Significance
Many animal communication signals arise from preexisting characteristics that can be detected by conspecifics. These signals may evolve, taking advantage of receivers’ sensory biases and avoiding communication with heterospecifics. Prostaglandin F2α is a hormone that drives fish egg laying, and when water borne, it attracts males of some species. We find this olfactory signal evolved ≥200 million years ago, but an ancestor to many fishes lost its olfactory receptor. This gene loss explains divergent reproductive signaling, and we provide insights into characteristics of the signal that has taken its place. This work shows that a preexisting indicator of reproductive status can initiate alternative signaling that coevolves with the sensory system to a derived state.
Keywords: cichlid, prostaglandin, pheromone, olfactory receptor, hormone
Abstract
Pheromones play essential roles in reproduction in many species. Prostaglandin F2α (PGF2α) acts as a female reproductive hormone and as a sex pheromone in some species. An olfactory receptor (OR) for PGF2α was recently discovered in zebrafish, but this signaling pathway is evolutionarily labile. To understand the evolution of signals that attract males to fertile females, we used the African cichlid Astatotilapia burtoni and found that adult males strongly prefer fertile female odors. Injection of a prostaglandin synthesis inhibitor abolishes this attractivity of fertile females, indicating these hormones are necessary for pheromonal signaling. Unlike zebrafish, A. burtoni males are insensitive to PGF2α, but they do exhibit strong preference for females injected with PGF2α. This attractiveness is independent of the PGF2α hormonal receptor Ptgfr, indicating that this pheromone signaling derives from PGF2α metabolization into a yet-undiscovered pheromone. We further discovered that fish that are insensitive to PGF2α lack an ortholog for the OR Or114 that zebrafish use to detect PGF2α. These results indicate that PGF2α itself does not directly induce male preference in cichlids. Rather, it plays a vital role that primes females to become attractive via an alternative male OR.
Sexual selection has generally been considered as competition among less choosy males for mating opportunities with choosy females (1, 2) because mate choice models predict that the selectivity of an individual during mate choice depends on their parental investment (3, 4). Females typically invest more in parental care at both the pre- and postzygotic stages and so are expected to be selective for the best-quality males. In contrast, since males typically invest less in parental care, they are expected to be less choosy. Nevertheless, increasing evidence has suggested that this model might underestimate the cost and benefit of male mating investment (5–10). For instance, courtship behavior is a time-consuming and energetically costly investment, which comes at the expense of other essential behavior like foraging, and it increases predation risk (11, 12). Moreover, sperm and ejaculates may limit male reproductive rate due to their depletion (10, 13–15). Due to these constraints on mating investment, males may exhibit more selective mate choice than previously appreciated. From a male perspective, female quality (6, 10, 16–19) and female reproductive status (19–22) are key factors, which influence male mating preference. Hence, choosy males would benefit from the ability to discern information about female condition, resulting in higher fitness (7, 9).
In African cichlids, renowned for their rapid adaptive radiation and highly diversified of social behavior including mating and parental care, most studies focus on female mate choice, while relatively little is understood about male mating preference. For example, previous studies in cichlid species have shown that females exhibit strong preference for conspecific mates, and male nuptial color morphology is sufficient to convey this information (23, 24). Additionally, females choose high-quality conspecific males based on their visible ornamentation (e.g., coloration and size of fins), behavior (e.g., courtship display quality or frequency) and social status (25–27). All studies mentioned above focus on female association or mating preference and demonstrate that females use visual cues to recognize high-quality conspecific males. However, whether male cichlids show a preference for conspecific females or choose females at different reproductive stages is less clear. Werner and Lotem (28, 29) provided the first behavioral evidence showing that male haplochromine cichlids (Astatotilapia flaviijosephi) prefer to court large females compared with small females. Another study in a biparental cichlid species (Pelvicachromis taeniatus) shows that males prefer females with a larger pelvic fin, which is a conspicuous sexual ornament and correlated with individual condition (30). These studies suggest that male cichlids are capable of visually assessing female quality and exhibit mating preference for higher-quality mates.
These prior studies mainly focus on preference driven by visual cues because these morphological and behavioral traits are correlated with mate quality (in males) and fecundity (in females). However, the effects of chemosensory cues are often underestimated and may also be key information for mating choice (31). Electrophysiological recordings from the olfactory epithelium (OE) reveal that cichlids can perceive and distinguish social cues from odorants. For instance, in male Mozambique tilapia (Oreochromis mossambicus), electroolfactogram (EOG) data indicate that the OE can discriminate odors from dominant versus subordinate males (32). Also, odor cues from females with high reproductive value (prespawning) induced significantly higher olfactory response in males than those from females with low reproductive value (postspawning), suggesting that the olfactory system can discriminate social information, including reproductive status from conspecifics (32, 33). Nevertheless, whether this olfactory response is translated into behavior preference in the males and what the key chemical components released from females to induce this response remain unknown.
Prostaglandin F2α (PGF2α) acts as a reproductive hormone in females to induce ovulation, mating, and spawning in the teleost fish that have been studied (34–37). In addition to its evolutionarily conserved function as a reproductive hormone, PGF2α serves as a sex pheromone in several fish species. Sorensen et al. first showed that in goldfish, PGF2α (and its metabolite, 15-keto PGF2α) is released by ovulated females, and it can induce preference and courtship behavior in males (38). Additionally, electrophysiological studies from several different fish species, including salmon (39–42), carp (43), zebrafish, and other cyprinid species (44), suggest the role of PGF2α as a sex pheromone. Therefore, PGF2α has a dual role as a female reproductive hormone and a pheromone that transmits female reproductive information. Yabuki et al. discovered two olfactory receptor (OR) paralogs, Or114-1 and Or114-2, which are responsible for detecting PGF2α in zebrafish (45). Male zebrafish null for or114-1 exhibit severe defects in courting female conspecifics. Although female cichlids produce PGF2α prior to spawning (27) and its signaling is necessary for females to reproduce (34), male cichlid olfactory systems appear insensitive to PGF2α or 15-keto PGF2α (46–49). Despite this, no behavioral studies have tested for an olfactory preference for PGF2α or its metabolites. Therefore, it is still unclear about what the role of PGF2α is in female reproductive pheromone signaling in cichlids.
In this study, we find that A. burtoni male cichlids exhibit a strong preference for fertile female odors, but unlike zebrafish, they do not use raw PGF2α as a pheromone cue to discriminate the reproductive status of females. However, PGF2α initiates attraction to females: It is necessary and sufficient to prime the females to become attractive to conspecific males. We further discover that cichlids and other clades of fish species are insensitive to raw PGF2α due to the loss of an ortholog for Or114 used to detect PGF2α in cypriniform fish species. These results indicate that, in African cichlids, PGF2α drives reproductive female pheromone signaling through an evolutionarily divergent pathway.
Results
Males Prefer Olfactory Cues from Fertile Females.
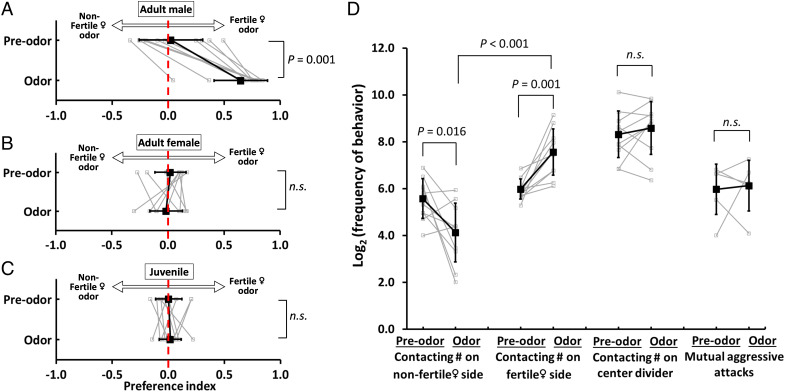
We asked whether A. burtoni males are attracted to olfactory cues from fertile females. Here, we define fertile status as the female fish carrying mature eggs and are ready to spawn within 24 h. We designed a dichotomous choice paradigm (Fig. 1 A and B) that tests preference for olfactory cues without any visual or mechanosensory contribution. We find that male cichlids exhibit a strong preference for odor cues from fertile females compared with nonfertile females (Fig. 2A and SI Appendix, Fig. S1). We find that males increase contact frequency with the perforated divider at fertile female odor sources and decreased the contact at the nonfertile female odor side (Fig. 2D), suggesting that males actively search for reproductive female cues. Furthermore, we tested whether female odor cues influence interactions between males by quantifying the contact frequency and mutual aggressive events across the central divider between two focal males. We found that male–male interaction rates were not influenced by the presence of female odors (Fig. 2D). We also asked whether attraction to reproductive females is a general phenomenon in A. burtoni. We found neither adult females (Fig. 2B) nor sexually immature juvenile males (Fig. 2C) prefer fertile female odors implying this is a sexually mature male preference and it may play a key role in reproduction.
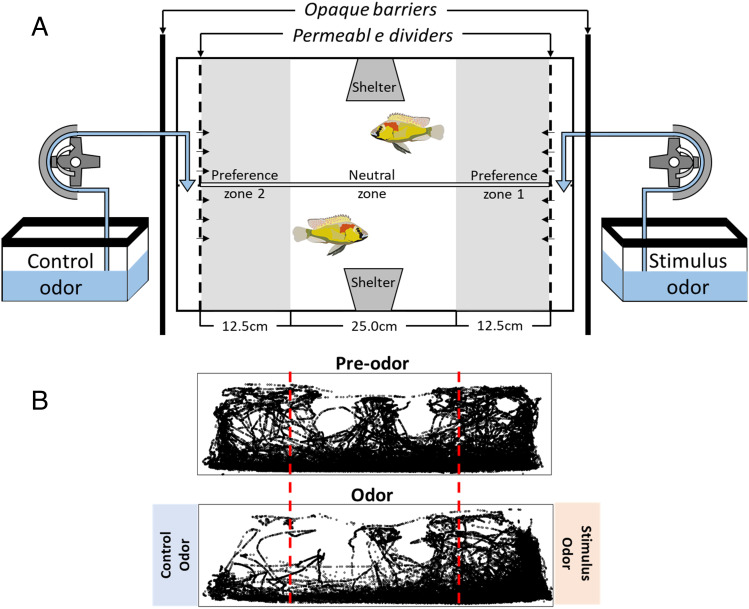
Fig. 1.
(A) Depiction of the tank setup for olfaction preference test. Stimulus odors are delivered by peristaltic pumps to opposing sides of a tank, and fish locations are tracked over time. Note that the two focal individuals in the arena are separated by a transparent divider so that they can see but will not be able to physically interact with each other. (B) Representative fish positions before and during odor delivery. In these plots, dots represent the position of fish in every video frame during the trials. Note that the distribution of the dots is skewed to the side of stimulus odor, indicating the fish are attracted to this odor cue.
Fig. 2.
Adult males, but not females or sexually immature juvenile males, show an olfactory preference for reproductive female cues. (A) Male A. burtoni exhibit a strong side preference for fertile female odors over nonfertile female (control) odors, suggesting that they can perceive the differences between these two types of females solely by odor cues. (B) Adult female and (C) sexually immature juvenile male cichlids do not prefer fertile female odors. (D) Male cichlids contact the perforated divider associated with fertile female odors more frequently than the divider associated with nonfertile female odor. Contact on the central divider between males and mutual aggressive acts are similar between the preodor and odor stages. Mutual aggressive attack is counted when the pair of focal fish exhibit aggressive display simultaneously across the central divider. Gray squares and lines represent individual behavior performance, while black squares and lines represent mean ± SD. Mann–Whitney U test, n = 10 fish per experiment. n.s., nonsignificant in statistical analysis.
Prostaglandin F2α Is Necessary and Sufficient for Female Reproductive Pheromonal Signaling.
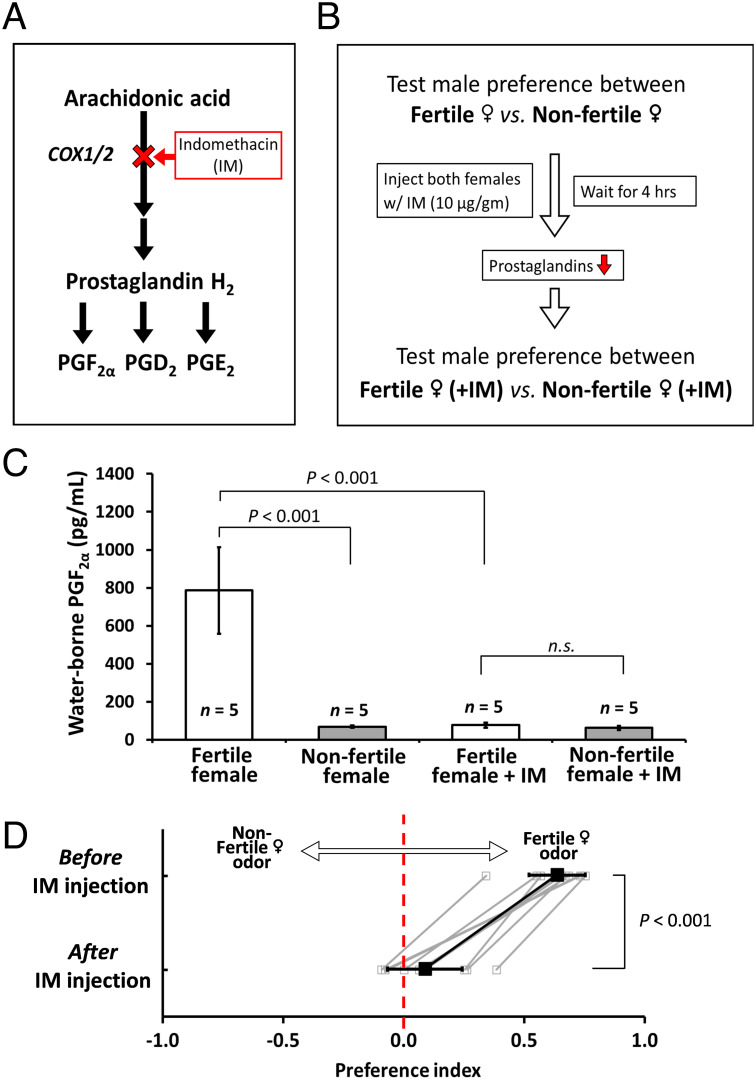
PGF2α is an essential signaling molecule for reproduction and in some species acts as a pheromone itself (36). We asked whether PGF2α participates in cichlid female-to-male pheromonal signaling. We first tested the necessity of PGF2α in female reproductive pheromone signaling by inhibiting prostaglandin synthesis in fertile females using indomethacin (IM) (Fig. 3 A and B). We designed a two-stage preference test: In an initial test, we assessed male preference between fertile and nonfertile females; in a subsequent test, we injected both females with IM and then retested the male preference for the same pair of females. Before IM injection, fertile females release more PGF2α into tank water (Fig. 3C). However, 4 h after IM injection, PGF2α released into tank water by fertile females was reduced to a level similar to that of nonfertile females, indicating that IM effectively blocks prostaglandin synthesis. In keeping with a key role for PGF2α in pheromonal signaling, IM injection significantly reduced the attractiveness of fertile female odors to focal males (Fig. 3D and SI Appendix, Fig. S2). Thus, one or more prostaglandins are necessary to initiate female pheromone signaling to males.
Fig. 3.
Prostaglandin signaling is necessary for production of attractive fertile female cue. (A) IM inhibits the prostaglandin synthesis pathway by antagonizing cyclooxygenases (COX1 and COX2), thereby preventing the conversion of arachidonic acid to prostaglandin H2, precursor for PGF2α, PGD2, and PGE2. (B) An experimental flowchart for testing the necessity of prostaglandins in mediating female reproductive pheromone signaling. (C) In fertile females, IM treatment significantly reduced waterborne PGF2α to a level similar to that of nonfertile females 4 h after injection. Mean ± SEM; the ANOVA test followed by Tukey’s test for post hoc analysis. (D) Before IM injection, male cichlids show strong preference to fertile female odors; after IM injection, this preference is significantly reduced. Gray squares and lines represent individual behavior performance, while black squares and lines represent mean ± SD. Mann–Whitney U test, n = 10 fish per experiment. n.s., nonsignificant in statistical analysis.
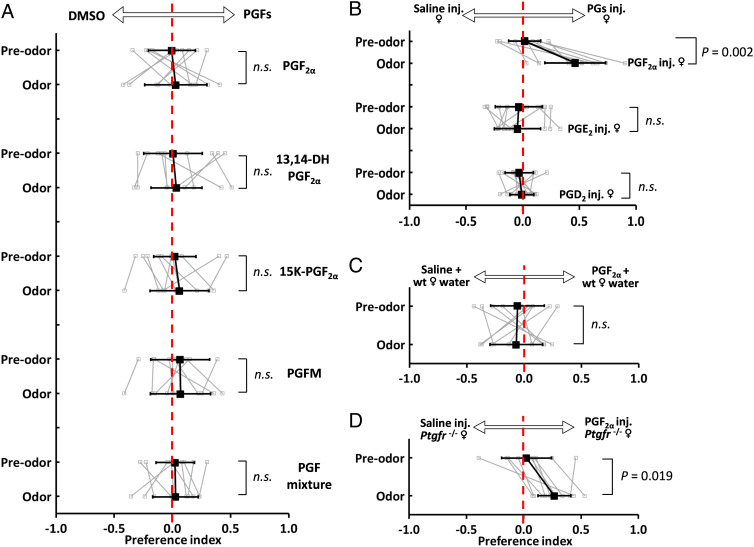
To ask whether PGF2α is the key component that attracts males, we tested whether males prefer PGF2α. We found that males do not spend more time near a source of PGF2α compared with vehicle (Fig. 4A). This result contrasts with the strong attraction observed in several species of cyprinid fish (38, 44, 45) but is consistent with electrophysiological experiments that did not detect a response to PGF2α in the A. burtoni olfactory system (50). Because male cyprinid fish are also sensitive to PGF2α metabolites (38, 51), we tested the attractivity of three known PGF2α metabolites (15-keto PGF2α [15K-PGF2α], 13,14-dihydro-15-keto PGF2α [PGFM], and 13,14-dihydro PGF2α [13,14-DH PGF2α]). We found no attraction to any molecule alone (Fig. 4A and SI Appendix, Fig. S3A). As carp have been shown to produce a blend of PGF2α metabolites (52), we also tested for a male preference for a mixture of PGF2α plus five known metabolites from mammals (15K-PGF2α, PGFM, 13,14-DH PGF2α, 19(R)-hydroxy PGF2α, and 20-hydroxy PGF2α). However, our data showed that neither PGF2α nor a mixture of the five known PGF2α metabolites is sufficient to attract male cichlids (Fig. 4A).
Fig. 4.
PGF2α induces attractivity but is not itself attractive. (A) Males do not prefer PGF2α, three major known metabolites individually, or the mixture of PGF2α plus five commercially available metabolites. Control is DMSO vehicle. 13,14-DH PGF2α: 13,14-dihydro PGF2α; 15K-PGF2α: 15-keto PGF2α; PGFM: 13,14-dihydro-15-keto PGF2α. (B) Males prefer odors from wild-type, nonfertile females which are injected with PGF2α but not odors from females which are injected with PGD2 or PGE2. (C) Males do not prefer odor from nonfertile female-conditioned water spiked with PGF2α. (D) Males show a mild preference for the odor from the PGF2α hormonal receptor knockout (ptgfr−/−) females which were injected with PGF2α. Gray squares and lines represent individual behavior performance, while black squares and lines represent mean ± SD. Mann–Whitney U test, n = 10 fish per experiment. n.s., nonsignificant in statistical analysis.
Although male cichlids are not attracted to raw PGF2α, the timing of PGF2α level rises at fertility, and the essential role of this molecule for reproduction drove us to ask whether PGF2α may still contribute to pheromone signaling. We therefore injected PGF2α into nonfertile females and tested their attractiveness to males. Surprisingly, we found that male cichlids exhibit a strong preference for the odor cues from females injected with PGF2α compared with vehicle-injected control females (Fig. 4B and SI Appendix, Fig. S3B). This attractive pheromone was only produced by PGF2α-injected females but not by females injected with PGE2 or PGD2 (Fig. 4B and SI Appendix, Fig. S3B). Taken together with the reduced attraction to PGF2α-depleted females after IM treatment, these results indicate that PGF2α is necessary and sufficient to induce female-to-male pheromone signaling.
The failure of PGF2α alone to attract males may also result from the absence of ancillary chemicals released by females. Thus, we spiked water conditioned by nonfertile females with raw PGF2α and found no preference compared with the same water spiked with vehicle (Fig. 4C). This result indicates that PGF2α must be processed in females prior to the release of the attractive compound(s). Two models for this process include the metabolization of PGF2α to an attractive molecule(s) or the activation of PGF2α hormonal signaling in females that causes the release of a pheromone(s). To test the second model, we took advantage of females carrying a nonfunctional receptor (Ptgfr) for this hormone. We have previously shown that females null for ptgfr are insensitive to PGF2α, and as a result fail to initiate reproductive behaviors (34). We injected PGF2α into ptgfr−/− females, and found that males still prefer ptgfr−/− females injected with PGF2α compared with vehicle-injected ptgfr−/− females (Fig. 4D and SI Appendix, Fig. S3B), suggesting that this female reproductive pheromone is produced independent of its hormonal signaling. Together, these results suggest that PGF2α produced at the time of fertility is metabolized into a yet-undiscovered metabolite(s).
PGF2α-Sensitive Receptor Or114 Is Absent from Most Fish Species.
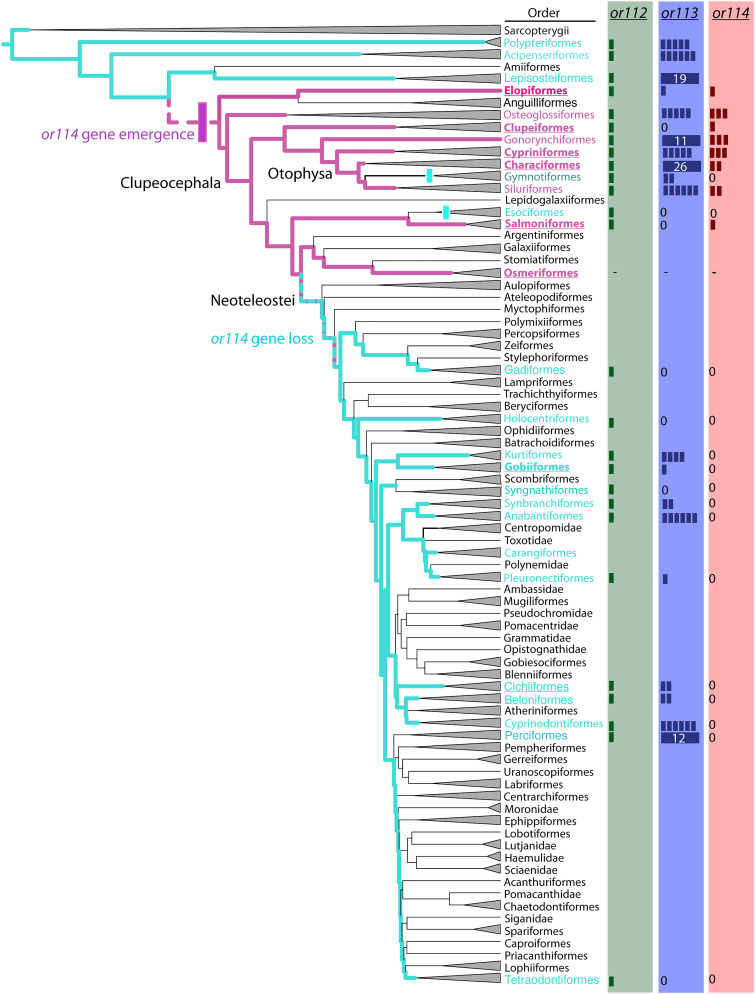
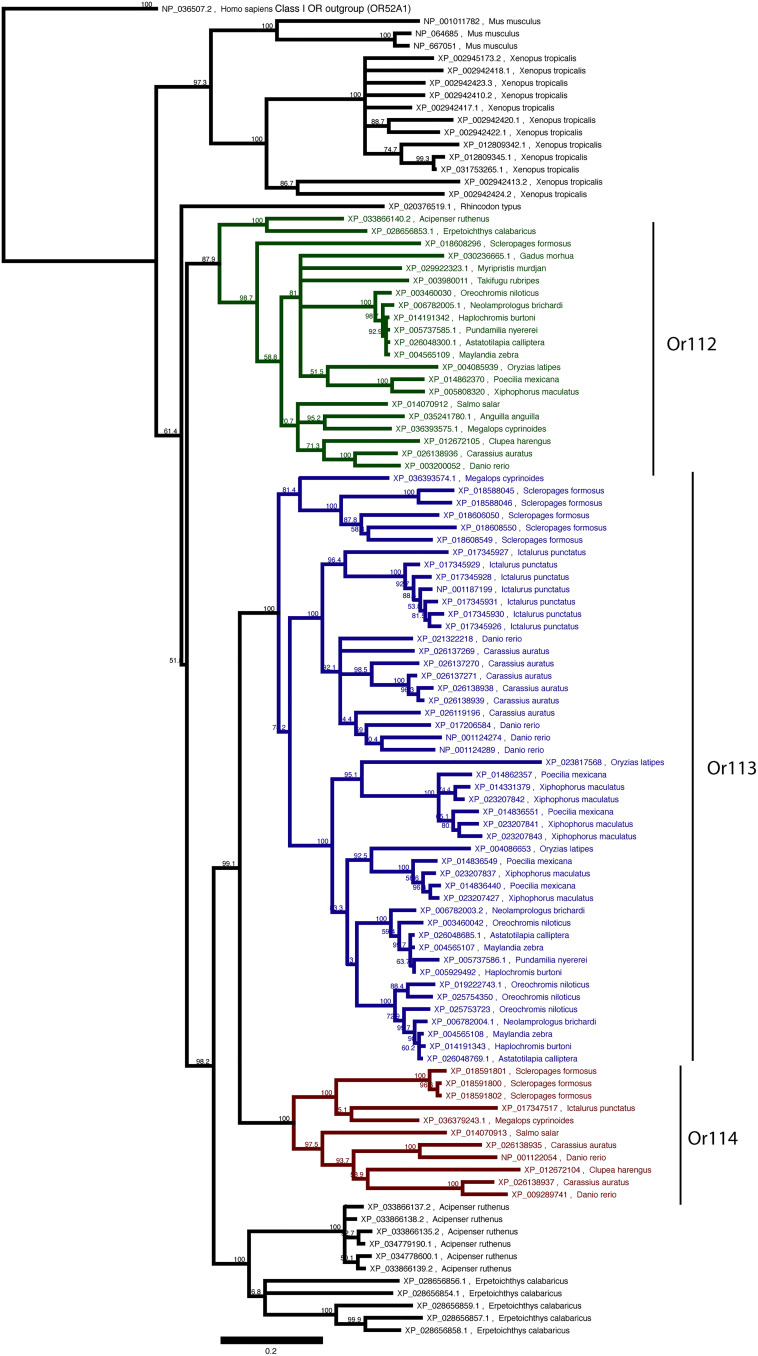
We sought to understand why fish species exhibit different PGF2α sensitivities by comparing the complement of OR genes present in a variety of fish species. Cypriniform and salmoniform fish can detect trace levels of PGF2α (38–40, 53). Despite the apparent ubiquity of PGF2α as a hormone that controls spawning behavior, the ability to detect this pheromone is not universal. The OE in a variety of neoteleost species appears insensitive to PGF2α (47, 48, 50, 54). This finding could result from a difference in the OR repertoire encoded by the genome, differences in expression in the OE, or changes in the signaling pathways downstream of PGF2α–OR binding. The ORs that detect PGF2α in zebrafish have been identified as the ORs or114-1 and or114-2 (45), so we focused on the clade of ORs which contain zebrafish or112, or113, and or114 genes (the β clade of ORs), which include mouse class I receptor genes Olfr543, Olfr544, and Olfr545 (55). We asked whether this gene family has changed across fish species. A. burtoni has three β ORs: one ortholog of or112 and two of or113. Other haplochromine cichlid genomes also carry the same three orthologs, indicating that this low number is not a consequence of incomplete genome assembly. or112 exhibits a remarkable conservation across fish species. Whereas most ORs are rapidly gained and lost over evolutionary time, or112 (unlike or113 and or114) is present in every species we examined (Fig. 5). Furthermore, nearly every species carries exactly one or112 ortholog, indicating that duplications or losses of this receptor are deleterious.
Fig. 5.
Phylogeny of teleost fish reveals that olfactory sensitivity to PGF2α arose after the split of modern teleosts from the basal Lepisosteiformes (gar) species, and was subsequently lost, as inferred by olfactory responses to PGF2α and the presence of an or114 gene paralog. Fish in six orders, underlined, have been previously tested for an olfactory response to PGF2α. Colored bars at right indicate the number of gene paralogs for each β OR. Zero indicates that no paralog was detected. Absence of data indicates either no genome sequence available or sequences were not tested. Order names and their lineages are colored based on the presence (red) or absence (green) of an or114 gene paralog. For species that were tested for PGF2α response, the presence of an or114 gene paralog successfully predicted a positive response. Dotted lineage refers to incomplete or ambiguous data. Paralog count is determined for representative species for each order; for details, see Table 1. Phylogeny adapted from ref. 58.
We found that each species demonstrated to be sensitive to PGF2α by EOG (38, 39, 42, 43, 45) carries ≥1 gene ortholog of zebrafish or114-1. Conversely, no species with an olfactory system shown to be insensitive to PGF2α (47, 48, 50, 54), including A. burtoni, carries an or114 ortholog (Fig. 6 and Table 1). These results indicate that the presence of an or114 ortholog determines olfactory sensitivity to the raw PGF2α. Thus, the absence of an electrophysiological or behavioral response to PGF2α in A. burtoni may be explained by the lack of an or114 gene. Our results also predict that many otophysan species are sensitive to PGF2α, while no species outside Otophysa and Salmoniformes detect PGF2α via Or114. Surprisingly, or114-like genes are not restricted to a single clade; they are present in salmon (Salmoniformes), tarpon (Elopiformes), and bonytongue (Osteoglossiformes). This suggests that or114 was present in teleost fish by the split of the elopomorpha and clupeocephala lineages ~250 Mya, likely arising from an ancestral OR encoding an Or113-like receptor. Although this gene appears to arise around the time of the teleost whole-genome duplication, its presence in a multi-OR cluster with other β ORs indicates it arose as the result of a direct tandem duplication. This gene was later lost in the lineage leading to all neoteleost fish, around 150 Mya, which is consistent with the loss of sensation of PGF2α in this fish taxon containing the majority of fish species.
Fig. 6.
Genomic search for putative PGF2α ORs. We identified β ORs through BLASTp searches of vertebrate genomes and then assembled phylogenetic trees. Teleost ORs clustered into OR clades corresponding to Or112, Or113, and Or114 proteins named per zebrafish nomenclature. Basally branching fishes (Reedfish, Erpetoichthys calabaricus, a polypteriform, and sturgeon, Acipenser ruthenus, an acipenseriform) carry ORs that do not cluster with Or113 or Or114, consistent with a model in which Or114 arose following the divergence of their lineages from teleostei. We observed that each species demonstrated to be sensitive to PGF2α by EOG carries a predicted protein sequence similar to zebrafish Or114. Conversely, no species with an olfactory system shown to be insensitive to PGF2α has an Or114 ortholog. (Scale bar, 0.2 substitutions per residue.)
Table 1.
List of literature regarding PGF2α sensitivity (by EOG), the presence of or114 gene ortholog, and male preference for PGF2α in fish species
| Order | Species | Common name | PGF2α sensitivity by EOG | or114 ortholog? | ♂ preference for PGF? |
|---|---|---|---|---|---|
| Cichliformes | Astatotilapia burtoni | Burton’s mouthbrooder | No (50) | No | No* |
| Oreochromis niloticus | Nile tilapia | No (48) | No | – | |
| Oreochromis mossambicus | Mozambique tilapia | No (48) | No | – | |
| Maylandia zebra | Zebra mbuna | – | No | – | |
| Gobiiformes | Neogobius melanostomus | Mudskipper goby | No (54) | No | – |
| Cypriniformes | Danio rerio | Zebrafish | Yes (44, 45) | Yes | Yes (42) |
| Carassius auratus | Goldfish | Yes (38) | Yes | – | |
| Cyprinus carpio | Common carp | Yes (43) | Yes | – | |
| Barbonymus schwanenfeldii | Tinfoil barbs | Yes (51) | – | – | |
| Epalzeorhynchos frenatus | Red fin sharks | Yes (59) | – | – | |
| Barilius bendelisis | Hamilton’s barila | Yes (60) | – | – | |
| Zacco temminckii | Dark chub | Yes (43) | – | – | |
| Zacco platypus | Freshwater minnow | Yes (43) | – | – | |
| Misgurnus anguillicaudatus | Pond loach | Yes (43) | – | – | |
| Clupeiformes | Plecoglossus altivelis | Sweetfish | Yes (43) | Yes | – |
| Salmoniformes | Salmo salar | Atlantic salmon | Yes (39, 42) | Yes | – |
| Salvelinus alpinus | Arctic char | Yes (39) | Yes | – | |
| Salmo trutta | Brown trout | Yes (39–41) | – | – | |
| Coregonus clupeaformis | Lake whitefish | Yes (39, 40) | – | – | |
| Oncorhynchus mykiss | Rainbow trout | No (39, 40, 43) | – | – | |
| Oncorhynchus rhodurus | Biwa trout | No (43) | – | – | |
| Osmeriformes | Plecoglossus altivelis | Ayu | Yes (43) | – | |
| Characiformes | Astyanax mexicanus | Mexican cavefish | Yes (53) | Yes | – |
| Elopiformes | Megalops cyprinoides | Tarpon | Yes (61) | Yes | – |
| Acipenseriformes | Huso huso x Acipenser ruthenus | Bester sturgeon | No (43) | No | – |
| Beloniformes | Oryzias latipes | Medaka | – | No | – |
| Esociformes | Esox lucius | Northern pike | – | No | – |
| Osteoglossiformes | Scleropages formosus | Asian bonytongue | – | Yes | – |
*The result is presented in current study.
Discussion
PGF2α is Vital for Female Reproductive Pheromonal Signaling in African Cichlids.
Previous research in mate choice of fish has largely focused on visual cues (e.g., body size and nuptial colors), but other sensory information may also play an important role in mate preference. Here, we demonstrate that olfactory cues are important in female-to-male reproductive signaling in a Lake Tanganyika cichlid species, A. burtoni. Although some previous electrophysiological studies have shown that cichlids can sense the social information via the OE (32, 33), we further confirmed that these olfaction signals are translated into a behavioral response. We found that male cichlids can assess reproductive status in females and exhibit preference for fertile females solely by odor cues. Interestingly, we did not observe courtship behavior (e.g., body quivers, tail waggles, and leads to shelter) during the preference trials despite males were actively searching for female odors (Fig. 2D). This finding is consistent with a previous study showing that male cichlids spend significantly more time searching and exploring the tank when exposed to odor cues from fertile females but do not perform courtship behaviors in the absence of visual cues (56). Other studies in tilapia also show that although males can discriminate female reproductive status through olfactory cues (32, 33), visual cues are necessary for the full display of reproductive behaviors including nesting, courtship, and spawning (57). These results, together with our data, imply that olfaction and visual cues play different but complementary roles during mating. Thus, we speculate that olfactory cues in cichlids could be important during the initial stages of conspecific recognition, seeking mates and mating location, when distance and turbidity in the lake would limit visual detection, while visual signals stimulate courtship and mating behavior during the late stages.
The preference for fertile female odors was found in adult males but not in adult females or in sexually immature males, suggesting that this is a sexually mature male-specific preference, which should be related to reproduction. Sex and age differences in olfactory responses to reproductive odors have been reported in several fish species (44), but it is still unclear what mechanisms mediate these differences. One explanation is that only male cichlids express the OR(s) responsible for detecting fertile female cues. An alternative explanation is that females and juveniles each detect the odor, but the salience of this cue is low due to differences in downstream processing by central neural circuits. Cardwell et al. (51) showed in tinfoil and silver barb, androgen implants increase OE sensitivity, specifically to PGF2α, in juvenile males. The implanted silver barb juveniles also performed courtship behavior in the presence of PGF2α-injected females. Thus, circulating hormones, such as androgens, in the mature males may modulate olfactory sensitivity or the neural circuits, which mediate preference behavior.
In probing the basis of male olfactory preference for fertile female cues, we discovered that PGF2α is a necessary and sufficient driver of female reproductive pheromone signaling. Although male cichlids are physiologically (48, 50) and behaviorally insensitive to PGF2α or known PGF2α metabolites, they exhibit a strong preference for nonfertile females injected with PGF2α, indicating that PGF2α primes the female to become attractive to conspecific males. We propose the following three models to explain these data:
-
1)
One or more PGF2α metabolites acts as a pheromone.
-
2)
PGF2α is metabolized and activates a non-Ptgfr–dependent pathway in females, resulting in the release of a distinct pheromone.
-
3)
PGF2α or its metabolite interacts with a cofactor in the female prior to release.
Our data under model 1 suggest the role of a distinct PGF2α metabolite(s) since we show that known metabolites are not attractive to male A. burtoni alone or in combination. Steroids and lipid hormones usually are low-polarity molecules, which are difficult to be excreted; these hormones are often converted to metabolites with higher polarity (e.g., glucuronated or sulfated form) in order to be rapidly released and subsequently signal to conspecifics. Previous studies with cypriniform fishes have demonstrated sensitivity to PGF2α or its metabolites at the nanomolar (or greater) range (44, 62), while we use concentrations 1 to 3 orders of magnitude higher. Thus, we do not believe that a failure of males to seek synthetic PGF2α metabolites results from insufficient stimulation. In some species, the ratio of the metabolites produced has been suggested to be important for attraction (52, 63). We delivered chemicals in approximately equimolar ratios, so we cannot exclude a model in which cichlids compare concentrations of individual compounds within a blend. Although our study focuses on PGF2α, the fertile females may release multiple distinct cues, including non-PGF2α derivatives, as a pheromone bouquet to induce male preference. Further biochemical analysis will be necessary to determine the identity of this molecule(s).
Our data rule out a simple version of model 2 in which PGF2α activates its receptor Ptgfr in the females, leading to the release of another pheromone since females null for ptgfr retain attractivity after PGF2α injection despite losing behavioral sensitivity to this hormone (34). It remains possible that PGF2α acts as another female receptor, which then triggers the release of a distinct pheromone. However, cichlids have only one ptgfr gene ortholog (34), and injection of females with the structurally similar PGE2 or PGD2 does not induce attractivity, so other known prostaglandin receptors are unlikely to mediate this response. Moreover, females null for ptgfr fail to initiate sexual behavior (34), suggesting that no other redundant PGF2α receptor can compensate for the loss of ptgfr. Thus, any non-Ptgfr mechanism that senses PGF2α must be selective for PGF2α and structurally distinct from known prostaglandin receptors. We note that the degree of attraction to ptgfr−/− females injected with PGF2α appears reduced compared with that in WT females, although this does not reach statistical significance (P = 0.131, Mann–Whitney U test). Thus, Ptgfr is not essential for PGF2α-initiated attraction, but we cannot exclude the possibility that its signaling contributes to full attraction. Future experiments will show whether metabolites produced by animals injected with PGF2α differ by age, sex, or species, leading to insights into the pathways responsible and their evolution.
We also considered that PGF2α may act in combination with other female odorants as many pheromones require context to be attractive. However, PGF2α in the context of conditioned water from a female does not attract males. Thus, under model 3, we propose that if PGF2α is to act in combination with a cofactor, it must interact within the female prior to release into the water. One class of cofactor may be pheromone-binding proteins, which are critical in conspecific signaling because they act as solubilizers and carriers to protect the pheromones from enzymatic degradation (64); they also serve as cofactors in the activation of pheromonal receptors (65). In mice, major urinary proteins and other lipocalins are thought to bind small, volatile pheromone molecules and thus prolong the effectiveness of compounds contained in scent marks (66). Thus, it is possible that this pheromonal signal may require PGF2α binding to a pheromone-binding protein prior to release.
Proximate and Ultimate Causes of Losing Sensitivity to PGF2α in African Cichlids.
PGF2α is an ancient molecule with evolutionarily conserved function as a reproductive hormone in both invertebrates (67) and vertebrates (37, 68). Thus, its release is a reliable and efficient cue to transmit female reproductive status. Why then are cichlids insensitive to PGF2α or its major known metabolites? In our OR genomic search, we find that species that are sensitive to PGF2α (38, 39, 42, 43, 45) carry a gene paralog to zebrafish or114-1, a PGF2α OR (45). On the other hand, species insensitive to PGF2α (47, 48, 50, 54) do not have an or114 ortholog (Fig. 5 and Table 1). A. burtoni does not have or114, which provides a parsimonious proximate answer to why males are insensitive to raw PGF2α.
Our or114 phylogenetic analysis agrees with results of previous studies showing that the ability to detect PGFs is only found within a few clades (summarized by ref. 69). Or114 orthologs are found in limited fish families: Otophysa (including Cypriniformes and Characiformes), Clupeiformes, Salmoniformes, Elopiformes, and Osteoglossiformes (Table 1). This suggests that or114 was present in early teleost fish but that it was subsequently lost in most euteleost lineages. But why would a eutelost ancestor lose a receptor for detecting PGF2α, a ubiquitous female reproductive signal? One explanation is that during sympatric speciation in ancient environments, relying on a common female reproductive cue makes it difficult to find conspecific mates or avoid fertile predators. Thus, recognition of conspecific fertile females may require combinatorial cues or species-specific metabolites. Studies in silver carp (Hypophthalmichthys molitrix) show that females release odor mixtures with a blend of PGF2α and two metabolites. Although PGF2α or its metabolites alone only have modest attractiveness to silver carp, this PGF2α odor mixture is highly attractive to conspecific males (52). Interestingly, silver carp did not appear to respond to the PGF mixture released by heterospecific carp (52), suggesting the ratio of metabolites carries information about species identity.
In African cichlids, olfactory cues also guide identification of conspecifics. In two closely related Lake Malawi haplochromine species (Pseudotropheus), preference for conspecific males only occurs when olfactory cues are present (31), suggesting that rapid divergence of olfactory signals (or their sensation) contributes to the radiation of the African cichlid species. Our results support the idea that African cichlids utilize olfactory cues to recognize conspecifics and assess reproductive status of potential mates, but this occurs via a distinct pheromone sensory pathway from that of cypriniform fishes.
We note that although this study focuses on olfactory cues, it is also possible that fish use multisensory systems to identify an appropriate mate. For instance, different cichlids may use a PGF2α-derived odor cue but utilize other species-specific factors, such as color, morphology, or behavior patterns to recognize conspecific mates. Among haplochromine cichlids of Lake Victoria, color differences among closely related conspecifics are sufficient to guide mating decisions (23, 24). Combinations of visual and olfactory cues provide sufficient stimulus to activate key brain regions and drive reproductive behaviors (56). Further studies to identify the sensory receptors and perceptual mechanisms will provide a better understanding of central mechanisms for the initiation of mating behavior.
Our data show that PGF2α is central to production of an attractive pheromone in cichlids. What olfactory mechanisms might transduce a PGF2α-activated signal? The hormonal receptor Ptgfr is an unlikely candidate because Ptgfr−/− males mate normally with fertile females (34), and ptgfr mRNA is not expressed in the OE of zebrafish (45). While Ptgfr could be expressed in sensory structures, it is unclear how it would selectively sense a PGF2α metabolite but not raw PGF2α. As the PGF2α-sensitive or114 is absent from the cichlid genome, an alternate model is that a paralog (ie, Or112 or Or113) senses a structurally related PGF2α metabolite. The sensitivity of β ORs in fish (aside from zebrafish Or114) has not been tested, but the related mouse Olfr543, 544, and 545 have been shown to bind short-chain fatty acids. Consistent with this, a mouse mutant that reduces expression of these and other class I ORs lose sensitivity to carboxylic acids (70). As prostaglandins exhibit fatty acid motifs, and β ORs are present in all fish species we screened, another β OR may be responsible for binding a PGF2α metabolite pheromone. An important role for β ORs would appear likely as these receptors are found in all fish species. Furthermore, or112 is found as a single ortholog in every teleost we examined, implying deleterious effects of its duplication or deletion. Among OR gene families, the β OR class is the only one to be conserved from fish to mammals. This deep conservation suggests an essential role for these receptors. While the evolution of chemosensors for mate recognition among insects has been well studied (71, 72), much less is known in fish. We suggest that an understanding of the evolution of the β OR family will provide important insights into pheromones that guide mating of this speciose taxon.
In conclusion, we find that male A. burtoni perceive female reproductive status solely by odor cues and exhibit a strong preference for fertile females over nonfertile females. PGF2α is a necessary component for initiating this female pheromone signaling, and it is sufficient to prime nonfertile females to become attractive. Unlike cyprinids, most fish species are insensitive to PGF2α which can be explained by the lack of or114 orthologs. However, cichlids still exhibit a strong preference for the odor cues from females which are injected with PGF2α, suggesting that they detect a female pheromone downstream of PGF2α through an evolutionarily divergent pathway.
Materials and Methods
Study Organism.
Mixed-sex groups of adult A. burtoni derived from a population from Lake Tanganyika (73) were raised at the University of Maryland. Fish were maintained in 32- to 80-liter community tanks with pH ~8.0, 25 °C water temperature, and a 12:12-h light:dark cycle with 15-min dusk and dawn periods. Gravel and halved terracotta pots provided substrate that facilitates the establishment and maintenance of territories for reproduction. Fish were fed daily with cichlid food pellets (Zeigler Bros Inc.) between 0900 h and 1100 h. All animal maintenance and experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Maryland (protocol 1046257-1).
Tank Setup for Olfaction Preference Test.
Male preference was assessed using a dichotomous choice paradigm. On the day before preference assay, two adult males (> 1 y old and SL > 5 cm) were placed in the central compartment of a 120-liter tank divided into three sections by transparent, perforated acrylic dividers (Fig. 1A). Ten holes (2 mm diameter) on the left and right side of dividers allowed odor cues to diffuse into the central compartment. An additional transparent divider separated the two focal males to prevent direct interactions. Two three-sided opaque shelters with a transparent roof were placed in each tank to mimic a spawning site and allow tracking. In pilot tests, we found that single males usually hid in the shelter or corners, which could potentially bias the results. Because A. burtoni is a social species, we therefore used two males in each preference trial to increase explorative activity by focal males. Two peristaltic pumps were set up underneath the preference tank to deliver odor cues (at ~0.2 mL/s) from two separate 8-L tanks into the left and right compartments. The 8-L tanks containing stimulus odor or control cues were set underneath the preference tank and covered with black curtains to prevent any visual information being revealed to the focal males. The sides of odor cues were randomly decided by a coin flip. Focal males were acclimatized in this preference tank for at least 3 h (ranging from 3 to 16 h) until they freely swam and explored the tank environment. On the day of the trial, a Raspberry Pi camera (Raspberry Pi 3 Model B and Camera Module v2) recorded behavior performance of focal fish. In each trial, we first recorded 90 min of behavior (“preodor” stage, when no odor cue was delivered into the tank) to assess baseline preference. We then turned on the pumps and recorded 90 min of behavior (“odor” stage, when odor cue was delivered into the left and right compartments). We also assessed preference in nonfertile adult females (age > 1 y old and SL > 4 cm) and juvenile males (sexually immature fish and age between 4 and 6 wk using 30-L tanks). All behavior trials were conducted between 0900 h and 1700 h.
Behavioral Tracking and Assessing Preference.
IdTracker.es v1.0 (74) was used to track an individual’s coordinates on a frame-by-frame basis (Fig. 1B). We tracked fish with the following parameters: number of individuals = 2, intensity threshold = 0.75, minimum size = 600, resolution reduction = 1, interval = 1 to 135,000, and number of frames for references = 3,000. Focal fish preference for a particular side was scored as the amount of time spent in the “association zone”: within 15 cm of the left or right perforated dividers. To assess the preference, we calculated the following indices:
1) Preference index:
2) Percentage of time spent in stimulus odor zone
We compare the preference index in the preodor and odor stages to assess whether fish exhibit preference for stimulus odor. In addition to tracking fish position, we also used Behavioral Observation Research Interactive Software [BORIS (75)] to score the following behaviors: 1) contact frequency in preference zone: focal fish use their mouth to touch or bite the perforated divider associated with stimulus odor or control odor; 2) contact frequency on the central divider: focal fish used their mouth to touch or bite the central divider; and 3) mutual aggressive events: both focal fish exhibit aggressive display and attack simultaneously across the central divider.
Collection of Fertile Female Odors.
To identify fertile females, five all-female communities were established in 30-L aquaria, with 3 to 4 females and 1 half terracotta pot shard territories each. All females were tagged with Visible Implant Elastomer tags (Northwest Marine Technology) for identification. All five tanks were observed for 30 min daily to check if any female showed signs of fertility, which include 1) distended abdomen; 2) territorial aggression toward other females and frequent spawn site visits. To further confirm whether the female is ready to spawn, the potential fertile female is introduced into the tank with a dominant male and tested if the females exhibited the circling behavior for 30 min. One day before preference trials, after confirming the reproductive status in communities, one fertile female and one size-matched (Δ standard length [SL] < 5 mm) nonfertile female were transferred into two 8-L tanks which contained 6 L of clean aquarium water (23 g cichlid salt and 375 mL saturated baking soda solution, each dissolved in 150 L reverse osmosis water). These females were acclimatized overnight (~16 h) to allow for accumulation of odor cues in the water. After finishing the trials, fertile females were transferred into a tank with a dominant male to confirm it spawned within 24 h. If the female did not spawn within 24 h, the trials corresponding with this female were not included in the final data analysis.
Preference Test for IM-Injected Fertile Female Odors.
Similar to the first experiment, one fertile female and one size-matched (Δ SL < 5 mm) nonfertile female were transferred into two 8-L tanks, which contained 6 L of clean aquarium water. These females were acclimatized in 8-L tanks for 2 h to accumulate odor cues in the water. We then performed a 90-min pretest to confirm the fertile female odor is preferred by males. The two females were then injected with IM (Sigma-Aldrich, I7318, 10 µg/g of body mass) and returned to two separate housing tanks for 4 h to ensure the IM injection decreased the levels of prostaglandins (76). The two females were then transferred to new tanks containing 6 L of clean aquarium water and accumulated odor cues for 2 h. This water was used to test if the fertile female odor is still preferred by males after IM injection. The sides of fertile or nonfertile female cues were swapped between pre- and posttests to prevent the males from associating the cues with side of the tank. We also saved 1 L of conditioned water from each female before and after IM injection to quantify the level of waterborne PGF2α as a post hoc test to confirm the effects of IM injection. Blood samples were collected for measuring serum PGF2α levels, and body and gonad mass were measured to calculate the gonadosomatic index (ovary mass/body mass * 100). The levels of waterborne PGF2α were quantified by the Cayman EIA kit (product no. 516011). Procedures for PGF2α hormone sample collection and analysis were adapted from Li et al. (77). Detailed procedures are described in section “Waterborne Hormone Measurement.”
Preference Test for Single and Mixture of PGFs.
One milligram of prostaglandin F molecules (PGF2α, 13,14-dihydro PGF2α [13,14-DH PGF2α], 15-keto PGF2α [15K-PGF2α], and 13,14-dihydro-15-keto PGF2α [PGFM]; Cayman Chemical, MI, USA) was resuspended in DMSO to 1 µg/µL as stock solution and stored in −20 °C freezer. On the day of trials, after the preodor stage, 200 µL of prostaglandin stock solution (stimulus odor) or DMSO (control odor) was then added into 500 mL clean aquarium water. We then turned on the peristaltic pumps to deliver PGF cues or DMSO from bottles to each side of the preference tank. Due to the limited amount of chemicals, we only ran a 30-min test in the preodor and odor stages for these tests. We also tested male preference for a mixture of PGF2α plus five known metabolites (PGF2α, 13,14-DH PGF2α, 15K-PGF2α, PGFM, 19(R)-hydroxy PGF2α, and 20-hydroxy PGF2α). The final concentration of each chemical is ~10−6 M, which is at least 100-fold higher than detectable concentration in other species of fish including goldfish (62) and zebrafish (44). These concentrations are also higher than the natural-borne PGF levels that fertile females released into the water.
Preference Test for PGF2α-Injected Female Odors.
To test whether males exhibit preference to nonfertile females which were injected with PGF2α, prostaglandin E2 (PGE2), or prostaglandin D2 (PGD2), 1 mg of each prostaglandin (Cayman Chemical) was resuspended in DMSO to 30 µg/µL as stock solution. Working solution (3 µg/µL) was freshly prepared right before injection by mixing 8 µL saline (0.5%), 1 µL Fast Green FCF dye (0.1%), and 1 µL of prostaglandin stock solution. On the day of trials, after the preodor stage, each female was either given an intraperitoneal injection of 1.5 μg/g body mass of prostaglandins or saline. The prostaglandin- and saline-injected females were immediately returned to the stimulus and control odor tanks, respectively, and then, the odor stage began 30 min following injection. To test whether females’ attractiveness is mediated through the PGF2α hormonal receptor, Ptgfr, we injected homozygous ptgfr−/− females (34) with PGF2α or saline.
Preference Test for Female-Conditioned Water Plus PGF2α.
One day before preference trials, two size-matched, nonfertile females were selected from the female communities and transferred into two 8-L tanks to acclimatize overnight (~16 h). On the day of trials, after the preodor stage, both females were removed from 8-L tank. Eight hundred microliters of PGF2α stock solution or DMSO were then added into 2 L of water conditioned by nonfertile females, and then, the odor stage began by turning on the pumps.
Waterborne Hormone Measurement.
Individual fish were placed in 8-L tanks containing 6 L of clean aquarium water for 2 h, and 1 L of water sample was fast frozen. Waters Sep-Pak C18 columns (3 cc Vac Cartridge, 500 mg, WAT020805) were fitted to a 24-port manifold. Before use, the columns were first primed twice with 2 mL HPLC-grade methanol (MeOH) followed by two washes with 2 mL ultrapure water. After finishing behavior experiments, water samples were passed through the column. After hormone binding, columns were thawed and purged once with 2 mL washes of ultrapure water. The free fraction of hormone was eluted from the columns by 2 × 2 mL washes with HPLC-grade MeOH. The eluted solvent was evaporated at 37 °C under a gentle stream of nitrogen (~10 bar), which was passed over the samples through an evaporating manifold. The resulting hormone residue was resuspended in 300 μL enzyme immunoassay (EIA) buffer supplied with the kits, and the samples were stored at −20 °C until assay. Serum samples were diluted 1:30 in EIA buffer before assay. Cayman Chemical EIA kits were used to quantify PGF2α following the manufacturer’s recommended procedures. Plates were read at 405 nm on an Absorbance Microplate Reader (EPOCH, BioTek). All hormone data are presented as picograms per milliliter. Intraassay coefficient of variation was 2.83%.
OR Genomic Search.
To identify the repertoire of potential PGF2α-sensitive ORs across fish species, we searched the NCBI RefSeq database for sequences similar to zebrafish Or114-1 (XP_009289721) using BLASTp. We queried annotated genes in high-quality genomes for species in Table 1. We analyzed further the protein sequences with the 20 lowest E values for each species. We aligned these sequences from each fish (Geneious; MUSCLE algorithm) and then built a phylogenetic tree (Geneious; Jukes–Cantor neighbor joining). Some species (i.e., Mexican cavefish and gar) have >20 OR genes that encode β ORs; we therefore included additional genes from these species in our analysis to ensure that we included all β ORs. The final tree presented includes a representative set of species, and fish genes more distantly related than the mouse β ORs have been removed. The fish species phylogeny is an edited version of Fig. 2 from ref. 58, with Or114 existence inferred from either detection of the gene or from experimental evidence of PGF2α sensitivity (Table 1). Paralog numbers are derived from genome searches in the following representative species. Polypteriformes, Erpetoichthys calabaricus; Acipenseriformes, Acipenser ruthenus; Lepisosteiformes, Lepisosteus oculatus; Elopiformes, Megalops cyprinoides; Osteoglossiformes, Scleropages formosus; Clupeiformes, Clupea harengus; Gonorynchiformes, Chanos chanos; Cypriniformes, Danio rerio; Characiformes, Astyanax mexicanus; Gymnotiformes, Electrophorus electricus; Siluriformes, Ictalurus punctatus; Esociformes, Esox lucius; Salmoniformes, Salmo salar; Gadiformes, Gadus morhua; Holocentriformes, Myripristis murdjan; Kurtiformes, Sphaeramia orbicularis; Gobiiformes, Boleophthalmus pectinirostris; Syngnathiformes, Hippocampus comes; Synbranchiformes, Mastacembelus armatus; Anabantiformes, Anabas testudineus; Pleuronectiformes, Cynoglossus semilaevis; Cichliformes, A. burtoni; Beloniformes, Oryzias latipes; Cyprinodontiformes, Poecilia mexicana; Perciformes, Lates calcarifer; and Tetraodontiformes, Takifugu rubripes.
Statistical Analysis.
We used JMP Pro (v. 14; SAS Institute) for all statistical analyses. Due to the small sample size of these preference tests, we are unable to achieve the normal distribution assumption for parametric statistical analysis. We therefore used Mann–Whitney tests on data for the preference index, contact frequency, and mutual aggressive events between the preodor and odor stages. The differences in the levels of waterborne and serum PGF2α before and after IM injection were also examined by the ANOVA test followed by Tukey’s test for post hoc analysis. The chi-squared test was used to examine the difference in the percentage of time focal fish spent in the stimulus odor zone between the preodor and odor stages.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We thank the Juntti, Carleton, and Kocher laboratories for helpful comments on this manuscript. All animal experiments were performed under a study protocol approved by the University of Maryland Institutional Animal Care and Use Committee. Funding to S.A.J. was provided by Human Frontiers in Science Program (RGY0079), the NSF (IOS-1825723), and the NIH (R35 GM142872).
Author contributions
C.-Y.L. and S.A.J. designed research; C.-Y.L., K.L., and J.M.-C. performed research; K.L. contributed new reagents/analytic tools; C.-Y.L. and S.A.J. analyzed data; and C.-Y.L. and S.A.J. wrote the paper.
Competing interest
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
All study data necessary to evaluate the conclusions of this paper are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Bateman A. J., Intra-sexual selection in Drosophila. Heredity (Edinb). 2, 349–368 (1948), 10.1038/hdy.1948.21. [DOI] [PubMed] [Google Scholar]
- 2.Lehtonen J., Parker G. A., Scharer L., Why anisogamy drives ancestral sex roles. Evolution. 70, 1129–1135 (2016), 10.1111/evo.12926. [DOI] [PubMed] [Google Scholar]
- 3.Johnstone R. A., Reynolds J. D., Deutsch J. C., Mutual mate choice and sex differences in choosiness. Evolution. 50, 1382–1391 (1996), 10.1111/j.1558-5646.1996.tb03912.x. [DOI] [PubMed] [Google Scholar]
- 4.Owens I. P., Thompson D. B., Sex differences, sex ratios and sex roles. Proc. Biol. Sci. 258, 93–99 (1994), 10.1098/rspb.1994.0148. [DOI] [PubMed] [Google Scholar]
- 5.Amundsen T., Forsgren E., Male mate choice selects for female coloration in a fish. Proc. Natl. Acad. Sci. U.S.A. 98, 13155–13160 (2001), 10.1073/pnas.211439298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonduriansky R., The evolution of male mate choice in insects: A synthesis of ideas and evidence. Biol. Rev. Camb. Philos. Soc. 76, 305–339 (2001), 10.1017/s1464793101005693. [DOI] [PubMed] [Google Scholar]
- 7.Edward D. A., Chapman T., Measuring the fitness benefits of male mate choice in Drosophila melanogaster. Evolution. 66, 2646–2653 (2012), 10.1111/j.1558-5646.2012.01648.x. [DOI] [PubMed] [Google Scholar]
- 8.Fitzpatrick C. L., Servedio M. R., Handling editor: Ingo S. The evolution of male mate choice and female ornamentation: A review of mathematical models. Curr. Zool. 64, 323–333 (2018), 10.1093/cz/zoy029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer T. B., Uetz G. W., Complex male mate choice in the brush-legged wolf spider Schizocosa ocreata (Hentz). Behav. Ecol. 30, 27–38 (2019), 10.1093/beheco/ary172. [DOI] [Google Scholar]
- 10.Wedell N., Gage M. J. G., Parker G. A., Sperm competition, male prudence and sperm-limited females. Trends Ecol. Evol. 17, 313–320 (2002), 10.1016/s0169-5347(02)02533-8. [DOI] [Google Scholar]
- 11.Edomwande C., Barbosa F., The influence of predation risk on mate signaling and mate choice in the lesser waxmoth Achroia grisella. Sci. Rep. 10, 524 (2020), 10.1038/s41598-020-57481-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoglund J., Kalas J. A., Fiske P., The costs of secondary sexual characters in the lekking great snipe (Gallinago-Media). Behav. Ecol. Sociobiol. 30, 309–315 (1992), 10.1007/BF00170596. [DOI] [Google Scholar]
- 13.Galvani A., Johnstone R., Sperm allocation in an uncertain world. Behav. Ecol. Sociobiol. 44, 161–168 (1998), 10.1007/s002650050528. [DOI] [Google Scholar]
- 14.Preston B. T., Stevenson I. R., Pemberton J. M., Wilson K., Dominant rams lose out by sperm depletion. Nature 409, 681–682 (2001), 10.1038/35055617. [DOI] [PubMed] [Google Scholar]
- 15.Warner R. R., Shapiro D. Y., Marcanato A., Petersen C. W., Sexual conflict: Males with highest mating success convey the lowest fertilization benefits to females. Proc. Biol. Sci. 262, 135–139 (1995), 10.1098/rspb.1995.0187. [DOI] [PubMed] [Google Scholar]
- 16.Barry K. L., Influence of female nutritional status on mating dynamics in a sexually cannibalistic praying mantid. Anim. Behav. 80, 405–411 (2010), 10.1016/j.anbehav.2010.05.024. [DOI] [Google Scholar]
- 17.Maxwell M. R., Gallego K. M., Barry K. L., Effects of female feeding regime in a sexually cannibalistic mantid: Fecundity, cannibalism, and male response in Stagmomantis limbata (Mantodea). Ecol. Entomol. 35, 775–787 (2010), 10.1111/j.1365-2311.2010.01239.x. [DOI] [Google Scholar]
- 18.Scharf I., The multifaceted effects of starvation on arthropod behaviour. Anim. Behav. 119, 37–48 (2016), 10.1016/j.anbehav.2016.06.019. [DOI] [Google Scholar]
- 19.Thomas M. L., Detection of female mating status using chemical signals and cues. Biol. Rev. 86, 1–14 (2011), 10.1111/j.1469-185X.2010.00130.x. [DOI] [PubMed] [Google Scholar]
- 20.Burris Z. P., Dam H. G., Female mating status affects mating and male mate-choice in the copepod genus Acartia. J. Plankton Res. 37, 183–196 (2015), 10.1093/plankt/fbu090. [DOI] [Google Scholar]
- 21.Aisenberg A., Costa F. G., González M., Male sexual cannibalism in a sand-dwelling wolf spider with sex role reversal. Biol. J. Linn. Soc. 103, 68–75 (2011), 10.1111/j.1095-8312.2011.01631.x. [DOI] [Google Scholar]
- 22.Belliure J., Fresnillo B., Cuervo J. J., Male mate choice based on female coloration in a lizard: The role of a juvenile trait. Behav. Ecol. 29, 543–552 (2018), 10.1093/beheco/ary005. [DOI] [Google Scholar]
- 23.Seehausen O., van Alphen J. J. M., The effect of male coloration on female mate choice in closely related Lake Victoria cichlids (Haplochromis nyererei complex). Behav. Ecol. Sociobiol. 42, 1–8 (1998), 10.1007/s002650050405. [DOI] [Google Scholar]
- 24.Selz O. M., Pierotti M. E. R., Maan M. E., Schmid C., Seehausen O., Female preference for male color is necessary and sufficient for assortative mating in 2 cichlid sister species. Behav. Ecol. 25, 612–626 (2014), 10.1093/beheco/aru024. [DOI] [Google Scholar]
- 25.Clement T. S., Grens K. E., Fernald R. D., Female affiliative preference depends on reproductive state in the African cichlid fish, Astatotilapia burtoni. Behav. Ecol. 16, 83–88 (2005), 10.1093/beheco/arh134. [DOI] [Google Scholar]
- 26.Karino K., Female mate preference for males having long and symmetric fins in the bower-holding cichlid Cyathopharynx furcifer. Ethology 103, 883–892 (2010), 10.1111/j.1439-0310.1997.tb00130.x. [DOI] [Google Scholar]
- 27.Kidd M. R., et al. , Female preference for males depends on reproductive physiology in the African cichlid fish Astatotilapia burtoni. Gen. Comp. Endocrinol. 180, 56–63 (2013), 10.1016/j.ygcen.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 28.Werner N. Y., Lotem A., Choosy males in a haplochromine cichlid: First experimental evidence for male mate choice in a lekking species. Anim. Behav. 66, 293–298 (2003), 10.1006/anbe.2003.2208. [DOI] [Google Scholar]
- 29.Werner N. Y., Lotem A., Experimental evidence for male sequential mate preference in a lekking species. Ethology 112, 657–663 (2006), 10.1111/j.1439-0310.2006.01202.x. [DOI] [Google Scholar]
- 30.Baldauf S. A., Bakker T. C., Herder F., Kullmann H., Thunken T., Male mate choice scales female ornament allometry in a cichlid fish. BMC Evol. Biol. 10, 301 (2010), 10.1186/1471-2148-10-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plenderleith M., van Oosterhout C., Robinson R. L., Turner G. F., Female preference for conspecific males based on olfactory cues in a Lake Malawi cichlid fish. Biol. Lett. 1, 411–414 (2005), 10.1098/rsbl.2005.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simoes J. M., et al. , Social odors conveying dominance and reproductive information induce rapid physiological and neuromolecular changes in a cichlid fish. BMC Genomics 16, 114 (2015), 10.1186/s12864-015-1255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miranda A., Almeida O. G., Hubbard P. C., Barata E. N., Canario A. V., Olfactory discrimination of female reproductive status by male tilapia (Oreochromis mossambicus). J. Exp. Biol. 208, 2037–2043 (2005), 10.1242/jeb.01584. [DOI] [PubMed] [Google Scholar]
- 34.Juntti S. A., et al. , A neural basis for control of cichlid female reproductive behavior by prostaglandin F2alpha. Curr. Biol. 26, 943–949 (2016), 10.1016/j.cub.2016.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kidd M. R., et al. , Prostaglandin F2α facilitates female mating behavior based on male performance. Behav. Ecol. Sociobiol. 67, 1307–1315 (2013), 10.1007/s00265-013-1559-9. [DOI] [Google Scholar]
- 36.Kobayashi M., Sorensen P. W., Stacey N. E., Hormonal and pheromonal control of spawning behavior in the goldfish. Fish Physiol. Biochem. 26, 71–84 (2002), 10.1023/A:1023375931734. [DOI] [Google Scholar]
- 37.Stacey N. E., Goetz F. W., Role of prostaglandins in fish reproduction. Can. J. Fish Aquat. Sci. 39, 92–98 (1982), 10.1139/f82-011. [DOI] [Google Scholar]
- 38.Sorensen P. W., Hara T. J., Stacey N. E., Goetz F. W., F prostaglandins function as potent olfactory stimulants that comprise the postovulatory female sex pheromone in goldfish. Biol. Reprod. 39, 1039–1050 (1988), 10.1095/biolreprod39.5.1039. [DOI] [PubMed] [Google Scholar]
- 39.Hara T. J., Zhang C., Topographic bulbar projections and dual neural pathways of the primary olfactory neurons in salmonid fishes. Neuroscience 82, 301–313 (1998), 10.1016/s0306-4522(97)00279-0. [DOI] [PubMed] [Google Scholar]
- 40.Laberge F., Hara T. J., Behavioural and electrophysiological responses to F-prostaglandins, putative spawning pheromones, in three salmonid fishes. J. Fish Biol. 62, 206–221 (2003), 10.1046/j.1095-8649.2003.00020.x. [DOI] [Google Scholar]
- 41.Moore A., Olsen K. H., Lower N., Kindahl H., The role of F-series prostaglandins as reproductive priming pheromones in the brown trout. J. Fish Biol. 60, 613–624 (2002), 10.1006/jfbi.2002.1879. [DOI] [Google Scholar]
- 42.Moore A., Waring C. P., Electrophysiological and endocrinological evidence that F-series prostaglandins function as priming pheromones in mature male Atlantic salmon (Salmo salar) Parr. J. Exp. Biol. 199, 2307–2316 (1996), 10.1242/jeb.199.10.2307. [DOI] [PubMed] [Google Scholar]
- 43.Kitamura S., Ogata H., Takashima F., Olfactory responses of several species of teleost to F-Prostaglandins. Comp. Biochem. Physiol. Comp. Physiol. 107, 463–467 (1994), 10.1016/0300-9629(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 44.Belanger R. M., Pachkowski M. D., Stacey N. E., Methyltestosterone-induced changes in electro-olfactogram responses and courtship behaviors of cyprinids. Chem. Senses 35, 65–74 (2010), 10.1093/chemse/bjp085. [DOI] [PubMed] [Google Scholar]
- 45.Yabuki Y., et al. , Olfactory receptor for prostaglandin F2alpha mediates male fish courtship behavior. Nat. Neurosci. 19, 897–904 (2016), 10.1038/nn.4314. [DOI] [PubMed] [Google Scholar]
- 46.Frade P., Hubbard P. C., Barata E. N., Canario A. V. M., Olfactory sensitivity of the Mozambique tilapia to conspecific odours. J. Fish Biol. 61, 1239–1254 (2002), 10.1006/jfbi.2002.2140. [DOI] [Google Scholar]
- 47.Hubbard P. C., Mota V. C., Keller-Costa T., da Silva J. P., Canario A. V., Chemical communication in tilapia: A comparison of Oreochromis mossambicus with O. niloticus. Gen. Comp. Endocrinol. 207, 13–20 (2014), 10.1016/j.ygcen.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 48.Keller-Costa T., Canario A. V., Hubbard P. C., Olfactory sensitivity to steroid glucuronates in Mozambique tilapia suggests two distinct and specific receptors for pheromone detection. J. Exp. Biol. 217, 4203–4212 (2014), 10.1242/jeb.111518. [DOI] [PubMed] [Google Scholar]
- 49.Keller-Costa T., Canario A. V., Hubbard P. C., Chemical communication in cichlids: A mini-review. Gen. Comp. Endocrinol. 221, 64–74 (2015), 10.1016/j.ygcen.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 50.Cole T. B., Stacey N. E., Olfactory responses to steroids in an African mouth-brooding cichlid, Haplochromis burtoni (Gunther). J. Fish Biol. 68, 661–680 (2006), 10.1111/j.0022-1112.2006.00944.x. [DOI] [Google Scholar]
- 51.Cardwell J. R., Stacey N. E., Lang S. L. C., Tan E. S. P., McAdam D. S. O., Androgen increases olfactory receptor response to a vertebrate sex pheromone. J. Comp. Physiol. A 176, 55–61 (1995), 10.1007/bf00197752. [DOI] [Google Scholar]
- 52.Sorensen P. W., Rue M. C. P., Leese J. M., Ghosal R., Lim H., A blend of F prostaglandins functions as an attractive sex pheromone in silver carp. Fishes 4, 27 (2019), 10.3390/fishes4020027. [DOI] [Google Scholar]
- 53.Stacey N., Sorensen P., “Hormonal pheromones in fish” in Hormones, Brain and Behavior, Pfaff D. W., Arnold A. P., Fahrbach S. E., Etgen A. M., Rubin R. T., Eds. (Elsevier, 2002), pp. 375–434. [Google Scholar]
- 54.Murphy C. A., Stacey N. E., Corkum L. D., Putative steroidal pheromones in the round goby, Neogobius melanostomus: Olfactory and behavioral responses. J. Chem. Ecol. 27, 443–470 (2001), 10.1023/a:1010376503197. [DOI] [PubMed] [Google Scholar]
- 55.Niimura Y., Nei M., Extensive gains and losses of olfactory receptor genes in mammalian evolution. PLoS One 2, e708 (2007), 10.1371/journal.pone.0000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Field K. E., McVicker C. T., Maruska K. P., Sexually-relevant visual and chemosensory signals induce distinct behaviors and neural activation patterns in the Social African Cichlid, Astatotilapia burtoni. Front. Behav. Neurosci. 12, 267 (2018), 10.3389/fnbeh.2018.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Castro A. L., Goncalves-de-Freitas E., Volpato G. L., Oliveira C., Visual communication stimulates reproduction in Nile tilapia, Oreochromis niloticus (L.). Braz. J. Med. Biol. Res. 42, 368–374 (2009), 10.1590/s0100-879x2009000400009. [DOI] [PubMed] [Google Scholar]
- 58.Hughes L. C., et al. , Comprehensive phylogeny of ray-finned fishes (Actinopterygii) based on transcriptomic and genomic data. Proc. Natl. Acad. Sci. U.S.A. 115, 6249–6254 (2018), 10.1073/pnas.1719358115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stacey N., Chojnacki A., Narayanan A., Cole T., Murphy C., Hormonally derived sex pheromones in fish: Exogenous cues and signals from gonad to brain. Can. J. Physiol. Pharmacol. 81, 329–341 (2003), 10.1139/y03-024. [DOI] [PubMed] [Google Scholar]
- 60.Bhatt J. P., Kandwal J. S., Nautiyal R., Water temperature and pH influence olfactory sensitivity to pre-ovulatory and post-ovulatory ovarian pheromones in male Barilius bendelisis. J. Biosci. 27, 273–281 (2002), 10.1007/BF02704916. [DOI] [PubMed] [Google Scholar]
- 61.Stacey N. E., Cardwell J. R., “Hormones as sex pheromones in fish: Widespread distribution among freshwater species” in Proceedings of the Fifth International Symposium on the Reproductive Physiology of Fish (The University of Texas at Austin, Austin, Texas, USA, 2–8 July 1995), pp. 244–248. [Google Scholar]
- 62.Sato K., Sorensen P. W., The chemical sensitivity and electrical activity of individual olfactory sensory neurons to a range of sex pheromones and food odors in the goldfish. Chem. Senses 43, 249–260 (2018), 10.1093/chemse/bjy016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smadja C., Butlin R. K., On the scent of speciation: The chemosensory system and its role in premating isolation. Heredity (Edinb). 102, 77–97 (2009), 10.1038/hdy.2008.55. [DOI] [PubMed] [Google Scholar]
- 64.Zhang J., Walker W. B., Wang G., Pheromone reception in moths: From molecules to behaviors. Prog. Mol. Biol. Transl. Sci. 130, 109–128 (2015), 10.1016/bs.pmbts.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 65.Zhang G., Chen J., Yu H., Tian X., Wu J., Molecular and functional characterization of pheromone binding protein 1 from the Oriental Fruit Moth, Grapholita molesta (Busck). Sci. Rep. 8, 2276 (2018), 10.1038/s41598-018-20719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hurst J. L., Beynon R. J., Scent wars: The chemobiology of competitive signalling in mice. Bioessays. 26, 1288–1298 (2004), 10.1002/bies.20147. [DOI] [PubMed] [Google Scholar]
- 67.Edmonds J. W., et al. , Insulin/FOXO signaling regulates ovarian prostaglandins critical for reproduction. Dev. Cell 19, 858–871 (2010), 10.1016/j.devcel.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Unezaki S., Sugatani J., Masu Y., Watanabe K., Ito S., Characterization of prostaglandin F2 alpha production in pregnant and cycling mice. Biol. Reprod. 55, 889–894 (1996), 10.1095/biolreprod55.4.889. [DOI] [PubMed] [Google Scholar]
- 69.Stacey N., “Hormonally derived pheromones in teleost fishes” in Fish Pheromones and Related Cues, Sorensen P. W., Wisenden B. D., Eds. (John Wiley & Sons, 2014), pp. 33–88. [Google Scholar]
- 70.Cichy A., Shah A., Dewan A., Kaye S., Bozza T., Genetic depletion of class I odorant receptors impacts perception of carboxylic acids. Curr. Biol. 29, 2687–2697 (2019), 10.1016/j.cub.2019.06.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cande J., Prud’homme B., Gompel N., Smells like evolution: The role of chemoreceptor evolution in behavioral change. Curr. Opin. Neurobiol. 23, 152–158 (2013), 10.1016/j.conb.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 72.Wyatt T. D., Pheromones and signature mixtures: Defining species-wide signals and variable cues for identity in both invertebrates and vertebrates. J. Comp. Physiol. A 196, 685–700 (2010), 10.1007/s00359-010-0564-y. [DOI] [PubMed] [Google Scholar]
- 73.Fernald R. D., Hirata N. R., Field study of Haplochromis burtoni: Quantitative behavioural observations. Anim. Behav. 25, 964–975 (1997), 10.1016/0003-3472(77)90048-3. [DOI] [Google Scholar]
- 74.Perez-Escudero A., Vicente-Page J., Hinz R. C., Arganda S., de Polavieja G. G., idTracker: Tracking individuals in a group by automatic identification of unmarked animals. Nat. Methods 11, 743–748 (2014), 10.1038/nmeth.2994. [DOI] [PubMed] [Google Scholar]
- 75.Friard O., Gamba M., BORIS: A free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 7, 1325–1330 (2016), 10.1111/2041-210x.12584. [DOI] [Google Scholar]
- 76.Sorensen P. W., Appelt C., Stacey N. E., Goetz F. W., Brash A. R., High levels of circulating prostaglandin F2alpha associated with ovulation stimulate female sexual receptivity and spawning behavior in the goldfish (Carassius auratus). Gen. Comp. Endocrinol. 267, 128–136 (2018), 10.1016/j.ygcen.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 77.Li C. Y., Earley R. L., Huang S. P., Hsu Y., Fighting experience alters brain androgen receptor expression dependent on testosterone status. Proc. Biol. Sci. 281, 20141532 (2014), 10.1098/rspb.2014.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data necessary to evaluate the conclusions of this paper are included in the article and/or SI Appendix.