Abstract
The ongoing SARS-CoV-2 pandemic represents a brutal reminder of the continual threat of mucosal infectious diseases. Mucosal immunity may provide robust protection at the predominant sites of SARS-CoV-2 infection. However, it remains unclear whether respiratory mucosal administration of DNA vaccines could confer protective immune responses against SARS-CoV-2 challenge due to insurmountable barriers posed by the airway. Here, we applied self-assembled peptide-poloxamine nanoparticles with mucus-penetrating properties for pulmonary inoculation of a COVID-19 DNA vaccine (pSpike/PP-sNp). The pSpike/PP-sNp not only displays superior gene transfection and favorable biocompatibility in the mouse airway, but also promotes a tripartite immunity consisting of systemic, cellular, and mucosal immune responses that are characterized by mucosal IgA secretion, high levels of neutralizing antibodies, and resident memory phenotype T-cell responses in the lungs of mice. Most importantly, immunization with pSpike/PP-sNp completely eliminates SARS-CoV-2 infection in both upper and lower respiratory tracts and enables 100% survival rate of mice following lethal SARS-CoV-2 challenge. Our findings indicate PP-sNp is a promising platform in mediating DNA vaccines to elicit all-around mucosal immunity against SARS-CoV-2.
Keywords: Mucosal vaccine, SARS-CoV-2, DNA vaccine, Pulmonary delivery, Nonviral delivery system
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has posed a huge and continual threat to the world. Although there are already several authorized COVID-19 vaccines, they are inoculated via the injectable approach which predominantly elicits systemic immunity dominated by serum IgG antibodies without conferring mucosal immunity [1]. SARS-CoV-2, which invades the host through the mucosa of the respiratory tract, may seed in the initial reservoir and continue to spread between individuals [2]. Recent studies have demonstrated that respiratory mucosal vaccinated adenoviral vaccines could overcome the drawbacks of intramuscularly administered counterparts via stimulation of broad local immune responses in the airways that in turn block both infection and spread from this reservoir [[3], [4], [5], [6]]. Secretory immunoglobulin A (sIgA) and pulmonary resident memory T cells (TRM) are suggested to be key components in this first-line of defense [[7], [8], [9]]. There are compelling evidences indicating that sIgA in the respiratory mucosa contributed to SARS-CoV-2 neutralization to a greater extent than IgG equivalents [10,11], and lung-TRM cells are critical in mediating protection against respiratory pathogens [12]. Since the mucosal route of immunization is considered by the research community as the most straightforward approach to induce potent mucosal immunity [13], safe and efficient delivery of vaccine to the respiratory mucosa would be needed in order to reliably stimulate sIgA secretion and engender protective mucosal immunity against SARS-CoV-2 [14].
Despite the great potential of mucosal immunity, the major advances seen with injectable vaccines (such as adjuvanted subunit antigens, DNA, and more recently RNA vaccines) have not been translated into licensed mucosal vaccines [15]. There continues to be a dearth of safe and effective respiratory mucosal vaccine platforms despite decades of investigations [16]. Of the nine mucosal vaccines approved for use in humans (one intranasal and eight oral) all are either live attenuated or whole-cell inactivated vaccines [15]. The dichotomy in intramuscular and mucosal approaches is, in part, due to the existence of multiple barriers (e.g., the mucus layer that traps exogenously inhaled substances) and natural defense mechanisms (such as mucociliary clearance) keeping the foreign substances out [13]. Viruses have evolved for millions of years to proficiently overcome these barriers, underpinning their popularity among the studies with the topic of airway-administered vaccines against SARS-CoV-2 [6,[17], [18], [19]]. Nevertheless, these virus vaccines are often compromised by preexisting immunity and safety issues, some adenovirus-based COVID-19 vaccines may cause cerebral venous sinus thrombosis [20], provoking strict limits by the US Food and Drug Administration (FDA). Indeed, it is difficult to straddle a delicate balance between immune efficacy and safety of these virus vaccines. On the other hand, cutting-edge advances and research into the nucleic acid-based vaccine technologies provide alternative options to solve the unmet needs for safe and efficient non-viral based mucosal vaccines.
DNA vaccines, having proven themselves in controlling the pandemic [21,22], would offer substantial advantages over virus-based vaccines, including safe profiles [23,24], ease of manufacture with low costs, and stability at room temperature [25]. However, DNA vaccines generally display limited immunogenicity in the airways because the most commonly used electroporation device is not applicable in the respiratory route of administration, and consequently require potent delivery systems to overcome the barriers within the respiratory tract [26]. Traditional DNA delivery vectors, such as cationic polymers and even lipid-based formulations that were tested in clinical trials, have turned out to be inefficient owing to the poor airway mucus penetrating properties [27,28]. Therefore, the pulmonary administration of conventional DNA nanoparticles is unlikely to shuttle DNA cargos efficiently to initiate robust immune responses in the respiratory tract. As revealed by a previous study, the intranasal COVID-19 DNA vaccination in mice only led to a modest induction of local T-cells secreting IFN-γ, without eliciting mucosal sIgA and circulating IgG against SARS-CoV-2 even after prime and two boost doses [29]. To the best of our knowledge, whether a COVID-19 DNA vaccine delivered via the airway could confer protective efficacy against SARS-CoV-2 remains unknown.
In a previous study, we developed a self-assembled peptide-poloxamine nanoparticle (PP-sNp) based carrier that is specifically designed for efficient delivery of plasmid DNA and in vitro transcribed mRNA across the mucus layer of the respiratory tract [30]. The composition and structure of PP-sNp are completely distinct from conventional lipid-based and cationic polymeric gene delivery systems. The neutral charged poloxamine component with amphiphilic properties confers the PP-sNp ability to efficiently penetrate mucus barriers, while the peptide component allows a versatile integration of different functional modules, such as targeting modules that enable the delivery of PP-sNp into specific cells (e.g. bronchial epithelial cells) [30]. PP-sNp carrying a Sleeping Beauty transposon system successfully enabled the genomic integration of CFTR gene in the airway epithelia of cystic fibrosis mice with a safe integration profile [30]. These results suggest that PP-sNp would be promising in mediating DNA vaccines to stimulate potent mucosal immune responses against SARS-CoV-2. To this end, we integrated an intercellular adhesion molecule-1 (ICAM-1/CD54) targeting moiety into the PP-sNp with the purpose of enhancing specific cellular uptake into pulmonary dendritic cells and airway epithelial cells. These cells, which abundantly express ICAM-1 [31], have been suggested to play important roles in both initiating potent respiratory immune responses and orchestrating innate immunity to maintain normal airway architecture [32,33]. A DNA vaccine encoding the wild-type spike protein of SARS-CoV-2 (pSpike, which has been tested in clinical trials [23]) was loaded into PP-sNp (pSpike/PP-sNp) for mucosal vaccine applications. Three vaccine doses of pSpike/PP-sNp via respiratory tract induced comprehensive and broad protective immune responses, including mucosal immunity (SARS-CoV-2 specific sIgA in the airways as well as lung-TRM cells) and systemic immunity (neutralizing serum IgG). All these merits conferred virtually full protection of vaccinated mice against lethal SARS-CoV-2 challenge and completely removed the virus in both the turbinates and lungs of mice. Thus, our study may serve as the first proof-of-concept demonstrating that respiratory mucosal immunization with DNA vaccines holds great potential to elicit robust protective immunity against SARS-CoV-2 infection in both the upper and lower respiratory tracts.
2. Results
2.1. Characterization and in vitro investigation of peptide-poloxamine nanoparticle (PP-sNp) containing plasmid DNA
The PP-sNp formulation is prepared by a simple self-assembly of multi-modular peptide (Table S1), poloxamine 704 (Fig. S1), and pDNA components (Fig. 1 a). This system has been described in our previous work [30]. Briefly, Poloxamine 704 is a kind of poloxamine-based copolymer with amphiphilic and nearly neutral properties, which can elevate the stability and biocompatible of nanoparticles. Poloxamine 704 is a block copolymer proven to be capable of mediating efficient DNA transfection in the airways to a level that is significantly better than “gold-standard” lipid-based GL67A formulation utilized in clinical trials [28,34,35]. The synthetic peptide (Table S1) consists of three functional moieties, namely: (1) an anchor moiety containing hydrophobic molecules used to interact with the hydrophobic blocks of Poloxamine 704; (2) a cationic moiety comprising several basic amino acids to condense pDNA and facilitate efficient endosomal escape; and (3) a targeting moiety that actively directs the pDNA payloads to target cells. The synthetic peptide is designed to spontaneously form ternary complexes with Poloxamine 704 copolymer and pDNA via a simple self-assembly process, thus addressing the extracellular barriers in the lung tissue. We engineered small (size: 116.6 ± 1.7 nm) and monodisperse (PDI: 0.183) PP-sNp nanoparticles with negative charge (ζ potential: 34.7 ± 0.5 mV) via an optimized procedure to best manage the small volumes and high concentrations required in vivo (Fig. 1b; Tables S2–3). The electron microscopy showed that PP-sNp has a spherical morphology with uniform size distribution (Fig. 1c and Fig. S2). PP-sNp can be stored at room or high temperatures and remains stable (Fig. S3). We then evaluated the uptake efficiency of PP-sNp in DC2.4 and MH-S cell lines (these cell lines were adopted as in vitro models of dendritic cells and alveolar macrophages, respectively), the results indicate an enhanced cellular uptake of PP-sNp containing Cy5 labeled pDNA (Cy5-pDNA) by both cell lines compared to the 25 kDa branched polyethyleneimine (PEI, a representative polymeric delivery vehicle in DNA mucosal vaccines [[36], [37], [38]])-based and naked Cy5-pDNA-based controls (Fig. 1d and S4). Confocal microscopy revealed that the majority of Cy5-fluorescence was observed in the nucleus of Cy5-pDNA/PP-sNp transfected DC2.4 cells with dispersed patterns, while the Cy5-fluorescence signal within the nucleus of cells transfected by Cy5-pDNA/PEI and naked Cy5-pDNA was very limited (Fig. 1e). Afterwards, pDNA encoding firefly luciferase (pFLuc) was applied as a reporter to evaluate transfection efficiency. As shown in Fig. 1f and S5, PP-sNp displayed the most efficient transfection in DC2.4 cells (mouse bone marrow-derived dendritic cells), Calu-3 cells (human lung adenocarcinoma cells), 16HBE cells (human bronchial epithelial cells), and BEAS-2B cells (human bronchial epithelial cells) compared to control formulations, such as lipofectamine2000 (lipo2000)-based lipoplex, PEI-based polyplex, and naked-pFLuc. We also synthesized a peptide without the targeting moiety (Table S1) to confirm the effect of target sequence on transfection efficiency. Transfection efficiency of a peptide with targeting moiety in PP-sNp was significantly higher than the formulations without targeting moiety in DC2.4 cells and 16HBE cells (Fig. S6). Furthermore, the transfection efficiency was also investgated after incubating with ICAM-1 inhibitor. The results indicate that the transfection efficiency mediated by PP-sNp was affected by the ICAM-1 inhibitor in DC2.4 and 16HBE cells. Transfection mediated by PEI, however, was unaffected (Fig. S7). The results indicate that the targeting moiety of the synthetic peptide is crucial for efficient PP-sNP-mediated gene transfection. Meanwhile, we evaluated the cytotoxicity of these formulations and found that PP-sNp-based formulations did not provoke significant cytotoxicity on the viability of these cell lines, whereas Lipo2000-based and PEI-based counterparts did (Fig. S8). Additionally, multiple particle tracking (MPT) assays were performed to evaluate the mucus penetrating ability of PP-sNp. The trajectories of the PP-sNp-based and PEI-based particle motions were captured (see Videos S1 and S2), and representative trajectories were mapped in Fig. 1g. PEI nanoparticles were almost trapped by the mucus network. In contrast, PP-sNp was able to move freely in a large area, displaying a better diffusion pattern. The mean square displacement (MSD) of PP-sNp was about 1000-fold higher than the PEI counterpart at 10 s (Fig. 1h). The effective diffusivities (Deff) of PP-sNp were consistently significantly higher than that of PEI (Fig. 1i). The distinct movement patterns suggest that PP-sNp hold the capability of efficient mucus penetrating. Meanwhile, we confirmed a deep penetration and widespread distribution of the intratracheally administered Cy5-pSpike/PP-sNp in the mucus-covered respiratory tract in vivo (Fig. S9). On the contrary, identically administered pSpike/PEI or naked-pSpike was almost undetectable (Fig. S9), demonstrating the superior mucus penetrating ability of PP-sNp.
Fig. 1.
Characterization and in vitro evaluation of pDNA/PP-sNp. a) Schematic showing the formation of pDNA/PP-sNp through a self-assembly process in aqueous solution. b) The dynamic light scattering results measured for particle sizes of pDNA/PP-sNp prepared by different methods. c) The transmission electron microscopy (TEM) micrograph pDNA/PP-sNp. Scale bar: 100 nm. d) Cellular uptake of Cy5-pDNA/PP-sNp, Cy5-pDNA/PEI and naked Cy5-pDNA in DC2.4 and MH-S cells. Cy5-fluorescence intensity within the cells was measured by flow cytometry. e) Subcellular fate of the Cy5-pDNA/PP-sNp, Cy5-pDNA/PEI, and naked Cy5-pDNA after 4 h incubation with DC2.4 cells detected by confocal laser scanning microscopy. Blue channel, nuclei stained by DAPI; Red channel, Cy5-pDNA; Merged, combination of the aforementioned channels. Scale bars: 20 μm. f) Transfection efficiency of pFLuc/PP-sNp in DC2.4 cells. DC2.4 cells were incubated with pFLuc/PP-sNp, pFLuc/PEI, pFLuc/lipo2000, or naked-pFLuc for 4 h, then subjected to detection of bioluminescence in cell lysate 48 h post transfection. Data in f represent mean ± SEM (n = 3 independent experiments). Statistical significance was calculated by one-way ANOVA with Dunnett's multiple comparisons tests (**P < 0.01, ****P < 0.0001). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Supplementary data related to this article can be found at https://doi.org/10.1016/j.biomaterials.2022.121907.
The following are the supplementary data related to this article:
Multiple particle tracking (MPT) studies were carried out to investigate the movement of PP-sNp in mucus mimicking gel, the motion of PEI nanoparticles was quantified for comparison as a control. g) Representative trajectories for particles in mucin solution (3%, w/v) during the 15-s movies. h) MSD of individual nanoparticle as a function of the time scale. i) Deff of individual nanoparticle (n > 100 nanoparticles per experiment). Data in h and i represent mean ± SEM of three independent experiments. A two-tailed unpaired t-test was used to determine the significance of the indicated comparisons (****P < 0.0001).
2.2. In vivo evaluation of the transfection properties and biocompatibility of PP-sNp
Based on our previous experience, intratracheally administered PP-sNp tends to result in more efficient gene transfection compared to intranasal counterpart. So we applied intratracheal route of dosing for all formulations in current study. As shown in Fig. 2a and S10, PP-sNp led to strong bioluminescence signals in the lungs of mice 24 h later, the signal reached peak at 48 h post-dosing and declined to the background level 7 days afterwards. Bioluminescence signals from live animals and excised organs reveal intratracheally administered PP-sNp exhibited superior efficacy compared to pFLuc/PEI or naked-pFLuc counterparts, and the lung was the most abundant FLuc-expressing site and no signals were detected in other organs (e.g., heart, live, spleen, kidneys and small intestines) after PP-sNp transfection (Fig. 2 b). Based on these results, we further evaluated the capability of PP-sNp in intratracheal delivery of a plasmid encoding the full-length spike protein of SARS-CoV-2 (pSpike) [23]. Considerable levels of spike gene-specific mRNA were detected in the lungs of mice transfected by pSpike/PP-sNp compared to pSpike/PEI or naked-pSpike counterparts (Fig. 2c). Histopathological analysis demonstrated that the lungs and other major organs (including heart, liver, spleen, and kidney) from pSpike/PP-sNp treated mice were indistinguishable from naked-pSpike and phosphate-buffered saline (PBS) controls, without severe histopathological signs of inflammation (Fig. S11). In contrast, administration of pSpike/PEI counterpart resulted in signs of interstitial oedema, damage to the epithelial stconsistent with previous findings indicating that the highly positively charged and non-degradable nature of PEI based formulation tends to provoke apoptosis and stress responses [39]. Meanwhile, we also observed that pSpike/PP-sNp may rapidly and robustly, but only transiently and locally, activate innate immunity. As shown in Fig. 2d, tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) expression within bronchoalveolar lavage fluid (BALF) and supernatants of lung homogenates peaked during 1–2 days after administration then immediately restored to background levels. However, no significant increase of cytokines was found in the spleen-based and serum-based counterparts (Fig. S12). The PEI-based formulation induced TNF-α significant and persistent expression in BALF and supernatants of lung homogenates that somehow reflected the heightened levels of lung inflammation, which was consistent with the results of Fig. S11. The PEI-based formulation was excluded in the following studies due to its poor transfection efficiency and toxicity profiles. Mature DCs are powerful antigen-presenting cells (APCs) and play key roles in initiating antigen-specific immune responses. We therefore evaluated whether the pulmonary DCs (CD11c+CD11b+) can efficiently uptake the pSpike/PP-sNp. Comparative analysis showed that 6.15 ± 0.65% of pulmonary DCs internalized Cy5-pSpike/PP-sNp with a mean fluorescence intensity (MFI) of 232.0 ± 20.60, which was significantly higher than naked Cy5-pSpike counterpart with a 1.23 ± 0.64% uptake rate (MFI: 62.5 ± 19.90) (Fig. 2e and S13). Immunofluorescence sections of airway region suggest that considerable pSpike expression mediated by PP-sNp could be clearly observed in pulmonary DCs and airway epithelial cells (AECs) with a widespread distribution pattern, while the spike protein signal within naked-pSpike treated group was very limited and neither co-localize with pulmonary DCs nor AECs (Fig. 2f). We further examined the ability of pSpike/PP-sNp in promoting DC maturation. Compared to naked-pSpike, pSpike/PP-sNp could induce two-fold expressions of CD11c+CD86+ on bone marrow derived cells (BMDCs) (Fig. 2g and S14). The expressions of CD86+MHC-II+ on CD11c+ cells were significantly elevated by more than 300% in pSpike/PP-sNp treated group compared to naked-pSpike control (Fig. 2h and S14).
Fig. 2.
In vivo transfection profiles and biocompatibility of pDNA/PP-sNp. a) Kinetics of transgene expression mediated by intratracheally administered pFLuc/PP-sNp over time in mice. Data represent mean ± SEM (n = 5 biologically independent samples). b) Images of in vivo bioluminescence induced by pFLuc/PP-sNP, pFLuc/PEI, and naked-pFLuc 48 h after intratracheal dosing in mice. c) The expression of the SARS-CoV-2 spike gene specific-mRNA in lung of mice intratracheally administered pSpike/PP-sNp, pSpike/PEI, and naked-pSpike, measured by RT-qPCR 48 h after transfection. d) Supernatants of lung homogenates and BALF samples were collected at indicated time points and were analyzed for cytokine levels via enzyme-linked immunosorbent assay (ELISA). Data represent mean ± SEM (n = 3 biologically independent samples). e) In vivo DC uptake of pSpike/PP-sNp and naked-pSpike 15 h following intratracheal administration. Mice were sacrificed to detect the fluorescence intensity of Cy5 in pulmonary DC cells via flow cytometry. f) Immunohistochemical staining of the spike protein (red channel) and CD11c (green channel) or ZOl (zonula occludens protein 1, which indicates the epithelium, green channel) in lung of mice treated by pSpike/PP-sNp and naked-pSpike via pulmonary route. Scale bars: 25 μm. g) Expression of CD11c+CD86+ or h) CD86+MHC-II+ (gated on CD11c+) on BMDCs after 24 h incubation with pSpike/PP-sNp, naked-pSpike and phosphate buffered saline (PBS) (n = 3 biologically independent samples). Each symbol in the bar chart of c, e, g, and h represents one sample from a biologically independent mouse. Data in c, e, g and h are shown as mean ± SEM. One-way ANOVA with Dunnett's multiple comparisons tests was used to determine significance (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3. pSpike/PP-sNp induces robust mucosal and humoral immunity
To determine the immunogenicity of mucosal vaccination, mice were immunized via intratracheal route with pSpike/PP-sNp, naked-pSpike, an empty vector pVax loaded PP-sNp (pVax/PP-sNp, served as Mock control) or PBS (Fig. 3 a). The body weight of mice treated by pSpike/PP-sNp and naked-pSpike transiently decreased during 2–3 days post the prime and 1st boost dosing, then progressively recovered to the levels displayed by other control groups (Fig. 3b). We verified neither pSpike/PEI with intratracheal immunization (Fig. S15) or pSpike/PP-sNp with intramuscular administration (Fig. S16) had succeeded to induce spike-specific IgG or sIgA antibodies in serum or BALF samples. We also confirmed the necessity of the 2nd boost dose by detection of IgG in serum and sIgA in BALF at pre-determined time points (Fig. S17 ). Intratracheal immunization of pSpike/PP-sNp, but neither pVax/PP-sNp, naked-pSpike nor PBS controls, induced high levels of spike-specific IgG antibodies in serum samples (Fig. 3c). The highest IgG titer within serum from pSpike/PP-sNp group reached 1/51,200 on day 35 (Fig. 3d). The titer ratio of IgG2a/IgG1 implies that pSpike/PP-sNp tends to induce a Type 1 T helper cell (Th1)-biased immune response (Fig. S18) [40,41]. Most importantly, only pSpike/PP-sNp induced high levels of spike-specific sIgA antibody in BALF with most samples reaching an endpoint titer of 1/128 (Fig. 3e and f). The neutralization titer (ND50) of serum and BALF samples from pSpike/PP-sNp group approached ∼1/1875 (Fig. 3g) and ∼1/73 (Fig. 3h), respectively. Whereas no neutralizing antibodies (NAb) could be detected in samples from other groups (naked-pSpike, pVax/PP-sNp and PBS).
Fig. 3.
Humoral immune responses after intratracheal immunization of pSpike/PP-sNp in mice. a) Schematic diagram of immunization, sample collection and challenge schedule. Mice were immunized on day 0 and boosted with the same dose on day 14 and day 28, respectively. b) Animal weights were recorded during the whole period of immunization. Data represent mean ± SEM (n = 10 mice/group). c) OD450 nm values of SARS-CoV-2 spike (S1 + S2) protein-specific IgG in serial diluted serum samples collected 35 days after initial vaccination. Data represent mean ± SEM (n = 10 biologically independent samples). d) The SARS-CoV-2 spike (S1 + S2) protein-specific IgG antibody titer in serum samples collected 35 days after initial vaccination. Each symbol represents one sample from a biologically independent mouse. e) OD450 nm values of SARS-CoV-2 spike (S1 + S2) protein-specific sIgA in serial diluted BALF samples collected 35 days after priming. Data represent mean ± SEM (n = 10 biologically independent samples). f) The SARS-CoV-2 spike (S1 + S2) protein-specific sIgA antibody titer in BALF samples collected 35 days after initial vaccination. Each symbol represents one sample from a biologically independent mouse. Pseudovirus neutralizing antibody titer in g) serum samples and h) BALF samples collected 35 days after priming. Data in g and h are shown as mean ± SEM of samples collected from three mice. One-way ANOVA with Dunnett's post-hoc test was used to determine significance within d and f (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
3.1. pSpike/PP-sNp elicits potent T cell responses and memory-biased immunity
In order to investigate cellular immune responses activated via mucosal immunization, we assessed the production of interferon-γ (IFN-γ) and interleukin-4 (IL-4) in pulmonary lymphocytes or splenocytes using ELISpot assay. A significantly higher level of IFN-γ and IL-4 secretion was detected in pulmonary lymphocytes from pSpike/PP-sNp group, while negligible levels of both cytokines were detected in samples from other groups (Fig. 4 a and b). A similar trend was also observed in splenocyte samples (Figs. S19 and S20). We next evaluated the intracellular IFN-γ and IL-4 production within CD4+ and CD8+ T cells after ex vivo re-stimulation. Flow cytometry analysis showed that pSpike/PP-sNp led to a significant secretion of IFN-γ+ by CD4+ (1.412 ± 0.077)% (Fig. 4c) and CD8+ T cells (1.212 ± 0.102)% (Fig. 4d) within pulmonary lymphocytes. However, there was no significant difference in IL-4 secretion by CD4+ T cells between pSpike/PP-sNp immunized mice and naked-pSpike treated ones (Fig. 4e). We also failed to detect significant secretion of IFN-γ or IL-4 in splenic CD8+ T and CD4+ T cells (Figs. S21 and S22). These data reveal a comprehensive SARS-CoV-2-specific Th1 and cytotoxic T cells activation in the lung of pSpike/PP-sNp-treated mice. To determine whether memory T cell responses within pulmonary mucosal sites were elicited by pSpike/PP-sNp, the effector memory T (TEM) cells or central memory T (TCM) cells, identified as CD44hiCD62Llo and CD44hiCD62Lhi, were detected by flow cytometry. We found both CD4+ TEM and CD8+ TEM cells in lung, but not splenic TEM cells or TCM cells, were significantly activated via the immunization of pSpike/PP-sNp (Fig. 4f and g, S23 and S24). Recent studies have suggested that tissue-resident memory T (TRM) cells play crucial roles in maintaining long-term protective immunity against mucosal pathogens [42,43]. We investigated the expression of the tissue-retention markers CD69 and CD103 on total CD4+ or CD8+ T cells isolated from the lung. As shown in Fig. 4h, CD4+CD69+ cells and CD8+CD69+ cells in pSpike/PP-sNp immunized group displayed 5.6- and 3.3-fold increase than naked-pSpike counterpart, respectively. Although the activation of CD103 maker was relatively limited, the presence of CD4+CD69+CD103+ cells and CD8+CD69+CD103+ cells within pSpike/PP-sNp immunized group could be identified and was significantly higher than other controls (Fig. 4i). These results denote the tremendous potential of pSpike/PP-sNp in inducing comprehensive mucosal immune responses and immune memory to impart a powerful anti-SARS-CoV-2 protection.
Fig. 4.
SARS-CoV-2-specific T cell immune responses and memory-biased immunity in pSpike/PP-sNP vaccinated mice. Enzyme-linked immunospot (ELISpot) analyses of a) IFN-γ and b) IL-4 spot-forming cells in pulmonary lymphocyte after re-stimulation with peptide pools of 14-mer overlapping peptides spanning the SARS-CoV-2 receptor binding domain (RBD) region. c) CD4+ T cells and d) CD8+ T cells in the lung were assayed for IFN-γ+ expression by flow cytometry after re-stimulation with the SARS-CoV-2 RBD peptide pool. e) CD4+ T cells in the lung were analyzed for IL-4+ expression via flow cytometry in the same way as describe above. f) Percentage of CD4+ effector memory T cells (TEM) co-expressing CD44hi and CD62Llo and central memory T cells (TCM) co-expressing CD44hi and CD62Lhi in the lung of mice 35 days after priming. g) Percentage of CD8+ TEM and TCM in lung of mice 35 days after priming. h) Percentage of CD4+ TRM and i) CD8+ TRM in lung of mice 35 days after initial vaccination. Data in a-i represent mean ± SEM (n = 5 biologically independent samples). One-way ANOVA with Dunnett's post-hoc test was used to determine significance (**P < 0.01, ***P < 0.001, ****P < 0.0001).
3.2. Immunization with pSpike/PP-sNp completely prevents lethal SARS-CoV-2 infection in the upper and lower respiratory tracts
The data described above prompted us to confirm if sufficient protection against SARS-CoV-2 could be achieved via mucosal immunization of pSpike/PP-sNp. To this end, a SARS-CoV-2 C57MA14 strain (which causes severe respiratory symptoms and mortality in mice) was applied in the challenge study. The pSpike/PP-sNp vaccine demonstrated remarkable protective efficacy as evidenced by an 100% survival rate at the predetermined end (14 days post-challenge), while all mice from control groups were deceased during 4–7 days after challenge (Fig. 5 a). The body weights of pSpike/PP-sNp vaccinated mice decreased slightly (<10%) in the first 3 days post-challenge but gradually recovered to a level displayed by the control group (without challenge). In contrast, pVax/PP-sNp and PBS groups led to a dramatic decrease in body weight until 6 days after challenge when all animals died (Fig. 5b). Some mice were randomly euthanized to analysis the viral RNA loads in the airways 3 days post-challenge. Significant lower levels of viral RNA were detected in the turbinates (Fig. 5c and e) and the lungs (Fig. 5d and f) of pSpike/PP-sNp vaccinated mice compared to control counterparts. Moreover, the viral RNA loads in turbinates and lungs of pSpike/PP-sNp treated mice decreased with time and were almost undetectable 14 days post-challenge (Fig. 5g and h), indicating pSpike/PP-sNp not only prevents virus infection but eventually eliminates the virus in respiratory tract completely. Additionally, lung sections were subjected to an immunohistochemistry assay aiming to explore the replication of SARS-CoV-2 by detecting its SARS-CoV-2 N protein expression in the lung 3 days post-challenge. Less SARS-CoV-2 N protein in lung sections from pSpike/PP-sNp immunized mice was detected (Fig. 5i). On the other hand, mice treated by pVax/PP-sNp or PBS not only been detected with obvious SARS-CoV-2 N protein (Fig. 5i), but also developed typical lung lesions characterized by denatured epithelial tissues, thickened alveolar septa, and activated inflammatory cell infiltration according to the histopathological assays (Fig. 5j). Whereas the pathological changes significantly alleviated in lung sections from pSpike/PP-sNp vaccinated mice (Fig. 5j). We further evaluated the potential roles of NAb and TRM cells in the process of eliminating SARS-CoV-2. As depicted in Fig. S25, significant numbers of CD4+TRM cells were identified exclusively in pulmonary sections of pSpike/PP-sNp treated mice after challenge, and the NAb titers against SARS-CoV-2 C57MA14 strain in serum of pSpike/PP-sNp immunized mice were elevated more than 5 times 14 days after challenge (Fig. S26), implying NAb and TRM mediated protective effects were probably involved in the protection of mice from lung lesions and the complete elimination of infected SARS-CoV-2 in the whole respiratory tract.
Fig. 5.
Pulmonary immunization with pSpike/PP-sNp confers complete protection against lethal SARS-CoV-2 challenge in mice. Forty days after the initial immunization, mice were challenged with a lethal dose (50 LD50) of SARS-CoV-2 C57MA14 strain via intranasal instillation, and the indicated tissues were collected at indicated time points after challenge to detect viral loads and the lung pathology. a) The survival rate of mice (n = 10 mice/group) intratracheally immunized with pSpike/PP-sNp, pVax/PP-sNp (Mock control) and PBS after the SARS-CoV-2 challenge. Untreated mice without challenge were served as a control (Control) b) The body weight changes of mice (n = 10 mice/group) intratracheally inoculated with pSpike/PP-sNp, pVax/PP-sNp and PBS after the SARS-CoV-2 challenging. Untreated mice without challenge were served as a control (Control, marked in green). Data represent mean ± SEM. Viral RNA loads in c) the turbinates and d) the lungs of mice treated by pSpike/PP-sNp, pVax/PP-sNp and PBS as described above and that in untreated counterpart (Control) 3 days after the SARS-CoV-2 challenge. Viral titers in e) the turbinates and f) the lungs of mice treated by indicated formulations as described above and that in untreated counterpart (Control) 3 days after the SARS-CoV-2 challenging. The viral loads in g) the turbinates and h) the lungs of mice treated by intratracheally vaccinated pSpike/PP-sNp 3, 7, 14 days post-challenge. i) Immunohistochemistry assay for SARS-CoV-2 N protein 3 days post-challenge. Scale bar, 100 μm. Positive signals are shown in red. j) Representative hematoxylin-eosin staining (H&E) staining of lung pathology 3 days after the SARS-CoV-2 challenge. Scale bar, 100 μm. Data in c-h represent mean ± SEM (n = 3 biologically independent samples). One-way ANOVA with Dunnett's post-hoc test was used to determine significance (ns represent not significant, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.3. PP-sNp induces innate and adaptive antiviral pathways in the lungs of mice
To further understand the function of pSpike/PP-sNp in pulmonary immunization, we performed RNA sequencing of lung tissues of pSpike/PP-sNp immunized mouse, with PBS-treated mice were used as control. A total of 623 differentially expressed genes (DEGs) were identified, including 427 up-regulated genes and 196 down-regulated genes (Fig. 6 a and b). A variety of cytokines (IL-12b, IL-17a, IL-27) and chemokines (CCL6, CCL8, CXCL3, CXCL9, CXCL10), which involved in immunoregulatory and inflammatory processes, were detected among the up-regulated genes (Fig. 6c). KEGG enrichment analysis showed that the up-regulated genes were significantly enriched in pro-inflammatory signalling (e.g. Cytokine-cytokine receptor interaction, Chemokine signalling pathway, Toll-like receptor signalling pathway and NOD-like receptor signalling pathway). Meanwhile, C-type lectin receptor (CLR) signalling pathway, which induce the expression of specific cytokines to determine T cell polarization fates [44], was significantly enriched as well (Fig. 6d). Notably, antiviral defence pathways, including viral protein interaction with cytokine and cytokine receptor and coronavirus disease-COVID-19, also participated in the PP-sNp induced pulmonary immunization (Fig. 6d). Multiple genes, such as NOD2, CLEC7A (also known as Dectin1), IL-17a, have been reported to participate in innate and adaptive immune response [[45], [46], [47], [48]]. We also found these genes are actively involved in the PP-sNp induced pulmonary immunization through a variety of pathways (Fig. 6e). The innate immune system play a key role in the recognition and early response to infectious agents. Innate immune cells, such as macrophages and dendritic cells (DCs), recognize viruses through extra- and intracellular receptors (including Toll-like and NOD-like receptors) and secrete inflammatory mediators (including chemokines and cytokines) [49], which enhance antigen processing and in turn active T and B cells [50]. We found that Toll-like receptor and NOD-like receptor signalling pathways were significantly enriched in pSpike/PP-sNp induced pulmonary immunization. Three key mediator genes, TLR2, TLR8 and NOD2, which were associated with pathogen-mediated inflammatory response to innate immunity [45,51], were significantly upregulated in lung tissues of pSpike/PP-sNp immunized mouse, indicating that PP-sNp can induce innate immune response. C-type lectin receptors (CLRs) expressed by dendritic cells are crucial for tailoring immune responses to pathogens and CLR signalling pathway play a key role in adaptive immune responses. Most CLRs induce adaptive immune responses via activate canonical NF-κB pathway, however, CLEC7A induce an unique signalling pathway that leads to the activation of the non-canonical NF-κB pathway [44,46]. Collectively, we inferred that PP-sNp mediated innate and adaptive immune responses through the recognition by pattern recognition receptors (PRRs) of pulmonary immune cells, but the molecular mechanism needs to be further verified in future studies. As an RNA sequencing analysis reveals, pSpike/PP-sNp successfully activated innate and adaptive anti-virus pathways in vaccinated mice (Fig. 6), which is in consistent with previous findings suggesting the transiently activated innate immunity is sufficient to augment subsequent adapted immune responses [52].
Fig. 6.
RNAseq analysis of mouse lung tissues immunized with pSpike/PP-sNp. a) Volcano plot and b) hierarchical clustering of differentially expressed genes (P < 0.05) in lung tissues treated with pSpike/PP-sNp, in comparison to genes expressed in lung tissues of mice treated by PBS. Downregulated genes (green or blue); upregulated genes (red) c) KEGG enrichment analysis of upregulated genes. Shown are the top 20 statistically significant pathways, calculated using an adjusted P value < 0.05. d) Heat map representing the upregulated cytokines, chemokines and receptors. e) Chord plot depicting the relationship between PP-sNp-mediated mouse pulmonary immunization-related genes and KEGG pathways. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
In this study, we demonstrate a COVID-19 DNA vaccine (pSpike/PP-sNp) delivered via pulmonary route could induce a robust protective immunity consisting of mucosal, humoral and cellular immune responses, which is sufficient to completely protect the upper and lower respiratory tracts against lethal SARS-CoV-2 challenge in mice. The development of mucosal COVID-19 vaccines that impart protective immunity would be highly desirable. Apart from extensive investigations using adenovirus-based vaccines [17,18,53] whether DNA vaccines could achieve this goal remains unclear. Early studies suggested that DNA vaccines are poorly immunogenic in the airways with low levels of antigen expression owing to the barriers posed by the respiratory tract. This could be reflected by the previous study indicating intranasal COVID-19 DNA vaccination were not able to elicit mucosal sIgA and circulating IgG against SARS-CoV-2 even after three times of repeated dosing [29]. Similar observations have been reported in another study showing that the serum neutralization IC50 of a COVID-19 DNA vaccine just reached 1/83.8 against SARS-CoV-2 pseudoviruses after seven-times repeated intranasal vaccinations [54].
Previous studies from both our group and others have clearly demonstrated that poloxamine-based delivery system mediates significantly better levels of DNA transfection and lower levels of associated inflammatory response in the airways of mouse and pig models than were achieved with the cutting-edge lipid-based formulations [28,34,35]. PEI was adopted as a control formulation because it is one of the most well-studied polymer-based gene carrier and has been applied in many mucosal DNA vaccine applications [55]. Although PEI appears to be promising in delivery of mucosal DNA vaccines encoding antigens of Influenza, HIV and SARS-CoV [55], we found it fails to mediate efficient DNA transfection in the mouse airway, perhaps owing to its poor mucus penetrating properties. We applied an improved method of preparing nanoparticles without aggregation to enhance the utility of PP-sNp for in vivo applications [56]. The optimized nanocomplexes appeared as evenly distributed spherical nanoparticles with sizes being smaller than mucus mesh pores (∼140 ± 50 nm) [57]. The unique high mobility in mucus gel facilitates deep penetration and widely spread of PP-sNp through the airway mucus layer (Fig. 7 a), thereby improving the probability of DNA payloads encounter and uptake by target cells. Subsequently, the targeting moiety within PP-sNp (which binds the ICAM-1/CD54) provides the possibility to specifically deliver the DNA cargos via receptor-mediated uptake, concurrent with efficient endosome escape and nucleus localization in these cells [30]. By virtue of these merits, PP-sNp efficiently mediates the transfection of antigen-expressing plasmid in pulmonary DCs and AECs (Fig. 7b). Being the most powerful APCs, pulmonary DCs are imperative in shaping antigen-specific immune responses [58]. The enhanced DNA vaccine uptake by pulmonary DCs and subsequent DC differentiation may lead to more profound and durable antigen presentation to T-cells [59]. Our finding is also in agreement with previous evidences demonstrating a positive correlation between the ratio of antigen-activated DCs and the magnitude of TEM-biased response [60]. Meanwhile, AECs appear to be essential for determining the potency of mucosal immune response and IgA production. Several studies have suggested that AECs provides a constant supply of cytokines (such as IL-6) which are essential for B cell proliferation and differentiation into IgA producing plasma cells [61,62]. The close proximity of AECs to DCs and B cells situated in respiratory mucosa probably ensures efficient antigen cross-presentation which directs B cells isotype switch towards IgA1 and IgA2 with the help of cytokines produced by AECs [62]. AECs also hold critical roles in orchestrating innate and adaptive immune responses in the respiratory system during viral infection [63,64]. As an RNA sequencing analysis reveals, pSpike/PP-sNp successfully activated innate and adaptive anti-virus pathways in vaccinated mice (Fig. 6), which is in consistent with previous findings suggesting the transiently activated innate immunity is sufficient to augment subsequent adapted immune responses [52].
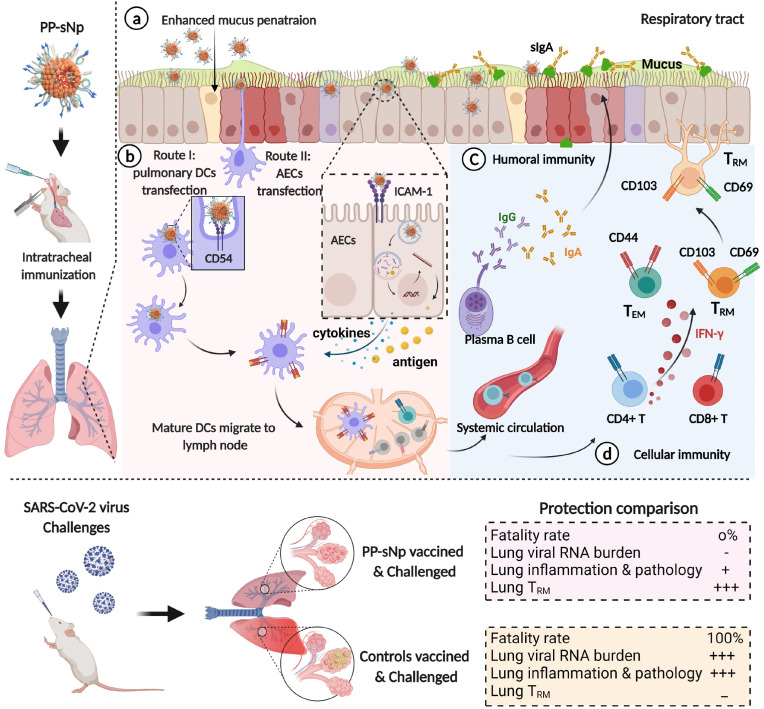
Fig. 7.
Schematic of the SARS-CoV-2 specific immune responses and subsequent protection established by pSpike/PP-sNp vaccine inoculated via respiratory route. a) After pulmonary inoculation, pSpike/PP-sNp is able to penetrate efficiently through the physical and biological barriers at the airway mucosal site. b) With the help of targeting moiety (ICAM-1/CD54 ligand) in PP-sNp, pSpike/PP-sNp is internalized and captured by airway epithelial cells (AECs) or pulmonary dendritic cells (DCs). The cationic moiety within PP-sNp mediates endosome escape and nucleus localization of pSpike, followed by the transcription and translation process. The SARS-CoV-2 derived antigens expressed in AECs are subsequently presented to other immune cells such as pulmonary DCs. AECs simultaneously orchestrate the adaptive immune responses via the transient secretion of cytokines. Mature pulmonary DCs then migrate to the bronchial-associated lymphoid tissues (e.g., mediastinal lymph node) and present antigens to naïve T cells and B cells for humoral and cellular immune responses. c) Activated B cells proliferate and differentiate into antibody-secreting plasma cells to generate secretory IgA (sIgA) and systemic IgG antibodies, the former of which efficiently neutralizes invading SARS-CoV-2 within the upper and lower respiratory tracts. d) Meanwhile, a portion of T cells obtains a tissue resident memory phenotype (TRM), enabling them to reside in the airway and respond rapidly when encountering SARS-CoV-2 virus. Other activated CD4+ or CD8+ T cells, including CD44hiCD62Llo T cells, CD4+IFN-γ T cells and CD8+IFN-γ T cells, take part in the process of eliminating SARS-CoV-2 infections as well. The robust and comprehensive immunity conferred by pulmonary vaccination of pSpike/PP-sNp probably controls SARS-CoV-2 replication and removes the viruses at the initial sites of infection, thus protects the vaccinated mice from lung lesions and death.
The complete protection against lethal SARS-CoV-2 challenge was largely due to the ability of pSpike/PP-sNp in stimulating comprehensive mucosal, humoral and cellular immunity, characterized by robust secretion of sIgA and NAb as well as potent T cell responses (especially TRM cells) in the respiratory system. pSpike/PP-sNp efficiently activated systemic immune response, concurrent with significant levels (ND50 at ∼1/1875) of NAb in serum. Previous findings suggest a serum NAb titer >300 was generally associated with protection against SARS-CoV-2 [65]. It is worth noting that pSpike/PP-sNp vaccinated mice displayed B cells secreting IgA and high levels of SARS-CoV-2-specific sIgA antibody titer (with a mean dilution titer of 1/102.4) in the BALF samples (Fig. 7c), which are even higher than those induced by intranasal vaccination of adjuvanted subunit vaccines according to previous publications [[66], [67], [68]]. Although the SARS-CoV-2-specific sIgA antibody titer in the BALF of intranasally vaccinated mice using adenovirus-vector is insurmountable when compared to non-viral vaccine induced counterpart, substantial NAb titers could be detected in BALF samples of pSpike/PP-sNp vaccinated mice with a level (∼1/73) that is comparable to the adenovirus-based counterpart (∼1/100) [19]. However, intramuscular dosing of a chimpanzee adenovirus-vectored vaccine encoding a prefusion stabilized spike protein does not confer sterilizing immunity, as evidenced by detection of viral RNA and induction of anti-nucleoprotein antibodies after SARS-CoV-2 challenge [69]. Meanwhile, high levels of spike-binding IgG and IgA in the nose and lung were only detected in intranasally vaccinated animals with SARS-CoV-2 subunit vaccine when compared with intramuscular vaccination [70]. Most importantly, a combination of the current mRNA vaccination plus mucosal adenovirus-S immunization induced strong neutralizing antibody responses (IgG and IgA from BALF), not only against the ancestral virus but also the Omicron BA.1.1 variant [71]. Therefore, the well-recognized limitations of i. m. vaccine delivery, along with our current findings and those from others, should bolster the global effort in developing respiratory mucosal SARS-CoV-2 vaccines.
Additionally, it has been long recognized that high-quality antigen-specific T cell responses are pivotal in combating various types of coronavirus infection [9]. pSpike/PP-sNp successfully mediated the presence of SARS-CoV-2 specific CD8+IFN-γ T cells (cytotoxic T cells) and elicited a Th1-biased cellular immune responses (Fig. 7d), both of which would be advantageous in eliminating coronavirus without adverse effects, since Th2 cell responses are suggested to be associated with enhancement of lung diseases [72]. pSpike/PP-sNp also generated a robust memory-based cellular immunity in the airways, including the significantly activated TEM cells and TRM cells with a resident memory phenotype (Fig. 7d). TEM-biased response created by pSpike/PP-sNp, which could immediately act on and rapidly removes respiratory pathogens, is particularly pronounced in the lung. But we failed to observe increased ratio of TCM cells, which is in line with previous findings indicating that TCM-biased response generally induced by conventional route of vaccination (e.g., electroporation) [73]. Accumulating evidences demonstrate that pulmonary CD4+TRM and CD8+TRM cells play crucial roles in resisting respiratory pathogen infections, including MERS-CoV, SARS-CoV and SARS-CoV-2 [3,74]. The airway TRM cells, which are exclusively generated through the respiratory route vaccination/infection, reside in the lung and does not recirculate [75], so that they can instantly recognize invading pathogens in the airway and efficiently prevent virus replication at the early stage of infection/re-infection.
Despite the promising results we observed, pSpike/PP-sNp is still far from successful clinical translation and more in-depth investigations are necessary. There are several limitations that we did not address in this study and will be useful topics for future studies, including the absence of data on the neutralization and protection efficiency elicited by pSpike/PP-sNp against emerging SARS-CoV-2 variants of concern. Similar to those cases of authorized COVID-19 vaccines [76], the neutralizing activity of NAb induced by the pSpike/PP-sNp vaccine may suffer a significant decrease within several months/years after vaccination, more boost doses may be necessary. For DNA vaccines, despite the ease with which preclinical studies revealed efficacy for a range of disease models, the efficacy of DNA vaccination in humans generally proved disappointing [77]. As a result, immunization and challenge studies with larger animals such as non-human primates should be carried out to confirm the extent of protective mucosal immunity conferred by pSpike/PP-sNp. Alternative approaches to overcome the inherent shortcomings of DNA vaccines would be the utilization of cutting-edge technologies such as in vitro transcribed mRNA, since mRNA vaccines offer huge advantages in terms of safety and efficacy as indicated by the COVID-19 mRNA clinical trials and real-world evidences [78,79]. Another limitation relates to the intratracheal dosing which is not appropriate to be applied in humans when considering its poor compliance. Most of the relevant studies chose the intranasal inoculation because of its noninvasive and convenient features, but there are still huge concerns and uncertainties regarding intranasal route of vaccination. For example, negative perception for nasal vaccines was generated from reported cases of Bell's palsy after intranasal dosing of influenza vaccines [80,81]. And nasal administration of nucleic acid formulations leads to large parts of drug dose being swallowed and degraded in the gastrointestinal tract [82]. Alternatively, the noninvasive nebulized formulations seem to be one of the most appropriate approaches in delivering mucosal vaccines to the human airway. However, the nebulized DNA formulations still face many challenges as indicated by a previous study showing that as little as 10% of the DNA payload in a nebulization device chamber could be successfully emitted [83], it thus requires advanced nebulization strategies and further optimization on the formulations to ensure the transfection efficiency [84]. Finally, the mechanism and pathways that involved in the pSpike/PP-sNp induced immune responses will be explored in detail with the help of cutting-edge technologies such as the single-cell RNA sequencing in order to further improve the vector/platform design and our understanding of DNA-based mucosal vaccines.
5. Conclusion
In summary, our dataset reveals that pSpike/PP-sNp with pulmonary mucus penetrating properties was capable of inducing comprehensive mucosal, humoral and cellular immune responses to provide complete protection against SARS-CoV-2 infection. The safe profile and ability to potentiate DNA vaccines for strong mucosal immunity make PP-sNp a promising platform for COVID-19 mucosal vaccines if its efficacy can be shown in large animal models and clinical trials, since robust protection at the respiratory mucosal site would be, at least theoretically, one of the most effective means to prevent the infection of SARS-CoV-2.
6. Materials and methods
6.1. Reagents
Poloxamine 704 (T704) was kindly provided by InCellArt (Nantes, France). All synthetic peptides were manually synthesized by Chinese Peptide Company (Hangzhou, China) with purity >95%. Branched PEI (average molecular weight at 25 kDa), Cell Counting Kit-8 (CA1210) and D-Luciferin (L6882) were all purchased from Sigma-Aldrich (Saint Louis, MO, USA). Lipofectamine2000 was purchased from Invitrogen (11668019, Carlsbad, USA). pGL4.51-Luciferase Reporter Vectors (pFLuc, E1320) was purchased from Promega (Madison, USA). Plasmids encoding SARS-CoV-2 S protein (pSpike) and pVax were kindly provided by Advaccine Biopharmaceuticals Co., Ltd (Suzhou, China). Purified full length S1 + S2 ECD spike protein of SARS-CoV-2 was purchased from Sino Biologics (40,589-v08B1, Beijing, China). For in vivo uptake and flow cytometric analysis, plasmid was fluorescently labeled with the Cy5 fluorophores using the Mirus Label IT® tracker intracellular nucleic acid localization kit (MIR 7021, Mirus Bio, Madison, USA) according to the manufacturer's instruction. Other reagents were obtained from Sigma-Aldrich (Saint Louis, MO, USA) as analytical grade or better. The ICAM-1 inhibitor (4-(4-methylphenyl)sulfanylthieno [2,3-c]pyridine-2-carboxamide, CAS: 251,992-66-2) was purchased from ShanghaiyuanyeBio-TechnologyCo.,Ltd.
6.2. Cell lines
MH-S cell line (Mice alveolar macrophages cells), DC2.4 cell line (Mouse bone marrow-derived dendritic cells), BEAS-2B (human bronchial epithelial cells) cell line and Calu-3 (human lung cancer cells) cell line were obtained from ATCC (Manassas, VA, USA). 16HBE (human bronchial epithelial cells) cell line was generously provided by Prof. Dr. Dieter C. Gruenert (University of California at San Francisco, CA, USA). ACE2-293T cells (ACE2-expressing cell line, constructed by hygromycin B screening) were purchased from PackGene (LV-2058, Guangzhou, China). Cells were maintained in medium DMEM (Gibco, USA) supplemented with 10% fetal bovine serum (Gibco, USA), penicillin (100 units/mL) and streptomycin (100 μg/mL) (complete medium) at 37 °C in 5% CO2. All cell lines used in current study were obtained from original providers who authenticated the cell lines using morphology, karyotyping and PCR-based approaches. No additional authentication has been performed. All cell lines tested negative for mycoplasma contamination. All experiments were performed on cells in the logarithmic growth phase.
6.3. Preparation and characterization of pDNA/PP-sNp formulations
The pDNA/PP-sNp formulations were prepared via a simple self-assembly process as described previously [30]. For in vivo applications, T704 stock solution (10 mg/mL) prepared in nuclease-free water was mixed with an equal volume of peptide solution (0.667 mg/mL) using a self-designed microfluidic mixer. pDNA solution (0.6 mg/mL) in double volumes were mixed with the above T704/peptide solution using the same mixer. The complex was incubated for 20 min at room temperature and filtered by 0.22 μm filter membrane before further use, which could optimize the particle size and morphology without significantly changing the concentration of components. For in vitro study, T704 solution at a certain concentration (defined as the w/w ratio between T704 and pDNA) and peptide solution at a specific concentration (defined as the N/P ratio; namely, the ratio between nitrogen residues in peptide and nucleic acid phosphate groups) were applied using similar methods. For PEI based formulation, brPEI (25 kDa, Sigma) at an optimum N/P ratio of 10 was mixed with pDNA solution in nuclease-free water, the resulting complex was incubated at room temperature for 20 min and served as a polyplex control. The Lipofectamine 2000 complex, serving as a lipoplex control, was prepared according to the manufacturer's instructions using the optimum concentration. Size measurements were performed using dynamic light scattering (DLS) on a Malvern Zetasizer Nano-ZS (Malvern). The morphology of pDNA/PP-sNp formulations were investigated by transmission electron microscope and scanning electron microscope (JEOL Ltd). The stability of pDNA/PP-sNp was investigated by DLS. Briefly, we parallelly prepared batches of pDNA/PP-sNp and measured particle sizes by DLS at 0, 4, 8, 24, 48 h after storage of the formulations at room temperature or 37 °C to investigate the stability of nanoparticles. Each sample of pDNA/PP-sNp was tested for once, 3 independent experiments were conducted for each time point.
6.4. Cell Counting Kit 8(CCK-8) assay
We used the Cell Counting Kit 8(CCK-8) assay to measure the cell viability of live cells, which is a common assay to investigate the toxicity of biomaterials. The kit uses a water-soluble tetrazolium salt to quantify the number of live cells by producing an orange formazan dye upon bio-reduction in the presence of an electron carrier. The CCK-8 assay described as follow. (1) Plate 5000 to 10,000 cells per well in 96 well plate with a clear bottom, the total volume is 100 μL. (2) Add pDNA/PP-sNp or other formulations into cells and incubate for 24 h in a 37 °C, 5% CO2 incubator. For blank wells (medium without cells), add the same amount of formulations. (3) Add 10 μL/well of CCK-8 solution to each well. Protect from the light and incubate for 1–4 h at 37 °C. (4) Measure the absorbance increase at 460 nm.
Cell viability = [(As-Ab)/(Ac-Ab)] × 100%
As represents OD460 of experimental group (including cells, medium, CCK-8 and pDNA/PP-sNp or pDNA/PEI); Ac represents OD460 of control group (including cells, medium and CCK-8); Ab represents OD460 of blank group (including cells and medium).
6.5. Cellular uptake and in vitro transfection
For cellular uptake investigation, the Cy5 conjugated pDNA was prepared according to manual instructions. Cells were incubated with Cy5-pDNA/PP-sNp or naked Cy5-pDNA for 4 h in Opti-MEM I Reduced Serum Medium (31985062, Invitrogen). The cells were further collected for the analysis of mean fluorescence intensity (MFI) by flow cytometry. To evaluate the transfection profile of PP-sNp, pDNA/PP-sNp complexes encoding firefly luciferase (i.e., pFLuc/PP-sNp) were prepared. pFLuc/PP-sNp and naked-pFLuc were incubated with cells for 4 h in Opti-MEM I Reduced Serum Medium, then were replaced by fresh complete medium. The protein expression of firefly luciferase in cells was observed by bioluminescence imaging at 48 h by Firefly Luciferase Reporter Gene Assay Kit (RG005, Beyotime). For investigating the effects of targeting moiety of peptide on gene transfection, the ICAM-1 inhibitor (final concentration was 15 nM) was pre-incubation with cells for 6 h before adding the pFLuc/PP-sNp.
6.6. Optical video recording of PP-sNp and the mean-squared displacement (MSD) analysis
Leica SP8 microscope equipped with a 40 × water objective was used to record the motion of PP-sNp. Cy5-DNA-containing PP-sNp were prepared as described above. PP-sNp (20 μL, 1.3 μg/μL) were mixed with mucin gel (400 μL, Mucin II solution 3%, w/v) mimicking mucus, followed by a 30 min incubation at 37 °C. DNA/PEI nanoparticles were also added as control. Fifteen-second Movies (Frame rate 23 fps) were captured using the LAS4.5 software (Leica). The trajectories of the nanoparticles were precisely quantified from the videos by software (TrackMate plugin in FIJI (ImageJ)), then the trajectory data was used to calculate the MSD and the corresponding diffusion coefficients (De) in MATLAB through the following equations, as implemented in MSD Analyzer.
De represents the effective diffusion coefficient and Δt represents the time interval.
7. Animals
6–8 weeks old specific pathogen-free (SPF) female BALB/c mice were purchased from the Beijing HFK Bioscience Co., Ltd (Beijing, China). All animal studies were approved by the Laboratory Animal Welfare and Ethics Committee of Third Military Medical University (AMUWEC20210929) and were performed in accordance with the institutional and national policies and guidelines for the use of laboratory animals. The mice were kept and vaccinated in SPF facilities, and provided with free access to sterile food and water. Animals were randomly divided into groups and conceded an adaption time of at least 7 days before the beginning of the experiments. For intratracheal dosing, the mice were sedated with isoflurane and received the formulations via a self-improved micro-injector.
7.1. Bioluminescence imaging
Mice were anaesthetized with isoflurane, followed by an injection of the substrate D-Luciferin (150 mg/kg) intraperitoneally. Bioluminescence was measured 10 min later using a Lumina Series III In Vivo Imaging System (PerkinElmer). To evaluated the in vivo delivery capability and visualize protein expression and tissue distribution of pDNA/PP-sNp formulations, pDNA encoding a firefly luciferase (pFLuc) was incorporated. The formulations were administered to mice via intratracheal (i.t.) route. The protein expression of firefly luciferase mediated by pFLuc/PP-sNp was observed by bioluminescence imaging at 24 h, 48 h, 72 h and 7 days post-dosing, respectively. The pFLuc/PEI (at N/P ratios of 10) and naked pFLuc plasmid counterparts were also injected via i. t. route with same dose of pFLuc to evaluate the bioluminescence in vivo.
7.1.1. Bone marrow derived dendritic cells (BMDC) maturation study
Bone marrow cells were isolated from the femurs of female BALB/c mice and cultured in RPMI 1640 complete medium (Gibco, USA) supplemented with 10% FBS, 1% penicillin/streptomycin, 10 ng/mL of Interleukin-4 (IL-4) and Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF). The culture media was replaced with fresh media on day 2 and 5 to remove the non-adherent and loosely adherent cells. The remaining cells continued to culture for another 2 days. To examine the maturation of BMDCs in vitro, BMDCs (1 × 106 mL−1) were co-cultured with pSpike/PP-sNp and naked-pSpike only for 24 h, respectively. Subsequently, FITC anti-mouse CD11c (117,305, Biolegend), PE-Cy7 anti-mouse MHC-II (25-5321-82, Invitrogen), and APC anti-mouse CD86 (105,011, Biolegend) were used to stain the cells in flow cytometry staining (FACS) buffer for 30 min at 4 °C before being washed and analyzed by BD FACS Array software™ on a BD FACS Array flow cytometer (BD Biosciences, USA).
7.2. Mouse vaccination and challenge experiments
Mice were immunized on day 0 and boosted with the same dose on day 14 and 28, respectively. Each anaesthetized mouse intratracheally received 50 μL of pSpike/PP-sNp formulation containing 15 μg pSpike. pVax/PP-sNp and phosphate buffered saline (PBS) was adopted as a mock control and a negative control, respectively. Mice were sacrificed on day 35 for assessing respiratory mucosal immune response, cellular immune response and memory establishment. Relevant tissues (lung and spleen) were harvested and processed for flow cytometry analysis. Bronchoalveolar lavage fluid (BALF) was collected by washing the lungs of euthanized mice with 500 μL of ice-cold PBS containing 0.05% Tween-20. Collected BALF was stored at −80 °C for further use.
The SARS-CoV-2 challenge model was based on a novel mouse-adapted SARS-CoV-2 strain, C57MA14 (NCBI GenBank number: OL913104.1, details can be found in: https://www.ncbi.nlm.nih.gov/nuccore/2167992552), that causes severe respiratory symptoms, and mortality to BALB/c mice. Immunized BALB/c mice were challenged intranasally with 50 LD50 SARS-CoV-2 C57MA14 on day 40 post initial immunization. On day 3 post challenge, 3 animals/group were sacrificed, and the lung and trachea tissues were collected for subsequent viral loads detection.
7.3. Quantification of viral by quantitative RT-PCR and TCID50 in challenged mouse tissues
Viral RNA in lung and turbinate tissues from challenged mice was detected by quantitative reverse transcription PCR (RT-qPCR). Briefly, tissue samples were homogenized with stainless steel beads in a Tissuelyser-24 (Shanghai jingxin Industrial Development CO., LTD) in 500 μL of DMEM. Viral RNA in tissues were divided into two parts. One part was extracted using the QIAamp Viral RNA Mini Kit (QIAGEN) according to the manufacturer's protocol. SARS-CoV-2 RNA quantification was performed by RT-qPCR targeting the S gene of SARS-CoV-2 using One Step PrimeScript RT-PCR Kit (Takara) with the following SARS-CoV-2 specific primers and probes: CoV-F3 (5′-TCCTGGTGATTCTTCTTCAGGT-30), CoV-R3 (5′-TCTGAGAGAGGGTCAAGTGC-30), and CoV-P3 (5′-FAM-AGCTGCAGCAC CAGCTGTCCA-BHQ1-30). Another part was serially diluted in DMEM and added into Vero E6 cells in 96-well plates. The plates were incubated 1 h at 37 °C with 5% CO2, the inoculation was replaced with DMEM containing 2% FBS and 1% penicillin-streptomycin. After incubating for 72 h, the median tissue culture infective dose (TCID50) was detected by the cytopathic effect (CPE).
7.3.1. Enzyme linked immunosorbent assay (ELISA)
SARS-CoV-2 S protein specific antibodies in mouse serum and BALF were measured by ELISA. Briefly, polystyrene microtiter 96-well plates were coated with full length SARS-CoV-2 spike protein (3 μg/mL in carbonate buffer, pH = 9.6) and incubated overnight at 4 °C. After blocking with 1% bovine albumin (BSA) in PBS, 100 μl/well pre-diluted samples were added into the plates with 1 h incubation at 37 °C. After three-times washes with PBST (PBS with 0.05% Tween-20), plates were added with horseradish peroxidase (HRP) conjugated goat anti-mouse IgG (Ab231712), IgA (Ab97235), IgG1 (ab97240), or IgG2a (Ab97245) (1:10,000, Abcam, UK) and incubated for 40 min at 37 °C. Plates were then washed three-times and added with peroxidase substrate (Ab171522, Abcam, UK), the reaction was terminated by stop solution (Ab171529, Abcam, UK) and the absorbance at 450 nm was read using a microplate reader (AID iSpot, Germany).
7.4. SARS-CoV-2 pseudovirus neutralization assay
Mouse serum samples from pSpike/PP-sNp vaccinated mice were serially diluted double fold starting at a 1:100 dilution with DMEM contain 2% FBS for the assay. Serum or BALF were incubated with 10 μl of Luc-SARS-Cov-2 pseudotyped virus (LV-2058, PackGene, China) for 60 min, then added to the HEK293T cells stably expressing ACE2 to incubate in a standard incubator (37 °C, 5% CO2) for 72 h. Post infection, cells were lysed and detected using a luminescence reporter gene assay system (RG006, Beyotime Biotechnology, China). The Luciferase activity was measured using the Promega GloMax Navigator Detection System (GloMax, Promega, USA) and expressed as relative light units (RLU). The 50% neutralization titers (NT50) were calculated as the serum dilution at which RLU were reduced by 50% compared with RLU in virus control wells after subtraction of background RLU in cell control wells.
7.5. Tissue processing and flow cytometry
Single cell suspensions of splenic and pulmonary lymphocytes were prepared from resected spleens and lungs of mice. The lung tissues were cut into scraps and digested with collagenase Ⅱ (0.5 mg/mL, C8150, Solarbio, China) in calcium chloride (1 mM) and magnesium chloride (1 mM) solution at 37 °C for 1 h with shaking at 220 rpm. Next, the samples were filtered through a 75 mm cell strainer to obtain a single cell suspension. The immune cells were obtained by density gradient centrifugation with Percoll (17-0891-09, GE Healthcare, USA) according to the manufacture's instruction. Splenic lymphocytes were collected by grinding spleen in PBS then passed through a 75 mm cell strainer, cell pellets were re-suspended in 5 mL of red blood cell lysis buffer (RT122-02, TIANGEN, China) for 5 min at RT for remove the red blood cell. PBS was added to wash the cells twice, then centrifuged at 1500×g for 5 min, the cell pellets were eventually re-suspended in RPMI1640 media supplemented with 10% FBS and 1% penicillin/streptomycin.
For flow cytometric study, cells were first stained with the LIVE/DEAD fixable cell stains kit (65-0865-14, Invitrogen, USA) according to the manufacturer's protocol. For surface markers, the cells were incubated with anti-mouse CD4 (11-0041-82, Invitrogen), anti-mouse CD8a (45-0081-82, Invitrogen), For intracellular cytokine staining, cells were stimulated with the overlapping peptide pool spanning of 14-mer peptides overlapping by nine amino acids from the SARS-CoV-2 RBD proteins (see Supplementary Notes) for 6 h at 37 °C, 5% CO2. Then the cells were incubated with anti-mouse IL-4 (17-7041-82, Invitrogen) and anti-mouse IFN-γ (12-7311-82, Invitrogen) after processing with the Cytofix/Cytoperm Fixation/Permeabilization Kit (554,714, BD Biosciences) according to the manufacturer's instructions. In order to detect the TEM/TCM and TRM cells, the cell samples were stained with the following indicated antibodies in FACS buffer: anti-CD62L (161,204, BioLegend), anti-CD44 (25-0441-82, BioLegend), anti-CD69 (104,506, BioLegend) and anti-CD103 (121,416, BioLegend). The antibodies were diluted 1:100 with stain buffer according to the manufacturer's protocol. All the samples were measured on a BD FACS Array flow cytometer (BD Biosciences). Data are analyzed with FlowJo software V10.
7.5.1. Enzyme linked immunospot (ELISpot) assay
Cellular immune responses in mice were performed using mouse IFN-γ/IL-4 ELISPOT PLUS plates (3321-4AST-2/3311-4APW-2, MABTECH, Sweden). 96-well ELISPOT plates were pre-treated as the manufacturer's instructions. 5 × 105 mouse splenocytes or pulmonary lymphocytes were plated into each well and stimulated with the above-mentioned peptide pools at a final concentration of 1 μg of each peptide per well. Additionally, PMA/Ionomycin were added as a positive control and RPMI 1640 media was used as a negative control. After incubation at 37 °C, 5% CO2 for 24 h, the plates were washed with PBS and incubation with biotinylated anti-mouse IFN-γ or IL-4 antibody for 2 h at RT. Finally, TMB substrate solution were added to visualize the spots. Spots were scanned and quantified by an ImmunoSpot CTL (Bio-Rad) reader. Spot-forming unit (SFU) per million cells was calculated by subtracting the negative control wells.
7.5.2. Immunohistochemical assay
The protocol of the immunohistochemical staining were briefly descripted as follow. The fresh lung tissues were prepared to paraffin embedded tissue sections (5–8 μm thick). Deparaffinize section in xylene and be transferred to 100% alcohol, for 2 times, 3 min each, and then transfer once through 95%, 70% and 50% alcohols respectively for 3 min each. Block endogenous peroxidase activity by incubating sections in 3% H2O2 solution in methanol at room temperature for 10 min to block endogenous peroxidase activity. Add 100 μL blocking buffer (10% fetal bovine serum in PBS) onto the sections of the slides and incubate in a humidified chamber at room temperature for 1 h. Drain off the blocking buffer from the slides. Apply 100 μL diluted primary antibody to the sections on the slides and incubate in a humidified chamber at room temperature for 1 h. Apply 100 μL diluted biotinylated secondary antibody to the sections on the slides and incubate in a humidified chamber at room temperature for 30 min. Apply 100 μL diluted HRP conjugates to the sections on the slides and incubate in a humidified chamber at room temperature for 30 min. Apply 100 μL DAB substrate solution to the sections on the slides to reveal the color of antibody staining. Allow the color development for <5 min until the desired color intensity is reached. Counterstain slides by immersing sides in Hematoxylin for 1–2 min. Rinse the slides in running tap water for 10 min. Dehydrate the tissue slides through 4 times of alcohol (95%, 95%, 100% and 100%), 5 min each. Clear the tissue slides in 3 times of xylene and coverslip using mounting solution. Wash slides with PBS for 3 times, 2 min each after each staining procedure. Anti-ZO1 antibody (GB111402); Anti-CD11c antibody (GB11059); Anti-F4/80 antibody (GB113373) were all purchased from Wuhan Servicebio Technology Co., Ltd. The SARS-CoV-2 (2019-nCoV) Spike Antibody (40,591-T62) was purchased from Sino Biological, Inc.
8. RNA sequencing
Lung tissues were collected 1 week after the last immunization. Total RNAs isolation, cDNA synthesis and the RNA-seq library was prepared by the Beijing Genomics Institute. Sequence reads were obtained using BGISEQ-500 (Illumina) and successfully mapped to mouse genome. Reads counts were normalized on the basis of fragments per kilobase per million (FPKM), fold changes were calculated for all possible comparisons, and a twofold cutoff with an adjusted P value < 0.05 was used to select genes with expression changes. The set of expressed genes were used as for functional enrichment analyses.
Statistics and analysis.
Statistical analyses were performed using the GraphPad Prism 8 (GraphPad Software, USA). Unless otherwise specified, data were expressed as mean ± Standard Error of Mean (SEM). Dual comparisons were made using Welch's t-test, and comparisons between multiple conditions were analyzed using analysis of variance (ANOVA) followed by the appropriate post-hoc tests. All the tests were two tailed. Differences were considered statistically significant when P < 0.05. All of the experiments were successfully repeated at least twice with three or more biological replicates to ensure the reproducibility of the data.
Credit author statement
S.G. and Q.Z. conceived and directed the project. Y.D., D.L., W.Z., P.L., P.C., B.P. and J.R. contributed experimental materials. S.S., J.T., Q.Z., L.C., C.X., C.S., Y.O., C.L., H.L., and Y.D. performed experiments and analyzed data. E.L., Y.G., C.T. and Y.L. designed and performed the challenge study and relevant end-point investigations. C.L. was responsible for the bioinformatics and RNA sequencing data analysis. S.G., G.Z., B.W., Y.L. and Q.Z. designed and supervised the research. S.G. and S.S. wrote the manuscripts with help and comments from all authors.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Gan Zhao, Cheng Su, Yuan Ding, Bin Wang reports a relationship with Advaccine (Suzhou) Biopharmaceuticals Co., Ltd that includes: board membership and employment. Bruno Pitard reports a relationship with In-Cell-Art that includes: board membership. Shan Guan, Si Sun, Quanming Zou, Chao Li has patent pending to Third Military Medical University. S.G., S.S., Q.Z. and C.L. have applied for patents related to this study. B.W. is a scientific co-founder of the biotechnology company Advaccine Biopharmaceuticals (Suzhou, China), which focuses on the development of DNA vaccines. G.Z., C.S. and Y.D. are employees of Advaccine Biopharmaceuticals Co., Ltd. B.P. is a scientific co-founder of In-Cell-Art (Nantes, France) and owns stock of In-Cell-Art, which commercializes tetra-functional block copolymers for DNA vaccines. The other authors declare that they have no competing financial interests.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC, Grant No. 82041045, 82173764 and 31972720), the major project of Study on Pathogenesis and Epidemic Prevention Technology System (2021YFC2302500) by the Ministry of Science and Technology of China, the Chongqing Talents: Exceptional Young Talents Project (CQYC202005027) and the Natural Science Foundation of Chongqing (cstc2021jcyj-msxmX0136). We also thank H. Zeng, Y. Zhuang, J. Gu, Jinyong Zhang, L. Peng, H. Sun, Kaixiao Zhang and Jianxiang Zhang (Third Military Medical University, Chongqing, China) for helpful discussions and careful proofreading of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.biomaterials.2022.121907.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Azzi L., Dalla Gasperina D., Veronesi G., Shallak M., Ietto G., Iovino D., et al. Mucosal immune response in BNT162b2 COVID-19 vaccine recipients. EBioMedicine. 2022;75 doi: 10.1016/j.ebiom.2021.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou D., Chan J.F., Zhou B., Zhou R., Li S., Shan S., et al. Robust SARS-CoV-2 infection in nasal turbinates after treatment with systemic neutralizing antibodies. Cell Host Microbe. 2021;29(4):551–563. doi: 10.1016/j.chom.2021.02.019. e555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afkhami S., D'Agostino M.R., Zhang A., Stacey H.D., Marzok A., Kang A., et al. Respiratory mucosal delivery of next-generation COVID-19 vaccine provides robust protection against both ancestral and variant strains of SARS-CoV-2. Cell. 2022;185(5):896–915. doi: 10.1016/j.cell.2022.02.005. e819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bricker T.L., Darling T.L., Hassan A.O., Harastani H.H., Soung A., Jiang X., et al. A single intranasal or intramuscular immunization with chimpanzee adenovirus-vectored SARS-CoV-2 vaccine protects against pneumonia in hamsters. Cell Rep. 2021;36(3) doi: 10.1016/j.celrep.2021.109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hassan A.O., Shrihari S., Gorman M.J., Ying B., Yaun D., Raju S., et al. An intranasal vaccine durably protects against SARS-CoV-2 variants in mice. Cell Rep. 2021;36(4) doi: 10.1016/j.celrep.2021.109452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King R.G., Silva-Sanchez A., Peel J.N., Botta D., Dickson A.M., Pinto A.K., et al. Single-dose intranasal administration of AdCOVID elicits systemic and mucosal immunity against SARS-CoV-2 and fully protects mice from lethal challenge. Vaccines. 2021;9(8) doi: 10.3390/vaccines9080881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y., Jin L., Chen T. The effects of secretory IgA in the mucosal immune system. BioMed Res. Int. 2020;2020 doi: 10.1155/2020/2032057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braun J., Loyal L., Frentsch M., Wendisch D., Georg P., Kurth F., et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature. 2020;587(7833):270–274. doi: 10.1038/s41586-020-2598-9. [DOI] [PubMed] [Google Scholar]
- 9.Sekine T., Perez-Potti A., Rivera-Ballesteros O., Stralin K., Gorin J.B., Olsson A., et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183(1):158–168. doi: 10.1016/j.cell.2020.08.017. e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z., Lorenzi J.C.C., Muecksch F., Finkin S., Viant C., Gaebler C., et al. Enhanced SARS-CoV-2 neutralization by dimeric IgA. Sci. Transl. Med. 2021;13(577) doi: 10.1126/scitranslmed.abf1555. eabf1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sterlin D., Mathian A., Miyara M., Mohr A., Anna F., Claër L., et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med. 2021;13(577):eabd2223. doi: 10.1126/scitranslmed.abd2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grau-Exposito J., Sanchez-Gaona N., Massana N., Suppi M., Astorga-Gamaza A., Perea D., et al. Peripheral and lung resident memory T cell responses against SARS-CoV-2. Nat. Commun. 2021;12(1):3010. doi: 10.1038/s41467-021-23333-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shakya A.K., Chowdhury M.Y.E., Tao W., Gill H.S. Mucosal vaccine delivery: current state and a pediatric perspective. J. Contr. Release. 2016;240:394–413. doi: 10.1016/j.jconrel.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mudgal R., Nehul S., Tomar S. Prospects for mucosal vaccine: shutting the door on SARS-CoV-2. Hum. Vaccines Immunother. 2020;16(12):2921–2931. doi: 10.1080/21645515.2020.1805992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lavelle E.C., Ward R.W. Mucosal vaccines - fortifying the frontiers. Nat. Rev. Immunol. 2022;22(4):236–250. doi: 10.1038/s41577-021-00583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miquel-Clopes A., Bentley E.G., Stewart J.P., Carding S.R. Mucosal vaccines and technology. Clin. Exp. Immunol. 2019;196(2):205–214. doi: 10.1111/cei.13285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu S., Zhong G., Zhang J., Shuai L., Zhang Z., Wen Z., et al. A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nat. Commun. 2020;11(1):4081. doi: 10.1038/s41467-020-17972-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nv Doremalen, Purushotham J.N., Schulz J.E., Holbrook M.G., Bushmaker T., Carmody A., et al. Intranasal ChAdOx1 nCoV-19/AZD1222 vaccination reduces viral shedding after SARS-CoV-2 D614G challenge in preclinical models. Sci. Transl. Med. 2021;13(607) doi: 10.1126/.abh0755. eabh0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.scitranslmed Hassan A.O., Kafai N.M., Dmitriev I.P., Fox J.M., Smith B.K., Harvey I.B., et al. A single-dose intranasal Chad vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell. 2020;183(1):169–184. doi: 10.1016/j.cell.2020.08.026. e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.See I., Su J.R., Lale A., Woo E.J., Guh A.Y., Shimabukuro T.T., et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, march 2 to april 21, 2021. JAMA. 2021;325(24):2448–2456. doi: 10.1001/jama.2021.7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mallapaty S. India's DNA COVID vaccine is a world first - more are coming. Nature. 2021;597(7875):161–162. doi: 10.1038/d41586-021-02385-x. [DOI] [PubMed] [Google Scholar]
- 22.Sheridan C. First COVID-19 DNA vaccine approved, others in hot pursuit. Nat. Biotechnol. 2021;39(12):1479–1482. doi: 10.1038/d41587-021-00023-5. [DOI] [PubMed] [Google Scholar]
- 23.Tebas P., Yang S., Boyer J.D., Reuschel E.L., Patel A., Christensen-Quick A., et al. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: a preliminary report of an open-label, Phase 1 clinical trial. EClinicalMedicine. 2021;31 doi: 10.1016/j.eclinm.2020.100689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Modjarrad K., Roberts C.C., Mills K.T., Castellano A.R., Paolino K., Muthumani K., et al. Safety and immunogenicity of an anti-Middle East respiratory syndrome coronavirus DNA vaccine: a phase 1, open-label, single-arm, dose-escalation trial. Lancet Infect. Dis. 2019;19(9):1013–1022. doi: 10.1016/s1473-3099(19)30266-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park K.S., Sun X., Aikins M.E., Moon J.J. Non-viral COVID-19 vaccine delivery systems. Adv. Drug Deliv. Rev. 2021;169:137–151. doi: 10.1016/j.addr.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silveira M.M., Moreira G., Mendonca M. DNA vaccines against COVID-19: perspectives and challenges. Life Sci. 2021;267 doi: 10.1016/j.lfs.2020.118919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suk J.S., Kim A.J., Trehan K., Schneider C.S., Cebotaru L., Woodward O.M., et al. Lung gene therapy with highly compacted DNA nanoparticles that overcome the mucus barrier. J. Contr. Release. 2014;178:8–17. doi: 10.1016/j.jconrel.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alton E.W.F.W., Armstrong D.K., Ashby D., Bayfield K.J., Bilton D., Bloomfield E.V., et al. Repeated nebulisation of non-viral CFTR gene therapy in patients with cystic fibrosis: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir. Med. 2015;3(9):684–691. doi: 10.1016/s2213-2600(15)00245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chandrasekar S.S., Phanse Y., Hildebrand R.E., Hanafy M., Wu C.W., Hansen C.H., et al. Localized and systemic immune responses against SARS-CoV-2 following mucosal immunization. Vaccines. 2021;9(2) doi: 10.3390/vaccines9020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guan S., Munder A., Hedtfeld S., Braubach P., Glage S., Zhang L., et al. Self-assembled peptide–poloxamine nanoparticles enable in vitro and in vivo genome restoration for cystic fibrosis. Nat. Nanotechnol. 2019;14(3):287–297. doi: 10.1038/s41565-018-0358-x. [DOI] [PubMed] [Google Scholar]
- 31.Bui T.M., Wiesolek H.L., Sumagin R. ICAM-1: a master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J. Leukoc. Biol. 2020;108(3):787–799. doi: 10.1002/JLB.2MR0220-549R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwasaki A., Foxman E.F., Molony R.D. Early local immune defences in the respiratory tract. Nat. Rev. Immunol. 2017;17(1):7–20. doi: 10.1038/nri.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braciale T.J., Sun J., Kim T.S. Regulating the adaptive immune response to respiratory virus infection. Nat. Rev. Immunol. 2012;12(4):295–305. doi: 10.1038/nri3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richard-Fiardo P., Hervouet C., Marsault R., Franken P.R., Cambien B., Guglielmi J., et al. Evaluation of tetrafunctional block copolymers as synthetic vectors for lung gene transfer. Biomaterials. 2015;45:10–17. doi: 10.1016/j.biomaterials.2014.12.051. [DOI] [PubMed] [Google Scholar]
- 35.Caballero I., Riou M., Hacquin O., Chevaleyre C., Barc C., Pezant J., et al. Tetrafunctional block copolymers promote lung gene transfer in newborn piglets. Mol. Ther. Nucleic Acids. 2019;16:186–193. doi: 10.1016/j.omtn.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shim B.-S., Park S.-M., Quan J.-S., Jere D., Chu H., Song M.K., et al. Intranasal immunization with plasmid DNA encoding spike protein of SARS-coronavirus/polyethylenimine nanoparticles elicits antigen-specific humoral and cellular immune responses. BMC Immunol. 2010;11(1):65. doi: 10.1186/1471-2172-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torrieri-Dramard L., Lambrecht B., Ferreira H.L., Van den Berg T., Klatzmann D., Bellier B. Intranasal DNA vaccination induces potent mucosal and systemic immune responses and cross-protective immunity against influenza viruses. Mol. Ther. 2011;19(3):602–611. doi: 10.1038/mt.2010.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bivas-Benita M., Gillard G.O., Bar L., White K.A., Webby R.J., Hovav A.H., et al. Airway CD8+ T cells induced by pulmonary DNA immunization mediate protective anti-viral immunity. Mucosal Immunol. 2013;6(1):156–166. doi: 10.1038/mi.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Regnstrom K., Ragnarsson E.G., Koping-Hoggard M., Torstensson E., Nyblom H., Artursson P. PEI - a potent, but not harmless, mucosal immuno-stimulator of mixed T-helper cell response and FasL-mediated cell death in mice. Gene Ther. 2003;10(18):1575–1583. doi: 10.1038/sj.gt.3302054. [DOI] [PubMed] [Google Scholar]
- 40.Li S., Chen D.Q., Ji L., Sun S., Jin Z., Jin Z.L., et al. Development of different methods for preparing acinetobacter baumannii outer membrane vesicles vaccine: impact of preparation method on protective efficacy. Front. Immunol. 2020;11:1069. doi: 10.3389/fimmu.2020.01069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hörner C., Schürmann C., Auste A., Ebenig A., Muraleedharan S., Dinnon K.H., 3rd, et al. A highly immunogenic and effective measles virus-based Th1-biased COVID-19 vaccine. Proc. Natl. Acad. Sci. U. S. A. 2020;117(51):32657–32666. doi: 10.1073/pnas.2014468117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilk M.M., Misiak A., McManus R.M., Allen A.C., Lynch M.A., Mills K.H.G. Lung CD4 tissue-resident memory T cells mediate adaptive immunity induced by previous infection of mice with bordetella pertussis. J. Immunol. 2017;199(1):233–243. doi: 10.4049/jimmunol.1602051. [DOI] [PubMed] [Google Scholar]
- 43.Busch D.H., Frassle S.P., Sommermeyer D., Buchholz V.R., Riddell S.R. Role of memory T cell subsets for adoptive immunotherapy. Semin. Immunol. 2016;28(1):28–34. doi: 10.1016/j.smim.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geijtenbeek T.B., Gringhuis S.I. Signalling through C-type lectin receptors: shaping immune responses. Nat. Rev. Immunol. 2009;9(7):465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim D., Kim Y.G., Seo S.U., Kim D.J., Kamada N., Prescott D., et al. Nod2-mediated recognition of the microbiota is critical for mucosal adjuvant activity of cholera toxin. Nat. Med. 2016;22(5):524–530. doi: 10.1038/nm.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saijo S., Fujikado N., Furuta T., Chung S.H., Kotaki H., Seki K., et al. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat. Immunol. 2007;8(1):39–46. doi: 10.1038/ni1425. [DOI] [PubMed] [Google Scholar]
- 47.Christensen D., Mortensen R., Rosenkrands I., Dietrich J., Andersen P. Vaccine-induced Th17 cells are established as resident memory cells in the lung and promote local IgA responses. Mucosal Immunol. 2017;10(1):260–270. doi: 10.1038/mi.2016.28. [DOI] [PubMed] [Google Scholar]
- 48.Shibui A., Shimura E., Nambu A., Yamaguchi S., Leonard W.J., Okumura K., et al. Th17 cell-derived IL-17 is dispensable for B cell antibody production. Cytokine. 2012;59(1):108–114. doi: 10.1016/j.cyto.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nachbur U., Stafford C.A., Bankovacki A., Zhan Y., Lindqvist L.M., Fiil B.K., et al. A RIPK2 inhibitor delays NOD signalling events yet prevents inflammatory cytokine production. Nat. Commun. 2015;6:6442. doi: 10.1038/ncomms7442. [DOI] [PubMed] [Google Scholar]
- 50.Hoebe K., Janssen E., Beutler B. The interface between innate and adaptive immunity. Nat. Immunol. 2004;5(10):971–974. doi: 10.1038/ni1004-971. [DOI] [PubMed] [Google Scholar]
- 51.Kumar H., Kawai T., Akira S. Toll-like receptors and innate immunity. Biochem. Biophys. Res. Commun. 2009;388(4):621–625. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 52.Wang J., Shah D., Chen X., Anderson R.R., Wu M.X. A micro-sterile inflammation array as an adjuvant for influenza vaccines. Nat. Commun. 2014;5:4447. doi: 10.1038/ncomms5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu S., Huang J., Zhang Z., Wu J., Zhang J., Hu H., et al. Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: preliminary report of an open-label and randomised phase 1 clinical trial. Lancet Infect. Dis. 2021;21(12):1654–1664. doi: 10.1016/s1473-3099(21)00396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumar U.S., Afjei R., Ferrara K., Massoud T.F., Paulmurugan R. Gold-nanostar-chitosan-mediated delivery of SARS-CoV-2 DNA vaccine for respiratory mucosal immunization: development and proof-of-principle. ACS Nano. 2021 doi: 10.1021/acsnano.1c05002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu K., Gao P., Liu S., Lu S., Lei W., Zheng T., Liu X., Xie Y., Zhao Z., Guo S., Tang C., Yang Y., Yu W., Wang J., Zhou Y., Huang Q., Liu C., An Y., Zhang R., Han Y., Duan M., Wang S., Yang C., Wu C., Liu X., She G., Liu Y., Zhao X., Xu K., Qi J., Wu G., Peng X.D.L., Wang P., Gao G.F. Protective prototype-Beta and Delta-Omicron chimeric RBD-dimer vaccines against SARS-CoV-2. Cell. 2022 doi: 10.1016/j.cell.2022.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vauthier C., Cabane B., Labarre D. How to concentrate nanoparticles and avoid aggregation? Eur. J. Pharm. Biopharm. 2008;69(2):466–475. doi: 10.1016/j.ejpb.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 57.Suk J.S., Lai S.K., Wang Y.Y., Ensign L.M., Zeitlin P.L., Boyle M.P., et al. The penetration of fresh undiluted sputum expectorated by cystic fibrosis patients by non-adhesive polymer nanoparticles. Biomaterials. 2009;30(13):2591–2597. doi: 10.1016/j.biomaterials.2008.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nguyen T.L., Yin Y., Choi Y., Jeong J.H., Kim J. Enhanced cancer DNA vaccine via direct transfection to host dendritic cells recruited in injectable scaffolds. ACS Nano. 2020;14(9):11623–11636. doi: 10.1021/acsnano.0c04188. [DOI] [PubMed] [Google Scholar]
- 59.Kim Y.C., Hsueh H.T., Kim N., Rodriguez J., Leo K.T., Rao D., et al. Strategy to enhance dendritic cell-mediated DNA vaccination in the lung. Adv. Ther. 2020;3(7) doi: 10.1002/adtp.202000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marzo A.L., Klonowski K.D., Le Bon A., Borrow P., Tough D.F., Lefrancois L. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat. Immunol. 2005;6(8):793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beagley K.W., Eldridge J.H., Lee F., Kiyono H., Everson M.P., Koopman W.J., et al. Interleukins and IgA synthesis. Human and murine interleukin 6 induce high rate IgA secretion in IgA-committed B cells. J. Exp. Med. 1989;169(6):2133–2148. doi: 10.1084/jem.169.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salvi S., Holgate S.T. Could the airway epithelium play an important role in mucosal immunoglobulin A production? Clin. Exp. Allergy. 1999;29(12):1597–1605. doi: 10.1046/j.1365-2222.1999.00644.x. [DOI] [PubMed] [Google Scholar]
- 63.Whitsett J.A., Alenghat T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat. Immunol. 2014;16(1):27–35. doi: 10.1038/ni.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saenz S.A., Taylor B.C., Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol. Rev. 2008;226(1):172–190. doi: 10.1111/j.1600-065X.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang L., Cao L., Gao X.-S., Zheng B.-Y., Deng Y.-Q., Li J.-X., et al. A proof of concept for neutralizing antibody-guided vaccine design against SARS-CoV-2. Natl. Sci. Rev. 2021;8(8) doi: 10.1093/nsr/nwab053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sui Y., Li J., Zhang R., Prabhu S.K., Andersen H., Venzon D., et al. Protection against SARS-CoV-2 infection by a mucosal vaccine in rhesus macaques. JCI Insight. 2021;6(10) doi: 10.1172/jci.insight.148494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Du Y., Xu Y., Feng J., Hu L., Zhang Y., Zhang B., et al. Intranasal administration of a recombinant RBD vaccine induced protective immunity against SARS-CoV-2 in mouse. Vaccine. 2021;39(16):2280–2287. doi: 10.1016/j.vaccine.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.An X., Martinez-Paniagua M., Rezvan A., Sefat S.R., Fathi M., Singh S., et al. Single-dose intranasal vaccination elicits systemic and mucosal immunity against SARS-CoV-2. iScience. 2021;24(9) doi: 10.1016/j.isci.2021.103037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hassan A.O., Kafai N.M., Dmitriev I.P., Fox J.M., Smith B.K., Harvey I.B., et al. A single-dose intranasal Chad vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell. 2020;183(1):169–184. doi: 10.1016/j.cell.2020.08.026. e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van der Ley P.A., Zariri A., van Riet E., Oosterhoff D., Kruiswijk C.P. An intranasal OMV-based vaccine induces high mucosal and systemic protecting immunity against a SARS-CoV-2 infection. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.781280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tang J., Zeng C., Cox T.M., Li C., Son Y.M., Cheon I.S., et al. Respiratory mucosal immunity against SARS-CoV-2 following mRNA vaccination. Sci. Immunol. 2022:eadd4853. doi: 10.1126/sciimmunol.add4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bolles M., Deming D., Long K., Agnihothram S., Whitmore A., Ferris M., et al. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J. Virol. 2011;85(23):12201–12215. doi: 10.1128/JVI.06048-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosati M., Valentin A., Jalah R., Patel V., von Gegerfelt A., Bergamaschi C., et al. Increased immune responses in rhesus macaques by DNA vaccination combined with electroporation. Vaccine. 2008;26(40):5223–5229. doi: 10.1016/j.vaccine.2008.03.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao J., Zhao J., Mangalam Ashutosh K., Channappanavar R., Fett C., Meyerholz David K., et al. Airway memory CD4+ T cells mediate protective immunity against emerging respiratory coronaviruses. Immunity. 2016;44(6):1379–1391. doi: 10.1016/j.immuni.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mueller S.N., Mackay L.K. Tissue-resident memory T cells: local specialists in immune defence. Nat. Rev. Immunol. 2016;16(2):79–89. doi: 10.1038/nri.2015.3. [DOI] [PubMed] [Google Scholar]
- 76.Cohn B.A., Cirillo P.M., Murphy C.C., Krigbaum N.Y., Wallace A.W. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science. 2021 doi: 10.1126/science.abm0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu M.A. A comparison of plasmid DNA and mRNA as vaccine technologies. Vaccines. 2019;7(2) doi: 10.3390/vaccines7020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., et al. BNT162b2 mRNA covid-19 vaccine in a nationwide mass vaccination setting. N. Engl. J. Med. 2021;384(15):1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mutsch M., Zhou W., Rhodes P., Bopp M., Chen R.T., Linder T., et al. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N. Engl. J. Med. 2004;350(9):896–903. doi: 10.1056/NEJMoa030595. [DOI] [PubMed] [Google Scholar]
- 81.Izurieta H.S., Haber P., Wise R.P., Iskander J., Pratt D., Mink C., et al. Adverse events reported following live, cold-adapted, intranasal influenza vaccine. JAMA. 2005;294(21):2720–2725. doi: 10.1001/jama.294.21.2720. [DOI] [PubMed] [Google Scholar]
- 82.Merkel O.M. Can pulmonary RNA delivery improve our pandemic preparedness? J. Contr. Release. 2022;345:549–556. doi: 10.1016/j.jconrel.2022.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Birchall J.C., Kellaway I.W., Gumbleton M. Physical stability and in-vitro gene expression efficiency of nebulised lipid–peptide–DNA complexes. Int. J. Pharm. (Amst.) 2000;197(1):221–231. doi: 10.1016/S0378-5173(00)00339-2. [DOI] [PubMed] [Google Scholar]
- 84.Guan S., Darmstadter M., Xu C., Rosenecker J. Vitro investigations on optimizing and nebulization of IVT-mRNA formulations for potential pulmonary-based alpha-1-antitrypsin deficiency treatment. Pharmaceutics. 2021;13(8) doi: 10.3390/pharmaceutics13081281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.