Abstract
Background
Rotavirus infection remains an important cause of morbidity and mortality in children. The introduction of vaccination programs in more than 100 countries has contributed to a decrease in hospitalizations and mortality. This study investigates the epidemiological impact of the rotavirus vaccine ROTAVAC® in the Palestinian Territories, the first country to switch from ROTARIX® to this new vaccine.
Methods
Clinical surveillance data was collected from children younger than 5 attending outpatient clinics throughout Gaza with diarrhea between 2015 and 2020. The incidence of all-cause diarrhea was assessed using an interrupted time-series approach.
Rotavirus prevalence was determined at the Caritas Baby Hospital in the West Bank using ELISA on stool specimen of children younger than 5 with diarrhea. Genotyping was performed on 325 randomly selected rotavirus-positive samples from January 2015 through December 2020 using multiplex PCR analysis.
Results
Average monthly diarrhea cases dropped by 16.7% annually from introduction of rotavirus vaccination in May 2016 to the beginning of the SARS-CoV-2 epidemic in March 2020 for a total of 53%. Case count declines were maintained after the switch to ROTAVAC® in October 2018. Rotavirus positivity in stool samples declined by 67.1% over the same period without change following the switch to ROTAVAC®.
The distribution of predominant genotypes in rotavirus-positive stool samples changed from a pre-vaccination G1P [8] to G9P[8] and G12P[8] during the ROTARIX® period and G2P[4] after the introduction of ROTAVAC®.
Conclusion
ROTAVAC® has shown epidemiological impact on par with ROTARIX® after its introduction to the national immunization schedule in the Palestinian Territories. A molecular genotype shift from a pre-vaccination predominance of G1P[8] to a current predominance of G2P[4] requires more long-term surveillance.
Keywords: Rotavirus, Vaccine impact, Genotyping
1. Introduction and background
Global childhood deaths from rotavirus (RV) infection have decreased from an estimate of more than 500,000 per year in 2000 to 125,000 in 2018 [1]. Nevertheless, this pathogen remains the leading cause of mortality related to diarrhea in children [2]. The introduction of RV vaccination in more than 100 countries has contributed significantly to the decrease in mortality and hospitalization related to diarrheal disease and a decline in the prevalence of RV [3], [4], [5].
Rotavirus is a double-stranded RNA virus of the Reoviridae family. Its RNA encodes 6 structural proteins (VP1-4, VP6 and VP7) as well as 6 non-structural proteins (NSP1-NSP6). Genotyping is based on RNA sequences for 2 outer capsid proteins; the glycoprotein VP7 determines the G-types, and the protease-cleaved protein VP4 the P types [6]. To date, 41 G types and 57P types have been described, but globally, the majority of RV disease cases are associated with 6 G types (G1-G4, G9 and G12) and 3P types (P[4], P[6] and P[8]) [7], [8]. Two-thirds of global RV infections could be attributed to the G1P[8] genotype prior to the introduction of vaccines. Genotypes considered less common have become more prevalent recently, and genotype composition variations occur between regions, within regions, and from season to season [8], [9], [10].
In 2006, two live, oral rotavirus vaccines were prequalified by the World Health Organization (WHO): RotaTeq® (Merck & Co., West Point, PA, USA), a pentavalent bovine-human reassortant vaccine and ROTARIX® (Glaxo Smith Kline, Rixenstart, Belgium), a G1P[8] monovalent human vaccine. Both vaccines showed greater than 90 % efficacy in preventing severe disease in low-mortality countries [11], [12], [13] and a somewhat lower 50–64 % efficacy in high-mortality environments [14], [15], [16], attributed to the influence of co-administered oral polio vaccine (OPV), the effects of transplacental anti-rotavirus IgG transmission in high prevalence environments [17], [18], the effects of concurrent intestinal infections [19], [20], and/or the influences of environmental enteric dysfunction [21].
In 2018, two additional live, oral rotavirus vaccines were prequalified by WHO: ROTAVAC® (Bharat Biotech, Hyderabad, India), a monovalent human-bovine G9P[11] vaccine, and ROTASIIL® (Serum Institute of India, Pune, India), a pentavalent bovine-reassortant vaccine. Both vaccines showed efficacy rates comparable to their competitors with potential advantages for reduced storage space requirements, cold chain footprints and cost [22].
Since the introduction of rotavirus vaccines in more than 100 national vaccination programs worldwide, and despite their modest efficacy in low- and middle-income countries (LMIC), RV immunization has had a significant impact on reduction of rotavirus hospitalization and death in multiple settings. The available vaccines have shown efficacy against non-vaccine genotypes, and true ‘vaccine-escape’ strains causing significant disease have not been observed [23]. In the last decade, the global prevalence of the G1P[8] genotype has decreased while others like G2P[4], G12P[8] or G3P[8] have become more prominent. Cyclic temporal variations in genotype prevalence occurred also in the pre-vaccine years, but the sustained decrease in G1P[8], previously the most common strain associated with RV disease in children, may be attributable to the effect of vaccination [16], [24].
In 2016, the Palestinian Ministry of Health (MoH) included ROTARIX® in the national immunization schedule of the occupied Palestinian Territories of Gaza and the West Bank with the help of the Rostropovich Vishnevskaya Foundation (RVF). Diarrhea is a leading cause of illness among children in the occupied Palestinian Territories with RV being prevalent in 28 % of stool samples of hospitalized children in a study from the Central Pediatric Hospital in Gaza [25]. The disease follows a seasonal pattern, typical for the Eastern Mediterranean region, with incidence peaks in the colder winter months [26], [27], [28].
Within 2 years of vaccine introduction, the Palestinian MoH and RVF reported a 32.2 % reduction in all-cause diarrhea incidence among children under age 5 throughout all outpatient clinics in Gaza and a 64.6 % drop in RV prevalence in stool samples analyzed at the Caritas Baby Hospital in the West Bank, while sustaining a 97.4 % vaccination rate throughout the territories [29]. In 2018, the Palestinian MoH decided to switch from ROTARIX® to ROTAVAC® in response to a cost and economic impact analysis [30].
The present study is designed to investigate the clinical impact of rotavirus vaccine introduction on all-cause diarrhea cases among children under 5 years of age throughout Gaza, as well as its effects on RV prevalence in stool samples collected from all children under 5 years of age presenting to inpatient and outpatient facilities associated with the Caritas Baby Hospital in Bethlehem, West Bank. The study also investigates the genotype profiles of RV positive stool specimens at the hospital comparing samples from the pre-vaccination era (before May 2016) with samples from the era of ROTARIX® implementation (May 2016 – September 2018) and the era of ROTAVAC® implementation (October 2018 – December 2020). Finally, the study investigates occurrences of intussusception and their potential association with ROTAVAC®, as a small increase in risk for intussusception during the first 7 days following the first dose of RV vaccination has been reported in some countries [31], [32], [33].
2. Material and methods
2.1. Surveillance
The occupied Palestinian Territories of Gaza (population 1.6 million) and the West Bank (population 2.6 million) are an LMIC in the Eastern Mediterranean region. Territorial fragmentation, and the lack of economic and political sovereignty have inhibited development [34]. Individuals younger than 15 years of age make up 41.6 % of the population, and the annual birth cohort is about 130,000. Birth statistics are maintained by the MoH based on the registration of BCG vaccine doses given to all newborn babies. Equally, the MoH maintains data on all RV vaccine doses given and discarded from multi-dose vials at the end of vaccination days. These public health surveillance data were used to compute vaccination and vaccine wastage rates.
Outpatient medical care in Gaza is provided in 22 clinics run by the United Nations Relief and Works Agency for Palestinian Refugees (UNRWA) and 28 MoH clinics. Both types of clinics report “watery diarrhea in children under five years of age” with diarrhea defined as three or more liquid or semi-solid non-bloody stools per day for a duration of less than 14 days [35]. UNRWA uses an electronic medical record system for data collection, while MoH clinics collect information in manually maintained paper case logs. Aggregate information from both systems is reported to the MoH Preventive Medicine Directorate for Gaza and entered into a unified spreadsheet for reporting purposes. The data was used to determine the monthly all-cause diarrhea incidence throughout the investigation period for all of Gaza. Similar surveillance data collection for the West Bank is less comprehensive and was not included in this analysis to maintain a consistent level of data validity.
To assess the potential risk for vaccine-related intussusception in the occupied Palestinian Territories, all suspected intussusception cases in children aged one year or younger were collected prospectively during 2019 and 2020 at the Shifa Hospital, which is the only pediatric surgery center for Gaza. In the West Bank, cases were collected retrospectively at Caritas Baby Hospital through searches of the electronic medical record system for the years 2015 through 2020. Infants up to the age of one year were included whose condition met the criteria for Level 1 diagnostic certainty developed by the Brighton Collaboration Intussusception Working Group [36]. Criteria included direct visualization of intussusception during surgery, specific radiologic findings with intussusception reduction by air or contrast enema, or confirmation of intussusception during autopsy.
2.2. Diagnostic and molecular analysis
RV prevalence data in stool samples was collected at the Caritas Baby Hospital in Bethlehem. The hospital is the only pediatric hospital in the Southern part of the West Bank covering the Bethlehem and Hebron districts. All stool samples collected from children under 5 years of age presenting to hospital in- and outpatient facilities were investigated for enteric pathogens by microscopy, microbial culture and RV antigen testing using the Rota Stick One-Step Assay® (Novamed, Israel).
All stool samples were stored at −80 °C at the Caritas Baby Hospital laboratory. RV genotyping was performed on 325 single patient RV-positive stool samples covering the period between January 2015 and the end of 2020. Stool samples were diluted in 1 mL H2O, vortexed for 30 s and centrifuged at 13,000 rpm for 1 min. A 200 µl aliquot from the supernatant was used to extract RV viral RNA using High Pure Viral RNA extraction Kit (Roche, Germany). Extracted RNA was stored at −80 °C pending RV genotyping.
The WHO recommended Gouvea/Iturriza-Gómara primers and amplification conditions for genotyping RV VP4 and VP7 were used [37]. The complete VP4 and VP7 genes were amplified using Qiagen One-Step RT-PCR kit (Qiagen, Germany) and further purified using Qiagen PCR purification kit (Qiagen, Germany). For genotyping, a multiplex PCR using GoTaq® Green Master Mix (Promega, USA) and the recommended WHO primers cocktail were used to amplify the specific VP4 and VP7 genotype amplicon. Genotypes were determined through size comparison of resulting amplicons from stool samples to control strains for each major G and P type following agarose gel electrophoresis.
2.3. Statistical analysis
To assess the potential impact of vaccine introduction on disease occurrence or proportion of RV-positive diarrhea, an interrupted time-series (ITS) approach was used, employing a generalized linear mixed model with a Poisson likelihood to estimate relative reductions and 95 % confidence intervals (CIs). Models for Gaza surveillance and Caritas Baby Hospital were fit separately. In both models, a generalized linear model was fit to the monthly time series data starting in January 2015, assuming diarrheal cases were Poisson distributed. Seasonality was incorporated via an adjustment for calendar month, and secular trends were accounted for with a linear monthly term in the model [38].
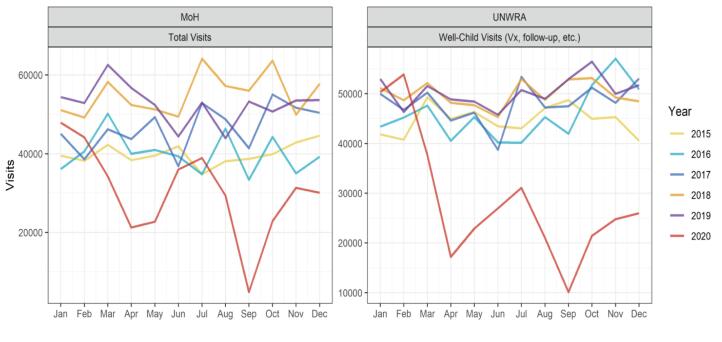
For the analysis of data from Caritas Baby Hospital, total diarrhea cases were accounted for by considering the log of rotavirus-negative diarrheal cases as the exposure [39]. In Gaza, catchment populations were not available. However, the Poisson regression approach facilitates estimation of percentage reductions in cases. For the ITS-specific parameterization, two impact frameworks were considered. First, three periods were considered, namely pre-introduction (before May 2016), ROTARIX® (May 2016 to September 2018), and ROTAVAC® (October 2018 to March 2020). In the second parameterization, two periods were considered, pre-introduction (before May 2016) and post-rotavirus vaccine introduction (May 2016 to March 2020). A significant decrease in clinic visits was observed from April through December 2020 during the height of the SARS-CoV-2 epidemic (Fig. 1). This likely impacted both the transmission of rotavirus and the probability of rotavirus cases being captured by the surveillance. Therefore, surveillance data after March 2020 were not considered in the evaluation of the vaccine introduction.
Fig. 1.
Monthly outpatient clinic visits for well and sick children throughout MoH and UNRWA clinic networks in Gaza 2015 through 2020. Note the significant drop in clinic visits after March 2020.
3. Results
Since the introduction of RV vaccination, MoH and UNRWA clinics were able to maintain a national vaccination rate of greater than 95 % for all vaccine doses at the appropriate ages of 2 and 4 months for ROTARIX® and 2,4 and 6 months for ROTAVAC®, respectively, for both Gaza and the West Bank. An interruption of vaccination efforts occurred during the first half of 2018 when ROTARIX® stores were depleted before ROTAVAC® vaccination could commence. Catch-up immunizations were initiated for children with missed doses, however, following MoH policy, such doses were not given to children who had reached beyond the age of 8 months.
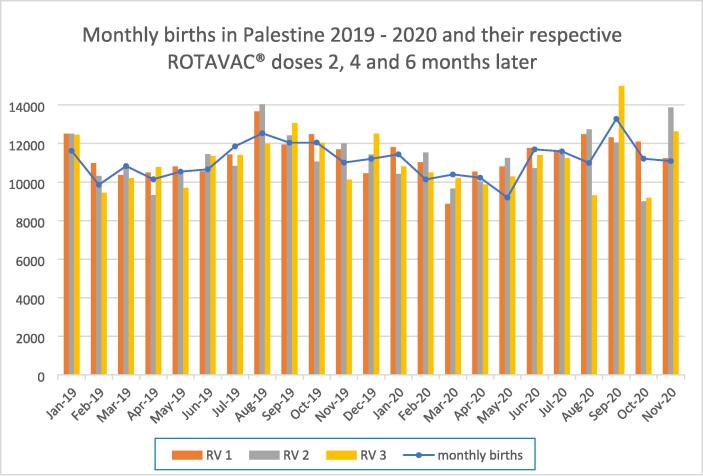
Minor fluctuations in vaccination coverage related to the SARS-CoV-2 epidemic occurred during the latter half of 2020 but were compensated for in subsequent months. Fig. 2 illustrates the monthly birth rates for 2019 and 2020 with the corresponding vaccinations given 2, 4 and 6 months later (Fig. 2). Vaccine wastage rates for ROTAVAC® multi-dose vials dropped from 14.4 % in December 2018 to 5.4 % by the end of 2020 with the introduction of an improved patient scheduling system.
Fig. 2.
Monthly births and ROTAVAC® doses given 2 (RV1), 4 (RV2) and 6 (RV3) months later for all infants born in the West Bank and Gaza during 2019 and 2020. The months indicated on the horizontal axis reflect birth dates rather than vaccination dates.
3.1. All-cause diarrhea case counts in Gaza
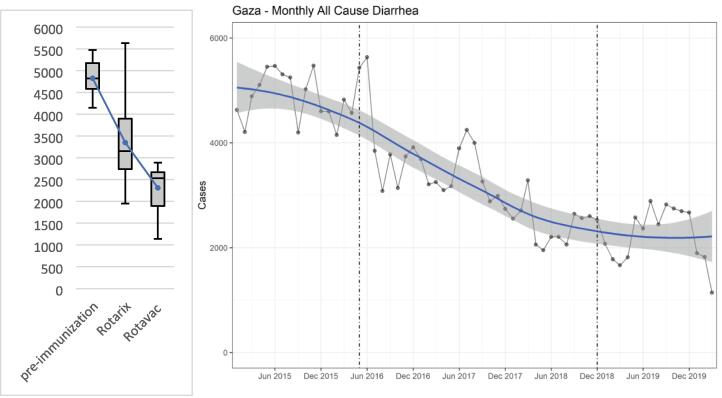
During the pre-introduction period, outpatient clinics in both the UNRWA and MoH networks of Gaza combined experienced an average of 4,856 (range: 4,149 – 5,473) cases of all-cause diarrhea per month. As vaccine was introduced in the ROTARIX® period, the average monthly count of all-cause diarrhea cases dropped to 3,364 (range: 1,952 – 5,634), a decrease of 30.3 %. After the transition to ROTAVAC®, monthly diarrhea cases dropped by an additional 32.1 % to an average of 2,284 (1,142–2,890) from December 2018 to the beginning of the SARS-CoV-2 impact in March 2020 for a total decline of case counts by 53 %. (Fig. 3a).
Fig. 3.
(a) Monthly average diarrhea cases in children under 5 in Gaza comparing pre-immunization period (Jan 2015 - Apr 2016, n = 16), ROTARIX® period (May 2016 – Sep 2018, n = 29), and ROTAVAC® period (Oct 2018 – Mar 2020, n = 18, illustrating means, highs, lows, and quartiles. (b) ITS with LOESS demonstrating diarrhea incidence before rotavirus vaccine (left of first dotted line), during ROTARIX® period (between dotted lines), and after transition to ROTAVAC® (right of second dotted line).
In the Poisson regression analysis, the annual reduction of diarrhea incidence was 21.4 % (CI 20.7, 22.1) after the introduction of ROTARIX®. The switch to ROTAVAC® did not change the diarrhea incidence significantly when looking at the pre-SARS-CoV-2 period up to March 2020, indicating a vaccine impact of ROTAVAC® comparable to that of ROTARIX®. In the two-period model, the diarrhea incidence following introduction of RV immunization of any kind was decreased by 16.7 % (CI 16.3, 17.12) per year when excluding the SARS-CoV-2 period (Table 1).The interrupted time-series as visualized by locally weighted scatterplot smoothing (LOESS) demonstrates the significant drop in diarrhea incidence with the introduction of ROTARIX®, followed by a minor further decrease with the transition to ROTAVAC® (Fig. 3b).
Table 1.
Annual percentage decreases (95% CI) in diarrhea incidence in Gaza related to the introduction of ROTARIX® and ROTAVAC® (left) and any RV vaccine (right).
|
Annual percentage decrease in diarrhea incidence |
||
|---|---|---|
| Introduction of both vaccines | Introduction of any RV vaccine | |
| Pre-introduction (JAN 2015-APR 2016) | 1.7 (0.0, 3.4) | 1.4 (-0.3, 3.1) |
| ROTARIX (MAY 2016-SEP 2018) | 21.4 (20.7, 22.1) | |
| ROTAVAC (OCT 2018-MAR 2020) | 2.6 (-0.5, 5.6) | |
| Post-introduction (MAY 2016 – MAR 2020) | 16.7 (16.3, 17.1) | |
3.2. RV prevalence in stool samples at Caritas Baby Hospital
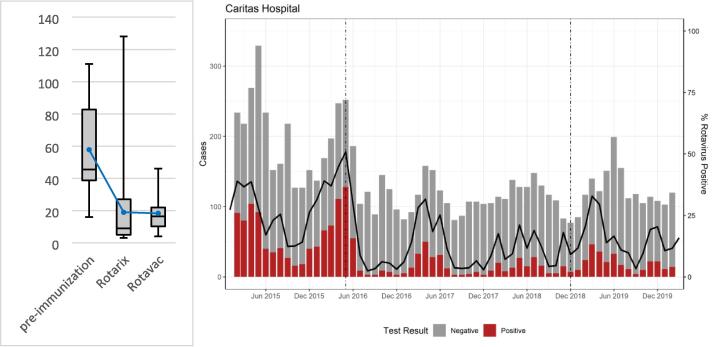
Analysis of the number of in- and out-patient diarrhea cases of children younger than 5 years of age at Caritas Baby Hospital in the West Bank showed a reduction in the monthly average case load for both RV and all-cause diarrhea cases as well as the proportion of RV positive cases following the introduction of RV vaccine. RV positive diarrhea cases decreased from a monthly average of 57.8 (range: 16–111) with a positivity rate of 38.2 % (range: 31.3–45.1) during the pre-introduction period to 19 cases (range: 3–128) per month with a positivity rate of 13 % (range: 2.5–50.8) following the introduction of ROTARIX®, a decline by 67.1 %. Monthly RV case averages plateaued after the transition to ROTAVAC® at 18.1 (range: 4–46)—a total decline by 68.7 %—with a positivity rate of 15 % (range: 3.9–32.9 (Fig. 4a). Fig. 4b also demonstrates the seasonality of rotavirus occurrences, which persisted at a less pronounced level after the introduction of vaccination (Fig. 4b).
Fig. 4.
(a) Monthly average RV positive cases in children under 5 at Caritas Baby Hospital comparing pre-vaccination period (Jan 2015 - Apr 2016, n = 16), ROTARIX® period (May 2016 – Sep 2018, n = 29), and ROTAVAC® period (Oct 2018 – Mar 2020, n = 18 illustrating means, highs, lows, and quartiles. (b) Monthly diarrhea case averages in children younger than 5 at Caritas Baby Hospital indicating RV positive (red bars), RV negative (gray bars) stool samples, as well as RV positivity rates (black line) between 2015 and 2020. Dotted lines indicate the introduction of ROTARIX® and ROTAVAC® vaccines, respectively. Seasonal variations of RV positivity are demonstrated. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The ITS analysis of the RV prevalence in stool samples showed an increase of RV positivity before vaccine introduction of 44.2 % per year, a decrease by 14.1 % per year with the introduction of ROTARIX® and a further decrease by 24.1 % annually after the transition to ROTAVAC® up to the effects of the SARS-CoV-2 epidemic. (Table 2). Here, small changes in absolute case numbers impact case rates more significantly as overall case numbers are low (Fig. 4a).
Table 2.
Annual percentage decreases (95% CI) in RV prevalence in stool samples at Caritas Baby Hospital related to the introduction of ROTARIX® and ROTAVAC® (left) and any RV vaccine (right).
|
Annual percentage decrease in RV positivity of stool samples |
||
|---|---|---|
| Introduction of both vaccines | Introduction of any RV vaccine | |
| Pre-introduction (JAN 2015 - APR 2016) | −44.2 (-65.3, −25.8) | −45.0 (-66.1, −26.5) |
| ROTARIX (MAY 2016 - SEP 2018) | 14.1 (1.9, 24.8) | |
| ROTAVAC (OCT 2018 - MAR 2020) | 24.1 (-5.6, 45.4) | |
| Post-introduction (MAY 2016 – MAR 2020) | −1.2 (-8.3, 5.4) | |
3.3. Molecular analysis of rotavirus positive stool samples
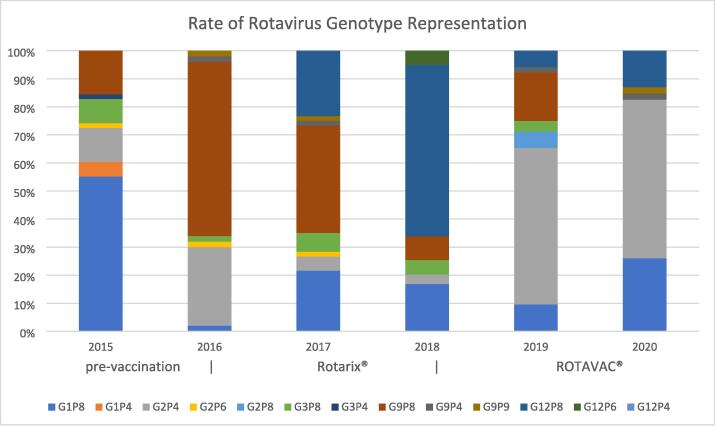
A total of 325 randomly selected RV stool samples collected between 2015 and 2020 were typed for their G- and P-genotypes. In 2015 (n = 58), before the introduction of RV vaccination in the territories, G1P[8] was most prevalent at 55.2 % of stool samples. The transitional year of 2016 (n = 50) saw a predominance of G9P[8] at 62 % and G2P[4] at 28 %. The ROTARIX® period of 2017 (n = 60) and 2018 (n = 59) showed a mixed genotype picture with G9P[8] at 38.3 % in 2017 and of G12P[8] at 61 % in 2018. The ROTAVAC® period of 2019 (n = 52) and 2020 (n = 46) was dominated by G2P[4] at 55.8 % and 56.5 % respectively (Fig. 5).
Fig. 5.
Proportional G and P genotype representation in RV positive stool samples randomly collected from 2015 through 2020 indicating the pre-vaccination, ROTARIX® and ROTAVAC® periods respectively.
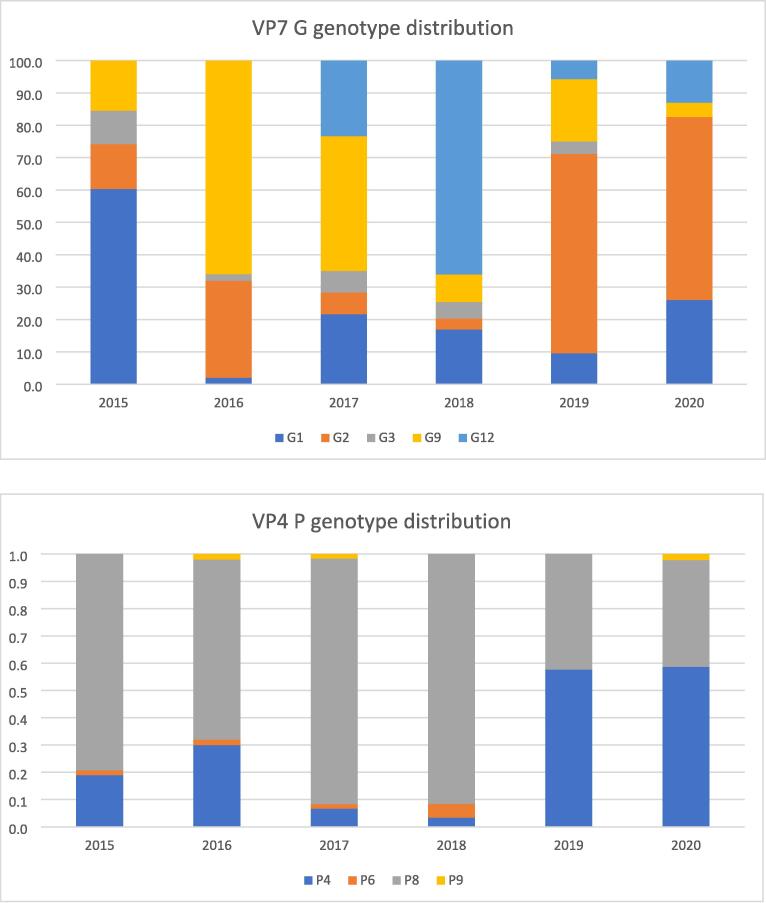
Analyzing the VP7 G genotypes and VP4 P genotypes separately, a transition from G1 to G9 was noted during the ROTARIX® implementation phase (from 60.3 % G1 in 2015 to 66 % G9 in 2016), followed by a shift towards G2 during the ROTAVAC® period (from 3.4 % in 2017 to 61.5 % in 2019 and 56.5 % in 2020) (Fig. 6a). VP4 P genotypes showed less variability. P[8] was predominant from 2015 through 2018 ranging from 66 % in 2016 to 91.5 % in 2018. With the implementation of ROTAVAC® a shift towards the P[4] genotype became apparent at 57.7 % in 2019 and 58.7 % in 2020 respectively (Fig. 6b).
Fig. 6.
A (top) and b (bottom): transition of VP7 G genotypes (top) and VP4 P genotypes (bottom) from 2015 through 2020 in response to ROTARIX® introduction in 2016 and transition to ROTAVAC® in 2018.
3.4. Intussusception
During 2019 and 2020, 51 cases of suspected intussusception in infants younger than 12 months of age were attended at the Shifa Hospital in Gaza. Of these, 48 were confirmed at surgery or through radiographic means. One case found at surgery had a polyp as the lead point for the intussusception. Three children had received ROTARIX®, 33 had completed three doses of ROTAVAC®, 8 had received 2 doses, and 3 had 1 dose. No cases of intussusception occurred during the 21 days following dose 1 of immunization, 1 case occurred within 21 days after dose 2, and 8 cases occurred within 21 days after the third dose of ROTAVAC®.
In the West Bank, 50 suspected cases of intussusception in infants were reported between December 2015 and the end of 2020. 47 of these were confirmed. 11 children had not received rotavirus vaccine, 9 had received both doses of ROTARIX®, 1 had received 1 dose, 4 had 2 doses and 8 had 3 doses of ROTAVAC®, respectively. No immunization information was recorded for 13 children. No cases of intussusception occurred within 21 days of the first dose of any vaccine. One case happened within 21 days of the second dose of ROTARIX® and two cases occurred within 21 days of the third dose of ROTAVAC®.
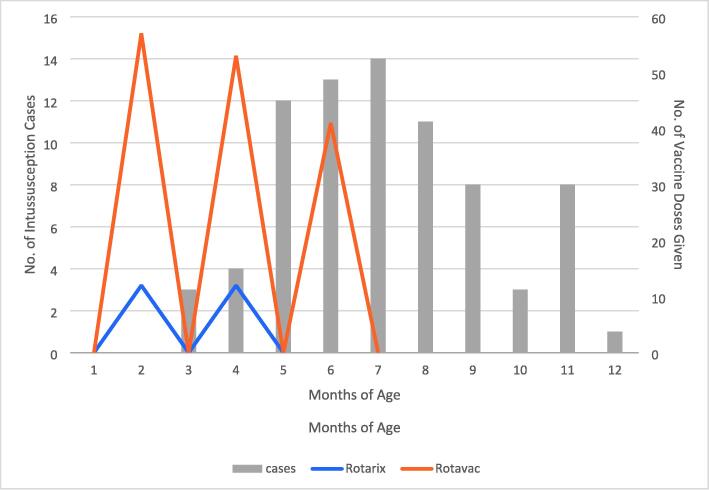
The age distribution of reported cases (Fig. 7) parallels the natural incidence of intussusception described elsewhere [40], [41], [42]. While the reported case numbers are too low to allow statistical analysis, these data provide reassurance that intussusception remains unrelated to vaccination.
Fig. 7.
Age distribution in months of intussusception cases in infants presenting at Shifa Hospital in Gaza (2019–2020) and Caritas Baby Hospital in the Southern West Bank (2015–2020) in relation to ROTARIX® doses given at 2 and 4 months (blue) and ROTAVAC® doses given at 2, 4 and 6 months (orange), respectively.
4. Discussion
This study was designed to demonstrate the clinical impact of RV vaccination on all-cause diarrhea and its effects on the prevalence of rotavirus in stool samples of children under 5 years of age in the Palestinian territories of Gaza and the West Bank. The country has been the first to switch to ROTAVAC® after an initial nationwide introduction of ROTARIX®. The study also aims to describe the prevalent genotype profiles of RV strains comparing the pre-vaccination period (January 2015 through April 2016) with the ROTARIX® (May 2016 through September 2018) and ROTAVAC® (October 2018 through December 2020) periods respectively. Lastly, the potential effects of ROTAVAC® on the occurrence of intussusception has been investigated.
The overall impact of RV vaccination on all-cause diarrhea showed a 53 % reduction of caseloads among children under 5 years of age during the study period. These results mirror the findings of a large meta-analysis including 101 studies from 47 countries estimating a 36 % reduction in all-cause diarrhea hospitalizations and deaths, accompanied by a 59 % reduction in rotavirus-related hospitalizations [3]. The impact of ROTARIX® on diarrhea caseloads was maintained by ROTAVAC® following the vaccine switch in 2018 at an overall annual case reduction rate of 16.7 %, indicative of the non-inferiority of ROTAVAC® compared with ROTARIX®.
Further case load decreases after March 2020 were not included in the statistical analysis as the onset of the SARS-CoV-2 epidemic affected health care utilization in the Palestinian territories as much as elsewhere, likely leading to underreporting of diarrhea cases. Between March and December 2020, monthly clinic visits at MoH and UNRWA clinics throughout Gaza dropped from an average of 94,515 for the years 2015 through 2019 to 48,740, a drop by 48.4 %. Elsewhere, since the onset of the epidemic, Taquechel, et al. reported an 87 % decrease in outpatient pediatric asthma care visits in the US [43], while separate reports indicate decreasing vaccination rates, and a decline in primary and urgent care visits globally [44], [45].
RV positivity rates dropped by 67.1 % (from 38.2 % to 13 %) after the introduction of ROTARIX® and were maintained at 15 % after the transition to ROTAVAC®, again confirming non-inferiority between the two vaccines. While this is the first study to describe vaccine impact for ROTARIX® and ROTAVAC® in the same population, a large metanalysis of 20 randomized clinical trials and 38 case-control studies by Sun et al [46] established a relative risk for rotavirus gastroenteritis in low-income countries of 0.653 (0.479–0.891) following ROTARIX® and 0.664 (0.548–0.804) following ROTAVAC® vaccination, suggesting comparable effectiveness for the two vaccines as well. Similarly, a 2021 Cochrane review [47] found that ROTARIX® reduced severe rotavirus cases in high-mortality countries (58 %) at a comparable rate as ROTAVAC® (54 %).
The effects of RV vaccination on genotype distribution and prevalence are not yet fully understood. While many countries have reported declines in G1P[8] strain prevalence following the introduction of ROTARIX® [48], [9], [49], [50], [51], which is a G1[P8] vaccine, others have reported a decline of G1P[8] strains even before the introduction of a national vaccination program [52], no changes [53] or even an increase in G1P[8] prevalence following the introduction of the vaccine [54]. Most studies, however, concur on the increasing relative prevalence of previously less frequent strains such as G2, G3, G9 or G12, and greater diversity of co-circulating strains since the introduction of vaccines [52], [53], [54], [55], [56]. In addition, less common genotype combinations like G2P[6], G12P[6], or G8P[4] have emerged [50] but there is no clear association between these and vaccine introduction.
This study indicates that G1P[8] strains have not dominated since rotavirus vaccine introduction, suggesting a level of selective pressure on circulating strains by the ROTARIX® vaccine. In the years post-ROTARIX® introduction, the predominant genotypes shifted to G9P[8] and G12P[8] and G2P[4] following the transition to ROTAVAC®. The effects of the G9P[11] ROTAVAC® on genotype diversity may be noticed by the decline of G9 genotypes that had emerged during the ROTARIX® period. The variability of VP7 G genotypes in response to vaccine strain exposure appears to be compensated to some extent by the less diverse presentation of VP4 P genotype prevalence [57]. Nevertheless, the emergence of G2P[4] strains following vaccination in the region [58] as well as globally [59] is concerning. Taking the global genotype fluctuations into account, these patterns may continue to change, and longer-term surveillance is advisable.
RV vaccination has been associated with an increased risk of intestinal intussusception in selected geographies, primarily high- and middle-income countries [31], [32], [33]. An increase in Intussusception risk was not observed during ROTAVAC® Phase 3 trials [60], [61] nor during post-introduction surveillance [62], [63]. In addition, a modeling analysis comparing the risk of intussusception with the reduction of mortality provided by RV vaccination in 135 lower-and middle-income countries found that vaccination showed a significantly positive benefit-risk profile [64]. While this study does not provide sufficient power for statistical analysis, no cases were observed during the previously identified highest-risk period of 7 days following the first dose of RV vaccine. The results should be seen as reassuring that ROTAVAC® does not carry an increased risk of intussusception.
There are limitations to this study. The effects of the SARS-CoV-2 epidemic on clinic visits has been discussed. Clinical surveillance data for the period of March through December 2020 has been excluded from the analysis to reduce the effects of reduced clinic visits on case identification. In addition, there are two transition periods in the longitudinal analysis of surveillance data; the year 2016 when ROTARIX® was introduced up to a time when the complete infant cohort of the year could be considered immunized, and 2018, when the transition from ROTARIX® to ROTAVAC® was undertaken. During this period some infants were immunized with one vaccine and others with the other. These periods cannot be used to interpret comparison data between the two vaccines. Therefore, the study serves to provide a trend of disease occurrence and rotavirus prevalence rather than a head-to-head comparison between the two vaccines. Thirdly, the areas of Gaza and the West Bank, while being situated in the same region, are geographically separate. Vaccination programs of the neighboring state of Israel may influence disease occurrence and rotavirus prevalence in the West Bank more than in Gaza where there is virtually no population exchange with Israel. Therefore, this study was not designed to compare disease occurrences and rotavirus prevalence between the Palestinian Territories, but rather to analyze trends over time within the respective territories.
5. Conclusion
The occupied Palestinian Territories were the first country outside of India to include ROTAVAC® in its national immunization program. Two years into the program, the vaccine has shown epidemiological impact, on par with ROTARIX® and measured decreases in the overall numbers of children under 5 years of age with acute gastroenteritis. No increases in the incidence of intussusception were noticed since the vaccine switch. Rotavirus genotype shifts from a pre-vaccination predominance of G1P[8] to a current predominance of G2P[4] requires longer-term surveillance to determine if they are secular or vaccine-induced variations.
Funding
This work was supported by the Bill & Melinda Gates Foundation [Grant ID OPP1199552]. Under the grant conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper: [Wolfgang Rennert MD reports financial support was provided by Bill & Melinda Gates Foundation].
Acknowledgements
The authors are grateful to Dr. Miren Iturriza-Gomara of PATH Geneva for her insightful inputs to the interpretation of results and review of this manuscript. The authors are also grateful to the Bill & Melinda Gates Foundation for its funding support.
Contributor Information
Wolfgang Rennert, Email: rennertw@gunet.georgetown.edu.
Musa Hindiyeh, Email: hindiyeh@yahoo.com.
Majd Allahham, Email: majdallahham11@gmail.com.
Laina D. Mercer, Email: lmercer@path.org.
Khalil I. Hamad, Email: Kh.Hamad@UNRWA.org.
Nedal I. Ghuneim, Email: ghuneimnedal@yahoo.com.
Zuheir A. M. Eljaro, Email: zohergar1972@gmail.com.
Fakhr Abu-Awwad, Email: dr-fakhr@live.com.
Yaser Bozya, Email: yaser515@gmail.com.
Diaa Hjaija, Email: pmdmoh@yahoo.com.
Niranjan Bhat, Email: nbhat@path.org.
Troy Leader, Email: troyleader@gmail.com.
Asad Ramlawi, Email: ramlawi_asad@hotmail.com.
Hiyam Marzouqa, Email: hiyam.marzouqa@cbh-beth.org.
Data availability
Data will be made available on request.
References
- 1.Troeger C., Khalil I.A., Rao P.C., et al. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr. 2018;172(10):958–965. doi: 10.1001/jamapediatrics.2018.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Troeger C., Blacker B.F., Khalil I.A., et al. Estimates of global, regional, and national morbidity, mortality, and aetiologias of diarrhea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18:1211–1228. doi: 10.1016/S1473-3099(18)30362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnett E., Parashar U.D., Tate J. Global impact of Rotavirus vaccination on diarrhea hospitalizations and deaths among children <5 years old: 2006–2019. JID. 2020;222:1731–2179. doi: 10.1093/infdis/jiaa081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnett E., Jonesteller C.L., Tate J.E., Yen C., Parashar U.D. Global impact of Rotavirus vaccination on childhood hospitalizations and mortality from diarrhea. JID. 2017;215(11):1666–1672. doi: 10.1093/infdis/jix186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aliabadi N., Antoni S., Mwenda J.M., et al. Global impact of rotavirus vaccine introduction on rotavirus hospitalisations among children under 5 years of age, 2008–16: findings from the Global Rotavirus Surveillance Network. Lancet Glob Health. 2019;7:e893–e903. doi: 10.1016/S2214-109X(19)30207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crawford S.E., Ramani S., Tate J.E., et al. Rotavirus infection Nat Rev Dis Primers. 2017;3:17083. doi: 10.1038/nrdp.2017.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.J, Matthijnssens, M, Ciarlet, S, McDonald et al. Uniformity of rotavirus strain nomenclature proposed by the Rotavirus Classification Working Group (RCWG). Arch Virol 2011; 156: 1397-13. https://doi.org/10.1007/s00705-011-1006-z. [DOI] [PMC free article] [PubMed]
- 8.Patton J. Rotavirus diversity and evolution in the post-vaccine world. Discov Med. 2012;13(68):85–97. PMID: 22284787. [PMC free article] [PubMed] [Google Scholar]
- 9.Giri S., Kumar C.P.G., Khakha S.A., et al. Diversity of rotavirus genotypes circulating in children < 5 years of age hospitalized for acute gastroenteritis in India from 2005 to 2016: analysis of temporal and regional genotype variation. BMC Infect Dis. 2020;20:740. doi: 10.1186/s12879-020-05448-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hungerford D., Allen D.J., Nawaz S., Collins S., Ladhani S., Vivancos R., et al. Impact of rotavirus vaccination on rotavirus genotype distribution and diversity in England, September 2006 to August 2016. Euro Surveill. 2019 Feb;24(6):1700774. doi: 10.2807/1560-7917.ES.2019.24.6.1700774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matthijnssens J., Bilcke J., Ciarlet M., Martella V., Banyai K., Rahman M., et al. Rotavirus disease and vaccination: impact on genotype diversity. Future Microbiol. 2009 Dec;4(10):1303–1316. doi: 10.2217/fmb.09.96. [DOI] [PubMed] [Google Scholar]
- 12.Cortese M.M., Immergluck L.C., Held M., Jain S., Chan T., Grizas A.P., et al. Effectiveness of monovalent and pentavalent rotavirus vaccine. Pediatrics. 2013 Jul;132(1):e25–e33. doi: 10.1542/peds.2012-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang F.T., Mast T.C., Glass R.J., Loughlin J., Seeger J.D. Effectiveness of the pentavalent rotavirus vaccine in preventing gastroenteritis in the United States. Pediatrics. 2010 Feb;125(2):e208–e213. doi: 10.1542/peds.2009-1246. [DOI] [PubMed] [Google Scholar]
- 14.Armah G.E., Sow S.O., Breiman R.F., Dallas M.J., Tapia M.D., Feikin D.R., et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010 Aug 21;376(9741):606–614. doi: 10.1016/S0140-6736(10)60889-6. [DOI] [PubMed] [Google Scholar]
- 15.Zaman K., Dang D.A., Victor J.C., Shin S., Yunus M., Dallas M.J., et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010 Aug 21;376(9741):615–623. doi: 10.1016/S0140-6736(10)60755-6. [DOI] [PubMed] [Google Scholar]
- 16.Jonesteller C.L., Burnett E., Yen C., Tate J.E., Parashar U.D. Effectiveness of rotavirus vaccination: a systematic review of the first decade of global postlicensure Data, 2006–2016. CID. 2017 Sep 1;65(5):840–850. doi: 10.1093/cid/cix369. [DOI] [PubMed] [Google Scholar]
- 17.Appaiahgari M.B., Glass R., Singh S., et al. Transplacental rotavirus IgG interferes with the immune response to live oral rotavirus vaccine ORV-116E in Indian infants. Vaccine. 2014;32(6):651–656. doi: 10.1016/j.vaccine.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 18.Otero C.E., Langel S.N., Blasi M., Permar S.R. Maternal antibody interference contributes to reduced rotavirus vaccine efficacy in developing countries. PLoS Pathog. 2020;16(11):e1009010. doi: 10.1371/journal.ppat.1009010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burnett E., Parashar U., Tate J. Rotavirus vaccines: effectiveness, safety and future directions. Pediatr Drugs. 2018 June;20(3):223–233. doi: 10.1007/s40272-018-0283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Praharaj I., Platts-Mills J.A., Taneja S., et al. Diarrheal etiology and impact of co-infections on Rotavirus vaccine efficacy estimates in a clinical trial of a monovalent human-bovine (116E) oral Rotavirus vaccine, Rotavac. India CID. 2019;69(2):243–250. doi: 10.1093/cid/ciy896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marie C., Ali A., Chandwe K., Petri W.A., Jr, Kelly P. Pathophysiology of environmental enteric dysfunction and its impact on oral vaccine efficacy. Mucosal Immunol. 2018;11:1290–1298. doi: 10.1038/s41385-018-0036-1. [DOI] [PubMed] [Google Scholar]
- 22.Rose J., Homa L., Meropol S., et al. Health impact and cost-effectiveness of a domestically-produced rotavirus vaccine in India: a model based analysis. PLoS One. 2017;12(11):e0187446. doi: 10.1371/journal.pone.0187446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bibera G.L., Chen J., Pereira P., Benninghoff B. Dynamics of G2P[4] strain evolution and rotavirus vaccination: a review of evidence for Rotarix. Vaccine. 2020;38:5591–5600. doi: 10.1016/j.vaccine.2020.06.059. [DOI] [PubMed] [Google Scholar]
- 24.H, Bergman, N, Henschke, D, Hungerford, F, Pitan, D, Ndwandwe, N, Cunliffe, K, Soares-Weiser, Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database Syst Rev 2021; 11: CD008521. https://doi.org/10.1002/14651858.CD008521.pub6. [Accessed 07 January 2022]. [DOI] [PMC free article] [PubMed]
- 25.Abu-Elamreen F.H., Abed A.A., Sharif F.A. Viral, bacterial and parasitic etiology of pediatric diarrhea in Gaza. Palestine Med Princ Pract. 2008;17(4):296–301. doi: 10.1159/000129609. [DOI] [PubMed] [Google Scholar]
- 26.Malek M.A., Teleb N., Abu-Elyazeed R., et al. The epidemiology of rotavirus diarrhea in countries in the Eastern Mediterranean region. JID. 2010;202(supl1):S12–S22. doi: 10.1086/653579. [DOI] [PubMed] [Google Scholar]
- 27.Shaheen M.N.F. Rotavirus gastroenteritis among hospitalized children under 5 years of age in the Eastern Mediterranean region: a review. East Mediterr Health J. 2019 Aug 19;25(6):422–430. doi: 10.26719/emhj.18.054. [DOI] [PubMed] [Google Scholar]
- 28.Zaraket H., Charide R., Kreidieh K., Ddaibo G., Melhem N.M. Update on the epidemiology of rotavirus in the Middle East and North Africa. Vaccine. 2017;35:6047–6058. doi: 10.1016/j.vaccine.2017.09.067. [DOI] [PubMed] [Google Scholar]
- 29.Rennert W.P., Hindiyeh M., Abu-Awwad F.M., Marzouqa H., Ramlawi A. Introducing rotavirus vaccine to the Palestinian territories: the role of public-private partnerships. J Public Health (Oxf) 2019 Mar 1;41(1):e78–e83. doi: 10.1093/pubmed/fdy101. [DOI] [PubMed] [Google Scholar]
- 30.Debellut F., Jaber S., Bouzya Y., et al. Introduction of rotavirus vaccination in Palestine: An evaluation of the costs, impact, and cost-effectiveness of ROTARIX and ROTAVAC. PLoS One. 2020 Feb 5;15(2):e0228506. doi: 10.1371/journal.pone.0228506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koch J., Harder T., von Kries R., Wichman O. Risk of intussuception after rotavirus vaccination. Dtsch Ärztebl Int. 2017;114:255–262. doi: 10.3238/arztebl.2017.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yih W.K., Lieu T.A., Kulldorff M., et al. Intussusception risk after rotavirus vaccination in U.S. children. NEJM. 2014;370(6):503–512. doi: 10.1056/NEJMoal1303164. [DOI] [PubMed] [Google Scholar]
- 33.Weintraub E.S., Baggs J., Duffy J., et al. Risk of intussusception after monovalent rotavirus vaccination. NEJM. 2014;370(6):513–519. doi: 10.1056/NEJMoal1311738. [DOI] [PubMed] [Google Scholar]
- 34.Hill P.S., Pavignani E., Michael M., Murru M., Beesley M. The, “empty void” is a crowded space: health service provision at the margins of fragile and conflict affected states. Confl Health. 2014 Oct;22(8):20. doi: 10.1186/1752-1505-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Gastroenterology Organization . World Gastroenterology organization; Milwaukee, WI: 2012. Global Guardian of Digestive health: WGO practice guideline – acute diarrhea; pp. 5–24. [Accessed 13 June 2022] [Google Scholar]
- 36.Bines J.E., Kohl K.S., Foster J., et al. Brighton Collaboration Intussusception Working Group. Acute intussusception in infants and children as an adverse event following immunization: case definition and guidelines of data collection, analysis, and presentation. Vaccine. 2004;22(5–6):569–574. doi: 10.1016/j.vaccine.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 37.WHO. Manual of rotavirus detection and characterization methods immunization, vaccines and biologicals. WHO/IVB/08.17 October 2019 [Accessed June 15, 2022]. http://apps.who.int/iris/bitstream/handle/10665/70122/WHO_IVB_08.17_eng.pdf.
- 38.Enane L.A., Gastañaduy P.A., Goldfarb D.M., et al. Impact of rotavirus vaccination on hospitalizations and deaths from childhood gastroenteritis in Botswana. CID. 2016;62(S2):S168–S174. doi: 10.1093/cid/civ1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Armah G., Pringle K., Enweronu-Laryea C.C., et al. Impact and effectiveness of monovalent rotavirus vaccine against severe rotavirus diarrhea in Ghana. CID. 2016;62(S2):S200–S207. doi: 10.1093/cid/ciw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hawken S., Duchamme R., Rosella L.C., et al. Assessing the risk of intussusception and rotavirus vaccine safety in Canada. Hum Vaccin Immunother. 2017;13(3):703–710. doi: 10.1080/21645515.2016.1240846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tate J.E., Mwenda J.M., Armah G., et al. Evaluation of intussusception after monovalent rotavirus vaccination in Africa. NEJM. 2018;378(16):1521–1528. doi: 10.1056/NEJMoa1713909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Groome M.J., Tate J.E., Arnold M., et al. Evaluation of intussusception after oral monovalent rotavirus vaccination in South Africa. CID. 2020;70(8):1606–1612. doi: 10.1093/cid/ciz431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taquechel K., Diwadkar A., Sayed S., et al. Pediatric asthma health care utilization, viral testing, and air pollution changes during the COVID-19 pandemic. J Allergy Clin Immunol Pract. 2020;8(10):3378–3387. doi: 10.1016/j.jaip.2020.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pingel E.S., Llovet A., Cosentino F., Lesser J. Committing to continuity: primary care practices during COVID-19 in an urban Brazilian neighborhood. Health Educ Behav. 2020;48(1):29–33. doi: 10.1177/1090198120979609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu Z., Fan J., Ding J., et al. The Impact of COVID-19 on primary care general practice consultations in a teaching hospital in Shanghai. China Front Med. 2021;8 doi: 10.3389/fmed.2021.642496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.ZW, Sun, Y, FU, RX, Yang, H, Goyal, Y, Jiang, HG, Xu Association of rotavirus vaccines with reduction in rotavirus gastroenteritis in children younger than 5 years: a systematic review and meta-analysis of randomized clinical trials and observational studies. JAMA Pediatrics 2021; 175(7): e210347. https://doi.org/10.1001/jamapediatrics.2021.0347. [DOI] [PMC free article] [PubMed]
- 47.H, Bergman, N, Henschke, D, Hungerford et al. Vaccines for preventing rotavirus diarrhoea: vaccines in use (review). Cochrane Database of Systemic Reviews 2021; 11: CD008521. https:doi.org/10.1002/14651858.CD008521.pub6. [DOI] [PMC free article] [PubMed]
- 48.Thomas S., Donato C.M., Covea S., et al. Genotype diversity before and after the introduction of a rotavirus vaccine into the national immunization program in Fiji. Pathogens. 2021;10:358. doi: 10.3390/pathogens10030358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mukhopadhya I., Murdoch H., Berry S., et al. Changing molecular epidemiology of rotavirus infection after introduction of monovalent rotavirus vaccination in Scotland. Vaccine. 2017;55:156–163. doi: 10.1016/j.vaccine.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 50.Mphahlele M.J., Groome M.J., Page N.A., Bhagwandin N., Mwenda J.M., Steele A.D. A decade of rotavirus vaccination in Africa – saving lives and changing the face of diarrhoel diseases: report of the 12th African rotavirus symposium. Vaccine. 2021;39:2319–2324. doi: 10.1016/j.vaccine.2021.03.014. [DOI] [PubMed] [Google Scholar]
- 51.Almalki S.S.R. Circulating rotavirus G and P strains post rotavirus vaccination in Eastern Mediterranean region. Saudi Med J. 2018;39(8):755–766. doi: 10.15537/smj.2018.6.21394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tapisiz A., Karahan Z.C., Çifçi E., Ince E., Doğru Ü. Changing patterns of rotavirus genotypes in Turkey. Curr Microbiol. 2011;63:517–522. doi: 10.1007/s00284-011-0014-2. [DOI] [PubMed] [Google Scholar]
- 53.Mwanga M.J., Owor B.E., Ochieng J., et al. Rotavirus group A genotype circulation patterns across Kenya before and after nationwide vaccine introduction, 2010–2018. BMC Infect Dis. 2020;20:504. doi: 10.1186/s12879-020-05230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.João E.D., Munlela B., Chissaque A., et al. Molecular epidemiology of rotavirus A strains pre- and post-vaccine (Rotarix) introduction in Mozambique, 2012–2019: emergence of genotypes G3P[4] and G3P[8] Pathogens. 2020;9:671. doi: 10.3390/pathogens9090671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muhsen K., Kassem E., Rubinstein U., et al. Incidence of rotavirus gastroenteritis hospitalizations and genotypes, before and five years after introduction of universal immunization in Israel. Vaccine. 2016;34:5916–5922. doi: 10.1016/j.vaccine.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 56.Armah G.E., Steele A.D., Binka F.N., et al. Changing patterns of rotavirus genotypes in Ghana: emergence of human rotavirus G9 as a major cause of diarrhea in children. J Clin Microbiol. 2003;6:2317–2322. doi: 10.1128/JCM.41.6.2317-2322.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trask S.D., McDonald S.M., Patton J.P. Structural insights into the coupling of virion assembly and rotavirus replication. Nat Rev Microbiol. 2012;10(3):165–177. doi: 10.1038/nrmicro2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harastani H.H., Reslan L., Sabra A., et al. Genetic diversity of human Rotavirus A among hospitalized children under-5 years in Lebanon. Front Immunol. 2020;11:317. doi: 10.3389/fimmu.2020.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dennis A.F., McDonald S.M., Payne D.C., et al. Molecular epidemiology of contemporary G2P[4] human rotaviruses circulating in a single U.S. community: footprints of a globally transitioning genotype. J Virol. 2014;88:3789–3801. doi: 10.1128/JVI.03516-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.John J., Kawade A., Rongsen-Chandola T., et al. Active surveillance for intussusception in a phase III efficacy trial of an oral monovalent rotavirus vaccine in India. Vaccine. 2014;32(S1):A104–A109. doi: 10.1016/j.vaccine.2014.03.036. [DOI] [PubMed] [Google Scholar]
- 61.Bhandari N., Rongsen-Chandola T., Bavdekar A., et al. Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian infants: a randomized, double-blind, placebo-controlled trial. Lancet. 2014;383:2136–2143. doi: 10.1016/S0140-6736(13)62630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reddy S.N., Nair N.P., Tate J.E., et al. Intussusception after rotavirus vaccine introduction in India. NEJM. 2020;383(20):1932–1940. doi: 10.1056/NEJMoa2002276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.INCLEN Intussusception Surveillance Network Study Group. Risk of intussusception after monovalent rotavirus vaccine (Rotavac) in Indian infants: a self-controlled case series analysis. Vaccine 2021; 39: 78-84. https://doi.org/10.1016/j.vaccine.2020.09.019. [DOI] [PMC free article] [PubMed]
- 64.Clark A., Tate J., Parashar U., et al. Mortality reduction benefits and intussusception risks of rotavirus vaccination in 135 low-income and middle-income countries: a modeling analysis of current and alternative schedules. Lancet Glob Health. 2019;7:e1541–e1552. doi: 10.1016/S2214-109X(19)30412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.