Abstract
Viral hemorrhagic fevers caused by arenaviruses are severe zoonotic diseases. In reservoirs, the presence of antibodies may indicate viral circulation in a population of a specific region, and these data can be used as an indicator for further investigations by molecular techniques. The present study aimed to detect the presence of arenavirus antibodies in wild rodents captured from 1998 to 2008 during epidemiological surveillance activities. A retrospective analysis of 2243 wild rodent blood samples using a broad cross-reactive in-house developed enzyme-linked immunosorbent assay (ELISA) revealed a 0.44% (10/2243) positive rate in wild rodents, which included Necromys lasiurus (6/1012), Calomys callosus (2/94), and Akodon sp. (2/273) species. These rodents were captured between 2002 to 2006 in Campo Alegre de Goiás/GO, Bodoquena/MS, Nuporanga/SP, and Mogi das Cruzes/SP. Our findings suggest the sylvatic circulation of arenavirus among wild rodents in the southeast region of Brazil. However, future virological and molecular studies are necessary to confirm the viral presence in these regions.
Keywords: Rodent-borne disease, ELISA, New World arenaviruses
Introduction
The Arenaviridae family, from the Bunyavirales order, is currently divided into four recognized genera: Antennavirus, Hartmanivirus, Mammarenavirus, and Reptarenavirus, which are responsible for infecting fish, reptiles, and mammals, respectively [1]. Some species belonging to Mammarenavirus are zoonotic and thus can cause severe illness in humans by a spillover event when viral aerosol particles from rodent excreta are inhaled as a result of human interaction with the natural reservoirs [2, 3]. The viral particle is composed of two segments, the large (L) and small (S) of a single-stranded RNA genome of an ambisense-like polarity [3]. The S segment encodes the nucleoprotein (NP) and the glycoprotein precursor complex (GPc) polyprotein, post-translational cleaved into the subunits GP-1 and GP-2. The L segment encodes to the large protein (L or RNA-dependent RNA polymerase [RdRp]) and to the zinc-binding protein (Z), which has multiple functions and acts at various stages during viral infections [4, 5].
The arenavirus viral particle has a pleomorphic morphology with an often-spherical shape and a diameter ranging from 50 to 300 nm. It also possesses a lipid bilayer envelope derived from the host plasma cell membrane, acquired during viral budding. Numerous ribosomes within viral particles give it a sandy appearance, and its denomination derives from the Latin script “arena” [4]. According to their genetic, geographic, and epidemiological characteristics, the genus Mammarenavirus is further subdivided into Old World or New World groups [6].
Hemorrhagic fevers caused by arenaviruses are considered endemic, occurring in a restricted geographic area, even when their respective rodent reservoirs have a wider geographic distribution [7]. The presence of antibodies in rodents may indicate viral circulation in a specific region. Therefore, it may be used as an indicator for subsequent investigations using molecular techniques [5]. Rodents (order Rodentia) are the main reservoirs of Mammarenaviruses, in which the viruses are most often maintained through a largely asymptomatic, persistent infection. Mammarenavirus are further classified into Old World group, with reservoirs belonging to family Muridae, and subfamily Murinae, and New World group, with reservoirs belonging to family Cricetidae, subfamilies Sigmodontinae and Neotominae [5]. The exception is Tacaribe virus, which is associated with frugivorous bats [8, 9].
In Brazil, nine Mammarenavirus were described: Sabiá (SABV), Amapari (AMAV), Cupixi (CPXV), Flexal (FLEV), Pinhal (PINV), Latino (LATV), Oliveiros (OLV), Xapuri (XAPV), and Aporé [10–16]. To date, only SABV has been associated with Brazilian hemorrhagic fever (BHF), presenting an acute, severe, and lethal disease whose reservoir remains unknown until the present moment [10, 17, 18]. In addition, the Flexal virus was the causative agent of two laboratory infections presenting disease [19].
As seroprevalence studies for arenavirus are scarce in the Brazilian territory, more information regarding viral circulation in wild rodents and other possible reservoirs is needed. To explore this question, we designed a retrospective study to detect the presence of immunoglobulin G (IgG) class antibodies against arenavirus in blood samples from rodents captured in central-west, southeast, and south regions of Brazil during epidemiological Hantavirus surveillance activities from 1998 to 2008.
Material and methods
Sample collection
Blood samples were obtained from wild rodents during fieldwork carried out by the Núcleo de Doenças de Transmissão Vetorial (NDTV) of the Adolfo Lutz Institute, during the Hantavirus surveillance program that started in 1993, following the Ministry of Health Guidelines [20]. Rodents were captured using Sherman live-capture traps (Sherman Traps Inc., Tallahassee, FL). Animals were sedated, and blood samples were collected from the retro-orbital area using heparinized capillaries and placed into cryogenic microtubes that were kept in liquid nitrogen. Rodents were identified by their taxonomy in the field, or their carcasses were preserved in a 10% formalin solution for further taxonomic confirmation. They were collected in different Brazilian regions according to CDC guidelines described in [21], comprising different biomes: Atlantic Forest and Cerrado (southeastern region), Atlantic Forest (south region ), and Cerrado and Pantanal (central-west region). All procedures using animals were conducted according to biosafety protocols, following ethical guidelines for animal research. Historical blood samples used herein were kept under −20°C. Sample selection was made randomly from a total of 14.614 blood samples using random.org to cover every expedition made (available at https://www.random.org/). Positive samples for Hantavirus by ELISA [22] and RT-PCR [23] and samples from rodent species Mus musculus and Rattus ratus, which are not associated with pathogenic arenavirus, were excluded from the study.
Antibody detection
To detect the presence of IgG antibodies for Mammarenaviruses, an in-house enzyme-linked immunosorbent assay (ELISA) for rodents was performed with JUNV strain XJ CL #3 lysate grown in Vero C76 used as antigen [24]. The plates have been coated with 100 µL of positive and negative Vero cultures of JUNV, which was used because of cross-reactivity among new world arenaviruses [25]. A mouse ascitic fluid (1:6400) was used as the positive control, while dilution buffer MASTER solution, consisting of PBS pH 7.4, skimmed milk 5% (Becton Dickinson, USA), and 0.1% Tween 20 (Sigma-Aldrich, USA), was the negative control. These reagents were produced and donated by the Instituto Nacional de Enfermedades Virales Humanas Dr. Julio I. Maiztegui (INEVH) - Pergamino/Argentina. Rodent samples were diluted 1/100 of the dilution buffer solution, and each microplate received 42 samples in a single-well-based format.
The revealing reaction system was composed of anti-Rat (anti-Rattus norvegicus), anti-Peromyscus (anti-Peromyscus leucopus), and anti-mouse conjugates, labeled with the enzyme peroxidase and ABTS substrate (2,2′-Azino-bis [3-ethylbenzthiazoline-6-sulfonic acid], Kirkegaard & Perry Laboratories, MD, USA). Results were obtained by the optical density (OD) value of each sample in an ELISA plate reader Multiskan FC with a 405-nm filter. The liquid OD was performed by calculating the difference in the OD value of each sample in the presence of the JUNV antigen to the values obtained from the same sample with the absence of antigen (Vero cells supernatant only). The following liquid ODs were considered for the evaluation of the cut off value, being cut off > 0.200 (fixed value): reagent; cut off < 0.100: non-reagent; and cut off = 0.100–0.199: inconclusive.
Results
A total of 2243 samples were analyzed in this study, with a total of 10 (0.44%) positive samples in the following species: Necromys lasiurus (6/1012) being 1 from Nuporanga-SP (2002), 1 from Campo Alegre de Goiás-GO (2003), 3 from Bodoquena-MS (2004), and 1 from Mogi das Cruzes (2006), and Calomys callosus (2/94) from Bodoquena-MS (2004) and Akodon sp. (2/723), being 1 from Nuporanga (2002) and 1 from Mogi das Cruzes (2006). A summary of the wild rodents tested by species is depicted in Table 1, while information regarding positive animals is shown in Table 2. Locations of the positive cases are depicted in Fig. 1.
Table 1.
Detection of IgG arenavirus antibodies in blood samples of wild rodents captured during 1998–2008
| Species of rodents | Total of specimens analyzed | Reagent (%*) |
|---|---|---|
| Akodon sp. | 723 | 2 (0.3%)* |
| Brucepattersonius soricinus | 06 | 0 |
| Calomys callosus | 94 | 2 (0.3%)* |
| Calomys laucha | 08 | 0 |
| Calomys tener | 185 | 0 |
| Cerradomys subflavus | 23 | 0 |
| Delomys sublineatus | 23 | 0 |
| Holochilus brasiliensis | 03 | 0 |
| Necromys lasiurus | 1012 | 6 (0.6%)* |
| Necromys sp. | 03 | 0 |
| Nectomys squamipes | 02 | 0 |
| Oligoryzomys flavescens | 02 | 0 |
| Oligoryzomys nigripes | 60 | 0 |
| Oryzomys ratticeps | 13 | 0 |
| Oligoryzomys delticola | 03 | 0 |
| Oligoryzomys microtis | 02 | 0 |
| Oligoryzomys stramineus | 01 | 0 |
| Oryzomys Grupo nitidus | 13 | 0 |
| Oryzomys capito | 04 | 0 |
| Oryzomys sp. | 01 | 0 |
| Oxymycterus quaestor | 23 | 0 |
| Oxymycterus rufus | 10 | 0 |
| Pseudoryzomys simplex | 06 | 0 |
| Rhipidomys mastacalis | 03 | 0 |
| Thaptomys nigrita | 20 | 0 |
| Total | 2243 | 10 |
*Percentage was calculated according to the total of each species analyzed.
Table 2.
Wild rodent species with IgG Mammarenavirus antibodies according to year and place of capture, age, and sex
| Rodent ID | Species | Year | Municipalities/states | Age | Sex | Final OD value |
|---|---|---|---|---|---|---|
| 14,342 | Necromys lasiurus | 2002 | Nuporanga/SP | Young | Female | 0.506 |
| 14,467 | Akodon sp | 2002 | Nuporanga/SP | Young | Female | 0.412 |
| 17,092 | Necromys lasiurus | 2003 | Campo Alegre de Goiás/GO | Adult | Female | 0.285 |
| 19,206 | Necromys lasiurus | 2004 | Bodoquena/MS | Adult | Female | 2,337 |
| 19,223 | Necromys lasiurus | 2004 | Bodoquena/MS | Young | Male | 0.203 |
| 19,235 | Necromys lasiurus | 2004 | Bodoquena/MS | Adult | Female | 0.886 |
| 19,238 | Calomys callosus | 2004 | Bodoquena/MS | Adult | Female | 0.268 |
| 19,240 | Calomys callosus | 2004 | Bodoquena/MS | Adult | Female | 0.376 |
| 23,139 | Akodon sp | 2006 | Mogi das Cruzes/SP | Young | Male | 0.289 |
| 23,150 | Necromys lasiurus | 2006 | Mogi das Cruzes/SP | Young | Male | 0.419 |
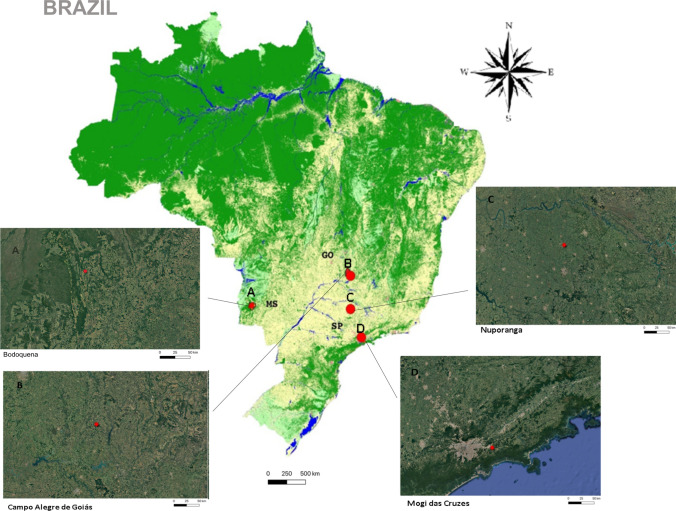
Fig. 1.
Locations where wild rodent species with the presence of IgG anti-Mammarenavirus antibodies were captured (2002–2006). Legend: Map of Brazil using Mapbiomas plug in (mapbiomas.org) showing vegetation coverage in 2006. Green areas refer to forest, yellow areas refer to crop, and blue areas refer to rivers. Red dots indicate cities where positive samples from wild rodents were captured in the States of Goiás (GO) and São Paulo (SP). Landscapes show locations where IgG-positive rodents were captured. Nuporanga/SP; Mogi das Cruzes/SP; Campo Alegre de Goiás/GO, Bodoquena (MS)*. Maps were generated using QGIS software version 3.16 Hannover. *no coordinates were obtained from these cases; only farm address was available in the record
Discussion
Using an in-house ELISA, we have identified IgG arenavirus antibodies in 10/2243 (0.44%) samples from the wild rodents Akodon sp., Calomys callosus, and Necromys lasiurus, with prevalences of 0.3%, 0.3%, and 0.6%, respectively. These samples were captured in different Brazilian states, including species from Cerrado, Pantanal, and Atlantic Forest. ELISA IgG test was performed due to its low complexity, reproducibility, sensitivity, and specificity [24]. Previous studies demonstrated cross-reactivity among arenavirus using complement fixation test (CF) and ELISA, including a laboratory-acquired case of Sabiá [19, 26]. In addition, it has been shown that JUNV presented cross-reactivity with New World arenaviruses [25].
Our results indicate that arenavirus may be widespread in several Brazilian biomes, differing from where most of the Mammarenavirus were previously detected. During surveys conducted in the 1960s, Mammarenavirus were mostly detected from wild rodents in the Brazilian Amazon, including the detection of Amapari virus (Neacomys guianae), Cupixi virus (Oryzomys megacephalus), and Flexal virus (Oryzomys spp.) [27–30]. In this study, Necromys lasiurus was the most prevalent species analyzed, with 1012 samples tested (45.1%). It inhabits open areas and forest formations in the Cerrado and Atlantic Forest, distributed within all Brazilian regions [13]. Previous serological studies using a JUNV recombinant nucleocapsid protein [31] have reported the presence of antibodies to Mammarenavirus in samples obtained from Necromys lasiurus captured between 2008 and 2009 in the northeastern region of the state of São Paulo. Another study [12] described the co-circulation of the nonpathogenic Mammarenavirus species Oliveiros and Latino, which were detected between 2005 and 2006 in Necromys lasiurus and Calomys callosus captured in two municipalities in the state of Mato Grosso. Our study detected antibodies anti- Mammarenavirus in 6 samples from Necromys lasiurus.
Furthermore, the genus Calomys inhabits forest, and open formations in the Caatinga, Cerrado, and Pantanal biomes, with the species Calomys callosus being widely distributed in the western region of Mato Grosso do Sul state [32]. Members of the genus Calomys are considered important reservoirs of pathogenic arenavirus, including the Calomys musculinus and Calomys callosus, which are the reservoirs of Junin and Machupo viruses, respectively [33, 34].
The presence of antibodies anti-Mammarenavirus in samples of Calomys callosus was also reported elsewhere (available at < https://www.arca.fiocruz.br/bitstream/icict/37332/2/jorlan_jesus_ioc_dout_2018.pdf>[35]), when from a total of 54 samples of wild rodents, 2 animals presented serum reactivity to arenavirus IgG antibodies, both from the municipality of Sidrolândia (MS). In addition, the detection of the Latino virus in Calomys genus has been previously reported in 2010 and 2015 in Mato Grosso State [12]. In agreement, we were able to detect anti-Mammarenavirus antibodies in 2 Calomys callosus that were captured in 2004 in Mato Grosso do Sul state.
Also, two positive samples were detected in Akodon genus, one from 2002 and another from 2006, both from São Paulo state. This genus inhabits forest formations, adjacent open areas, altitude fields throughout the Atlantic Forest, forest areas of the Caatinga, and open and closed vegetation formations of Cerrado [32]. The transmission of JUNV has been previously associated with Akodon azarae rodents from Argentina; however, there are no reports of the presence of arenavirus in this species in the Brazilian territory [33].
Although we observed a low frequency (0.44%) of positive anti-Mammarenavirus antibodies, our results demonstrate that arenavirus may be distributed in distinct regions of the country, possibly with a silent circulation. In Brazil, SABV is the causative agent of all Brazilian Mammarenavirus human cases, with three natural infection cases reported, all from São Paulo state. All three patients had severe hemorrhagic manifestations with fatal outcomes within days. The index case was detected in 1990 in Cotia city, while the second was confirmed in 1999 in Espírito Santo do Pinhal [10, 17]. Recently, in 2020, a third case was described in Sorocaba city. These results were obtained from metagenomics analysis in a patient with a Yellow Fever virus suspicion, whose diagnosis was negative. Sequencing revealed a Mammarenavirus with approximately 90% similarity to the 1990 SABV [18].
Emerging infectious diseases present one of the twenty-first century’s most significant public health challenges. Among these are zoonotic viruses that originate from reservoir species, often mammals. Central and South America are large sources of virus diversity, and hotspots of emergent zoonosis [36], and targeting surveillance studies to such regions provide better allocation of global resources to prevent emerging infectious diseases and implement control measures to deal with outbreaks [37]. More, such surveillance studies, when associated with viral genomics, can bring information about the genetic diversity of a virus population [38]. Despite its low occurrence, rodent-borne viruses have the potential to impose substantial lethality, morbidity, and economic loss for humans. However, despite many efforts to identify pathogenic Mammarenavirus reservoirs since the discovery of SABV in 1990, the species remains unknown. However, thanks to field-based studies, Pinhal virus (PINV), a new Mammarenavirus, was identified in a Calomys tener collected during investigations after the Santo Antônio do Pinhal human case. The virus clustered in a subclade within a lineage C, associated with the Mammarenavirus branch that is nonpathogenic to humans [15].
Although by using serological tests, it is impossible to determine which Mammarenavirus species is circulating among wild rodents, it may indicate hotspots of virus circulation, as little is known about the virus biology and ecology so far. Thus, considering the great biodiversity of viruses and fauna in Brazil and the difficulty of performing molecular and next-generation sequencing in the country, we encourage using in-house ELISA tests for seroprevalence studies. Considering the recent BHF human case caused by SABV, we emphasize the need for more studies of eco-epidemiological surveillance aimed at identifying and monitoring wild animals that can act as natural reservoirs of Mammarenavirus in the Brazilian territory.
Acknowledgements
The authors gratefully acknowledge the team at the National Institute of Human Viral Diseases Dr. Julio I. Maiztegui by providing antigens to perform this work and the team at Núcleo de Doenças de Transmissão Vetorial from Instituto Adolfo Lutz. We also gratefully acknowledge the contribution of Akemi Suzuki and Dr. Luiz Eloi Pereira (in memoriam) to rodents’ collection and identification. We also gratefully acknowledge Juliana Silva Nogueira’s assistance with the serological analysis and support during the manuscript review and Lucila Vilela for English review. We also thank the reviewers for their comments.
Author contribution
Manuscript preparation: ALRO, RPS, MSC. Obtained funding and study supervision: MCSTT. ELISA assay: ALRO and IB. Performed the analyses: ALRO, MCSTT. All authors reviewed, contributed to, and approved the final version of the manuscript.
Funding
Secretaria do Estado de Saúde de São Paulo (SES), Programa de Pós-graduação em Ciências. Mestrado em Pesquisa Laboratoriais em Saúde pública da Coordenadoria de Controle de Doenças da Secretaria de Estado da Saúde de São Paulo, São Paulo, Brazil.
Declarations
Ethics approval
Field activities concerning rodent trapping and handling were cleared by national legal provisions. The collection and handling of biological samples were carried out following international recommendations, applying human methods of anesthesia and euthanasia. Also, the research project was approved by the National Council for Technological and Scientific Development–CNPq, Proc. 403023/2004-1, which fully considered the ethical aspects in granting the research funds. The arbovirus and hantavirus surveillance program, conducted by Instituto Adolfo Lutz, was approved by CEUA protocol number 02/2011.
Conflict of interest
The authors declare no competing interests.
Footnotes
Responsible Editor: Luis Nero
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ana Lúcia Rodrigues de Oliveira, Email: alro@hotmail.com.
Mariana Sequetin Cunha, Email: masequetin@gmail.com.
References
- 1.Radoshitzky SR, Buchmeier MJ, Charrel RN, et al. ICTV virus taxonomy profile: Arenaviridae. J Gen Virol. 2019;100:1200–1201. doi: 10.1099/jgv.0.001280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radoshitzky SR, Bào Y, Buchmeier MJ, et al. Past, present, and future of arenavirus taxonomy. Arch Virol. 2015;160:1851–1874. doi: 10.1007/s00705-015-2418-y. [DOI] [PubMed] [Google Scholar]
- 3.Shao J, Liang Y, Ly H. Human hemorrhagic fever causing arenaviruses: molecular mechanisms contributing to virus virulence and disease pathogenesis. Pathog (Basel, Switzerland) 2015;4:283–306. doi: 10.3390/pathogens4020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchmeier M, Peters CJ, De la Torre C. Arenaviridae: the virus and their replication. Fields Virol. 2007;2:1792–1827. [Google Scholar]
- 5.Charrel RN, de Lamballerie X. Zoonotic aspects of arenavirus infections. Vet Microbiol. 2010;140:213–220. doi: 10.1016/j.vetmic.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 6.Hallam SJ, Koma T, Maruyama J, Paessler S. Review of Mammarenavirus biology and replication. Front Microbiol. 2018;9:1751. doi: 10.3389/fmicb.2018.01751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zapata JC, Salvato MS. Arenavirus variations due to host-specific adaptation. Viruses. 2013;5:241–278. doi: 10.3390/v5010241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Downs WG, Anderson CR, Spence L, et al. Tacaribe virus, a new agent isolated from Artibeus bats and mosquitoes in Trinidad, West Indies. Am J Trop Med Hyg. 1963;12:640–646. doi: 10.4269/ajtmh.1963.12.640. [DOI] [PubMed] [Google Scholar]
- 9.Cogswell-Hawkinson A, Bowen R, James S, et al. Tacaribe virus causes fatal infection of an ostensible reservoir host, the Jamaican fruit bat. J Virol. 2012;86:5791–5799. doi: 10.1128/JVI.00201-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lisieux T, Coimbra M, Nassar ES, et al. New arenavirus isolated in Brazil. Lancet (London, England) 1994;343:391–392. doi: 10.1016/s0140-6736(94)91226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charrel RN, Feldmann H, Fulhorst CF, et al. Phylogeny of New World arenaviruses based on the complete coding sequences of the small genomic segment identified an evolutionary lineage produced by intrasegmental recombination. Biochem Biophys Res Commun. 2002;296:1118–1124. doi: 10.1016/S0006-291X(02)02053-3. [DOI] [PubMed] [Google Scholar]
- 12.Fernandes J, de Oliveira RC, Guterres A, et al. Detection of Latino virus (Arenaviridae: Mammarenavirus) naturally infecting Calomys callidus. Acta Trop. 2018;179:17–24. doi: 10.1016/j.actatropica.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Fernandes J, de Oliveira RC, Guterres A, et al. Co-circulation of Clade C New World arenaviruses: new geographic distribution and host species. Infect Genet Evol. 2015;33:242–245. doi: 10.1016/j.meegid.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Fernandes J, Guterres A, de Oliveira RC, et al. Xapuri virus, a novel mammarenavirus: natural reassortment and increased diversity between New World viruses. Emerg Microbes Infect. 2018;7:120. doi: 10.1038/s41426-018-0119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bisordi I, Levis S, Maeda AY, et al. Pinhal virus, a new arenavirus isolated from Calomys tener in Brazil. Vector Borne Zoonotic Dis. 2015;15:694–700. doi: 10.1089/vbz.2014.1708. [DOI] [PubMed] [Google Scholar]
- 16.Fernandes J, Guterres A, de Oliveira RC, et al. Aporé virus, a novel mammarenavirus (Bunyavirales: Arenaviridae) related to highly pathogenic virus from South America. Mem Inst Oswaldo Cruz. 2019;114:e180586. doi: 10.1590/0074-02760180586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellwanger JH, Chies JAB. Keeping track of hidden dangers - the short history of the Sabiá virus. Rev Soc Bras Med Trop. 2017;50:3–8. doi: 10.1590/0037-8682-0330-2016. [DOI] [PubMed] [Google Scholar]
- 18.de Mello MF, Amgarten D, de Nastri AC, SS, , et al. Sabiá virus-like Mammarenavirus in patient with fatal hemorrhagic fever, Brazil, 2020. Emerg Infect Dis. 2020;26:1332–1334. doi: 10.3201/eid2606.200099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters CJ, Buchmeier M, Rollin PE, Ksiazek TG (1996) Arenaviruses. B.N. Fields, D.M. Knipe, P.M. Howley, R.M. Chanock, J.L. Melnick, T.P. Monath, R. Roizman, S.E. Straus (Eds.), Fields Virology, Lippincott-Raven Publishers
- 20.Brazilian Ministry of Health (2013) Manual de vigilância, prevenção e controle das hantaviroses. Avaiable at https://bvsms.saude.gov.br/bvs/publicacoes/manual_vigilancia_prevencao_controle_hantaviroses.pdf
- 21.Mills JN, Childs JE, Ksiazek T et al (1995) Methods for trapping and sampling small mammals for virologic testing. Avaiable at https://stacks.cdc.gov/view/cdc/11507. Accessed 9 Sept 2022
- 22.Ksiazek TG, Peters CJ, Rollin PE, et al. Identification of a new North American hantavirus that causes acute pulmonary insufficiency. Am J Trop Med Hyg. 1995;52:117–123. doi: 10.4269/ajtmh.1995.52.117. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki A, Bisordi I, Levis S, et al. Identifying rodent hantavirus reservoirs, Brazil. Emerg Infect Dis. 2004;10:2127–2134. doi: 10.3201/eid1012.040295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morales MA, Calderón GE, Riera LM, et al. Evaluation of an enzyme-linked immunosorbent assay for detection of antibodies to Junin virus in rodents. J Virol Methods. 2002;103:57–66. doi: 10.1016/S0166-0934(01)00452-9. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez A, Pifat DY, Kenyon RH, et al. Junin virus monoclonal antibodies: characterization and cross-reactivity with other arenaviruses. J Gen Virol. 1989;70(Pt 5):1125–1132. doi: 10.1099/0022-1317-70-5-1125. [DOI] [PubMed] [Google Scholar]
- 26.Vasconcelos PF, Travassos da Rosa AP, Rodrigues SG, et al. Laboratory-acquired human infection with SP H 114202 virus (Arenavirus: Arenaviridae family): clinical and laboratory aspects. Rev Inst Med Trop Sao Paulo. 1993;35:521–525. doi: 10.1590/s0036-46651993000600008. [DOI] [PubMed] [Google Scholar]
- 27.Pinheiro FP, Shope RE, de Andrade AHP, et al. Amapari, a new virus of the Tacaribe group from rodents and mites of Amapa Territory, Brazil. Proc Soc Exp Biol Med. 1966;122:531–535. doi: 10.3181/00379727-122-31182. [DOI] [Google Scholar]
- 28.Abudurexiti A, Adkins S, Alioto D, et al. Taxonomy of the order Bunyavirales: update 2019. Arch Virol. 2019;164:1949–1965. doi: 10.1007/s00705-019-04253-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowen MD, Peters CJ, Nichol ST. The phylogeny of New World (Tacaribe complex) arenaviruses. Virology. 1996;219:285–290. doi: 10.1006/viro.1996.0248. [DOI] [PubMed] [Google Scholar]
- 30.Charrel RN, Lemasson JJ, Garbutt M, et al. New insights into the evolutionary relationships between arenaviruses provided by comparative analysis of small and large segment sequences. Virology. 2003;317:191–196. doi: 10.1016/j.virol.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Sabino-Santos GJ, Maia FGM, Jonsson CB, et al. Serologic evidence of Mammarenaviruses among wild rodents in Brazil. J Wildl Dis. 2016;52:766–769. doi: 10.7589/2015-09-252. [DOI] [PubMed] [Google Scholar]
- 32.Bonvicino CR, De Oliveira JA, D’andrea PS. Guia dos roedores do Brasil, com chaves para Gêneros baseadas em caracteres externos. Rio de Janeiro: Centro Pan-Americano de febre aftosa; 2008. p. 120. [Google Scholar]
- 33.Mills JN, Ellis BA, McKee KTJ, et al. Junin virus activity in rodents from endemic and nonendemic loci in central Argentina. Am J Trop Med Hyg. 1991;44:589–597. doi: 10.4269/ajtmh.1991.44.589. [DOI] [PubMed] [Google Scholar]
- 34.Patterson M, Grant A, Paessler S. Epidemiology and pathogenesis of Bolivian hemorrhagic fever. Curr Opin Virol. 2014;5:82–90. doi: 10.1016/j.coviro.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tropical M (2018) Arenavirus no Brasil : eco-epidemiologia e os aspectos de sua ocorrência no processo de expansão da agricultura familiar arenavirus no Brasil : eco-epidemiologia e os aspectos de sua ocorrência no processo de expansão da agricultura familiar
- 36.Olival KJ, Hosseini PR, Zambrana-Torrelio C, et al. Host and viral traits predict zoonotic spillover from mammals. Nature. 2017;546:646–650. doi: 10.1038/nature22975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones KE, Patel NG, Levy MA, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grubaugh ND, Ladner JT, Lemey P, et al. Tracking virus outbreaks in the twenty-first century. Nat Microbiol. 2019;4:10–19. doi: 10.1038/s41564-018-0296-2. [DOI] [PMC free article] [PubMed] [Google Scholar]