Abstract
Cerebral visual disorders include a range of common and rare deficits. They can be divided into effects on low-, intermediate-, and high-level forms of visual processing. Low-level deficits are various forms of homonymous hemifield scotomata, which affect all types of vision within their borders. Intermediate-level deficits refer to impairments of colour or motion perception, which affect either one hemifield or the entire field when lesions are bilateral. High-level deficits are divided into those of the ventral (occipitotemporal) or dorsal (occipitoparietal) stream. Occipitotemporal lesions affect various aspects of object recognition, ranging from general visual agnosia to selective agnosias, such as prosopagnosia or topographagnosia from right or bilateral lesions, and pure alexia from left-sided lesions. Occipitoparietal lesions cause the various components of Bálint syndrome, namely, simultanagnosia, optic ataxia, and ocular motor apraxia. They can also cause other impairments of visuospatial or visuotemporal processing, such as astereopsis and sequence-agnosia. Because of anatomic proximity, certain deficits cluster together to form a number of cerebral visual syndromes. Treatment of these disorders remains challenging, with frequent reliance on strategic substitutions rather than restorative approaches.
Keywords: Agnosia, balint, hemianopia
Visual symptoms are common after brain lesions. Many occipital, temporal, and parietal regions have visual responses, and substantial white matter is devoted not only to relaying information from the eye to occipital cortex, but also between visual cortical regions. These regions are linked in networks specialized for different aspects of vision.
A GENERAL SCHEME FOR CEREBRAL VISUAL LOSS
Distinguishing effects due to white versus grey matter damage is not always necessary. More important is clarifying from the symptoms and signs the levels of the visual hierarchy affected. A useful scheme is to divide vision into low-, intermediate-, and high-level processes [Figure 1]. The latter two are also subdivided into ventral (occipitotemporal) or dorsal (occipitoparietal) components.
Figure 1.
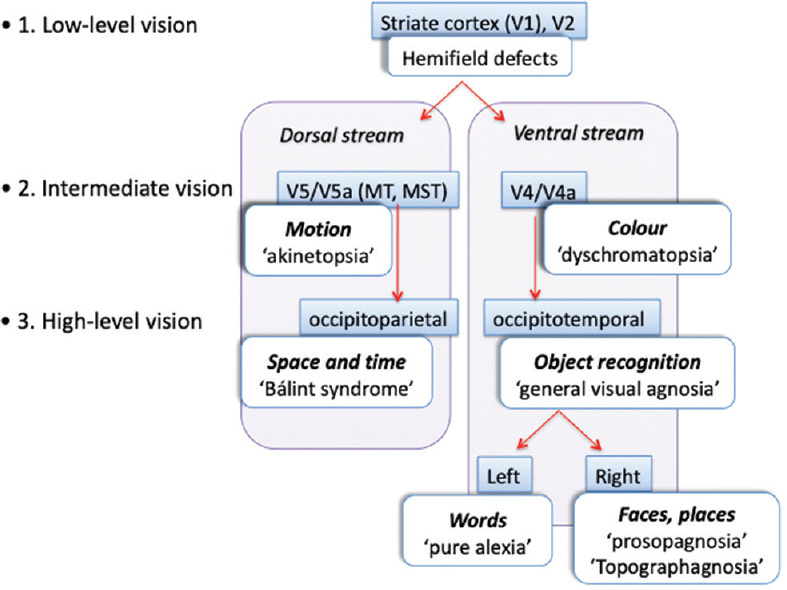
A framework for thinking about cerebral visual disorders. These can be divided into low-, intermediate-, and high-level problems. The latter two can be separated into distinct dorsal and ventral streams
Low-level vision refers to the information relay in the optic radiations, and the striate and peri-striate cortex. Damage to these structures causes general visual loss limited to the contralateral hemifield in both eyes. Therefore, the hallmarks of 'low-level' damage are homonymous field defects, ranging from small scotomata to the entire hemifield. The defects are 'general' in that all types of vision are lost within the affected area.
Intermediate-level vision refers to processing in regions with a coarser retinotopic code but now possessing some visual selectivity. In the ventral stream, areas V4 and V8 (V4a) are specialized for colour processing. In the dorsal stream, areas V5 and V5a are specialized for motion perception. Lesions of these regions cause achromatopsia or akinetopsia in the contralateral hemifield, sometimes just in one quadrant, but never a small scotoma. Their full expression almost always requires bilateral lesions.
High-level visual processing is even more specialized and cause deficits that are not limited to one part of the visual field. In the ventral stream, these are various object recognition problems, ranging from the broad (general visual agnosia) to the selective (prosopagnosia, topographagnosia, and alexia). The latter can also be divided by the hemisphere involved. The dorsal stream is directed at visuospatial processing, and damage here causes various aspects of Bálint's syndrome, neglect, and astereopsis.
EVALUATING THE PATIENT WITH CORTICAL VISUAL LOSS
Keep in mind two facts when evaluating a patient for cortical visual dysfunction: many lesions are large, and the different components of the visual system lie near each other. These two facts have three implications.
First, lesions can affect several levels of the visual hierarchy. For example, one can find superior field defects (low), dyschromatopsia (intermediate), and prosopagnosia (high) in one patient.
Second, lesions can affect several components at the same level. Thus, prosopagnosia and landmark agnosia, a form of topographagnosia, both high-level deficits, often co-exist.
Third, certain combinations of deficits occur more frequently than others, creating syndromes.
To start, when a patient describes difficulty with a complex task, ensure that the difficulty is not explained by a low-level deficit before concluding that there is a high-level one. Patients may have trouble recognizing faces because of macular degeneration, not prosopagnosia. Those struggling to read may have a homonymous central scotoma from an occipital pole infarct, not pure alexia. Careful perimetry and measures of visual acuity are always the first steps.
The corollary to this point, though, is that one should be familiar enough with the effects of low-level visual loss to realize which visual symptoms it cannot cause. For example, some patients with prosopagnosia blame it on their hemianopia, yet the experienced clinician knows that many patients with even macula-splitting hemianopia can still recognize faces.
Assessments of some non-visual functions are also important preliminary steps. Intact oral and auditory language is needed to understand the instructions for some complicated visual tests. Also, when pure alexia is suspected, testing non-visual language is key to excluding a broader aphasic syndrome that affects reading. Tests of auditory memory help exclude a general amnestic syndrome that may be behind impaired face or place recognition. Attention is a requisite for optimal visual function, and testing will be unreliable in the inattentive or confused patient.
LOW-LEVEL CEREBRAL VISUAL LOSS - HEMIFIELD DEFECTS
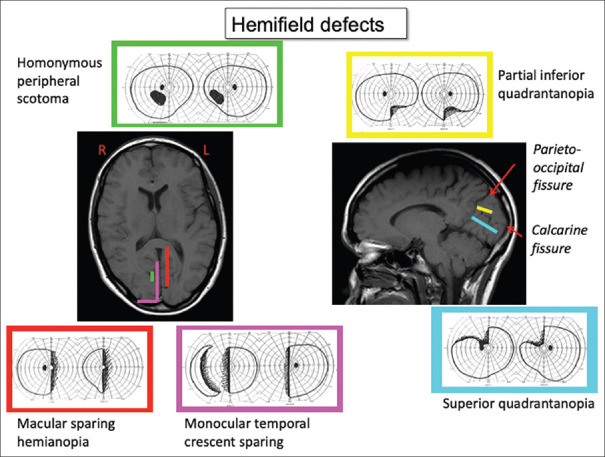
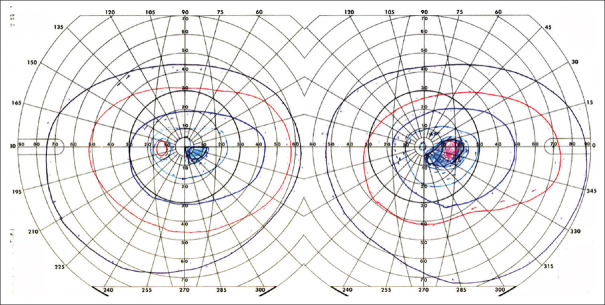
Visual field defects from brain damage are a) homonymous, meaning that both eyes have a similar defect, and b) limited to the contralateral hemifield, meaning that they do not cross the vertical meridian. Even with bilateral lesions, there will be a sharp distinction between the right and left aspects of the defect at the vertical meridian, unless visual loss is complete (cerebral blindness). With the latter, preservation of the pupil light reflex distinguishes cerebral blindness from bilateral ocular damage. The area of field affected in the two eyes is often slightly incongruous—meaning the area differs in size and shape—with optic radiation damage, and highly congruous with striate lesions. There is a precise correspondence between the anatomy of the lesion and the retinotopy of the visual defect [Figure 2]. Damage to the superior optic radiations or calcarine bank affects the lower visual field, and damage to the inferior aspects of these structures affects the upper field. In striate cortex, the occipital pole represents the central field and retrosplenial cortex the far peripheral field. Partial lesions cause homonymous scotomata. Cortical magnification refers to the fact that more cortex is devoted to the central than the peripheral field.[1] Hence, a lesion at the occipital pole causes a small hemi-scotoma limited to the central 5 degrees [Figure 3]—enough to disrupt reading but sometimes hard to detect.
Figure 2.
Hemifield defects from lesions of striate cortex. Defects are highly retinotopic, in that the part of the visual field affected correlates with the location of the lesion, indicated by colour coding. Damage that spares the occipital pole causes a hemianopia with macula-sparing, whereas sparing of striate cortex just behind the parieto-occipital fissure will cause a hemianopia with sparing of the monocular temporal crescent, the most peripheral part of the field. A lesion of the mid-portion of striate cortex will cause a homonymous peripheral scotoma. The right figure illustrates that damage to the superior calcarine bank will cause an inferior quadrantanopia, whereas damage to the inferior bank will be associated with a superior quadrantanopia
Figure 3.
A homonymous central hemifield scotoma in a patient with a left occipital pole hemorrhage
Homonymous hemifield defects can be detected by careful confrontation testing.[2] Rather than comparing the far periphery of the patient's vision with your own, as often taught in medical school, check the central 30°. As the patient watches your nose, ask if any part of your face is blurry or hard to see. At a conversational distance of 1 meter, the face spans the central 5°. Next, hold your two hands up side-by-side, one in each quadrant, and ask if one is harder to see than the other, or have them count your fingers while you flash one or two digits. This can be supplemented with coloured targets, asking if these are faded or less intense in any quadrant. Once a defect is found, move the target to find the edges of the defect. In particular, determine if the defect respects the vertical meridian (it is confined to one hemifield) or better yet, aligns on it (the defect abuts against the meridian). If you suspect a central defect and the patient denies any difficulty seeing your face, step back six feet and repeat central testing with your hands and coloured targets.
Bedside evaluation is often supplemented with perimetric testing. A Goldmann exam samples the entire field, but the results vary with the perimetrist and the exam is not detailed for the central 10°. If the latter is important, computerized perimetry targeting the central 10° or 30° is better, if the patient is attentive and cooperative.
INTERMEDIATE-LEVEL DEFICITS
Cerebral dyschromatopsia is impaired colour vision from a brain lesion.[3,4] Unlike congenital colour blindness, it is not specific for any type of colour.[5] A unilateral lesion of the lingual and fusiform gyri causes hemi-achromatopsia in the contralateral field, but this is often asymptomatic and seldom tested. Patients with bilateral lesions are aware of their colour problem. This can be achromatopsia, in which the world appears in greyscale, or dyschromatopsia, in which some degraded colour vision persists.
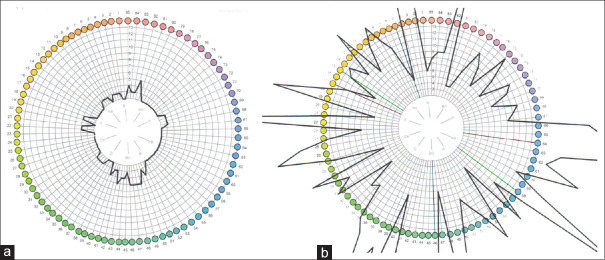
Testing for dyschromatopsia starts with Ishihara plates, but these were designed for congenital red-green defects and may not detect mild cerebral dyschromatopsia. Likewise, colour naming is coarse—'red' covers a lot of hues—and may seem normal in dyschromatopsic patients. On the other hand, patients with colour anomia cannot name colours but can still perceive colours well. The best test is to have patients sort hues [Figure 4], e.g., the Farnsworth Munsell 100-hue test.[5]
Figure 4.
Results of Farnsworth-Munsell 100-hue testing. The ring shows the different colour chips, and the error score for each chip is plotted as a thick black line. A perfect score would be indicated by a black line on the inner circle. The further the black line is away from this central circle, the greater the error in sorting for that colour. a) Normal performance. b) Performance of a patient with achromatopsia
Cerebral akinetopsia is impaired motion perception.[6,7] Hemi-akinetopsia from a unilateral lateral occipitotemporal lesion causes only subtle symptoms[8] and is rarely detected. Patients with akinetopsia are extremely rare. They complain of trouble judging the speed of oncoming cars or seeing motion in freeze-frames.[6] There are no standardized clinical tests. Diagnosis depends on experimental computer motion displays.
HIGH-LEVEL DEFICITS: VENTRAL STREAM
The ventral or occipitotemporal stream is sometimes referred to as the 'What' stream, as it plays a dominant role in object recognition.
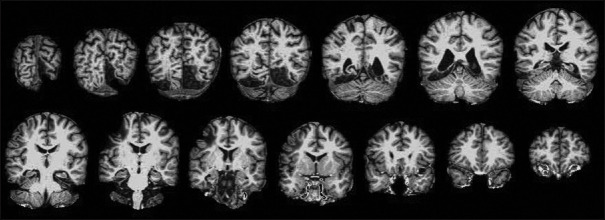
General visual agnosia is rare and usually requires bilateral occipital lesions [Figure 5]. The 'man who mistook his wife for a hat'[9] had this condition. The problem is modality-specific, meaning that the patient can recognize objects by sound or touch but not by sight. In the clinic, show the patient common objects such as keys and pens, and when they fail to recognize them, shake the keys to make a sound or place the pen in their hand. A more sensitive test is to show line drawings depicting objects, as these have fewer visual cues. Line drawings of overlapping figures may be particularly sensitive, as this probes their ability to segment and group elements appropriately [Figure 6].
Figure 5.
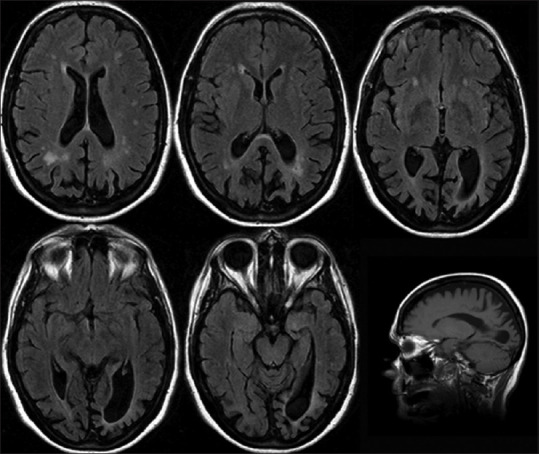
General visual agnosia. Axial FLAIR magnetic resonance images of a patient with posterior cortical atrophy. The occipital sulci and posterior horns of the lateral ventricles are enlarged, indicating localized atrophy
Figure 6.
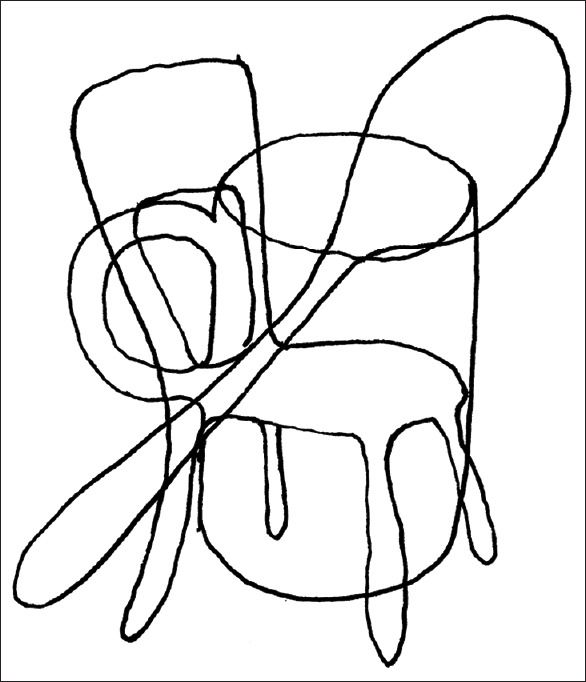
Overlapping line figures. Three objects are depicted superimposed. The subject must determine which line segments group together to identify the three objects, which is difficult for subjects with general visual agnosia to do
With more selective agnosias, patients can recognize the type of object but struggle with more specific identifications. Thus, a prosopagnosic patient knows that a face is not a hat, but cannot tell his wife's face from his sister's.
Prosopagnosia is the inability to recognize the identity of faces, and all faces look unfamiliar.[10] In some patients, this is due to an inability to perceive the subtle differences between faces, but in others, it is due to poor memory for faces. Affected patients can often process other types of facial information, such as expressions and lip-reading. In some patients, the identification problem may extend to other objects.[11] Most prosopagnosic patients know that they cannot recognize familiar faces. In the clinic, have them look at photographs on a family member's cellphone, to see if they can tell which faces they have seen before, and have the family member verify their response. Otherwise, have a file of photographs of anonymous and famous people that they can sort into familiar and unfamiliar piles. Tests like the Warrington Recognition Memory Test[12] and Cambridge Face Memory Test[13] are used by neuropsychologists.
Patients with topographagnosia get lost in familiar places. This can occur for several reasons, which are not mutually exclusive.[14] Landmark agnosia is the inability to recognize buildings and scenes. Patients with this can find their way around if they rely on street signs and numbers. However, that strategy will not help those with impaired cognitive map formation.[15] A mental map provides the most flexible means of navigation, because with it you can calculate the shortest route to get from any point A to any point B. Patients without a mental map get hopelessly lost if they deviate from a well-worn route. Computerized tests of cognitive map formation are available at www.gettinglost.ca.
Both prosopagnosia and topographagnosia occur in patients with right or bilateral occipitotemporal lesions [Figure 7]. Pure alexia (alexia without agraphia) occurs with left occipitotemporal lesions [Figure 8]. This ranges in severity from those who cannot read letters or digits to those who can read but more slowly than before. The latter show a word-length effect[16]: the more letters in the word, the slower their reading. Hence, they are called 'letter-by-letter readers'. Alexic patients can still write, although their spelling of irregular words may be impaired, i.e., 'surface dysgraphia'. Although they understand words they hear and their speech is intact, they may also struggle with lip-reading, another visual aspect of language.[17]
Figure 7.
Prosopagnosia. Coronal T1-weighted magnetic resonance images showing bilateral inferior occipitotemporal infarcts
Figure 8.
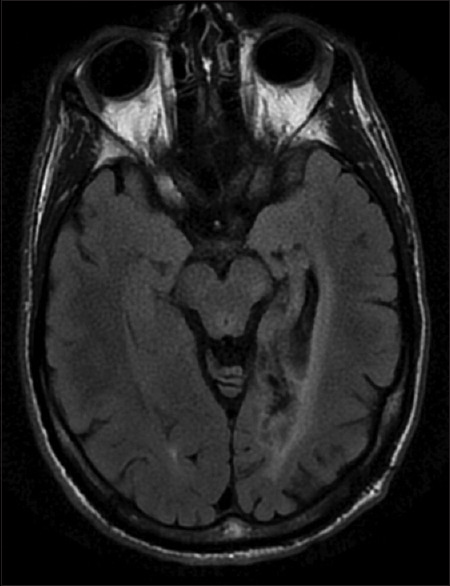
Pure alexia. Axial FLAIR magnetic resonance images of a patient with a posterior cerebral arterial infarct affecting the left medial occipitotemporal cortex, extending anteriorly to involve the visual word form area in the fusiform gyrus
HIGH-LEVEL DEFICITS: DORSAL STREAM
Occipitoparietal lesions impair various elements of visuospatial processing. Bálint syndrome[18] is the classic triad of simultanagnosia, optic ataxia, and ocular motor apraxia, which occur together often, but not always.
Simultanagnosia is a problem of spatial attention, primarily a limitation of capacity. With multiple stimuli, simultanagnosic patients cannot maintain awareness of more than a few. This can be tested by asking them to describe the Cookie theft picture, other scenes with multiple components, or even a desktop strewn with random objects. They cannot sustain attention across large regions of space.[19] With displays that have both a local (trees) and global (forest) structure, as with Navon letters [Figure 9] and Arcimboldo paintings, they often report only the stronger percept, whereas healthy subjects have no trouble seeing both.[20]
Figure 9.
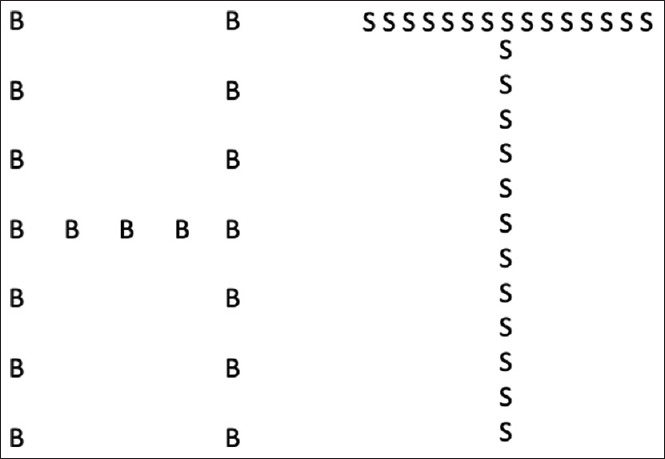
Navon figures. These show large 'global' letters (H, T), made up of small 'local' letters (B, S). Subjects with simultanagnosia have trouble seeing both, and often report just the local letters, which is called 'local capture'. If the global letter is made stronger by increasing the density of the local elements, as on the right, the probability that they will report the global letter increases
Optic ataxia is a problem of visuospatial guidance of reaching. This can be general or partial, affecting targets only in contralateral space or only with the contralateral arm. Impaired reaching to visual targets is contrasted with reaching to their own body parts with their eyes closed. If this too is affected, they have a more general problem with spatial orientation.
Ocular motor apraxia is a problem with saccadic generation. There can be two components. One is difficulty in initiating voluntary saccades. Thus, saccades on the examiner's command may be delayed, whereas their ability to look at a stimulus that suddenly appears is intact. The second is inaccurate targeting of stimuli. When severe, their saccades wander around until they stumble upon the stimulus.
There are other less well-known disturbances from dorsal stream damage. An extension of impaired visuospatial processing into the third dimension causes astereopsis, a form of impaired depth perception.[21] Temporal aspects of visual processing can be affected, so that some patients do not know the correct order in which visual stimuli occurred, which has been called 'sequence-agnosia' [Figure 10].[22]
Figure 10.
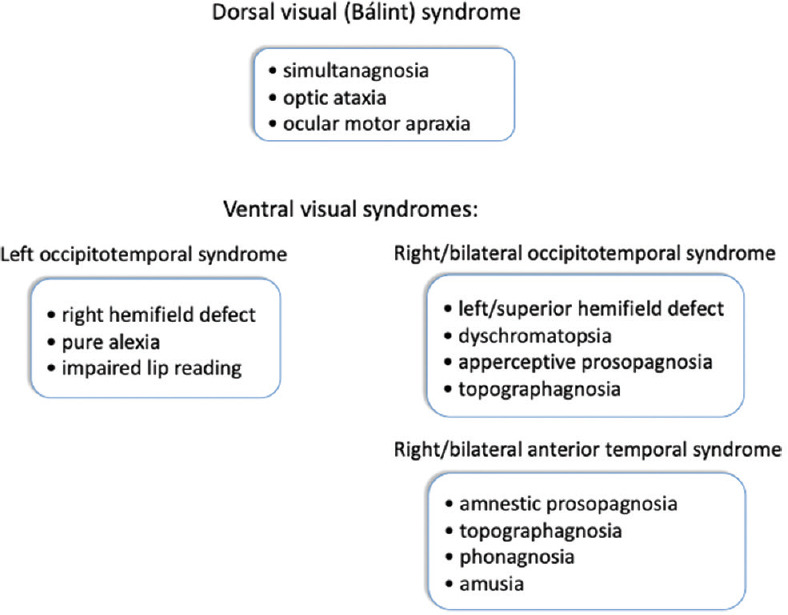
Cerebral visual syndromes. Certain deficits cluster together because of the anatomy, to form dorsal and ventral visual syndromes
VISUAL SYNDROMES
Because of anatomic proximity, various visual and non-visual deficits cluster together frequently—but not invariably—to form cortical syndromes. The most well-known is Bálint syndrome, the dorsal visual syndrome.
Right or bilateral occipitotemporal damage causes a ventral visual syndrome, consisting of upper visual field defects, the apperceptive type of prosopagnosia, topographagnosia, and dyschromatopsia. A left ventral visual syndrome consists of right hemifield defects, pure alexia, and impaired lip reading.
Because perceptual information from different modalities converges on the anterior temporal lobe, damage here can cause multimodal deficits, resulting in an anterior temporal syndrome of the amnestic type of prosopagnosia, landmark agnosia, phonagnosia (impaired voice recognition), and amusia (tone deafness).[15,23]
THERAPY FOR CEREBRAL VISUAL DISORDERS
When cerebral pathology is permanent, the visual deficits they cause often persist. This certainly seems the case for low-level hemifield defects, where attempts to train vision at the edge of the defect have produced controversial results.[24] If restoration is not possible, a 'strategic substitution' may help. Hemianopic patients can learn to use saccades to scan more into their blind side, to reduce their frequency of colliding with obstacles. Driving simulations have shown that their crash avoidance correlates with the amount of scanning.[25] For those with hemianopic dyslexia, making larger saccades towards the unseen portion of text can be promoted through training (www.readright.ucl.ac.uk).[26]
One might expect that intermediate- or high-level visual functions would show more plasticity, as these involve networks of regions, not all of which succumb to their lesion. Hence, it is theoretically possible that the surviving components can compensate with time or training. However, little is known about whether this actually happens. There are some early data showing that the face perception of prosopagnosic patients can improve with training,[27] though it is not clear how much this transfers into daily life.
Otherwise, strategic substitutions are the mainstay of managing these defects. For prosopagnosic patients, this could involve face recognition software or dependence on voice recognition instead, which many claim they do already. For topographagnosia, GPS technology is extremely helpful. Alexic subjects rely more on auditory communication and audio-books.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.McFadzean R, Brosnahan D, Hadley D, Mutlukan E. Representation of the visual field in the occipital striate cortex. Brit J Ophthalmol. 1994;78:185–90. doi: 10.1136/bjo.78.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shahinfar S, Johnson LN, Madsen RW. Confrontation visual field loss as a function of decibel sensitivity loss on automated static perimetry. Implications on the accuracy of confrontation visual field testing. Ophthalmology. 1995;102:872–7. doi: 10.1016/s0161-6420(95)30940-2. [DOI] [PubMed] [Google Scholar]
- 3.Zeki SM. A century of cerebral achromatopsia. Brain. 1990;113:1721–77. doi: 10.1093/brain/113.6.1721. [DOI] [PubMed] [Google Scholar]
- 4.Bouvier SE, Engel SA. Behavioral deficits and cortical damage loci in cerebral achromatopsia. Cereb Cortex. 2006;16:183–91. doi: 10.1093/cercor/bhi096. [DOI] [PubMed] [Google Scholar]
- 5.Moroz D, Corrow SL, Corrow JC, Barton AR, Duchaine B, Barton JJ. Localization and patterns of cerebral dyschromatopsia: A study of subjects with prospagnosia. Neuropsychologia. 2016;89:153–60. doi: 10.1016/j.neuropsychologia.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zihl J, von Cramon D, Mai N. Selective disturbance of movement vision after bilateral brain damage. Brain. 1983;106:313–40. doi: 10.1093/brain/106.2.313. [DOI] [PubMed] [Google Scholar]
- 7.Zeki S. Cerebral akinetopsia (visual motion blindness). A review. Brain. 1991;114:811–24. doi: 10.1093/brain/114.2.811. [DOI] [PubMed] [Google Scholar]
- 8.Vaina L, Grzywacz N, LeMay M, Bienfang D, Wolpow E. Perception of motion discontinuities in patients with selective motion deficits. In: Watanabe T, editor. High-Level Motion Processing-Computational, Physiological and Psychophysical Approaches. Cambridge: MIT Press; 1998. pp. 213–48. [Google Scholar]
- 9.Sacks O. The Man Who Mistook His Wife for a Hat and Other Clinical Tales. London: Gerald Duckworth; 1985 [Google Scholar]
- 10.Davies-Thompson J, Pancaroglu R, Barton J. Acquired prosopagnosia: Structural basis and processing impairments. Front Biosci (Elite Ed) 2014;6:159–74. doi: 10.2741/e699. [DOI] [PubMed] [Google Scholar]
- 11.Barton JJS, Albonico A, Susilo T, Duchaine B, Corrow SL. Object recognition in acquired and developmental prosopagnosia. Cogn Neuropsychol. 2019;36:54–84. doi: 10.1080/02643294.2019.1593821. [DOI] [PubMed] [Google Scholar]
- 12.Warrington E. Warrington Recognition Memory Test. Los Angeles: Western Psychological Services; 1984 [Google Scholar]
- 13.Duchaine BC, Nakayama K. The Cambridge face memory test: Results for neurologically intact individuals and an investigation of its validityt using inverted face stimuli and prosopagnosic patients. Neuropsychologia. 2006;44:576–85. doi: 10.1016/j.neuropsychologia.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Aguirre G, D'Esposito M. Topographical disorientation: A synthesis and taxonomy. Brain. 1999;122:1613–28. doi: 10.1093/brain/122.9.1613. [DOI] [PubMed] [Google Scholar]
- 15.Corrow JC, Corrow SL, Lee E, Pancaroglu R, Burles F, Duchaine B, et al. Getting lost: Topographic skills in acquired and developmental prosopagnosia. Cortex. 2016;76:89–103. doi: 10.1016/j.cortex.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barton JJ, Hanif HM, Eklinder Bjornstrom L, Hills C. The word-length effect in reading: A review. Cogn Neuropsychol. 2014;31:378–412. doi: 10.1080/02643294.2014.895314. [DOI] [PubMed] [Google Scholar]
- 17.Albonico A, Barton JJS. Face perception in pure alexia: Complementary contributions of the left fusiform gyrus to facial identity and facial speech processing. Cortex. 2017;96:59–72. doi: 10.1016/j.cortex.2017.08.029. [DOI] [PubMed] [Google Scholar]
- 18.Rizzo M. Bálint's syndrome and associated visuospatial disorders. In: Kennard C, editor. Bailliere's International Practice and Research. Philadelphia: W B Saunders; 1993. pp. 415–37. [PubMed] [Google Scholar]
- 19.Rizzo M, Hurtig R. Looking but not seeing: Attention, perception, and eye movements in simultanagnosia. Neurology. 1987;37:1642–8. doi: 10.1212/wnl.37.10.1642. [DOI] [PubMed] [Google Scholar]
- 20.Dalrymple KA, Kingstone A, Barton JJ. Seeing trees OR seeing forests in simultanagnosia: Attentional capture can be local or global. Neuropsychologia. 2007;45:871–5. doi: 10.1016/j.neuropsychologia.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Rizzo M, Damasio H. Impairment of stereopsis with focal brain lesions. Ann Neurol. 1985;18:147. [Google Scholar]
- 22.Malcolm GL, Barton JJ. “Sequence Agnosia” in Bálint's syndrome: Defects in visuotemporal processing after bilateral parietal damage. J Cogn Neurosci. 2007;19:102–8. doi: 10.1162/jocn.2007.19.1.102. [DOI] [PubMed] [Google Scholar]
- 23.Liu RR, Pancaroglu R, Hills CS, Duchaine B, Barton JJ. Voice recognition in face-blind patients. Cereb Cortex. 2016;26:1473–87. doi: 10.1093/cercor/bhu240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reinhard J, Schreiber A, Schiefer U, Kasten E, Sabel BA, Kenkel S, et al. Does visual restitution training change absolute homonymous visual field defects? A fundus controlled study. Br J Ophthalmol. 2005;89:30–5. doi: 10.1136/bjo.2003.040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papageorgiou E, Hardiess G, Mallot HA, Schiefer U. Gaze patterns predicting successful collision avoidance in patients with homonymous visual field defects. Vision Res. 2012;65:25–37. doi: 10.1016/j.visres.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Spitzyna GA, Wise RJ, McDonald SA, Plant GT, Kidd D, Crewes H, et al. Optokinetic therapy improves text reading in patients with hemianopic alexia: A controlled trial. Neurology. 2007;68:1922–30. doi: 10.1212/01.wnl.0000264002.30134.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davies-Thompson J, Fletcher K, Hills C, Pancaroglu R, Corrow SL, Barton JJ. Perceptual learning of faces: A rehabilitative study of acquired prosopagnosia. J Cogn Neurosci. 2017;29:573–91. doi: 10.1162/jocn_a_01063. [DOI] [PubMed] [Google Scholar]