Abstract

As the turnaround time of diagnosis becomes important, there is an increasing demand for rapid, point-of-care testing (POCT) based on polymerase chain reaction (PCR), the most reliable diagnostic tool. Although optical components in real-time PCR (qPCR) have quickly become compact and economical, conventional PCR instruments still require bulky thermal systems, making it difficult to meet emerging needs. Photonic PCR, which utilizes photothermal nanomaterials as heating elements, is a promising platform for POCT as it reduces power consumption and process time. Here, we develop a photonic qPCR platform using hydrogel microparticles. Microparticles consisting of hydrogel matrixes containing photothermal nanomaterials and primers are dubbed photothermal primer-immobilized networks (pPINs). Reduced graphene oxide is selected as the most suitable photothermal nanomaterial to generate heat in pPIN due to its superior light-to-heat conversion efficiency. The photothermal reaction volume of 100 nL (predefined by the pPIN dimensions) provides fast heating and cooling rates of 22.0 ± 3.0 and 23.5 ± 2.6 °C s–1, respectively, enabling ultrafast qPCR within 5 min only with optical components. The microparticle-based photonic qPCR facilitates multiplex assays by loading multiple encoded pPIN microparticles in a single reaction. As a proof of concept, four-plex pPIN qPCR for bacterial discrimination are successfully demonstrated.
Keywords: photonic PCR, hydrogel, reduced graphene oxide (rGO), real-time PCR, multiplex assay, bacteria
Polymerase chain reaction (PCR) is the gold standard for diagnosing infectious diseases, examining food quality, and monitoring the environment by virtue of its high sensitivity and specificity.1 PCR amplifies the target DNA through thermal cycling, which consists of repetitions of two or three temperature steps for denaturation, annealing and extension. Most conventional PCR assays utilize Peltier-based thermal cyclers that control the solution temperature using heat blocks and coolers.2 Even though the needs for point-of-care testing (POCT) based on real-time PCR (qPCR) are growing,3,4 heating units for thermal cyclers make qPCR less attractive for POCT due to its high-power consumption. To overcome these limitations, researchers have developed a photonic PCR system, which replaces Peltier elements with light sources such as lasers or light emitting diodes (LEDs) by the incorporation of various photothermal nanomaterials into the reaction.4,5 Until recently, metal, carbon, or organic nanoparticles or nanostructures (i.e., gold nanoparticles, carbon nanotubes (CNTs), indocyanine green, etc.) have been utilized as photothermal nanomaterials to convert light to heat.5−11 While noble metal nanomaterials convert the light to heat on the basis of photon-induced coherent oscillation of electrons creating the hot electrons, the photothermal effect of carbon-based materials relies on the atomic lattice vibration induced by electron–phonon coupling when the electrons excited by light irradiation are relaxed to the lower energy states.4,7,12−14
A series of nanomaterial-based photonic PCRs have achieved very fast amplification and are operated with compact hardware.5−7,15 The reaction time and power consumption mainly depend on the efficiency of the light-to-heat conversion of the photothermal nanomaterials. Previous studies enhanced the plasmonic properties of the nanomaterials by changing their size, shape, and the other factors related to localized surface plasmon resonance (LSPR) effect.7,13,14 Although modification of nanoparticles effectively improves photothermal efficiency, aggregation of nanoparticles during the reaction is another challenge because the aggregation might change the resonant absorbance shift owing to the change of the structure16 as well as nonuniform temperature distribution in the reaction. As an alternative to nanoparticles, metal thin films have been suggested as photothermal heaters. Metal thin film-based photonic systems successfully demonstrated very fast PCR (in about 10 min) by harnessing plasmon-assisted heat generation. However, thin film-based photonic PCR also must overcome the limited choices of photothermal materials and delicate deposition processes.5,6,17 For instance, the film material is mostly limited to gold among diverse photothermal materials due to its facile deposition process as well as the metal thin film should be passivated by another polymer layer on it to prevent PCR reaction inhibition.17,18
Here, we demonstrate a photonic real-time PCR platform in hydrogel microparticles, which function as reliable nanoliter photonic reactors. The photothermal hydrogel microparticles of primer-immobilized network (pPIN) are designed to preserve evenly distributed nanomaterials inside the hydrogel matrix as well as to prevent the disorder of nanomaterial dispersion during the reaction. Four different nanomaterials were investigated to find an ideal photothermal material in this study: gold nanorod (GNR), CNT, graphene oxide (GO), and reduced graphene oxide (rGO). Among them, rGO exhibited superior photothermal properties to the other in terms of the light-to-heat conversion efficiency in pPIN. Since rapid heat generation is localized to a nanoliter microparticle and the peripheral solution operates as a thermal reservoir, fast heating/cooling rates during thermal cycling were easily achieved even without cooling fans (heating rate, 22.0 ± 3.0 °C s–1; cooling rate, 23.5 ± 2.6 °C s–1). By integrating with real-time fluorescence measurements, pPIN qPCR was able to perform 40 cycles within 5 min. Moreover, the PCR reactor in the form of a microparticle where target-specific primers are preloaded facilitates multiplex assays. With assorted microparticles in a single chamber, the injection of the mixture of the target nucleic acid and PCR Mastermix is enough to detect multiple genes at the same time. As a proof of concept, we successfully demonstrated four-plex photonic qPCR for bacterial discrimination using four encoded pPINs.
Results and Discussion
Establishing Photonic Real-Time PCR System
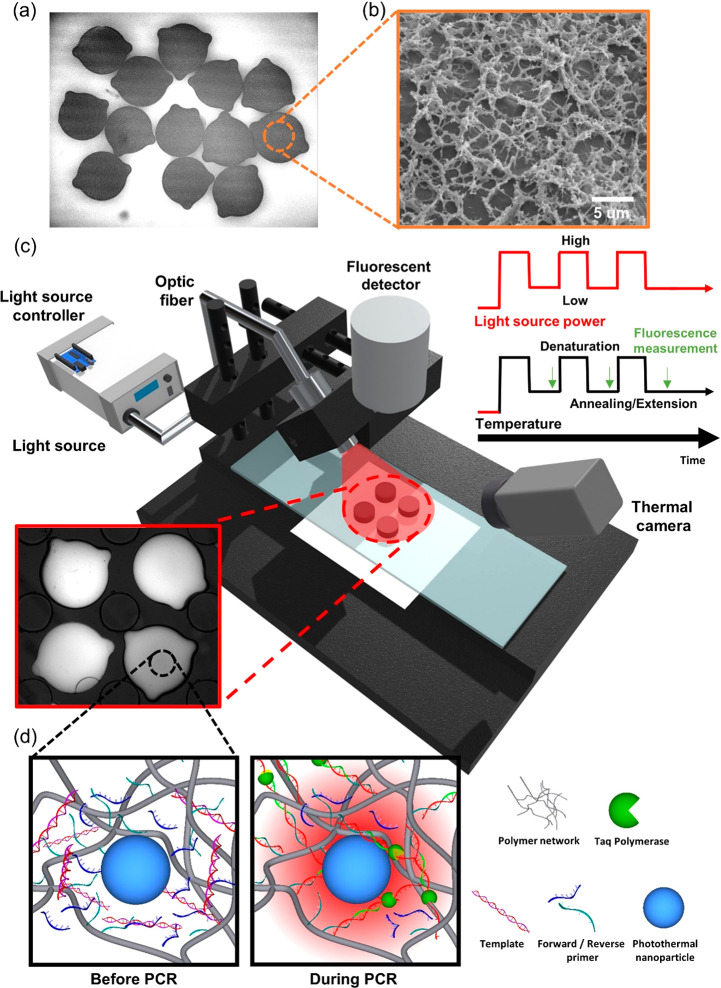
The photonic PCR system is composed of three major elements: photothermal microparticles, a light source, and thermal feedback control system. Photothermal microparticles were made of polyethylene glycol (PEG) hydrogels to confine the enzyme reaction in its internal volume. The photocurable characteristics of PEG hydrogels enable the easy production of encoded microparticles, and its highly porous structure facilitates internal mass transfer required for an effective reaction19 (Figure 1a,b and Figure S1). It is also efficient in physically embedding photothermal nanomaterials and maintaining uniform distribution.20 Based on the features described above, hydrogel microparticles with photothermal nanomaterials were utilized in this study as matrixes for confining photonic amplification reactions, which are named pPINs. In the case of pPIN PCRs, the microparticles were loaded on a planar plastic PCR chip under a customized microscope configuration as shown in Figure 1c and Figure S2. Light was irradiated to pPINs, and their temperature was monitored in real time via a thermal camera with the accurate calibration by the intensity change of thermoresponsive dye (Figure S3).21,22
Figure 1.
Schematic overview of pPIN qPCR. The microparticles in a plastic chip were irradiated to generate heat, and their fluorescent signals were measured in each cycle. Laser power is controlled for thermal cycling via temperature feedback from a thermal camera. (a) Microscope image of encoded pPINs. (b) Cryogenic SEM image of cross-section of pPIN. (c) pPIN qPCR system configuration. (d) Schematic of the photonic reaction in hydrogel matrix of pPIN.
Illumination power was controlled for thermal cycling by temperature feedback using customized software (Figure S4). As described in Figure 1d, the light modulated the heat generation from the nanomaterials in the hydrogel network. Since the photothermal nanomaterials were locally trapped in the pPIN, the reaction was locally confined in the volume of pPIN. Fluorescent images were automatically captured and recorded at the end of each annealing step for the detection of real-time amplification.
For photonic PCR, the light source type and spectrum have been selected to maximize the photothermal nanomaterial’s heat efficiency. Although a variety of light sources with different spectra can be implemented, an accurate laser system was chosen to precisely evaluate photothermal features of nanomaterials and pPINs for this study. Specifically, 800 nm was used as the wavelength of the photonic light source to avoid photobleaching or interference with the fluorescent signal.23 SYBR Green I, a most commonly used fluorescent dye in real-time PCR, emits a green spectrum (λmax = 520 nm) excited at the blue spectrum (λmax = 497 nm).24,25 Therefore, the light spectra of the fluorescence measurement and photonic light source do not overlap each other. In addition, the temperature of surrounding solution negligibly changed while remaining at 21–23 °C during 800 nm laser illumination (Figure S5a,b).
Selection of Photothermal Nanoparticles and Hydrogel Microparticle Volume
The absorbance and light-to-heat conversion efficiency of photothermal nanomaterials are most critical for effective photonic PCR. Various nanomaterials have been used in photonic applications. Among them, gold and carbon nanoparticles are known as excellent light-to-heat conversion materials.5−10 In this paper, GNR and three different carbon nanoparticles (CNT, GO, and rGO) were chosen as potential photothermal agents to be incorporated in pPINs. They all have effective light absorption at 800 nm.
For accurate comparison between nanomaterials, the concentration of four nanomaterials in all experiments was set to 0.25 mg mL–1. The absorbance characteristics of the nanomaterials were measured using UV/vis/NIR spectroscopy ranging from 400 to 1000 nm in deionized (DI) water. Among the four nanomaterials tested in this study, rGO showed a higher absorption than the other nanomaterials (Figure 2a). Upon irradiation at 800 nm, rGO consistently generated heat most effectively. (Figure 2b).26−28 To compare the photothermal conversion for identical absorption of photon, the concentration of the four nanomaterials was tuned to the absorbance of 0.2 at 800 nm (Figure S6). As a result, rGO and CNT transformed photon into more heat than GNR and GO. Thus, rGO has the best absorption per weight and, at the same time, the highest photothermal conversion efficiency per absorption.
Figure 2.
(a) Absorbance measurement of photothermal nanoparticles in DI water. (b) Photothermal heat generation curves of photothermal nanoparticles in DI water under the irradiation of an 1 W 800 nm laser. (c) Photothermal heat generation curves of pPIN under the irradiation of an 1 W 800 nm laser. (d) Temperature profiles with respect to the volume of the pPIN under same light dose of 0.28 W. Dash line showed the temperature profile of 100 nL of pPIN triggered by the irradiation with a light dose of 1.3 W.
To see the photothermal effect in the form of hydrogel microparticles, pPINs were prepared using each nanoparticle. In each case, a prepolymer solution was prepared by mixing PEG diacrylate (PEGDA), acrydited primer, and the nanoparticles, followed by spotting on the code mold, curing by UV light, and collecting the completed pPINs (detailed information is provided in the Materials and Methods section.) With the same illumination, pPIN with rGO showed the most efficient light-to-heat conversion, which is consistent with the experiments in solution (Figure 2c). Based on the results in solution and hydrogel microparticle, rGO was finally selected as the photothermal nanoparticle to create pPIN.
Apart from the light-to-heat conversion of the photothermal nanoparticles, reaction volume is also closely related to heating efficiency. The use of microparticles defined the exact reaction volume at the nanoliter scale because the reaction only occurs in the microparticles containing the photothermal nanomaterials. Using microparticles of various sizes, the heating rate was evaluated under identical light doses of 0.28 W (Figure 2d). Higher volumes of microparticles result in higher ramp rates and higher saturated temperatures due to the decrease in heat dissipation resulting from the decrease in surface-to-volume ratio. Under a higher dose of irradiation (1.32 W) in our PCR system, the pPIN microparticles of even 100 nL exceeded 95 °C in 6 s (Figure 2d). Considering the loading of the multiple microparticles for the multiplex assay in a single chamber afterward, 100 nL disk-type pPINs that were 650 μm in diameter and 300 μm in height were selected as the microparticles.
rGO as a Supplier of Primer As a Form of rGO–DNA Complex
For the multiplex assay with multiple pPINs afterward, the amplification on each pPIN should be target-specific as target-specific bidirectional primers are provided in the microparticles. Previous works confirmed that target-specific amplification occurred with high amplification efficiency comparable to that of the solution phase amplification when one primer is immobilized on the hydrogel matrix and the counterpart primer is freely provided.19,29,30 Moreover, carbon nanomaterials having dominant resonance structures on the surface such as CNT, GO, and rGO were effective in delivering the primers to the microparticle as complex forms, which were ready to be freely released for the reaction at the PCR temperature.20,30,31 Carbon nanomaterials can keep the DNA primers tightly adsorbed at room temperature and release them as free forms to participate in the reaction when the temperature increases during the amplification (Figure 3a). Based on this knowledge, rGO was conjugated with DNA primers through sonication for 30 min. To see if the amount of primer released from rGO is enough for the reaction, pPIN was compared to normal PIN with the addition of primer from the solution through qPCR on a Peltier thermal stage in a conventional assay format. Both amplification curves were well-superimposed, which indicates the primers supplied from rGO play the equivalent role for the effective PCR amplification, and the enzyme reaction is independent from the presence of rGO in PIN (Figure S7). Since both primers were completely integrated into the pPIN, specific and independent PCR can be achieved for a single target. This pPIN characteristic enables multiplex qPCR in a single chamber with a single fluorescent channel by simply adding pPIN microparticles. At the same time, the assay process becomes simpler since the primers are preloaded.
Figure 3.
(a) Schematic for photonic PCR of the rGO embedded pPIN. Primers adsorbed on rGO surface are released when PCR starts, and target DNA is amplified within the pPIN microparticle through photonic cycling. (b) Photothermal heat generation of pPINs made with various rGO concentrations. Fluorescent signals of (c) amplification and (d) its normalized values obtained with Peltier-based thermal cycler according to various rGO concentrations in pPIN. (e) Fluorescent images of the pPINs at the first and the last cycles on Peltier-based thermal cycler with varying concentrations of rGO. Concentration of rGO denotes the value after mixing with the primer (1:1, v/v).
To evaluate the photothermal effect according to the concentration of nanomaterials in the pPIN, pPIN microparticles were made using rGO suspensions of different concentrations. The laser was irradiated on the pPINs fabricated with rGO concentrations from 0.06 to 0.5 mg mL–1, and the temperature change of the pPINs was monitored in real time (Figure 3b). As the concentration of rGO increased, the microparticles were heated faster at the same light dose (0.48 W). With a higher irradiation (1.32 W) dose, pPINs with rGO of 0.13 mg mL–1 or more were heated to 95 °C, which is the denaturation temperature in PCR thermal cycling.
Most photothermal nanomaterials, including rGO, generate thermal energy through efficient absorption of light, but they also absorb fluorescence emission, resulting in the attenuation of the signal from PCR.31 Thus, this fluorescence quenching is less favored in real-time PCR. To assess only the quenching effect of rGO in microparticles, amplification was conducted with a conventional thermal plate-based PCR instrument instead of photonic PCR configuration. Consequently, the presence of rGO in the pPIN attenuated the fluorescent signal; however, Ct values were not affected by the concentration of rGO when it was less than 0.5 mg mL–1 (Figure 3c–e). This means that the concentration of rGO less than 0.5 mg mL–1 does not affect the amplification itself but only affects the transmission of the fluorescence signal due to its quenching characteristics. When rGO concentration is equal to or higher than 0.5 mg mL–1, a clear amplification curve was no longer observed, which is probably caused by more enhanced quenching effect or PCR inhibition due to high concentration of nanomaterial.32−35 Therefore, rGO concentration in pPINs was optimized to 0.25 mg mL–1 to appropriately perform photonic real-time PCR and avoid significant loss of the fluorescence signal.
With the pPINs of optimum size and composition, the light-to-heat conversion was evaluated for three independent batches of microparticles. The temperature of the microparticles exceeded 90 °C within 5 s after irradiation at a light dose of 1.32 W. Temperature variation was less than 3% in all three batches (Figure S8), which can easily be corrected by feedback control in practical use. Consequently, the hydrogel network in pPIN supported the reliable dispersion and trapping of rGO, resulting in practical and stable temperature control.
Thermal Cycling of pPIN qPCR
Accurate temperature control during PCR thermal cycling is an important factor for specific and effective target amplification. Unstable temperature during annealing/extension may lead to nonspecific amplification, and unstable temperature during denaturation may damage polymerases, nucleotides, and other reagents, resulting in a decrease in amplification efficiency. To confirm the stability of thermal cycling in pPIN, a 40-cycle thermal cycling test was conducted at a light dose of 1.52 W to pPIN microparticles. We found out that the photothermal temperature control of pPIN was stable for 40 cycles with standard deviations of 1.5 and 0.8 °C at 4 s denaturation and 20 s annealing/extension steps, respectively, as shown in Figure 4a,b.
Figure 4.
(a) Temperature profile for 40-cycle PCR. (b) Temperature stability on denaturation (black) and annealing/extension (red) steps. (c) Heating and (d) cooling rates during 40-cycle PCR.
Appropriate heating and cooling rates are essential to achieve fast, effective amplification. While most photonic PCRs have shown efficient heating, cooling takes longer than heating without additional heat dissipation settings.36 In this study, we successfully achieved both high heating and high cooling rates in pPIN PCR (average heating rate, 22.0 ± 3.0 °C s–1; cooling rate, 23.5 ± 2.6 °C s–1) as demonstrated in Figure 4c,d. Cooling was very rapid after switching off the light even without the aid of cooling fans or heat dissipation plates used by most other photonic PCR systems.5−7 This effective passive cooling was ascribed to the exclusive heating of pPIN and small reaction volume of pPIN microparticles surrounded by the liquid operating as a thermal reservoir. The temperature of the surrounding liquid was maintained around 30–40 °C throughout the 40-cycle thermal cycling (Figure S9). Although the photothermal heating can be affected by the thermal conductivity of surrounding liquid, it was relatively minor enough to be adjusted by feedback control software in our system (Figure S10). Thus, the microparticle-based photonic system enables the neat thermal cycling on the basis of stable temperature control and high ramping/cooling rates.
Ultrafast Real-Time pPIN PCR
Photonic real-time PCR using pPINs has been demonstrated based on the features of the pPINs evaluated above. Since both specific primers were already integrated in each particle, only a mixture of target DNA and PCR Mastermix was introduced into the chamber followed by the injection of mineral oil to isolate the pPINs. A hydrophilic matrix structure of pPINs facilitated the isolation of the PCR cocktail in the microparticles through the oil injection because PEG hydrogel prefers water to oil and penetration through pPIN has a higher fluid resistance than flowing around pPINs (Figure S11). Therefore, the simple oil injection easily achieved the reliable isolation of the nanoliter reaction. Two-step thermal cycling was adopted with denaturation for 4 s and annealing/extension for 20 s, resulting in a total assay time of 20 min for 40 cycles. The quantitative information on the target DNA was analyzed in real time by recording the fluorescent intensity of the pPIN microparticles at the end of each annealing/extension step. A 10-fold serial dilution from 108 copies μL–1 of E. coli DNA template was performed to see the quantitative reliability of pPIN qPCR. The results showed that the gap between each amplification curve was constant from 108 to 102 copies μL–1, yielding an amplification efficiency of 97.62%, which is comparable to that of the conventional solution-phase qPCR (Figure 5). Melting curve analyses of the pPINs after the amplification proved that the amplification signals come from the target (Figure S12). Furthermore, the amplification performance of pPIN during 4 weeks of storage showed an average standard deviation of 0.68 in Ct value, which indicates that the rGO or its photothermal characteristics did not deteriorate over time and that the primers adsorbed to rGO stayed fully functional during storage (Figure S13).
Figure 5.
Amplification performance of singleplex pPIN qPCR for E. coli. (a) Serial snapshots of pPIN qPCR for targeting E. coli 107 copies μL–1. (b) Ten-fold serial dilution of pPIN qPCR for targeting E. coli from 108 to 102 copies μL–1. NTC denotes no-template control. (c) Standard curve of pPIN qPCR for targeting E. coli.
Since the length of the amplicon is normally around 100 base pairs in real-time PCR applications, a few seconds would be sufficient for annealing/extension step.37 Also, previous works reported that a few milliseconds at 95 °C might be sufficient for the sake of the denaturation process.5 Based on these features, more challenging ultrafast pPIN qPCR was performed by gradually reducing the duration time for denaturation and annealing/extension. By reducing the duration time of each step to 0.1 and 3 s for denaturation and annealing/extension, respectively, the total reaction time was reduced from 20 to 5 min while consistently maintaining the Ct value (Figure 6a and Movie S1). Also, as shown in Figure 6b and Figure S14, all the cases with different cycling parameters showed successful amplification signals with target template, while they showed no signal for NTC, including 5 min ultrafast pPIN qPCR parameters. This indicates that 5 min ultrafast photonic qPCR amplified the target DNA, and the reaction time can be further reduced if more effective photothermal nanomaterials are integrated. To our knowledge, this is the demonstration of the fastest photonic PCR combined with fluorescence-based real-time monitoring.5−7,38
Figure 6.
Ultrafast pPIN qPCR with 107 copies μL–1 of E. coli. (a) Temperature profile and normalized amplification curves of 40-cycle ultrafast pPIN qPCR with varied duration time. (b) Serial snapshots of 5 min ultrafast pPIN qPCR with or without the DNA template (PTC and NTC).
Multiplex Photonic qPCR
As the number of target genes has rapidly increased, assay multiplicity has become essential in diagnostics.29,30,39 Although multiplex assays are typically achieved using different color channels of the fluorescence, it has been difficult to use multiple fluorescence channels across wavelengths to avoid interference from the light sources of photonic heating.7 pPIN qPCR is ideal for expanding the number of targets without concerns about spectral overlap because the targets are distinguished by geometric codes on pPINs corresponding to the target gene. pPIN qPCR enables the detection of multitarget amplification using only a single fluorescent channel. For multiplex analysis, the irradiation area was expanded, and the output power of laser was increased so that four pPIN microparticles were subject to heating at the same time with similar light doses as in singleplex qPCR. Prior to multiplex assay, a singleplex assay using 107 copies μL–1E. coli was conducted with four pPIN microparticles in a single chip to see the reaction uniformity between four pPINs. As a result, all four pPIN microparticles were equally amplified by negligible differences in Ct value and fluorescence intensity (Figure S15). Then, the multiplex pPIN assay was configured for the bacterial discrimination. Four different pPINs were prepared to target four different genes of the following bacterial species: A. baumannii, E. coli, K. pneumoniae, and P. aeruginosa. Each pPIN was encoded with a different geometry to distinguish the target gene (Figure 7a). In addition, each pPIN was fabricated with target-specific primers for the bacterial species as listed in Table S1. All four pPINs showed amplification curves with the target genes and showed a negligible signal without them (Figure S16). With this configuration, the multiplex assay was performed with the introduction of E. coli. As a result, only E. coli targeting pPIN showed the amplification curve, and the other pPINs showed no detectable change in fluorescence intensity (Figure 7b). In addition, the Ct value of E. coli targeting pPIN in this assay was ∼20, which is consistent with the value in the singleplex assay performed only with E. coli targeting pPIN. This indicates that the amplification was well-localized in pPIN as an independent reaction as if they were separated as singleplex assays. This result was also consistent with the other targets, K. pneumoniae (Figure 7c), P. aeruginosa (Figure 7d), and A. baumannii (Figure 7e). This means that the pPIN qPCR can easily achieve a reliable multiplex assay using a single fluorescence channel with scalability of the area for irradiation and detection. More recently, multiplex assays are not only necessary to discriminate targets but also widely used to identify multiple sites to ensure the accuracy of the diagnosis, especially in the current pandemic.40 Therefore, the pPIN qPCR provides photonic real-time PCR with expandable multiplexing capability enabling precise molecular diagnosis with the advantages of photonic control.
Figure 7.
Multiplex pPIN qPCR for bacterial discrimination. (a) Codes of pPINs for each bacterial target. Images and amplification curves of multiplex pPIN qPCR with the introduction of (b) E. coli, (c) K. pneumoniae, (d) P.aeruginosa, and (e) A. baumannii (the first and 40th cycles).
Discussion
The general advantages of photonic PCR include a reduction in the power consumption and the high speed of the assay as compared with conventional PCR. Diverse photothermal materials effectively reduced power consumption using high concentrations and improved light-to-heat conversion (Table S2). However, there are still limitations in that the materials should have good dispersity at high concentration and keep their dispersion uniformly during the reaction.7 Maintaining long-term dispersibility of the nanoparticles is challenging, but a sizable number of nanoparticles can achieve decent temporal dispersion by simple surface modification. For example, rGO in aqueous solutions is vulnerable to rapid aggregation and random condensation when exposed to light (Figure S17). However, DNA adsorption to rGO improves dispersion by repulsive force between negatively charged DNA backbones. When a prepolymer solution is spotted and cured for pPIN production, rGO maintain their dispersity in the prepolymer solution and the hydrogel networks, resulting in uniform temperature distribution in a single pPIN as well as reliability among pPINs. Since the production of the pPINs typically takes less than 10 min from the preparation of the prepolymer solution, a variety of superior photothermal nanomaterials can be applied to the pPIN assay as long as they have temporal dispersibility.
While several efforts in photothermal efficiency and cooling methods have been demonstrated to the reduce assay time in photonic PCR, the most effective way is to reduce the PCR volume. Reduction of the thermal volume to the nanoliter scale was very effective to reduce the time for thermal cycling due to the rapid passive cooling.4,5,41,42 However, accurate manipulation of a small reaction volume has been typically achieved with sophisticated equipment such as liquid handlers or microfluidic controllers, which negate the benefit of simplicity in photonic PCR.41 In contrast, the reaction volume of the pPIN assay was predefined by the dimension of the microparticle with locally distributed photothermal nanomaterials (100 nL in this study). Since the volume variation of pPIN fabricated through spotting is less than 10%,19 the nanoliter reaction volume for photothermal control is always consistently implemented with typical pipetting as in conventional qPCR without additional instruments. We believe that simple but secure nanoliter-scale pPIN qPCR will help reduce the assay time and increase the reliability of the assay.
Besides the reliable nanoliter reaction volume, total assay time is critical for point-of-care testing (POCT). Throughout this paper, 30 min was taken to ensure the diffusion of PCR mixture into pPIN microparticles, which is not suitable for POCT. To reduce the process time, the lyophilization of the chip where the pPINs were preloaded was tested. As soon as the PCR mixture reached to the lyophilized pPINs, they were fully swollen within 30 s thanks to their high hydrophilicity. After that, oil isolation and qPCR were sequentially conducted to see the effect of lyophilization. As a result, the Ct values for both the nonlyophilized pPINs with 30 min incubation and the lyophilized pPINs with 30 s swelling were almost identical and even the lyophilized pPINs showed higher fluorescent intensity than nonlyophilized ones (Figure S18). We think that this is because the capillary force during swelling is much more effective than diffusion to deliver PCR mixture into the pPIN microparticles. The amplification curve of pPINs showed no significant change even after vacuum storage for 5 days of the lyophilized microparticles. Hence, the chip containing lyophilized pPINs can be provided as a ready-to-use form in the field. Based on this observation, we believe that pPIN qPCR will be ideal for POCT if the further optimization such as lyophilization and vacuum packing protocol is added.
Conclusion
We have developed a microparticle-based photonic real-time PCR platform that reliably localizes the reaction into a nanoliter-scale volume. rGO was chosen as a photothermal nanomaterial embedded into the hydrogel microparticle showing the best photothermal performance. rGO was also used as a primer carrier for the reaction through forming an reversible complex with the DNA primer, rendering pPIN a fully target-specific reactor. Localization of the reaction in pPIN enabled ultrafast real-time PCR within 5 min even without the external fan due to the small reaction volume (∼100 nL) and allowed rapid passive cooling. Multiplex pPIN qPCR for bacterial discrimination was also achieved simply by loading multiple pPINs in a single reaction. Future work will be focused on the optimization and miniaturization of a pPIN photonic qPCR system, which will be the ideal platform for POCT.
Materials and Methods
Preparation of Nanomaterials
Gold nanorods (GNRs) were purchased from Nanopartz (AC12-10-808-CTAB-DIH-100–1), carbon nanotubes (CNTs) were purchased from Sigma-Aldrich (SKU: 775533), and graphene oxide (GO) was synthesized using a modified Hummers’ method.43 To prepare reduced graphene oxide (rGO), chemical reduction of GO was conducted using hydrazine. Initially, a GO solution was prepared at a 0.5 mg mL–1 concentration under ultrasonication, 700 μL of 28% ammonia solution and 100 μL of 35% hydrazine solution were added to 100 mL of GO solution. The mixture was stirred for 5 min and reacted in an oil bath for 1 h at 95 °C for reduction. After the reaction, the rGO solution was purified by extensive dialysis for 1 week to remove any byproducts and remaining reactants. Purified rGO solution was stored at 4 °C until use.
Absorbance Measurement of Nanomaterials
A mixture of 0.25 mg mL–1 of a photothermal nanomaterial solution was prepared, and 200 μL of solution was transferred to a 96-well plate. Absorbance was measured and analyzed from 400 to 1000 nm using a UV/vis/NIR spectrophotometer (Synergy MX, BioTek, USA).
Measurement of Photothermal Effect of Nanomaterials Suspension
A nanomaterial suspension (0.25 mg mL–1) was loaded on a 96-well plate. Light was introduced to each well from an 800 nm laser (Changchun New Industries Optoelectronics Technology Co., Ltd) through a fiber optic coupler (ø 400 μm, SMA90516060090) as temperature was monitored using a thermal camera (FLIR A325SC, FLIR System, INC.).
Preparation of Primers and Templates
The synthetic DNA templates and forward primers and reverse primers for E. coli, P. aeruginosa, A. baumannii, and K. pneumoniae were purchased from Integrated DNA Technologies (IDT). Forward primers were modified with acrydite at the end of 5′ to link them to PEG hydrogel during UV curing. The sequence information is described in Table S1.
Production of Nanomaterial-Embedded PINs
A prepolymer solution was prepared by mixing 40% v/v poly(ethylene glycol) (PEG, Sigma-Aldrich, Mn = 600), 35% v/v of the nanomaterial suspension solution with equivalent concentrations of 0.25 mg mL–1 (GNR, CNT, GO or rGO), 20% v/v poly(ethylene glycol)-diacrylate (PEGDA, Sigma-Aldrich, Mn = 700), and 5% v/v Darocur 1173 (Sigma-Aldrich). The prepolymer solution for the pPIN was finalized by mixing with 200 μM acrydited forward primer (the sequence information is described in Table S1) at a volume ratio of 9:1. The resulting mixed solution was spotted onto the code microparticle mold, which was made of polydimethylsiloxane (PDMS) replicated from microfabricated SU-8 mold; then, it was cured by UV light at 20 mW cm–2 for 10 s to solidify the prepolymer solution into the microparticles as the forward primer was covalently immobilized on their polymer network. After photocuring, the code mold with the cured microparticles was submerged into DI water with 0.2% sodium cholate to detach and collect cured pPINs from the mold. Then, the preparation of the pPINs was completed by rinsing with DI water with 0.2% sodium cholate and storing at 4 °C until use.
Production of pPIN with rGO–DNA Complex
First, 20 μL of 0.5 mg mL–1 rGO solution and 20 μL of 200 μM reverse primer (sequences in Table S1, IDT) were mixed and sonicated using a Branson 1510MT ultrasonic cleaner (Merck. Ltd.) in an ice bath for 30 min, leading to the rGO–DNA complex where the reverse primers are immobilized on the surface of rGO until PCR begins. To prepare a prepolymer solution, 40% v/v PEG, 35% v/v of rGO–DNA complex solution, 20% v/v PEGDA, and 5% v/v Darocur 1173 were mixed. Then, the prepolymer solution was mixed with 200 μM acrydited forward primer solution at a volume ratio of 9:1. The rest of the pPIN production steps followed the above description.
Photonic qPCR Platform Setup and Heating/Cooling Rate Measurement
A custom stage was used to hold the fiber optic coupler and place the plastic PCR chip (Genesystem Cat.# 9699100800) under the microscope. To control the thermal cycling by manipulating output power, a thermal camera was installed, and temperature values from thermal camera software were continuously sent to customized Python software for temperature feedback control during the thermal cycle. An Arduino UNO board (Arduino) was connected between the computer and 800 nm laser equipment, and a modified duty cycle signal was sent from the Python software to the laser equipment via an Arduino UNO board to control output power of the laser. Lastly, qPCR fluorescent images were recorded at each cycle using a fluorescent microscope (Zeiss Axio Plan 2).
To measure heating and cooling rates of photonic PCR, four pPINs were located on the plastic PCR chip, and 40-cycle thermal cycling was performed using the 800 nm laser. The temperature was measured and recorded in real time via a thermal camera. The heating rate was measured by calculating interval between the end of annealing step and the beginning of denaturation step, and the cooling rate was measured by calculating the interval between the end of denaturation step and the beginning of annealing step.
Photonic qPCR
For photonic qPCR, pPINs were first loaded on a plastic PCR chip. The PCR cocktail was prepared by mixing 8 μL of 2X SYBR Green I Mastermix, 7 μL of DI water, and 1 μL of template. This cocktail was introduced into the plastic PCR chip where the pPINs were loaded. The chip was kept at 4 °C for 30 min, which was sufficient time for the PCR cocktail to thoroughly diffuse inside the pPINs. After 30 min of incubation, 16 μL of mineral oil was introduced into the chip to replace the PCR cocktail surrounding the pPINs with, which isolates individual pPINs while maintaining ∼100 nL of PCR cocktail inside the pPIN. After that, the inlet and outlet were sealed using sealing tape. A two-step amplification process was carried out with the initiation of predenaturation step at 95 °C for 8 s and cycling between denaturation step at 95 °C for 4 s and annealing/extension at 65 °C for 20 s. At the end of the annealing step, fluorescent images were taken automatically under a fluorescent microscope, and ImageJ (image processing freeware, http://imagej.nih.cov/ij/) was used to create an amplification curve from the saved images. The temperatures of the pPINs were monitored by a thermal camera and were saved for further analysis. For the 5 min ultrafast real-time PCR, a two-step amplification process was carried out with the initiation of the predenaturation step at 95 °C for 8 s and cycling between the denaturation step at 95 °C for 0.1 s and annealing/extension at 65 °C for 3 s. The rest of the experimental steps were the same as described above.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (2018R1A2A1A0577112) and the R&D Convergence Program of the National Research Council of Science & Technology of the Republic of Korea (CRC-20-02-KIST).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsnano.2c07017.
Movie of ultrafast photonic qPCR (MP4)
Figures of cryo-SEM images of hydrogel microparticle, photonic qPCR chip, photonic PCR platform temperature calibration, photonic PCR temperature step, interferential analysis of the photothermal light source in photonic qPCR system, photothermal heat generation curves of nanomaterials with the identical absorbance, evaluation of primer amount supplied from rGO, batch-to-batch variation of pPINs, thermal reservoir effect of the surrounding solution of pPINs, effect of surrounding liquid in localized photothermal heating, oil isolation effect, photonic qPCR melting analysis, long-term storage stability of pPIN, ultrafast pPIN qPCR with varied cycling parameters, singleplex photonic qPCR using four pPINs, singleplex pPIN qPCR for each bacterium, rGO dispersion before and after laser exposure, and lyophilization of pPIN and tables of sequence information on the DNA templates and primers and photonic PCR platform comparison (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Mullis K. B. The unusual origin of the polymerase chain reaction. Sci. Am. 1990, 262 (4), 56–65. 10.1038/scientificamerican0490-56. [DOI] [PubMed] [Google Scholar]
- Qiu X.; Yuan J.. Temperature control for PCR thermocyclers based on Peltier-effect thermoelectric. 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, 2006; IEEE: pp 7509–7512. [DOI] [PubMed] [Google Scholar]
- Niemz A.; Ferguson T. M.; Boyle D. S. Point-of-care nucleic acid testing for infectious diseases. Trends Biotechnol. 2011, 29 (5), 240–250. 10.1016/j.tibtech.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You M.; Li Z.; Feng S.; Gao B.; Yao C.; Hu J.; Xu F. Ultrafast Photonic PCR Based on Photothermal Nanomaterials. Trends Biotechnol. 2020, 38 (6), 637–649. 10.1016/j.tibtech.2019.12.006. [DOI] [PubMed] [Google Scholar]
- Son J. H.; Cho B.; Hong S.; Lee S. H.; Hoxha O.; Haack A. J.; Lee L. P. Ultrafast photonic PCR. Light: Science & Applications 2015, 4 (7), e280–e280. 10.1038/lsa.2015.53. [DOI] [Google Scholar]
- Son J. H.; Hong S.; Haack A. J.; Gustafson L.; Song M.; Hoxha O.; Lee L. P. Rapid Optical Cavity PCR. Adv. Healthcare Mater. 2016, 5 (1), 167–174. 10.1002/adhm.201500708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.-H.; Cheglakov Z.; Yi J.; Cronin T. M.; Gibson K. J.; Tian B.; Weizmann Y. Plasmonic photothermal gold bipyramid nanoreactors for ultrafast real-time bioassays. J. Am. Chem. Soc. 2017, 139 (24), 8054–8057. 10.1021/jacs.7b01779. [DOI] [PubMed] [Google Scholar]
- Kim S.-E.; Lee B.-R.; Lee H.; Jo S. D.; Kim H.; Won Y.-Y.; Lee J. Near-Infrared Plasmonic Assemblies of Gold Nanoparticles with Multimodal Function for Targeted Cancer Theragnosis. Sci. Rep 2017, 7 (1), 17327. 10.1038/s41598-017-17714-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. l.; Gu M. Gold-Nanoparticle-Enhanced Cancer Photothermal Therapy. Selected Topics in Quantum Electronics, IEEE Journal of 2010, 16, 989–996. 10.1109/JSTQE.2009.2030340. [DOI] [Google Scholar]
- Huang X.; El-Sayed M. A. Plasmonic photo-thermal therapy (PPTT). Alexandria Journal of Medicine 2011, 47 (1), 1–9. 10.1016/j.ajme.2011.01.001. [DOI] [Google Scholar]
- Roche P. J. R.; Beitel L. K.; Khan R.; Lumbroso R.; Najih M.; Cheung M. C. K.; Thiemann J.; Veerasubramanian V.; Trifiro M.; Chodavarapu V. P.; et al. Demonstration of a plasmonic thermocycler for the amplification of human androgen receptor DNA. Analyst 2012, 137 (19), 4475–4481. 10.1039/c2an35692a. [DOI] [PubMed] [Google Scholar]
- Li T.-J.; Chang C.-M.; Chang P.-Y.; Chuang Y.-C.; Huang C.-C.; Su W.-C.; Shieh D.-B. Handheld energy-efficient magneto-optical real-time quantitative PCR device for target DNA enrichment and quantification. NPG Asia Materials 2016, 8 (6), e277–e277. 10.1038/am.2016.70. [DOI] [Google Scholar]
- Kadu P.; Pandey S.; Neekhra S.; Kumar R.; Gadhe L.; Srivastava R.; Sastry M.; Maji S. K. Machine-Free Polymerase Chain Reaction with Triangular Gold and Silver Nanoparticles. J. Phys. Chem. Lett. 2020, 11 (24), 10489–10496. 10.1021/acs.jpclett.0c02708. [DOI] [PubMed] [Google Scholar]
- Kim J.; Kim H.; Park J. H.; Jon S. Gold Nanorod-based Photo-PCR System for One-Step, Rapid Detection of Bacteria. Nanotheranostics 2017, 1 (2), 178–185. 10.7150/ntno.18720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho B.; Lee S. H.; Song J.; Bhattacharjee S.; Feng J.; Hong S.; Song M.; Kim W.; Lee J.; Bang D.; et al. Nanophotonic Cell Lysis and Polymerase Chain Reaction with Gravity-Driven Cell Enrichment for Rapid Detection of Pathogens. ACS Nano 2019, 13 (12), 13866–13874. 10.1021/acsnano.9b04685. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Gao Z.; Han Z.; Liu Y.; Yang H.; Akkin T.; Hogan C. J.; Bischof J. C. Aggregation affects optical properties and photothermal heating of gold nanospheres. Sci. Rep 2021, 11 (1), 898. 10.1038/s41598-020-79393-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B.-H.; Lee Y.; Yu E.-S.; Na H.; Kang M.; Huh H. J.; Jeong K.-H. Ultrafast and Real-Time Nanoplasmonic On-Chip Polymerase Chain Reaction for Rapid and Quantitative Molecular Diagnostics. ACS Nano 2021, 15 (6), 10194–10202. 10.1021/acsnano.1c02154. [DOI] [PubMed] [Google Scholar]
- Lee Y.; Kang B. H.; Kang M.; Chung D. R.; Yi G. S.; Lee L. P.; Jeong K. H. Nanoplasmonic On-Chip PCR for Rapid Precision Molecular Diagnostics. ACS Appl. Mater. Interfaces 2020, 12 (11), 12533–12540. 10.1021/acsami.9b23591. [DOI] [PubMed] [Google Scholar]
- Jung S.; Kim J.; Lee D. J.; Oh E. H.; Lim H.; Kim K. P.; Choi N.; Kim T. S.; Kim S. K. Extensible Multiplex Real-time PCR of MicroRNA Using Microparticles. Sci. Rep 2016, 6 (1), 22975. 10.1038/srep22975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. M.; Jung S.; Jeon E. J.; Kim B. K.; No J. Y.; Kim M. J.; Kim H.; Song C. S.; Kim S. K. Highly Selective Multiplex Quantitative Polymerase Chain Reaction with a Nanomaterial Composite Hydrogel for Precise Diagnosis of Viral Infection. ACS Appl. Mater. Interfaces 2021, 13 (26), 30295. 10.1021/acsami.1c03434. [DOI] [PubMed] [Google Scholar]
- Chauhan V. M.; Hopper R. H.; Ali S. Z.; King E. M.; Udrea F.; Oxley C. H.; Aylott J. W. Thermo-optical characterization of fluorescent rhodamine B based temperature-sensitive nanosensors using a CMOS MEMS micro-hotplate. Sens. Actuators, B 2014, 192, 126–133. 10.1016/j.snb.2013.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arata H. F.; Löw P.; Ishizuka K.; Bergaud C.; Kim B.; Noji H.; Fujita H. Temperature distribution measurement on microfabricated thermodevice for single biomolecular observation using fluorescent dye. Sens. Actuators, B 2006, 117 (2), 339–345. 10.1016/j.snb.2005.11.017. [DOI] [Google Scholar]
- Terazono H.; Hattori A.; Takei H.; Takeda K.; Yasuda K. Development of 1480 nm photothermal high-speed real-time polymerase chain reaction system for rapid nucleotide recognition. Jpn. J. Appl. Phys. 2008, 47 (6S), 5212. 10.1143/JJAP.47.5212. [DOI] [Google Scholar]
- Walsh T.; Lee J.; Park K. Laser-assisted photothermal heating of a plasmonic nanoparticle-suspended droplet in a microchannel. Analyst 2015, 140 (5), 1535–1542. 10.1039/C4AN01750A. [DOI] [PubMed] [Google Scholar]
- Demirbas U.; Acar D. A. E. Continuous-wave, quasi-continuous-wave, gain-switched, and femtosecond burst-mode operation of multi-mode diode-pumped Cr: LiSAF lasers. JOSA B 2016, 33 (10), 2105–2113. 10.1364/JOSAB.33.002105. [DOI] [Google Scholar]
- Bisoyi H. K.; Urbas A. M.; Li Q. Soft Materials Driven by Photothermal Effect and Their Applications. Advanced Optical Materials 2018, 6 (15), 1800458. 10.1002/adom.201800458. [DOI] [Google Scholar]
- Chala T.; Wu C.-M.; Chou M.-H.; Gebeyehu M.; Cheng K.-B. Highly efficient near infrared photothermal conversion properties of reduced tungsten oxide/polyurethane nanocomposites. Nanomaterials 2017, 7 (7), 191. 10.3390/nano7070191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.-J.; Chen D.-H. Preparation and near-infrared photothermal conversion property of cesium tungsten oxide nanoparticles. Nanoscale Res. Lett. 2013, 8 (1), 57. 10.1186/1556-276X-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.; Jung S.; Byoun M. S.; Yoo C.; Sim S. J.; Lim C. S.; Kim S. W.; Kim S. K. Multiplex real-time PCR using temperature sensitive primer-supplying hydrogel particles and its application for malaria species identification. PLoS One 2018, 13 (1), e0190451 10.1371/journal.pone.0190451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S.; Kim J.; Kim J.; Yang S.; Kim S. K. Extensible multiplex real-time PCR for rapid bacterial identification with carbon nanotube composite microparticles. Biosens. Bioelectron. 2017, 94, 256. 10.1016/j.bios.2017.02.049. [DOI] [PubMed] [Google Scholar]
- Roh K.; Kim D.-M.; Lee E. H.; Kim H.; Park H. S.; Jang J.-H.; Hwang S.-H.; Kim D.-E. A simple PCR-based fluorometric system for detection of mutant fusion DNAs using a quencher-free fluorescent DNA probe and graphene oxide. Chem. Commun. 2015, 51 (32), 6960–6963. 10.1039/C5CC00263J. [DOI] [PubMed] [Google Scholar]
- Bai Y.; Cui Y.; Paoli G. C.; Shi C.; Wang D.; Shi X. Nanoparticles Affect PCR Primarily via Surface Interactions with PCR Components: Using Amino-Modified Silica-Coated Magnetic Nanoparticles as a Main Model. ACS Appl. Mater. Interfaces 2015, 7 (24), 13142–13153. 10.1021/am508842v. [DOI] [PubMed] [Google Scholar]
- Zhong Y.; Huang L.; Zhang Z.; Xiong Y.; Sun L.; Weng J. Enhancing the specificity of polymerase chain reaction by graphene oxide through surface modification: zwitterionic polymer is superior to other polymers with different charges. Int. J. Nanomedicine 2016, 11, 5989–6002. 10.2147/IJN.S120659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M.; Luo C.; Zhang F.; Liu F.; Zhang J.; Guo S. Interactions of the primers and Mg2+ with graphene quantum dots enhance PCR performance. RSC Adv. 2015, 5 (91), 74515–74522. 10.1039/C5RA12729G. [DOI] [Google Scholar]
- Sanabria N. M.; Gulumian M. The presence of residual gold nanoparticles in samples interferes with the RT-qPCR assay used for gene expression profiling. J. Nanobiotechnol. 2017, 15 (1), 72. 10.1186/s12951-017-0299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You M.; Cao L.; Xu F. Plasmon-Driven Ultrafast Photonic PCR. Trends Biochem. Sci. 2020, 45 (2), 174–175. 10.1016/j.tibs.2019.11.007. [DOI] [PubMed] [Google Scholar]
- Gelfand D. H.Taq DNA Polymerase. In PCR Technology: Principles and Applications for DNA Amplification, Erlich H. A., Ed.; Palgrave Macmillan UK, 1989; pp 17–22. [Google Scholar]
- Neuzil P.; Zhang C.; Pipper J.; Oh S.; Zhuo L. Ultra fast miniaturized real-time PCR: 40 cycles in less than six minutes. Nucleic Acids Res. 2006, 34 (11), e77 10.1093/nar/gkl416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J.; Jung C. Hydrogels for Efficient Multiplex PCR. Biotechnology and Bioprocess Engineering 2020, 25 (4), 503–512. 10.1007/s12257-020-0134-2. [DOI] [Google Scholar]
- Liu R.; Han H.; Liu F.; Lv Z.; Wu K.; Liu Y.; Feng Y.; Zhu C. Positive rate of RT-PCR detection of SARS-CoV-2 infection in 4880 cases from one hospital in Wuhan, China, from Jan to Feb 2020. Clin. Chim. Acta 2020, 505, 172–175. 10.1016/j.cca.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.; Wang P.; Tong L.; Zhang L. Gold nanorod-facilitated localized heating of droplets in microfluidic chips. Opt. Express 2013, 21 (1), 1281–1286. 10.1364/OE.21.001281. [DOI] [PubMed] [Google Scholar]
- Ahrberg C. D.; Manz A.; Chung B. G. Polymerase chain reaction in microfluidic devices. Lab Chip 2016, 16 (20), 3866–3884. 10.1039/C6LC00984K. [DOI] [PubMed] [Google Scholar]
- Hummers W. S.; Offeman R. E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80 (6), 1339–1339. 10.1021/ja01539a017. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.