Abstract
Background
Peutz-Jeghers syndrome (PJS) is a rare, autosomal dominant disorder caused by germline mutations of STK11/LKB1, with an increased risk of tumors at multiple sites. Intraductal oncocytic papillary neoplasm (IOPN) is a unique subtype of intraductal papillary neoplasm of the bile duct (IPNB) defined by a premalignant neoplasm with intraductal papillary or villous growth of biliary-type epithelium. IOPN has a distinct mutation profile compared with both IPNB and intraductal papillary mucinous neoplasm (IPMN).
Case presentation
We herein describe the case of a 44-year-old woman who presented as polyps in the intestinal lumen of sigmoid colon and a 3.1 × 2.1 cm mass in the left lobe of liver. Gross feature revealed a cystic papillary mass and the neoplasm had a clear boundary with the surrounding liver tissue. Histology revealed complex papillary structures, a small amount of fine fibrovascular cores and immunohistochemistry showed extensive positive for MUC5AC, MUC6, CD117. Therefore, histological and immunohistochemical examination of the liver tumor suggested the diagnosis of IOPN. Next-generation sequencing (NGS) revealed other than STK11 germline mutation, the tumor also harbors GNAS somatic mutation at codon 478 and EGFR amplification.
Conclusion
To our knowledge, this is the first report of IOPN arising in PJS. This case enlarges the spectrum of PJS related tumors and genetic rearrangements in IOPN.
Keywords: Peutz-Jeghers syndrome, Intraductal oncocytic papillary neoplasm, GNAS, STK11, EGFR
Background
Peutz-Jeghers syndrome (PJS) is an autosomal dominant hereditary disease characterized by hamartomatous polyps of the gastrointestinal tract and mucocutaneous melanin deposits [1]. PJS is commonly caused by germline mutations in the tumor suppressor gene LKB1/STK11 on chromosome 19 [2]. Previous studies have reported that patients with PJS had a significantly increased risk of gastrointestinal and extra-intestinal malignancies [1, 2]. Common extra-intestinal malignancies are breast cancer, ovarian cancer, cervical cancer, pancreatic cancer. Among them, the case of intraductal papillary mucinous neoplasm (IPMN) arising in Peutz-Jeghers syndrome is extremely rare [3]. Furthermore, intraductal papillary neoplasm of the bile duct (IPNB) with PJS has not been reported before. IPNB is an epithelial neoplasm with a tendency to progress into invasive cholangiocarcinoma and considered the biliary counterpart of IPMN [4]. Histologically, IPNB can be divided into four subtypes: intestinal, gastric, pancreatobiliary and oncocytic [4]. The oncocytic subtype of IPNB is also known as Intraductal oncocytic papillary neoplasm (IOPN) [5].
Previous studies have discovered genetic mutations in IPNB and IPMN through next-generation sequencing (NGS) method, such as GNAS, KRAS, TP53, STK11, CTNNB1, RNF43, APC, SMAD4, EGFR, etc. [4]. Recently, however, few reports suggest a distinct mutation profile of IOPN compared with IPNB and IPMN [5]. For example, GANS mutations are frequently found in both IPNB and IPMN [5]. While only one literature described IOPN could also harbor GNAS mutation [6]. As for EGFR mutations or amplifications, which are already uncommon in IPNB and IPMN, has not yet been reported in IOPN [7]. On the contrary, STK11 mutations are more frequently seen in IOPN than IPNB and IPMN [8].
Here we report an extraordinarily rare case of IOPN arising in PJS, harboring GNAS somatic mutation at codon 478, EGFR amplification and STK11 germline mutation. To the best of our knowledge, this is the first case to report in literature. Therefore, the case report increases the spectrum of PJS related tumors and provides new genetic arrangements in IOPN.
Case presentation
Clinical history
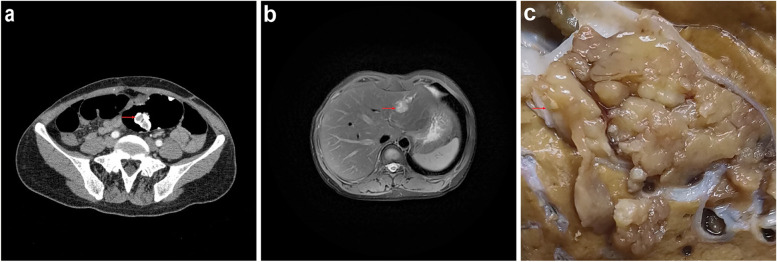
A 44-year-old Chinese woman was admitted to our hospital due to liver occupation during physical examination. Her suspected identical twin was a patient with PJS and died of colon cancer. Her other relatives do not suffer from PJS. And the patient had more than 30 years with pigmentation on the lip and intermittent abdominal pain for more than 10 years. Pelvic computed tomography (CT) scans revealed nodular soft tissue tumor in the intestinal lumen of sigmoid colon, about 2.3 × 1.0 cm in size, surrounded by high-density contrast agent, suggesting polyps (Fig. 1a). Abdominal color ultrasound showed the high echo area in the left lobe of liver with clear boundary and regular shape. Abdominal CT scans revealed a round low-density mass in the left lobe of liver with a size of 3.1 × 2.1 cm and a clear boundary. Abdominal magnetic resonance imaging (MRI) indicated mild expansion of the distal bile duct of the lesion in addition to the above mass (Fig. 1b). Abdominal color ultrasound, CT and MRI prompted suspicion of primary liver tumor.
Fig. 1.
Pelvic CT and Abdominal MRI from the case and gross features of the neoplasm. a Pelvic CT showed nodular soft tissue shadow in the intestinal lumen of sigmoid colon, surrounded by high-density contrast agent. Red arrow indicates designate polyps. b Abdominal MRI showed a round low-density mass in the left lobe of liver with a clear border. Red arrow indicates designate IOPN. c Macroscopically, the neoplasm had a clear boundary with the surrounding liver tissue. Red arrow indicates designate IOPN
Pathological findings of resected specimens
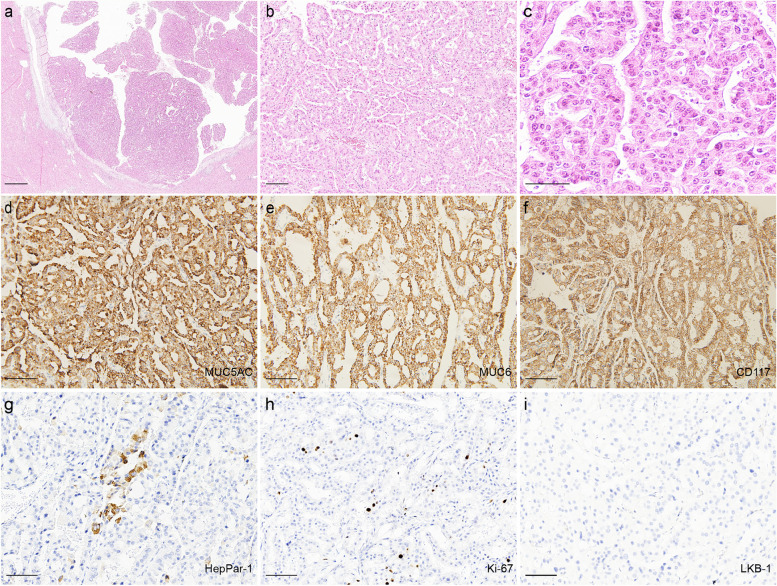
Grossly, a cystic papillary mass was seen in the hepatic bile duct, and the mass size was 2.1 × 2.8 × 1.7 cm. The distal hepatic bile duct was dilated. Macroscopically, the neoplasm had a clear boundary with the surrounding liver tissue, and did not invade the bile duct wall and surrounding liver tissue (Fig. 1c). Histology showed the mass was located in the hepatic bile duct. The bile duct wall and surrounding liver tissue were not invaded (Fig. 2a). The mass presented as complex papillary structures, a small amount of fine fibrovascular cores lined by 2 to 5 layers of cuboidal to columnar cells (Fig. 2b). And a small amount of mucus could be seen in the lumen. The neoplastic cells were abundant eosinophilic granular cytoplasm, with round or oval nuclei, clear nucleolus and the mitotic figure is rarely seen (Fig. 2c).
Fig. 2.
Histological and Immunohistochemical features of the neoplasm. a The bile duct wall and surrounding liver tissue were not invaded. (Scale bar = 1000 μm). b The neoplasm shows complex papillary structures, fine fibrovascular stalks. (Scale bar = 100 μm). c The tumor cells were abundant eosinophilic granular cytoplasm, round or oval nuclei, clear nucleoli. (Scale bar = 50 μm). d-h Tumor cells were positive for MUC5AC (d), MUC6 (e), CD117 (f), HepPar-1 (g) and the Ki-67 (h) index is relatively low. (All scale bars = 100 μm). i LKB1 staining was negative in the tumor cells. (Scale bar = 100 μm)
Immunohistochemical examination showed that tumor cells showed diffusely and intensely positive for MUC5AC, MUC6, CD117 (Fig. 2d–f), focal and patchy positivity of HepPar-1(Fig. 2g), multifocal positivity of S100P, MUC1 and negative for LKB1(Fig. 2h), CK20, MUC2, CDX-2. The Ki-67 proliferating index was 8% (Fig. 2i). Conclusively, our diagnosis of the hepatic lesion in this patient was “IOPN arising in PJS bile duct.”
Molecular findings of resected specimens
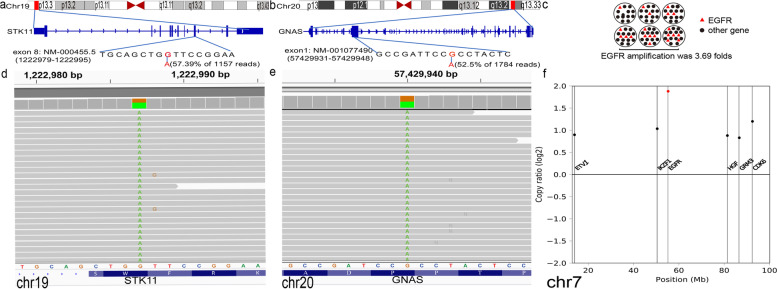
Mutations included germline STK11 c.924G > A (p.W308*), exon 8 in 57.39% of 1157 reads, GNAS c.1433G > A (p.R478H), exon 1 in 52.5% of 1784 reads, and EGFR amplification with 3.69 folds in the patient (Fig. 3a-c) (Results of complete NGS were summarized in Table 1) were detected by NGS through TruSight Oncology 500 from Illumina.
Fig. 3.
Molecular profile of the IOPN described in this case. a-c Schematic illustration of STK11 (a) mutation, GNAS (b) mutation and EGFR (c) amplification. d The sequencing result showed STK11 c.924G > A (p.W308*), exon 8 in 57.39% of 1157 reads mutation. e The sequencing result showed GNAS c.1433G > A (p.R478H), exon 1 in 52.5% of 1784 reads mutation. f The sequencing result showed EGFR amplification was 3.69 folds. (Data presented as log2 fold change at the FDR < 0.05)
Table 1.
Results of NGS in the patient
| Mutation | Reads |
|---|---|
| STK11 c.924G > A (p.W308*), exon 8 | 57.39%/1157 |
| GNAS c.1433G > A (p.R478H), exon 1 | 52.50%/1782 |
| FOXP1 c.174-176del (p. Q59del), exon 6 | 1.80%/3219 |
| SF3B1 c.1849 A > G (p.I617V), exon 14 | 1.90%/1263 |
| TRAF7 c.759-2 A > G, exon 10 | 3.08%/843 |
| TRAF7 c.805 A > G (p.1269 V), exon 10 | 3.90%/950 |
| TRAF7 c.815 A > C (p.Q272P), exon 10 | 4.53%/1016 |
| EGFR amplification with 3.69 | NA |
| NA indicates not available |
Treatments and outcome
The patient underwent hepatic left lateral lobectomy due to liver neoplasm. After 18 months of follow-up by CT and MRI surveillance, no evidence of tumor recurrence or progression was observed.
Discussion and conclusion
IOPN is an epithelial neoplasm derived from the pancreatobiliary system. In contrast to the other types of IPNB, IOPN is composed of complex, arborizing papillae lined by one to several stratified layers of cuboidal to columnar cells, and the cells are abundant eosinophilic granular cytoplasm, hyperchromatic, round, large, and fairly uniform nuclei [9]. Symptoms of IOPN are not specific. IOPN is frequently presented with intermittent biliary obstruction, recurrent pyogenic cholangitis, jaundice, intermittent abdominal pain, nausea, and weight loss [9]. Molecular studies of IOPN are scant [5, 6, 8, 10–18]. Here, we have summarized previous publications of molecular alterations of IOPN (both pancreatic and biliary) in Table 2. As we can see from Table 2, IOPN rarely harbors GNAS mutations, which are frequently found in both IPMN and IPNB. Other than gene mutations, recent publications have discovered recurrent rearrangements in PRKACA and PRKACB in IOPN [5, 17, 18]. These fusions result in increased PRKACA or PRKACB expression and, consequently, an increase in Protein Kinase A (PKA) activity [5, 17, 18].
Table 2.
Review of reported molecular alterations of IOPN
| References | Upregulated gene |
Fusion | Loss of heterozygosity |
Amplification | Gene mutation | Origin |
|---|---|---|---|---|---|---|
| Xiao et al. [10] | KRAS, BRAF | Pancreas | ||||
| Mohri et al. [11] | KRAS | Pancreas | ||||
| Schlitter et al. [12] | P16 | Bile Duct | ||||
| Amato et al. [6] | GNAS, KRAS | Pancreas | ||||
| Basturk et al. [13] | ARHGAP26, ASXL1, EPHA8, ERBB4 | Pancreas | ||||
| Singhi et al. [5] |
ATP1B1-PRKACA, ATP1B1-PRKACB, DNAJB1-PRKACA |
Pancreas, Bile Duct | ||||
| Vyas et al. [18] |
DNAJB1-PRKACA, PRKACA-ATP1B1 |
Pancreas, Bile Duct | ||||
| Aoki et al. [14] | TP53, KRAS, PBRM1, ELF3, NF1, BAP1, EPHA6, ERBB3, KMT2D, CDKN2A | Bile Duct | ||||
| Nakahodo et al. [15] |
Follistatin (FST) |
Pancreas, Bile Duct | ||||
| Chang et al. [16] | KRAS | Pancreas | ||||
| Omori et al. [8] | P16 | STK11, KRAS, P53, SMAD4 | Pancreas | |||
| Maimaitiaili et al. [17] |
ATP1B1-PRKACA, DNAJB1-PRKACA ATP1B1-PRKACB |
KRAS | Pancreas, Bile Duct | |||
| This case | EGFR | STK11, GNAS | Bile Duct |
NGS revealed the patient in our study mainly had STK11 mutation, GNAS mutation and EGFR amplification. STK11 is a tumor suppressor gene, which acts to restrict cell growth via mTOR inactivation and induction of other AMP(adenosine monophosphate)-activated protein kinase (AMPK)-related kinases [19]. GNAS mutation leads to constitutive activation of the cyclic adenosine monophosphate (cAMP)-PKA signaling pathway [20]. This causes upregulation of epidermal growth factor receptor (EGFR) [21]. EGFR is a 170-kDa monomeric glycoprotein [22].When EGFR is amplified, it stimulates cell proliferation via EGFR-mechanistic target of rapamycin (mTOR) Pathway [23]. On the one hand, STK11 defection promotes cell growth by activating mTOR pathway. On the other hand, GNAS mutation and EGFR amplification promote cell growth by activation of PKA, overexpression of EGFR and stimulation of mTOR. Therefore, we propose that STK11 mutation, GNAS mutation and EGFR amplification together promote tumorigenesis of IOPN. Both GNAS mutations and PRKACA and PRKACB fusions result in an increase in PKA activity [5, 17, 18]. This suggest that in our case, the function of GNAS mutation might be “mimicking” PRKACA and PRKACB fusions in the development of IOPN.
In this case, the tumor showed GNAS gene mutation at codon 478 (exon 1, c.1433G > A). While previous research only reported that IPNB and IPMN have GNAS mutations at codon 201 [4, 24]. This novel mutation locus adds to the spectrum of genetic mutations associated to IPNB. Simultaneously, it suggests that the GNAS mutation locus in IOPN may be different from other subtypes of IPNB and IPMN.
In conclusion, we have described for the first time a rare case of IOPN arising in PJS. Current surveillance programs for PJS are enforcement both for prevention of gastrointestinal complications and for early detection of relevant malignancies [2]. IOPN is not part of the known spectrum of PJS, thus we suggest that the early screening of pancreatobiliary system could also be important in the prevention of PJS related malignancies.
Acknowledgements
To anyone who has participated in the care of this patient directly or indirectly.
Abbreviations
- PJS
Peutz-Jeghers syndrome
- IOPN
intraductal oncocytic papillary neoplasm
- IPNB
intraductal papillary neoplasm of the bile duct
- IPMN
intraductal papillary mucinous neoplasm
- NGS
next-generation sequencing
- CT
computed tomography
- MRI
magnetic resonance imaging
- FST
follistatin
- PKA
protein kinase A, cAMP-dependent protein kinase
- AMP
adenosine monophosphate
- AMPK
AMP-activated protein kinase
- cAMP
cyclic adenosine monophosphate
- EGFR
epidermal growth factor receptor
- mTOR
mechanistic target of rapamycin
Author’s contributions
Qingyue Liu drafted the manuscript. Teng Li conceived the study and reviewed the manuscript. Zhiyu Wang drafted the figures. Chaoran Yu and Jianping Zhu prepared the histological and immunohistochemical images. Li Ren performed histopathological evaluations. Chengli Liu performed the operation and collected clinical information. Xiangsheng Li examined the patient with CT, MRI and provided related data. The author(s) read and approved the final manuscript.
Funding
This work was supported by Beijing Nova Program (Z181100006218065).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The ethical approval and documentation for the participation and publication of this case report were waived with approval of the Institutional Review Board at Air Force Medical Center, PLA.
Consent for publication
Written informed consent was obtained from the patient for the publication of this case report and any accompanying images.
Competing interests
The authors have no conflicts of interest to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sengupta S, Bose S. Peutz-Jeghers Syndrome. N Engl J Med. 2019;380:472. doi: 10.1056/NEJMicm1806623. [DOI] [PubMed] [Google Scholar]
- 2.Boland CR, Idos GE, Durno C, Giardiello FM, Anderson JC, Burke CA, et al. Diagnosis and management of Cancer Risk in the gastrointestinal hamartomatous polyposis syndromes: recommendations from the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2022;162:2063–85. doi: 10.1053/j.gastro.2022.02.021. [DOI] [PubMed] [Google Scholar]
- 3.Goh PG, Moon HS, Sung JK, Jeong HY, Song KS. [A case of Peutz-Jeghers syndrome with intraductal papillary mucinous carcinoma of pancreas] Korean J gastroenterology = Taehan Sohwagi Hakhoe chi. 2010;55:73–7. doi: 10.4166/kjg.2010.55.1.73. [DOI] [PubMed] [Google Scholar]
- 4.Yang CY, Huang WJ, Tsai JH, Cheng A, Chen CC, Hsu HP, et al. Targeted next-generation sequencing identifies distinct clinicopathologic and molecular entities of intraductal papillary neoplasms of the bile duct. Mod pathology: official J United States Can Acad Pathol Inc. 2019;32:1637–45. doi: 10.1038/s41379-019-0306-9. [DOI] [PubMed] [Google Scholar]
- 5.Singhi AD, Wood LD, Parks E, Torbenson MS, Felsenstein M, Hruban RH, et al. Recurrent rearrangements in PRKACA and PRKACB in Intraductal Oncocytic Papillary Neoplasms of the pancreas and bile Duct. Gastroenterology. 2020;158:573–82.e2. doi: 10.1053/j.gastro.2019.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amato E, Molin MD, Mafficini A, Yu J, Malleo G, Rusev B, et al. Targeted next-generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas. J Pathol. 2014;233:217–27. doi: 10.1002/path.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chadwick B, Willmore-Payne C, Tripp S, Layfield LJ, Hirschowitz S, Holden J. Histologic, immunohistochemical, and molecular classification of 52 IPMNs of the pancreas. Appl Immunohistochem Mol morphology: AIMM. 2009;17:31–9. doi: 10.1097/PAI.0b013e31817c02c6. [DOI] [PubMed] [Google Scholar]
- 8.Omori Y, Ono Y, Morikawa T, Motoi F, Higuchi R, Yamamoto M, et al. Serine/Threonine kinase 11 plays a canonical role in malignant progression of KRAS-mutant and GNAS-wild-type Intraductal Papillary Mucinous Neoplasms of the pancreas. Ann Surg. 2021. 10.1097/SLA.0000000000004842. Epub ahead of print. [DOI] [PubMed]
- 9.Wang T, Askan G, Adsay V, Allen P, Jarnagin WR, Memis B, et al. Intraductal Oncocytic Papillary Neoplasms: clinical-pathologic characterization of 24 cases, with an emphasis on Associated Invasive Carcinomas. Am J Surg Pathol. 2019;43:656–61. doi: 10.1097/PAS.0000000000001226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao HD, Yamaguchi H, Dias-Santagata D, Kuboki Y, Akhavanfard S, Hatori T, et al. Molecular characteristics and biological behaviours of the oncocytic and pancreatobiliary subtypes of intraductal papillary mucinous neoplasms. J Pathol. 2011;224:508–16. doi: 10.1002/path.2875. [DOI] [PubMed] [Google Scholar]
- 11.Mohri D, Asaoka Y, Ijichi H, Miyabayashi K, Kudo Y, Seto M, et al. Different subtypes of intraductal papillary mucinous neoplasm in the pancreas have distinct pathways to pancreatic cancer progression. J Gastroenterol. 2012;47:203–13. doi: 10.1007/s00535-011-0482-y. [DOI] [PubMed] [Google Scholar]
- 12.Schlitter AM, Born D, Bettstetter M, Specht K, Kim-Fuchs C, Riener MO, et al. Intraductal papillary neoplasms of the bile duct: stepwise progression to carcinoma involves common molecular pathways. Mod pathology: official J United States Can Acad Pathol Inc. 2014;27:73–86. doi: 10.1038/modpathol.2013.112. [DOI] [PubMed] [Google Scholar]
- 13.Basturk O, Tan M, Bhanot U, Allen P, Adsay V, Scott SN, et al. The oncocytic subtype is genetically distinct from other pancreatic intraductal papillary mucinous neoplasm subtypes. Mod pathology: official J United States Can Acad Pathol Inc. 2016;29:1058–69. doi: 10.1038/modpathol.2016.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aoki Y, Mizuma M, Hata T, Aoki T, Omori Y, Ono Y, et al. Intraductal papillary neoplasms of the bile duct consist of two distinct types specifically associated with clinicopathological features and molecular phenotypes. J Pathol. 2020;251:38–48. doi: 10.1002/path.5398. [DOI] [PubMed] [Google Scholar]
- 15.Nakahodo J, Fukumura Y, Saito T, Hirabayashi K, Doi R, Hayashi T, et al. Upregulation of follistatin and low apoptotic activity in intraductal oncocytic papillary neoplasm of the pancreatobiliary system. Sci Rep. 2020;10:8179. doi: 10.1038/s41598-020-64920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang XY, Wu Y, Jiang Y, Wang PY, Chen J. RNF43 mutations in IPMN cases: a potential prognostic factor. Gastroenterol Res Pract. 2020;2020:1457452. doi: 10.1155/2020/1457452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maimaitiaili Y, Fukumura Y, Hirabayashi K, Kinowaki Y, Naito Y, Saito A, et al. Investigation of -PRKACA/-PRKACB fusion genes in oncocytic tumors of the pancreatobiliary and other systems. Virchows Archiv. 2022;481:865–76. [DOI] [PubMed]
- 18.Vyas M, Hechtman JF, Zhang Y, Benayed R, Yavas A, Askan G, et al. DNAJB1-PRKACA fusions occur in oncocytic pancreatic and biliary neoplasms and are not specific for fibrolamellar hepatocellular carcinoma. Mod pathology: official J United States Can Acad Pathol Inc. 2020;33:648–56. doi: 10.1038/s41379-019-0398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kottakis F, Bardeesy N. LKB1-AMPK axis revisited. Cell Res. 2012;22:1617–20. doi: 10.1038/cr.2012.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe K, Nakamura T, Onodera S, Saito A, Shibahara T, Azuma T. A novel GNAS-mutated human induced pluripotent stem cell model for understanding GNAS-mutated tumors. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2020;42:1010428320962588. doi: 10.1177/1010428320962588. [DOI] [PubMed] [Google Scholar]
- 21.Evaul K, Hammes SR. Cross-talk between G protein-coupled and epidermal growth factor receptors regulates gonadotropin-mediated steroidogenesis in Leydig cells. J Biol Chem. 2008;283:27525–33. doi: 10.1074/jbc.M803867200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharifi J, Khirehgesh MR, Safari F, Akbari B. EGFR and anti-EGFR nanobodies: review and update. J Drug Target. 2021;29:387–402. doi: 10.1080/1061186X.2020.1853756. [DOI] [PubMed] [Google Scholar]
- 23.Lin H, Zhang C, Zhang H, Xia YZ, Zhang CY, Luo J, et al. Physakengose G induces apoptosis via EGFR/mTOR signaling and inhibits autophagic flux in human osteosarcoma cells. Phytomedicine: Int J phytotherapy phytopharmacology. 2018;42:190–8. doi: 10.1016/j.phymed.2018.03.046. [DOI] [PubMed] [Google Scholar]
- 24.Ohtsuka T, Tomosugi T, Kimura R, Nakamura S, Miyasaka Y, Nakata K, et al. Clinical assessment of the GNAS mutation status in patients with intraductal papillary mucinous neoplasm of the pancreas. Surg Today. 2019;49:887–93. doi: 10.1007/s00595-019-01797-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.