Abstract
Background
Brucellosis, caused by Brucella spp., is a major zoonotic public health threat. Although several Brucella vaccines have been demonstrated for use in animals, Brucella spp. can cause human infection and to date, there are no human‐use vaccines licensed by any agency. Recently, methods in vaccine informatics have made major breakthroughs in peptide‐based epitopes, opening up a new avenue of vaccine development.
Objectives
The purpose of this article was to identify potential antigenic peptides in Brucella by proteome and peptidome analyses.
Methods
Mouse infection models were first established by injection with Brucella and spleen protein profiles were then analysed. Subsequently, the major histocompatibility complex class I or II (major histocompatibility complex [MHC]‐I/II)‐binding peptides in blood samples were collected by immunoprecipitation and peptides derived from Brucella proteins were identified through liquid chromatography–mass spectrometry (LC‐MS/MS). These peptides were then evaluated in a variety of ways, such as in terms of conservation in Brucella and synchronicity in predicted peptides (similarity and coverage), which allowed us to more effectively measure their antigenic potential.
Results
The expression of the inflammatory cytokines IL1B and IFN‐γ was significantly altered in the spleen of infected mice and some Brucella proteins, such as Muri, AcpP and GroES, were also detected. Meanwhile, in blood, 35 peptides were identified and most showed high conservation, highlighting their potential as antigen epitopes for vaccine development. In particular, we identified four proteins containing both MHC‐I‐ and MHC‐II‐binding peptides including AtpA, AtpD, DnaK and BAbS19_II02030. They were also compared with the predicted peptides to estimate their reliability.
Conclusions
The peptides we screened could bind to MHC molecules. After being stimulated with antigen T epitopes, Memory T cells can stimulate T cell activation and promote immune responses. Our results indicated that the peptides we identified may be good candidate targets for the design of subunit vaccines and these results pave the way for the study of safer vaccines against Brucella.
Keywords: Brucella abortus, mass spectrometry, MHC, proteomics
Based on proteomics and peptidomics, we identified 35 peptides from the Brucella proteome, which have the ability to bind MHC molecules and have the potential to be used in vaccine design. In addition, we also found four Brucella proteins containing the identified MHC‐I and MHC‐II binding peptides, which may be good candidate targets for designing subunit vaccines.

1. INTRODUCTION
Brucella is a gram‐negative facultative intracellular bacterium, that causes a chronic zoonotic infection called brucellosis (Glynn and Lynn, 2008). There are 12 recognised species of Brucella, which in humans is rarely fatal but can be severely debilitating or disabling (Rajendhran, 2021). Skin wounds and mucous membranes are the primary targets of Brucella infection in humans, and such infections can damage the reproductive system and joints, resulting in genitourinary complications and peripheral arthritis. In endemic areas, Brucella is usually transmitted through the consumption of unpasteurised dairy products and fresh soft cheeses made from unpasteurised milk (Glynn and Lynn, 2008). In the grassland, Brucella species can also infect other mammals in addition to their preferred hosts. For example, B. abortus and B. melitensis can both infect cows and contaminate their milk, resulting in huge economic losses to grazing lands. Therefore, Brucella has the potential to cause great harm to human health and seriously hinder the development of grazers.
Vaccination is an effective method to reduce the incidence of brucellosis in livestock, which can reduce the risk of Brucella infections in humans. At present, several kinds of live attenuated vaccines have been used in livestock. For example, B. abortus strain 19 (S19) is currently the most effective vaccine used in cattle. Researchers have found that vaccination with S19 provide 70%–91% protection against bovine abortion caused by infection with B. abortus (Confer et al., 1985; Wyckoff et al., 2005) and subcutaneous inoculation of S19 in 3–6‐month‐old female calves yields high immune efficacy. In addition, some other live attenuated vaccines, such as RB51 and Rev 1, also have been shown to provide protection to animals. Although these live attenuated vaccines have been widely used in animals, the safety of them still needs to be considered due to their pathogenicity in humans. With the development of subunit vaccines, a variety of Brucella protective antigens, such as BLS, Omp19, DnaK and Bp26, have been used as candidate vaccine targets (Pan et al., 2021; Yang et al., 2013). At the same time, single component antigens provide an opportunity for the introduction of widely used delivery carriers, which could greatly enhance specific immune responses. To date, many promising delivery systems, such as viral vectors, bacterial vectors, chitosan derivatives, liposomes, polymers and inorganics, have been used to explore Brucella vaccines (Pan et al., 2021). Considering the stability of these delivery systems, loading simpler antigen epitopes, which have less interference with the carrier, may be a promising strategy for producing safe and efficient brucellosis vaccines.
Generally speaking, bacterial vaccines are internalised by phagocytes through different mechanisms in an organism. In the traditional research paradigm, major histocompatibility complex class I major histocompatibility complex (MHC‐I)‐binding peptides are mostly derived from intracellular antigens, while MHC class II (MHC‐II)‐binding peptides are derived from external antigens. Both antigens are processed and presented by antigen‐presenting cells (APCs) to trigger subsequent immune processes. MHC‐I‐antigenic peptides are generated from the proteasome, then translocated into the endoplasmic reticulum (ER) and loaded onto MHC‐I molecules (López et al., 2001). MHC‐II molecules are synthesised and transferred from the ER to the endomembrane compartment, where these derived peptides bind to their respective antigen‐binding domains. These MHC‐II‐peptide complexes exist on the surface of APCs and can be recognised by CD4+ T cells, which secrete cytokines to further activate humoral immunity. By contrast, MHC‐I molecules bind to antigen‐binding domains on the surface of most cells and present antigen fragments to CD8+ T cells, which may play an important role in the eradication of Brucella by cell lysis of infected macrophages (Oliveira and Splitter, 1995). Therefore, the affinity between antigen epitopes and MHC‐I/II molecules impact the type of immune response and the identification of antigen epitopes is great significance for vaccine design and development.
Subunit vaccines use certain surface structural components from microorganisms to make a vaccine that does not contain nucleic acids and can induce a patient to produce antibodies. Subunit vaccines have only a few major surface proteins, avoiding the production of antibodies induced by many unrelated antigens, thereby reducing the incidence of related diseases caused by vaccines. Polypeptide vaccines are a tape of subunit vaccine prepared using chemical synthesis technology, mainly based on known or predicted amino acid sequences for an antigen epitope from a pathogen. The greatest advantage of peptide vaccines lies in the aggregation of immune epitopes, which not only improves the immunogenicity of these vaccines, but also reduces their side effects (Gong et al., 2022). The prediction and screening of epitopes is therefore the most important step in their production. Epitope screening methods include nuclear magnetic resonance and X‐ray crystallography for the structural identification of antigen and monoclonal antibody complexes, and enable atomic level interactions to be determined. There are also methods to predict T and B cell epitopes using specific computer algorithms, which can also identify monoclonal antibody epitopes by mass spectrometry (MS), or identify epitopes containing known T cell epitopes or peptides. As peptide‐based vaccines are completely synthesised, there is no reversion to virulence or inactivation deficiencies, which might be the major drawback in live attenuated vaccines. Furthermore, compared with these subunit vaccines, the design of peptide‐based vaccines is convenient and fast. Peptide‐based vaccines have also been developed to treat dengue fever (Chen et al., 2021), malaria, influenza (López et al., 2001), multiple sclerosis (Shahsavandi et al., 2015), tuberculosis (Gong et al., 2022) and even cancers, such as colorectal cancer, myeloid leukemia and stomach cancer (Bourdette et al., 2005; Maslak et al., 2018; Sundar et al., 2018). Based on the above research background, the purpose of this study was to identify potential antigenic peptides in Brucella by proteome and peptidome analyses.
2. METHODS
2.1. Infection of mice
A total of six female BALB/c mice (6‐ to 8‐week old) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). All animal procedures were approved by The Academy of Military Medical Sciences Institutional Animal Care and Use Committee (Ethics Approval Code IACUC‐DWZX‐2021‐008) and performed in accordance with commonly accepted procedures.
The six female BALB/c mice were randomly divided into infected and uninfected groups, three per group. S19 was inoculated into a sterile Tryptic Soy Broth (TSB) liquid medium and cultured at 37°C. After 16 h, the concentration of Brucella was measured with a spectrophotometer. Using TSB medium as a blank control to zero the instrument and measuring the absorbance of four folds diluted bacterial solution. When the OD600 was 2.0, the number of colonies on plates was counted. A total of 100 μl of each bacterial solution was taken and added to 900 μl of normal saline, mixed well, and diluted to make the dilution 10−5. A total of 100 μl of diluted liquid was taken and spread on Tryptic Soy Agar medium in triplicate. The plates were placed at 37°C, and after 3 days, the number of colonies was counted. The calculation formula was as follows: total viable bacteria per ml = average number of colonies from three counts at the same dilution × dilution ratio × 50. Based on the calculation results, 2OD = 1 * 108 CFU/ml. A total of 20 μl of each bacterial solution was taken and added to 380 μl of normal saline to prepare a premix. The injection dose was 5 × 105 CFU/100 μl in the infected group, while 100 μl of sterile saline was used in the uninfected group, and both groups were injected intraperitoneally. Six days after infection, blood was collected after mice were anesthetised by subcutaneous injection of sodium barbital at a dose of 200 mg/kg. On the same day, the mice were sacrificed by cervical dislocation, and then the spleens were collected for further analysis.
2.2. Isolation of mouse spleen cells
All spleens were individually dry‐pulverised using an automated cryogenic grinding system (Shanghai Jingxin Industrial Development Co., Ltd.). All tissues were homogenised in lysis buffer (4% SDS) supplemented with a protease inhibitor cocktail (A32953, Thermo Scientific). Spleen homogenates were subsequently incubated at 4°C for 30 min and then centrifuged at 16,000 g and 4oC for 20 min. The supernatant was transferred to a new tube, and the protein concentration was then determined using a BCA protein assay kit (23225, Pierce).
2.3. Liquid chromatography tandem‐mass spectrometry (LC‐‐MS/MS)‐based spleen proteome
Peptides were resolved by capillary chromatography using an Easy nano‐LC‐1200 nano‐capillary UHPLC, coupled with an electrospray interface, on a Q Exactive HF‐X mass spectrometer (Thermo Fisher Scientific). The peptides were eluted with a linear, 120‐min, 6%–38 % acetonitrile gradient in 0.1% formic acid, at a flow rate of 0.25 μl/min. Data‐dependent acquisition (DDA) was performed using the following parameters: MS1 resolution was set to 60,000 with the automatic gain control (AGC) target set to 750%. Dynamic exclusion was set to 30 s. For precursor fragmentation in high energy collision dissociation (HCD), the collision energy was set to 27%. For MS2, the resolution was set to 15,000 with an AGC target of 200% and a maximum inject time of 50 ms. Label‐free quantification was also performed using Peaks Studio based on results with a 1% false discovery rate (FDR). The relative abundances of peptide features (precursor peak area) were detected using multiple samples. Feature detection was performed separately on each sample, and then the features of the same peptide from different samples were reliably aligned together using a high‐performance retention time alignment algorithm. Normalisation was performed using the total ion current of each sample, and the normalised abundance was calculated from the raw abundance divided by the normalisation factor.
Peaks Studio was used to process all data. The settings for the database searching were Orbitrap for identified instrument, HCD fragmentation and no digestion enzyme. Precursor and fragment mass tolerances of 10 ppm and 0.02 Da, respectively, were used. Peptide identification was performed against a combination of mouse and S19 Uniprot database (updated on December 2019, URL of mouse Uniprot database: https://rest.uniprot.org/proteomes/stream?compressed= true&fields = upid%2Corganism%2Corganism_id%2Cprotein_count%2Cbusco%2Ccpd&format = tsv&query = upid%3AUP000000589; URL of S19 Uniprot database: https://rest.uniprot.org/proteomes/stream?compressed= true&fields = upid%2Corganism%2Corganism_id%2Cprotein_count%2Cbusco%2Ccpd&format = tsv&query = upid%3AUP000002565). Analysis was carried out with oxidation [+15.99] and deamidation [+0.98] set as variable peptide modifications, with a maximum of three modifications per peptide. The peptide‐spectrum match (PSM) level threshold was set to 1%.
2.4. Peripheral blood mononuclear cell (PBMC) isolation
Whole blood was collected into EDTA tubes (366643, BD Vacutainer) 6 days after infection. PBMCs were isolated using Ficoll–Paque gradient centrifugation according to the manufacturer's instruction. Briefly, 3 ml of Ficoll–Paque plus (17144003, Cytiva) was preloaded into a 10 ml LeucoSep tube (163288, Greiner Bio‐One) by centrifugation for 1 min at 1000 g. The whole blood was then diluted 1:1 with phosphate‐buffered saline (PBS) and added to a Ficoll–Paque preloaded LeucoSep tube. The tubes were then centrifuged for 15 min at 1000 g at room temperature. The PBMC layer was collected, and washed twice with PBS.
2.5. MHC‐bound peptides purification
MHC‐I‐ and ‐II‐bound peptide libraries were generated based on the isolation of MHC complexes and subsequent dissociation of bound peptides using an immunoprecipitation protocol as previously described (Rijensky et al., 2020). Briefly, cells were lysed and homogenised by incubation with rotation for 45 min at 4°C. Immunoprecipitation was performed using a crosslink IP kit (26147, Pierce) according to the manufacturers’ recommendations. A primary anti‐MHC‐I antibody (MA5‐18004, Invitrogen) and an anti‐MHC‐II antibody (14‐5321‐82, eBioscience) were covalently bound to mouse protein A/G sepharose to immunoprecipitate MHC‐I and MHC‐II complexes, respectively. The immunoprecipitated peptides were eluted twice with 1% TFA and were subjected to desalting using a SepPak (WAT054955, Waters).
2.6. LC‐MS/MS analysis of MHC‐binding peptides
Peptides were resolved by capillary chromatography using an easy nano LC‐1200 nano‐capillary UHPLC, coupled by electrospray interface, to a Oritrap Fusion Lumos MS (Thermo Fisher Scientific). The MHC peptides were eluted with a linear, 60 min, 6%–36 % acetonitrile gradient in 0.1 % formic acid, at a flow rate of 0.3 μl/min. DDA was performed using the following parameters: the MS1 resolution was set to120,000 with a normalised AGC target 200%. And the dynamic exclusion was set to 30 s. Mass tolerance of ±10 ppm was allowed. For precursor fragmentation in HCD, collision energies of 25%, 30%, and 35% were used. For MS2, the resolution was set to 50,000 with a normalised AGC target of 200%, and a maximum injection time of 86 ms.
Proteins were identified and quantified with Peaks Studio. The parameters were similar to the previous proteome database search as highlighted in the previous section. Briefly, precursor and fragment mass tolerances, as well as peptide modifications, were the same, and the target database was the S19 Uniprot database (updated in December 2019), the PSM and protein level FDR threshold were set to 1%.
2.7. Statistical analysis
After MS analysis, the count number of proteins was obtained. The R package ‘DESeq2’ was used for differential expression analysis to determine the expression changes of each protein. | log2FoldChange | ≥1 was used as the threshold, an adjusted p value (FDR) < 0.05 was regarded as significant, and then differentially expressed genes (DEGs) were obtained. We then summarised the upregulated and downregulated genes using the R packet “Clusterprofiler” for GO analysis and KEGG pathway enrichment, respectively, in which the GO analysis included secondary‐level term and full‐level term analyses. R (v4.1.2) was used for statistical analyses in this study, and a p (adjusted p) value < 0.05 was regarded as significant.
2.8. Immunoinformatics analysis
In this study, the prediction of MHC‐binding antigenic peptides from bacterial proteins was performed using the Immune Epitope Database (IEDB) (Buus et al., 2003; Wang et al., 2008). A half‐limiting dose (IC50) rank < 2% was regarded as strong binding, and MHC‐I‐binding antigenic peptides were predicted based on the IEDB–ANN method with a 9‐mer. The prediction of MHC‐II‐binding antigenic peptides was performed using the IEDB‐recommended method with a 12‐mer, and an adjusted rank <2% was regarded as strong binding. VaxiJen was used for immunogenicity analysis with the ‘bacteria’ mode as the default setting.
3. RESULTS
3.1. Differential protein expression analysis from the spleens of infected mice
There has been a great development in epitope‐based peptide vaccines in recent years. A short antigenic epitope is important for vaccine rational design and delivery. To obtain reliable Brucella antigen peptides, we infected mice with Brucella, and the proteomic data from spleen and blood samples were then analysed. MHC‐binding peptides were obtained by immunoprecipitation and identified through MS analysis (Figure 1).
FIGURE 1.

Schematic diagram of this study . Mice were injected with Brucella abortus intraperitoneally and then the spleen cells and PBMCs were isolated 6 days after infection. Proteins from spleen were extracted and assessed by LC–MS/MS. MHC‐binding peptides in PBMCs were extracted by immunocoprecipitation and further detected through proteome analysis.
As known, the spleen is the main target of Brucella infection. When mice are infected with Brucella, Splenomegaly is one of the most obvious phenotypic changes. At the same time, as the most important immune organ, the spleen is usually used to analyse the degree of infection and the immune response (Jacob and Curtiss, 2021); Wang et al., 2020). Therefore, to determine the reliability of the infection model and further comprehensively analyse the immunological changes after infection, we used the spleen of uninfected and infected mice. Since the spleen is the main target of Brucella infection and the main organ of the immune response, we first analysed the changes in the spleen proteome of infected mice. These MS proteomics data were deposited in the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the iProX partner repository (Ma et al., 2019) with the dataset identifier PXD032856. The data from the infected group and the control group were obtained by LC–MS/MS. A total of 2914 mouse DEGs were identified in a comparison of gene expression levels between the infected group and the control group (Figure 2a). The expression levels of 1628 genes were significantly increased, while 1286 genes were significantly decreased (Figure 2b). Specifically, there were significant changes in the expression of several inflammatory cytokines in infected mice, such as IL1B and IFN‐γ, indicating a serious inflammatory reaction caused by Brucella spp. (Figure 2c). In addition, three Brucella proteins, MurI, AcpP and GroES, were detected in the infected group, but not in the control group (Figure S1). The MurI protein is mainly involved in the biosynthesis of peptidoglycans and the regulation of cell shape. AcpP presents substrates to enzymes involved in fatty acid biosynthesis or in polyketide secondary metabolite biosynthesis. GroES, related to bacterial metabolism processes, is a well‐known Brucella immunogen from B. melitensis 16 M. Furthermore, APCs in the spleen can process and present foreign antigens. Thus, by analysing the level of MHC‐related proteins, we found that both MHC‐I‐and MHC‐II‐mediated immune responses were enhanced (Figure 2d).
FIGURE 2.

Differential expression analysis of murine proteins in the spleens of infected mice. (a) Proteomics results from infected and uninfected mice were compared. Fold changes indicate the degree of changes in the infected group compared with the control group. | log2FoldChange | ≥ 1 was used as the threshold, and the adjusted p value (FDR) < 0.05 was regarded as significant. Each dot represents a gene, red dots represent an upregulated gene, green dots represent a downregulated gene and black dots represent nonsignificant genes. In particular, the blue dots represent immune‐related genes, and the brown dots represent histocompatibility antigen related genes. (b) Each cell represents the gene expression level relative to another group. This value comes from the mass spectrometer, where the count of one protein in each sample is divided by the average of another group and the logarithm is taken. The level of inflammatory cytokines (c) and MHC‐related proteins (d) identified in infected and controlled mice
3.2. GO analysis and KEGG pathway enrichment analysis
To further analyse the function of these DEGs, GO analysis was performed. Secondary‐level GO analysis results from upregulated genes including biological process (BP) and molecular function (MF) and a total of nine terms were enriched, such as the “detoxification” process, which has a close relationship with infection (Figure 3a). The downregulated genes were enriched into seven terms in BP and MF by secondary‐level GO analysis. However, all of them were routine cellular processes and functions (Figure 3b). In our secondary‐level GO analysis, we noticed that both upregulated genes and downregulated genes were enriched in BP and MF, but no cellular component terms were found. This phenomenon may indicate that the infection process affects gene expression in these mice, but the cellular structure is relatively stable.
FIGURE 3.

GO analysis and KEGG pathway enrichment of DEGs. (a–b) Secondary‐level GO analysis, including upregulated genes (a) and downregulated genes (b). Each column represents a term, and its length represents the number of DEGs contained under each term. The green terms are BP ontologies, and orange terms are molecular function ontologies. (c, d) KEGG pathway enrichments, including upregulated genes (c) and downregulated genes (d). Each bubble represents a pathway, its size being proportional to the number of genes, and the colour represents the level of statistical significance. The abscissa is the gene ratio, that is, the proportion of genes in DEGs that are enriched in each pathway. Abbreviations: Ont, ontology; BP, biological process; MF, molecular function
By performing KEGG pathway enrichment analysis on upregulated genes, these genes were found to be enriched in ‘biosynthetic of antibiotics’, ‘bacterial invasion of epithelial cells’ and ‘antigen processing and presentation’ pathways (Figure 3c). The downregulated genes were enriched in the ‘bacterial invasion of epithelial cells’ pathway and correlated significantly with the ‘B cell receptor signaling pathway’ (Figure 3d). These pathways are closely related to bacterial infection, indicating that Brucella infection impacted the physiological condition of infected mice.
3.3. MHC‐binding peptides
After immunoprecipitation and elution, samples were analysed by MS to identify MHC‐I‐binding peptides. The MS proteomics data were also deposited in the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the iProX partner repository (Ma et al., 2019) with the dataset identifier PXD032855. After searching the Brucella protein sequence database, 19 peptides were identified, which matched 12 proteins in the Brucella S19. The shortest peptide consisted of 8 amino acids and the longest contained 17 amino acids. In addition, multiple peptides derived from different regions of the same protein could be found (Table 1). MHC‐II‐binding peptides were also identified in the same way. After searching this database, 16 peptides were matched to 11 proteins in the Brucella S19 (Table 2). The shortest peptide consisted of 7 amino acids and the longest contained 21 amino acids. Consistent with the well‐known different spatial structures between the two classes of MHC molecules (the peptide‐binding clefts are more open in MHC‐II molecules compared with MHC‐I molecules), the length range of MHC‐II‐binding peptides identified was wider than for MHC‐I‐binding peptides. Interestingly, we found four proteins (including AtpA, AtpD, DnaK and BAbS19_II02030) that had both MHC‐I‐ and MHC‐II‐binding peptides, indicating the potential to simultaneously activate humoral and cellular immunity. It has been reported that DnaK can be used as an antigen for Brucella vaccine development and provides good protection against Brucella infection (Delpino et al., 2007; Minhas et al., 2021). However, the other three candidate targets had not been reported yet and their immunogenicity needed to be further verified.
TABLE 1.
MHC‐I‐binding peptides identified in this study
| Serial number | Gene | Protein accession | Peptide | Length |
|---|---|---|---|---|
| I‐01 | AtpA | B2S7M5 | KLAVNQVG | 8 |
| I‐02 | AtpA | B2S7M5 | HALILYDDLSK | 11 |
| I‐03 | AtpD | A0A0F6ASF0 | VVDLLAPYAK | 10 |
| I‐04 | AtpD | A0A0F6ASF0 | FTQAGSEVSALLGR | 14 |
| I‐05 | BAbS19_I13440 | A0A0F6ARK0 | LLPGMILSNE | 10 |
| I‐06 | BAbS19_I14960 | A0A0F6ARY6 | VVFTALVLVAIFAP | 14 |
| I‐07 | BAbS19_II02030 | A0A0F6ATS5 | GSKFTTAK | 8 |
| I‐08 | BAbS19_II04710 | A0A0F6AUF9 | SLDPLIRTEMQD | 12 |
| I‐09 | BAbS19_II06640 | A0A0F6AUY5 | VVARADNHVIL | 11 |
| I‐10 | BAbS19_II10320 | A0A0F6AVW6 | TFAVFTGEK | 9 |
| I‐11 | BAbS19_II10320 | A0A0F6AVW6 | TFAVFTGEKG | 10 |
| I‐12 | BAbS19_II10320 | A0A0F6AVW6 | TLTLGTATPT | 10 |
| I‐13 | DnaK | A0A0F6AT48 | IINEPTAAALAY | 12 |
| I‐14 | DnaK | A0A0F6AT48 | IINEPTAAALAYGLDK | 16 |
| I‐15 | DnaK | A0A0F6AT48 | IINEPTAAALAYGLDKR | 17 |
| I‐16 | DnaK | A0A0F6AT48 | VTHAVVTVPAYFDDAQR | 17 |
| I‐17 | GyrA | A0A0F6AQX3 | EDEGDEILSV | 10 |
| I‐18 | LeuB | A0A0F6AU32 | ALAHSSSL | 8 |
| I‐19 | RuvB | B2S7D9 | LQRTPRGR | 8 |
TABLE 2.
MHC‐II‐binding peptides identified in this study
| Serial number | Gene | Protein accession | Peptide | Length |
|---|---|---|---|---|
| II‐01 | AtpA | B2S7M5 | HALILYDDLSK | 11 |
| II‐02 | AtpA | B2S7M5 | GSSAQIKAMKQVAGSIKGELA | 21 |
| II‐03 | AtpD | A0A0F6ASF0 | FTQAGSEVSALLGR | 14 |
| II‐04 | BAbS19_I01280 | A0A0F6ANP8 | DTRAIVLTGY | 10 |
| II‐05 | BAbS19_I01780 | A0A0F6ANU0 | LRHIAGTKIMR | 11 |
| II‐06 | BAbS19_I14740 | A0A0F6ARW4 | LIILDRY | 7 |
| II‐07 | BAbS19_II02030 | A0A0F6ATS5 | GSKFTTAK | 8 |
| II‐08 | BAbS19_II10850 | A0A0F6AW15 | PPQLGYL | 7 |
| II‐09 | DnaK | A0A0F6AT48 | IINEPTAAALAYGLDK | 16 |
| II‐10 | DnaK | A0A0F6AT48 | IINEPTAAALAYGLDKR | 17 |
| II‐11 | DnaK | A0A0F6AT48 | VTHAVVTVPAYFNDAQR | 17 |
| II‐12 | Eno | B2S5Y3 | VNQIGSLSSTLDAVET | 16 |
| II‐13 | Lon | A0A0F6AQY1 | VPLFVGR | 7 |
| II‐14 | Lon | A0A0F6AQY1 | NLSDYLGVEK | 10 |
| II‐15 | MutS | A0A0F6ANQ7 | VVKRDVIRLVTP | 12 |
| II‐16 | MutS | A0A0F6ANQ7 | GIASLLDGALLPDELAAARE | 20 |
3.4. Conservation analysis of proteins and peptides identified in this study
Basic Local Alignment Search Tool (BLAST) was used to analyse the sequence similarity of these proteins against the RefSeq Select proteins database. We would like to introduce this analysis to explore the possibility of its widespread application. Our previous analysis has yielded certain peptides in several proteins, so we further analysed them at both protein and peptide levels. The conservation among multiple Brucella strains was calculated for each identified protein by BLAST method (each protein in different strains). Then, we further amplified the details of the target peptides to obtain individual conservation of each peptide (each peptide on one protein in different strains). Most of the proteins had high conservation among Brucella spp., with about 90% similarity (Figure S2). Yet, there were a few proteins, such as BAbS19‐II02030, that showed relatively lower similarities, which may have narrow‐range protection between Brucella. The same scenario was observed in a conservation analysis of peptides identified (Figure 4). For instance, ‘VVDLLAPYAK’ (I‐03) was a highly conserved sequence in Brucella spp., while ‘VVARADNHVIL’ (I‐09) was less conserved.
FIGURE 4.
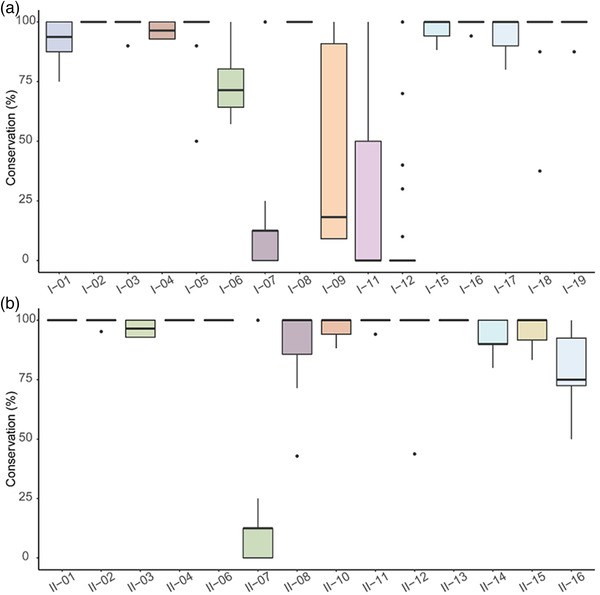
Conservation of MHC‐I‐binding peptides (a) and MHC‐II‐binding peptides (b) in Brucella spp. The horizontal axis represents each peptide, and the vertical axis represents their conservation. Overlapping sequences were merged to show only the longer sequences from each.
3.5. Comparison of MHC‐binding peptide sequences experimentally identified with those predicted by bioinformatics methods
Multiple sequence alignment (MSA) was performed to analyse the common patterns of MHC‐I‐binding peptides experimentally identified using the ‘muscle’ method (https://www.drive5.com/muscle/). Given that the MHC‐I‐binding peptides identified in the previous studies were mainly 9‐mers, we extracted the 9‐mer core amino acids of MHC‐I‐binding peptides according to MSA and identified the motif of MHC‐I‐binding peptides (Figure 5a). The sequences of MHC‐II‐binding peptides were also analysed by the ‘muscle’ method. Due to the wide range of MHC‐II‐binding peptides lengths, we extracted the core amino acids, and after MSA, these sites were covered by at least half of these peptides, and then we obtained amotif for MHC‐II‐binding peptides (Figure 5b). The related proteins of these two groups of MHC‐binding peptides were predicted, and we then obtained MHC‐predictive peptides (Figure 5c,d).
FIGURE 5.

Comparison of MHC‐binding peptide sequences experimentally identified with those predicted by bioinformatics methods. The ordinate represents the MHC‐binding capacity of different amino acids at this site, where the icon above the abscissa indicates that this amino acid at this site will promote MHC binding, while the icon below the abscissa indicates that this amino acid at this site will inhibit MHC binding. (a) Identified MHC‐I‐binding peptides. (b) Identified MHC‐II‐binding peptides. (c) MHC‐I‐predictive peptides. (d) MHC‐II‐predictive peptides
3.6. Correlation of MHC‐binding peptides with MHC‐predictive peptides
In this study, a sequence correlating MHC‐predictive peptides at more than three amino acid repeats to an MHC‐binding peptide was regarded as a relevant peptide. We compared the identified MHC‐binding peptides with the predictive results obtained above, and found that there were five MHC‐binding peptides, which could match the relevant predictive peptides, and their corresponding proteins were AtpD, LeuB, MutS, BAbS19_II02030 and BAbS19 II10850 (Table 3). These five peptides cannot only be detected through immunoprecipitation, but also can be found in the predictive peptides. In other words, they are approved both in silico calculates and biological tests, which effectively reducing the false positives of their immune capacities. In particular, the peptide of ‘FTQAGSEVSALLGR’ (AtpD) was found in both MHC‐I‐ and MHC‐II‐predictive peptides, and its overlap sequence ‘FTQAGSEV’ showed antigenic potential in immunogenicity analyses. The other four binding peptides from BAbS19_II02030, BAbS19_II10850, LeuB and MutS only have relevant MHC‐I‐predictive peptides. In particular, the ‘FTQAGSEVSALLGR’ peptide (AtpD) was found in the prediction of both MHC‐I and MHC‐II, and its repeat sequence ‘FTQAGSEV’ showed antigen potential in immunogenicity analyses. The other four binding peptides from BAbS19_II02030, BAbS19_II10850, LeuB and MutS only have relevant peptides in only one class of MHC.
TABLE 3.
Correlation of MHC‐binding peptides with MHC predictive peptides
| Gene | MHC‐binding peptide | MHC‐I predictive peptide | IC50 rank (%) | MHC‐II predictive peptide | Adjusted rank (%) |
|---|---|---|---|---|---|
| AtpD | FTQAGSEVSALLGR | RFTQAGSEV | 1.3 | IFRFTQAGSEVS | 1.99 |
| BAbS19_II02030 | GSKFTTAK | TTAKRESAF | 1.5 | – | |
| BAbS19_II10850 | PPQLGYL | – | ‐ | QLGYL TLTALSA | 1.72 |
| LeuB | ALAHSSSL | CYPALAHSS | 1.9 | – | |
| MutS | GIASLLDGALLPDELAAARE | – | ‐ | GFEAAGGIASLL | 1.96 |
Note: Repeated amino acid sequences are highlighted in bold.
4. DISCUSSION
In contrast to experiments using a specific cell type (such as PBMCs) to identify potential bacterial epitopes in vitro, the spleens of infected mice were collected and analysed in this in vivo study. We first compared the spleen proteomic changes between infected and control mice. In the spleen of infected mice, we detected three Brucella proteins, MurI, AcpP and GroES, it may be that our samples came from mouse spleens, which are not easy for Brucella to multiply. They involved in common functions, such as amino acid racemic, fatty acid chain synthesis or molecular chaperone, so they may be high in content and easy to detect. After confirming the infection status and the activation of spleen immune responses, MHC‐binding peptides were isolated and identified by LC–MS/MS. Due to their different conservation, certain screening could be carried out. Through MHC epitope antigen prediction, some relevant peptides also showed MHC affiliative abilities, suggesting that the peptides obtained in this research could be used as candidate antigenic peptides with great potential for the development of Brucella vaccines. We found that the proteins were encoded by five genes, including AtpD, LeuB, MutS, BAbS19_II02030 and BAbS19_II10850. AtpD, has proton‐transporting ATP synthase activity (https://www.ebi.ac.uk/QuickGO/term/GO:0046933), and LeuB has been shown to be involved in the leucine biosynthetic process (Essenberg and Sharma, 1993; Rajasekaran et al., 2008). MutS is involved in the repair of mismatches in DNA (Bellacosa, 2001), BAbS19_II02030 with transmembrane transporter activity (https://www.ebi.ac.uk/QuickGO/term/GO:0022857) and BAbS19_II10850 is related to the regulation of transcription (https://www.ebi.ac.uk/QuickGO/term/GO:0006355). Among these proteins, the ‘FTQAGSEV’ sequence has been found both in the binding and predictive peptides of MHC‐I and MHC‐II. In general, in this study, we identified 35 MHC‐binding peptides by experimental methods and provide candidate antigen targets for further vaccine design.
At present, several studies on Brucella epitope vaccines have been published (Chen et al., 2021; Oliveira et al., 2021; Sha et al., 2020), and in most of which epitopes were predicted using only bioinformatic software. In this study, the MHC‐binding peptides in infected mice were combined with predicted peptides to obtain more reliable MHC‐binding antigenic peptides. Brucella mainly infects livestock, rather than traditional laboratory animals such as mice, so there have been no suitable animal models presented date. MHC molecules vary greatly between species, and polymorphisms in MHC loci have also been observed within species, suggesting that specific epitopes may only cover part of a population. However, studies based on this method can further explore the antigenic peptides of interest in MHC alleles. Previous studies have shown that human leukocyte antigen (HLA) transgenic mice can be used to effectively screen epitope peptides from specific alleles (Weingarten‐Gabbay et al., 2021). Thus, in the future, we will carry out similar studies to identify antigenic peptides with binding abilities in human MHC molecules using HLA transgenic mice. Furthermore, these peptides can be also loaded onto nanoparticles to improve the immunogenicity of these peptides to produce high‐performance nanovaccines (Pan et al., 2021).
5. CONCLUSION
Although several Brucella vaccines have been used in animals, they can cause human infection and as yet, there are no licenced human‐used vaccines. Based on proteomics and peptidomics, we identified 35 peptides from the Brucella proteome, which have the ability to bind with MHC molecules and have potential for vaccine design. In addition, we also found four Brucella proteins containing both identified MHC‐I‐ and MHC‐II‐binding peptides, which might be good candidate targets for the design of subunit vaccines. Thus, these results pave the way for the study of safer vaccines against Brucella.
AUTHOR CONTRIBUTIONS
Data curation (lead); formal analysis (lead); investigation (lead); methodology (lead); and writing–original draft (lead): Meijuan Pei. Data curation (equal); formal analysis (equal); investigation (lead); methodology (equal); and writing–original draft (equal): Ao Dong. Data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); supervision (equal); and writing–original draft (equal): Xueping Ma. Formal analysis (equal) and investigation (equal): Shulei Li. Data curation (equal) and investigation (equal): Yan Guo. Formal analysis (equal) and investigation (equal): Menglan Li. Formal analysis (equal) and investigation (equal): Zhenghui Wang. Conceptualisation (equal); formal analysis (equal); investigation (equal); and writing–review & editing (equal): Hengliang Wang. Conceptualisation (equal); formal analysis (equal); funding acquisition (equal); investigation (equal); and writing–review & editing (equal): Li Zhu. Conceptualisation (equal); data curation (equal); funding acquisition (equal); and writing–review & editing (equal): Chao Pan. Conceptualisation (lead); formal analysis (lead); funding acquisition (lead); investigation (lead); methodology (lead); project administration (lead); and writing–review & editing (lead): Yufei Wang.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received. All animal procedures were approved by Academy of Military Medical Sciences Institutional Animal Care and Use Committee (Ethics Approval Code IACUC‐DWZX‐2021‐008) and performed in accordance with relevant guidelines. A total of 6 female BALB/c mice (6–8‐week old) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China).
Supporting information
Figure S1 Brucella gene expression in the infected group and control group.
Figure S2 Conservation of related proteins in Brucella.
ACKNOWLEDGEMENTS
We thank Dr. Xiaofeng Xie for her support with the experiment. This work was supported by the National Natural Science Foundation of China (No. U20A20361 and 82171819)) and the National Key Research and Development Program of China (2018YFC1603705).
Pei, M. , Dong, A. , Ma, X. , Li, S. , Guo, Y. , Li, M. , Wang, Z. , Wang, H. , Zhu, L. , Pan, C. , & Wang, Y. (2023). Identification of potential antigenic peptides of Brucella through proteome and peptidome. Veterinary Medicine and Science, 9, 523–534. 10.1002/vms3.1048
Meijuan Pei, Ao Dong and Xueping Ma are co‐authors of this paper.
Contributor Information
Chao Pan, Email: panchaosunny@163.com.
Yufei Wang, Email: tosya@163.com.
REFERENCES
- Bellacosa, A. (2001). Functional interactions and signaling properties of mammalian DNA mismatch repair proteins. Cell Death Differ, 8(11), 1076–92. [DOI] [PubMed] [Google Scholar]
- Bourdette, D. , Edmonds, E. , Smith, C. , Bowen, J. D. , Guttmann, C. R. , Nagy, Z. P. , Simon, J. , Whitham, R. , Lovera, J. , Yadav, V. , Mass, M. , Spencer, L. , Culbertson, N. , Bartholomew, R. M. , Theofan, G. , Milano, J. , Offner, H. , & Vandenbark, A. A. (2005). A highly immunogenic trivalent T cell receptor peptide vaccine for multiple sclerosis. Multiple Sclerosis, 11(5), 552–561. 10.1191/1352458505ms1225oa [DOI] [PubMed] [Google Scholar]
- Buus, S. , Lauemoller, S. L. , Worning, P. , Kesmir, C. , Frimurer, T. , Corbet, S. , Fomsgaard, A. , Hilden, J. , Holm, A. , & Brunak, S. (2003). Sensitive quantitative predictions of peptide‐MHC binding by a ‘Query by Committee’ artificial neural network approach. Tissue Antigens, 62(5), 378–384. 10.1034/j.1399-0039.2003.00112.x [DOI] [PubMed] [Google Scholar]
- Chen, Z. , Zhu, Y. , Sha, T. , Zhu, Y. , Sha, T. , Li, Z. , Li, Y. , Zhang, F. , & Ding, J. (2021). Design of a new multi‐epitope vaccine against Brucella based on T and B cell epitopes using bioinformatics methods. Epidemiology and Infection, 149, e136. 10.1017/S0950268821001229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Confer, A. W. , Hall, S. M. , Faulkner, C. B. , Espe, B. H. , Deyoe, B. L. , Morton, R. J. , & Smith, R. A. (1985). Effects of challenge dose on the clinical and immune‐responses of cattle vaccinated with reduced doses of Brucella abortus strain‐19. Veterinary Microbiology, 10(6), 561–575. 10.1016/0378-1135(85)90065-3 [DOI] [PubMed] [Google Scholar]
- Delpino, M. V. , Estein, S. M. , Fossati, C. A. , Baldi, P. C. , & Cassataro, J. (2007). Vaccination with Brucella recombinant DnaK and SurA proteins induces protection against Brucella abortus infection in BALB/c mice. Vaccine, 25(37–38), 6721–6729. 10.1016/j.vaccine.2007.07.002 [DOI] [PubMed] [Google Scholar]
- Essenberg, R. C. , & Sharma, Y. K. (1993). Cloning of genes for proline and leucine biosynthesis from Brucella abortus by functional complementation in Escherichia‐coli. Journal of General Microbiology, 139, 87–93. 10.1099/00221287-139-1-87 [DOI] [PubMed] [Google Scholar]
- Glynn, M. K. , & Lynn, T. V. (2008). Brucellosis. Journal of the American Veterinary Medical Association, 233(6), 900–908. 10.2460/javma.233.6.900 [DOI] [PubMed] [Google Scholar]
- Gong, W. , Pan, C. , Cheng, P. , Wang, J. , Zhao, G. , & Wu, X. (2022). Peptide‐based vaccines for tuberculosis. Frontiers in Immunology, 13, 830497. 10.3389/fimmu.2022.830497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob, J. M. , & Curtiss, R. (2021). Characterization of Brucella abortus S19 as a challenge strain for use in a mouse model of brucellosis. Microbes and Infection, 23(4–5), 104809. 10.1016/j.micinf.2021.104809 [DOI] [PubMed] [Google Scholar]
- López, J. A. , Weilenman, C. , Audran, R. , Roggero, M. A. , Bonelo, A. , Tiercy, J. M. , Spertini, F. , & Corradin, G. (2001). A synthetic malaria vaccine elicits a potent CD8(+) and CD4(+) T lymphocyte immune response in humans: Implications for vaccination strategies. European Journal of Immunology, 31(9), 2839–2839. [DOI] [PubMed] [Google Scholar]
- Ma, J. , Chen, T. , Wu, S. , Yang, C. , Bai, M. , Shu, K. , Li, K. , Zhang, G. , Jin, Z. , He, F. , Hermjakob, H. , & Zhu, Y. (2019). iProX: An integrated proteome resource. Nucleic Acids Research, 47(D1), D1211–D1217. 10.1093/nar/gky869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslak, P. G. , Dao, T. , Bernal, Y. , Chanel, S. M. , Zhang, R. , Frattini, M. , Rosenblat, T. , Jurcic, J. G. , Brentjens, R. J. , Arcila, M. E. , Rampal, R. , Park, J. H. , Douer, D. , Katz, L. , Sarlis, N. , Tallman, M. S. , & Scheinberg, D. A. (2018). Phase 2 trial of a multivalent WT1 peptide vaccine (galinpepimut‐S) in acute myeloid leukemia. Blood Advances, 2(3), 224–234. 10.1182/bloodadvances.2017014175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minhas, P. , Kumar, B. V. S. , & Verma, R. (2021). Expression of recombinant DnaK of Brucella abortus and its evaluation as immuno‐modulator. Archives of Microbiology, 203(5), 2719–2725. 10.1007/s00203-021-02190-0 [DOI] [PubMed] [Google Scholar]
- Oliveira, K. C. , Brancaglion, G. A. , Santos, N. C. M. , Araújo, L. P. , Novaes, E. , Santos, R. L. , Oliveira, S. C. , Corsetti, P. P. , & de Almeida, L. A. (2021). Epitope‐based vaccine of a Brucella abortus putative Small RNA target induces protection and less tissue damage in mice. Frontiers in Immunology, 12, 778475. 10.3389/fimmu.2021.778475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira, S. C. , & Splitter, G. A. (1995). CD8(+) type‐1 CD44(HI) CD45 RB(LO) T‐lymphocytes control intracellular Brucella abortus infection as demonstrated in major histocompatibility complex class‐I‐deficient and class‐II‐deficient mice. European Journal of Immunology, 25(9), 2551–2557. 10.1002/eji.1830250922 [DOI] [PubMed] [Google Scholar]
- Pan, C. , Yue, H. , Zhu, L. , Ma, G. H. , & Wang, H. L. (2021). Prophylactic vaccine delivery systems against epidemic infectious diseases. Advanced Drug Delivery Reviews, 176, 113867. 10.1016/j.addr.2021.113867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran, P. , Seleem, M. N. , Contreras, A. , Purwantini, E. , Schurig, G. G. , Sriranganathan, N. , & Boyle, S. M. (2008). Brucella abortus strain RB51 leucine auxotroph as an environmentally safe vaccine for plasmid maintenance and antigen overexpression. Applied and Environmental Microbiology, 74(22), 7051–7055. 10.1128/AEM.01511-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendhran, J. (2021). Genomic insights into Brucella. Infection, Genetics and Evolution, 87, 104635. 10.1016/j.meegid.2020.104635 [DOI] [PubMed] [Google Scholar]
- Rijensky, N. M. , Blondheim Shraga, N. R. , Barnea, E. , Barnea, E. , Peled, N. , Rosenbaum, E. , Popovtzer, A. , Stemmer, S. M. , Livoff, A. , Shlapobersky, M. , Moskovits, N. , Perry, D. , Rubin, E. , Haviv, I. , & Admon, A. (2020). Identification of tumor antigens in the HLA peptidome of patient‐derived xenograft tumors in mouse. Molecular & Cellular Proteomics, 19(8), 1360–1374. 10.1074/mcp.RA119.001876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha, T. , Li, Z. , Zhang, C. , Zhao, X. , Chen, Z. , Zhang, F. , & Ding, J. (2020). Bioinformatics analysis of candidate proteins Omp2b, P39 and BLS for Brucella multivalent epitope vaccines. Microbial Pathogenesis, 147, Article 104318. 10.1016/j.micpath.2020.104318 [DOI] [PubMed] [Google Scholar]
- Shahsavandi, S. , Ebrahimi, M. M. , Sadeghi, K. , & Mahravani, H. (2015). Design of a heterosubtypic epitope‐based peptide vaccine fused with hemokinin‐1 against influenza viruses. Virologica Sinica, 30(3), 200–207. 10.1007/s12250-014-3504-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar, R. , Rha, S. Y. , Yamaue, H. , Katsuda, M. , Kono, K. , Kim, H. S. , Kim, C. , Mimura, K. , Kua, L. F. , & Yong, W. P. (2018). A phase I/Ib study of OTSGC‐A24 combined peptide vaccine in advanced gastric cancer. BMC Cancer, 18(1), Article 332. 10.1186/s12885-018-4234-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, P. , Sidney, J. , Dow, C. , Mothé, B. , Sette, A. , & Peters, B. (2008). A systematic assessment of MHC class II peptide binding predictions and evaluation of a consensus approach. PLoS Computational Biology, 4(4), Article e1000048. 10.1371/journal.pcbi.1000048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S. L. , Li, Z. Q. , Zhang, J. L. , Xi, L. , Cui, Y. , Zhang, W. , Zhang, J. , & Zhang, H. (2020). A safe non‐toxic Brucella abortus ghosts induce immune responses and confer protection in BALB/c mice. Molecular Immunology, 124, 117–124. 10.1016/j.molimm.2020.06.002 [DOI] [PubMed] [Google Scholar]
- Weingarten‐Gabbay, S. , Klaeger, S. , Sarkizova, S. , Klaeger, S. , Sarkizova, S. , Pearlman, L. R. , Chen, D. Y. , Gallagher, K. M. E. , Bauer, M. R. , Taylor, H. B. , Dunn, W. A. , Tarr, C. , Sidney, J. , Rachimi, S. , Conway, H. L. , Katsis, K. , Wang, Y. , Leistritz‐Edwards, D. , Durkin, M. R. , … Rivera, K. D. (2021). Profiling SARS‐CoV‐2 HLA‐I peptidome reveals T cell epitopes from out‐of‐frame ORFs. Cell, 184(15), 3962–3980. 10.1016/j.cell.2021.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff, J. H. , Howland, J. L. , Scott, C. M. O. , Smith, R. A. , & Confer, A. W. (2005). Recombinant bovine interleukin 2 enhances immunity and protection induced by Brucella abortus vaccines in cattle. Veterinary Microbiology, 111(1–2), 77–87. 10.1016/j.vetmic.2005.09.004 [DOI] [PubMed] [Google Scholar]
- Yang, X. , Skyberg, J. A. , Cao, L. , Clapp, B. , Thornburg, T. , & Pascual, D. W. (2013). Progress in Brucella vaccine development. Frontiers in Biology, 8(1), 60–77. 10.1007/s11515-012-1196-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Brucella gene expression in the infected group and control group.
Figure S2 Conservation of related proteins in Brucella.


