Abstract
Background: Associations between the microbiome–gut–brain axis and dementia have attracted considerable attention in research literature. This study examined the microbiome–gut–brain axis and dementia-related research from a bibliometric perspective. Methods: A search for original research and review articles on the microbiome–gut–brain axis and dementia was conducted in the Web of Science Core Collection (WOSCC) database. The R package “bibliometrix” was used to collect information on countries, institutions, authors, journals, and keywords. VOSviewer software was used to visualize the co-occurrence network of keywords. Results: Overall, 494 articles met the study inclusion criteria, with an average of 29.64 citations per article. Corresponding authors of published articles were mainly from China, the United States and Italy. Zhejiang University in China and Kyung Hee University in Korea were the most active institutions, while the Journal of Alzheimer’s Disease and Nutrients published the most articles in this field. Expected main search terms, “Parkinson disease” and “chain fatty-acids” were high-frequency keywords that indicate current and future research directions in this field. Conclusions: This bibliometric study helped researchers to identify the key topics and trends in the microbiome–gut–brain axis and dementia-related research. High-frequency keywords identified in this study reflect current trends and possible future directions in this field related to methodologies, mechanisms and populations of interest.
Keywords: brain-gut axis, dementia, Alzheimer’s disease, bibliometrics, hotspots
1. Introduction
A microbiome is a community of microorganisms, including bacteria, viruses, fungi, and protozoa, that forms in a specific environment [1]. In healthy human intestinal tracts, there are diverse and dynamic populations of microbiomes, most of which reside in the ileum and colon [2]. Previous studies have found that alterations of gut microbiota are associated with various intestinal disorders (e.g., irritable bowel syndrome) and brain function [3]. As such, the concept of “microbiome–gut–brain axis” has garnered growing attention and addresses complex bidirectional interactions between brain and gut [4]. For instance, the alteration or absence of an intestinal microbiome may trigger systemic immune activation that contributes to intestinal barrier defects, damage to the blood–brain barrier, the promotion of neuroinflammation, and eventual brain damage and degeneration [5].
Dementia is a chronic syndrome characterized by progressive deterioration of cognitive abilities [6]. Dementia can affect memory, thinking, language, behavior, and daily activities and is a major source of disability among older people worldwide [7]. Current estimates suggest that around 55 million people suffer from dementia globally and the number is projected to rise to 78 million by 2030 and 139 million by 2050 [8]. In addition, there are nearly 10 million new cases of dementia per year [8]. Alzheimer’s disease (AD) is the most common form of dementia and accounts for 60–80% of total cases [9]. Other forms of dementia include vascular dementia, Lewy body dementia, dementia in Parkinson’s disease, and frontotemporal dementia [10].
Some studies have implicated gut microbiota in the development or progression of dementia [11,12]. Longitudinal research has also shown that individuals with inflammatory bowel disease linked with gut microbiome were diagnosed with dementia at an earlier age on average compared with healthy samples [13]. Moreover, the microbiome–gut–brain axis has emerged as a potential diagnostic and therapeutic target in a wide range of psychiatric and neurologic disorders [4]. Relatedly, altering gut microbiota with antibiotic therapy or probiotic treatment can improve performance on learning and memory tests [14,15].
Understanding progress and trends within a particular research field is important. Bibliometric analysis is a widely used approach designed to identify key features of relevant publications including core research themes, methodologies, authors, institutions, and countries [16]. For instance, bibliometric analysis can provide information on citations of articles that reflect the academic impact of publications. In addition, the compilation of keyword frequencies can help researchers to identify past foci and future trends on specific research topics [17]. Bibliometric analysis also provides network maps of co-authorship and co-occurrence analysis that elucidate collaborations between countries, thus allowing researchers to seek potential interdisciplinary collaborators [18].
Previous bibliometric manuscripts have explored associations between gut microbiota and Parkinson’s disease [19], associations between the gut–brain axis and depression [20], and the microbiome–gut–brain axis [21]. However, to date, links between the microbiome–gut–brain axis and dementia have not been analyzed using the bibliometric method. Therefore, this study examined characteristics of relevant research on the microbiome–gut–brain axis and dementia from a bibliometric perspective.
2. Materials and Methods
2.1. Data Collection
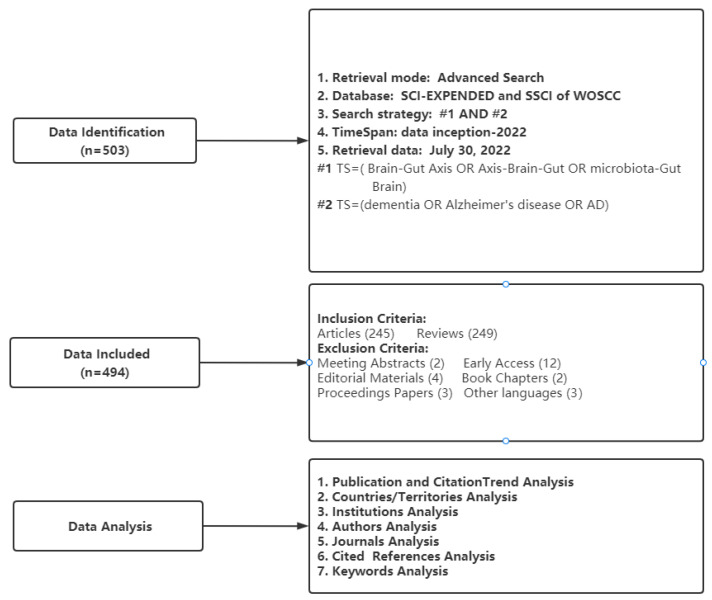
Relevant studies on the microbiome–gut–brain axis and dementia were extracted from the Web of Science Core Collection (WOSCC) database; the editions were limited to Science Citation Index Expanded (SCI-EXPANDED) and Social Sciences Citation Index (SSCI), which are the most widely used sources for bibliometric analyses. Following previous studies [21,22], the key terms “microbiome–gut–brain axis” and “dementia” and its synonyms were used as search terms, with the search strategy: TS = (microbiome–gut–brain axis OR axis–brain–gut OR microbiota–gut–brain) AND TS = (dementia OR Alzheimer OR Alzheimer’s disease OR AD). Original articles and reviews published in English were searched from dates of inception to 30 July 2022. Bibliometric data were exported as “plain text” documents with the full record and cited references. Overall, 494 publications on microbiome–gut–brain axis and dementia were included for analyses; 245 were original articles and 249 were reviews.
2.2. Data Analysis
Bibliometrix is an R-based tool for comprehensive bibliometric mapping analysis [23] that can be used for (1) number of publications, (2) citation analysis including summaries of citation information for countries or regions, affiliations, journals, and authors, and (3) the conduct of Three-Fields Plots from the keywords Plus analysis. VOSviewer is a software program based on the Java environment [24] used to (1) create clusters of keywords and (2) perform co-occurrence analyses of keywords. We also used an online bibliometric analysis approach (https://bibliometric.com/, accessed on 30 July 2022) to generate a string plot of research collaborations between countries. Microsoft Excel 2019 was used to map the annual number of publications and depict a circle plot of country contributions and bar plot of keyword frequencies. Moreover, we used a knowledge synthesis method to summarize specific keywords used in this literature [25]. Figure 1 presents a flowchart of publication selection and analysis.
Figure 1.
Flowchart of data selection and analysis.
3. Results
3.1. General Features of Publications
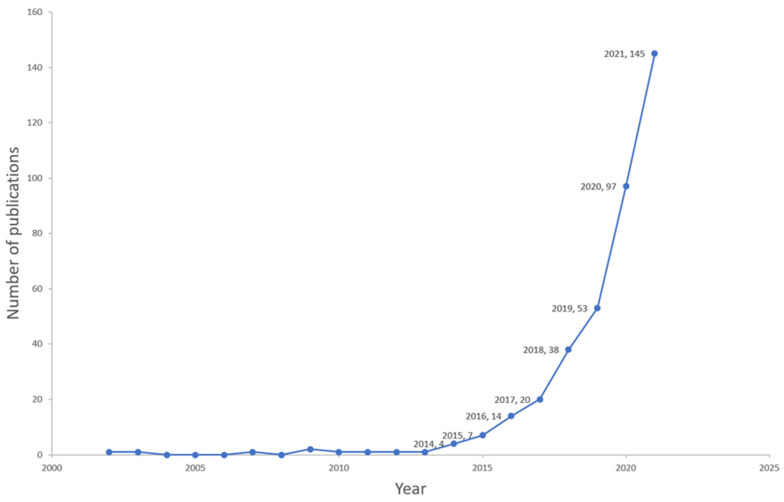
Overall, 494 publications on microbiome–gut–brain axis and dementia-related research were published between 2002 and 2022, with an annual growth rate of 25.6%. These publications included 245 (49.6%) original articles and 249 (50.4%) reviews involving 246 sources (Journals and Books), 2986 authors, and 34,963 references; the average number of citations for each publication was 29.64. As shown in Figure 2, the publication total increased substantially, particularly in recent years, from 20 publications in 2017 to 145 in 2021.
Figure 2.
Year-wise distribution of the documents between 2002–2021.
3.2. Top Cited Papers
Table 1 presents the 10 most cited articles in the field of microbiome–gut–brain axis and dementia-related research, each of which had total citation counts (TC) of at least 200. The two most highly cited articles were general review papers on the microbiome-gut brain axis. First, was a review entitled, “The Microbiota-Gut-Brain Axis” [26] that was published in Physiological Reviews and generated 894 citations. Second, was a review, “Interactions between the microbiota, immune and nervous systems in health and disease”, published in Nature Neuroscience, that garnered 764 citations. The 8 remaining most highly cited papers were all review papers as well but focused more specifically on links between the microbiome–gut–brain axis and Alzheimer’s disease or neurogenerative diseases; each had generated roughly 200–330 citations to date. In terms of total citations per year (TC/Y), “The Microbiota-Gut-Brain Axis” also ranked first with a TC/Y of 223.5, followed by “Interactions between the microbiota, immune and nervous systems in health and disease” (TC/Y = 127.33) and “Brain-Gut-Microbiota Axis in Alzheimer’s Disease” (TC/Y = 69.25) [27] published in the Journal of Neurogastroenterology and Motility.
Table 1.
Top 10 most cited articles on microbiome–gut–brain axis and dementia related research.
| SCR | Title (N = 494) | Year | Journal | FA | CA | TC | TC/Y |
|---|---|---|---|---|---|---|---|
| 1 | The Microbiota-Gut-Brain Axis | 2019 | Psychological Review | John F. Cryan | Timothy G. Dinan | 894 | 223.50 |
| 2 | Interactions between the microbiota, immune and nervous systems in health and disease | 2017 | Nature Neuroscience | Thomas C Fung | Elaine Y Hsiao | 764 | 127.33 |
| 3 | The Gut Microbiota and Alzheimer’s Disease | 2017 | Journal of Alzheimers Disease | Chunmei Jiang | Bin Zhao | 329 | 54.83 |
| 4 | Brain-Gut-Microbiota Axis in Alzheimer’s Disease | 2019 | Journal of Neurogastroenterology and Motility | Karol Kowalski | Agata Mulak | 277 | 69.25 |
| 5 | Microbiota-Microbiome-Gut-Brain Axis and Neurodegenerative Diseases | 2017 | Current Neurology and Neuroscience Reports | Eamonn M M Quigley | Eamonn M M Quigley | 263 | 43.83 |
| 6 | Gut microbiome in health and disease: Linking the microbiome-gut-brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases | 2016 |
Pharmacology
& Therapeutics |
Shivani Ghaisas | Anumantha Kanthasamy | 252 | 36.00 |
| 7 | Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease | 2016 | Nutrition Review | Francesca Pistollato | Maurizio Battino | 250 | 35.71 |
| 8 | The bowel and beyond: the enteric nervous system in neurological disorders | 2016 | Psychiatry Research | Meenakshi Rao | Michael D Gershon | 230 | 32.86 |
| 9 | Neuropeptide Y: A stressful review | 2016 | Neuropeptides | Florian Reichmann | Peter Holzer | 227 | 32.43 |
| 10 | Microbiome, probiotics and neurodegenerative diseases: deciphering the gut brain axis | 2017 | Cellular and Molecular Life Sciences | Susan Westfall | Satya Prakash | 211 | 35.17 |
Notes: SCR: Standard competition ranking; TC: Total Citations; TC/Y: Average per Year Total Citations; FA: First author; CA: Corresponding author.
3.3. Journal Analysis
Table 2 presents the 15 most cited journals out of 246 journals on microbiome–gut–brain axis and dementia-related research. The Journal of Alzheimer’s Disease was ranked first in both total citations (TC = 620) and number of publications (N = 23), followed by Nutrients (TC = 423, N = 19). However, Nutrients and Frontiers in Aging Neuroscience had the highest H-index (9) compared with other journals; over half of the 15 most cited journals had H-indexes ranging between 2 and 4. With respect to impact factors (IF), Frontiers in Immunology ranked highest (IF = 8.786), followed by Antioxidants (IF = 7.765) and Nutrients (IF = 6.706). Of the 15 most highly cited journals, 5 are in the category of Medicine, 4 are in Agricultural and Biological Sciences, and two are in the field of Neuroscience. All journals are located in quartiles 1 or 2 according of their respective Journal Citation Reports categories (2021–2022), except for Microorganisms, a Q3 journal with an IF of 4.926.
Table 2.
Top 15 most cited journal contributed to microbiome–gut–brain axis and dementia related research.
| SCR | Journal (N = 246) | h_index | TC | NP | IF | JCR | Research Domain |
|---|---|---|---|---|---|---|---|
| 1 | Journal of Alzheimers Disease | 8 | 620 | 23 | 4.160 | Q1 | Psychology |
| 2 | Nutrients | 9 | 423 | 19 | 6.706 | Q1 | Agricultural and Biological Sciences |
| 3 | International Journal of Molecular Sciences | 7 | 237 | 18 | 6.208 | Q1 | Chemistry |
| 4 | Frontiers in Aging Neuroscience | 9 | 386 | 14 | 5.702 | Q1 | Neuroscience |
| 5 | Frontiers in Neuroscience | 5 | 103 | 12 | 5.152 | Q2 | Neuroscience |
| 6 | Microorganisms | 6 | 83 | 8 | 4.926 | Q3 | Medicine |
| 7 | Antioxidants | 4 | 45 | 7 | 7.675 | Q1 | Agricultural and Biological Sciences |
| 8 | Current Alzheimer Research | 4 | 155 | 7 | 3.040 | Q1 | Medicine |
| 9 | Frontiers in Immunology | 3 | 82 | 7 | 8.786 | Q1 | Medicine |
| 10 | Aging-Us | 4 | 159 | 6 | 5.955 | Q2 | Biochemistry, Genetics and Molecular Biology |
| 11 | Frontiers in Cellular and Infection Microbiology | 4 | 164 | 6 | 6.073 | Q2 | Medicine |
| 12 | Frontiers in Nutrition | 4 | 32 | 6 | 6.590 | Q1 | Food Science |
| 13 | Frontiers in Pharmacology | 2 | 49 | 6 | 5.988 | Q1 | Medicine |
| 14 | Journal of Agricultural and Food Chemistry | 3 | 62 | 6 | 5.895 | Q1 | Agricultural and Biological Sciences |
| 15 | Scientific Reports | 4 | 385 | 6 | 4.996 | Q1 | Multidisciplinary |
Notes: SCR: Standard competition ranking; TC: Total citations; NP: Number of publications; IF: Impact factor (2021–2022); JCR: Journal Citation Reports category (2021–2022).
3.4. Author Analysis
Table 3 summarizes the 10 most influential authors in the field of microbiome–gut–brain axis and dementia-related research. Dr. Katerina Johnson from Oxford University had the highest total citation (TC = 552) and average total citation (ATC = 138) counts, followed by Dr. Sunmin Park from Hoseo University (TC = 178, ATC = 35.6). There is a marked difference between Dr. Johnson’s ATC and that of other top authors. Of the top 10 authors, nine had published 5 papers while Dr. Johnson had a much higher number of citations from just 4 publications. In terms of H-index rates, there was little difference between the 10 authors; 6 authors had an H-index of 5, 3 authors had an H-index of 4 and one author had an H-index of 3.
Table 3.
Top 10 authors contributed to microbiome–gut–brain axis and dementia related research.
| Name | Institution | h_index | NP | TC | ATC |
|---|---|---|---|---|---|
| Johnson K Katerina V.-A. Johnson |
University of Oxford | 4 | 4 | 552 | 138 |
| Park S Sunmin Park |
Hoseo University | 4 | 5 | 178 | 35.6 |
| Goebel Stengel M Miriam Goebel-Stengel |
Martin-Luther-Krankenhaus | 5 | 5 | 105 | 21.0 |
| Kobelt P Peter Kobelt |
Charité—Universitätsmedizin Berlin | 5 | 5 | 105 | 21.0 |
| Rose M | Philipps-Universität | 5 | 5 | 105 | 21.0 |
| Stengel A Andreas Stengel |
University Hospital Tübingen | 5 | 5 | 105 | 21.0 |
| Liu X Xiaofei Liu |
South China University of Technology | 4 | 5 | 96 | 19.2 |
| Perry G George Perry |
The University of Texas at San Antonio | 5 | 5 | 89 | 17.8 |
| Obrenovich M Mark Obrenovich |
Cleveland State University | 5 | 5 | 82 | 16.4 |
| Zhang M Meng Zhang |
Beijing Gene Tangram Technology | 3 | 5 | 78 | 15.6 |
Notes: NP: Number of publications; TC: Total citations; ATC: Average Total citations.
3.5. Country/Territory Analysis
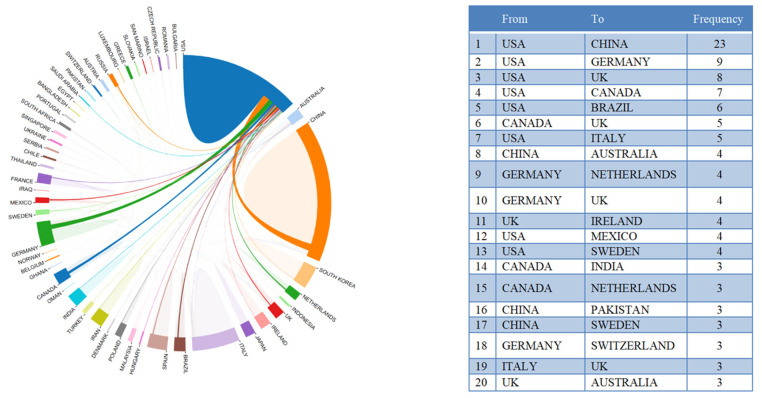
Figure 3 shows country/territory contributions to microbiome–gut–brain axis and dementia-related research. A total of 46 countries/territories had published at least one paper in this field. Figure 3 suggests that China had the highest number of publications (NP) with 136 articles, followed by the United States (U.S.) (92 articles), and Italy (46 articles). Countries with more than 10 publications included Korea, Spain, Germany, Poland, the United Kingdom, Australia, and Canada. Figure 3 shows the top 15 most cited countries/territories. Of the 46 countries/territories with publications on microbiome–gut–brain axis and dementia, four had no citations to date. Of the top 10 most cited countries/territories with at least 340 total citations (TC), four had 1000 citations or more, including the U.S. (TC = 3301), China (TC = 2767), Italy (TC = 1857), and Ireland (TC = 1036). Regarding differences between the ranking of average article citations (AAC) and TC, Ireland showed the largest gap with 148 AAC and a rank of fourth in TC. Austria was ranked second in AAC (117.33), but ninth on the TC rankings. Inconsistencies between AAC and TC may be due to differences in high quality research articles between these countries/territories. Figure 4 shows the international collaborations in microbiome–gut–brain axis and dementia related research. China and the U.S. had the most extensive cooperation in microbiome–gut–brain axis and dementia research, with 23 publications. U.S. institutions also worked closely with those in Germany (N = 9), UK (N = 8), Canada (N = 7), and Brazil (N = 6).
Figure 3.
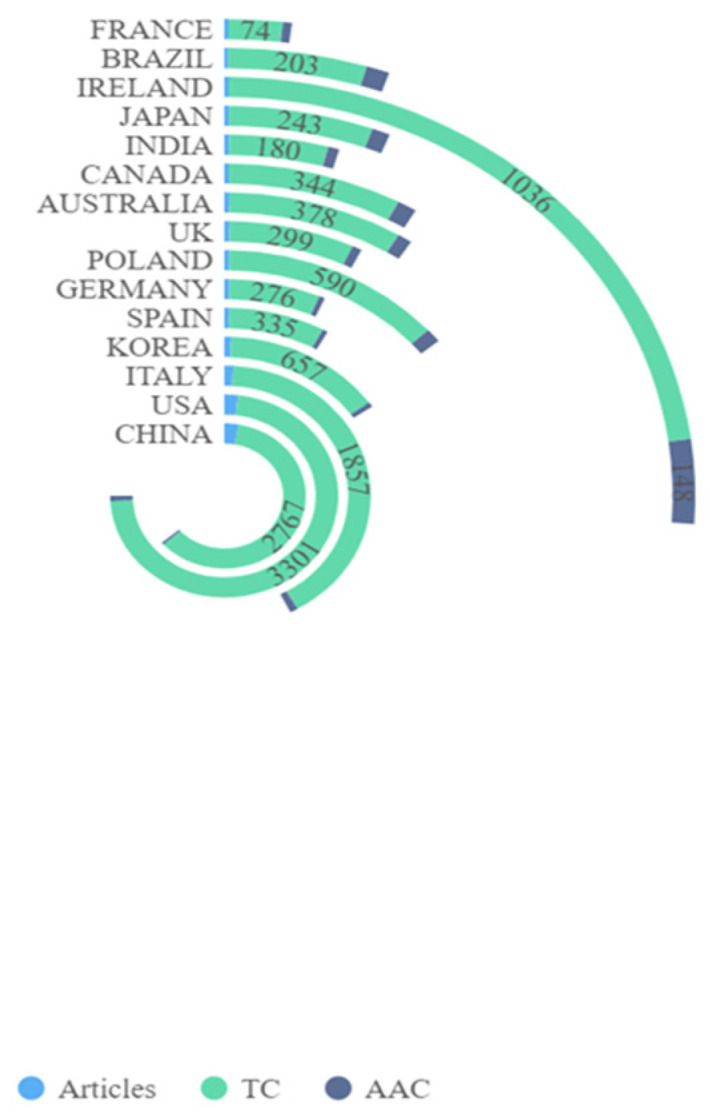
Top 15 contributing countries on microbiome–gut–brain axis and dementia-related research. Notes: TC: Total number of citations; AAC: Average Article Citations.
Figure 4.
International collaboration analysis and the top 20 collaborations between countries.
3.6. Institution Analysis
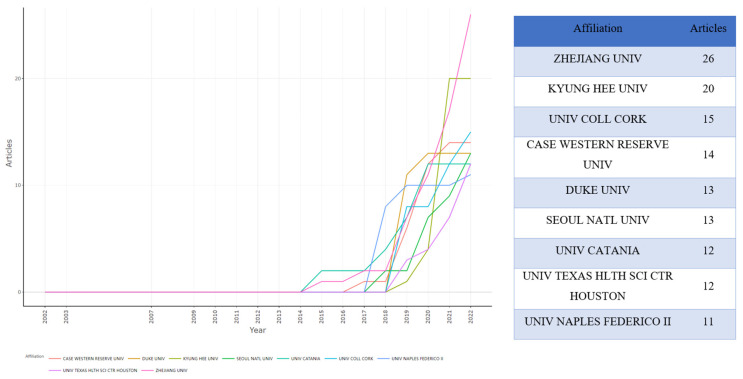
Figure 5 illustrates institution contributions to microbiome–gut–brain axis and dementia-related research. In total, 924 institutions contributed to this research area. The right and left panels, respectively, show the number of publications by institution and annual publications for each institution. Zhejiang University was ranked first and showed a rapid rate of growth in annual publications from two papers in 2014 to 26 articles in 2022. Kyung Hee University was ranked second, followed by University of College Cork. The top nine most active institutions began publishing related articles after 2014, with Duke University and the University of Texas Health Science Center, Houston both publishing their first related articles in 2018.
Figure 5.
Top 9 contributing institutions and production over time on microbiome–gut–brain axis and dementia-related research.
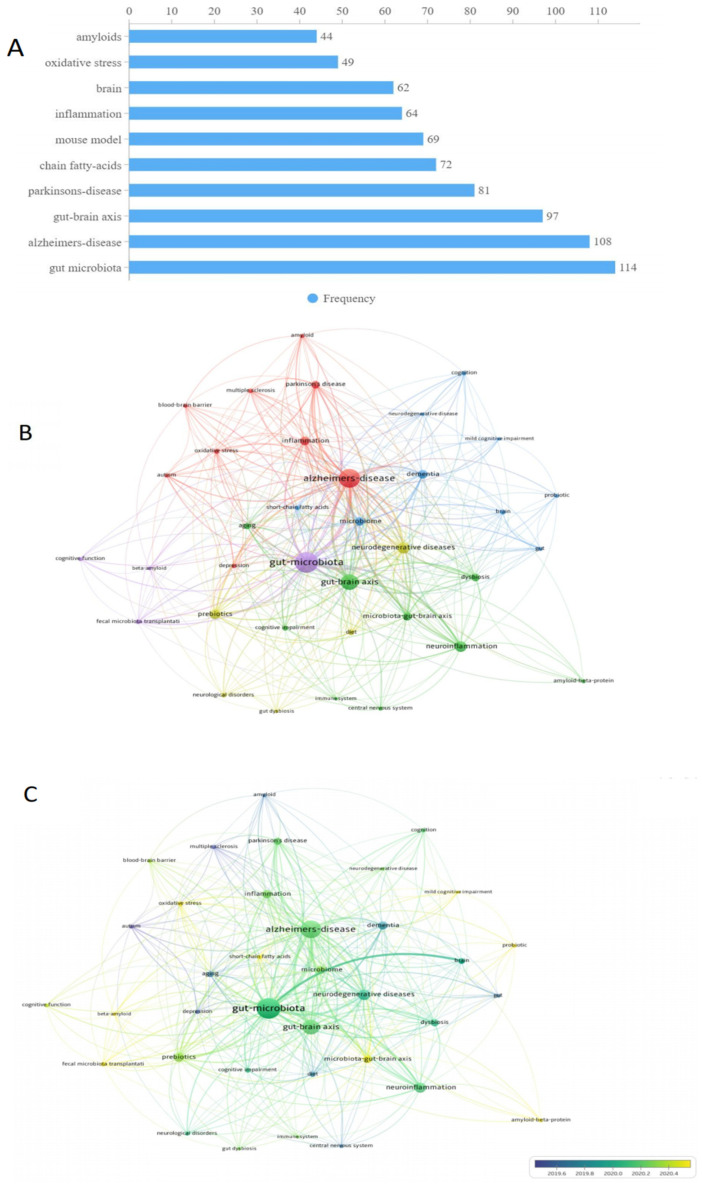
3.7. Keyword Analysis
Figure 6 shows the most frequently used keywords in the field. Of 1116 unique keywords, “gut microbiota” and “Alzheimer’s disease” were used most frequently, with 114 and 108 occurrences, respectively, followed by “gut-brain axis” with 97 occurrences. “Parkinson’s disease” as a common neurodegenerative disease had 81 occurrences. “Chain fatty acids” and “Amyloids” as major features of AD pathology had 72 and 44 occurrences, respectively. Several keywords related to hypothesized dementia mechanisms, including “inflammation” and “oxidative stress”, were also used as frequent keywords, with 64 and 49 occurrences, respectively. The middle and bottom panels of Figure 6 present the co-occurrence analysis of keywords, with a minimum number of 8 occurrences. After keywords with similar meanings were merged, 37 keywords were retained for analyses. The size of nodes in Figure 6 represents the frequency of keywords while the color of nodes represents the correlation between nodes. All keywords could be divided into 3 main clusters. The red cluster was the largest one and included several neurodegenerative diseases such as “Alzheimer’s disease”, “Parkinson disease”, “multiple sclerosis” as well as inflammation related terms such as “inflammation” and “oxidative stress”. The green cluster included two axes (“gut–brain axis” and “microbiota–gut- brain axis”) and two systems (“immune system” and “central nervous system”). The blue cluster included “mild cognitive impairment” and “cognition”, both of which were related to cognitive function.
Figure 6.
Co-occurrence analysis of author keywords and top 10 keywords on COVID-19 and sleep related research. ((A): the most frequently used keywords. (B): Co-occurrence network analysis of author keywords. (C): Co-occurrence overlay analysis of author keywords).
4. Discussion
This is the first bibliometric analysis of research on the microbiome–gut–brain axis and dementia. Of 494 publications identified from the WOSCC database search, around half were original research articles and the other half were review articles. The results showed a substantial growth of relevant publications, particularly during the period from 2017 to 2021.
The total number of citations can be used as an important indicator of interest in a particular research area [28]. The top 10 most cited articles in this study were all review papers that emphasized the importance of the microbiota–gut–brain axis and/or its links with neurodegenerative disease development. Sharp increases in the number of papers and citations over the past several years provide evidence of the rapid growth in interest in this field. The brain–gut axis reflects bidirectional communication between central and enteric nervous systems [26]; as one of the main regulators of the brain–gut axis, the distribution of neuroactive compounds released by microbiota around the axis may lead to the changes of cognitive function that contribute to the development of dementia [29]. Specifically, dementia is hypothesized to arise when gut bacteria activate immune activation through a defective intestinal barrier and lead to systemic inflammation, that, in turn, disrupts the blood–brain barrier and promotes neuroinflammation, ultimately leading to nerve damage and degeneration [30,31,32]. Evidence based on a nationwide population-based cohort study supports this hypothesis, as irritable bowel syndrome patients are at higher risk of dementia compared to healthy controls [33].
Regarding publication outlets for microbiome–gut–brain axis and dementia research, different dimensions were explored to identify journal contributions to the field. The H-index is an indicator of research performance, incorporating quantity and impact of publications into a ratio [34]. For the journal Nutrients, the H-index of 9 indicates 9 papers, each having at least 9 citations, having been published to date. Regarding the IF value and JCR classification of journals, higher IF values do not always represent higher JCR classifications; for example, the Q2 journal Aging-US has a higher IF than the Q1 journal Current Alzheimer Research, because the journals are included in different research domains. Notably, compared with other bibliometric analyses of journals typically found in a single research domain [35], research on the microbiome–gut–brain axis and dementia is not limited to medicine and extends to other fields including Agricultural and Biological Sciences, Nutrition and Dietetics, and Neuroscience. This pattern could reflect relatively high levels of interdisciplinary focus in the field. As such, researchers pursuing this field may need to be literate in the content and typical methodologies of different fields.
China is currently the most productive country in the number of publications on microbiome–gut–brain axis and dementia research, followed by the U.S. Generally, the economic situation of a country affects the level and priorities in research funding support, thus influencing its academic capability [36]. For instance, the National Institute of Health in the U.S. launched a strategically focused research program called the Human Microbiome Project in 2007; the program has provided financial support and attracted scholars in microbiome related fields [37] as a result. Many countries have joined this project and developed collaborations with U.S.-based institutions, especially China and Germany. Microbiome–gut–brain axis research had become more popular globally as a result of such initiatives [38,39].
Keywords can be regarded as core content of a specific article. Hence, keyword frequencies provide important clues about major trends in a given research area [40]. Apart from “gut microbiota”, “Alzheimer’s disease” was the most frequently used keyword in the field of microbiome–gut–brain axis and dementia. As the most common form of dementia, AD has far-reaching implications for brain health and function in the overall population [41]. A core feature of AD is the severe accumulation of senile plaques involving amyloid β protein (Aβ) and neurofibrillary tangles [42]. Notably, “amyloids” were another frequently used keyword in this research field and reflect a trend in research linking changes in gut microbiota strains with the progress of amyloid deposition [43]. Compared with healthy controls, AD patients display less microbial diversity of gut microbiota including deficits in fusobacteriaceae, firmicutes, and actinobacteria [44,45,46]. However, mechanisms underlying connections between gut microbiome and AD are still not clear. Some authors assume that inflammation plays a vital role [46,47]. The intestinal gut is involved in regulating the development and function of microglia. Presumably, its repeated activation has a wide range of proinflammatory effects that may lead to the progression of AD [48]. In addition, “Chain fatty acids” was another high frequency keyword; short chain fatty acids (SCFA) are key gut microbiota metabolites that typically grow in the large intestine [49]. Previous studies have found that SCFAs play an important role in the microbiota–gut–brain axis and have been linked with neurodegenerative diseases including AD [50]. One metabolomics study found that the concentration of propionate (a type of SCFA) was higher in the saliva of AD patients than healthy controls [51]. Similarly, compared with healthy controls, acetic acids level may be elevated in AD patients [52].
Intestinal gut dysbiosis is also associated with various diseases including irritable bowel syndrome (IBS). As another commonly used keyword in microbiome–gut–brain axis and dementia research, “oxidative stress” has been proposed as a potentially important risk factor for IBS in the field [53]. Over time several studies documented the co-occurrence of IBS and AD [13,54,55]. From this foundation, a recent genome-wide cross-trait analysis indicated AD and gastrointestinal tract disorders share several loci including PDE4B and BRINP3 that are promising candidates as underlying gut–brain mechanisms related to AD and IBD [56]. As another high frequency keyword, “Parkinson’s disease” (PD) is a progressive neurodegenerative disorder displaying an altered α-synucleinopathy that may be distributed along the brain–gut axis [57]. Dysregulation of the microbiota–brain–gut axis is common among PD patients [58] and is a concomitant symptom for over 80% of these cohorts [59]. A meta-analysis involving more than 30,000 participants revealed implicated helicobacter pylori infection as an agent associated with increased PD risk [60]. Furthermore, a randomized control trial found that eradicating Helicobacter pylori with L-dopa can improve PD symptoms [61].
Another trend identified in the keyword analysis was “mouse model”. Animal models have been widely used to examine associations of the gut microbiome with dementia [62]. One of the most widely used mouse models in this field is the APP/PS1 double transgenic mice, an AD-like model [63]. In this type of mouse, the presence of mutated transgenes can induce Aβ plaque deposition, resulting in memory deficits similar to those observed among humans with AD [64]. Alternately, a murine AD model posits that gut microbiome composition influences the build-up of amyloid plaque deposition, and oral antibiotic treatments might reduce such progress [47]. Still other animal studies have found that prebiotics, such as Lactobacillus strains, have benefits for brain function through the gut–heart–brain axis and can be used as a therapeutic agent for cognitive impairment [65].
Notwithstanding its implications for research, this study has several limitations. First, considering bibliometric analysis recommendations [66] that using a single database is preferable [67], data were only searched and extracted exclusively from the most widely used source, the WOSCC database. Second, bibliometric methods cannot provide a nuanced summary of salient subject content based on a large number of studies, as sometimes the document type defined by the WOSCC may be inaccurate [21].
5. Conclusions
In conclusion, this bibliometric analysis of microbiome–gut–brain axis and dementia has shown a rapid increase in the number of associated publications during the past 10 years; in particular, there has been a sharp rise in papers published during the two most recent years that underscores the current popularity of this field. Zhejiang University in China and Kyung Hee University in Korea were the most active institutions, while the Journal of Alzheimer’s Disease and Nutrients were the most active journals in microbiome–gut–brain axis and dementia research. Keyword analysis results revealed “Alzheimer’s Disease”, “Parkinson disease”, “chain fatty-acids”, “inflammation”, and “mouse model” as high-frequency keywords that reflect current trends and possible future directions in this field related to populations of interest, mechanisms, and methodologies.
Author Contributions
Study design: H.-L.S., S.S. and Y.-T.X. Data collection, analysis and interpretation: H.-L.S., Y.F., Q.Z., J.-X.L., Y.-Y.W., Z.S. and T.C. Drafting of the manuscript: H.-L.S. and Y.-T.X. Critical revision of the manuscript: T.J. Approval of the final version for publication: all co-authors. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The study was supported by the National Science and Technology Major Project for investigational new drug development (2018ZX09201-014), the Beijing Municipal Science & Technology Commission (No. Z181100001518005), and the University of Macau (MYRG2019-00066-FHS; MYRG2022-00187-FHS) and Ministry of Education Frontiers Science Center for Precision Oncology, University of Macau (MoE-FSCPO)—Understanding the occurrence and development of cancer for precision oncology (No.: SP2022-00001-FSCPO).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Claesson M.J., O’Toole P.W. Evaluating the latest high-throughput molecular techniques for the exploration of microbial gut communities. Gut Microbes. 2010;1:277–278. doi: 10.4161/gmic.1.4.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parker A., Fonseca S., Carding S.R. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes. 2020;11:135–157. doi: 10.1080/19490976.2019.1638722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angelucci F., Cechova K., Amlerova J., Hort J. Antibiotics, gut microbiota, and Alzheimer’s disease. J. Neuroinflamm. 2019;16:108. doi: 10.1186/s12974-019-1494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin C.R., Osadchiy V., Kalani A., Mayer E.A. The Brain-Gut-Microbiome Axis. Cell Mol. Gastroenterol. Hepatol. 2018;6:133–148. doi: 10.1016/j.jcmgh.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharon G., Sampson T.R., Geschwind D.H., Mazmanian S.K. The Central Nervous System and the Gut Microbiome. Cell. 2016;167:915–932. doi: 10.1016/j.cell.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandilyan M.B., Dening T. Evidence-Based Practice in Dementia for Nurses and Nursing Students. Jessica Kingsley; London, UK: 2019. What is dementia. [Google Scholar]
- 7.Gale S.A., Acar D., Daffner K.R. Dementia. Am. J. Med. 2018;131:1161–1169. doi: 10.1016/j.amjmed.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization . Global Action Plan on the Public Health Response to Dementia 2017–2025. World Health Organization; Geneva, Switzerland: 2017. [Google Scholar]
- 9.Alzheimer’s Disease Facts and Figures 2020 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2020;16:391–460. doi: 10.1002/alz.12068. [DOI] [Google Scholar]
- 10.Li W., Guo J., Shen Y., Huang L., Leng B., Fan D., Shui L., Chen C. Probiotics, prebiotics, and synbiotics for the treatment of dementia: Protocol for a systematic review. Medicine. 2020;99:e18608. doi: 10.1097/MD.0000000000018608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins S.M., Surette M., Bercik P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012;10:735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- 12.Sampson T.R., Mazmanian S.K. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe. 2015;17:565–576. doi: 10.1016/j.chom.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang B., Wang H.E., Bai Y.M., Tsai S.J., Su T.P., Chen T.J., Wang Y.P., Chen M.H. Inflammatory bowel disease is associated with higher dementia risk: A nationwide longitudinal study. Gut. 2021;70:85–91. doi: 10.1136/gutjnl-2020-320789. [DOI] [PubMed] [Google Scholar]
- 14.Fröhlich E.E., Farzi A., Mayerhofer R., Reichmann F., Jačan A., Wagner B., Zinser E., Bordag N., Magnes C., Fröhlich E., et al. Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain Behav. Immun. 2016;56:140–155. doi: 10.1016/j.bbi.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang T., Hu X., Liang S., Li W., Wu X., Wang L., Jin F. Lactobacillus fermentum NS9 restores the antibiotic induced physiological and psychological abnormalities in rats. Benef. Microbes. 2015;6:707–717. doi: 10.3920/BM2014.0177. [DOI] [PubMed] [Google Scholar]
- 16.Durieux V., Gevenois P.A. Bibliometric indicators: Quality measurements of scientific publication. Radiology. 2010;255:342–351. doi: 10.1148/radiol.09090626. [DOI] [PubMed] [Google Scholar]
- 17.Kokol P., Blažun Vošner H., Završnik J. Application of bibliometrics in medicine: A historical bibliometrics analysis. Health Info. Libr. J. 2021;38:125–138. doi: 10.1111/hir.12295. [DOI] [PubMed] [Google Scholar]
- 18.Guo J., Gu D., Zhao T., Zhao Z., Xiong Y., Sun M., Xin C., Zhang Y., Pei L., Sun J. Trends in Piezo Channel Research Over the Past Decade: A Bibliometric Analysis. Front. Pharmacol. 2021;12:668714. doi: 10.3389/fphar.2021.668714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cabanillas-Lazo M., Quispe-Vicuña C., Barja-Ore J., Fernandez-Giusti A., Munive-Degregori A., Retamozo-Siancas Y., Guerrero M.E., Mayta-Tovalino F. A 10-Year Bibliometric Analysis of Global Research on Gut Microbiota and Parkinson’s Disease: Characteristics, Impact, and Trends. BioMed Res. Int. 2022;2022:4144781. doi: 10.1155/2022/4144781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu X., Hu J., Deng S., Tan Y., Qiu C., Zhang M., Ni X., Lu H., Wang Z., Li L., et al. Bibliometric and Visual Analysis of Research on the Links Between the Gut Microbiota and Depression From 1999 to 2019. Front. Psychiatry. 2020;11:587670. doi: 10.3389/fpsyt.2020.587670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H., Long T., You J., Li P., Xu Q. Bibliometric Visualization Analysis of Microbiome-Gut-Brain Axis from 2004 to 2020. Med. Sci. Monit. 2022;28:e936037. doi: 10.12659/MSM.936037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prince M., Bryce R., Albanese E., Wimo A., Ribeiro W., Ferri C.P. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimer’s Dement. 2013;9:63–75.e2. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Aria M., Cuccurullo C. bibliometrix: An R-tool for comprehensive science mapping analysis. J. Informetr. 2017;11:959–975. doi: 10.1016/j.joi.2017.08.007. [DOI] [Google Scholar]
- 24.van Eck N.J., Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84:523–538. doi: 10.1007/s11192-009-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whittemore R., Chao A., Jang M., Minges K.E., Park C. Methods for knowledge synthesis: An overview. Heart Lung. 2014;43:453–461. doi: 10.1016/j.hrtlng.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Cryan J.F., O’Riordan K.J., Cowan C.S.M., Sandhu K.V., Bastiaanssen T.F.S., Boehme M., Codagnone M.G., Cussotto S., Fulling C., Golubeva A.V., et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019;99:1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 27.Kowalski K., Mulak A. Brain-Gut-Microbiota Axis in Alzheimer’s Disease. J. Neurogastroenterol. Motil. 2019;25:48–60. doi: 10.5056/jnm18087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aksnes D.W., Langfeldt L., Wouters P. Citations, citation indicators, and research quality: An overview of basic concepts and theories. Sage Open. 2019;9:2158244019829575. doi: 10.1177/2158244019829575. [DOI] [Google Scholar]
- 29.Bhattacharjee S., Lukiw W.J. Alzheimer’s disease and the microbiome. Front. Cell Neurosci. 2013;7:153. doi: 10.3389/fncel.2013.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dinan T.G., Cryan J.F. Gut instincts: Microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 2017;595:489–503. doi: 10.1113/JP273106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Itzhaki R.F., Lathe R., Balin B.J., Ball M.J., Bearer E.L., Braak H., Bullido M.J., Carter C., Clerici M., Cosby S.L., et al. Microbes and Alzheimer’s Disease. J. Alzheimer’s Dis. 2016;51:979–984. doi: 10.3233/JAD-160152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray E.R., Kemp M., Nguyen T.T. The Microbiota-Gut-Brain Axis in Alzheimer’s Disease: A Review of Taxonomic Alterations and Potential Avenues for Interventions. Arch. Clin. Neuropsychol. 2022;37:595–607. doi: 10.1093/arclin/acac008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen C.H., Lin C.L., Kao C.H. Irritable Bowel Syndrome Is Associated with an Increased Risk of Dementia: A Nationwide Population-Based Study. PLoS ONE. 2016;11:e0144589. doi: 10.1371/journal.pone.0144589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bornmann L., Daniel H.D. What do we know about the h index? J. Am. Soc. Inf. Sci. Technol. 2007;58:1381–1385. doi: 10.1002/asi.20609. [DOI] [Google Scholar]
- 35.Sun H.L., Bai W., Li X.H., Huang H., Cui X.L., Cheung T., Su Z.H., Yuan Z., Ng C.H., Xiang Y.T. Schizophrenia and Inflammation Research: A Bibliometric Analysis. Front. Immunol. 2022;13:907851. doi: 10.3389/fimmu.2022.907851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiraz M. A Holistic Investigation of Global Outputs of COVID-19 Publications in Neurology and Neurosurgery. 2020, 4, 506–512. Eurasian J. Med. Investig. 2020;4:506–512. [Google Scholar]
- 37.Peterson J., Garges S., Giovanni M., McInnes P., Wang L., Schloss J.A., Bonazzi V., McEwen J.E., Wetterstrand K.A., Deal C., et al. The NIH Human Microbiome Project. Genome Res. 2009;19:2317–2323. doi: 10.1101/gr.096651.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding T., Schloss P.D. Dynamics and associations of microbial community types across the human body. Nature. 2014;509:357–360. doi: 10.1038/nature13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ley R.E., Lozupone C.A., Hamady M., Knight R., Gordon J.I. Worlds within worlds: Evolution of the vertebrate gut microbiota. Nat. Rev. Microbiol. 2008;6:776–788. doi: 10.1038/nrmicro1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang X., Lai H., Zhang F., Wang Y., Zhang L., Yang N., Wang C., Liang Z., Zeng J., Yang J. Visualization and Analysis in the Field of Pan-Cancer Studies and Its Application in Breast Cancer Treatment. Front. Med. 2021;8:635035. doi: 10.3389/fmed.2021.635035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lane C.A., Hardy J., Schott J.M. Alzheimer’s disease. Eur. J. Neurol. 2018;25:59–70. doi: 10.1111/ene.13439. [DOI] [PubMed] [Google Scholar]
- 42.Murphy M.P., LeVine H., 3rd Alzheimer’s disease and the amyloid-beta peptide. J. Alzheimer’s Dis. 2010;19:311–323. doi: 10.3233/JAD-2010-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Megur A., Baltriukienė D., Bukelskienė V., Burokas A. The Microbiota-Gut-Brain Axis and Alzheimer’s Disease: Neuroinflammation Is to Blame? Nutrients. 2020;13:37. doi: 10.3390/nu13010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salminen S., Bouley C., Boutron-Ruault M.C., Cummings J.H., Franck A., Gibson G.R., Isolauri E., Moreau M.C., Roberfroid M., Rowland I. Functional food science and gastrointestinal physiology and function. Br. J. Nutr. 1998;80((Suppl. 1)):S147–S171. doi: 10.1079/BJN19980108. [DOI] [PubMed] [Google Scholar]
- 45.Goswami A., Wendt F.R., Pathak G.A., Tylee D.S., De Angelis F., De Lillo A., Polimanti R. Role of microbes in the pathogenesis of neuropsychiatric disorders. Front. Neuroendocrinol. 2021;62:100917. doi: 10.1016/j.yfrne.2021.100917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cattaneo A., Cattane N., Galluzzi S., Provasi S., Lopizzo N., Festari C., Ferrari C., Guerra U.P., Paghera B., Muscio C., et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging. 2017;49:60–68. doi: 10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 47.Minter M.R., Zhang C., Leone V., Ringus D.L., Zhang X., Oyler-Castrillo P., Musch M.W., Liao F., Ward J.F., Holtzman D.M., et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci. Rep. 2016;6:30028. doi: 10.1038/srep30028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bagyinszky E., Giau V.V., Shim K., Suk K., An S.S.A., Kim S. Role of inflammatory molecules in the Alzheimer’s disease progression and diagnosis. J. Neurol. Sci. 2017;376:242–254. doi: 10.1016/j.jns.2017.03.031. [DOI] [PubMed] [Google Scholar]
- 49.Silva Y.P., Bernardi A., Frozza R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020;11:25. doi: 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qian X.H., Xie R.Y., Liu X.L., Chen S.D., Tang H.D. Mechanisms of Short-Chain Fatty Acids Derived from Gut Microbiota in Alzheimer’s Disease. Aging Dis. 2022;13:1252–1266. doi: 10.14336/AD.2021.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Figueira J., Jonsson P., Nordin Adolfsson A., Adolfsson R., Nyberg L., Öhman A. NMR analysis of the human saliva metabolome distinguishes dementia patients from matched controls. Mol. Biosyst. 2016;12:2562–2571. doi: 10.1039/C6MB00233A. [DOI] [PubMed] [Google Scholar]
- 52.Yilmaz A., Geddes T., Han B., Bahado-Singh R.O., Wilson G.D., Imam K., Maddens M., Graham S.F. Diagnostic Biomarkers of Alzheimer’s Disease as Identified in Saliva using 1H NMR-Based Metabolomics. J. Alzheimer’s Dis. 2017;58:355–359. doi: 10.3233/JAD-161226. [DOI] [PubMed] [Google Scholar]
- 53.Rezaie A., Parker R.D., Abdollahi M. Oxidative Stress and Pathogenesis of Inflammatory Bowel Disease: An Epiphenomenon or the Cause? Dig. Dis. Sci. 2007;52:2015–2021. doi: 10.1007/s10620-006-9622-2. [DOI] [PubMed] [Google Scholar]
- 54.Momtaz Y.A., Hamid T.A., Ibrahim R. Gastritis May Boost Odds of Dementia. Am. J. Alzheimer’s Dis. Other Demen. 2014;29:452–456. doi: 10.1177/1533317513518654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doulberis M., Kotronis G., Gialamprinou D., Polyzos S.A., Papaefthymiou A., Katsinelos P., Kountouras J. Alzheimer’s disease and gastrointestinal microbiota; impact of Helicobacter pylori infection involvement. Int. J. Neurosci. 2021;131:289–301. doi: 10.1080/00207454.2020.1738432. [DOI] [PubMed] [Google Scholar]
- 56.Adewuyi E.O., O’Brien E.K., Nyholt D.R., Porter T., Laws S.M. A large-scale genome-wide cross-trait analysis reveals shared genetic architecture between Alzheimer’s disease and gastrointestinal tract disorders. Commun. Biol. 2022;5:691. doi: 10.1038/s42003-022-03607-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caputi V., Giron M.C. Microbiome-Gut-Brain Axis and Toll-Like Receptors in Parkinson’s Disease. Int. J. Mol. Sci. 2018;19:1689. doi: 10.3390/ijms19061689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fasano A., Visanji N.P., Liu L.W., Lang A.E., Pfeiffer R.F. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2015;14:625–639. doi: 10.1016/S1474-4422(15)00007-1. [DOI] [PubMed] [Google Scholar]
- 59.Pfeiffer R.F. Gastrointestinal dysfunction in Parkinson’s disease. Parkinsonism Relat. Disord. 2011;17:10–15. doi: 10.1016/j.parkreldis.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 60.Shen X., Yang H., Wu Y., Zhang D., Jiang H. Meta-analysis: Association of Helicobacter pylori infection with Parkinson’s diseases. Helicobacter. 2017;22:e12398. doi: 10.1111/hel.12398. [DOI] [PubMed] [Google Scholar]
- 61.Bjarnason I.T., Charlett A., Dobbs R.J., Dobbs S.M., Ibrahim M.A., Kerwin R.W., Mahler R.F., Oxlade N.L., Peterson D.W., Plant J.M., et al. Role of chronic infection and inflammation in the gastrointestinal tract in the etiology and pathogenesis of idiopathic parkinsonism. Part 2: Response of facets of clinical idiopathic parkinsonism to Helicobacter pylori eradication. A randomized, double-blind, placebo-controlled efficacy study. Helicobacter. 2005;10:276–287. doi: 10.1111/j.1523-5378.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- 62.Brandscheid C., Schuck F., Reinhardt S., Schäfer K.H., Pietrzik C.U., Grimm M., Hartmann T., Schwiertz A., Endres K. Altered Gut Microbiome Composition and Tryptic Activity of the 5xFAD Alzheimer’s Mouse Model. J. Alzheimer’s Dis. 2017;56:775–788. doi: 10.3233/JAD-160926. [DOI] [PubMed] [Google Scholar]
- 63.Huang H., Nie S., Cao M., Marshall C., Gao J., Xiao N., Hu G., Xiao M. Characterization of AD-like phenotype in aged APPSwe/PS1dE9 mice. Age. 2016;38:303–322. doi: 10.1007/s11357-016-9929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ferguson S.A., Sarkar S., Schmued L.C. Longitudinal behavioral changes in the APP/PS1 transgenic Alzheimer’s Disease model. Behav. Brain Res. 2013;242:125–134. doi: 10.1016/j.bbr.2012.12.055. [DOI] [PubMed] [Google Scholar]
- 65.Liu Y.W., Liong M.T., Tsai Y.C. New perspectives of Lactobacillus plantarum as a probiotic: The gut-heart-brain axis. J. Microbiol. 2018;56:601–613. doi: 10.1007/s12275-018-8079-2. [DOI] [PubMed] [Google Scholar]
- 66.Donthu N., Kumar S., Mukherjee D., Pandey N., Lim W.M. How to conduct a bibliometric analysis: An overview and guidelines. J. Bus. Res. 2021;133:285–296. doi: 10.1016/j.jbusres.2021.04.070. [DOI] [Google Scholar]
- 67.Kiraz S., Demir E. Global Scientific Outputs of Schizophrenia Publications From 1975 to 2020: A Bibliometric Analysis. Psychiatr. Q. 2021;92:1725–1744. doi: 10.1007/s11126-021-09937-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.