Abstract
Malaria-associated cardiac injury has been reported to be the primary cause of death due to severe malaria. The discovery of substances showing a protective effect on cardiac injury during malaria infection is urgently needed. Hence, the purpose of this study was to evaluate the efficacy of Gymnema inodorum leaf extract (GIE) on cardiac function in mice infected with Plasmodium berghei. ICR mice were treated with 1 × 107 infected red blood cells of P. berghei ANKA (PbANKA), administered orally with GIE in 100, 250 and 500 mg/kg body weight of mice. Creatine phosphokinase (CPK) and echocardiography were carried out. It was found that CPK and heart-weight to body-weight (HW/BW) ratios were significantly higher in untreated mice than the healthy control. Moreover, impaired cardiac function in the untreated group was observed as indicated by changes in echocardiography. Interestingly, GIE exerted a protective effect on cardiac injury induced by PbANKA infection. Our results demonstrated that the parasitemia percentage, CPK, HW/BW ratio, and echocardiography in GIE treated mice were improved. However, there was no significant difference between GIE dosages. Therefore, GIE possessed a cardio-protective effect during malaria infection in mice.
Keywords: gymnema inodorum, malaria, pbANKA, cardiac function
Introduction
Malaria caused by protozoa called Plasmodium and transmitted by infected female Anopheles mosquito, is a major public health problem in tropical and subtropical countries. In 2020, they were an estimated 241 million cases of malaria and 627,000 malaria deaths, with children under five years old still the most vulnerable group affected. In 2020, malaria cases shot up by an additional 14 million and malaria deaths spiked by 47,000 due to the impact of COVID-19 on healthcare services, compared with pre-pandemic levels.1 Severe malaria can occur when the infection is complicated by serious multiple organ failures or abnormalities in blood or metabolism. It has been increasingly reported that the causes of severe manifestations of malaria, including cerebral malaria and severe anemia, are due to hemolysis, acute respiratory distress syndrome, acute kidney injury, thrombocytopenia, and abnormalities in blood coagulation, hypoglycemia, metabolic acidosis, liver failure, and jaundice.2,3 One of them is impaired cardiac function, which is the common cause of death in patients with malaria.4,5 The impairment of cardiovascular involvement in malaria is multifactorial and several hypotheses suggested the involvement of parasite sequestration and obstruction in microvascular coronary, myocardial injury caused by parasite-released proteins and inflammatory cytokines, and anemia.6,7 Endothelial dysfunction with impaired nitric oxide bioavailability by parasite compromised vascular permeability of cardiovascular system.8,9 This has prompted research toward the finding of new and affordable chemotherapies. In this respect, plant extracts are potential targets of research for alterative malarial drugs.
Gymnema inodorum (Lour.) Decne belongs to the family of Apocynaceae and is traditionally used for the management of various diseases. It is found ubiquitously throughout Southeast Asia, and its leaves and stems have been used as vegetable ingredients in many cuisines. This plant is also recognized as one of the major botanicals in diabetes treatment using the Ayurvedic system of medicine. It has been found that leaf extracts of G. inodorum in both water and ethanol showed high antioxidant activity with polyphenols and flavonoids as the active compounds.10 Gymnema sylvestre, also a member of Apocynaceae, is a wild herb found in India, Africa, Australia and China. The investigation of G. sylvestre in both in vitro and in vivo found pharmacological properties, including antioxidant, anti-inflammatory, anti-cancer, anti-diabetic, hypoglycemic, immunosuppressive, anti-bacterial, anti-fungal, anti-parasitic, anti-malarial, hepatoprotective and nephroprotective activities.11 In addition, the cardio-protective effect of G. sylvestre has also been reported. The active chemical ingredients of G. sylvestre have been investigated, such as polyphenols, flavonoids, triterpenoid saponin, tannins, quinones, anthraquinones, cardiac glycosides and gymnemic acid.12 However, very few studies on the effects of G. inodorum leaf extract have been conducted and cardio-protective activity in malaria model of this plant has not yet been reported. Hence, this study was aimed at investigating the cardio-protective effect of G. inodorum leaf extract in mice infected with Plasmodium berghei as in vivo model.
Materials and Methods
Plant Material Preparation and Extraction
The leaves of Gymnema inodorum were collected from the Chiangda Organic Garden, Chiang Mai, Thailand. Identification and authentication of the plant was done by a taxonomist at Chiang Mai University, where a voucher specimen was coded and deposited for further studies. For extraction, fresh leaves of G. inodorum were thoroughly washed with tap water and dried in a hot air oven with a temperature of 50 °C overnight. The dried powdered leaves were then prepared using an electric blender and extracted in distilled water (DW) at a proportion of 5 g%. To facilitate the extraction process, the mixture was incubated at 60 °C with a mechanical shaker for 15 mins. After centrifugation at 2500/min for 5 min, supernatant was collected and lyophilization was subsequently performed using Beta 1-8 LSCplus (Martin Christ Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany). Aqueous crude extract of G. inodorum leaves (GIE) was stored at −20 °C for further experiments. The percentage yield after freeze-drying was 17.43% (w/w).
Experimental Animals
Thirty-five healthy male ICR mice aged 6−8 weeks, weighing 30−35 g, obtained from Nomura Siam International Co., Ltd., were used. Mice were kept at room temperature (22 °C-26 °C) with a 12-h light/dark cycle and fed with pellet food and drinking water, ad libitum. They were acclimatized for 1 week before being used for the experiments. The first set of mice (n = 5) was infected with PbANKA and monitored for parasitemia percentage and CPK level for 10 days. The second set of mice (n = 30) was randomly divided into 6 groups: (i) healthy (H, n = 5); (ii) PbANKA-infected (untreated, UN, n = 5); (iii) PbANKA-infected + GIE 100 mg/kg body weight of mice (GI100, n = 5); (iv) PbANKA-infected + GIE 250 mg/kg body weight of mice (GI250, n = 5); (v) PbANKA-infected + GIE 500 mg/kg body weight of mice (GI500, n = 5); (vi) PbANKA-infected + chloroquine 10 mg/kg body weight of mice (CQ, n = 5). The animal care and handling was per the international guidelines for use and maintenance of experimental animals. The animal ethical approval was obtained from the Animal Ethics Committee Walailak University (EC no. WU-AICUC-63-031).
The activity of GIE on mice infected with PbANKA was carried out with the standard Peter's test as previously described. On the first day of treatment (Day 1), naïve ICR mice were inoculated with 1 × 107 infected RBC by intraperitoneal injection to generate PbANKA-infected mice. Three hours post-infection, the untreated control (UN) received DW 10 ml/kg body weight of mice, the treatment groups received GIE at 100, 250 and 500 mg/kg body weight of mice (GI100, GI250, GI500), and the positive control received chloroquine-CQ (10 mg/kg). GIE extracts at 100, 200 and 500 mg/kg body weight of mice and CQ at a dose of 10 mg/kg body weight of mice were freshly prepared. The dosages were adjusted at the time of administration according to the body weight of each mouse. The treatment was administered orally by gavage once a day for 4 consecutive days. On Day 4, echocardiography was performed. Blood smear was prepared from the tail of each mouse for parasitemia determination. Mice were sacrificed and the blood samples were then collected by cardiac puncture into heparinized vacuum tubes, and plasma was subsequently prepared for the measurement of CPK. Hearts were collected for cardiac histology.
Rodent Malaria Parasite and Parasite Inoculation
Plasmodium berghei strain ANKA (PbANKA) obtained from Malaria Research and Reference Reagent Resource Center (MR4) was used. Parasite was maintained by serial weekly passage in mice through intraperitoneal inoculation with 1 × 107 infected red blood cells (RBC). Parasitemia was monitored daily by microscopic examination of Giemsa-stained blood smear.
Parasitemia Determination
Determination of parasitemia was carried out by microscopic examination of Giemsa-stained blood smear. Tail vein of PbANKA-infected mice was smeared on microscopic slides and fixed with 100% methanol for 10s. Staining was then performed using 10% Giemsa solution and smears were incubated at room temperature for 10 mins. PbANKA-infected RBC was counted under light microscope with 100x oil immersion lens. Percent parasitemia was calculated according to the formula indicated below:
Echocardiography
To study the effect of GIE on cardiac function, different doses of GIE extract (100, 250 and 500 mg/kg body weight) and CQ were orally administered daily to the different groups for 4 days after malaria infection. Echocardiography was performed under 1−2% isoflurane anesthesia of PbANKA-infected mice with a Mindray DC-70 Ultrasound System (Mindray, Shenzhen, China). Left ventricular (LV) regional wall motion was scored in LV short axis view at the level of the papillary muscle for the 16 segments viewed. LV internal diameter end systole (LVIDs) and end diastole (LVIDd) were measured. End-systolic volume (ESV), LV fractional shortening (FS) and ejection fraction (EF) were obtained. The average of 3 consecutive cardiac cycles was used for each measurement, with the operator being blinded to treatment assignment.
Creatine Phosphokinase Measurement
After echocardiography was performed, mice were allowed to recover for a couple of hours, then mice were deeply anesthetized with Zoletil (50 mg/kg); chest was opened and blood was then collected from the hepatic vein in vacuum tubes containing heparin as anticoagulant. Plasma was subsequently prepared by centrifugation at 5000/min for 10 mins. Creatine phosphokinase (CPK) was measured using a clinical chemistry automated analyzer (Mindray Biochemistry BS Series, Shenzhen, China) with specific reagent kit from Mindray (Shenzhen, China).
Histological Examination
After blood collection, hearts were excised, briefly washed in cold 0.9% saline solution and preserved with 10% buffer formalin for paraffin embedment. Transverse sections of whole heart (3-μm thickness) were stained with hematoxylin and eosin (H&E) by Gemini AS tissue staining (Thermo Scientific). Evidence of malaria pigment deposit and vacuolar degeneration was visualized by 100x image Olympus cellSens software in 15−20 microscopic areas from 3 mice per group. Then, the pathological changes were analyzed using a score from 0 to 3 as follows: 0 = normal tissue without evidence of malaria pigment deposit and vacuolar degeneration; 1 = evidence of pathological features less than 25% of the field of view; 2 = evidence of pathological features 25% to 50% of the field of view; 3 = evidence of pathological features greater than 50% of the field of view.13
Data Analysis
All data were organized and analyzed using GraphPad Prism version 9.0 (GraphPad Prism software, Inc., USA). Results were presented as mean ± SEM (standard error of mean). Analysis at 95% confidence interval and p-value less than 0.05, using one-way analysis of variance (ANOVA), followed by Tukey's post-hoc test was considered to be statistically significant.
Results
Malaria-Associated Cardiac Injury Development During PbANKA-Infected Mice
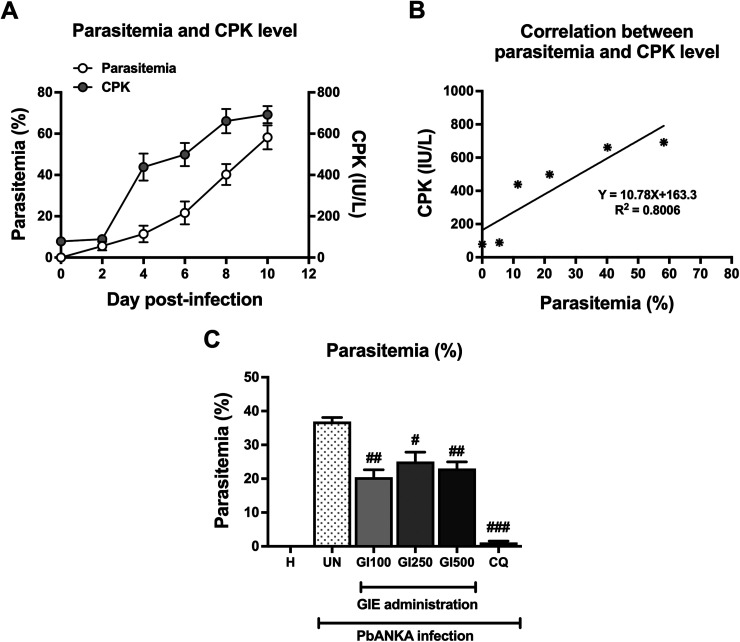
As shown in Figure 1A (Y left axis), parasitemia was first detected on Day 1 post-infection and reached 58 ± 5.8% on Day 10. Next, we observed that CPK level showed a progressive increase in response to the presence of PbANKA propagation, which reached notable levels starting on Day 4 post-infection (Figure 1A, Y right axis). Additionally, a positive correlation (R2 = 0.8006) between parasitemia and CPK level was observed (Figure 1B). Interestingly, on Day 4 of GIE treatment in PbANKA-infected mice, parasitemia percentage decreased significantly to 20 ± 1.0%, 25 ± 1.3% and 23 ± 0.9% in the GIE-treated groups of 100, 250 and 500 mg/kg body weight, respectively, compared with the untreated group (Figure 1C). There was no significant difference among GIE-treated groups, while CQ-treated group decreased the parasitemia to 1.0 ± 0.2%, compared with untreated group (p < 0.001, Figure 1C).
Figure 1.
Malaria-associated cardiac injury and effect of GIE on parasitemia. (A) Monitoring parasitemia percentage and CPK level during 10 days post-infection. (B) Positive correlation between parasitemia and CPK level. (C) GIE treatment reduced the parasitemia percentage in 4-day PbANKA-infected mice. Results were expressed as mean + SEM. H: healthy; UN: untreated; GI100, GI250, and GI500: GIE treatment of 100, 250 and 500 mg/kg body weight of mice, respectively; CQ: CQ treatment of 10 mg/kg body weight of mice.
#p < 0.05, ##p < 0.01 and ###p < 0.001, compared with UN.
Effect of GIE on Protection of Cardiac Injury in Mice with PbANKA Infection
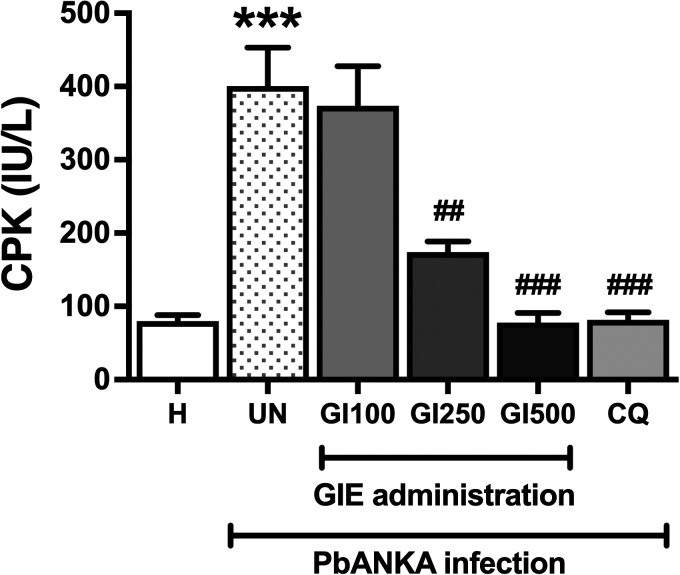
The effect of GIE treatment on malaria-induced cardiac injury was presented in Figure 2. CPK levels were observed to be significantly (p < 0.01) elevated in the untreated mice and the infected mice treated with GIE 100 mg/kg body weight, as compared with the healthy control. However, significant (p < 0.01) decreases of CPK levels were observed in the infected mice treated with GIE 250 and 500 mg/kg body weight, and CQ, compared with the untreated group. No significant difference among GIE treated mice (250 and 500 mg/kg) and CQ treated mice was observed.
Figure 2.
Effect of GIE on CPK level in PbANKA infection in mice. H: healthy; UN: untreated; GI100, GI250, and GI500: GIE treatment of 100, 250 and 500 mg/kg body weight of mice, respectively; CQ: CQ treatment of 10 mg/kg body weight of mice.
***p < 0.001 compared with H. ## p < 0.01 and ###p < 0.001, compared with UN.
Effect of GIE on LV Dysfunction in Mice with PbANKA Infection
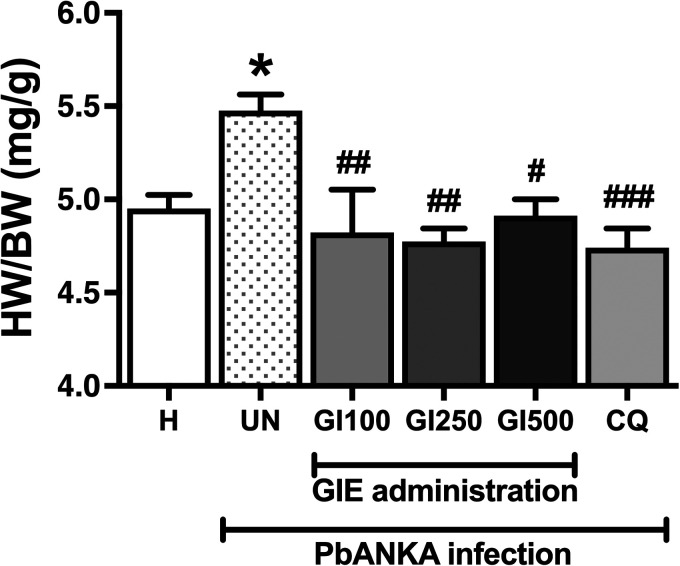
The heart-weight to body-weight (HW/BW) ratio averaged 5.0 ± 0.08 mg/g in healthy control and was significantly increased in the untreated group (to 5.5 ± 0.09 mg/g, p < 0.05, Figure 3). The administration of GIE at any dose significantly prevented PbANKA-induced increase in the HW/BW ratio. The efficiency of GIE administration was similar to CQ administration. However, there were no significant differences between GIE-treated groups and the CQ-treated group at any dose (100, 250 and 500 mg/kg).
Figure 3.
Effect of GIE on HW/BW in PbANKA infection in mice. H: healthy; UN: untreated; GI100, GI250 and GI500: GIE treatment of 100, 250 and 500 mg/kg body weight of mice, respectively; CQ: CQ treatment of 10 mg/kg body weight of mice.
*p < 0.05 compared with H. #p < 0.05, ##p < 0.01, and ###p < 0.001 compared with UN.
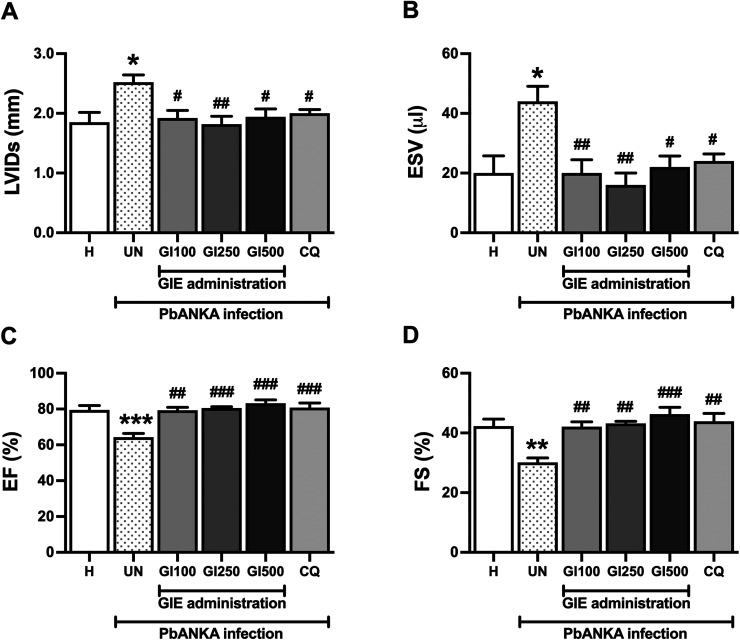
To study the effects of GIE on hemodynamic parameters, echocardiography was carried out. In PbANKA-infected mice, cardiac functions were deteriorated. LVIDs and ESV were increased (36% and 120% increase, respectively) while EF and FS were decreased (29% and 19% reduction, respectively) compared with the healthy control. GIE administration at doses of 100, 250 and 500 mg/kg improved malaria-induced LV dysfunction. However, there was no significant change among GIE groups. The effects of GIE administration were similar to the CQ-treated group (Figure 4).
Figure 4.
Effect of GIE on cardiac function in PbANKA infection in mice. Echocardiography was performed, as indicated by the parameters, including (A) LVIDs, (B) ESV, (C) EF, and (D) FE. Results were expressed as mean + SEM. H: healthy; UN: untreated; GI100, GI250 and GI500: GIE treatment of 100, 250 and 500 mg/kg body weight of mice, respectively; CQ: CQ treatment of 10 mg/kg body weight of mice.
*p < 0.05, **p < 0.01, and ***p < 0.001 compared to H. #p < 0.05, ##p < 0.01, and ###p < 0.001 compared with UN. LVIDs, left ventricular internal diameter end systole; LVIDd, left ventricular internal diameter end diastole; ESV, end-systolic volume; FS, fractional shortening (FS); EF, ejection fraction.
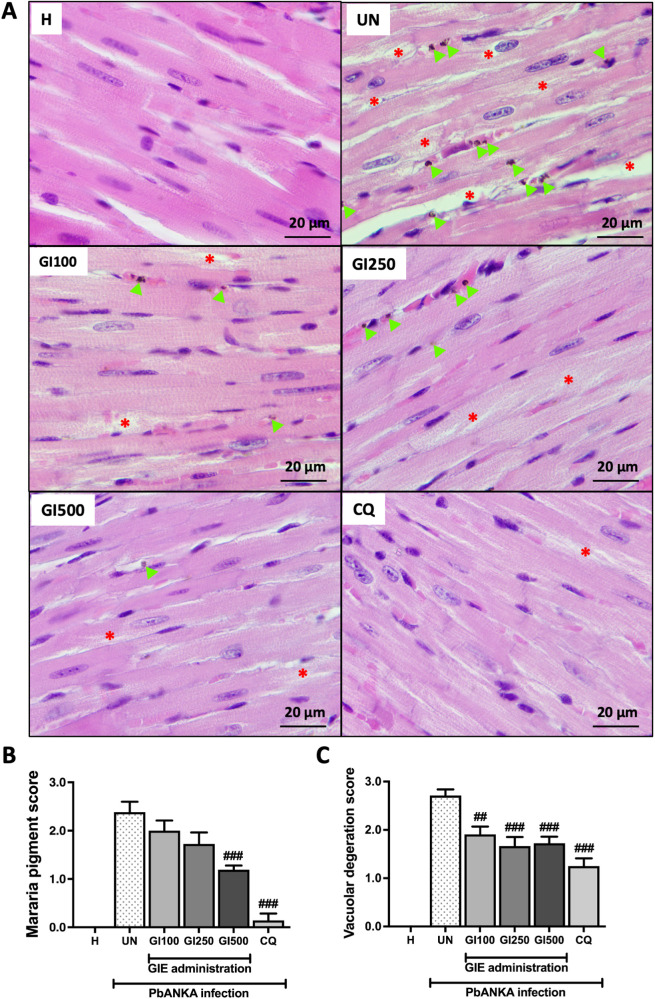
Effect of GIE on Pathological Changes in Hearts of Mice with PbANKA Infection
After 4 days of PbANKA infection, malaria deposit and vacuolar degeneration were observed in the heart using H&E staining. The score was categorized from 0 to 3. Malaria pigment deposit was detected in the transverse section of whole heart in PbANKA-infected mice (Figure 5A and B). Administration of GIE at 500 mg/kg and CQ significantly decreased parasite deposit compared with untreated group. Vacuolar degeneration was observed in untreated group (Figure 5A). Administration of GIE at any dose significantly decreased vacuolar degeneration (100, 250 and 500 mg/kg) compared with untreated group, similar to CQ administration (Figure 5C).
Figure 5.
Microscopic observation of H&E staining. (A) Transverse sections of whole heart observed malaria pigment deposit (green arrow) and vacuolar degeneration (star) under 100x microscope in healthy (H), untreated (UN), GIE-treated (GI100, GI250 and GI500) and CQ-treatment. (B) Represents score of malaria pigment deposit and (C) shows score of vascular degeneration from 3 mice. Scale bar: 20 μm.
##p < 0.01, and ###p < 0.001 compared with UN.
Discussion
In this study, we investigated cardiac function using CPK as a cardiac biomarker and echocardiography in PbANKA-infected mice with GIE administered orally. Malaria-associated cardiac injury and dysfunction during PbANKA infection were observed as indicated by a significant elevation of CPK, HW/BW ratio and alteration of echocardiography.
In severe malaria with complicated falciparum, higher circulation of cardiac proteins, such as troponin T (TnT), myoglobin and CPK levels, as well as lactic acidosis and hypoglycemia, have been observed in myocardial injury.14 The inflammatory characteristic of PbANKA infection increases cytokine levels, such as TNF, IL-1β, and IFN-γ, in combination with high oxidative stress induced by malaria, leading to tissue damage, muscle weakness and ischemia.15–18 Together, this information could be used for improved monitoring of disease progression and development of specific interventions for the protection of cardiac muscles during malaria infection. In the present study, CPK level positively correlated with parasitemia. GIE-treated mice infected with PbANKA showed diminished myocardial damage and restored CPK levels. In the present study, we observed that GIE demonstrated antimalarial activity by inhibiting parasitemia. This result was also supported by GIE at lower concentrations, previously shown to have the potential to suppress parasitemia in the 4-day suppressive test,19 which may in turn reduce malaria deposit and vacuolar degeneration in the heart. Additionally, GIE has been shown to exhibit potential antioxidant properties by scavenging intracellular ROS production, subsequently reducing inflammatory cytokines.20 Our results demonstrated that at any doses of GIE reduced parasitemia and improved cardiac functions. However, there was no significant difference between GIE dosages. At higher dose of GIE contains higher quantities of its active compounds, such as polyphenols, flavonoids, alkaloids, triterpenoids, saponins, quercetins and gymnemic acids.20–22 This cardio-protective effect may result from individual or synergistic effects of these active compounds, according to GIE treatment in PbANKA-infected mice.12,23
Evidence of pathological changes in acute malaria infection, including tissue inflammation, architectural loss and malarial pigment deposit, was observed in brain, liver, spleen, kidney, lung and heart.13 This complication is related to parasite RBC sequestration, microvascular obstruction, endothelial damage and tissue hypoxia, leading to cardiac dysfunction.17,24,25 In this study, PbANKA acute infection presented an enlarged heart and left ventricular dilation with reduced systolic function, which may result from sequestration of infected RBC in cardiac capillaries and subsequent blood flow obstruction.26 Interestingly, significantly decreased ejection fraction and fractional shortening were present in the PbANKA-infected group (UN). The cause may not be entirely due to increased after-load, which results from malaria-related increased vascular resistance, but other factors involved, most likely sequestration in the myocardial vessels with secondary ischemic change and impaired myocardial function.27–29 Alterations in cardiac echocardiography parameters were also observed in patients with non-severe Plasmodium vivax with impaired cardiac structures and function.30 Our finding demonstrated that GIE administration produced strong cardioprotective effects against left ventricular dysfunction in PbANKA-infected mice.
Conclusion
According to the results in this study, evidence is provided that GIE is highly potent in treating PbANKA-induced cardiac injury and dysfunction in mice, thereby validating the use of GIE in traditional medicine to treat malaria and related symptoms. Therefore, this plant performs promising cardio-protective activities and further studies should be conducted to isolate and identify the active compounds responsible for these activities.
Acknowledgements
The authors gratefully acknowledge the small animal hospital, Akkhraratchakumari Veterinary College, and the Research Institute for Health Sciences, Walailak University, for their support in the animal experiments. We thank the B. Sc. students from the School of Allied Health Sciences, Walailak University, for supporting this study.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was partially supported by the Research Institute for Health Sciences, Chiang Mai University, (grant number 2019-2020) and the Research Institute for Health Sciences, Walailak University, grant number WU-IRG-63-074 (S.S.).
Ethical Approval: The animal care and handling was per the international guidelines for use and maintenance of experimental animals. The animal ethical approval was obtained from the Animal Ethics Committee, Walailak University, Thailand (EC no. WU-AICUC-63-031).
Author Contributions: Sakaewan Ounjaijean provided research background, problem statement, objectives and hypothesis, and performed main research, collected data, analyzed and interpretated the results.
Rujikorn Rattanatham and Voravuth Somsak prepared experimental malaria mice model and plant extraction, collected data, analyzed and interpretated the results.
Worakan Boonhoh performed echocardiography in mouse model, collected and analyzed the results.
Sirirat Surinkaew designed and approved the protocol, discussed and concluded the main idea, and prepared and submitted the manuscript.
ORCID iD: Sirirat Surinkaew https://orcid.org/0000-0001-9161-8567
References
- 1.World Health O. World malaria report 2021. World Health Organization, 2021. [Google Scholar]
- 2.Luzolo AL, Ngoyi DM. Cerebral malaria. Brain Res Bull 2019; 145: 53-58. DOI: 10.1016/j.brainresbull.2019.01.010 [DOI] [PubMed] [Google Scholar]
- 3.White NJ. Anaemia and malaria. Malar J 2018; 17(1): 371. DOI: 10.1186/s12936-018-2509-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yacoub S, Lang HJ, Shebbe M, et al. Cardiac function and hemodynamics in Kenyan children with severe malaria. Crit Care Med 2010; 38(3): 940-945. DOI: 10.1097/CCM.0b013e3181cd114a [DOI] [PubMed] [Google Scholar]
- 5.Kotlyar S, Olupot-Olupot P, Nteziyaremye J, et al. Assessment of myocardial function and injury by echocardiography and cardiac biomarkers in African children with severe plasmodium falciparum malaria. Pediatr Crit Care Med 2018; 19(3): 179-185. DOI: 10.1097/PCC.0000000000001411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Genrich GL, Guarner J, Paddock CD, et al. Fatal malaria infection in travelers: Novel immunohistochemical assays for the detection of plasmodium falciparum in tissues and implications for pathogenesis. Am J Trop Med Hyg 2007; 76(2): 251-259. [PubMed] [Google Scholar]
- 7.Barber BE, William T, Grigg MJ, et al. Parasite biomass-related inflammation, endothelial activation, microvascular dysfunction and disease severity in vivax malaria. PLoS Pathog 2015; 11(1): e1004558. DOI: 10.1371/journal.ppat.1004558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alencar Filho AC, Lacerda MV, Okoshi K, et al. Malaria and vascular endothelium. Arq Bras Cardiol 2014; 103(2): 165-169. DOI: 10.5935/abc.20140088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeo TW, Lampah DA, Kenangalem E, et al. Decreased endothelial nitric oxide bioavailability, impaired microvascular function, and increased tissue oxygen consumption in children with falciparum malaria. J Infect Dis 2014; 210(10): 1627-1632. DOI: 10.1093/infdis/jiu308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.An JP, Park EJ, Ryu B, et al. Oleanane triterpenoids from the leaves of Gymnema inodorum and their insulin mimetic activities. J Nat Prod 2020; 83(4): 1265-1274. DOI: 10.1021/acs.jnatprod.0c00051 [DOI] [PubMed] [Google Scholar]
- 11.Di Fabio G, Romanucci V, Di Marino C, et al. Gymnema sylvestre R. Br., an Indian medicinal herb: Traditional uses, chemical composition, and biological activity. Curr Pharm Biotechnol 2015; 16(6): 506-516. DOI: 10.2174/138920101606150407112903 [DOI] [PubMed] [Google Scholar]
- 12.Khan F, Sarker MMR, Ming LC, et al. Comprehensive review on phytochemicals, pharmacological and clinical potentials of Gymnema sylvestre. Front Pharmacol 2019; 10: 1223. 20191029. DOI: 10.3389/fphar.2019.01223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bello RO, Abdullah MA, Abd Majid R, et al. IL35 Modulation altered survival, cytokine environment and histopathological consequences during malaria infection in mice. Malar J 2019; 18(1): 434. DOI: 10.1186/s12936-019-3070-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehrhardt S, Mockenhaupt FP, Anemana SD, et al. High levels of circulating cardiac proteins indicate cardiac impairment in African children with severe plasmodium falciparum malaria. Microbes Infect 2005; 7(11-12): 1204-1210. DOI: 10.1016/j.micinf.2005.04.007 [DOI] [PubMed] [Google Scholar]
- 15.Wu X, Gowda NM, Gowda DC. Phagosomal acidification prevents macrophage inflammatory cytokine production to malaria, and dendritic cells are the Major source at the early stages of infection: IMPLICATION FOR MALARIA PROTECTIVE IMMUNITY DEVELOPMENT. J Biol Chem 2015; 290(38): 23135-23147. DOI: 10.1074/jbc.M115.671065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyke KE, Burges R, Cissoko Y, et al. Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect Immun 2004; 72(10): 5630‐55637. DOI: 10.1128/iai.72.10.5630-5637.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park M-K, Ko E-J, Jeon K-Y, et al. Induction of angiogenesis by malarial infection through hypoxia dependent manner. Korean J Parasitol 2019; 57(2):117-125. DOI: 10.3347/kjp.2019.57.2.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vasquez M, Zuniga M, Rodriguez A. Oxidative Stress and Pathogenesis in Malaria. Front Cell Infect Microbiol. 2021; 11: 768182. DOI: 10.3389/fcimb.2021.768182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ounjaijean S, Romyasamit C, Somsak V. Evaluation of antimalarial potential of aqueous crude Gymnema Inodorum leaf extract against plasmodium berghei infection in mice. Evid Based Complement Alternat Med 2021; 2021: 9932891. 20210427. DOI: 10.1155/2021/9932891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunkhunthod B, Talabnin C, Murphy M, et al. Gymnema inodorum (lour.) decne. Extract alleviates oxidative stress and inflammatory mediators produced by RAW264.7 macrophages. Oxid Med Cell Longev 2021; 2021: 8658314. 20210204. DOI: 10.1155/2021/8658314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimizu K, Ozeki M, Iino A, et al. Structure-Activity relationships of triterpenoid derivatives extracted from Gymnema inodorum leaves on glucose absorption. Jpn J Pharmacol. 2001; 86(2): 223-229. DOI: 10.1254/jjp.86.223 [DOI] [PubMed] [Google Scholar]
- 22.Srinuanchai W, Nooin R, Pitchakarn P, et al. Inhibitory effects of Gymnema inodorum (lour.) decne leaf extracts and its triterpene saponin on carbohydrate digestion and intestinal glucose absorption. J Ethnopharmacol. 2021; 266: 113398. DOI: 10.1016/j.jep.2020.113398 [DOI] [PubMed] [Google Scholar]
- 23.Kang MH, Lee MS, Choi MK, et al. Hypoglycemic activity of Gymnema sylvestre extracts on oxidative stress and antioxidant status in diabetic rats. J Agric Food Chem. 2012; 60(10): 2517-2524. DOI: 10.1021/jf205086b [DOI] [PubMed] [Google Scholar]
- 24.de Souza JB, Riley EM. Cerebral malaria: The contribution of studies in animal models to our understanding of immunopathogenesis. Microbes Infect 2002; 4(3): 291-300. DOI: 10.1016/s1286-4579(02)01541-1 [DOI] [PubMed] [Google Scholar]
- 25.Sadoh WE, Uduebor JO. Electrocardiographic changes and troponin T levels in children with severe malaria anemia and heart failure. Niger J Clin Pract 2017; 20(5): 552-556. DOI: 10.4103/1119-3077.187313 [DOI] [PubMed] [Google Scholar]
- 26.Costenaro P, Benedetti P, Facchin C, et al. Fatal myocarditis in course of plasmodium falciparum infection: Case report and review of cardiac complications in malaria. Case Rep Med 2011; 2011: 202083. 20110414. DOI: 10.1155/2011/202083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dondorp AM, Ince C, Charunwatthana P, et al. Direct in vivo assessment of microcirculatory dysfunction in severe falciparum malaria. J Infect Dis 2008; 197(1): 79-84. DOI: 10.1086/523762 [DOI] [PubMed] [Google Scholar]
- 28.Kondo H, Imai Y, Ishikawa T, et al. Hemodynamic analysis of microcirculation in malaria infection. Ann Biomed Eng 2009; 37(4):702-709. DOI: 10.1007/s10439-009-9641-1 [DOI] [PubMed] [Google Scholar]
- 29.Herr J, Mehrfar P, Schmiedel S, et al. Reduced cardiac output in imported plasmodium falciparum malaria. Malar J. 2011; 10: 160. DOI: 10.1186/1475-2875-10-160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alencar-Filho AC, Ferreira J, Salinas JL, et al. Cardiovascular changes in patients with non-severe plasmodium vivax malaria. Int J Cardiol Heart Vasc 2016; 11: 12-16. DOI: 10.1016/j.ijcha.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]