Abstract
Malignant pleural mesothelioma (MPM) is a rare aggressive cancer. This study investigated the growth-inhibitory effects of the combination of carbon ion beam irradiation (IR) and cisplatin (CDDP) on MPM xenografts. Carbon-ion beam IR at 15 Gy effectively inhibited tumor growth and decreased the tumor volume more than 90% after 9 weeks. However, tumor regrowth was observed after 17 weeks. The combination of carbon-ion beam IR (15 Gy) and CDDP significantly suppressed tumor growth after 9 weeks, with tumor regression being observed for more than 18 weeks. In contrast, X-ray IR (30 Gy) alone or in combination with CDDP effectively suppressed tumor growth and decreased the tumor volume after 11 weeks, but tumor growth was observed after 15 weeks. Carbon-ion beam IR at 25 Gy resulted in complete tumor regression without tumor regrowth in the 20-week follow-up period. Histopathological analysis revealed that combination of carbon-ion beam IR and CDDP exerted effective cytotoxic effects on MPM xenograft tumor cells and significantly promoted tumor cell necrosis, cavitation, and fibrosis when compared with individual treatment with carbon-ion beam, X-ray IR, or CDDP. Immunohistochemical analysis revealed that the expression levels of tumor cell migration and invasion-related proteins such as CXCL12, MMP2 and MMP9 were not significantly affected upon low dose (15 Gy) carbon-ion beam IR alone or in combination with CDDP but were markedly upregulated upon treatment with CDDP alone relative to control. However, IR with a high dose (25 Gy) carbon-ion beam inhibited tumor growth without upregulating these proteins. In conclusion, the combination of IR with a low dose (15 Gy) carbon ion beam and CDDP effectively suppressed MPM tumor in vivo without significantly upregulating CXCL12, MMP2 and MMP9, suggesting that combination therapy of carbon ion beam IR and chemotherapy is a promising therapeutic strategy for MPM.
Keywords: Carbon-ion irradiation, cisplatin, mesothelioma, tumor control
Introduction
The etiological factor for malignant pleural mesothelioma (MPM), a rare highly aggressive cancer, is long-term asbestos exposure [1]. The diagnosis of MPM is difficult at early stages [2,3]. The therapeutic strategy for patients with MPM includes surgical resection, radiation therapy, and chemotherapy [4-6]. As most patients with MPM are diagnosed with late-stage disease, treatment options are limited, primarily chemotherapy or immunotherapy [7-9]. Carbon-ion beam, a novel promising radiation therapy can target radiation-resistant tumors with high efficacy [10-12]. Previously, we had elucidated the molecular mechanisms underlying the cytotoxic effects of carbon ion beam irradiation (IR) on radioresistant cancer stem cells (CSCs) in colorectal, breast and pancreatic cancers. In particular, the combination of carbon-ion beam IR and DNA damaging drugs exert potent cytotoxic effects against CSCs [13-17]. Additionally, our previous basic biological study demonstrated that carbon-ion beam IR in combination with cisplatin (CDDP) effectively exerted cytotoxic effects against MPM cells including CSCs [18]. However, in addition to evaluating the effect of the combination of carbon-ion beam IR and CDDP on MPM in vitro, the in vivo cytotoxic effects of this combination must be examined to provide basic data for future clinical trials.
In the last 28 years, 15,000 patients with various cancer types have been treated with carbon-ion radiation therapy (CIRT) and achieved promising results [12,19,20]. Furthermore, we have previously developed a superconducting carbon ion beam gantry, which can target tumors from all directions [21]. This gantry is especially suitable for treating complex-shaped tumors such as MPM. This study aimed to elucidate the growth-inhibitory effects of carbon-ion beam IR alone or in combination with CDDP on MPM xenograft tumors in vivo. To the best of our knowledge, this is the first study to report the effectiveness of carbon-ion beam IR alone or in combination with CDDP in targeting MPM xenograft. The findings of this study may provide useful information for the development of novel treatment strategies for this refractory cancer.
Materials and methods
Cell lines and reagent
Human MPM cell lines (H226 and MESO1) which purchased from American Type Culture Collection (Manassas, VA), were cultured in Dulbecco’s Modified Eagle’s medium supplemented with 10% heat-inactivated fetal bovine serum (Beit-HaEmek, Israel), 100 U/mL penicillin and 100 μg/mL streptomycin (Invitrogen) at 37°C and 5% CO2. The culture medium was replaced every other day. The stock solution of cisplatin (CDDP, Takara Bio Japan) was diluted in saline immediately before use. The dosage of CDDP used in this study was 5.0 mg/kg body weight, which was determined based on previous reports [22], and is suitable to evaluate its effects on mesothelioma in vivo.
Mice and irradiation
Nonobese diabetic-severe immunodeficiency (NOD-SCID) mice (male, aged 6-8 weeks, Charles River Laboratories, Yokohama, Japan) were maintained under defined conditions at the QST animal facility. The mice were divided into non-IR control (n = 5), CDDP group (n = 5), and IR groups (X-30 Gy; X-30 Gy + CDDP; C-15 Gy; C-15Gy + CDDP; C-25 Gy; each group n = 7) for tumor growth delay assay. The mice were subcutaneously injected with 50 μL solution containing 1 × 106 viable H226 or MESO1 cells into the right thigh. Mice were irradiated with carbon-ion beams (accelerated by the HIMAC, 290 MeV/n, center of 6 cm Spread-Out Bragg Peak (SOBP)) when the xenograft tumor size was approximately 7-9 mm. Tumors irradiated with conventional X-ray (200 kVp, TITAN-320, GE, USA) served as a reference. Mice bearing tumors with diameter of 7-9 mm were injected intraperitoneally with CDDP (50 mg/kg body weight) alone or at times after a single fraction of carbon-ion beam IR, twice a week for 2 weeks. All animal experiments were performed according to the QST institutional animal welfare guidelines.
Morphological and histopathological analysis
Morphological changes were followed up to 18 weeks after a single carbon ion beam, or X-ray IR alone or in combination with CDDP. The tumors were resected at week 8 or 12 post-carbon ion beam, or X-ray IR alone or in combination with CDDP, and subjected to histopathological examination. Xenograft tumors from different groups were fixed with 10% neutral formalin, embedded in paraffin, and sectioned (thickness: 4 μm). The sections were stained with hematoxylin and eosin (HE) and observed under a microscope.
Immunohistochemical (IHC) analysis
IHC staining was performed according to the manufacturer’s protocol (Vectastain ABC Elite kit; Vector Laboratories) (8). Briefly, formalin-fixed, paraffin-embedded tissue sections were deparaffinized, rehydrated in a graded series of ethanol, and incubated with 0.3% hydrogen peroxidase in methanol to block endogenous peroxidase. The sections were incubated in boiling 10 mmol/L citrate buffer (pH 6.0) to retrieve the antigen and preincubated with normal horse serum (1:50, Vector Laboratories). Next, the sections were incubated with anti-CXCL12, anti-MMP2 and anti-MMP9 (human monoclonal; Miltenyi Biotec; 1:2000 dilution) iantibodies overnight at 4°C. After washing sections, they were incubated with universal secondary antibodies (Vectastain ABC Elite kit; Vector Laboratories) containing anti-mouse/anti-goat IgG for 30 min, stained with diaminobenzidine (Vectastain ABC Elite kit; Vector Laboratories) for 10 min and counterstained with hematoxylin. Ten fields were selected and the expression of targeted proteins in 1,000 tumor cells was evaluated under a high magnification (400 ×) microscope. As a negative control, the sections were stained without primary antibodies to monitor background staining levels. The CXCL12, MMP2, and MMP9 signals detected in the cytoplasmic and/or membrane were considered immunoreactive. Each section was evaluated for immunostaining intensity, background staining, and percentage of cells expressing the target protein.
Feasibility study of MPM
A feasibility study of treating MPM with a superconducting carbon ion beam gantry was performed using CTV and GTV simulations according to the evaluated doses and tumor margins.
Statistical analysis
Means between the groups were compared using one-way analysis of variance, followed by Bonferroni multiple comparison tests. All statistical analyses were performed using StatView software (SAS Institute, Inc., Cary, NC). Differences were considered significant at P < 0.05.
Results
Carbon ion beam IR alone or in combination with CDDP delays tumor growth
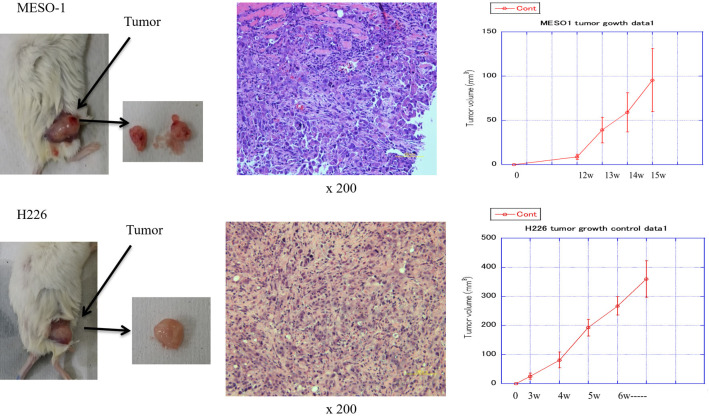
In this study, both MESO1 and H226 cells could form xenograft tumors after transplantation into NOD-SCID mice. The time for the MESO1 and H226 cell-derived tumors to reach 7-9 mm was approximately 14-15 weeks and 4-5 weeks, respectively (Figure 1). This study primarily used H226 xenograft tumors because the growth of MESO1 cell-derived tumors is too slow for use in this experiment.
Figure 1.
Tumorigenicity of MESO1 and H226 cells after transplantation into nonobese diabetic-severe immunodeficiency (NOD-SCID) mice.
To evaluate the growth-inhibitory effects of carbon-ion beam IR alone or in combination with CDDP, H226 xenograft tumors were treated when they achieved a dimeter of 7-9 mm. The growth of H226 xenograft tumors was initially slow. However, the tumor volume was more than 300 mm3 at week 5 post-subcutaneous implantation.
Carbon-ion beam IR at 15 Gy effectively inhibited tumor growth with the tumor volume decreasing by more than 90% at week 9 post-IR. However, the regrowth of tumor was observed at week 17 post-IR. Carbon-ion beam IR (15 Gy) in combination with CDDP significantly suppressed tumor growth at week 9 post-treatmen. The decreased tumor size and volume were observed for more than 18 weeks. In contrast, X-ray IR at 30 Gy alone or in combination with CDDP effectively suppressed tumor growth and reduced the tumor size and volume by more than 90% at week 11 post-IR, but the regrowth of tumors was observed at week 15 post-IR. Carbon-ion beam IR at 25 Gy resulted in complete regression without tumor regrowth in the 20-week follow-up period (Figures 2, 3). The time for tumor to regrow to volume 20 mm3 was 80 days after low dose (15 Gy) of carbon-ion beam IR alone treatment, which was delayed more than 17 days compared to X-30 Gy treatment. It was 27 days further delayed when combined with CDDP. In contrast, treatment with high-dose (25 Gy) carbon ion beam IR alone resulted in complete tumor disappearance without regrowth. The tumor growth delay in detail after carbon-ion beam, X-ray IR alone or in combination with CDDP is shown in Table 1.
Figure 2.

Morphological changes in the H226 xenograft tumor after irradiation with carbon ion beam or X-ray irradiation alone or in combination with cisplatin (CDDP).
Figure 3.

Irradiation with carbon ion beam or X-ray irradiation alone or in combination cisplatin (CDDP) delayed H226 xenograft tumor growth.
Table 1.
H226 xenograft tumor growth delay after carbon-ion beam or X-ray IR alone or in combination with CDDP
| Time for tumor grow to volume (80 mm3) | Time for tumor to shrink to minimum volume (0-5 mm3) | Time for tumor to regrow to volume (20 mm3) | Time for tumor to regrow to volume (70 mm3) | Time for tumor to regrow to volume (80 mm3) | |
|---|---|---|---|---|---|
| Control | 34 days | ||||
| X-30 Gy | 52 days | 63 days | 77 days | 84 days | |
| X-30 Gy + CDDP | 35 d (-17 days) | 72 d (+9 days) | 90 d (+13 days) | ||
| C-15 Gy | 25 d (-27 days) | 80 d (+17 days) | -- | ||
| C-15 Gy + CDDP | 25 d (-27 days) | 90 d (+27 days) | -- | ||
| C-25 Gy | 21 d (-31 days) | -- | -- |
Effect of carbon-ion beam alone or in combination with CDDP on histopathology in xenograft tumors
Histopathological analysis revealed that the combination of carbon ion beam IR and CDDP effectively inhibited growth of MPM xenograft tumor cells and induced significant tumor cell necrosis, cavitation, and fibrosis when compared with treatment with CDDP alone. This indicated that the combination of carbon ion beam IR and CDDP exerts potent cytotoxic effect against tumor cells. IR with high dose of carbon ion beam induced severe tumor cell cavitation, eliminate tumors, and prevented tumor recurrence (Figure 4).
Figure 4.

Effects of carbon ion beam or X-ray irradiation alone or in combination with cisplatin (CDDP) on histopathological features of the H226 xenograft tumor.
IHC analysis of the effect of carbon-ion beam IR alone or in combination with CDDP on CXCL12, MMP2, and MMP9 expression levels
To clarify the effects of carbon-ion beam IR alone or in combination with CDDP on tumor migration and invasion-related proteins, the expression levels of CXCL12, MMP2 and MMP9 expression were examined using IHC. IR with low dose (15 Gy) carbon-ion beam IR alone did not significantly affect expression of CXCL12. In contrast, treatment with CDDP alone or with high dose (30 Gy) X-ray IR alone or in combination with CDDP markedly upregulated the CXCL12 levels. Furthermore, the CXCL12 expression level in the groups treated with the combination of carbon-ion beam IR and CDDP was slightly higher than that in control. IR with high dose (25 Gy) carbon-ion beam completely inhibited tumor growth without upregulating CXCL12 expression. Similar to CXCL12, MMP2 expression was not significantly affected upon IR with 15 Gy carbon-ion beam alone but was upregulated upon treatment with CDDP alone or IR with X-ray or carbon-ion beam in combination with CDDP. In contrast, IR with high dose of carbon-ion beam (25 Gy) alone effectively eradicated tumors without inducing MMP2 expression. The expression levels of MMP9 in the group treated with CDDP alone or with high dose (30 Gy) of X-ray IR alone or in combination with CDDP were markedly higher than those in the group treated with the combination of carbon-ion beam IR and CDDP. IR with a low dose (15 Gy) of carbon-ion beam slightly downregulatged MMP9 expression. The expression of MMP9 was not detected in the group irradiated with a high dose (25 Gy) of carbon-ion beam (Figure 5).
Figure 5.

Effects of irradiation with carbon ion beam or X-ray irradiation alone or in combination with cisplatin (CDDP) on the expression levels of CXCL12, MMP2, and MMP9 in H226 xenograft tumor.
Feasibility study of MPM
The treatment of MPM, which is a complex-shaped tumor, using conventional radiation therapy is challenging. Previously, we had developed a high-LET superconducting carbon ion beam gantry system with fast scanning and fast respiration gating, which can target tumors from all directions with a smooth IR electric field expansion [21]. Thus, a feasibility study was performed. Based on the accumulated data of clinical outcomes of CIRT for non-small cell lung cancer (NSCLC), a dose of 68.4 Gy/RBE achieved a local control rate of 95% [23,24]. MPM cells are reported to be more sensitive to carbon ion IR than NSCLC cells [18,25]. Hence, an appropriate total dose of 64.4 GyE can be used to target the MPM with a certain margin to the GTV, CTV and PTV, and the carbon ion beam can be delivered from four coplanar beam directions for diseased lung plus one non-coplanar beam direction for interlobar pleura (Figure 6).
Figure 6.

Feasibility study of using superconducting carbon ion beam gantry to treat malignant pleural mesothelioma. The carbon ion beam can be delivered from four coplanar beam directions for diseased lung plus one non-coplanar beam direction for interlobar pleura.
Discussion
This study demonstrated that H226 xenograft tumors were significantly suppressed by more than 90% at week 8 post-carbon-ion beam IR (15 Gy) alone. However, tumor regrowth was observed at week 17 post-IR. Carbon-ion beam IR at 15 Gy in combination with CDDP effectively inhibited tumor growth and decreased the tumor size and volume. Tumor regrowth was not observed for more than 18 weeks post-IR. In contrast, X-ray IR at 30 Gy effectively suppressed tumor growth and decreased the tumor size and volume at week 10 post-IR, but tumor regrowth was observed at week 12 post-IR. IR with 25 Gy carbon-ion beam resulted in complete regression without tumor regrowth in the 20-week follow-up period. IR with high dose of carbon-ion beam or the combination of carbon-ion beam IR and CDDP exerted higher growth-inhibitory effects against MPM tumors than carbon-ion beam IR alone or X-ray IR in combination with CDDP. This finding suggests that combination strategies are important for improving tumor control when it is difficult to increase the dose of carbon-ion beam IR, but it is better to treat the tumor with a dose high enough to achieve a complete cure. This is consistent with the results of previous studies, which reported that DNA damaging anticancer drugs can effectively induce cell death in tumors including MPM [26-28]. Furthermore, the combination of carbon ion beam IR and CDDP achieved a significantly higher regression of tumor size than X-ray, carbon ion beam IR alone, or the combination of X-ray IR and CDDP, suggesting that CDDP significantly sensitized the MPM tumor to carbon ion beam IR.
Histopathological analysis revealed that the growth-inhibitory efficacy of the combination of carbon-ion beam IR and CDDP against MPM cells with significant induction of tumor cell necrosis, cavitation and fibrosis was higher than that of carbon-ion beam IR, X-ray IR, or CDDP treatment alone. A high dose of carbon ion beam IR appeared to induce severe tumor cell cavitation and eliminated tumor cells. This finding indicates that a relatively low dose of carbon-ion beam IR combined with CDDP, or a high dose of carbon ion beam IR can efficiently inhibit MPM growth. These findings are partially consistent with those of in vivo (16) and clinical trials studies, which reported that combination of carbon ion beam IR and chemotherapy effectively suppresses cancer growth [29,30].
Furthermore, the effects of the combination of carbon ion beam IR and CDDP on tumor migration and invasion-related proteins, such as CXCL12, MMP2, and MMP9 were examined. CXCL12, a chemotactic factor for T cells, is reported to be associated with tumor cell proliferation, motility, and metastasis [31,32]. MMP2 and MMP9 play pivotal roles in the processes of inflammation, and tumor invasion and metastasis [33,34]. The expression levels of CXCL12 in the group irradiated with a relatively low dose (15 Gy) of carbon-ion beam alone or in combination with CDDP were not significantly different from those in the control. However, the CXCL12 expression levels were upregulated upon treatment with CDDP alone or with high dose (30 Gy) X-ray IR alone or in combination with CDDP, indicating that chemotherapy or conventional X-ray IR alone or its combination upregulates CXCL12 expression. However, IR with a high dose (25 Gy) of carbon-ion beam completely inhibited tumor growth without inducing CXCL12 expression. We speculate that carbon-ion beam IR destroyed tumor cells and abolished CDDP-induced increases in migration and invasion. This finding suggests that chemotherapy or conventional X-ray IR alone or its combination enhances the ability of migration and invasion, whereas the combination of carbon-ion beam IR and CDDP suppresses tumor regrowth but does not markedly promote tumor migration and invasion. MMP2 expression was not significantly affected upon IR with 15 Gy carbon-ion beam alone or in combination with CDDP but was upregulated upon treatment with CDDP alone or with 30 Gy X-ray IR alone or in combination with CDDP. In contrast, IR with a high dose (25 Gy) of carbon-ion beam alone effectively eradicated tumors without inducing MMP2 expression. The MMP9 expression levels in the group treated with CDDP alone or with 30 Gy X-ray IR alone or in combination with CDDP were higher than those in the group treated with the combination of carbon-ion beam IR and CDDP. However, IR with a low dose (15 Gy) of carbon-ion beam slightly downregulated MMP9 expression. IR with a high dose (25 Gy) of carbon-ion beam completely suppressed the expression of MMP9. This suggests that the combination of carbon-ion beam IR and CDDP may inhibit MPM growth without significantly upregulating tumor cell migration and invasion.
The results of a feasibility study of MPM revealed that superconducting carbon ion beam gantry can target tumors flexibly from any angle with fast scanning and smooth IR field expansion. An appropriate dose of carbon ion beam can be delivered to MPM without exposing the surrounding healthy organs, such as the heart and lungs to radiation.
In summary, a low dose (15 Gy) of carbon ion beam in combination with CDDP effectively suppressed MPM tumor in vivo without significantly upregulating tumor migration and invasion-related proteins. Thus, combination therapy with carbon ion beam IR and chemotherapy is a promising treatment for MPM.
Acknowledgements
We thank Mr Hirakawa K, Mrs Fang YQ and Mrs Maruyama F for providing technical support.
Disclosure of conflict of interest
None.
References
- 1.Scherpereel A, Wallyn F, Albelda SM, Munck C. Novel therapies for malignant pleural mesothelioma. Lancet Oncol. 2018;19:e161–e172. doi: 10.1016/S1470-2045(18)30100-1. [DOI] [PubMed] [Google Scholar]
- 2.Bibby AC, Tsim S, Kanellakis N, Ball H, Talbot DC, Blyth KG, Maskell NA, Psallidas I. Malignant pleural mesothelioma: an update on investigation, diagnosis and treatment. Eur Respir Rev. 2016;25:472–486. doi: 10.1183/16000617.0063-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsao AS, Pass HI, Rimner A, Mansfield AS. New era for malignant pleural mesothelioma: updates on therapeutic options. J. Clin. Oncol. 2022;40:681–692. doi: 10.1200/JCO.21.01567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bueno R, Opitz I IASLC Mesothelioma Taskforce. Surgery in malignant pleural mesothelioma. J Thorac Oncol. 2018;13:1638–1654. doi: 10.1016/j.jtho.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Davis AP, Kao SC, Clarke SJ, Boyer M, Pavlakis N. Emerging biological therapies for the treatment of malignant pleural mesothelioma. Expert Opin Emerg Drugs. 2021;26:179–192. doi: 10.1080/14728214.2021.1924670. [DOI] [PubMed] [Google Scholar]
- 6.Remon J, Reguart N, Corral J, Lianes P. Malignant pleural mesothelioma: new hope in the horizon with novel therapeutic strategies. Cancer Treat Rev. 2015;41:27–34. doi: 10.1016/j.ctrv.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Banerji S, Meyers DE, Harlos C, Dawe DE. The role of immunotherapy in the treatment of malignant pleural mesothelioma. Curr Oncol. 2021;28:4542–4551. doi: 10.3390/curroncol28060385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho BCJ, Donahoe L, Bradbury PA, Leighl N, Keshavjee S, Hope A, Pal P, Cabanero M, Czarnecka K, McRae K, Tsao MS, de Perrot M. Surgery for malignant pleural mesothelioma after radiotherapy (SMART): final results from a single-centre, phase 2 trial. Lancet Oncol. 2021;22:190–197. doi: 10.1016/S1470-2045(20)30606-9. [DOI] [PubMed] [Google Scholar]
- 9.de Gooijer CJ, Baas P, Burgers JA. Current chemotherapy strategies in malignant pleural mesothelioma. Transl Lung Cancer Res. 2018;7:574–583. doi: 10.21037/tlcr.2018.04.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakano T, Suzuki Y, Ohno T, Kato S, Suzuki M, Morita S, Sato S, Oka K, Tsujii H. Carbon beam therapy overcomes the radiation resistance of uterine cervical cancer originating from hypoxia. Clin Cancer Res. 2006;12:2185–2190. doi: 10.1158/1078-0432.CCR-05-1907. [DOI] [PubMed] [Google Scholar]
- 11.Rosenzweig KE. Radiation therapy for malignant pleural mesothelioma. Thorac Surg Clin. 2020;30:473–480. doi: 10.1016/j.thorsurg.2020.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Tsujii H, Kamada T. A review of update clinical results of carbon ion radiotherapy. Jpn J Clin Oncol. 2012;42:670–685. doi: 10.1093/jjco/hys104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui X, Oonishi K, Tsujii H, Yasuda T, Matsumoto Y, Furusawa Y, Akashi M, Kamada T, Okayasu R. Effects of carbon ion beam on putative colon cancer stem cells and its comparison with X-rays. Cancer Res. 2011;71:3676–3687. doi: 10.1158/0008-5472.CAN-10-2926. [DOI] [PubMed] [Google Scholar]
- 14.Kim EH, Kim MS, Furusawa Y, Uzawa A, Han S, Jung WG, Sai S. Metformin enhances the radiosensitivity of human liver cancer cells to gamma-rays and carbon ion beams. Oncotarget. 2016;7:80568–80578. doi: 10.18632/oncotarget.12966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oonishi K, Cui X, Hirakawa H, Fujimori A, Kamijo T, Yamada S, Yokosuka O, Kamada T. Different effects of carbon ion beams and X-rays on clonogenic survival and DNA repair in human pancreatic cancer stem-like cells. Radiother Oncol. 2012;105:258–265. doi: 10.1016/j.radonc.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Sai S, Vares G, Kim EH, Karasawa K, Wang B, Nenoi M, Horimoto Y, Hayashi M. Carbon ion beam combined with cisplatin effectively disrupts triple negative breast cancer stem-like cells in vitro. Mol Cancer. 2015;14:166. doi: 10.1186/s12943-015-0429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sai S, Wakai T, Vares G, Yamada S, Kamijo T, Kamada T, Shirai T. Combination of carbon ion beam and gemcitabine causes irreparable DNA damage and death of radioresistant pancreatic cancer stem-like cells in vitro and in vivo. Oncotarget. 2015;6:5517–5535. doi: 10.18632/oncotarget.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sai S, Suzuki M, Kim EH, Hayashi M, Vares G, Yamamoto N, Miyamoto T. Effects of carbon ion beam alone or in combination with cisplatin on malignant mesothelioma cells in vitro. Oncotarget. 2018;9:14849–14861. doi: 10.18632/oncotarget.23756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamada T, Tsujii H, Blakely EA, Debus J, De Neve W, Durante M, Jakel O, Mayer R, Orecchia R, Potter R, Vatnitsky S, Chu WT. Carbon ion radiotherapy in Japan: an assessment of 20 years of clinical experience. Lancet Oncol. 2015;16:e93–e100. doi: 10.1016/S1470-2045(14)70412-7. [DOI] [PubMed] [Google Scholar]
- 20.Okada T, Kamada T, Tsuji H, Mizoe JE, Baba M, Kato S, Yamada S, Sugahara S, Yasuda S, Yamamoto N, Imai R, Hasegawa A, Imada H, Kiyohara H, Jingu K, Shinoto M, Tsujii H. Carbon ion radiotherapy: clinical experiences at National Institute of Radiological Science (NIRS) J Radiat Res. 2010;51:355–364. doi: 10.1269/jrr.10016. [DOI] [PubMed] [Google Scholar]
- 21.Mori S, Sakata Y, Hirai R, Furuichi W, Shimabukuro K, Kohno R, Koom WS, Kasai S, Okaya K, Iseki Y. Commissioning of a fluoroscopic-based real-time markerless tumor tracking system in a superconducting rotating gantry for carbon-ion pencil beam scanning treatment. Med Phys. 2019;46:1561–1574. doi: 10.1002/mp.13403. [DOI] [PubMed] [Google Scholar]
- 22.Shirmanova MV, Druzhkova IN, Lukina MM, Dudenkova VV, Ignatova NI, Snopova LB, Shcheslavskiy VI, Belousov VV, Zagaynova EV. Chemotherapy with cisplatin: insights into intracellular pH and metabolic landscape of cancer cells in vitro and in vivo. Sci Rep. 2017;7:8911. doi: 10.1038/s41598-017-09426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paz AE, Yamamoto N, Sakama M, Matsufuji N, Kanai T. Tumor control probability analysis for single-fraction carbon-ion radiation therapy of early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2018;102:1551–1559. doi: 10.1016/j.ijrobp.2018.07.2009. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto N, Miyamoto T, Nakajima M, Karube M, Hayashi K, Tsuji H, Tsujii H, Kamada T, Fujisawa T. A dose escalation clinical trial of single-fraction carbon ion radiotherapy for peripheral stage I non-small cell lung cancer. J Thorac Oncol. 2017;12:673–680. doi: 10.1016/j.jtho.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki M, Kase Y, Kanai T, Ando K. Change in radiosensitivity with fractionated-dose irradiation of carbon-ion beams in five different human cell lines. Int J Radiat Oncol Biol Phys. 2000;48:251–258. doi: 10.1016/s0360-3016(00)00606-4. [DOI] [PubMed] [Google Scholar]
- 26.Denis I, Cellerin L, Gregoire M, Blanquart C. Cisplatin in combination with Phenethyl Isothiocyanate (PEITC), a potential new therapeutic strategy for malignant pleural mesothelioma. Oncotarget. 2014;5:11641–11652. doi: 10.18632/oncotarget.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Cano J, Ambroise G, Pascual-Serra R, Carrion MC, Serrano-Oviedo L, Ortega-Muelas M, Cimas FJ, Sabater S, Ruiz-Hidalgo MJ, Sanchez Perez I, Mas A, Jalon FA, Vazquez A, Sanchez-Prieto R. Exploiting the potential of autophagy in cisplatin therapy: a new strategy to overcome resistance. Oncotarget. 2015;6:15551–15565. doi: 10.18632/oncotarget.3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee YJ, Lee GJ, Yi SS, Heo SH, Park CR, Nam HS, Cho MK, Lee SH. Cisplatin and resveratrol induce apoptosis and autophagy following oxidative stress in malignant mesothelioma cells. Food Chem Toxicol. 2016;97:96–107. doi: 10.1016/j.fct.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 29.Okonogi N, Wakatsuki M, Kato S, Karasawa K, Miyasaka Y, Murata H, Nakano T, Kamada T, Shozu M Working Group of Gynecological Tumors. A phase 1/2 study of carbon ion radiation therapy with concurrent chemotherapy for locally advanced uterine cervical squamous cell carcinoma (Protocol 1302) Int J Radiat Oncol Biol Phys. 2019;104:631–639. doi: 10.1016/j.ijrobp.2019.02.042. [DOI] [PubMed] [Google Scholar]
- 30.Shinoto M, Yamada S, Terashima K, Yasuda S, Shioyama Y, Honda H, Kamada T, Tsujii H, Saisho H Working Group for Pancreas Cancer. Carbon ion radiation therapy with concurrent gemcitabine for patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2016;95:498–504. doi: 10.1016/j.ijrobp.2015.12.362. [DOI] [PubMed] [Google Scholar]
- 31.Otsuka S, Bebb G. The CXCR4/SDF-1 chemokine receptor axis: a new target therapeutic for non-small cell lung cancer. J Thorac Oncol. 2008;3:1379–1383. doi: 10.1097/JTO.0b013e31818dda9d. [DOI] [PubMed] [Google Scholar]
- 32.Shi A, Wang T, Jia M, Dong L, Shi H. Effects of SDF-1/CXCR7 on the migration, invasion and epithelial-mesenchymal transition of gastric cancer cells. Front Genet. 2021;12:760048. doi: 10.3389/fgene.2021.760048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang Y, Lv P, Sun Z, Han L, Zhou W. 14-3-3beta promotes migration and invasion of human hepatocellular carcinoma cells by modulating expression of MMP2 and MMP9 through PI3K/Akt/NF-kappaB pathway. PLoS One. 2016;11:e0146070. doi: 10.1371/journal.pone.0146070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Webb AH, Gao BT, Goldsmith ZK, Irvine AS, Saleh N, Lee RP, Lendermon JB, Bheemreddy R, Zhang Q, Brennan RC, Johnson D, Steinle JJ, Wilson MW, Morales-Tirado VM. Inhibition of MMP-2 and MMP-9 decreases cellular migration, and angiogenesis in in vitro models of retinoblastoma. BMC Cancer. 2017;17:434. doi: 10.1186/s12885-017-3418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]