromley M.J., Wang W., Wu J., Kopp A., Ackermann L., et al. Rational design of Phe-BODIPY amino acids as fluorogenic building blocks for peptide-based detection of urinary tract candida infections. Angew. Chem. Int. Ed. Engl. 2022;61:e202117218. doi: 10.1002/anie.202117218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J., Muto M., Yatabe R., Tahara Y., Onodera T., Tanaka M., Okochi M., Toko K. Highly selective rational design of peptide-based surface plasmon resonance sensor for direct determination of 2,4,6-trinitrotoluene (TNT) explosive. Sens. Actuators B. 2018;264:279–284. doi: 10.1016/j.snb.2018.02.075. [DOI] [Google Scholar]
- 52.Liu X., Zhang Q., Knoll W., Liedberg B., Wang Y. Rational design of functional peptide-gold hybrid nanomaterials for molecular interactions. Adv. Mater. 2020;32:e2000866. doi: 10.1002/adma.202000866. [DOI] [PubMed] [Google Scholar]
- 53.Levin A., Hakala T.A., Schnaider L., Bernardes G.J.L., Gazit E., Knowles T.P.J. Biomimetic peptide self-assembly for functional materials. Nat. Rev. Chem. 2020;4:615–634. doi: 10.1038/s41570-020-0215-y. [DOI] [Google Scholar]
- 54.Hou S., Ma J., Cheng Y., Wang Z., Yan Y. Overview-gold nanoparticles-based sensitive nanosensors in mycotoxins detection. Crit. Rev. Food Sci. Nutr. 2022;62:1–16. doi: 10.1080/10408398.2022.2095973. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y., Zhang C., Wang J., Knopp D. Recent progress in rapid determination of mycotoxins based on emerging biorecognition molecules: A review. Toxins. 2022;14:73. doi: 10.3390/toxins14020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu R., Yu Z., He Q., Xu Y. An immunoassay for ochratoxin A without the mycotoxin. Food Control. 2007;18:872–877. doi: 10.1016/j.foodcont.2006.05.002. [DOI] [Google Scholar]
- 57.He Z., He Q., Xu Y., Li Y., Liu X., Chen B., Lei D., Sun C. Ochratoxin A Mimotope from Second-Generation Peptide Library and Its Application in Immunoassay. Anal. Chem. 2013;85:10304–10311. doi: 10.1021/ac402127t. [DOI] [PubMed] [Google Scholar]
- 58.Xu Y., He Z., He Q., Qiu Y., Chen B., Chen J., Liu X. Use of Cloneable Peptide-MBP Fusion Protein as a Mimetic Coating Antigen in the Standardized Immunoassay for Mycotoxin Ochratoxin. J. Agric. Food. Chem. 2014;62:8830–8836. doi: 10.1021/jf5028922. [DOI] [PubMed] [Google Scholar]
- 59.Rahi S., Lanjekar V., Ghormade V. Development of a rapid dot-blot assay for ochratoxin A (OTA) detection using peptide conjugated gold nanoparticles for bio-recognition and detection. Food Control. 2022;136:108842. doi: 10.1016/j.foodcont.2022.108842. [DOI] [Google Scholar]
- 60.Zou X., Chen C., Huang X., Chen X., Wang L., Xiong Y. Phage-free peptide ELISA for ochratoxin A detection based on biotinylated mimotope as a competing antigen. Talanta. 2016;146:394–400. doi: 10.1016/j.talanta.2015.08.049. [DOI] [PubMed] [Google Scholar]
- 61.Zou W., Shi R., Wang G., Zhao Z., Zhao F., Yang Z. Rapid and sensitive noncompetitive immunoassay for detection of aflatoxin B1 based on anti-immune complex peptide. Food Chem. 2022;393:133317. doi: 10.1016/j.foodchem.2022.133317. [DOI] [PubMed] [Google Scholar]
- 62.Zhao F., Tian Y., Shen Q., Liu R., Shi R., Wang H., Yang Z. A novel nanobody and mimotope based immunoassay for rapid analysis of aflatoxin B1. Talanta. 2019;195:55–61. doi: 10.1016/j.talanta.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 63.Mukhtar H., Ma L., Pang Q., Zhou Y., Wang X., Xu T., Hammock B.D., Wang J. Cyclic peptide: A safe and effective alternative to synthetic aflatoxin B1-competitive antigens. Anal. Bioanal. Chem. 2019;411:3881–3890. doi: 10.1007/s00216-019-01862-7. [DOI] [PubMed] [Google Scholar]
- 64.Liu B., Peng J., Wu Q., Zhao Y., Shang H., Wang S. A novel screening on the specific peptide by molecular simulation and development of the electrochemical immunosensor for aflatoxin B1 in grains. Food Chem. 2022;372:131322. doi: 10.1016/j.foodchem.2021.131322. [DOI] [PubMed] [Google Scholar]
- 65.Yan J., Shi Q., You K., Li Y., He Q. Phage displayed mimotope peptide-based immunosensor for green and ultrasensitive detection of mycotoxin deoxynivalenol. J. Pharm. Biomed. Anal. 2019;168:94–101. doi: 10.1016/j.jpba.2019.01.051. [DOI] [PubMed] [Google Scholar]
- 66.Xu Y., Yang H., Huang Z., Li Y., He Q., Tu Z., Ji Y., Ren W. A peptide/maltose-binding protein fusion protein used to replace the traditional antigen for immunological detection of deoxynivalenol in food and feed. Food Chem. 2018;268:242–248. doi: 10.1016/j.foodchem.2018.06.096. [DOI] [PubMed] [Google Scholar]
- 67.He Q., Xu Y., Zhang C., Li Y., Huang Z. Phage-borne peptidomimetics as immunochemical reagent in dot-immunoassay for mycotoxin zearalenone. Food Control. 2014;39:56–61. doi: 10.1016/j.foodcont.2013.10.019. [DOI] [Google Scholar]
- 68.He Q., Xu Y., Huang Y.-H., Liu R.-R., Huang Z.-B., Li Y.-P. Phage-displayed peptides that mimic zearalenone and its application in immunoassay. Food Chem. 2011;126:1312–1315. doi: 10.1016/j.foodchem.2010.11.085. [DOI] [Google Scholar]
- 69.Chen Y., Zhang S., Hong Z., Lin Y., Dai H. A mimotope peptide-based dual-signal readout competitive enzyme-linked immunoassay for non-toxic detection of zearalenone. J. Mater. Chem. B. 2019;7:6972–6980. doi: 10.1039/C9TB01167F. [DOI] [PubMed] [Google Scholar]
- 70.Chen Y., Zhang S., Huang Y., Lv L., Dai H., Lin Y. A bio-bar-code photothermal probe triggered multi-signal readout sensing system for nontoxic detection of mycotoxins. Biosens. Bioelectron. 2020;167:112501. doi: 10.1016/j.bios.2020.112501. [DOI] [PubMed] [Google Scholar]
- 71.Liu R., Shi R., Zou W., Chen W., Yin X., Zhao F., Yang Z. Highly sensitive phage-magnetic-chemiluminescent enzyme immunoassay for determination of zearalenone. Food Chem. 2020;325:126905. doi: 10.1016/j.foodchem.2020.126905. [DOI] [PubMed] [Google Scholar]
- 72.Peltomaa R., Fikacek S., Benito-Peña E., Barderas R., Head T., Deo S., Daunert S., Moreno-Bondi M.C. Bioluminescent detection of zearalenone using recombinant peptidomimetic Gaussia luciferase fusion protein. Microchim. Acta. 2020;187:547. doi: 10.1007/s00604-020-04538-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peltomaa R., Farka Z., Mickert M.J., Brandmeier J.C., Pastucha M., Hlaváček A., Martínez-Orts M., Canales Á., Skládal P., Benito-Peña E., et al. Competitive upconversion-linked immunoassay using peptide mimetics for the detection of the mycotoxin zearalenone. Biosens. Bioelectron. 2020;170:112683. doi: 10.1016/j.bios.2020.112683. [DOI] [PubMed] [Google Scholar]
- 74.Zhou J., Wang X., Li Y., Chen Y., Liu Y., Liu H., Liang C., Zhu X., Qi Y., Wang A. Fluorescence immunoassay based on phage mimotope for nontoxic detection of Zearalenone in maize. J. Food Saf. 2022;42:e12982. doi: 10.1111/jfs.12982. [DOI] [Google Scholar]
- 75.Liu X., Xu Y., He Q., He Z., Xiong Z. Application of mimotope peptides of fumonisin B1 in Peptide ELISA. J. Agric. Food. Chem. 2013;61:4765–4770. doi: 10.1021/jf400056p. [DOI] [PubMed] [Google Scholar]
- 76.Xu Y., Chen B., He Q., Qiu Y., Liu X., He Z., Xiong Z. New approach for development of sensitive and environmentally friendly immunoassay for mycotoxin fumonisin B1 based on using peptide-MBP fusion protein as substitute for coating antigen. Anal. Chem. 2014;86:8433–8440. doi: 10.1021/ac502037w. [DOI] [PubMed] [Google Scholar]
- 77.Peltomaa R., Benito-Peña E., Barderas R., Sauer U., González Andrade M., Moreno-Bondi M.C. Microarray-based immunoassay with synthetic mimotopes for the detection of fumonisin B1. Anal. Chem. 2017;89:6216–6223. doi: 10.1021/acs.analchem.7b01178. [DOI] [PubMed] [Google Scholar]
- 78.Peltomaa R., Agudo-Maestro I., Más V., Barderas R., Benito-Peña E., Moreno-Bondi M.C. Development and comparison of mimotope-based immunoassays for the analysis of fumonisin B1. Anal. Bioanal. Chem. 2019;411:6801–6811. doi: 10.1007/s00216-019-02068-7. [DOI] [PubMed] [Google Scholar]
- 79.You T., Ding Y., Chen H., Song G., Huang L., Wang M., Hua X. Development of competitive and noncompetitive immunoassays for clothianidin with high sensitivity and specificity using phage-displayed peptides. J. Hazard. Mater. 2022;425:128011. doi: 10.1016/j.jhazmat.2021.128011. [DOI] [PubMed] [Google Scholar]
- 80.Fu H.J., Chen Z.J., Wang H., Luo L., Wang Y., Huang R.-M., Xu Z.L., Hammock B. Development of a sensitive non-competitive immunoassay via immunocomplex binding peptide for the determination of ethyl carbamate in wine samples. J. Hazard. Mater. 2021;406:124288. doi: 10.1016/j.jhazmat.2020.124288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen H., You T., Zong L., Mukhametova L.I., Zherdev D.O., Eremin S.A., Ding Y., Wang M., Hua X. Competitive and noncompetitive fluorescence polarization immunoassays for the detection of benzothiostrobin using FITC-labeled dendrimer-like peptides. Food Chem. 2021;360:130020. doi: 10.1016/j.foodchem.2021.130020. [DOI] [PubMed] [Google Scholar]
- 82.González-Techera A., Kim H.J., Gee S.J., Last J.A., Hammock B.D., González-Sapienza G. Polyclonal antibody-based noncompetitive immunoassay for small analytes developed with short peptide loops isolated from phage libraries. Anal. Chem. 2007;79:9191–9196. doi: 10.1021/ac7016713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yuan Q., Pestka J.J., Hespenheide B.M., Kuhn L.A., Linz J.E., Hart L.P. Identification of mimotope peptides which bind to the mycotoxin deoxynivalenol-specific monoclonal antibody. Appl. Environ. Microbiol. 1999;65:3279–3286. doi: 10.1128/AEM.65.8.3279-3286.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang D.T., Fu H.J., Huang J.J., Luo L., Lei H.T., Shen Y.D., Chen Z.J., Wang H., Xu Z.L. Mimotope-based immunoassays for the rapid analysis of mycotoxin: A review. J. Agric. Food. Chem. 2021;69:11743–11752. doi: 10.1021/acs.jafc.1c04169. [DOI] [PubMed] [Google Scholar]
- 85.Bazin I., Andreotti N., Hassine A.I.H., De Waard M., Sabatier J.M., Gonzalez C. Peptide binding to ochratoxin A mycotoxin: A new approach in conception of biosensors. Biosens. Bioelectron. 2013;40:240–246. doi: 10.1016/j.bios.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 86.Yan J.X., Hu W.J., You K.H., Ma Z.E., Xu Y., Li Y.P., He Q.H. Biosynthetic mycotoxin conjugate mimetics-mediated green strategy for multiplex mycotoxin immunochromatographic assay. J. Agric. Food. Chem. 2020;68:2193–2200. doi: 10.1021/acs.jafc.9b06383. [DOI] [PubMed] [Google Scholar]
- 87.Hua X., Shi H., Wang M. Phage display peptide library technology and its research progress in immunoassay of pesticide residue. J. Food Saf. Qual. 2014;5:3955–3961. doi: 10.19812/j.cnki.jfsq11-5956/ts.2014.12.036. [DOI] [Google Scholar]
- 88.Zhao F., Shi R., Liu R., Tian Y., Yang Z. Application of phage-display developed antibody and antigen substitutes in immunoassays for small molecule contaminants analysis: A mini-review. Food Chem. 2021;339:128084. doi: 10.1016/j.foodchem.2020.128084. [DOI] [PubMed] [Google Scholar]
- 89.Rossotti M.A., Carlomagno M., González-Techera A., Hammock B.D., Last J., González-Sapienza G. Phage anti-immunocomplex assay for clomazone: Two-site recognition increasing assay specificity and facilitating adaptation into an on-site format. Anal. Chem. 2010;82:8838–8843. doi: 10.1021/ac101476f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.González-Techera A., Vanrell L., Last J.A., Hammock B.D., González-Sapienza G. Phage anti-immune complex assay: general strategy for noncompetitive immunodetection of small molecules. Anal. Chem. 2007;79:7799–7806. doi: 10.1021/ac071323h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim H.J., McCoy M., Gee S.J., González-Sapienza G., Hammock B.D. Noncompetitive phage anti-immunocomplex real-time polymerase chain reaction for sensitive detection of small molecules. Anal. Chem. 2011;83:246–253. doi: 10.1021/ac102353z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lassabe G., Rossotti M., González-Techera A., González-Sapienza G. Shiga-like toxin b subunit of escherichia coli as scaffold for high-avidity display of anti-immunocomplex peptides. Anal. Chem. 2014;86:5541–5546. doi: 10.1021/ac500926f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu S., Du P., Sun H., Yu H.Y., Wang Z.G. Bioinspired supramolecular catalysts from designed self-Assembly of DNA or peptides. ACS Catal. 2020;10:14937–14958. doi: 10.1021/acscatal.0c03753. [DOI] [Google Scholar]
- 94.Hamley I.W. Biocatalysts based on peptide and peptide conjugate nanostructures. Biomacromolecules. 2021;22:1835–1855. doi: 10.1021/acs.biomac.1c00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li X., Li J., Zhu J., Hao S., Fang G., Liu J., Wang S. Degradation of phthalic acid esters (PAEs) by an enzyme mimic and its application in the degradation of intracellular DEHP. Chem. Commun. 2019;55:13458–13461. doi: 10.1039/C9CC06794A. [DOI] [PubMed] [Google Scholar]
- 96.Ding Y., Li Z., Jiang W., Yuan B., Huang T., Wang L., Tang J. Degradation of phenol using a peroxidase mimetic catalyst through conjugating deuterohemin-peptide onto metal-organic framework with enhanced catalytic activity. Catal. Commun. 2020;134:105859. doi: 10.1016/j.catcom.2019.105859. [DOI] [Google Scholar]
- 97.Makam P., Yamijala S.S.R.K.C., Bhadram V.S., Shimon L.J.W., Wong B.M., Gazit E. Single amino acid bionanozyme for environmental remediation. Nat. Commun. 2022;13:1505. doi: 10.1038/s41467-022-28942-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wariishi H., Kabuto M., Mikuni J., Oyadomari M., Tanaka H. Degradation of water-insoluble dyes by microperoxidase-11, an effective and stable peroxidative catalyst in hydrophilic organic media. Biotechnol. Progr. 2002;18:36–42. doi: 10.1021/bp010124v. [DOI] [PubMed] [Google Scholar]
- 99.He X.X., Zhang F.Y., Liu J., Fang G., Wang S. Homogenous graphene oxide-peptide nanofiber hybrid hydrogel as biomimetic polysaccharide hydrolase. Nanoscale. 2017;9:18066–18074. doi: 10.1039/C7NR06525F. [DOI] [PubMed] [Google Scholar]
- 100.Hatakawa Y., Tanaka A., Furubayashi T., Nakamura R., Konishi M., Akizawa T., Sakane T. Direct Delivery of ANA-TA9, a peptide capable of Aβ hydrolysis, to the brain by intranasal administration. Pharmaceutics. 2021;13:1673. doi: 10.3390/pharmaceutics13101673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu Q., Wan K., Shang Y., Wang Z.G., Ding B. Cofactor-free oxidase-mimetic nanomaterials from self-assembled histidine-rich peptides. Nat. Mater. 2021;20:395–402. doi: 10.1038/s41563-020-00856-6. [DOI] [PubMed] [Google Scholar]
- 102.Wang Q., Yang Z., Zhang X., Xiao X., Chang C., Xu B. A supramolecular-hydrogel-encapsulated hemin as an artificial enzyme to mimic peroxidase. Angew. Chem. 2007;46:4285–4289. doi: 10.1002/anie.200700404. [DOI] [PubMed] [Google Scholar]
- 103.Wang Q., Yang Z., Ma M., Chang C.K., Xu B. High catalytic activities of artificial peroxidases based on supramolecular hydrogels that contain heme models. Chem. Eur. J. 2010;14:5073–5078. doi: 10.1002/chem.200702010. [DOI] [PubMed] [Google Scholar]
- 104.Kim M.C., Lee S.Y. Peroxidase-like oxidative activity of a manganese-coordinated histidyl bolaamphiphile self-assembly. Nanoscale. 2015;7:17063–17070. doi: 10.1039/C5NR04893A. [DOI] [PubMed] [Google Scholar]
- 105.Zhang Y., Tian X., Li X. Supramolecular assemblies of histidine-containing peptides with switchable hydrolase and peroxidase activities through Cu (II) binding and co-assembling. J. Mater. Chem. B. 2022;10:3716–3722. doi: 10.1039/D2TB00375A. [DOI] [PubMed] [Google Scholar]
- 106.Korendovych I., Zozulia O., Marshall L., Kohn E., Kim I. Self-assembling catalytic peptidenanomaterials capable of highly efficient peroxidase activity. Chem. Eur. J. 2021;27:5388–5392. doi: 10.1002/chem.202100182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zastrow M.L., Peacock A., Stuckey J.A., Pecoraro V.L. Hydrolytic catalysis and structural stabilization in a designed metalloprotein. Nat. Chem. 2011;4:118–123. doi: 10.1038/nchem.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dorian J.M., Heier J.L., Schade B., Koksch B. Catalytic activity of peptide-nanoparticle conjugates regulated by a conformational change. Biomacromolecules. 2017;18:3557–3562. doi: 10.1021/acs.biomac.7b00887. [DOI] [PubMed] [Google Scholar]
- 109.Zhao Y., Lei B., Wang M., Wu S., Qi W., Su R., He Z. A supramolecular approach to construct a hydrolase mimic with photo-switchable catalytic activity. J. Mater. Chem. B. 2018;6:2444. doi: 10.1039/C8TB00448J. [DOI] [PubMed] [Google Scholar]
- 110.Singh A., Joseph J.P., Gupta D., Miglani C., Pal A. Photothermally switchable peptide nanostructures towards modulating catalytic hydrolase activity. Nanoscale. 2021;13:13401–13409. doi: 10.1039/D1NR03655F. [DOI] [PubMed] [Google Scholar]
- 111.Sarkhel B., Chatterjee A., Das D. Covalent catalysis by cross β amyloid nanotubes. J. Am. Chem. Soc. 2020;142:4098–4103. doi: 10.1021/jacs.9b13517. [DOI] [PubMed] [Google Scholar]
- 112.Huang K.Y., Yu C.C., Horng J.C. Conjugating catalytic polyproline fragments with a self-assembling peptide produces efficient artificial hydrolases. Biomacromolecules. 2020;21:1195–1201. doi: 10.1021/acs.biomac.9b01620. [DOI] [PubMed] [Google Scholar]
- 113.Wang M., Lv Y., Liu X., Wei Q., He Z. Enhancing the activity of peptide-based artificial hydrolase with catalytic ser/his/asp triad and molecular imprinting. ACS Appl. Mater. Interfaces. 2016;8:14133–14141. doi: 10.1021/acsami.6b04670. [DOI] [PubMed] [Google Scholar]
- 114.Zhang C., Xue X., Luo Q., Li Y., Yang K., Zhuang X., Jiang Y., Zhang J., Liu J., Zou G. Self-assembled peptide nanofibers designed as biological enzymes for catalyzing ester hydrolysis. ACS Nano. 2014;8:11715–11723. doi: 10.1021/nn5051344. [DOI] [PubMed] [Google Scholar]
- 115.Mikolajczak D.J., Koksch B. Peptide-gold nanoparticle conjugates as artificial carbonic anhydrase mimics. Catalysts. 2019;9:903–910. doi: 10.3390/catal9110903. [DOI] [Google Scholar]
- 116.Makam P., Yamijala S.S.R.K.C., Tao K., Shimon L.J.W., Gazit E. Non-proteinaceous hydrolase comprised of a phenylalanine metallo-supramolecular amyloid-like structure. Nat. Catal. 2019;2:977–985. doi: 10.1038/s41929-019-0348-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang C., Shafi R., Lampel A., MacPherson D., Pappas C.G., Narang V., Wang T., Maldarelli C., Ulijn R.V. Switchable hydrolase based on reversible formation of supramolecular catalytic site using a self-assembling peptide. Angew. Chem. Int. Ed. 2017;56:14511–14515. doi: 10.1002/anie.201708036. [DOI] [PubMed] [Google Scholar]
- 118.Al-Garawi Z.S., Mcintosh B., Neill-Hall D., Hatimy A., Serpell L. The amyloid architecture provides a scaffold for enzyme-like catalysts. Nanoscale. 2017;9:10773–10783. doi: 10.1039/C7NR02675G. [DOI] [PubMed] [Google Scholar]
- 119.Guler M.O., Stupp S.I. A self-assembled nanofiber catalyst for ester hydrolysis. J. Am. Chem. Soc. 2007;129:12082–12083. doi: 10.1021/ja075044n. [DOI] [PubMed] [Google Scholar]
- 120.Huang Z., Guan S., Wang Y., Shi G., Cao L., Gao Y., Dong Z., Xu J., Luo Q., Liu J. Self-assembly of amphiphilic peptides into bio-functionalized nanotubes: A novel hydrolase model. J. Mater. Chem. B. 2013;1:2297–2304. doi: 10.1039/c3tb20156b. [DOI] [PubMed] [Google Scholar]
- 121.Der B.S., Edwards D.R., Kuhlman B. Catalysis by a de novo zinc-mediated protein interface: Implications for natural enzyme evolution and rational enzyme engineering. Biochemistry. 2012;51:3933–3940. doi: 10.1021/bi201881p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nan G., Kai D., Zhao A., Sun H., Ying W., Ren J., Qu X. Polyoxometalate-based nanozyme: Design of a multifunctional enzyme for multi-faceted treatment of Alzheimer’s disease. Nano Res. 2016;9:1079–1090. doi: 10.1007/s12274-016-1000-6. [DOI] [Google Scholar]
- 123.Nakamura R., Konishi M., Taniguchi M., Hatakawa Y., Akizawa T. The discovery of synthetic proteolytic peptide. Preprints. 2018;1:0106. doi: 10.20944/preprints201801.0106.v1. [DOI] [PubMed] [Google Scholar]
- 124.Nakamura R., Konishi M., Sakaguchi Y., Hatakawa Y., Tanaka A., Sakane T., Saito M., Akizawa T. JAL-TA9 inhibits aggregation of hPrP180-192 through the cleavage reaction. J. Pharm. Pharmacol. Res. 2020;4:23–33. doi: 10.26502/fjppr.029. [DOI] [Google Scholar]
- 125.Nakamura R., Konishi M., Hatakawa Y., Saito M., Akizawa T. The novel catalytic peptide, a synthetic nona-peptide (JAL-TA9) derived from Tob1 protein, digests the amyloid-β peptide. J. R. Sci. 2019;1:30–35. [Google Scholar]
- 126.Nakamura R., Konishi M., Taniguchi M., Hatakawa Y., Akizawa T. The discovery of shorter synthetic proteolytic peptides derived from Tob1 protein. Peptides. 2019;116:71–77. doi: 10.1016/j.peptides.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 127.Hatakawa Y., Nakamura R., Konishi M., Sakane T., Saito M., Akizawa T. Catalytides derived from the Box A region in the ANA/BTG3 protein cleave amyloid-β fragment peptide. Heliyon. 2019;5:e02454. doi: 10.1016/j.heliyon.2019.e02454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hatakawa Y., Nakamura R., Konishi M., Sakane T., Tanaka A., Matsuda A., Saito M., Akizawa T. Amyloid beta cleavage by ANA-TA9, a synthetic peptide from the ANA/BTG3 Box A region. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2021;7:e12146. doi: 10.1002/trc2.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tanaka F., Barbas Iii C.F. Phage display selection of peptides possessing aldolase activity. Chem. Commun. 2001;8:769–770. doi: 10.1039/b100479b. [DOI] [Google Scholar]
- 130.Tanaka F., Barbas C.F. A modular assembly strategy for improving the substrate specificity of small catalytic peptides. J. Am. Chem. Soc. 2002;124:3510–3511. doi: 10.1021/ja0171815. [DOI] [PubMed] [Google Scholar]
- 131.Rufo C.M., Moroz Y.S., Moroz O.V., Stöhr J., Smith T.A., Hu X., DeGrado W.F., Korendovych I.V. Short peptides self-assemble to produce catalytic amyloids. Nat. Chem. 2014;6:303–309. doi: 10.1038/nchem.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Choi D.W., Semrau J.D., Antholine W.E., Hartsel S.C., Anderson R.C., Carey J.N., Dreis A.M., Kenseth E.M., Renstrom J.M., Scardino L.L., et al. Oxidase, superoxide dismutase, and hydrogen peroxide reductase activities of methanobactin from types I and II methanotrophs. J. Inorg. Biochem. 2008;102:1571–1580. doi: 10.1016/j.jinorgbio.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 133.Begum S.Z., Nizam N., Muhamad A., Saiman M.I., Rahman M. Imidazole-rich copper peptides as catalysts in xenobiotic degradation. PLoS ONE. 2020;15:e0238147. doi: 10.1371/journal.pone.0238147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xin J.-Y., Li C.-Y., Zhang S., Wang Y., Zhai W., Xia C.-G. Cu-induced assembly of methanobactin-modified gold nanoparticles and its peroxidase mimic activity. IET Nanobiotechnol. 2018;12:915–921. doi: 10.1049/iet-nbt.2018.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gkaniatsou E., Serre C., Mahy J.-P., Steunou N., Ricoux R., Sicard C. Enhancing microperoxidase activity and selectivity: Immobilization in metal-organic frameworks. J. Porphyr. Phthalocyanines. 2019;23:718–728. doi: 10.1142/S1088424619300106. [DOI] [Google Scholar]
- 136.Lykourinou V., Chen Y., Wang X.S., Meng L., Hoang T., Ming L.J., Musselman R.L., Ma S. Immobilization of MP-11 into a mesoporous metal-organic framework, MP-11@mesoMOF: A new platform for enzymatic catalysis. J. Am. Chem. Soc. 2011;133:10382–10385. doi: 10.1021/ja2038003. [DOI] [PubMed] [Google Scholar]
- 137.Polgár L. The catalytic triad of serine peptidases. Cell. Mol. Life Sci. 2005;62:2161–2172. doi: 10.1007/s00018-005-5160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen Y., Yang Y., Orr A.A., Makam P., Redko B., Haimov E., Wang Y., Shimon L.J., Rencus-Lazar S. Self-assembled peptide nano-superstructure towards enzyme mimicking hydrolysis. Angew. Chem. 2021;60:17164–17170. doi: 10.1002/anie.202105830. [DOI] [PubMed] [Google Scholar]
- 139.Li X., Li J., Hao S., Han A., Yang Y., Fang G., Liu J., Wang S. Enzyme mimics based membrane reactor for di (2-ethylhexyl) phthalate degradation. J. Hazard. Mater. 2021;403:123873. doi: 10.1016/j.jhazmat.2020.123873. [DOI] [PubMed] [Google Scholar]
- 140.Razkin J., Nilsson H., Baltzer L. Catalysis of the cleavage of uridine 3′-2,2,2-trichloroethylphosphate by a designed helix-loop-helix motif peptide. J. Am. Chem. Soc. 2007;129:14752–14758. doi: 10.1021/ja075478i. [DOI] [PubMed] [Google Scholar]
- 141.Hahn K.W., Klis W.A., Stewart J.M. Design and synthesis of a peptide having chymotrypsin-like esterase activity. Science. 1990;248:1544–1547. doi: 10.1126/science.2360048. [DOI] [PubMed] [Google Scholar]
- 142.Zaramella D., Scrimin P., Prins L.J. Self-assembly of a catalytic multivalent peptide–nanoparticle complex. J. Am. Chem. Soc. 2012;134:8396–8399. doi: 10.1021/ja302754h. [DOI] [PubMed] [Google Scholar]
- 143.Burton A.J., Thomson A.R., Dawson W.M., Brady R.L., Woolfson D.N. Installing hydrolytic activity into a completely de novo protein framework. Nat. Chem. 2016;8:837–844. doi: 10.1038/nchem.2555. [DOI] [PubMed] [Google Scholar]
- 144.He X., Zhang F., Zhang L., Zhang Q., Fang G., Liu J., Wang S., Zhang S. Probing the structure–activity relationship of a novel artificial cellobiose hydrolase. J. Mater. Chem. B. 2017;5:5225–5233. doi: 10.1039/C7TB01426K. [DOI] [PubMed] [Google Scholar]
- 145.Adler-Abramovich L., Vaks L., Carny O., Trudler D., Magno A., Caflisch A., Frenkel D., Gazit E. Phenylalanine assembly into toxic fibrils suggests amyloid etiology in phenylketonuria. Nat. Chem. Biol. 2012;8:701. doi: 10.1038/nchembio.1002. [DOI] [PubMed] [Google Scholar]
- 146.Peme T., Brady D., Juma W., Makatini M. Development of fructose-1,6-bisphosphate aldolase enzyme peptide mimics as biocatalysts in direct asymmetric aldol reactions. RSC Adv. 2021;11:36670–36681. doi: 10.1039/D1RA06616A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang B., Xu L., Ding J., Wang M., Ge R., Zhao H., Zhang B., Fan J. Natural antimicrobial lipopeptides secreted by Bacillus spp. and their application in food preservation, a critical review. Trends Food Sci. Technol. 2022;127:26–37. doi: 10.1016/j.tifs.2022.06.009. [DOI] [Google Scholar]