Abstract
A large part of the central nervous system is involved in the normal functioning of the vision, and hence vision can be affected in a stroke patient. Transient visual symptoms can likewise be a harbinger of stroke and prompt rapid evaluation for the prevention of recurrent stroke. A carotid artery disease can manifest as transient monocular visual loss (TMVL), central retinal artery occlusion (CRAO), anterior ischemic optic neuropathy or ocular ischemic syndrome (OIS). Stroke posterior to the optic chiasm can cause sectoranopias, quadrantanopias, or hemianopias, which can be either congruous or incongruous. Any stroke involving the dorsal stream (occipito-parietal lobe), or ventral stream (occipito-temporal lobe) can manifest with visuospatial perception deficits. Similarly, different ocular motility abnormalities can result from a stroke affecting the cerebrum, cerebellum, or brainstem. Among these deficits, vision and perception disorders are more difficult to overcome. Clinical, experimental, and neuroimaging studies have helped us to understand the anatomical basis, physiological dysfunction, and the underlying mechanisms of these neuro-ophthalmic signs.
Keywords: Dorsal stream, neuro-ophthalmic signs, quadrantanopias, vision
INTRODUCTION
Neurologists or physicians may be the first point of medical contact for patients presenting with neuro-ophthalmic disorders. The eye is a “window to the brain,” and a thorough ocular examination can assist clinicians to understand the mechanism of acute neurologic deficits. Poststroke visual impairment is common. It is seen in 48% stroke admissions and 60% of stroke survivors with 56% presenting impaired central vision, 40% eye movement abnormalities, 28% visual field loss, 27% visual inattention, and 5% with visual perceptual disorders.[1]
Purpose of review
The objective of this review was to help the clinician to identify common neuro-ophthalmic signs in stroke patients, which will aid in ordering appropriate investigations and making a therapeutic and rehabilitation plan.
METHOD
We searched the literature for studies describing “Eye signs and stroke” from Jan 2002 to March 2022 in PubMed databases. PubMed search was done using ('Eye signs in stroke'[Title/Abstract]) OR ('Eye signs and stroke'[Title/Abstract]) OR ('Neuro ophthalmic manifestations of stroke'[Title/Abstract]) OR ('Neuro ophthalmic manifestations and stroke'[Title/Abstract]) OR 'Higher cortical visual disorders'[Title/Abstract]). We included review articles, original articles, case series, and case reports. Conference presentations were excluded. A search with the keywords mentioned above led to 1,924 articles from PubMed for “Eye signs and stroke”,1026 articles for 'Higher cortical visual disorders' and 129 articles for 'Neuro ophthalmic manifestations of stroke' The abstracts were reviewed by the authors and articles relevant for this review were selected. Thirty-five articles and three textbook references were included for this review.
RESULTS
This review will cover common eye signs in stroke patients, anatomical localization, underlying neural mechanisms and how to elicit them. The algorithm for examining eye signs in a patient with stroke has been depicted in Figure 1.
Figure 1.
Algorithm for examining eye signs in a patient with stroke
Eye signs have been divided into the following categories
Eye signs related to afferent visual system
Eye signs related to higher cortical function
Eye signs related to efferent visual system
I. EYE SIGNS RELATED TO AFFERENT VISUAL SYSTEM
Ophthalmic symptoms and signs may occasionally be the only manifestation of a stroke/transient ischemic attack (TIA) due to carotid artery disease. Hemianopia may be a clue to a stroke involving the retro chiasmal visual pathway and rarely the chiasm.
Transient monocular visual loss or amaurosis fugax
Emboli originating in the carotid artery can enter the ophthalmic artery and can cause either a transient monocular visual loss (TMVL) or amaurosis fugax. A “curtain” or “shade” dropping over the vision is the traditional description.[2] This is because the arteries supplying the inferior retina are more susceptible to ischemia. The duration of these episodes ranges from a few seconds to a few minutes, and they rarely exceed 15 minutes.
Iris diaphragm type of TMVL
It should be noted that the visual symptoms experienced with internal carotid artery (ICA) hypoperfusion unlike in amaurosis fugax results in the constriction of the visual fields start from the periphery and moving toward the centre.[2]
Retinal hypoperfusion due to internal carotid stenosis can also cause Bright light amaurosis or transient amaurosis after exposure to bright light (retinal claudication).[3] Transient amaurosis brought on by a change in head position, a heavy meal or exercise may be other features.[4]
Examination of the face
Prominent pulsation of the superficial temporal artery can occur due to compensatory hyper-perfusion of the ECA with a subjective bruit over the ipsilateral ear. It can be reduced by compressing the temporal artery (Olivares' external carotid sign).[5] Dilated episcleral arteries may be seen due to retrograde filling of the ophthalmic arteries.[6]
Visual field examination
Paracentral Scotoma
Central retinal artery branch occlusions (CRABO) where only the upper, lower, nasal, or temporal portion of the retina may be involved, and a small central or paracentral scotoma may be the only manifestation of a carotid disease.[7]
Contralateral homonymous superior quadrantanopia, (pie-in-the-sky) along with seizure, memory deficits and sensory aphasia can occur with inferior temporal lobe infarction (Meyer's Loop) [Figure 2, lesion D].
Figure 2.

Visual pathway from retina to occipital cortex. The visual field defects associated with damage to different parts of the visual pathway are shown
Homonymous inferior quadrantanopia (pie-on-the-floor) can occur on the contralateral side along with hemineglect with superior parietal lobe infarction [Figure 2, lesion E].
Bitemporal hemianopia. Strokes involving the optic chiasma (supplied by branches from the Circle of Willis) are rare and can cause bitemporal hemianopia [Figure 2, lesion B].
Incongruous hemianopias can arise from the optic tract and lateral geniculate body (LGB) ischemia, and they become more congruous with more posteriorly placed lesions since the fibers of the visual pathway converge in the occipital lobe.[8]
Congruous hemianopia with macular sparing can occur with occipital lobe lesions. The central field of vision is spared due to the dual blood supply at the macula from the posterior cerebral artery (PCA) and middle cerebral artery (MCA) branches.[9] Kinetic testing using a 5 mm red bead and static finger wiggle testing is more sensitive to pick up field deficits than the confrontation visual field testing, and a formal perimetry may be needed if the clinical suspicion is high [Figure 2, lesion G].[10]
Fundus examination
Different types of micro-emboli may be seen in the retinal arterioles, which may give a clue to the stroke etiology.
Hollenhorst plaques: Cholesterol crystals from eroded atheromas. Irregular, bright glistening retractile, and orange-yellow
Platelet-fibrin emboli: Grayish-white nonrefractile plugs
Calcific emboli: Greyish-white
Cherry-red spot and box car appearance
In central retinal artery occlusion (CRAO) an emboli from the carotid can get lodged at the lamina cribrosa (the narrowest part of ophthalmic artery) and can manifest as a sudden, painless visual loss with an afferent pupillary defect. Subsequently, a slow streaming of flow in the retinal veins causing a box car appearance can be appreciated on fundus examination.[11]
Ocular ischemic syndrome (OIS) or “venous stasis retinopathy.” It can occur due to chronic ipsilateral carotid stenosis and present with pain (which may be relieved on assuming a supine position) or gradual visual loss. Most of the changes caused by OIS can be seen with diabetes and hypertension, but the presence of marked asymmetry in the findings can be a clue that the changes are more likely due to carotid narrowing.[12]
II. EYE SIGNS RELATED TO HIGHER CORTICAL FUNCTIONS
Alexia without agraphia:
It is defined as a selective (pure) reading impairment, as it occurs in the absence of more general language deficits, dementia, or general visual agnosia.[13] Writing is preserved, although patients may have trouble writing longer paragraphs as they cannot read back what they have immediately written.
Lesion anatomy in pure alexia:
Dejerine and Bub et al. had identified the left mesial occipital cortex, posterior ventral occipito-temporal cortex (vOT) cortex and splenium as the site of lesion in patients with pure alexia.[14] Since reading depends on centers within the left brain, visual input to those centers can be disrupted by a combination of a left occipital lesion causing right hemianopia and a callosal lesion disrupting the transfer of information [Figure 3].[15]
Figure 3.

Alexia without agraphia localization and anatomy. (Adapted from: Alexia without Agraphia as a Manifestation of Posterior Reversible Encephalopathy Syndrome. Can J Neurol Sci. 2021 Sep; 48 (5):727-9.)[15]
Etiology: Most common cause of this syndrome is PCA territory stroke. Other causes are 1) multiple sclerosis 2) glioblastoma 3) acute encephalopathy, and 4) posterior reversible encephalopathy syndrome (PRES).[15]
Anton Syndrome
The main features of this syndrome are agnosia, anosognosia and confabulation. As with all cases of post-geniculate vision loss, the pupillary responses and optic nerve appearance on fundoscopy remain normal. Bilateral PCA occlusion is the most common cause of cortical blindness.
Visual agnosia
It is defined as a neuropsychological disorder characterized by failure to recognize familiar objects in patients who apparently have adequate sensory functions (e.g., normal vision and normal sensitivity to luminance in their visual fields), and this disorder cannot be explained by other cognitive deficits like aphasia, poor semantic memory, or low intellectual capacity.[16] Visual Agnosia has been divided into 1) Apperceptive visual agnosia 2) Associative visual agnosia
Differentiating two forms of agnosia is shown in Table 1
Table 1.
Differentiating the forms of visual agnosia
| Apperceptive | Associative | |
|---|---|---|
| Object feature description | × | ✓ |
| Visual identification | × | × |
| Copying line drawings | × | ✓ |
| Object matching | × | ✓ |
| Object knowledge (from name) | ✓ | × |
| Tactile naming | ✓ | × |
(Adapted from John R. Hodges. Testing cognitive function at the bedside. In: Cognitive Assessment for Clinicians. 3rd Ed. Oxford University Press; Oct 2017)[17]
Testing visual agnosia
Visual agnosia is diagnosed by examining the patient's capacity to name an object, describe its use, and pantomime the usage of visually presented objects.
Matching Test:
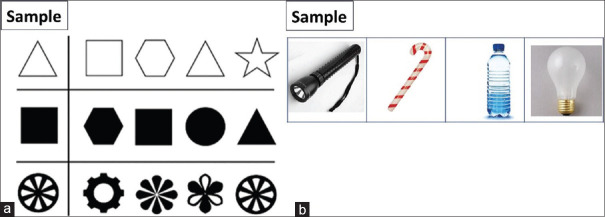
(a) View-matching task: In this test, the patient has to match the sample to the target image, which is presented along with similar-looking objects. Patients with apperceptive agnosia cannot complete this task correctly as they are not able to generate a coherent visual perception of an object [Figure 4].
Figure 4.
Illustration of matching tasks in which agnostic patients depending on type of agnosia typically show deficits. (a) View-matching task: (b) Function-match task
(b) Function-match task: The subject has to match those two pictures that share a common function. Deficits in performing this task are distinguishing features of associative agnostic patients [Figure 4].
Cerebral Achromatopsia
These deficits arise when a lesion disrupts area V4 in the inferior occipital cortex, which is specialized for color vision processing in the contralateral hemifield [Figure 5a]. The area V4 also plays an important role in processing forms, such as the aspect ratio and spatial frequencies of a visual stimulus.[18]
Figure 5.

A lesion circumscribed to area V4 in the inferior occipital cortex [area highlighted in red]. (5a) Loss of color vision perception in the contralateral homonymous visual field. (5b)
Clinical Presentation: Patients with hemiachromatopsia presents with altered color perception in which the deficit is limited to one homonymous visual field. It is an isolated disorder of color perception, without other visual deficits (visual acuity, brightness, etc.) [Figure 5b].
Testing: Hemiachromatopsia is tested by moving a colored object (a pin with a red ball at the top) from one visual field to the other and asking the patients if there are any changes in the color of the object.[19]
Prosopagnosia
It is a specific form of visual agnosia in which face perception is impaired while elementary aspects of vision, such as acuity and visual field, remain intact. More recently, studies using Positron-emission tomography (PET) and functional magnetic resonance imaging (fMRI) have demonstrated that face recognition areas are located in the fusiform face area, which is part of the fusiform (occipitotemporal) gyrus.[20]
Etiology: 1) Bilateral posterior cerebral infarcts 2) head trauma 3) herpes encephalitis, particularly if bilateral 4) tumors 5) lobar hemorrhages, and 6) surgical resections for epilepsy. Progressive forms can occur with focal temporal atrophy from frontotemporal degeneration.
Testing of Prosopagnosia
To diagnose prosopagnosia, the clinician should first confirm that the patient does not have a general visual agnosia, by administering a basic object recognition test. The Boston Naming Test is commonly used for this purpose. Patients are presented with images of celebrities and unknown people and asked to indicate which faces look familiar. A patient with prosopagnosia has decreased ability to make this distinction.[21]
Bálint syndrome
Diagnosis of the Bálint syndrome requires the presence of all three core symptoms: simultanagnosia, optic ataxia, and ocular apraxia. Each symptom can be found in patients without the others, indicating that they result from damage to independent processing networks that have in common a spatial analysis of vision. Clinically, it is recognized by deficits in simultaneously processing multiple items resulting in the poor interpretation of complex visual scenes, deficits in visually guided limb movements, eye movements, and sensory inattention.[22]
Tests for simultanagnosia: Navon letter and lady cooking on chullah can be used to test simultanagnosia. A patient with simultanagnosia see only small letter and ignore large letter when asked to describe a Navon letter, and similarly, the interpretation of the number of people present is slow and often inadequate when they are asked to describe a lady cooking on chullah [See Figure 6].[21,23]
Figure 6.

(a) Navon letter (b) lady cooking on challah. (Adapted from Prasad K, Dash D, Kumar A. Validation of the Hindi version of National Institute of Health Stroke Scale. Neurol India. 2012 Jan-Feb; 60 (1):40-4.)[23]
Optic ataxia
It is an inability to accurately point to or clench objects under visual guidance. The lesions that cause optic ataxia are present in the occipitoparietal area involving the junction between the superior occipital cortex and the inferior parietal lobule.[21]
Acquired Ocular Motor Apraxia
Bilateral lesions can cause a defect in the initiation and guidance of saccades to visual targets. Patients with severe acquired ocular motor apraxia may have trouble generating any saccades in response to a visual stimulus on command, however saccades can sometimes be helped by blinking; this difficulty is also known as psychic paralysis of gaze.[21]
Cerebral Akinetopsia
Akinetopsia is a condition in which a moving item appears to “jump” from one stationary position to another due to an inability to detect motion. Cerebral akinetopsia is a relatively unusual condition caused by bilateral lesions in the human visual motion area (V5/MT).
III. EYE SIGNS RELATED TO EFFERENT VISUAL SYSTEM
Stroke can affect the efferent visual system, including the extraocular muscles, cranial nerves or the supranuclear pathways, either individually or in combination producing a spectrum of ophthalmological symptoms.
1. Ptosis:
Pure midbrain strokes constitute about 0.6-2.3% of total strokes.[24] Infarction of the paramedian midbrain or the oculomotor fasciculus can manifest with isolated unilateral ptosis without ophthalmoplegia or pupillary involvement.
2. Oculomotor dysmotility:
-
Oculomotor nerve (CN III) palsy was observed to have the highest rate of occurrence in patients with stroke, according to a retrospective review.[25] Ptosis, diplopia, mydriasis and gaze palsies, along with hemiparesis or cerebellar signs may occur. Midbrain infarction results in a constellation of ipsilateral third nerve palsy with contralateral hemiparesis called Weber's syndrome.
An aneurysm of the posterior communicating artery (PCOM), which lies in close proximity to the CN III can result in external compression resulting in mydriasis and ophthalmoparesis.
Diplopia is a common presenting complaint after stroke. According to a prospective study, diplopia was seen in 16.5% of patients after stroke.[26] Involvement of the IIIrd, IVth, or VIth cranial nerves can result in horizontal or vertical ocular misalignment presenting as diplopia. The rate of occurrence of diplopia is equal with the involvement of either cerebral hemisphere.
Internuclear Ophthalmoplegia: INO is characterized by ipsilateral impaired adduction, partial or complete, along with a contralateral horizontal nystagmus of the abducting eye. It occurs due to a lesion of the medial longitudinal fasciculus, which connects the ipsilateral CN III nucleus to the contralateral paramedian pontine reticular formation (PPRF) and the adjacent CN VI nucleus [Figure 7].[27] Infarction of the MLF is the most common etiology of INO in the elderly.[26]
-
Gaze Palsies: The most common ocular motility dysfunction seen after a stroke is gaze palsy reported in 18-44% of patients.[28] Horizontal gaze palsy is more common than vertical gaze palsy, and complete palsy is more than partial.[29] A stroke involving the PPRF at the pons can result in an ipsilateral horizontal gaze palsy, whereas that involving the frontal cortex rostral to the precentral cortex can result in an interruption of input from the frontal eye fields with a subsequent contralateral gaze palsy.
The Rostral interstitial nuclei of the medial longitudinal fasciculus (riMLF) are the final center for the integrated neural pathways for vertical gaze. Midbrain and thalamic infarcts can result in vertical gaze defects.[29] The constellation of impaired upgaze, lid retraction (Collier sign), convergence-retraction nystagmus, downward gaze preference (setting-sun sign), light-near dissociation (poor response of pupils to light but better to accommodation) is called Parinaud syndrome or dorsal midbrain syndrome. It occurs usually due to a pineal tumor compressing the midbrain, or an infarct or tumor of the midbrain pretectum. Complete vertical gaze palsy is indicative of bilateral riMLF lesions.
-
One-and-a-half syndrome:
The combination of an ipsilateral horizontal gaze palsy (one) with ipsilateral INO (a half) is termed one and a half syndrome. This in combination with ipsilateral facial nerve involvement is called eight and a half syndrome.[30] Brainstem infarction followed by demyelinating disorders like multiple sclerosis are among the most common causes.
Saccades: Saccades are rapid, conjugate, voluntary eye movements that help shift your gaze toward an object of interest. A right frontal lobe lesion will result in a transient left gaze palsy with a right gaze preference, which resolves when the PPRF regains saccadic control. Brainstem lesions can also cause saccadic defects. Ipsilateral hypermetric saccades with contralateral hypometric saccades can occur with infarctions of the brainstem and cerebellum.
Pursuits: The pursuit system allows the eyes to smoothly follow a moving object. The neural circuit of the smooth pursuit is a subject of debate. There is some evidence to suggest that descending projections from the primary visual cortex, frontal eye fields, temporo-parieto-occipital region, are likely involved in the initiation of pursuit,[31] with inputs from pons and cerebellum. Ipsilateral occipitoparietal lesions can result in ipsilateral impaired smooth pursuit. Cerebellar infarctions involving the vermis have also been reported to inhibit smooth pursuits.[32]
Nystagmus: Cerebellar stroke is reported to have the highest association with acute nystagmus.[33] One study reported a 24% prevalence of nystagmus in patients with posterior circulation stroke.[34] Posteroinferior cerebellum infarction can result in bidirectional horizontal nystagmus, whereas the involvement of the superior vermis causes an upbeating nystagmus.
Strabismus and Vergence: Strabismus has been reported to occur in 16.5- 50% of patients[35,36] with stroke, with a higher prevalence (69%) in cortical strokes. Exotropia is the most common (74%) type of strabismus noted.[37] Convergence insufficiency has been reported in almost 55% patients after stroke.[38]
Figure 7.

Neural pathway of horizontal eye movements. (Adapted from Alberstone CD, Benzel EC, Najm IM, Steinmetz MP, editors. Anatomic Basis of Neurologic Diagnosis. 1st ed. New York, NY: Thieme Medical Publishers; 2009. p. 427.)[27]
SUMMARY
The first contact physician needs to be vigilant as visual symptoms and signs may be the only manifestation of stroke. Visual symptoms in stroke patients can occur as vision loss, visual perceptual disorders, or disorders of ocular motility. Elicitation of different eye signs in stroke patients not only helps in anatomical localization and ascertaining etiology but also guide in making a diagnostic and therapeutic plan.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rowe FJ, Hepworth LR, Howard C, Hanna KL, Cheyne CP, et al. High incidence and prevalence of visual problems after acute stroke: An epidemiology study with implications for service delivery. PLoS One. 2019;14:e0213035. doi: 10.1371/journal.pone.0213035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruno A, Corbet JJ, Biller J. Transient monocular visual loss patterns and associated vascular abnormalities. Stroke. 1990;21:34–9. doi: 10.1161/01.str.21.1.34. [DOI] [PubMed] [Google Scholar]
- 3.Furlan AJ, Whisnant JP, Kearns TP. Unilateral visual loss in bright light. An unusual symptom of carotid artery occlusive disease. Arch Neurol. 1979;36:675–6. doi: 10.1001/archneur.1979.00500470045007. [DOI] [PubMed] [Google Scholar]
- 4.Arthur A, Alexander A, Bal S, Sivadasan A, Aaron S. Ophthalmic masquerades of the atherosclerotic carotids. Indian J Ophthalmol. 2014;62:472–6. doi: 10.4103/0301-4738.121183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gregory YC. Elimination of subjective bruit with compression of temporal artery: New physical sign indicative of internal carotid artery occlusion. Stroke. 1984;15:903–4. doi: 10.1161/01.str.15.5.903. [DOI] [PubMed] [Google Scholar]
- 6.Countee RW, Gnanadev A, Chavis P. Dilated episcleral arteries: A significant physical finding in assessment of patients with cerebrovascular insufficiency. Stroke. 1978;9:42–5. doi: 10.1161/01.str.9.1.42. [DOI] [PubMed] [Google Scholar]
- 7.Chawluk JB, Kushner MJ, Bank WJ, Silver FL, Jamieson DG, Bosley TM, et al. Atherosclerotic carotid artery disease in patients with retinal ischemic syndromes. Neurology. 1988;38:858–63. doi: 10.1212/wnl.38.6.858. [DOI] [PubMed] [Google Scholar]
- 8.Goodwin D. Homonymous hemianopia: challenges and solutions. Clin Ophthalmol. 2014;8:1919–27. doi: 10.2147/OPTH.S59452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glisson CC. Visual loss due to optic chiasm and retrochiasmal visual pathway lesions. Continuum. 2014;20:907–21. doi: 10.1212/01.CON.0000453312.37143.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerr NM, Chew SS, Eady EK, Gamble GD, Danesh-Meyer HV. Diagnostic accuracy of confrontation visual field tests. Neurology. 2010;74:1184–90. doi: 10.1212/WNL.0b013e3181d90017. [DOI] [PubMed] [Google Scholar]
- 11.Sharma S, ten Hove MW, Pinkerton RM, Cruess AF. Interobserver agreement in the evaluation of acute retinal artery occlusion. Can J Ophthalmol. 1997;32:441–4. [PubMed] [Google Scholar]
- 12.Mizener JB, Podhajsky P, Hayreh SS. Ocular ischemic syndrome. Ophthalmology. 1997;104:859–64. doi: 10.1016/s0161-6420(97)30221-8. [DOI] [PubMed] [Google Scholar]
- 13.Starrfelt R, Shallice T. What's in a name? The characterization of pure alexia. Cogn Neuropsychol. 2014;31:367–77. doi: 10.1080/02643294.2014.924226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Starrfelt R, Woodhead Z. Reading and alexia. Handb Clin Neurol. 2021;178:213–32. doi: 10.1016/B978-0-12-821377-3.00010-6. [DOI] [PubMed] [Google Scholar]
- 15.Cheema I, Chen T. Alexia without agraphia as a manifestation of posterior reversible encephalopathy syndrome. Can J Neurol Sci. 2021;48:727–9. doi: 10.1017/cjn.2020.251. [DOI] [PubMed] [Google Scholar]
- 16.Gerlach C, Robotham RJ. Object recognition and visual object agnosia. Handb Clin Neurol. 2021;178:155–73. doi: 10.1016/B978-0-12-821377-3.00008-8. [DOI] [PubMed] [Google Scholar]
- 17.Hodges JR. Cognitive Assessment for Clinicians. 3rd. Oxford, UK: Oxford University Press; 2017. [Last accessed on 2022 Feb 14]. Testing cognitive function at the bedside. Available from: https://oxfordmedicine_com/view/10.1093/med/9780198749189.001.0001/med-9780198749189-chapter-5 . [Google Scholar]
- 18.Desimone R, Schein SJ. Visual properties of neurons in area V4 of the macaque: sensitivity to stimulus form. J Neurophysiol. 1987;57:835–68. doi: 10.1152/jn.1987.57.3.835. [DOI] [PubMed] [Google Scholar]
- 19.Hart J., Jr . The Neurobiology of Cognition and Behavior. Oxford, UK: Oxford University Press; 2015. [Last accessed on 2022 Feb 14]. Higher-Order visual processing. Available from: https://oxfordmedicine_com/view/10.1093/med/9780190219031.0010.0001/med-9780190219031-chapter-7 . [Google Scholar]
- 20.Tsao DY, Livingstone MS. Mechanisms of face perception. Annu Rev Neurosci. 2008;31:411–37. doi: 10.1146/annurev.neuro.30.051606.094238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barton JJ. Higher cortical visual deficits. Continuum. 2014;20:922–41. doi: 10.1212/01.CON.0000453311.29519.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chechlacz M. Bilateral parietal dysfunctions and disconnections in simultanagnosia and Bálint syndrome. Handb Clin Neurol. 2018;151:249–67. doi: 10.1016/B978-0-444-63622-5.00012-7. [DOI] [PubMed] [Google Scholar]
- 23.Prasad K, Dash D, Kumar A. Validation of the hindi version of national institute of health stroke scale. Neurol India. 2012;60:40–4. doi: 10.4103/0028-3886.93587. [DOI] [PubMed] [Google Scholar]
- 24.Sugawara E, Nakamura H, Endo M, Tanaka F, Takahashi T. Isolated unilateral ptosis due to paramedian midbrain infarction. J Stroke Cerebrovasc Dis. 2015;24:e121–3. doi: 10.1016/j.jstrokecerebrovasdis.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 25.Ciuffreda KJ, Kapoor N, Rutner D, Suchoff IB, Han ME, Craig S. Occurrence of oculomotor dysfunctions in acquired brain injury: A retrospective analysis. Optometry. 2007;78:155–61. doi: 10.1016/j.optm.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Rowe F. VIS group UK. The profile of strabismus in stroke survivors. Eye. 2010;24:682–5. doi: 10.1038/eye.2009.138. [DOI] [PubMed] [Google Scholar]
- 27.Alberstone CD, Benzel EC, Najm IM, Steinmetz MP, editors. 1st. New York, NY: Thieme Medical Publishers; 2009. Anatomic Basis of Neurologic Diagnosis; p. 427. [Google Scholar]
- 28.Singer OC, Humpich MC, Laufs H, Lanfermann H, Steinmetz H, Neumann-Haefelin T. Conjugate eye deviation in acute stroke: Incidence, hemispheric asymmetry, and lesion pattern. Stroke. 2006;37:2726–32. doi: 10.1161/01.STR.0000244809.67376.10. [DOI] [PubMed] [Google Scholar]
- 29.Rowe FJ, Wright D, Brand D, Jackson C, Harrison S, Maan T, et al. Profile of gaze dysfunction following cerebrovascular accident. ISRN Ophthalmol. 2013;2013:264604. doi: 10.1155/2013/264604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bocos-Portillo J, Ruiz Ojeda J, Gomez-Beldarrain M, Vazquez-Picon R, Garcia-Monco JC. Eight-and-a-half syndrome. JAMA Neurol. 2015;72:830. doi: 10.1001/jamaneurol.2015.0255. [DOI] [PubMed] [Google Scholar]
- 31.Pedersen RA, Troost BT. Abnormalities of gaze in cerebrovascular disease. Stroke. 1981;12:251–4. doi: 10.1161/01.str.12.2.251. [DOI] [PubMed] [Google Scholar]
- 32.Baier B, Stoeter P, Dieterich M. Anatomical correlates of ocular motor deficits in cerebellar lesions. Brain. 2009;132:2114–24. doi: 10.1093/brain/awp165. [DOI] [PubMed] [Google Scholar]
- 33.Baier B, Dieterich M. Incidence and anatomy of gaze-evoked nystagmus in patients with cerebellar lesions. Neurology. 2011;76:361–5. doi: 10.1212/WNL.0b013e318208f4c3. [DOI] [PubMed] [Google Scholar]
- 34.Searls DE, Pazdera L, Korbel E, Vysata O, Caplan LR. Symptoms and signs of posterior circulation ischemia in the New England medical center posterior circulation registry. Arch Neurol. 2012;69:346–51. doi: 10.1001/archneurol.2011.2083. [DOI] [PubMed] [Google Scholar]
- 35.Maeshima S, Osawa A, Miyazaki Y, Takeda H, Tanahashi N. Functional outcome in patients with pontine infarction after acute rehabilitation. Neurol Sci. 2012;33:759–64. doi: 10.1007/s10072-011-0812-0. [DOI] [PubMed] [Google Scholar]
- 36.Su C-H, Young Y-H. Clinical significance of pathological eye movements in diagnosing posterior fossa stroke. Acta Otolaryngol. 2013;133:916–23. doi: 10.3109/00016489.2013.783716. [DOI] [PubMed] [Google Scholar]
- 37.Rowe F, VIS Group UK. Prevalence of ocular motor cranial nerve palsy and associations following stroke. Eye (Lond) 2011;25:881–7. doi: 10.1038/eye.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clisby C. Visual assessment of patients with cerebrovascular accident on the elderly care wards. Br Orthop J. 1995;52:38–40. [Google Scholar]