Abstract
Williams–Beuren syndrome (WBS) is a genetic condition frequently requiring interventions for associated congenital heart disease (CHD). Long-term survival data after cardiac interventions for children with WBS are sparse. This is a retrospective cohort study aiming to describe the 30-year survival outcomes of children with WBS after interventions for CHD using the Pediatric Cardiac Care Consortium (PCCC), a large North American-based registry of interventions for pediatric heart diseases, between 1982 and 2009. Outcomes were obtained from the PCCC and by linkage with the National Death Index through 2020. Survival of patients with WBS and their major subgroups was assessed by Kaplan-Meier survival curves and Cox regression. A total of 200 patients met the inclusion criteria of having their first intervention for CHD at a US PCCC center and age <21 years at time of intervention. The most common lesions were left heart obstructive lesions (LHOL), either in isolation (37%) or in combination with right heart obstructive lesions (RHOL) (49.0%), whereas isolated RHOL accounted for 11% of the total. The first procedure was surgery for 85.5% of the group, and the remainder underwent a transcatheter procedure. There were 5 in-hospital deaths (2.5%), and among survivors to hospital discharge, 164 had sufficient identifiers for National Death Index linkage. Over a median period of postdischarge follow-up of 23.7 years (interquartile range 18.7 to 27.3), 16 deaths occurred, with an overall 30-year survival rate of 90%. Survival rates ranged from 96.1% for isolated LHOL or RHOL to 83.4% for patients with combined disease (adjusted hazard ratio 4.7, 95% confidence intervals 1.35 to 16.59).
Williams–Beuren syndrome (WBS) is a multisystem autosomal dominant condition1–3 with a distinct cardiovascular (CV) phenotype caused by a microdeletion of the 7q11.23 region that includes the elastin gene (ELN).4,5 Congenital heart diseases (CHDs) and vascular anomalies are present in up to 90% of all patients with WBS, with the most common lesions being supravalvar aortic stenosis (SVAS) and pulmonary artery stenosis (PAS).1,2 These often coexist and contribute significantly to the overall morbidity and mortality of the syndrome. The non-CV features of WBS include mild intellectual disability, distinctive facies, and abnormal endocrine findings such as hypercalcemia and hypothyroidism.6 Approximately 2/3 of patients with WBS require cardiac surgery and/or transcatheter interventions, most often before the age of 5 years.7 The risk of CV events and death in these patients is very high during hospitalizations that involve surgery, catheterization, or anesthesia/sedation for any reason.1,8–10 However, the long-term outcomes after CV interventions in patients with WBS are less well studied,1 primarily because the condition is rare and there are limited opportunities for systematic follow-up. In this study, we describe the long-term outcomes of patients with WBS from the time of first surgical or transcatheter intervention for congenital CV disease.
Methods
We performed a retrospective cohort study of patients with WBS who underwent cardiac interventions between 1982 and 2009 in the PCCC (Pediatric Cardiac Care Consortium) registry,11,12 a large US-based registry for pediatric CV interventions, enriched with follow-up data after linkage with the US National Death Index (NDI). The study was approved by Emory University’s Institutional Review Board through waivers of informed consent for patients enrolled in the PCCC until April 15, 2003, at which date the stricter regulations of the Health Insurance Portability and Accountability Act became effective.
The foundation, purpose, and activities of the PCCC registry have been previously described.11,12 We queried the PCCC registry for patients who were coded as having WBS and were enrolled for their first congenital heart surgery or transcatheter procedure at <21 years of age, between 1982 and 2003. Additional inclusion criteria were defined as (1) US residency at the time of surgery and (2) intervention conducted at a US center. Long-term outcomes were provided by linkage with the NDI for patients enrolled up to April 15, 2003. Those enrolled after that date did not have direct identifiers and could not be linked with NDI datasets. This linkage has been shown to provide satisfactory estimates of long-term survival outcomes in this cohort.13
We collected data including age, birth weight (kg), sex, cardiac diagnoses, date and type of intervention (surgery or transcatheter), and discharge status. Variables for analysis included type of CHD including SVAS, aortic stenosis (AS) or sub-AS, coarctation of the aorta, pulmonary valvar stenosis or pulmonary arterial stenosis (PAS), mitral valve disease, atrial septal defect, ventricular septal defect, or other. Lesions were organized in major subgroups based on the type and location of the lesion: (1) right heart obstructive lesions (RHOL) including pulmonary valvar stenosis and PAS, (2) left heart obstructive lesions (LHOL) including SVAS, AS, or sub-AS in addition to coarctations of the aorta, (3) combined RHOL + LHOL, and (4) other conditions without right or left heart obstructive pathology. Categorization was determined by the respective PCCC diagnosis codes along with manual review of operative or catheterization notes when ambiguity existed. Type of intervention (surgery or transcatheter) was also collected as assigned by the respective surgical and interventional codes used by the PCCC.
Death ascertainment was confirmed from the PCCC database records and by linkage to NDI records through December 31, 2020. The NDI Plus provided underlying and multiple (contributing) causes of death14 in the form of International Classification of Disease codes.14
Descriptive statistics were obtained for variables of interest. Continuous variables were reported as medians with interquartile range (Interquartile range [IQR] quartile 1 to quartile 3) and compared using Wilcoxon rank-sum tests. Frequencies and percentages along with 95% confidence intervals (CIs) were presented for categorical information and compared using chi-square tests (or Fisher’s exact test when expected cell counts were <5). Kaplan-Meier (KM) survival plots were created for the long-term study cohort to estimate 30-year survival from the discharge date of first intervention censored at the date of death, or the end of follow-up (December 31, 2020), whichever occurred first. Survival data were compared across groups of interest using the log-rank test. The proportional hazards assumption was assessed using KM survival plot estimates by main subgroups, and no violations were found. We performed univariable and multivariable Cox regression modeling to explore main predictors of death after initial intervention. Hazard ratio (HR)/adjusted HRs and 95% CI for variables of interest were estimated using Cox proportional hazard models after adjustment for variables with HR >0.2 in the univariable analysis. The time to event was censored at the date of death or end of follow-up, whichever occurred first. Statistical significance was assessed at a 0.05 level. Statistical analyses were performed using SAS version 9.4 (Gary, North Carolina).
Results
We identified 200 patients with WBS who met the inclusion criteria of being <21 years old at the time of intervention, having their first intervention within a US-based PCCC center, and having US residency at the time of surgery (Figure 1). Most patients were male (n = 118, 59%, p = 0.011), most of them having an LHOL (61.6%) either in isolated form or in combination with RHOL (Table 1). Patients with RHOL were mostly female (66.7%) in comparison with 40.5% female in the LHOL group (p=0.011) and 36.7% in those with LHOL + RHOL (p = 0.034). A complete description of the patients anatomy is provided in Supplementary Table 1. There were no significant differences in type of lesions or age of intervention between male and female patients (Supplementary Table 2).
Figure 1.

Study flow chart illustrating outcomes for the 200 patients who met inclusion criteria. Long-term outcomes are reported for the 164 patients who had adequate identifiers and survived to discharge after first intervention.
Table 1.
Characteristics of the WBS cohort at the time of first intervention
| Total (n = 200) | LHOL (n = 74) | LHOL + RHOL (n = 98) | RHOL (n = 21) | Other (n = 7) | |
|---|---|---|---|---|---|
| Sex (male), n (%) | 118 (59.0%) | 44 (59.5%)* | 62 (63.3%)* | 7 (33.3%)* | 5 (71.4%) |
| Median age, years (IQR) | 2.6 (0.7, 7.4) | 6.4 (3.1, 9.0)† | 0.9 (0.4, 4.1)† | 1.1 (0.7, 3.2)† | 1.3 (0.9, 2.8) |
| Age group, n (%) | |||||
| 1 year & above | 132 (66.0%) | 67 (90.5%) | 48 (49.0%) | 12 (57.1%) | 5 (71.4%) |
| Under 1 year | 68 (34.0%) | 7 (9.5%) | 50 (51.0%) | 9 (42.9%) | 2 (28.6%) |
| Era of first intervention, n (%) | |||||
| 1984 – 1992 | 60 (30.0%) | 28 (37.8%) | 22 (22.4%) | 5 (23.8%) | 5 (71.4%) |
| 1993 – 1997 | 65 (32.5%) | 23 (31.1%) | 33 (33.7%) | 8 (38.1%) | 1 (14.3%) |
| 1998 – 2009 | 75 (37.5%) | 23 (31.1%) | 43 (43.9%) | 8 (38.1%) | 1 (14.3%) |
| Type of intervention, n (%)‡ | |||||
| Transcatheter | 29 (14.5%) | 2 (2.7%)‡ | 18 (18.4%)‡ | 9 (42.9%)‡ | 0 (0.0%) |
| Surgery | 171 (85.5%) | 72 (97.3%)‡ | 80 (81.6%)‡ | 12 (57.1%)‡ | 7 (100.0%) |
| In-hospital death, n (%) | 5 (2.5%) | 0 (0.0%) | 4 (4.1%) | 1 (4.8%) | 0 (0.0%) |
LHOL = left heart obstructive lesion, RHOL = right heart obstructive lesion.
p<0.05 comparing sex proportions in RHOL vs LHOL and RHOL vs combination lesions.
p<0.05 comparing age at intervention in LHOL vs RHOL and LHOL vs combination lesions.
p<0.05 comparing type of first intervention in LHOL vs combination and LHOL vs RHOL.
Values are presented as numbers with % by column given within parentheses, unless otherwise indicated.
The most common conditions involved combination of LHOL + RHOL (n = 98, 49%), followed by isolated LHOL (n = 74, 37%) or RHOL (n = 21, 11%), whereas the remaining were various CHD without right or left heart obstruction and categorized as “other” (n=7, 4%) (Supplementary Table 1, Table 1). In terms of specific individual lesions, SVAS was the most common overall diagnosis (n = 135, 67.5%), followed by PAS (n = 90, 45%). Patients with LHOL were operated on at older age than were those with RHOL either in isolation or in combination RHOL + LHOL (p <0.0001).
Patients with isolated LHOL almost always required surgery as their first intervention (97.3%), but the percentage of surgery as first intervention decreased in patients with combined LHOL + RHOL (81.6%) or isolated RHOL (57.1%) (p <0.05). Relief of the LHOL was the most common first surgical procedure (n = 139, 81.3%) (Supplementary Table 3). Concomitant procedures were performed in 67 of the operated cases (39.1%) (Supplementary Table 4). The most common transcatheter intervention was pulmonary arterioplasty, occurring in 23 of the 29 patients (79.3%) treated with transcatheter procedures (Supplementary Table 5). Of the patients discharged alive after they underwent surgery, 17 (9.94%) required reoperations within the PCCC in the subsequent follow-up period (Supplementary Table 6). The median time to reoperation was 3.2 years (IQR 0.64 to 4.23). The most common indication for reoperation was recurrent SVAS in 58.9% of the reoperated cases (n = 10). In many cases, the subsequent procedures involved a different site or lesion than in the original operation (n = 10, 58.9%).
Five patients died in hospital after their first cardiac intervention; of the 195 patients who survived to discharge, 164 (84%) had direct identifiers, which allowed tracking of postdischarge deaths by matching with the NDI. There were no significant differences between the groups with and without identifiers, except that as expected, patients without identifiers were more likely to have had intervention in the later era of 1998 to 2009 (results not shown).
Over a median period of postdischarge follow-up of 23.7 years (IQR 18.7 to 27.3) and up to 30 years later, 16 deaths were recorded. The overall KM survival plot after discharge after the first CHD intervention is shown in Figure 2. Attrition was high in the first year of follow-up but remained relatively constant during the remaining follow-up period, and the overall 30-year survival rate after discharge reached 90.1% (95% CI 85.6% to 94.8%). KM survival plots by main diagnostic category (Figure 2) reveal significantly higher mortality in patients with combined LHOL + RHOL (83.4%, 95% CI 75.5% to 92.1%) than in patients with the isolated forms of LHOL or RHOL (96.1%, 95% CI 91.9 to 100.0) (log-rank p = 0.0075). The LHOL or RHOL groups combined had only 3 death events in total. Adjusted analysis identified the combined LHOL + RHOL diagnostic group to be associated with 6.85 times the hazard of death (95% CI 1.52 to 30.84) as the group with isolated LHOL. We also detected an era effect with a hazard of death at least 5 times greater in the 1982 to 1992 and 1993 to 1997 eras than in the 1998 to 2009 era (Table 2). The effects of gender, age, and weight at intervention on the hazard of death were not significant.
Figure 2.
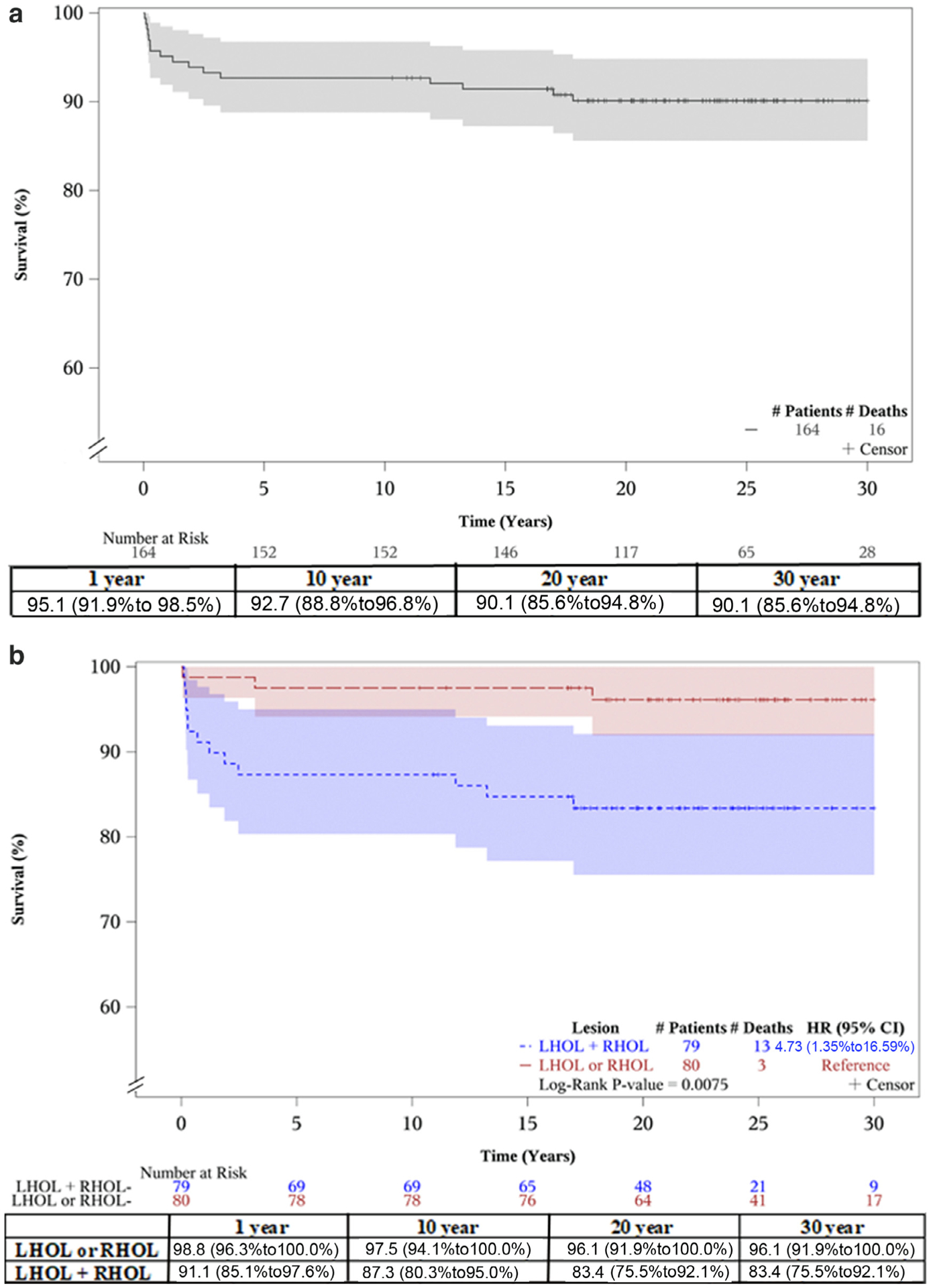
Kaplan-Meier plots showing 30-year survival of patients with WBS after their first cardiac intervention (at the bottom survival table with 95% CI). (A) and by main diagnostic category (B). The unadjusted HR demonstrates lower survival rates in patients with combined LHOL + RHOL compared with those with isolated LHOL or RHOL.
Table 2.
Univariable and multivariable analysis of all-cause mortality after first cardiac intervention for patients with WBS
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR | p-Value | aHR | p-Value | |
| Age at intervention in years | 0.98 | 0.678 | – | – |
| (0.88 – 1.09) | ||||
| Weight at intervention in kg | 0.99 | 0.708 | – | – |
| (0.96 – 1.03) | ||||
| Sex | 0.80 | 0.797 | – | – |
| Male vs female | (0.29 – 2.19) | |||
| Era (decades) | ||||
| 1982–1992 | 3.21 | 0.15 | 5.84 | 0.035 |
| (0.65 – 15.93) | (1.13 – 30.24) | |||
| 1993–1997 | 3.42 | 0.12 | 5.18 | 0.043 |
| (0.73 – 16.13) | (1.05 – 25.45) | |||
| 1998–2009 | Ref | Ref | Ref | Ref |
| Lesion | ||||
| LHOL + RHOL | 5.65 | 0.023 | 6.85 | 0.012 |
| (1.27 – 25.02) | (1.52 – 30.84) | |||
| RHOL | 1.95 | 0.585 | 2.27 | 0.503 |
| (0.18 – 21.52) | (0.21 – 25.20) | |||
| LHOL | Ref | Ref | Ref | Ref |
aHR = adjusted hazard ratio; numbers in parenthesis display the 95% confidence intervals (CI).
In the 16 patients with postdischarge death, CHD was the most common underlying cause of death (65%); CV conditions accounted for 15% of the deaths; and the remaining deaths (20%) were attributed to other non-CHD/non-CV disease conditions. Additional analysis after incorporating the multiple causes of death information, in which death was attributed directly or indirectly to the cause of interest, revealed that cardiovascular diseases contributed to 70% of the postdischarge deaths (Supplementary Table 7). Five of the deaths (31%) were attributed to ischemic heart disease and 4 (25%) to heart failure or arrhythmias.
Discussion
In this registry-based study, the overall 30-year survival rate after CV interventions for children with WBS was 90.1%, but there was significant early differential attrition between the group with combined left and right-sided cardiac obstructive lesions compared with patients with unilateral right or left obstruction. Therefore, the 5-year survival rate for the LHOL + RHOL group was 10% lower than for the latter group with unilateral pathology (87.3% vs 97.5%). After that time point, both groups remained stable, and their 30-year survival rate reached 83.4% and 96.1%, respectively.
Our findings extend the experience from previously published studies that were mostly limited to 10 to 15 years of follow-up.15,16 A more recent study reported good outcomes in patients with WBS specifically after SVAS repair, with a 94.3% survival rate at 5 and 10 years but with a mean follow-up of only 9 years.17 To the best of our knowledge, the data from our study capture a much longer time of follow-up than previously reported, and for 1 of the largest WBS cohorts with CV interventions. These data provide reassurance about WBS prognosis up to an older age when the interaction of their impaired CV background and emerging adult risk factors is expected to occur.
Similarly to findings from previous studies by other investigators,18–21 SVAS and PAS were the most commonly occurring lesions, with most patients having a combination of LHOL+RHOL. Overall, there were slightly more male than female patients with WBS who were operated on for CV disease, in keeping with experiences from other studies,22 although some attribute such differences to male patients tending to present at a younger age than do female patients.16 Our data show a preponderance of males among patients with WBS and LHOL, which may be related to the well-known association of male gender with LHOL in the general nonsyndromic population.23 This male preponderance for LHOL in association with the estradiol protective effects on vascular injury raises the possibility of a sex hormone modification effect on the impaired circumferential growth that has been reported as the underlying mechanism for the obstructive aortic disease in elastin-deficient animal models.24,25
The major strengths of our study are the large sample size from one of the oldest and largest US databases for CHD interventions (PCCC), along with the length of the follow-up period spanning up to 30 years. In addition, the linkage of the PCCC database with the “gold standard” NDI gives significant information regarding the long-term outcomes of the study group. An important limitation is the lack of information on potentially significant contributing factors that are not contained in the PCCC registry. These include socioeconomic variables, details about medications, and subsequent procedures performed outside the PCCC.
In conclusion, our data reveal favorable long-term outcomes in patients with WBS after CV interventions conditioning survival for 5 years after the initial CHD procedure, with survival rates >90% after 30 years from the first intervention. It also informs the differential effect of gender on the type of heart lesion, and how patients with combination lesions display significantly higher attrition after CHD interventional procedures than do patients with isolated LHOL or RHOL. Future studies with larger cohorts could help better understand the complications and pathology in these patients that place them at a higher risk of death.
Supplementary Material
Acknowledgments
This study was supported by Grant R01-HL122392 from National Heart, Lung, and Blood Institute, Bethesda, Maryland and Grant PR180683 from the Department of Defence, Washington, District of Columbia.
Footnotes
Disclosures
The authors have no conflicts of interest to declare.
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.amjcard.2022.10.037.
References
- 1.Pober BR. Williams–Beuren syndrome. N Engl J Med 2010;362: 239–252. [DOI] [PubMed] [Google Scholar]
- 2.Collins RT. Cardiovascular disease in Williams syndrome. Circulation 2013;127:2125–2134. [DOI] [PubMed] [Google Scholar]
- 3.Strømme P, Bjørnstad PG, Ramstad K. Prevalence estimation of Williams syndrome. J Child Neurol 2002;17:269–271. [DOI] [PubMed] [Google Scholar]
- 4.Bayés M, Magano LF, Rivera N, Flores R, Pérez Jurado LA. Mutational mechanisms of Williams-Beuren syndrome deletions. Am J Hum Genet 2003;73:131–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Francke U Williams-Beuren syndrome: genes and mechanisms. Hum Mol Genet 1999;8:1947–1954. [DOI] [PubMed] [Google Scholar]
- 6.Morris CA. Williams syndrome. In: Adam MP, Everman DB, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A, eds. GeneReviews®. Seattle: University of Washington; 1999. [PubMed] [Google Scholar]
- 7.Brown JW, Ruzmetov M, Vijay P, Turrentine MW. Surgical repair of congenital supravalvular aortic stenosis in children. Eur J Cardiothorac Surg 2002;21:50–56. [DOI] [PubMed] [Google Scholar]
- 8.Burch TM, McGowan FX, Kussman BD, Powell AJ, DiNardo JA. Congenital supravalvular aortic stenosis and sudden death associated with anesthesia: what’s the mystery? Anesth Analg 2008;107:1848–1854. [DOI] [PubMed] [Google Scholar]
- 9.Horowitz PE, Akhtar S, Wulff JA, Al Fadley F, Al Halees Z. Coronary artery disease and anesthesia-related death in children with Williams syndrome. J Cardiothorac Vasc Anesth 2002;16:739–741. [DOI] [PubMed] [Google Scholar]
- 10.Matisoff AJ, Olivieri L, Schwartz JM, Deutsch N. Risk assessment and anesthetic management of patients with Williams syndrome: a comprehensive review. Paediatr Anaesth 2015;25:1207–1215. [DOI] [PubMed] [Google Scholar]
- 11.Pyles LA, Hills CM, Larson VE, Moller JH. Pediatric Cardiac Care Consortium: an instrument for evidence-based clinical decision support. J Cardiovasc Transl Res 2009;2:219–224. [DOI] [PubMed] [Google Scholar]
- 12.Vinocur JM, Moller JH, Kochilas LK. Putting the pediatric cardiac care consortium in context: evaluation of scope and case mix compared with other reported surgical datasets. Circ Cardiovasc Qual Outcomes 2012;5:577–579. [DOI] [PubMed] [Google Scholar]
- 13.Spector LG, Menk JS, Vinocur JM, Oster ME, Harvey BA, St. Louis JD, Moller J, Kochilas LK. In-hospital vital status and heart transplants after intervention for congenital heart disease in the pediatric cardiac care consortium: completeness of ascertainment using the National Death Index and United Network for Organ Sharing datasets. J Am Heart Assoc 2016;5:e003783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Division of Vital Statistics, National Center for Health Statistics, Centers for Disease Control and Prevention. NDI PLUS: coded causes of death. Available at: https://catalog.hathitrust.org/Record/003543640. Accessed on June 3, 2021.
- 15.Fricke TA, d’Udekem Y, Brizard CP, Wheaton G, Weintraub RG, Konstantinov IE. Surgical repair of supravalvular aortic stenosis in children with Williams syndrome: a 30-year experience. Ann Thorac Surg 2015;99:1335–1341. [DOI] [PubMed] [Google Scholar]
- 16.Collins RT, Kaplan P, Somes GW, Rome JJ. Long-term outcomes of patients with cardiovascular abnormalities and Williams syndrome. Am J Cardiol 2010;105:874–878. [DOI] [PubMed] [Google Scholar]
- 17.Wu FY, Mondal A, Del Nido PJ, Gauvreau K, Emani SM, Baird CW, Kaza AK. Long-term surgical prognosis of primary supravalvular aortic stenosis repair. Ann Thorac Surg 2019;108:1202–1209. [DOI] [PubMed] [Google Scholar]
- 18.Pham PP, Moller JH, Hills C, Larson V, Pyles L. Cardiac catheterization and operative outcomes from a multicenter consortium for children with Williams syndrome. Pediatr Cardiol 2009;30:9–14. [DOI] [PubMed] [Google Scholar]
- 19.Bruno E, Rossi N, Thüer O, Córdoba R, Alday LE. Cardiovascular findings, and clinical course, in patients with Williams syndrome. Cardiol Young 2003;13:532–536. [PubMed] [Google Scholar]
- 20.Zalzstein E, Moes CAF, Musewe NN, Freedom RM. Spectrum of cardiovascular anomalies in Williams-Beuren syndrome. Pediatr Cardiol 1991;12:219–223. [DOI] [PubMed] [Google Scholar]
- 21.Del Pasqua AD, Rinelli G, Toscano A, Iacobelli R, Digilio C, Marino B, Saffirio C, Mondillo S, Pasquini L, Sanders SP, de Zorzi A. New Findings concerning cardiovascular Manifestations emerging from Long-term Follow-up of 150 patients with the Williams-Beuren-Beuren Syndrome. Cardiol Young 2009;19:563–567. [DOI] [PubMed] [Google Scholar]
- 22.Sadler LS, Pober BR, Grandinetti A, Scheiber D, Fekete G, Sharma AN, Urbán Z. Differences by sex in cardiovascular disease in Williams syndrome. J Pediatr 2001;139:849–853. [DOI] [PubMed] [Google Scholar]
- 23.Miller-Hance WC, Tacy TA. Gender differences in pediatric cardiac surgery: the cardiologist’s perspective. J Thorac Cardiovasc Surg 2004;128:7–10. [DOI] [PubMed] [Google Scholar]
- 24.Jiao Y, Li G, Korneva A, Caulk AW, Qin L, Bersi MR, Li Q, Li W, Mecham RP, Humphrey JD, Tellides G. Deficient circumferential growth is the primary determinant of aortic obstruction attributable to partial elastin deficiency. Arterioscler Thromb Vasc Biol 2017;37: 930–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iafrati MD, Karas RH, Aronovitz M, Kim S, Sullivan TR Jr, Lubahn DB, O’Donnell TF Jr, Korach KS, Mendelsohn ME. Estrogen inhibits the vascular injury response in estrogen receptor α-deficient mice. Nat Med 1997;3:545–548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


