Abstract
Social decision-making is critically influenced by neurocircuitries that regulate stress responsiveness. Adaptive choices, therefore, are altered by stress-related neuromodulatory peptide systems, such as corticotropin releasing factor (CRF). Experimental designs that take advantage of ecologically salient fear-inducing stimuli allow for revelation of neural mechanisms that regulate the balance between pro- and anti-stress responsiveness. To accomplish this, we developed a social stress and conditioning protocol, the Stress Alternatives Model (SAM), that utilizes a simple dichotomous choice, and produces distinctive behavioral phenotypes (Escape or Stay). The experiments involve repeated social aggression, a potent unconditioned stimulus (US), from a novel larger conspecific (a 3X larger Rainbow trout). Prior to the social interaction, the smaller test fish is presented with an auditory conditioning stimulus (water off = CS). During the social aggression, an escape route is available, but is only large enough for the smaller test animal. Surprisingly, although the new aggressor provides vigorous attacks each day, only 50% of the test fish choose Escape. Stay fish, treated with the CRF1 antagonist antalarmin, a potent anxiolytic drug, on day 4, promotes Escape behavior for the last 4 days of the SAM protocol. The results suggest that the decision to Escape, required a reduction in stress reactivity. The Stay fish that chose Escape following anxiolytic treatment, learned how to use the escape route prior to stress reduction, as the Escape latency in these fish was significantly faster than first time escapers. In Escape fish, the use of the escape route is learned over several days, reducing the Escape latency over time in the SAM. Fear conditioning (water off + aggression) resulted in elevated hippocampal (DL) Bdnf mRNA levels, with coincident reduction in the AMPA receptor subunit Glua1 expression, a result that is reversed following a one-time treatment (during SAM aggression on day 4) with the anxiolytic CRF1 receptor antagonist antalarmin.
Keywords: Corticotropin-releasing factor, Social aggression, Fear conditioning, Stress-Alternatives Model, SAM, PTSD, Anxiety, Depression
1. Introduction
Fear learning (associative learning that produces adaptive behavior to aversive stimuli) is often examined using artificial experimental protocols, such as foot shock [1, 2], but is also an important component of natural systems, resulting from such diverse stimuli as predators [3], prey, and aggressive conspecifics, as well as the smells [4, 5] and sounds that come from them [6–9]. Significant emotional involvement adds salience to memories and drives learning mechanisms to encode important contextual and social cues for future reference [10]. Acute fearful stimuli activate specific central, sympathetic, and neuroendocrine (HPA) responses, in humans and other vertebrates, which together mobilize bodily resources for stress responses [6, 11]. These responses are adaptive, acting in the limbic brain to strengthen memory formation of fearful conditions [12], but may also be maladaptive when the stressor is chronic or traumatic, leading to hippocampal atrophy, compromised immune function and development of post-traumatic stress disorder (PTSD) [13, 14].
Social aggression is among the most potent fear-inducing stimuli [15], due to its unpredictable nature and potential payoffs, which may include resources, territory and mating [16, 17]. We have developed a model of fear learning that utilizes a social defeat protocol to produce stress, and offers a simple dichotomous behavioral choice, escaping or not, to facilitate examination of neural regulation of fear learning and decision-making. The Stress Alternatives Model (SAM) [18], was designed for rodents [19, 20] and rainbow trout (Oncorhynchus mykiss) [6, 21], and takes into account ecologically and evolutionarily relevant stimuli and adaptive behavioral responses [22, 23]., Fear-eliciting stimulus in the SAM is social aggression from a much larger, novel conspecific territorial intruder, from which the test animal has the choice to utilize escape routes, thus two distinct, evenly distributed, and stable behavioral phenotypes emerge: Escape (active avoidance) or Stay (acceptance of subordination). Pavlovian Fear Conditioning is added by applying an auditory cue, the conditioned stimulus (CS, cessation of water flow) prior to the interaction, is paired with the unconditioned stimulus (US, aggression) over several days of training. However, in fish, only individuals of the Stay phenotype demonstrate the conditioned responses (CR; defined here as a physiological response: elevated cortisol stress hormone secretion, but also includes increased monoaminergic activity in amygdala, hypothalamus and raphé [6]) elicited in the absence of the US, to the CS alone. Understanding the mechanisms of fear learning in relation to real-world scenarios elucidates how appropriate behavioral and physiological responses are selected. These ecologically relevant mechanisms may have important clinical implications for a wide variety of psychiatric maladies, including anxiety disorders, depression, and PTSD [24–26].
Thus, Escape and Stay phenotypes differentially take advantage of social avoidance learning (Escape) or Pavlovian fear conditioning (Stay) [6, 21], as stress coping strategies similar to those of rodents and numerous other species [27–30]. During stress, the neuropeptide corticotrophin releasing factor (CRF), initiates central stress actions and the Hypothalamic-Pituitary-Adrenal stress cascade (HPA; HPI in fish) via CRF1 receptors in the pituitary [31–37]. These CRF1 receptors are also found in limbic brain regions (including preoptic area, amygdala (Dm and Vc/Vl), hippocampus (Dl), and raphé) [32, 38], such that intracerebroventricular (icv) CRF treatment in rainbow trout stimulates locomotion, anxiogenic behavior, and influences the nature and outcome of aggressive interactions [33, 39]. Additionally, while CRF has been demonstrated to elevate anxiety and indecision [21, 33, 39, 40], and to induce a stereotypical behavior, snap shake [21, 33, 40, 41], that is associated with indecision regarding escape, the selective anxiolytic (anxiety reducing) CRF type 1 (CRF1) receptor antagonist Antalarmin not only blocks snap shake, but in doing so appears to facilitate decision-making and faster escape [21]. To investigate the role of CRF1 antagonism directly on learned escape in our Rainbow trout model, both Escape and Stay fish were administered Antalarmin (or saline) orally on day 4 of the 7-day training period. Antalarmin was selected for its specificity to the CRF1 receptor, its proven efficacy in teleost fish and that orally administered drug crosses the blood-brain barrier to influence central CRF binding [42, 43]. Furthermore, formation of long-lasting memories requires hippocampal gene transcription, synthesis of new proteins, and long-term potentiation [44–46]. Included among genes necessary for specific types of learning are brain-derived neurotrophic factor (BDNF, Bdnf gene), and the NMDA and AMPA ionotropic glutamate receptors, specifically the AMPA receptor subunits GluA 1–4 (Glua1, Glua2, Glua3, Glua3 genes)[47–52]. The Bdnf gene is rapidly and selectively induced in response to external stimuli [53] via region specific promoters in the hippocampus [54, 55] during contextual learning [56, 57], and promotes the synaptic incorporation of GluA1 subunits increasing molecular plasticity [58]. Additionally, when Glua1 translation is repressed, BDNF-induced neuronal activity is blocked [58], and when GluA1–4 subunits are deleted from the CA1 region of the hippocampus, spatial learning tasks are inhibited [59–61]. These genes and their products play an important role in synaptic plasticity, modulation of neurotransmitter release and, when absent, produce deficits in learning and memory [48]. Importantly for our model, hippocampal BDNF levels in mice are positively correlated with ability to learn a spatial task [62, 63].
To test whether stress-resilient behavior, such as learned Escape [64–66], is regulated by social stress via CRF1 receptor activity, and concomitantly produces enhanced Bdnf plus Glua1 expression, we used the trout SAM paradigm coupled with Antalarmin treatment to examine behavioral and gene expression effects following learned escape and classical conditioning [20, 64]. Learned escape is a complex spatial task that includes finding (and using) a previously unknown escape route during aggressive social conflict, plus learning and remembering the location of this escape route, to limit vulnerability to future attacks. Conversely, Stay fish that never use the escape hole are subject to daily social aggression and exhibit robust fear conditioning [6]. More advanced analysis of fear conditioning in mouse SAM experiments demonstrates that both Stay and Escape mice exhibit enhanced freezing in response to CS (tone) plus US (aggression) pairing [64, 66, 67]. We hypothesized that hippocampal Bdnf gene expression would be differentially elevated in response to the spatial “Learned Escape” task compared to Stay fish, which experience more robust fear conditioning. Similarly, we hypothesized that commensurately elevated expression of Glua1 in Escape and Stay phenotypes. Finally, we hypothesize that pharmacologically induced (CRF1) receptor antagonism will induce behavioral and gene expression reversal.
2. Methods
2.1. Animals
Rainbow trout (Oncorhynchus mykiss) raised from eggs at Gavins Point National Fish Hatchery were reared in 6 foot circular fiberglass tanks under natural light conditions [6, 21]. Prior to experimentation, small (125–150 g) test fish (N = 40) were netted out of the group tank and placed individually into one of three separate compartments (3 opaque plexiglass dividers, one with an escape hole) of glass aquaria, which were lit and aerated, and held 75 gallons; where each was fed and allowed to acclimate (home space) for 10 days (Figure 1A). On day seven of acclimation, a blood sample was taken from the caudal vein of each fish to determine baseline levels of circulating cortisol (F). Following testing on experimental day 8, fish were anaesthetized by placement in a 10L bucket of water treated with MS-222 (Sigma) at a concentration of 500mg/L until loss of equilibrium (~12–15 seconds), and an additional blood sample and intact brains were collected. Plasma cortisol and regional monoamine data from this SAM + fear conditioning protocol have been published previously [6]. Large (350–450 g) adult hatchery brood-stock (N = 8) were used as the aggressive social stimulus; a novel aggressor presented as unconditioned stimulus (US) each day. These large fish were housed separately prior to experimentation, rotated and rested throughout the experiment to insure a high level of aggression towards the test fish. One larger aggressor was added to an empty compartment of each aquarium 1h prior to social interaction. All experiments were conducted in a manner that minimized suffering and the number of animals used, in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23), under a protocol approved by University of South Dakota IACUC.
Fig. 1.
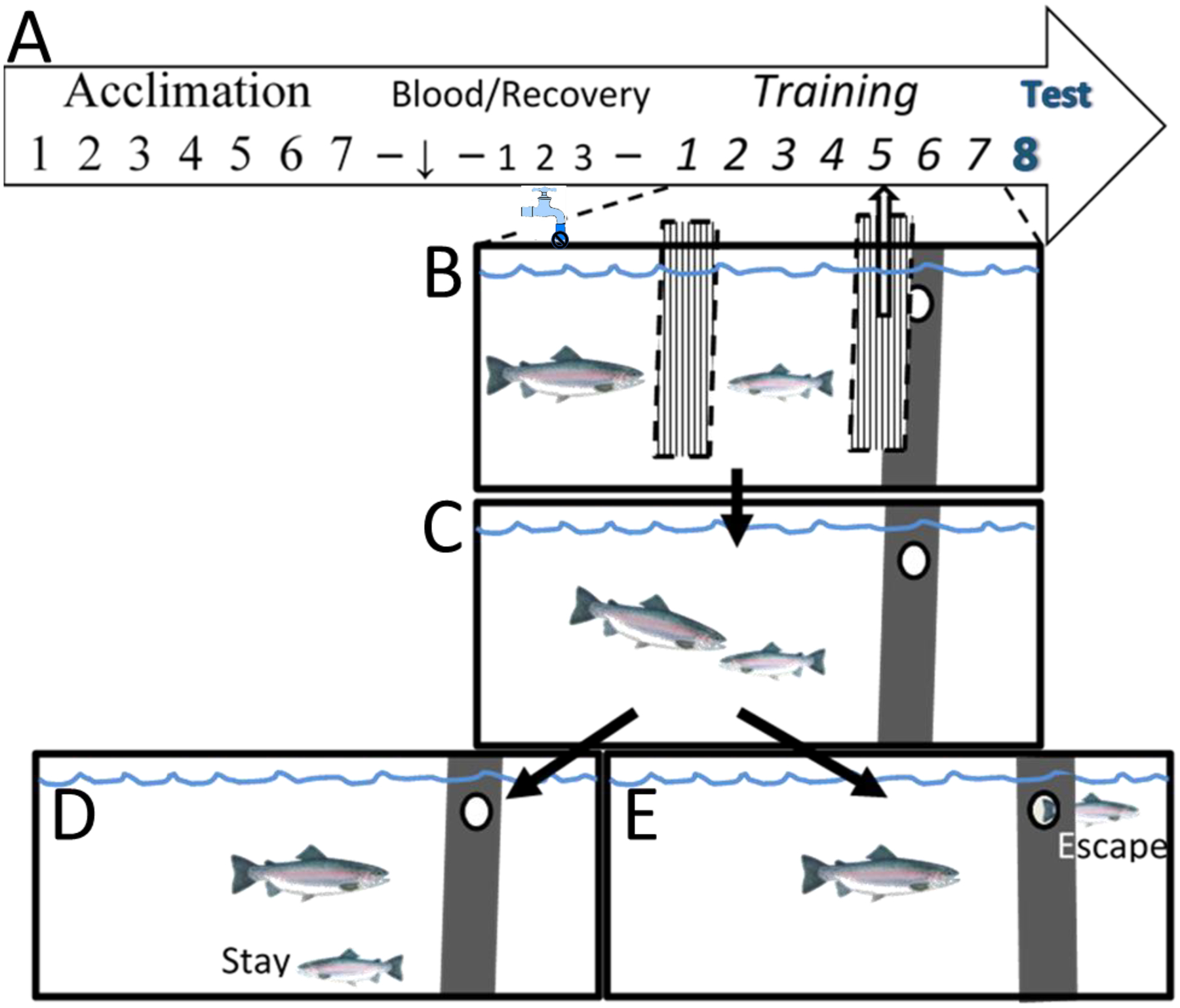
Experimental design and SAM aquatic social fear conditioning and behavioral arena. (A) The investigation design timeline included a week of acclimation to experimental tanks, followed by pretest blood sample 3 days of recovery, and then SAM conditioning. (B) Pulling the opaque dividers obscuring combatants and escape route, following CS presentation (water off), allowed for (C) social interactions with a novel large aggressor (US) occurred daily in the SAM over the next week, with CR testing on the 8th day. Fifteen minutes after the initiation of testing terminal blood and brain samples were collected for analysis. Individual trout exhibiting (D) Stay or (E) Escape phenotypes were reliably stable across the training period.
2.2. Experimental design - SAM
Following the 10 day acclimation period, on experimental day 1, water inflow to the tank was turned off (CS), and 15 seconds later, the barrier separating the large and small fish was removed, along with the barrier that covered the escape hole, making an empty chamber available to the test fish (Figure 1B). Importantly, the escape hole was large enough for only the test fish to pass through. Following the CS presentation for fear conditioning, fish were allowed to interact for 15 minutes (Figure 1C), and latency to first attack, aggressive/submissive behavior, and escape time (if applicable) were recorded. If the test fish did not escape within 15 minutes following the first attack (Figure 1D), the fish were separated, barriers re-inserted, water inflow was turned back on, and the interaction was over. If the test fish did escape (Figure 1E), the water was turned back on, the big fish moved back to his chamber, and the small fish was allowed to remain in the empty compartment for five minutes before being ushered back into the home chamber. Pairings of CS:US occurred once daily over seven days, and two stable phenotypes developed by day 2 during social interaction: active avoidance (Escape) or accepting confrontation (Stay) [29, 68]. On day 4 of training, both Escape (n = 10) and Stay (non-escaping; n = 8) individuals were orally administered either saline or the CRF1 receptor antagonist Antalarmin (2mg/Kg) in food, to produce 4 treatment groups: 1. Saline Control-Escape, 2. Saline Control-Stay, 3. Antalarmin-Escape, 4. Antalarmin-Stay. Antalarmin crosses the blood brain barrier. On day eight, test fish were presented with the CS only (water inflow turned off and barriers removed) with no large fish present, and fish were observed for 15 minutes. Immediately following this observation, test fish tissues were rapidly collected for analysis.
For purposes of determining CR, two other groups were included in the design that did not experience CS:US pairing. In the first of those groups, test fish (n = 12) were exposed to the aggression (US) alone, over 7 days of SAM interaction, with no access to the escape hole. These fish were included as an aggression only controls, to compare the effects of daily aggression on CR, without pairing to the water-off CS. The interactions and timeline for these fish were the same as described above. The final group of test fish (n = 12) was included as a CS only control group and was exposed only to the water off CS for 15 minutes once a day. These fish participated in no aggressive interactions over the course of the experiment but followed the same timeline as that described above. These two control groups, either US alone, or CS alone, were compared for hormonal and gene expression to that of CS:US paired Escape and Stay fish.
2.3. Sequencing of rainbow trout Bdnf, Glua1, Glua2 and Gapdh
Total RNA was isolated from the DL of the Rainbow trout Oncorhynchus mykiss using the RNeasy Mini kit System (Qiagen). During the process of isolation, samples were treated by RNase-free DNase (Qiagen) to eliminate DNA contamination of the samples according to the manufacturer’s protocol. Reverse transcribed RNA, was made using the SuperScript® First-Strand Synthesis System for RT-PCR (Invitrogen) with random hexamer according to the manufacturer’s instructions.
Degenerated primers were designed for the conserved regions of Bdnf, Glua1 and Glua2 and Gapdh using the known sequences from Mus musculus, Xenopus tropicalis, Gallus gallus, and Danio rerio. The following primers were used to amplify the coding sequences of Bdnf forward primer (5’- CCTKTTCSTTACTATGGTTATYTCAT −3’) and reverse primer (5’- CTGCCCCTCTTAA TGGTYARTGTRC −3’); AMPA receptor subunits Glua1 and Glua2: Glua1 forward primer (5’- YATMGTYGGHGGYGTCTGGTGGTTCTT −3’) and reverse primer (5’- YTTCAT GGTGTCACARGGYTTBC −3’), and Glua2 forward primer (5’- TCTAGAGGAGT TTTTGCCATTTTTG −3’) and reverse primer (5’- TYCTRACTAGGAAYARGACCAC −3’), and Gapdh: forward primer (5’- CCYTTCATCGACCTGSASTA −3’) and reverse primer (5’- GGATGACCTTGCCSACAG −3’). Fragments for PCR were purified from agarose gels with Zymoclean Gel DNA Recovery kit (Zymo Research), cloned into pGEM®-T System plasmid (Promega) according to instructions from the manufacturer and sequenced (Iowa State University Sequencing Facility). All sequences were submitted to and published in GenBank, and the Accession numbers for Bdnf, Gapdh, Glua1 and Glua2 are: bankit1276240 GU108573, bankit1276246 GU108574, bankit1276252 GU108575 and bankit1276255 GU108576 respectively.
2.4. Total RNA isolation and quantitative PCR (qPCR)
Fish crania were sliced coronally at 300 μm in a temperature controlled (−12°C) cryostat (Leica-Jung 1800). Brain slices were thaw-mounted onto glass microscope slides and individual tissue samples were microdissected with a 500 μm diameter punch [6, 33, 39]. The dorsolateral pallium (DL) was chosen for analysis as this region is homologous to the hippocampus in higher vertebrates, including mammals and birds [69–73]. As our model utilizes trace conditioning, and learning to use the escape hole during aggressive social interaction is a spatial learning task, both hippocampally dependent tasks, the dorsolateral pallium represents the brain region most likely involved.
Total RNA was isolated from the DL of the Rainbow trout Oncorhynchus mykiss using the RNeasy Mini kit System (Qiagen), and RNA was reverse transcribed with the SuperScript® First-Strand Synthesis System for RT-PCR (Invitrogen) with random hexamer according to the manufacturer’s instructions. Water was used instead of RNA in the negative control no-template reaction. Water was used instead of enzyme in the negative control no-enzyme reactions. Gene-specific primers and probes were created for Oncorhynchus mykiss Bdnf, Glua1, Glua2 and Gapdh mRNAs using the Primer Express Software (Applied Biosystems, Inc.; Table 1). Specificity of designed qPCR assays was tested by agarose gel electrophoresis. Assays for efficiency for qPCR were tested by serial dilutions of templates, and was 97.8% for Bdnf, 96.3% for Glua1, 98.2% for Glua2 and 102.3% for Gapdh.
Table 1.
Sequences of primers and probes used for analysis of Rainbow trout mRNA expression by quantitative real-time PCR. Polymerase chain reaction fragments from degenerate primer reactions were purified from agarose gels, cloned into plasmids and sequenced. From these sequences, gene-specific primers and probes were created for genes of interest using Primer Express Software (Applied Biosystems, Inc.).
| Target | Forward primer | Reverse primer | MGB Probe |
|---|---|---|---|
| Gapdh | ATGTATGAAGCCCCATGAATCC | GAAATGGGAAGAGGCCTTGTC | CCGGTGCTGATTACGTCGTTGAGTCC |
| Bdnf | GATCAGCAACCAAGTGCCTTTA | GCCGCCGTACCCTCATG | CCACCGCTGCTTTTTCTCCT |
| Glua1 | AAGGAGTTTTTCAGGAGGTCCAA | CCCCTCCGCTGTGGTTTT | CCGTATTTGAGAAGATGTGGTCTTACA |
| Glua2 | ATACGAGGGCTACTGTGTCGATTT | CTCGGGCTCCATATTTTCCA | CCGCTGAGATAGCCAAGCACTGTGG |
Real time RT-PCR was performed using 50 ng of cDNA per reaction combined with primer/probe sets and TaqMan® Gold RT-PCR Master Mix (Applied Biosystems, Inc.). Real time assays were run on an ABI 7000 (Applied Biosystems, Inc.). The real-time PCR profile consisted of one cycle at 50°C for 2 min and 95°C for 10 min, followed by 45 cycles at 95°C for 15 s and 60°C for 1 min. All reactions were run in duplicate and results from individuals averaged. Water was used instead of cDNA in the negative no-template control reactions. No-template and no-enzyme negative controls from the reverse transcription step were used to eliminate false-positive results.
Gene expression was normalized to Gapdh, and the relative quantity of mRNAs was calculated based on the comparative Ct method. TaqMan RT-PCR values for Gapdh were subtracted from Bdnf [74], Glua1 and Glua2 values, and fold expression calculated by the 2−ΔΔCT method [75]. The resulting corrected values were used for comparisons across the experimental groups.
2.5. Statistical Analyses
Statistical analyses and experimental designs were based on a priori hypotheses, for the purpose of avoiding combinatorial exponential expansion of error from multiple tests [76]. This statistical pre-planning allows for a wider range of multiple comparison analyses across hypothetical designs. A level of 0.05 was set as the limit for statistical significance. Two-way ANOVA (Drug [Antalarmin or saline] × Phenotype [Escape, Stay] design) was used to examine the contribution of drug effects relative to behavioral phenotype expression (Stay × Escape). For behavioral results, comparison of latency to escape was made using repeated measures one-way ANOVA. Evaluations of gene expression among un-treated groups were assessed by one-way ANOVA. Comparisons between two conditions within a given phenotype (Escape or Stay) were investigated by Student’s t-tests.
Each animal was a singular sample source, from which multiple measures and analyses were taken. Five assumptions of parametric statistics were applied to the data (Random and Equal samples, Normal distribution of data, Homogeneity of variance [similar homoscedasticity], Independence of data for different groups, Interval level data – linearity), which were transformed, when necessary, but also compared to non-parametric analyses, and graphed in their raw form. Analyses with both non-parametric and parametric statistics were performed along with examination for multiple comparisons using the Holm-Sidak method, and when the statistical analyses match, as they do for the data herein, we report the parametric results without α adjustment [77–82] based on a priori hypothesis driven exclusion from combinatorial effects [76]. Significant effects between groups for one-way analyses were examined with Student–Newman–Keuls post hoc analyses (to minimize Type I error) and Duncan’s Multiple Range Test (to minimize Type II error).
3. Results
3.1. Expression of Escape and Stay phenotypes
As with previous experiments in trout and mice, the division of behavioral phenotypes, Escape and Stay, was near 50% for each [6, 18, 19] (Figure 2A). However, when considering just Stay trout after treatment with the CRF1 antagonist, which on days prior to Antalarmin exhibited 0% Escape behavior, now include 67% Escape animals (Day 4, χ2: p < 0.022; Figure 2B). There were 33% of Antalarmin-treated Stay trout that did not make use of the escape route.
Fig. 2.

The CRF1 antagonist Antalarmin shifts pro-stress behavior and neuroendocrine responsiveness toward anti-stress phenotypes and outcomes. (A) Individual trout originally exhibiting Stay or Escape phenotypes were roughly equivalent: 45% Escape, 55% Stay. While antalarmin treatment did not affect the behavioral phenotype of Escape fish (data not shown), (B) most Stay animals (67%) began, and continued, using the escape route following CRF1 antagonist treatment on day 4. A minority (33%) of Stay fish continued to Stay following antalarmin treatment.
3.2. The effect of CRF1 antagonist on plasma cortisol concentrations
Measuring blood samples taken 3 days prior to SAM social interaction (left bars, Figure 3) all fish had similar plasma cortisol concentrations ([F], ~15 ng/ml), without significant differences between future group status (Escape vs Stay: F1,20 = 0.057, p > 0.81; Saline Control vs Antalarmin: F1,20 = 2.63, p ≥ 0.12; Interaction: F1,20 = 0.19, p > 0.73; Figure 3). After 7 days of social interaction, and blood samples taken again on day 8, when no large aggressor was present (US−), but the cue (CS+, water off) was given, plasma cortisol was significantly elevated (~50 ng/ml; Escape vs Stay: F1,20 = 25.6, p < 0.001) in Stay trout (right bars, Figure 3). This conditioned response (CR) is not due to elevated cortisol remaining from social aggression on day 7, but has been previously demonstrated to result from Pavlovian Conditioning, a response to the CS on day 8 (when the US is absent) [6]. After treatment with the CRF1 antagonist, Escape fish had cortisol concentrations on day 8 that were not significantly greater than in fish measured 3 days prior to social interaction. What is more, Stay fish that became Escape fish following Antalarmin treatment, also did not show elevated plasma cortisol concentrations (~15 ng/ml). However, Stay trout that remained Stay, did have statistically elevated plasma cortisol (~50 ng/ml) compared with pretreatment Stay saline control [F] (t10 = 4.73, p < 0.0008), compared to all controls (t31 = 4.73, p < 0.000001) and compared to trout treated with Antalarmin that Escape (t7 = 3.64, p < 0.008; Figure 3).
Fig. 3.

Plasma cortisol concentration [F] was similar in all fish before treatments or group placement (blood drawn 3 days prior to SAM exposure and 7 days before drug treatment; left bars). In the same animals, following all 7 days of SAM exposure, saline-treated control Stay fish without antalarmin treatment (hatched white bar) produced a significant physiological conditioned response (CR), elevated plasma [F], in response to the CS only (water off; no aggression) on day 8 (from a second blood draw), compared to saline-treated control Escape fish (white bar). Day 4 CRF1 receptor antagonist treatment (gray bars) eliminated increased [F] in Stay fish that Escape from day 4 onward (St/Esc; gray right-down hatched bar), but not in Stay fish that retained the Stay phenotype (St/St), which had elevated plasma [F] compared to fish treated with Antalarmin that Escape (*), and fish that began as Stay trout then Escape after Antalarmin pretreatment (*), Pre-SAM Stay controls [F] (+), and compared to all controls (#).
3.3. Latency to escape
In fish initially learning to escape, latency to escape dramatically improves with each trial, regardless of treatment with CRF1 antagonist (Figure 4 solid line, F6,57 = 24.37, p < 0.001; compared to dashed line, F6,22 = 13.25, p < 0.001). However, a significant portion (66%) of Stay trout showed Escape behavior following CRF1 antagonist exposure (Figure 4 dotted line). Importantly, initial Antalarmin-induced Escape (on day 5) in Stay fish was more rapid than any Escape on day 1, with or without drug treatment. Day 5 Escape latencies are identical in Stay Antalarmin-treated, Escape Antalarmin-treated, and saline-treated control Escapers (Figure 4 compare dotted, dashed, and solid lines). This suggests that non-escaping fish (Stay) learn the location of the escape hole during the first 4 training sessions, but do not utilize the option. Treatment with the CRF1 antagonist allowed expression of escape behavior. Importantly, treatment with this anxiolytic drug also allowed a trade-off in learning modalities, with fish switching from submission to learned escape.
Fig. 4.
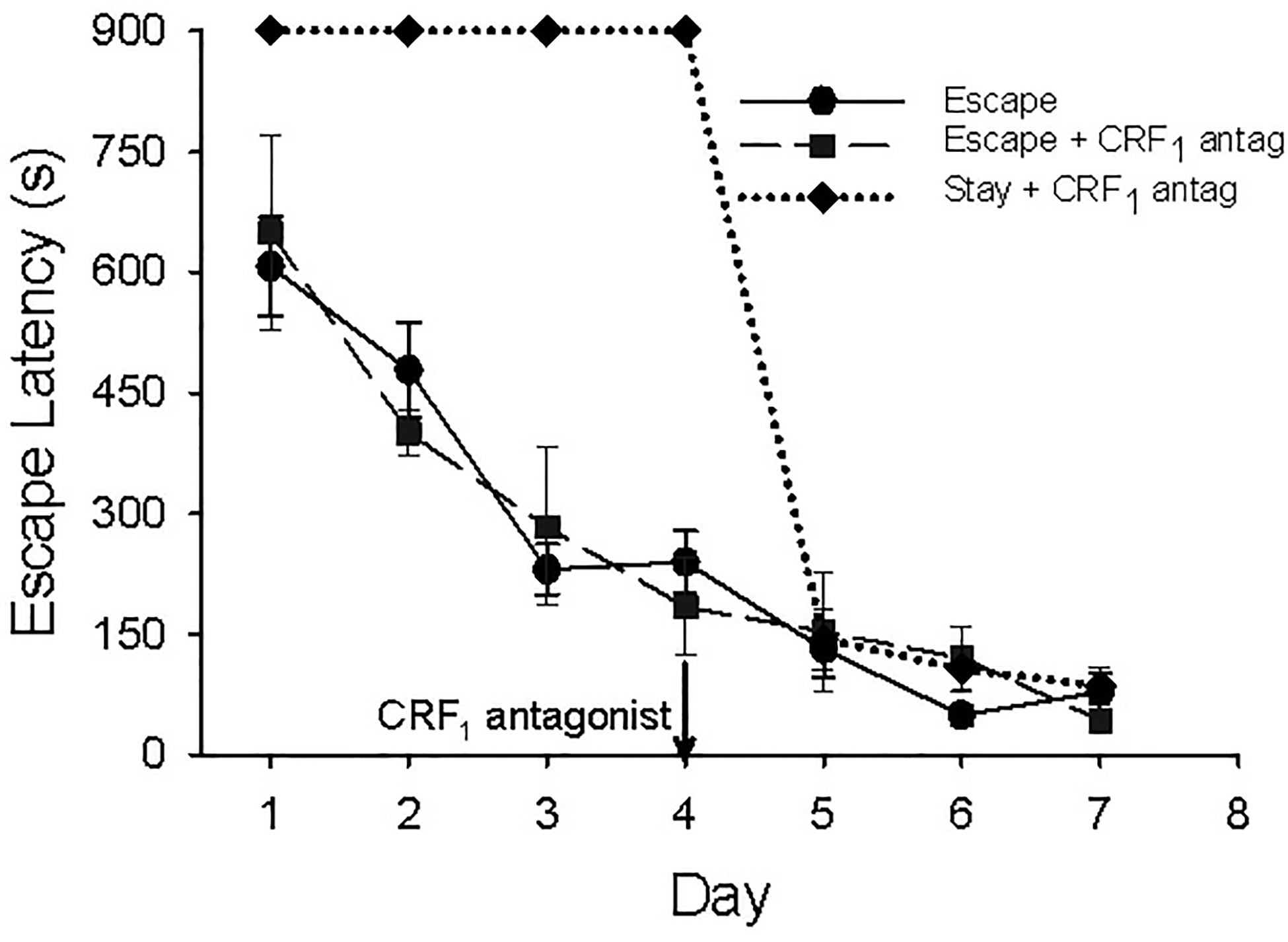
Latency to escape (mean ± SEM) over 7 days SAM social interaction with CS:US pairings. Black line with circles indicates escape latency in seconds for saline-treated control fish that escaped during social interactions. Black hashed line with squares indicates fish that escaped during the first 4 training sessions and were treated with CRF1 receptor antagonist on day 4. Diamonds with dotted line indicates fish that did not escape during the first 4 training sessions, were treated with CRF1 receptor antagonist on day 4 and began escaping.
3.4. Gene expression
After 7 days of SAM social interaction, on day 8 following presentation of the CS alone on test day, hippocampal levels of Bdnf mRNA were significantly higher in escaping fish (two way ANOVA-Phenotype: F1,17 = 6.64, p ≤ 0.02; -saline vs CRF1 antagonist treatment: F1,17 = 64.98, p < 0.001; -phenotype X saline or CRF1 antagonist interaction: F1,17 = 6.13, p ≤ 0.024; one way ANOVA-CS & US controls vs saline-treated: F3,14 = 20.09, p < 0.001; one way ANOVA- CS & US controls vs Antalarmin-treated: F3,17 = 0.55, p > 0.65; Figure 5A). In addition, because control groups on day 8 for the CS (water off) and US also exhibited unelevated Bdnf expression relative to the escaping fish, aggression (experienced by US only, Escape and Stay animals) did not promote Bdnf transcription. Pairing of the CS with aggression (US) occurred only in saline- or Antalarmin-treated Stay and Escape groups; the only groups for which elevated Bdnf mRNA are elevated are saline-treated (Figure 5A). Thus, conditioning is evident for elevated Bdnf expression in both Escape and Stay phenotypes [64]. It is important to note, that elevated Bdnf gene expression was blocked by anxiolytic treatment by means of CRF1 antagonism.
Fig. 5.
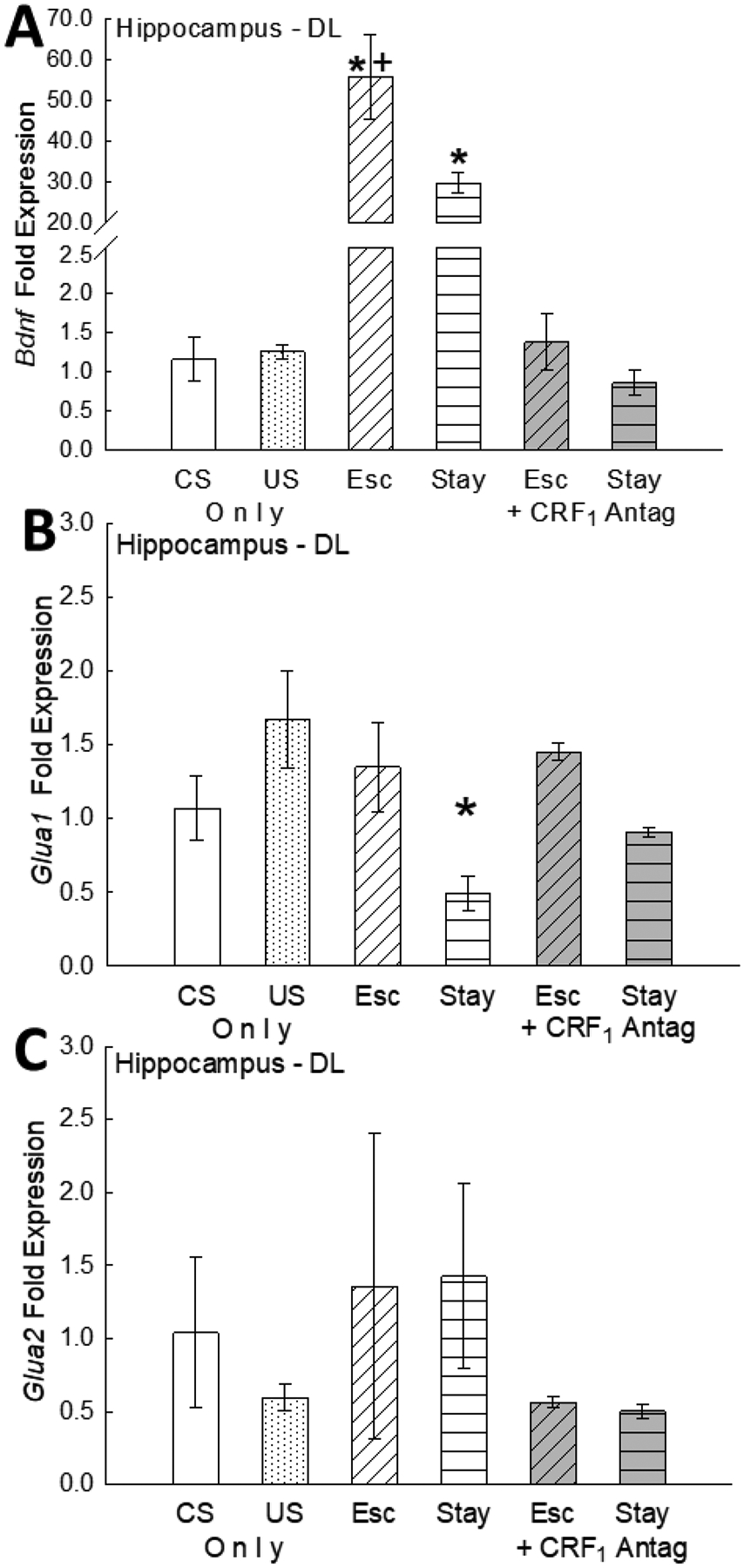
(A) Hippocampal Bdnf gene expression (mean fold ± SEM) is significantly but differentially enhanced in saline-treated control Escape (Esc; white tilt-hatched bar) vs saline-treated control Stay (white flat-hatched bar) fish following CS only presentation on test day (day 8). Escape and Stay fish expressed significantly elevated (*) Bdnf gene expression compared to individuals that received the conditioned stimulus only (CS; water off; white bar), to individuals receiving daily exposure to the unconditioned stimulus only (US; aggression from larger, novel conspecific; white dotted bar), and to Escape and Stay fish treated (day 4) with the CRF1 antagonist antalarmin (gray bars). Importantly, Escape Bdnf mRNA levels were also significantly (+) elevated compared to Stay fish; and stimulated hippocampal Bdnf gene expression is reversed by treatment with the CRF1 antagonist antalarmin. (B) Hippocampal Glua1 gene expression is significantly (*) reduced in saline-treated control Stay fish following presentation of the CS alone on test day compared to all other groups. Importantly Reduced hippocampal Glua1 mRNA expression is reversed following treatment with the CRF1 antagonist antalarmin. (C) Hippocampal Glua2 gene expression is not influenced by presentation of the CS only on test day. Following presentation of the CS only on test day (day 8), no differences in Glua2 mRNA were detected between groups. Additionally, treatment with the CRF1 antagonist did not influence Hippocampal GluA2 mRNA expression following presentation of the CS only on test day (day 8).
Levels of the AMPA receptor subunit Glua1 expression were significantly lower in Stay (non-escaping) fish compared to Escape fish (two way ANOVA-Phenotype: F1,21 = 14.0, p < 0.001; -saline or CRF1 drug treatment: F1,21 = 1.9, p > 0.18; -phenotype X saline or CRF1 antagonist interaction: F1,21 = 0.69, p > 0.41; one way ANOVA- CS & US controls vs saline-treated: F3,22 = 3.77, p ≤ 0.025; one way ANOVA- CS & US controls vs Antalarmin-treated: F3,19 = 1.42, p > 0.28; Figure 5B), while no difference was recorded in Glua2 subunit gene expression (two way ANOVA-Phenotype: F1,18 = 0.000, p > 0.99; - saline or CRF1 drug treatment: F1,18 = 2.13, p > 0.16; -phenotype X saline or CRF1 antagonist interaction: F1,18 = 0.012, p > 0.91; one way ANOVA- CS & US controls vs saline-treated: F3,20 = 0.27, p > 0.84; one way ANOVA- CS & US controls vs saline-treated: F3,20 = 0.73, p > 0.54; Figure 5C). Classical conditioning also appears to be also true for inhibited expression of Glua1 (Figure 5B). Additionally, inhibited Glua1 gene expression was blocked by CRF1 antagonism, an anxiolytic treatment.
4. Discussion
The Stress Alternatives Model is chronic-repetitive social stress paradigm that produces two distinctive phenotypes, Escape and Stay, through behavioral, genetic, and signaling neuroplasticity. Behaviorally, active avoidance (Escape) or passively accepting confrontation (Stay) fits well with stress coping strategies present numerous species [27–30, 83]. The Escape and Stay phenotypes appear to be evolutionarily conserved behavioral representations of a balance between pro-stress and anti-stress circuitries in the limbic regions of the brain [21, 64, 66, 67]. Similar outcomes for behavior and neuroendocrine regulation derived from SAM experimentation occur in fish (Figures 1–5)[6, 21], hamsters [84], mice [19, 20, 64–66, 85, 86], and rats [18]. The similarities in these outcomes include: early behavioral phenotypic stability, even distribution of Stay and Escape phenotypes, classically conditioned stress-related responses in both Stay and Escape animals, but with demonstrably greater conditioning, both cued and contextual, in the Stay phenotype, and numerous learned responses underlying specific phenotypic behavior. Another important evolutionarily conserved quality of outcomes derives from plasticity in the stress-related neurocircuitry that produces the phenotypes, such that anxiolytic treatments (including CRF1 or Orx1 receptor antagonism, Orx2 receptor stimulation, Neuropeptide S, exercise) transforms Stay into Escape animals, or modifies behavior toward pro-Escape consequences (such as reducing escape latency) in mice [20, 64, 66] and trout (see Figures 2–4) [6, 21]. In mice, anxiogenic treatments (including the α2-adrenoreceptor antagonist yohimbine, and Orx1 receptor stimulation) transforms Escape into Stay animals, or modifies behavior to be more Stay-oriented [20, 64]. We posit that the critical elements producing the evolutionarily conservative phenotypes, Escape and Stay, devolve from neuroplasticity of stress circuitry signaling and gene expression.
Here we show that both saline-treated stress-resilient Escape and stress-susceptible Stay fish exhibit elevated Bdnf gene expression in response to socially stressful interaction, and both groups show learning. Classical conditioning and spatial learning are clearly evident for both groups, although Stay fish only express the spatial Escape task upon relief of social aggression-induced stress responsiveness, via the CRF1 receptor antagonist antalarmin [20]. We have previously also demonstrated social learning in fish using this model [21]. Additionally, SAM social stress plus classical conditioning modifies hippocampal gene expression in a phenotype dependent manner (Figure 5). Similar results from recent work on mice from our lab demonstrates phenotype-dependent gene expression neuroplasticity in the basolateral amygdala (BLA) and hippocampus following SAM + conditioning [64, 86]. In submissive-Stay trout conditioned to an aggressive social interaction, hippocampal BDNF gene expression increases while GluA1 expression is reduced, and both effects disappear following CRF1 receptor antagonism-induced behavioral reversal: when Stay fish become Escape fish. At the same time, while saline-treated Escape fish have significantly elevated Bdnf expression, these mRNA levels are also statistically less than those of saline-treated Stay fish. Additionally, Escape fish show no change in GluA1 mRNA. Interestingly, the concentration of the stress hormone cortisol ([F]), low in all groups prior to SAM interaction (Figure 3), and elevated by social aggression [39] in the SAM, is only increased in plasma concentration on day 8 following the CS alone [= conditioned response, CR [6]] in saline-treated Stay trout. After CRF1 receptor antagonist treatment, Escape animals [F] remains low, and Stay animals that become Escape fish, also have un-stimulated [F]. However, plasma cortisol concentrations in Stay fish treated with Antalarmin that do not switch to Escape behavior are also significantly elevated (Figure 3).
As CRF1 antagonism has a response dependent on phenotype × behavior with respect to plasma [F], we are left to surmise that the stress neurocircuitry that regulates behavior and that controls the neuroendocrine stress response are tightly linked and synchronized. Trout that do not show a behaviorally anxiolytic response to Antalarmin (Escape), and remain Stay animals, appear to be unable to limit stress hormone responsiveness in response to the CS (Figure 3). On the other hand, fish that were stress-susceptible Stay animals, who in response to the CRF1 antagonist treatment switched phenotypes to Escape, also no longer required elevated plasma [F], and did not exhibit this stress response (Figure 3). This distinction in [F] response, suggests that some neuroendocrine trigger, perhaps [CRF], CRF2 receptors, or arginine vasotocin (AVT), must have remained capable of stimulating a neuroendocrine stress response [87–89] despite the inhibition of CRF1 receptors. The results also suggest that a subset of the trout Stay phenotype population remains somewhat insensitive to anxiolytic or antidepressive treatments, similar to human populations [90–96].
It is important that elevation of BDNF expression is measured in both groups of saline-treated and conditioned Escape and Stay fish. However, only saline-treated Stay fish exhibit a significant decrease in GluA1 gene expression. Similarly, the plasma stress hormone cortisol is also only elevated in Stay trout. In all cases the anti-stress CRF1 receptor antagonist Antalarmin blocked the effect (Figures 3, 5). For plasma [F], we have verified a CR in elevated concentrations [6], but the elevation in BDNF and reduction in GluA1 gene expression resulted from the same protocol. This protocol, however, did not affect GluA2, at the times measured. The data suggest that conditioned responses in BDNF and GluA1 mRNA are coincident with the CR for [F]. The role for BDNF and GluA1 in classical conditioning have been demonstrated in other species [50, 51, 97–104]. During turtle eye blink conditioning, BDNF and extracellular signal-regulated kinases (ERK) are necessary for early and late acquisition, leading to upregulation, trafficking, and synaptic localization of GluA1 subunits, followed by GluA4 subunits [50, 51, 97, 103–105]. By the time of retention and memory expression, GluA1 subunit expression is decreasing, while GluA4 expression remains elevated [50, 51, 97, 103–105]. These results fit well with our experiments, since BDNF and GluA1 gene expression was measured at the time of the conditioned response (day 8), but also suggests that future experiments should examine trafficking and expression of GluA4 subunits. Our own recent work in mice demonstrates behavioral conditioned responses in both Stay and Escape mice, with BDNF expression elevated in both phenotypes, but regulated specifically by phenotype through orexin 1 or orexin 2 receptor mechanisms [64]. Our data in trout suggest that similar mechanisms for BDNF-regulated neuroplasticity and AMPA receptor trafficking may occur during fear conditioning.
Since both Escape and Stay groups learned the position of the escape hole, and experienced significantly greater elevation of BDNF coincident with expression of conditioning, potentially, the anxiolytic effect of the CRF1 antagonist treatment inhibits the conditioned stimulation of BDNF expression and inhibition of GluA1 expression (Figure 5). The CRF1 antagonism can release behavioral expression of learned escape, even while this treatment blocks the conditioned responses of increasing BDNF and reduced GluA1 expression levels in newly escaping fish. This suggests that the development of fear conditioning both promoted the expression of BDNF and inhibited expression of GluA1.
Initially, we hypothesized that reduced, or smaller increase in, hippocampal BDNF expression was directly related to the absence of learned escape. However, following treatment with the CRF1 antagonist on day 4, most treated Stay fish begin escaping, with latencies equivalent to the original escaping group after 5 days of learning (Figure 4 dotted line). This suggests Stay (non-escapers) fish learn the location of the escape hole, but do not express escape behavior until after treatment with the CRF1 antagonist. By the time these antalarmin-treated fish would potentially express the BDNF-related CR, following presentation of the CS only on day 8, BDNF mRNA is not different from controls. In all Stay fish treated with the CRF1 antagonist, both BDNF and GluA1 mRNA levels are similar to those in CS only controls (Figure 5). Therefore, inhibiting this stress neuromodulator at CRF1 receptors reverses not only behavior, but also the conditioned stimulation or inhibition of genes associated with neural plasticity and learning. We have argued that inhibitory modulation of the pro-stress elements of emotional neurocircuitry, via CRF or orexin receptor agents, has implications for affective disorders such as PTSD [64, 66, 67, 85]. In our model, fish [or mice [20]] effectively learn an adaptive spatial task that allows for relief from aggressive trauma, but stress-susceptible Stay individuals are impaired from accessing this relief due to unremitting stress responsiveness, similar to what occurs in post-traumatic stress disorder (PTSD) patients [106, 107]. Anxiolytic drugs, that act directly on specific CRF or orexinergic elements of the stress neurocircuitry involved, have been demonstrated in our SAM protocol to allow stress-vulnerable animals to avoid stressful conditions and responses [20, 64–66], which may prove useful for patients with PTSD, anxiety, and depression.
It is important to note that BDNF expression is rapidly and selectively induced by learning dependent on the hippocampus [45], as is synaptic GluA1, which are influenced by glucocorticoid stress hormones and their receptors [108, 109]. Additionally, hippocampal BDNF controls synaptic increases in GluA1 subunits necessary for memory consolidation in inhibitory avoidance [110]. In our study, elevated [F] is measured only in saline-treated, non-escaping Stay fish and reversed by CRF1 antagonism in those that begin to Escape, but not by those that don’t. Concurrent with increased [F], BDNF mRNA levels are elevated by social stress, more in Escape than Stay fish, and also reversed by CRF1 antagonism in both groups. Finally, stress induced by social aggression alone (which does promote elevated glucocorticoid levels) does not influence BDNF mRNA levels (Fig. 4A; US only, dotted bar).
5. Conclusions
Utilizing the behavioral strategy of “learned escape” circumvents conditioned increases in plasma cortisol and regionally specific central monoamine activity seen in non-escaping individuals [6]. Concurrent conditioned stress-responsive cortisol concentrations, as well as stimulation of BDNF and inhibition of GluA1 gene expression in the hippocampus, suggest a suite of molecular, neural, and hormonal changes specific to expression of particular learning modalities. It is significant that this distinctive gene expression of both BDNF and GluA1, but not GluA2, is coincident with adoption of mutually exclusive, but equally adaptive, behavioral coping strategies. Additionally, CRF1 antagonist treatment reverses these conditioned responses, while stimulating this shift in behavioral strategy, from submissive non-escape (Stay), to learned Escape. Taken together, these data suggest that the CRF system, via the CRF1 receptor, mediates behavioral response to aggressive social interaction [39] and influences gene expression in the hippocampus during conditioning to a socially relevant and powerful fear-inducing stimulus. Moreover, the conditioned responses to a social context in this aquatic vertebrate suggest evolutionary conservation of fear-learning systems and strategies.
Acknowledgements
We would like to thank Craig Bockholt and the Gavins Point Fish Hatchery for their donation of time and resources, without which, these experiments would not have been possible. Further, we would like to thank Drs. R. Parrish Waters and Pat Ronan for their comments on this manuscript. Additionally, we acknowledge and commend efforts in the scientific community that stand up against discrimination and social injustices. Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health, USA, under Award Numbers R15 MH125306, R15 MH104485 and P20 RR15567, through an National Science Foundation (NSF) Doctoral Dissertation Improvement Grant to REC UA0900114, by an NSF grant IOS 0950602. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, NSF, the Department of Veterans Affairs or the United States Government. The opinions and assertions expressed herein are those of the author(s) and do not reflect the official policy or position of the Uniformed Services University of the Health Sciences or the Department of Defense.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT authorship contribution statement
Author Contributions
Russ E. Carpenter: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing – Original Draft, Writing – Review & Editing, Visualization.
Boris Sabirzhanov: Methodology, Validation, Formal analysis, Investigation, Writing – Review & Editing.
Tangi R Summers Conceptualization, Methodology, Validation, Formal analysis, Writing – Original Draft, Writing – Review & Editing, Visualization.
Timothy G. Clark: Conceptualization, Methodology, Validation, Supervision, Project Administration, Funding Acquisition.
Joyce Keifer: Methodology, Validation, Formal analysis, Writing – Review & Editing, Supervision, Project Administration, Funding Acquisition.
Cliff H Summers: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing – Original Draft, Writing – Review & Editing, Visualization, Supervision, Project Administration, Funding Acquisition.
All authors approved the final version for publication.
Conflict of Interest
Authors report no conflict of interest
References
- [1].Alvarez RP, Biggs A, Chen G, Pine DS, Grillon C, Contextual fear conditioning in humans: cortical-hippocampal and amygdala contributions, The Journal of neuroscience : the official journal of the Society for Neuroscience 28(24) (2008) 6211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rosen JB, Fanselow MS, Young SL, Sitcoske M, Maren S, Immediate-early gene expression in the amygdala following footshock stress and contextual fear conditioning, Brain research 796(1–2) (1998) 132–42. [DOI] [PubMed] [Google Scholar]
- [3].Blanchard RJ, Nikulina JN, Sakai RR, McKittrick C, McEwen B, Blanchard DC, Behavioral and endocrine change following chronic predatory stress, Physiology & behavior 63(4) (1998) 561–9. [DOI] [PubMed] [Google Scholar]
- [4].Wallace KJ, Rosen JB, Predator odor as an unconditioned fear stimulus in rats: elicitation of freezing by trimethylthiazoline, a component of fox feces, Behavioral neuroscience 114(5) (2000) 912–922. [DOI] [PubMed] [Google Scholar]
- [5].Endres T, Fendt M, Conditioned behavioral responses to a context paired with the predator odor trimethylthiazoline, Behavioral neuroscience 121(3) (2007) 594–601. [DOI] [PubMed] [Google Scholar]
- [6].Carpenter RE, Summers CH, Learning strategies during fear conditioning, Neurobiology of learning and memory 91(4) (2009) 415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Korzan WJ, Hoglund E, Watt MJ, Forster GL, Overli O, Lukkes JL, Summers CH, Memory of opponents is more potent than visual sign stimuli after social hierarchy has been established, Behavioural brain research 183(1) (2007) 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Takemoto M, Song WJ, Cue-dependent safety and fear learning in a discriminative auditory fear conditioning paradigm in the mouse, Learning & memory 26(8) (2019) 284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Takahashi LK, Olfactory systems and neural circuits that modulate predator odor fear, Front Behav Neurosci 8 (2014) 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Radley JJ, Arias CM, Sawchenko PE, Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress, The Journal of neuroscience : the official journal of the Society for Neuroscience 26(50) (2006) 12967–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jackson ED, Payne JD, Nadel L, Jacobs WJ, Stress differentially modulates fear conditioning in healthy men and women, Biological psychiatry 59(6) (2006) 516–22. [DOI] [PubMed] [Google Scholar]
- [12].Kozicz T, Bordewin LA, Czeh B, Fuchs E, Roubos EW, Chronic psychosocial stress affects corticotropin-releasing factor in the paraventricular nucleus and central extended amygdala as well as urocortin 1 in the non-preganglionic Edinger-Westphal nucleus of the tree shrew, Psychoneuroendocrinology 33(6) (2008) 741–54. [DOI] [PubMed] [Google Scholar]
- [13].Dunmore E, Clark DM, Ehlers A, Cognitive factors involved in the onset and maintenance of posttraumatic stress disorder (PTSD) after physical or sexual assault, Behav Res Ther 37(9) (1999) 809–29. [DOI] [PubMed] [Google Scholar]
- [14].Diamond DM, Rose GM, Stress impairs LTP and hippocampal-dependent memory, Ann.N.Y.Acad.Sci 746 (1994) 411–414. [DOI] [PubMed] [Google Scholar]
- [15].Koolhaas JM, De Boer SF, De Rutter AJ, Meerlo P, Sgoifo A, Social stress in rats and mice, Acta physiologica Scandinavica. Supplementum 640 (1997) 69–72. [PubMed] [Google Scholar]
- [16].Summers CH, Forster GL, Korzan WJ, Watt MJ, Larson ET, Overli O, Hoglund E, Ronan PJ, Summers TR, Renner KJ, Greenberg N, Dynamics and mechanics of social rank reversal, Journal of comparative physiology. A, Neuroethology, sensory, neural, and behavioral physiology 191(3) (2005) 241–52. [DOI] [PubMed] [Google Scholar]
- [17].Yehuda R, Cai G, Golier JA, Sarapas C, Galea S, Ising M, Rein T, Schmeidler J, Muller-Myhsok B, Holsboer F, Buxbaum JD, Gene expression patterns associated with posttraumatic stress disorder following exposure to the World Trade Center attacks, Biological psychiatry 66(7) (2009) 708–11. [DOI] [PubMed] [Google Scholar]
- [18].Robertson JM, Prince MA, Achua JK, Carpenter RE, Arendt DH, Smith JP, Summers TL, Summers TR, Summers CH, Nuance and behavioral cogency: How the Visible Burrow System inspired the Stress-Alternatives Model and conceptualization of the continuum of anxiety, Physiology & behavior 146 (2015) 86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Smith JP, Achua JK, Summers TR, Ronan PJ, Summers CH, Neuropeptide S and BDNF gene expression in the amygdala are influenced by social decision-making under stress, Front Behav Neurosci 8(121) (2014) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Smith JP, Prince MA, Achua JK, Robertson JM, Anderson RT, Ronan PJ, Summers CH, Intensity of anxiety is modified via complex integrative stress circuitries, Psychoneuroendocrinology 63 (2016) 351–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Summers TR, Summers TL, Carpenter RE, Smith JP, Young SL, Meyerink B, Orr TZ, Arendt DH, Summers CH, Learning and CRF-Induced Indecision during Escape and Submission in Rainbow Trout during Socially Aggressive Interactions in the Stress-Alternatives Model, Frontiers in neuroscience 11 (2017) 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Blanchard DC, Summers CH, Blanchard RJ, The role of behavior in translational models for psychopathology: functionality and dysfunctional behaviors, Neuroscience and biobehavioral reviews 37(8) (2013) 1567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Keifer J, Summers CH, Putting the “Biology” Back into “Neurobiology”: The Strength of Diversity in Animal Model Systems for Neuroscience Research, Front Syst Neurosci 10 (2016) 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Shansky RM, Sex differences in PTSD resilience and susceptibility: Challenges for animal models of fear learning, Neurobiology of stress 1 (2015) 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Armstrong T, Federman S, Hampson K, Crabtree O, Olatunji BO, Fear Learning in Veterans With Combat-Related PTSD Is Linked to Anxiety Sensitivity: Evidence From Self-Report and Pupillometry, Behav Ther 52(1) (2021) 149–161. [DOI] [PubMed] [Google Scholar]
- [26].Conoscenti MA, Fanselow MS, Dissociation in Effective Treatment and Behavioral Phenotype Between Stress-Enhanced Fear Learning and Learned Helplessness, Front Behav Neurosci 13 (2019) 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Koolhaas JM, de Boer SF, Buwalda B, van Reenen K, Individual variation in coping with stress: a multidimensional approach of ultimate and proximate mechanisms, Brain, behavior and evolution 70(4) (2007) 218–26. [DOI] [PubMed] [Google Scholar]
- [28].Koolhaas JM, de Boer SF, Coppens CM, Buwalda B, Neuroendocrinology of coping styles: towards understanding the biology of individual variation, Frontiers in neuroendocrinology 31(3) (2010) 307–21. [DOI] [PubMed] [Google Scholar]
- [29].Koolhaas JM, Korte SM, de Boer SF, van der Vegt BJ, van Reenen CG, Hopster H, de Jong IC, Ruis MA, Blokhuis HJ, Coping styles in animals: current status in behavior and stress physiology, Neuroscience and biobehavioral reviews 23(7) (1999) 925–935. [DOI] [PubMed] [Google Scholar]
- [30].Øverli Ø, Sorensen C, Pulman KG, Pottinger TG, Korzan W, Summers CH, Nilsson GE, Evolutionary background for stress-coping styles: Relationships between physiological, behavioral, and cognitive traits in non-mammalian vertebrates, Neurosci.Biobehav.Rev 31(3) (2007) 396–412. [DOI] [PubMed] [Google Scholar]
- [31].Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF, Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development, Neuron 20(6) (1998) 1093–102. [DOI] [PubMed] [Google Scholar]
- [32].Carpenter RE, Maruska KP, Becker L, Fernald RD, Social opportunity rapidly regulates expression of CRF and CRF receptors in the brain during social ascent of a teleost fish, Astatotilapia burtoni, PloS one 9(5) (2014) e96632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Carpenter RE, Watt MJ, Forster GL, Overli O, Bockholt C, Renner KJ, Summers CH, Corticotropin releasing factor induces anxiogenic locomotion in trout and alters serotonergic and dopaminergic activity, Hormones and behavior 52(5) (2007) 600–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Backström T, Winberg S, Central corticotropin releasing factor and social stress, Frontiers in neuroscience 7 (2013) 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Huising MO, Metz JR, van Schooten C, Taverne-Thiele AJ, Hermsen T, Verburg-van Kemenade BM, Flik G, Structural characterisation of a cyprinid (Cyprinus carpio L.) CRH, CRH-BP and CRH-R1, and the role of these proteins in the acute stress response, J Mol Endocrinol 32(3) (2004) 627–48. [DOI] [PubMed] [Google Scholar]
- [36].Walker CD, Perrin M, Vale W, Rivier C, Ontogeny of the stress response in the rat: role of the pituitary and the hypothalamus, Endocrinology 118(4) (1986) 1445–1451. [DOI] [PubMed] [Google Scholar]
- [37].Potter E, Behan DP, Linton EA, Lowry PJ, Sawchenko PE, Vale WW, The central distribution of a corticotropin-releasing factor (CRF)- binding protein predicts multiple sites and modes of interaction with CRF, Proc.Natl.Acad.Sci.U.S.A 89(9) (1992) 4192–4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Vindas MA, Gorissen M, Hoglund E, Flik G, Tronci V, Damsgard B, Thornqvist PO, Nilsen TO, Winberg S, Overli O, Ebbesson LOE, How do individuals cope with stress? Behavioural, physiological and neuronal differences between proactive and reactive coping styles in fish, The Journal of experimental biology 220(Pt 8) (2017) 1524–1532. [DOI] [PubMed] [Google Scholar]
- [39].Carpenter RE, Korzan WJ, Bockholt C, Watt MJ, Forster GL, Renner KJ, Summers CH, Corticotropin releasing factor influences aggression and monoamines: modulation of attacks and retreats, Neuroscience 158(2) (2009) 412–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Backström T, Pettersson A, Johansson V, Winberg S, CRF and urotensin I effects on aggression and anxiety-like behavior in rainbow trout, The Journal of experimental biology 214(Pt 6) (2011) 907–14. [DOI] [PubMed] [Google Scholar]
- [41].Abbott JC, Dill LM, Patterns of Aggressive Attack in Juvenile Steelhead Trout (Salmo-Gairdneri), Canadian Journal of Fisheries and Aquatic Sciences 42(11) (1985) 1702–1706. [Google Scholar]
- [42].Lastein S, Hoglund E, Overli O, Doving KB, Effects of antalarmin, a CRF receptor 1 antagonist, on fright reaction and endocrine stress response in crucian carp (Carassius carassius), J Comp Physiol A Neuroethol.Sens.Neural Behav Physiol 194(12) (2008) 1007–1012. [DOI] [PubMed] [Google Scholar]
- [43].McCarthy JR, Heinrichs SC, Grigoriadis DE, Recent advances with the CRF1 receptor: design of small molecule inhibitors, receptor subtypes and clinical indications, Curr.Pharm.Des 5(5) (1999) 289–315. [PubMed] [Google Scholar]
- [44].Ross RT, Orr WB, Holland PC, Berger TW, Hippocampectomy disrupts acquisition and retention of learned conditional responding, Behavioral neuroscience 98(2) (1984) 211–25. [DOI] [PubMed] [Google Scholar]
- [45].Hall J, Thomas KL, Everitt BJ, Rapid and selective induction of BDNF expression in the hippocampus during contextual learning, Nature neuroscience 3(6) (2000) 533–5. [DOI] [PubMed] [Google Scholar]
- [46].Squire LR, Stjohn S, Davis HP, Inhibitors of Protein-Synthesis and Memory - Dissociation of Amnesic Effects and Effects on Adrenal Steroidogenesis, Brain research 112(1) (1976) 200–206. [DOI] [PubMed] [Google Scholar]
- [47].Igaz LM, Bekinschtein P, Vianna MM, Izquierdo I, Medina JH, Gene expression during memory formation, Neurotox Res 6(3) (2004) 189–204. [DOI] [PubMed] [Google Scholar]
- [48].Hyman C, Hofer M, Barde YA, Juhasz M, Yancopoulos GD, Squinto SP, Lindsay RM, BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra, Nature 350(6315) (1991) 230–2. [DOI] [PubMed] [Google Scholar]
- [49].Liu S, Zheng D, Peng XX, Cabeza de Vaca S, Carr KD, Enhanced cocaine-conditioned place preference and associated brain regional levels of BDNF, p-ERK1/2 and p-Ser845-GluA1 in food-restricted rats, Brain research 1400 (2011) 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Li W, Keifer J, BDNF-induced synaptic delivery of AMPAR subunits is differentially dependent on NMDA receptors and requires ERK, Neurobiol.Learn.Mem 91(3) (2009) 243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Li W, Keifer J, Coordinate action of pre- and postsynaptic brain-derived neurotrophic factor is required for AMPAR trafficking and acquisition of in vitro classical conditioning, Neuroscience 155(3) (2008) 686–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Keifer J, Regulation of AMPAR trafficking in synaptic plasticity by BDNF and the impact of neurodegenerative disease, J Neurosci Res 100(4) (2022) 979–991. [DOI] [PubMed] [Google Scholar]
- [53].Keifer J, Comparative Genomics of the BDNF Gene, Non-Canonical Modes of Transcriptional Regulation, and Neurological Disease, Mol Neurobiol 58(6) (2021) 2851–2861. [DOI] [PubMed] [Google Scholar]
- [54].Timmusk T, Metsis M, Regulation of BDNF promoters in the rat hippocampus, Neurochem Int 25(1) (1994) 11–5. [DOI] [PubMed] [Google Scholar]
- [55].Tian F, Marini AM, Lipsky RH, NMDA receptor activation induces differential epigenetic modification of Bdnf promoters in hippocampal neurons, Amino Acids 38(4) (2010) 1067–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Mizuno K, Dempster E, Mill J, Giese KP, Long-lasting regulation of hippocampal Bdnf gene transcription after contextual fear conditioning, Genes, brain, and behavior 11(6) (2012) 651–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Liu IY, Lyons WE, Mamounas LA, Thompson RF, Brain-derived neurotrophic factor plays a critical role in contextual fear conditioning, The Journal of neuroscience : the official journal of the Society for Neuroscience 24(36) (2004) 7958–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Fortin DA, Srivastava T, Dwarakanath D, Pierre P, Nygaard S, Derkach VA, Soderling TR, Brain-derived neurotrophic factor activation of CaM-kinase kinase via transient receptor potential canonical channels induces the translation and synaptic incorporation of GluA1-containing calcium-permeable AMPA receptors, The Journal of neuroscience : the official journal of the Society for Neuroscience 32(24) (2012) 8127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Reisel D, Bannerman DM, Schmitt WB, Deacon RM, Flint J, Borchardt T, Seeburg PH, Rawlins JN, Spatial memory dissociations in mice lacking GluR1, Nature neuroscience 5(9) (2002) 868–73. [DOI] [PubMed] [Google Scholar]
- [60].Schmitt WB, Deacon RM, Reisel D, Sprengel R, Seeburg PH, Rawlins JN, Bannerman DM, Spatial reference memory in GluR-A-deficient mice using a novel hippocampal-dependent paddling pool escape task, Hippocampus 14(2) (2004) 216–23. [DOI] [PubMed] [Google Scholar]
- [61].Schmitt WB, Sprengel R, Mack V, Draft RW, Seeburg PH, Deacon RM, Rawlins JN, Bannerman DM, Restoration of spatial working memory by genetic rescue of GluR-A-deficient mice, Nature neuroscience 8(3) (2005) 270–2. [DOI] [PubMed] [Google Scholar]
- [62].Francia N, Cirulli F, Chiarotti F, Antonelli A, Aloe L, Alleva E, Spatial memory deficits in middle-aged mice correlate with lower exploratory activity and a subordinate status: role of hippocampal neurotrophins, Eur.J Neurosci 23(3) (2006) 711–728. [DOI] [PubMed] [Google Scholar]
- [63].Adlard PA, Cotman CW, Voluntary exercise protects against stress-induced decreases in brain-derived neurotrophic factor protein expression, Neuroscience 124(4) (2004) 985–92. [DOI] [PubMed] [Google Scholar]
- [64].Yaeger JDW, Krupp KT, Jacobs BM, Onserio BO, Meyerink BL, Cain JT, Ronan PJ, Renner KJ, DiLeone RJ, Summers CH, Orexin 1 Receptor Antagonism in the Basolateral Amygdala Shifts the Balance From Pro- to Antistress Signaling and Behavior, Biological psychiatry 91(9) (2022) 841–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Staton CD, Yaeger JDW, Khalid D, Haroun F, Fernandez BS, Fernandez JS, Summers BK, Summers TR, Sathyanesan M, Newton SS, Summers CH, Orexin 2 receptor stimulation enhances resilience, while orexin 2 inhibition promotes susceptibility, to social stress, anxiety and depression, Neuropharmacology 143 (2018) 79–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Yaeger JDW, Krupp KT, Gale JJ, Summers CH, Counterbalanced microcircuits for Orx1 and Orx2 regulation of stress reactivity, Medicine in Drug Discovery 100059 (2020) 1–20. [Google Scholar]
- [67].Summers CH, Yaeger JDW, Staton CD, Arendt DH, Summers TR, Orexin/hypocretin receptor modulation of anxiolytic and antidepressive responses during social stress and decision-making: Potential for therapy, Brain research 1731 (2020) 146085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Yaeger JDW, Krupp KT, Summers TR, Summers CH, Contextual generalization of social stress learning is modulated by orexin receptors in basolateral amygdala, Neuropharmacology 215 (2022) 109168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Salas C, Broglio C, Duran E, Gomez A, Ocana FM, Jimenez-Moya F, Rodriguez F, Neuropsychology of Learning and Memory in Teleost Fish, Zebrafish 3(2) (2006) 157–171. [DOI] [PubMed] [Google Scholar]
- [70].Northcutt RG, Davis RE, Telencephalic organization in ray-finned fishes, in: Davis RE, Northcutt RG (Eds.), Fish Neurobiology Volume 2, Higher Brain Areas and Functions, University of Michigan Press, Ann Arbor, 1983, pp. 203–236. [Google Scholar]
- [71].Portavella M, Vargas JP, Torres B, Salas C, The effects of telencephalic pallial lesions on spatial, temporal, and emotional learning in goldfish, Brain research bulletin 57(3–4) (2002) 397–399. [DOI] [PubMed] [Google Scholar]
- [72].Rodriguez F, Lopez JC, Vargas JP, Gomez Y, Broglio C, Salas C, Conservation of spatial memory function in the pallial forebrain of reptiles and ray-finned fishes, Journal of Neuroscience 22(7) (2002) 2894–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Rodriguez F, Lopez JC, Vargas JP, Broglio C, Gomez Y, Salas C, Spatial memory and hippocampal pallium through vertebrate evolution: Insights from reptiles and teleost fish, Brain research bulletin 57(3–4) (2002) 499–503. [DOI] [PubMed] [Google Scholar]
- [74].Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR, Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity, Journal of neurophysiology 88(5) (2002) 2187–2195. [DOI] [PubMed] [Google Scholar]
- [75].Livak KJ, Schmittgen TD, Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method, Methods 25(4) (2001) 402–8. [DOI] [PubMed] [Google Scholar]
- [76].Veazie PJ, When to combine hypotheses and adjust for multiple tests, Health services research 41(3 Pt 1) (2006) 804–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Feise RJ, Do multiple outcome measures require p-value adjustment?, BMC Med Res Methodol 2 (2002) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Jennions MD, Moller AP, A survey of the statistical power of research in behavioral ecology and animal behavior, Behav Ecol 14(3) (2003) 438–445. [Google Scholar]
- [79].Moran MD, Arguments for rejecting the sequential Bonferroni in ecological studies, Oikos 100(2) (2003) 403–405. [Google Scholar]
- [80].Nakagawa S, A farewell to Bonferroni: the problems of low statistical power and publication bias, Behav Ecol 15(6) (2004) 1044–1045. [Google Scholar]
- [81].Perneger TV, What’s wrong with Bonferroni adjustments, BMJ 316(7139) (1998) 1236–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Rothman KJ, No adjustments are needed for multiple comparisons, Epidemiology 1(1) (1990) 43–6. [PubMed] [Google Scholar]
- [83].Korzan WJ, Summers CH, Behavioral diversity and neurochemical plasticity: selection of stress coping strategies that define social status, Brain, behavior and evolution 70(4) (2007) 257–66. [DOI] [PubMed] [Google Scholar]
- [84].Arendt DH, Smith JP, Bastida CC, Prasad MS, Oliver KD, Eyster KM, Summers TR, Delville Y, Summers CH, Contrasting hippocampal and amygdalar expression of genes related to neural plasticity during escape from social aggression, Physiology & behavior 107(5) (2012) 670–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Pang T, Yaeger JDW, Summers CH, Mitra R, Cardinal role of the environment in stress iInduced changes across life stages and generations, Neuroscience & Biobehavioral Reviews 124 (2021) 137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Robertson JM, Achua JK, Smith JP, Prince MA, Staton CD, Ronan PJ, Summers TR, Summers CH, Anxious behavior induces elevated hippocampal Cb2 receptor gene expression, Neuroscience 352 (2017) 273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Backström T, Schjolden J, Overli O, Thornqvist PO, Winberg S, Stress effects on AVT and CRF systems in two strains of rainbow trout (Oncorhynchus mykiss) divergent in stress responsiveness, Hormones and behavior 59(1) (2011) 180–6. [DOI] [PubMed] [Google Scholar]
- [88].Lema SC, Population divergence in plasticity of the AVT system and its association with aggressive behaviors in a Death Valley pupfish, Hormones and behavior 50(2) (2006) 183–193. [DOI] [PubMed] [Google Scholar]
- [89].Semsar K, Kandel FL, Godwin J, Manipulations of the AVT system shift social status and related courtship and aggressive behavior in the bluehead wrasse, Hormones and behavior 40(1) (2001) 21–31. [DOI] [PubMed] [Google Scholar]
- [90].Flannery RB Jr., Towards stress-resistant persons: a stress management approach to the treatment of anxiety, Am J Prev Med 3(1) (1987) 25–30. [PubMed] [Google Scholar]
- [91].Ipser JC, Carey P, Dhansay Y, Fakier N, Seedat S, Stein DJ, Pharmacotherapy augmentation strategies in treatment-resistant anxiety disorders, The Cochrane database of systematic reviews (4) (2006) CD005473. [DOI] [PubMed] [Google Scholar]
- [92].Schmieder RE, Grassi G, Kjeldsen SE, Patients with treatment-resistant hypertension report increased stress and anxiety: a worldwide study, J Hypertens 31(3) (2013) 610–5; discussion 615. [DOI] [PubMed] [Google Scholar]
- [93].Sufka KJ, White SW, Identification of a treatment-resistant, ketamine-sensitive genetic line in the chick anxiety-depression model, Pharmacology, biochemistry, and behavior 113 (2013) 63–7. [DOI] [PubMed] [Google Scholar]
- [94].Bentley S, Artin H, Mehaffey E, Liu F, Sojourner K, Bismark A, Printz D, Lee EE, Martis B, De Peralta S, Baker DG, Mishra J, Ramanathan D, Response to intravenous racemic ketamine after switch from intranasal (S)-ketamine on symptoms of treatment-resistant depression and post-traumatic stress disorder in Veterans: A retrospective case series, Pharmacotherapy (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Albott CS, Lim KO, Forbes MK, Erbes C, Tye SJ, Grabowski JG, Thuras P, Batres YCTM, Wels J, Shiroma PR, Efficacy, Safety, and Durability of Repeated Ketamine Infusions for Comorbid Posttraumatic Stress Disorder and Treatment-Resistant Depression, The Journal of clinical psychiatry 79(3) (2018). [DOI] [PubMed] [Google Scholar]
- [96].Kaplan MJ, Klinetob NA, Childhood emotional trauma and chronic posttraumatic stress disorder in adult outpatients with treatment-resistant depression, The Journal of nervous and mental disease 188(9) (2000) 596–601. [DOI] [PubMed] [Google Scholar]
- [97].Keifer J, Sabirzhanov BE, Zheng Z, Li W, Clark TG, Cleavage of proBDNF to BDNF by a tolloid-like metalloproteinase is required for acquisition of in vitro eyeblink classical conditioning, The Journal of neuroscience : the official journal of the Society for Neuroscience 29(47) (2009) 14956–14964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Li W, Zheng Z, Keifer J, Transsynaptic EphB/Ephrin-B signaling regulates growth of presynaptic boutons required for classical conditioning, The Journal of neuroscience : the official journal of the Society for Neuroscience 31(23) (2011) 8441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Zheng Z, Sabirzhanov B, Keifer J, Oligomeric amyloid-β inhibits the proteolytic conversion of brain-derived neurotrophic factor (BDNF), AMPA receptor trafficking, and classical conditioning, The Journal of biological chemistry 285(45) (2010) 34708–34717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Faria RS, Sartori CR, Canova F, Ferrari EA, Classical aversive conditioning induces increased expression of mature-BDNF in the hippocampus and amygdala of pigeons, Neuroscience 255 (2013) 122–33. [DOI] [PubMed] [Google Scholar]
- [101].Janke KL, Cominski TP, Kuzhikandathil EV, Servatius RJ, Pang KC, Investigating the Role of Hippocampal BDNF in Anxiety Vulnerability Using Classical Eyeblink Conditioning, Front Psychiatry 6 (2015) 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Li W, Keifer J, Rapid enrichment of presynaptic protein in boutons undergoing classical conditioning is mediated by brain-derived neurotrophic factor, Neuroscience 203 (2012) 50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Zheng Z, Keifer J, Sequential delivery of synaptic GluA1- and GluA4-containing AMPA receptors (AMPARs) by SAP97 anchored protein complexes in classical conditioning, The Journal of biological chemistry 289(15) (2014) 10540–10550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Zheng Z, Sabirzhanov B, Keifer J, Two-stage AMPA receptor trafficking in classical conditioning and selective role for glutamate receptor subunit 4 (tGluA4) flop splice variant, Journal of neurophysiology 108(1) (2012) 101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Mokin M, Zheng Z, Keifer J, Conversion of silent synapses into the active pool by selective GluR1–3 and GluR4 AMPAR trafficking during in vitro classical conditioning, Journal of neurophysiology 98(3) (2007) 1278–1286. [DOI] [PubMed] [Google Scholar]
- [106].van der Kolk BA, Clinical implications of neuroscience research in PTSD, Annals of the New York Academy of Sciences 1071 (2006) 277–93. [DOI] [PubMed] [Google Scholar]
- [107].Yehuda R, LeDoux J, Response variation following trauma: a translational neuroscience approach to understanding PTSD, Neuron 56(1) (2007) 19–32. [DOI] [PubMed] [Google Scholar]
- [108].Chen DY, Bambah-Mukku D, Pollonini G, Alberini CM, Glucocorticoid receptors recruit the CaMKIIalpha-BDNF-CREB pathways to mediate memory consolidation, Nature neuroscience 15(12) (2012) 1707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Arango-Lievano M, Borie AM, Dromard Y, Murat M, Desarmenien MG, Garabedian MJ, Jeanneteau F, Persistence of learning-induced synapses depends on neurotrophic priming of glucocorticoid receptors, Proceedings of the National Academy of Sciences of the United States of America 116(26) (2019) 13097–13106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Slipczuk L, Bekinschtein P, Katche C, Cammarota M, Izquierdo I, Medina JH, BDNF activates mTOR to regulate GluR1 expression required for memory formation, PLoS.One 4(6) (2009) e6007. [DOI] [PMC free article] [PubMed] [Google Scholar]


