Abstract
Background:
Patients with atopic dermatitis (AD) frequently use healthy lifestyle behaviors, although their benefits are unclear. This study's aim was to investigate the effectiveness of hypnotherapy, fasting with diet adjustments, and exercise in AD patients.
Methods:
In a four-armed randomized controlled monocenter open explorative clinical trial, adult patients with mild-to-moderate severe AD underwent, over 16 weeks, a five-session hypnotherapy group program (HTP), a five-session intermittent fasting with diet adjustment group program (IFDP), a five-session exercise group program (EP), or no study intervention (control) as add-on to topical corticosteroid use if required. Endpoints included subjectively perceived itching on a visual analogue scale (VAS, 0–100 mm); disease severity by SCORing Atopic Dermatitis (SCORAD); and adverse events (AEs). Endpoints were analyzed descriptively in the Full Analysis Set (FAS). Due to the coronavirus disease 2019 (COVID-19) pandemic, relevant changes to the study protocol included online in addition to “in-presence” group interventions, closing the study arm EP and premature trial termination before randomization of 120 intended patients.
Results:
During the COVID-19 pandemic, study recruitment was poor. The FAS included 20 patients (17 female) with 35.0 ± 12.1 (mean ± standard deviation [SD]) years of age. At baseline, mean ± SD for HTP (n = 6), IFDP (n = 4), EP (n = 1), and control (n = 9) were VAS itching 63.2 ± 18.0, 65.0 ± 13.9, 43.0 mm, 62.1 ± 17.3; SCORAD 43.0 ± 13.6, 47.0 ± 21.0, 60.3, 39.1 ± 15.6. After 16 weeks, endpoints were VAS itching 26.0 ± 16.4, 31.7 ± 9.9, 23.0 mm, 39.3 ± 27.0; SCORAD 24.1 ± 12.2, 29.1 ± 19.1, 49.1, 25.5 ± 14.4. No serious AEs related to the interventions were observed.
Conclusion:
Despite very small groups, study results indicated potential beneficial changes to baseline in perceived itching intensity, disease severity, and disease-specific quality of life for HTP and IFDP. Therefore, further clinical trials should be performed investigating the effectiveness and safety of all interventions.
Clinical Trial Registration:
January 31, 2020 German Clinical Trials Register (DRKS): DRKS00020557, Universal Trial Number (UTN): U1111-1247-1512.
Keywords: atopic dermatitis, hypnosis, fasting, diet, exercise, randomized controlled trial, complementary medicine
Introduction
Atopic dermatitis (AD) is associated with a high personal, social, and economic burden.1 Even first-line symptomatic treatment with topical corticosteroids (TCS) entails a risk of relevant adverse events (AEs).2,3 Therefore, patients worry about the treatment and engage in potentially healthy lifestyle behaviors.4,5 Relaxation techniques, diet, and exercise can diminish AD symptoms and are recommended.6–12 In a Berlin subsample of 58 (50.4% response rate) AD patients, 41.4% engaged in a relaxation technique, 75.9% in nutrition adjustment, and 93.1% in exercise.13 Therefore, the authors decided to further investigate related lifestyle behaviors. In animal models, case reports, and in a few mostly nonrandomized clinical trials, they found indications for benefits related to AD for hypnotherapy,14–18 intermittent fasting, plant-based or arachidonic acid-restricted food,19–31 and exercise.32–35
Treatments in a group setting might reduce costs, have beneficial group effects, and may improve quality of life (QoL) and dermatological symptoms.36,37 The rationale for this trial was that a hypnotherapy group program (HTP), intermittent fasting with diet adjustment group program (IFDP), and an exercise group program (EP) for outpatients could provide new treatment strategies for AD patients, which could subsequently be implemented in health care practice.
The aim of this study was to exploratively investigate the effectiveness of hypnotherapy, fasting with diet adjustments, and exercise in adult AD patients with a control group, and to gather experience and data for future confirmatory trials.
Methods
Study design, setting, and mitigation strategies due to the COVID-19 pandemic
In a four-armed, randomized controlled, single-center, open explorative clinical trial, adult AD patients received HTP, IFDP, EP, or control (nonintervention, waiting list, control). This study followed the standards of the Declaration of Helsinki,38 and the ICH-GCP guidelines.39 The positive decision of the ethics commission at Charité - Universitätsmedizin Berlin (EA1/315/19) was obtained and the study was registered prospectively (https://www.drks.de/, DRKS00020557). All patients provided oral and written informed consent before their inclusion in the study.
A randomization list was generated by a data manager as a computer-generated block randomization with variable block length. The randomization list was concealed by use of a computer interface, implemented in an MS ACCESS database and managed by the study nurse. The study physician contacted the study nurse by telephone, and after entering participants' inclusion information, participants were assigned to the respective study arm, and randomization results were reported to the study physician. The study interventions HTP and IFDP were performed at the Outpatient Clinic for Integrative Medicine at Campus Mitte Charité - Universitätsmedizin Berlin, EP was performed at the Center for Outpatient Rehabilitation (ZAR), Berlin.
Changes to the protocol
Since March 2020, the coronavirus disease 2019 (COVID-19) pandemic40 seriously impacted clinical research. This study was started at the same time and thus affected by the pandemic-related restrictions. Resulting modifications and mitigations are reported considering the CONSERVE statement (Fig. 1).41 Extenuating circumstances were changing Berlin's pandemic laws and confinements, with strict in-house regulations prohibiting research “in presence” during many months. Impacts as a result included the near-impossible participant recruitment, difficulty in performing physical examination for study inclusion, and “in-presence” endpoint measures or interventions.
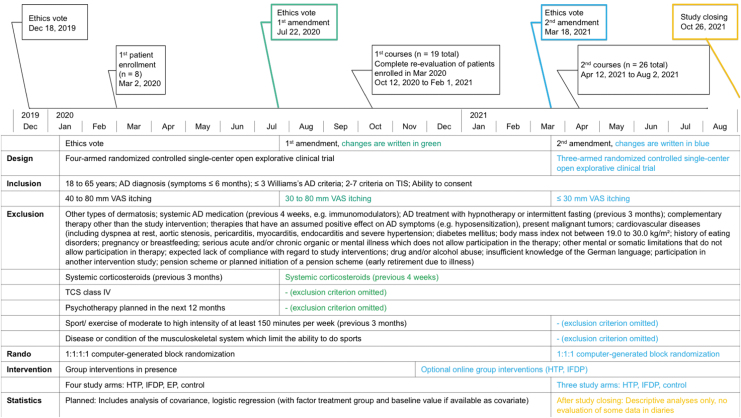
FIG. 1.
Modifications and Mitigations CAMATOP II. AD, atopic dermatitis; EP, exercise program; HTP, hypnotherapy group program; IFDP, intermittent fasting with diet adjustment group program; TCS, topical corticosteroids class I–III; TIS, three-item severity score; VAS, visual analogue scale.
Mitigating strategies were the constantly updated COVID-19 hygiene concepts, risk–benefit evaluations, and timing adjustments. As a consequence, at the beginning of the study, enrolled patients could not receive their allocated interventions in time. The authors constantly attempted restarts. In October 2020, these patients were re-evaluated by the study physician, and thereafter started together with newly recruited participants, the (now mostly online) group interventions (October 2020 to February 2021). Two ethics commission amendments were obtained for the following: Broadening of inclusion criteria (because of unusually poor recruitment), implementation of online group interventions (HTP and IFDP), recruitment stop, and, by this, closing the study arm EP (because of mandatory “in-presence” exercise monitoring including blood pressure, heart rate).
After passing the foreseen two years for the study project, and with the prospect of renewed stricter research limitations, the study was closed on October 26, 2021. These are important modifications because the protocol changes and the small sample size impaired effectiveness evaluations.
Patients
Patients were recruited in Berlin by public transport advertisements, posters, and digital media. The inclusion and exclusion criteria with their modifications are provided in Figure 1.
Study interventions
Group intervention programs
In addition to a literature review (trials, therapy books, German Society for Nutrition42), stakeholder engagements (six HTP, six IFDP, five EP experts) identified beneficial therapeutic components. Respective experts consented on closed groups with sequential scheduling for group effects. The results were then coordinated for comparability: Each program consisted of five sessions lasting 90 min in groups of 5–10 patients with approximately 2-week intervals. Furthermore, each program (HTP, IFDP, and EP) included supported self-treatments (audio recordings in HTP, a handout with instructions on intermittent fasting with diet adjustments in IFDP, and a handout with exercise instructions in EP). Group interventions were performed by well-educated and clinically experienced hypnotherapists (a physician and a psychologist), one dietician, or two sports scientists, respectively. Each group program was based on intervention-specific health education, training in the intervention, and resource activation. The concepts are displayed in Supplementary Table S1a–c, key characteristics are described below.
Hypnotherapy group program
The hypnosis exercises aimed for deep relaxation, increasing well-being and calmness, individual resource activation for skin healing and improving the skin function, strengthening physiological and psychological barrier functions, and to reduce itching.
Intermittent fasting with diet adjustment group program
Plant-based food was recommended with at least three daily portions (handful) of vegetables, two portions of fruits, three portions of low-processed cereals. A small amount of animal-derived food was allowed. The time-restricted feeding comprised a 16 (±1)-hour overnight fasting period, with optional modification of 16:8 fasting in one day per week.
Exercise group program
Mobility and coordination exercises included games (e.g., with balls, hoops) and walking. A 30-min endurance training complemented each session. The recommended home training combined an intensive-to-moderate endurance training, a moderate strength training (13/20 on a Borg scale),43 and stretching exercises of about 150 min per week.
Control
The patients could continue their routine care but received no study intervention during the first 16 weeks. Due to COVID-19-derived extenuating circumstances, patients allocated to control could only participate in HTP or IFDP after 26 weeks outside of the study.
Patients in all groups were allowed to use routine care, such as TCS and emollients, but no other AD medication (e.g., immunomodulators, calcineurin inhibitors).
Endpoints
No primary endpoint was defined, due to the exploratory nature of this trial. The most important endpoint was considered the average subjectively perceived itching intensity within the last seven days measured after 16 weeks on a horizontal visual analogue scale (VAS; 0–100 mm), as it is validated and broadly used in AD trials.44,45
The measurements at baseline and after 8, 16, and 26 weeks were as follows: The average subjectively perceived itching intensity within the last seven days on a VAS44; the average subjectively perceived total severity of all AD symptoms within the last seven days on a VAS (nonvalidated); number of TCS applications within the last seven days (TCS use, nonvalidated); general QoL using the 12-item Short Form Health Survey (validated)46–49; QoL disease-specific by the Dermatology Life Quality Index (DLQI, validated)50,51; affects by the Positive and Negative Affect Schedule (validated)52; incapacity to work due to AD (hours/during 8 weeks, nonvalidated); the average subjectively perceived sleep disturbance within the last seven days on a VAS (validated)53; the average subjectively perceived skin condition within the last seven days on a VAS (nonvalidated).
At baseline and after 8 and 16 weeks, independent raters (physicians and nurses who are neurodermatitis trainers and specialized in SCORing Atopic Dermatitis [SCORAD] rating) performed a group allocation-blinded assessment of the disease severity using the broadly used and validated SCORAD and Eczema Area and Severity Index (EASI).54,55
At baseline, patients provided sociodemographic data and rated their expectations regarding study interventions on a numeric rating scale (nonvalidated). After 8, 16, and 26 weeks, patients allocated to HTP, IFDP, or EP reported their perceived efficacy of the respective treatment and all patients reported their self-perceived change of complaints. During the first 16 weeks, all patients recorded their medication and data on compliance to allocated interventions in diaries.
Safety was assessed by AEs recorded in patients' diaries and/or in the study center.
Further details on endpoints (range, minimal clinically important difference) are provided in Table 2.
Table 2.
Baseline Values and Endpoints After 8, 16, and 26 Weeks (Patients Who Completed Questionnaires After Week 8), n = 20
| Endpoint | HTP (n = 6), mean ± SD/n (%) | IFDP (n = 4), mean ± SD/n (%) | Control (n = 9), mean ± SD/n (%) | EP (n = 1), mean ± SD/n (%) | ||||
|---|---|---|---|---|---|---|---|---|
| VAS itching [0–100 mm],a MCID 13.4 | ||||||||
| Baseline | 6 | 63.2 ± 18.0 | 4 | 65.0 ± 13.9 | 9 | 62.1 ± 17.3 | 1 | 43.0 |
| 8 Weeks | 6 | 25.0 ± 15.2 | 4 | 30.3 ± 19.2 | 9 | 49.0 ± 19.7 | 1 | 40.0 |
| 16 Weeks | 6 | 26.0 ± 16.4 | 3 | 31.7 ± 9.9 | 8 | 39.3 ± 27.0 | 1 | 23.0 |
| 26 Weeks | 4 | 29.5 ± 20.1 | 3 | 40.0 ± 28.2 | 9 | 48.1 ± 24.5 | 1 | 27.0 |
| SCORAD [0–103],b MCID 8.7 | ||||||||
| Baseline | 6 | 43.0 ± 13.6 | 4 | 47.0 ± 21.0 | 9 | 39.1 ± 15.6 | 1 | 60.3 |
| 8 Weeks | 6 | 32.9 ± 15.0 | 4 | 22.7 ± 7.8 | 9 | 32.2 ± 15.5 | 1 | 38.9 |
| 16 Weeks | 5 | 24.1 ± 12.2 | 2 | 29.1 ± 19.1 | 6 | 25.5 ± 14.4 | 1 | 49.1 |
| EASI [0–72],b MCID 6.6 | ||||||||
| Baseline | 6 | 12.4 ± 11.0 | 4 | 12.4 ± 7.6 | 9 | 10.6 ± 8.0 | 1 | 28.5 |
| 8 Weeks | 6 | 7.4 ± 5.8 | 4 | 4.6 ± 4.9 | 9 | 9.1 ± 8.6 | 1 | 18.9 |
| 16 Weeks | 5 | 4.7 ± 3.9 | 2 | 5.4 ± 6.5 | 6 | 7.6 ± 6.8 | 1 | 35.7 |
| VAS total AD symptoms [0–100 mm],c no MCID | ||||||||
| Baseline | 6 | 65.7 ± 18.8 | 4 | 72.5 ± 19.5 | 9 | 59.6 ± 14.7 | 1 | 40.0 |
| 8 Weeks | 6 | 28.0 ± 9.9 | 4 | 31.8 ± 17.8 | 9 | 48.4 ± 19.9 | 1 | 34.0 |
| 16 Weeks | 6 | 27.8 ± 17.0 | 3 | 32.7 ± 8.1 | 8 | 38.6 ± 27.2 | 1 | 24.0 |
| 26 Weeks | 4 | 27.0 ± 22.7 | 3 | 40.7 ± 25.4 | 9 | 45.1 ± 22.9 | 1 | 25.0 |
| Patients using AD medication (systemic or topic) | ||||||||
| Baseline (last 3 months) | 6 | 5 (83.3) | 4 | 4 (100) | 9 | 9 (100) | 1 | 1 (100) |
| 8 Weeks (last 8 weeks) | 6 | 4 (66.7) | 4 | 4 (100) | 9 | 9 (100) | 1 | 1 (100) |
| 16 Weeks (last 8 weeks) | 6 | 4 (66.7) | 3 | 3 (100) | 8 | 6 (75.0) | 1 | 1 (100) |
| 26 Weeks (last 8 weeks) | 4 | 2 (50.0) | 3 | 3 (100) | 9 | 9 (100) | 1 | 1 (100) |
| Patients using TCS | ||||||||
| Baseline (last 4 weeks) | 5 | 2 (40.0) | 4 | 2 (50.0) | 9 | 8 (88.9) | 1 | 1 (100) |
| 8 Weeks (last 8 weeks) | 4 | 1 (0.25) | 3 | 2 (66.7) | 8 | 7 (87.5) | 1 | 1 (100) |
| 16 Weeks (last 8 weeks) | 4 | 0 | 3 | 0 | 6 | 5 (83.3) | 1 | 1 (100) |
| 26 Weeks (last 8 weeks) | 2 | 0 | 3 | 2 (66.7) | 9 | 7 (77.8) | 1 | 1 (100) |
| SF-12 PCS,d MCID 5 | ||||||||
| Baseline | 6 | 49.8 ± 3.7 | 4 | 52.1 ± 8.5 | 8 | 50.6 ± 7.8 | 1 | 57.9 |
| 8 Weeks | 6 | 53.1 ± 7.8 | 4 | 52.5 ± 5.4 | 9 | 52.0 ± 7.5 | 1 | 41.7 |
| 16 Weeks | 6 | 55.0 ± 6.5 | 3 | 53.3 ± 3.2 | 8 | 50.2 ± 6.5 | 1 | 46.8 |
| 26 Weeks | 4 | 56.8 ± 6.6 | 2 | 49.3 ± 9.4 | 9 | 52.3 ± 7.6 | 1 | 58.3 |
| SF-12 MCS,d MCID 5 | ||||||||
| Baseline | 6 | 42.7 ± 4.3 | 4 | 43.7 ± 9.8 | 8 | 40.9 ± 9.1 | 1 | 39.3 |
| 8 Weeks | 6 | 36.3 ± 7.5 | 4 | 51.1 ± 4.0 | 9 | 43.0 ± 6.4 | 1 | 29.6 |
| 16 Weeks | 6 | 40.9 ± 7.8 | 3 | 50.9 ± 4.4 | 8 | 40.1 ± 7.3 | 1 | 50.0 |
| 26 Weeks | 4 | 35.1 ± 2.5 | 2 | 53.4 ± 9.3 | 9 | 33.3 ± 8.5 | 1 | 33.7 |
| DLQI [0–30],b MCID 3.3 | ||||||||
| Baseline | 6 | 12.8 ± 6.3 | 4 | 11.8 ± 11.7 | 9 | 11.3 ± 6.8 | 1 | 13.0 |
| 8 Weeks | 6 | 6.0 ± 2.8 | 4 | 5.3 ± 4.7 | 8 | 9.9 ± 4.9 | 1 | 15.0 |
| 16 Weeks | 6 | 7.0 ± 4.1 | 3 | 5.7 ± 4.0 | 7 | 9.4 ± 5.6 | 1 | 7.0 |
| 26 Weeks | 4 | 10.3 ± 4.9 | 3 | 9.0 ± 11.3 | 9 | 9.6 ± 7.1 | 1 | 10.0 |
| PANAS, positive affect dimension [1–5]b | ||||||||
| Baseline | 6 | 2.9 ± 0.2 | 4 | 3.2 ± 0.4 | 9 | 2.7 ± 0.9 | 1 | 3.1 |
| 8 Weeks | 6 | 2.9 ± 0.6 | 4 | 3.8 ± 0.2 | 9 | 2.7 ± 0.7 | 1 | 2.5 |
| 16 Weeks | 6 | 3.1 ± 0.6 | 3 | 3.6 ± 0.5 | 8 | 2.7 ± 0.7 | 1 | 2.6 |
| 26 Weeks | 4 | 3.0 ± 0.7 | 3 | 3.5 ± 0.7 | 9 | 2.4 ± 0.4 | 1 | 2.9 |
| PANAS, negative affect dimension [1–5]d | ||||||||
| Baseline (8 weeks) | 6 | 2.3 ± 0.8 | 4 | 1.9 ± 0.2 | 9 | 2.0 ± 0.5 | 1 | 1.6 |
| 8 Weeks | 6 | 2.0 ± 0.6 | 4 | 1.9 ± 0.5 | 9 | 1.8 ± 0.4 | 1 | 1.7 |
| 16 Weeks | 6 | 2.0 ± 0.3 | 3 | 1.6 ± 0.3 | 8 | 2.0 ± 0.4 | 1 | 2.0 |
| 26 Weeks | 4 | 2.2 ± 0.3 | 2 | 1.5 ± 0.3 | 9 | 2.0 ± 0.4 | 1 | 1.5 |
| Incapacity to work due to AD (hours/during 8 weeks) | ||||||||
| Baseline (yes n = 9) | 6 | 41.7 ± 67.8 | 1 | 96 | 9 | 55.5 ± 61.5 | 1 | 8 |
| 8 Weeks (yes n = 9) | 3 | 114.0 ± 192.3 | 1 | 120.0 | 3 | 129.3 ± 220.6 | 1 | 34.0 |
| 16 Weeks (yes n = 9) | 3 | 18.3 ± 25.7 | 1 | 26.0 | 4 | 97.3 ± 101.0 | 1 | 8.0 |
| 26 Weeks (yes n = 8) | 1 | 4.0 | 1 | 120.0 | 5 | 136.2 ± 121.8 | 1 | 10.0 |
| VAS sleep disturbances [0–100 mm],e MCID 10 | ||||||||
| Baseline | 5 | 34.8 ± 19.9 | 3 | 57.7 ± 21.0 | 6 | 46.0 ± 20.9 | 1 | 19.0 |
| 8 Weeks (yes n = 11) | 3 | 16.0 ± 12.1 | 2 | 32.5 ± 6.4 | 5 | 35.2 ± 27.1 | 1 | 19.0 |
| 16 Weeks (yes n = 8) | 1 | 31.0 | 3 | 17.0 ± 12.3 | 4 | 39.5 ± 39.2 | ||
| 26 Weeks (yes n = 14) | 4 | 14.8 ± 11.7 | 3 | 28.7 ± 38.6 | 6 | 29.0 ± 29.4 | 1 | 18.0 |
| VAS skin condition [0–100 mm],f no MCID | ||||||||
| Baseline | 6 | 58.7 ± 27.3 | 4 | 63.3 ± 19.6 | 9 | 55.9 ± 21.4 | 1 | 42.0 |
| 8 Weeks | 6 | 27.2 ± 11.8 | 4 | 29.0 ± 18.0 | 9 | 49.0 ± 19.5 | 1 | 40.0 |
| 16 Weeks | 6 | 28.7 ± 19.6 | 3 | 23.7 ± 5.8 | 8 | 39.8 ± 28.3 | 1 | 25.0 |
| 26 Weeks | 4 | 24.5 ± 14.3 | 3 | 45.0 ± 24.6 | 9 | 40.3 ± 27.4 | 1 | 27.0 |
| Patient efficacy (HTP, IFDP, EP) | ||||||||
| 8 Weeks | 6 | 4 | 1 | |||||
| Very effective | 0 | 1 (25.0) | 0 | |||||
| Effective | 4 (66.7) | 2 (50.0) | 0 | |||||
| Less effective | 2 (33.3) | 1 (25.0) | 1 (100) | |||||
| Ineffective | 0 | 0 | 0 | |||||
| 16 Weeks | 6 | 3 | 1 | |||||
| Very effective | 0 | 1 (33.3) | 0 | |||||
| Effective | 2 (33.3) | 1 (33.3) | 0 | |||||
| Less effective | 3 (50.0) | 1 (33.3) | 1 (100) | |||||
| Ineffective | 1 (16.7) | 0 | 0 | |||||
| 26 Weeks | 4 | 3 | 1 | |||||
| Very effective | 0 | 1 (33.3) | 0 | |||||
| Effective | 1 (25.0) | 0 | 0 | |||||
| Less effective | 3 (75.0) | 0 | 1 (100) | |||||
| Ineffective | 0 | 2 (66.7) | 0 | |||||
| Change of complaints | ||||||||
| 8 Weeks | 6 | 4 | 9 | 1 | ||||
| Very much improved | 0 | 1 (25.0) | 0 | 0 | ||||
| Greatly improved | 4 (66.7) | 2 (50.0) | 1 (11.1) | 0 | ||||
| Minimally improved | 1 (16.7) | 0 | 1 (11.1) | 0 | ||||
| Not changed | 0 | 1 (25.0) | 4 (44.4) | 1 (100) | ||||
| Minimally worsened | 0 | 0 | 3 (33.3) | 0 | ||||
| Very much worsened | 1 (16.7) | 0 | 0 | 0 | ||||
| 16 Weeks | 6 | 3 | 8 | 1 | ||||
| Very much improved | 0 | 0 | 0 | 0 | ||||
| Greatly improved | 3 (50.0) | 1 (33.3) | 1 (12.5) | 0 | ||||
| Minimally improved | 2 (33.3) | 2 (67.7) | 1 (12.5) | 1 (100) | ||||
| Not changed | 1 (16.7) | 0 | 4 (50.0) | 0 | ||||
| Minimally worsened | 0 | 0 | 2 (25.0) | 0 | ||||
| Very much worsened | 0 | 0 | 0 | 0 | ||||
| 26 Weeks | 4 | 3 | 9 | 1 | ||||
| Very much improved | 0 | 0 | 0 | 0 | ||||
| Greatly improved | 1 (25.0) | 0 | 0 | 0 | ||||
| Minimally improved | 0 | 1 (33.3) | 1 (11.1) | 0 | ||||
| Not changed | 2 (50.0) | 2 (67.7) | 7 (77.8) | 1 (100) | ||||
| Minimally worsened | 1 (25.0) | 0 | 1 (11.1) | 0 | ||||
| Very much worsened | 0 | 0 | 0 | 0 | ||||
Values are absolute numbers (N), column percentages or mean ± SD.
0 = no itching, 100 = extreme itching.
Lower values indicate better status.
0 = no symptoms, 100 = extreme symptoms.
Higher values indicate better status.
0 = no sleep disturbance, 100 = extreme sleep disturbance.
0 = undisturbed skin, 100 = extremely disturbed skin.
AD, atopic dermatitis; DLQI, Dermatology Life Quality Index; EASI, Eczema Area and Severity Index; EP, exercise program; HTP, hypnotherapy group program; IFDP, intermittent fasting with diet adjustment group program; MCID, minimal clinically important difference; MCS, mental component scale; PANAS, Positive and Negative Affect Schedule; PCS, physical component scale; SCORAD, SCORing Atopic Dermatitis; SD, standard deviation; SF-12, 12-item Short Form Health Survey; TCS, topical corticosteroids class I–III; VAS, visual analogue scale.
All data were collected pseudonymously by standardized paper forms and entered in the SoSci Survey.56
Statistical analyses
As this was an explorative clinical trial, no sample size calculation was performed. A total of 120 patients (30 per intervention group) were considered logistically manageable and sufficient to achieve the study's exploratory objectives. Because of insufficient recruitment, the statistical analyses were changed: Instead of analysis of covariance, data were analyzed only descriptively using mean values, standard deviations (SDs), medians, absolute and relative frequencies, and graphically using boxplots. Baseline values were calculated for the whole allocated population and for the Full Analysis Set (FAS) including patients who could be analyzed also at the 8-week follow-up. Missing data were not imputed. Statistical analyses were performed with IBM SPSS for Mac Version 26, IBM SPSS for Windows Version 27.57
Results
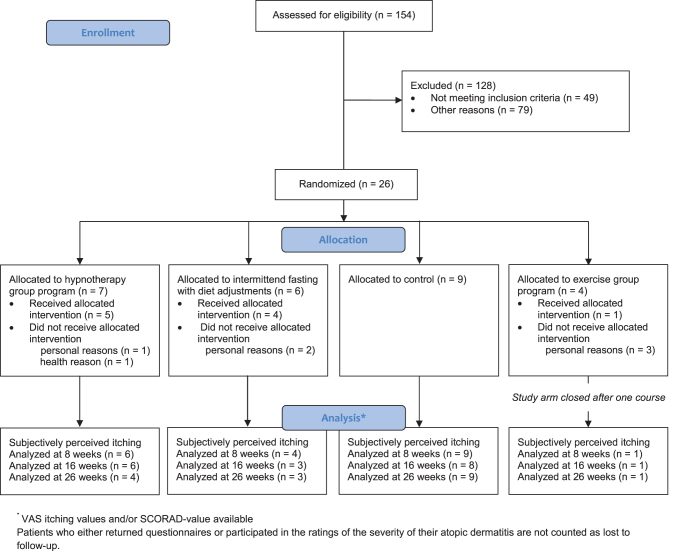
Between March 2020 and April 2021, of 154 eligible patients, 26 patients were randomized (HTP n = 7, IFDP n = 6, EP n = 4, control n = 9). Because a sample size increase was unlikely due to the COVID-19 pandemic, the study was stopped prematurely. Before the first treatment, five patients dropped out (HTP n = 1, IFDP n = 1, EP n = 3, for further details see Fig. 2). In addition, allocated treatment was not received by one patient randomized to HTP (health reason, AE, data until the follow-up at 16 weeks provided) and another patient randomized to IFDP (personal reason).
FIG. 2.
Recruitment, treatment, and follow-up of patients.
The FAS included 20 patients (85.0% female) with 35.0 ± 12.1 (mean ± SD) years of age. The authors found relevant intergroup baseline differences in patients' age, duration of AD disease, number of patients applying TCS during the last four weeks, hours of incapacity to work, and perceived sleep disturbance measured by a VAS (Tables 1 and 2).
Table 1.
Baseline Characteristics of Participants Who Completed Questionnaires After Week 8 (n = 20)
| N | HTP (n = 6), mean ± SD/n (%) | IFDP (n = 4), mean ± SD/n (%) | Control (n = 9), mean ± SD/n (%) | EP (n = 1), mean ± SD/n (%) | Total, mean ± SD/(n = 20), n (%) | |
|---|---|---|---|---|---|---|
| Age (years) | 20 | 29.3 ± 4.0 | 26.0 ± 12.1 | 39.6 ± 11.5 | 56.0 | 35.0 ± 12.1 |
| Sex (female) | 20 | 6 (100) | 3 (75.0) | 8 (88.9) | 0 | 17 (85.0) |
| Sex (male) | 20 | 0 | 1 (25.0) | 1 (11.1) | 1 (100) | 3 (15.0) |
| BMI (kg/m2) | 20 | 21.4 ± 2.6 | 21.7 ± 1.4 | 23.7 ± 3.0 | 25.2 | 22.7 ± 2.7 |
| German university entrance qualification | 20 | 6 (100) | 3 (75.0) | 8 (88.9) | 1 (100) | 18 (90.0) |
| Duration of AD (years) | 20 | 22.2 ± 6.4 | 21.5 ± 15.3 | 38.1 ± 13.0 | 54.0 | 30.8 ± 14.7 |
| Three-item severity score [0–9]a | 20 | 4.5 ± 1.0 | 4.8 ± 2.6 | 5.9 ± 0.8 | 5.0 | 5.2 ± 1.4 |
| Concomitant disease | 20 | 2 (33.3) | 2 (50.0) | 5 (55.6) | 0 | 9 (45.0) |
| Allergic asthma | 20 | 2 (33.3) | 2 (50.0) | 2 (22.2) | 0 | 6 (30.0) |
| History of allergy | 20 | 6 (100) | 2 (50.0) | 7 (77.8) | 1 (100) | 16 (80.0) |
| Food allergy | 20 | 4 (66.7) | 2 (50.0) | 3 (33.3) | 1 (100) | 10 (50.0) |
| Influence by climate | 20 | 6 (100) | 4 (100) | 7 (77.8) | 1 (100) | 18 (90.0) |
| Previous psychotherapy | 20 | 3 (50.0) | 2 (50.0) | 8 (88.9) | 0 | 13 (65.0) |
| Past treatment with hypnotherapy | 20 | 0 | 0 | 2 (22.2) | 0 | 2 (10.0) |
| Due to AD | 20 | 0 | 0 | 0 | 0 | 0 |
| Due to other diagnosis | 20 | 0 | 0 | 2 (22.2) | 0 | 2 (10.0) |
| Past fasting | 20 | 4 (66.7) | 1 (25.0) | 5 (55.6) | 0 | 10 (50.0) |
| Due to AD | 20 | 1 (16.7) | 0 | 2 (22.2) | 0 | 3 (15.0) |
| Due to other diagnosis | 20 | 3 (50.0) | 1 (25.0) | 3 (33.3) | 0 | 7 (35.0) |
| Incapacity to work due to AD (last 8 weeks) | 20 | 3 (50.0) | 1 (25.0) | 4 (44.4) | 1 (100) | 9 (45.0) |
| Sleep disturbances due to AD | 20 | 5 (83.3) | 3 (75.0) | 6 (66.7) | 1 (100) | 15 (75.0) |
| Patient expectations for HTP [0–10]b | 20 | 7.2 ± 1.2 Median 7.0 |
5.8 ± 2.2 Median 6.0 |
5.1 ± 2.4 Median 5.0 |
3.0 | 5.1 ± 2.4 Median 6.0 |
| Patient expectations for IFDP [0–10]b | 20 | 5.7 ± 2.3 Median 5.5 |
5.5 ± 2.4 Median 6.5 |
6.6 ± 3.2 Median 7.0 |
4.0 | 6.0 ± 2.6 Median 7.0 |
| Patient expectations for EP [0–10]b | 20 | 3.0 ± 2.5 Median 2.5 |
5.3 ± 2.2 Median 6.0 |
5.0 ± 2.5 Median 6.0 |
2.0 | 4.3 ± 2.5 Median 4.5 |
Values are absolute numbers (N), column percentages, or mean ± SD.
Lower values indicate better status.
0 = no improvement, 10 = complete recovery.
AD, atopic dermatitis; BMI, body mass index; EP, exercise program; HTP, hypnotherapy group program; IFDP, intermittent fasting with diet adjustment group program; SD, standard deviation.
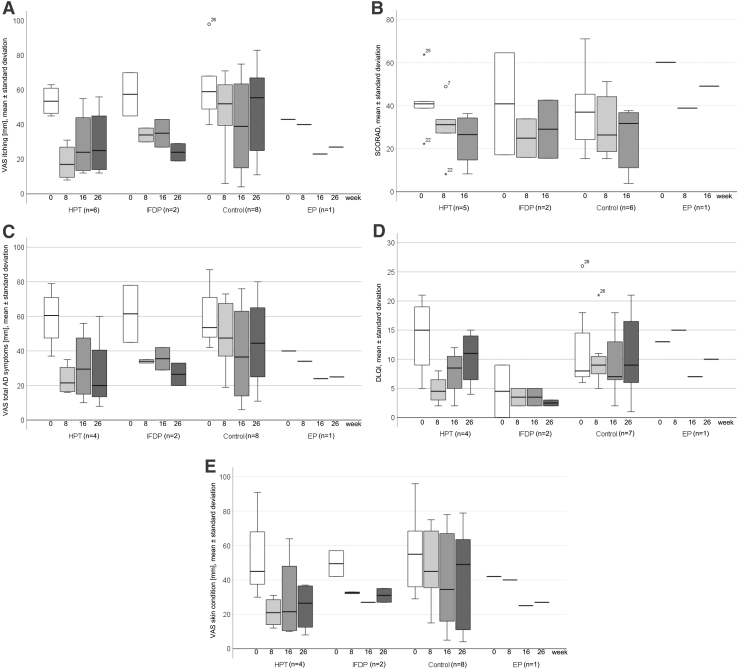
In the comparison of baseline with 8 or 16 weeks, the authors observed indications for beneficial effects in perceived itching intensity on VAS, disease severity by SCORAD, perceived total severity of all AD symptoms on VAS, disease-specific QoL by DLQI, and perceived skin condition on VAS for HTP, IFDP, and smaller for control (Table 2; Fig. 3A–E). The medication regimens were comparable between groups over time.
FIG. 3.
(A) Perceived itching intensity on a VAS through 26 weeks in HTP, IFDP, control, and EP. Values are means and standard deviations. (B) Disease severity by SCORAD through 26 weeks in HTP, IFDP, control, and EP. Values are means and standard deviations. (C) Perceived total severity of all AD symptoms on a VAS through 26 weeks in HTP, IFDP, control, and EP. Values are means and standard deviations. (D) Disease-specific quality of life by the DLQI through 26 weeks in HTP, IFDP, control, and EP. Values are means and standard deviations. (E) Perceived skin condition on a VAS through 26 weeks in HTP, IFDP, control, and EP. Values are means and standard deviations. AD, atopic dermatitis; DLQI, Dermatology Life Quality Index; EP, exercise group program; HTP, hypnotherapy group program; IFDP, intermittent fasting with diet adjustment group program; SCORAD, SCORing Atopic Dermatitis; VAS, visual analogue scale.
Regarding safety, one serious AE (SAE) was reported by one patient allocated to IFDP (AD symptom worsening, hospitalization before study intervention start) and one patient allocated to control (psychological symptoms, hospitalization). One patient allocated to HTP reported AD symptom worsening (AE) after the first and second session, with unsure relation to HTP. The patient used a topical immunomodulator (pimecrolimus) during weeks 1–3, which managed most symptoms. The patient provided data until the 16-week follow-up, and in additional interviews she reported persisted worsening of symptoms.
The authors found no anamnestic red flag or indication as to why this patient reacted the way she did. Another patient reported short duration of increased itching and psychological symptoms directly after HTP (by patient-related AE) in diary entries at weeks 1 and 3. One patient allocated to IFDP reported skin impurities and digestive problems (by patient-related AE) at week 2. The patient allocated to EP reported no AE.
Regarding feasibility, one HTP and one IFDP “in-presence” session (October 2020) and the subsequent online group meetings (HTP, IFDP) were feasible, but therapists reported to the study coordinator impaired perceived group feelings. At the beginning of the respective fifth sessions, the therapists got positive feedback regarding performance, comfort of online meetings for patients not living in Berlin, and perceived overall study effects. Patients allocated to HTP mostly applied self-hypnotherapy and used other relaxation methods. Patients allocated to IFDP reported mostly good adherence to the 16:8 fasting protocol. The EP was reduced to a single setting program joining in-house rehabilitation patients with five sessions. Therapists reported good exercise and home exercise feasibility, and patients' symptom reduction. Data of these patients are provided in tables and figures without further comments. Baseline data for the whole included sample are provided in Supplementary Table S2.
Discussion
The CAMATOP II trial was strongly impacted by confinements and research restrictions due to the COVID-19 pandemic. However, in the 20 included AD patients, the authors observed partly relevant beneficial changes over time in perceived itching intensity, disease severity, and disease-specific QoL for HTP, IFDP, and partly also for control. One AE with aggravation of itching and eczema with the use of a topical immunomodulator was unsurely related to HTP; other AEs by patients related to HTP or IFDP were minor and short-lived, probably by focusing the patients' attention on the skin. Reported SAEs were not related to the interventions. The HTP and IFDP “in-presence” and online meetings were feasible and can be utilized for further research. The “in-presence” EP was only applied to a single AD patient, but a group setting seemed feasible. In online meetings, special group feelings were not perceived by therapists, but patients gave positive feedback.
The COVID-19 pandemic meant that substantial modifications of the study methodology were required. Participant recruitment was insufficient despite multiple advertisements. In a former trial including adult AD patients,58 Berlin public transport advertisements were the most effective recruitment strategy. During the COVID-19 confinements, this advertisement strategy was ineffective, as people avoided public transport. In addition, following the experience in study recruitment, the months October to April were expected to be the best time for participant inclusion, but during these months in 2019/2020 and 2020/2021, the COVID-19 pandemic waves in Germany and the ensuing research restrictions were at their height. Mitigating strategies (protocol changes) were applied to enable recruitment and study performance. The closed-group design could have been an obstacle if more AD patients would have been interested in the study.
For larger studies, the authors advise different parallel sessions for each intervention. Finally, the study project could not be performed within the foreseen two-year period and was closed facing renewed research restrictions. From an ethical point of view, results of even few participants should be published to contribute to the scientific evidence. This applies especially for this study, as patients and the study team invested multiple extra efforts.
To our knowledge, this was the first study investigating group programs in hypnotherapy, intermittent fasting with diet adjustments, and exercise in AD patients compared with a nonintervention control, gathering experience and first data. Intergroup comparisons could not be performed. However, the use of hypnotherapy in atopic diseases has only been tested in few exploratory studies, and very rarely within the past 20 years.14–18 Senser et al (2004) randomized 33 adult AD patients to 12 single hypnotherapy sessions á 1 h, or to the nonintervention control.14 Patients allocated to hypnotherapy were reported to show improvements pre–post and in comparison with control patients regarding the severity of AD by SCORAD, subjectively perceived itching intensity (VAS), subjectively perceived skin condition (VAS), and QoL disease-specifically by the DLQI.14 The authors interpreted that 12 sessions of single hypnotherapy is an effective treatment. This study reported no AE. Earlier trials did not report AEs.59
In contrast, and in addition to minor AEs, in this study there was one AE unsurely related to HTP with worsening of symptoms (using a topical immunomodulator). The authors found no other explanation for the individual worsening. However, the possibility of symptom worsening is important for patient information and shared decision-making in hypnotherapy. Delaitre et al performed a retrospective data analysis in 27 AD outpatients (mean age 34.5 years) with symptom persistence despite local AD therapy treated with 2–16 individual hypnotherapy sessions á 20 min. The authors found a reduction in disease severity by EASI (measured by the treating physician) and patients' self-assessments, but reported no AE assessment. They concluded that hypnotherapy might be useful in AD patients.60 Regarding nutrition, single-armed trials reported AD symptom improvements after time-restricted feeding with a low-energy diet,21 and after a calorie-reduced vegan diet.22
The authors found no further clinical trials in adult AD patients. Although they found the medication regimens comparable between groups, they could not correlate endpoints to medication use, and therefore, they cannot exclude that medication regimens could have influenced the clinical endpoints. The authors can draw no conclusions from the one patient allocated to EP, as outcome changes might be by chance as in prospective case reports.
For the first time, group interventions such as HTP, IFDP, and EP were developed for adult AD patients in a university outpatient setting “in-presence” and as online group interventions. The strengths of this study include the implementation of blinded-rated and patient-reported endpoints, and the reporting of mitigations due to the COVID-19 pandemic based on the CONSERVE statement. The main limitations were caused by the COVID-19 pandemic. The change in the trial design (closing one study arm) and the small sample size substantially impair the effectiveness evaluations. However, as this is an exploratory trial, the feasibility of the study interventions could be investigated to a certain extent and the interventions proved practicable, as “in-presence” and as online meetings.
Further study limitations include the monocenter setting and the female predominance, which further limits the generalizability of the results. The study design had potential sources of bias: Patients performing HTP, IFDP, or EP received more therapist and/or peer group time and attention than control patients, and blinding of patients and therapists was impossible. Consequently, the observed indications for improvements may have been overestimated. Emerging trends should be verified by confirmatory studies. Further studies should consider to use the subjectively perceived itching intensity (VAS) as a primary endpoint, as this is to patients a meaningful and validated measurement.
Even with a modified study design, the study results are meaningful as they provide data and methodology for the group interventions, in presence and online. As patients participated in studies during the COVID-19 pandemic, they think it is ethical to publish the results. They provided an example, how to report a changed study design and results according to the CONSERVE statement.
The study results suggest that study interventions are feasible, acceptable, and might be effective, providing justification for the progression to confirmatory clinical studies on the interventions. For larger clinical trials, the authors advise different parallel sessions for each intervention, so that patients can choose convenient timeslots.
Conclusion
The study interventions were feasible and acceptable for patients with mild-to-moderate severe AD, however, the recruitment was difficult due to the COVID-19 pandemic. Despite very small groups, study results indicated potential beneficial changes to baseline in perceived itching intensity, disease severity, and disease-specific QoL for HTP and IFDP. Therefore, further high-quality clinical trials should be performed investigating the effectiveness and safety of hypnotherapy, fasting with diet adjustments, as well as exercise.
Supplementary Material
Acknowledgments
The authors thank their patients, especially for their flexibility and for staying with them despite adverse circumstances during the COVID-19 pandemic. They thank the expert in allergology/AD Professor Dr. Torsten Zuberbier from Charité - Universitätsmedizin Berlin for his respected general advice. The authors also thank the experts in hypnotherapy Dr. med. Nikola Aufmkolk, Prof. Mark Jensen, and Dipl.-Psych. Dr. Silvia Fisch for the comprehensive contribution to the concept of the HTP. They thank the experts in nutrition and fasting, Alexandra Prüß, for the contribution to the concept of the intermitted fasting with diet adjustment group program.
The authors thank Marco Spiegel and the Center for Outpatient Rehabilitation (ZAR) at Gartenstrasse 5, 10115 Berlin, for their cooperation. They thank the sports experts and experts in rehabilitation Mario Jossa and Igor Borsow and the author of the Erlangen studies on group exercise in AD patients Dr. Bernd Salzer for the comprehensive contribution to the EP. They also thank Petra Wagner, Marion Trentmann, and Lea Jerzynski for their support and cooperation in the SCORAD and EASI rating. The authors also thank their study nurse Margit Cree and the whole team at the Outpatient Clinic for Integrative Medicine at Campus Mitte Charité - Universitätsmedizin Berlin. The authors sincerely thank the German Neurodermatitis Foundation for kindly supporting the study.
Authors' Contributions
G.R. (study coordinator) and B.B. (principal investigator) conceived the study, raised the funding, and designed the study. G.R. designed and coordinated the study, interpreted the data, and drafted the article. M.T., R.S., and G.R. developed the HTP. M.D.O., M.O., B.B., A.M., and G.R. developed the intermitted fasting with diet adjustment group program. G.R. and B.W. developed the group exercise program. S.R. and T.T.D. provided statistical expertise in clinical trial design. S.B. and G.R. conducted the statistical analysis. S.B. prepared and performed the data management. All the authors contributed to the study methods, have read and approved the final version of the article, and agree with the order of presentation.
Availability of Data and Materials
The data sets used and/or analyzed during the current study are not publicly available due to concerns that the individual privacy of the participants could be compromised.
Consent for Publication
Within the scope of patient consent, all study participants' consent for publication was obtained.
Author Disclosure Statement
The authors declare no conflicts of interest regarding this study.
Funding Information
This study was an investigator-initiated trial that was funded partly by the German Neurodermatitis Foundation (Deutsche Neurodermitis Stiftung) and partly by funds from the investigating institute.
Supplementary Material
References
- 1. Jenner N, Campbell J, Marks R. Morbidity and cost of atopic eczema in Australia. Australas J Dermatol 2004;45(1):16–22; doi: 10.1111/j.1440-0960.2004.00046.x [DOI] [PubMed] [Google Scholar]
- 2. Hengge UR, Ruzicka T, Schwartz RA, et al. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol 2006;54(1):1–15; quiz 16–18; doi: 10.1016/j.jaad.2005.01.010 [DOI] [PubMed] [Google Scholar]
- 3. Hajar T, Leshem YA, Hanifin JM, et al. A systematic review of topical corticosteroid withdrawal (“steroid addiction”) in patients with atopic dermatitis and other dermatoses. J Am Acad Dermatol 2015;72(3):541.e2–549.e2; doi: 10.1016/j.jaad.2014.11.024 [DOI] [PubMed] [Google Scholar]
- 4. Aubert-Wastiaux H, Moret L, Le Rhun A, et al. Topical corticosteroid phobia in atopic dermatitis: A study of its nature, origins and frequency. Br J Dermatol 2011;165(4):808–814; doi: 10.1111/j.1365-2133.2011.10449.x [DOI] [PubMed] [Google Scholar]
- 5. Zuberbier T, Orlow SJ, Paller AS, et al. Patient perspectives on the management of atopic dermatitis. J Allergy Clin Immunol 2006;118(1):226–232; doi: 10.1016/j.jaci.2006.02.031 [DOI] [PubMed] [Google Scholar]
- 6. Werfel T, Kapp A. Environmental and other major provocation factors in atopic dermatitis. Allergy 1998;53(8):731–739; doi: 10.1111/j.1398-9995.1998.tb03968.x [DOI] [PubMed] [Google Scholar]
- 7. King RM, Wilson GV. Use of a diary technique to investigate psychosomatic relations in atopic dermatitis. J Psychosom Res 1991;35(6):697–706; doi: 10.1016/0022-3999(91)90120-d [DOI] [PubMed] [Google Scholar]
- 8. Stefanovic N, Flohr C, Irvine AD. The exposome in atopic dermatitis. Allergy 2020;75(1):63–74; doi: 10.1111/all.13946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vestergaard C. Atopic dermatitis and metabolic syndrome: Lifestyle or systemic inflammation? J Eur Acad Dermatol Venereol 2019;33(9):1629; doi: 10.1111/jdv.15831 [DOI] [PubMed] [Google Scholar]
- 10. Wehrmann J, Hinsch K-D, Nürnberg W. S1 Guideline. Dermatological inpatient rehabilitation for adult atopic dermatitis-update 2015 [German]. AWMF-Register: 013/026. AWMF online: 2015. [DOI] [PubMed] [Google Scholar]
- 11. (AWMF) AdWMF. Guidelines of the German Dermatological Society (DDG) and the Professional Association of German Dermatologists (BVDD): Guideline Neurodermatitis [atopic eczema; atopic dermatitis] [German], AWMF Register Number: 013–027. 2014. [Google Scholar]
- 12. Rustad AM, Nickles MA, Bilimoria SN, et al. The role of diet modification in atopic dermatitis: Navigating the complexity. Am J Clin Dermatol 2022;23(1):27–36; doi: 10.1007/s40257-021-00647-y [DOI] [PubMed] [Google Scholar]
- 13. Rotter G, Teut M, Binting S, et al. Lifestyle behaviors in patients with atopic dermatitis—Results of a cross-sectional study following a randomized controlled trial. Complement Med Res 2022; doi: 10.1159/000527107 [DOI] [PubMed] [Google Scholar]
- 14. Senser C, Habermüller M, Revenstorf D. Hypnotherapy in atopic dermatitis. Akt Dermatol 2004;30(04):103–108; doi: 10.1055/s-2004-814483 [DOI] [Google Scholar]
- 15. Shenefelt PD. Hypnosis in dermatology. Arch Dermatol 2000;136(3):393–399; doi: 10.1001/archderm.136.3.393 [DOI] [PubMed] [Google Scholar]
- 16. Shenefelt PD. Use of hypnosis, meditation, and biofeedback in dermatology. Clin Dermatol 2017;35(3):285–291; doi: 10.1016/j.clindermatol.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 17. Stewart AC, Thomas SE. Hypnotherapy as a treatment for atopic dermatitis in adults and children. Br J Dermatol 1995;132(5):778–783; doi: 10.1111/j.1365-2133.1995.tb00726.x [DOI] [PubMed] [Google Scholar]
- 18. Twerski AJ, Naar R. Hypnotherapy in a case of refractory dermatitis. Am J Clin Hypnosis 1974;16(3):202–205; doi: 10.1080/00029157.1974.10403677 [DOI] [PubMed] [Google Scholar]
- 19. Lithell H, Bruce A, Gustafsson IB, et al. A fasting and vegetarian diet treatment trial on chronic inflammatory disorders. Acta Derm Venereol 1983;63(5):397–403. [PubMed] [Google Scholar]
- 20. Nakamura H, Shimoji K, Kouda K, et al. An adult with atopic dermatitis and repeated short-term fasting. J Physiol Anthropol Appl Human Sci 2003;22(5):237–240; doi: 10.2114/jpa.22.237 [DOI] [PubMed] [Google Scholar]
- 21. Kouda K, Tanaka T, Kouda M, et al. Low-energy diet in atopic dermatitis patients: Clinical findings and DNA damage. J Physiol Anthropol Appl Human Sci 2000;19(5):225–228; doi: 10.2114/jpa.19.225 [DOI] [PubMed] [Google Scholar]
- 22. Tanaka T, Kouda K, Kotani M, et al. Vegetarian diet ameliorates symptoms of atopic dermatitis through reduction of the number of peripheral eosinophils and of PGE2 synthesis by monocytes. J Physiol Anthropol Appl Human Sci 2001;20(6):353–361; doi: 10.2114/jpa.20.353 [DOI] [PubMed] [Google Scholar]
- 23. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev 2017;39:46–58; doi: 10.1016/j.arr.2016.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harvie M, Howell A. Potential benefits and harms of intermittent energy restriction and intermittent fasting amongst obese, overweight and normal weight subjects—A narrative review of human and animal evidence. Behav Sci (Basel) 2017;7(1):4; doi: 10.3390/bs7010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tinsley GM, Horne BD. Intermittent fasting and cardiovascular disease: Current evidence and unresolved questions. Future Cardiol 2018;14(1):47–54; doi: 10.2217/fca-2017-0038 [DOI] [PubMed] [Google Scholar]
- 26. Patterson RE, Sears DD. Metabolic effects of intermittent fasting. Annu Rev Nutr 2017;37:371–393; doi: 10.1146/annurev-nutr-071816-064634 [DOI] [PubMed] [Google Scholar]
- 27. Antoni R, Johnston KL, Collins AL, et al. Effects of intermittent fasting on glucose and lipid metabolism. Proc Nutr Soc 2017;76(3):361–368; doi: 10.1017/s0029665116002986 [DOI] [PubMed] [Google Scholar]
- 28. Bragazzi NL, Sellami M, Salem I, et al. Fasting and its impact on skin anatomy, physiology, and physiopathology: A comprehensive review of the literature. Nutrients 2019;11(2):249; doi: 10.3390/nu11020249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Longo VD, Mattson MP. Fasting: Molecular mechanisms and clinical applications. Cell Metab 2014;19(2):181–192; doi: 10.1016/j.cmet.2013.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Anton SD, Moehl K, Donahoo WT, et al. Flipping the metabolic switch: Understanding and applying the health benefits of fasting. Obesity (Silver Spring) 2018;26(2):254–268; doi: 10.1002/oby.22065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Martin SA, Brash AR, Murphy RC. The discovery and early structural studies of arachidonic acid. J Lipid Res 2016;57(7):1126–1132; doi: 10.1194/jlr.R068072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hornstein OP, Gall K, Salzer B, et al. Group exercise in neurodermatitis-clinical, psychometric, and skin physiological studies [German]. Deutsche Zeitschrift für Sportmedizin 1998;49(2):39–45. [Google Scholar]
- 33. Salzer B, Rupprecht M, Hornstein OP. Sport in patients with atopic eczema [German]. Therapiewoche/TW-Sport and Medicine 1993;5(2):137–140. [Google Scholar]
- 34. Gall K, Salzer B, Hornstein OP. Group sports as therapeutic adjuvant in atopic dermatitis [German]. Deutsches Ärzteblatt 1997;94(40):A-2564–A-2568. [Google Scholar]
- 35. Orita K, Hiramoto K, Inoue R, et al. Strong exercise stress exacerbates dermatitis in atopic model mice, NC/Nga mice, while proper exercise reduces it. Exp Dermatol 2010;19(12):1067–1072; doi: 10.1111/j.1600-0625.2010.01130.x [DOI] [PubMed] [Google Scholar]
- 36. Weber R, Strauss B. Group Psychotherapy in Germany. Int J Group Psychother 2015;65(4):513–525; doi: 10.1521/ijgp.2015.65.4.513 [DOI] [PubMed] [Google Scholar]
- 37. Stangier U, Ehlers A, Gieler U. Predicting long-term outcome in group treatment of atopic dermatitis. Psychother Psychosom 2004;73(5):293–301; doi: 10.1159/000078846 [DOI] [PubMed] [Google Scholar]
- 38. World Medical Association. Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. 2008.
- 39. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). ICH Guidelines. 2020. Available from: https://www.ich.org/products/guidelines.html [Last accessed: December 2, 2020].
- 40. World Health Organization (WHO). WHO Director-General's opening remarks at the media briefing on COVID-19—11 March 2020. 2020. Available from: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—-11-march-2020 [Last accessed: August 20, 2021].
- 41. Orkin AM, Gill PJ, Ghersi D, et al. Guidelines for reporting trial protocols and completed trials modified due to the COVID-19 pandemic and other extenuating circumstances: The CONSERVE 2021 statement. JAMA 2021;326(3):257–265; doi: 10.1001/jama.2021.9941 [DOI] [PubMed] [Google Scholar]
- 42. German Society for Nutrition (DGE). Specialized information [German]. Rheumatism diet. 2022. Available from: https://www.dge.de/wissenschaft/weitere-publikationen/fachinformationen/rheumadiaet/ [Last accessed: March 10, 2022].
- 43. Löllgen H. Borg-Skala. Standards of sports medicine [German]. The perception of exertion (RPE, Borg scale). GDeutsche Zeitschrift für Sportmedizin 2004;55(11):299–300. [Google Scholar]
- 44. Reich A, Heisig M, Phan NQ, et al. Visual analogue scale: Evaluation of the instrument for the assessment of pruritus. Acta Derm Venereol 2012;92(5):497–501; doi: 10.2340/00015555-1265 [DOI] [PubMed] [Google Scholar]
- 45. Riepe C, Osada N, Reich A, et al. Minimal clinically important difference in chronic pruritus appears to be dependent on baseline itch severity. Acta Derm Venereol 2019;99(13):1288–1290; doi: 10.2340/00015555-3332 [DOI] [PubMed] [Google Scholar]
- 46. Bullinger M, Kirchberger I, Ware J. The German SF-36 Health Survey translation and psychometric testing of a disease-spanning instrument to assess health-related quality of life [German]. Zeitschrift für Gesundheitswissenschaften 1995;3(1):21–36. [Google Scholar]
- 47. Bullinger M, Kirchberger I. SF-36 health status questionnaire [German]. Göttingen: Hogrefe; 1998. [Google Scholar]
- 48. Ware J Jr., Kosinski M, Keller SD.. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med Care 1996;34(3):220–233; doi: 10.1097/00005650-199603000-00003 [DOI] [PubMed] [Google Scholar]
- 49. Resnick B, Nahm ES. Reliability and validity testing of the revised 12-item Short-Form Health Survey in older adults. J Nurs Measurem 2001;9(2):151–161. [PubMed] [Google Scholar]
- 50. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—A simple practical measure for routine clinical use. Clin Exp Dermatol 1994;19(3):210–216; doi: 10.1111/j.1365-2230.1994.tb01167.x [DOI] [PubMed] [Google Scholar]
- 51. Basra MK, Salek MS, Camilleri L, et al. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): Further data. Dermatology 2015;230(1):27–33; doi: 10.1159/000365390 [DOI] [PubMed] [Google Scholar]
- 52. Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol 1988;54(6):1063–1070; doi: 10.1037//0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- 53. Zisapel N, Nir T. Determination of the minimal clinically significant difference on a patient visual analog sleep quality scale. J Sleep Res 2003;12(4):291–298; doi: 10.1046/j.0962-1105.2003.00365.x [DOI] [PubMed] [Google Scholar]
- 54. Hill MK, Kheirandish Pishkenari A, Braunberger TL, et al. Recent trends in disease severity and quality of life instruments for patients with atopic dermatitis: A systematic review. J Am Acad Dermatol 2016;75(5):906–917; doi: 10.1016/j.jaad.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 55. Schram ME, Spuls PI, Leeflang MM, et al. EASI, (objective) SCORAD and POEM for atopic eczema: Responsiveness and minimal clinically important difference. Allergy 2012;67(1):99–106; doi: 10.1111/j.1398-9995.2011.02719.x [DOI] [PubMed] [Google Scholar]
- 56. Leiner DJ. SoSci Survey (Version 2.6.00) [Computer software]. 2016. Available from: https://www.soscisurvey.de [Last accessed: November 14, 2022].
- 57. IBM Corp. IBM SPSS Statistics. IBM Corp: Armonk, NY. [Google Scholar]
- 58. Rotter G, Ahnert MW, Geue AV, et al. Acupuncture and Osteopathic Medicine for Atopic Dermatitis—A Three-armed Randomized Controlled Explorative Clinical Trial. Clin Exp Dermatol 2022; doi: 10..1111/ced.15340 [DOI] [PubMed] [Google Scholar]
- 59. Vieira BL, Lim NR, Lohman ME, et al. Complementary and alternative medicine for atopic dermatitis: An evidence-based review. Am J Clin Dermatol 2016;17(6):557–581; doi: 10.1007/s40257-016-0209-1 [DOI] [PubMed] [Google Scholar]
- 60. Delaitre L, Denis J, Maillard H. Hypnosis in treatment of atopic dermatitis: A clinical study. Int J Clin Exp Hypn 2020;68(4):412–418; doi: 10.1080/00207144.2020.1788391 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets used and/or analyzed during the current study are not publicly available due to concerns that the individual privacy of the participants could be compromised.