Abstract
Soil-cultivation presents environmental limitations and requires considerable labor, space, and water supply. Alternatively, hydroponically-cultured ginseng (HG) was improved its productivity, availability, and functionality. Improvement of bio-functionality by probiotic fermentation also has been studied. Therefore, in this study, HG was fermented using probiotics to enhance antioxidant and anti-inflammatory activities. Soil-cultivated ginseng (SG), 1 and 2-year HG (HG1, HG2) were extracted using 70% ethanol and fermented by Lactobacillus brevis B7. After fermentation, the phenolic and flavonoid contents, and antioxidant and NO scavenging activities were increased, and HG showed higher bioactivities than SG. Particularly, fermented HG2 showed the highest antioxidant and anti-inflammatory activities and significantly decreased the level of inflammatory mediators. Furthermore, fermented HG2 also effectively inhibited NF-κB signaling pathway. These results suggested that fermented HG significantly enhanced functionality compared to SG and non-fermented HG. This suggests that fermented HG is a potentially useful ingredient for developing health-functional foods or pharmaceutical materials.
Keywords: Hydroponic ginseng, Probiotics, Fermentation, Antioxidant activity, Anti-inflammatory activity
Introduction
Panax ginseng Meyer has been widely utilized as medicinal plants because of its therapeutic efficacy attributed by different compositions of ginsenosides, phenolic, and flavonoid compounds (Hwang et al., 2019a; Lee et al., 2020). Ginsenosides are representative active compounds in ginseng because of their versatile pharmaceutical activities (Cui et al., 2016). Ginsenosides are briefly classified as protopanaxadiol (PPD; Rb1, Rb2, Rc, Rd, Rg3, Rh2, and compound K) and protopanaxatriol (PPT; Re, Rf, Rg1, and Rg2) according to the number of hydroxyl groups in aglycones (Wan et al., 2021). Additionally, ginsenosides can be divided into major (Rb, Rc, Rd, and so forth) and minor (Rg3, Rh, F2, compound K, and so far) ginsenosides based on the glycosylation state. Because of the absence of glycosylation, minor ginsenosides present higher bioavailability and permeability within the human body. Therefore, minor ginsenosides exhibit higher medicinal activities (Xu et al., 2003).
The hydroponic system requires only the roots and water enriched with minerals and nutrients and enables the growth of plants by conferring protection against insects, climate, or pollutants. Additionally, temperature, moisture, and light can be controlled. Furthermore, this system facilitates the growth of plants throughout the year and is not limited to the conditions of soil, thus obstacles of soil cultivation could be overcome (Magwaza et al., 2020).
Hydroponic systems can increase the productivity of ginseng considerably. Only 4-months was required until the size of root is comparable to 2-year soil-cultivated ginseng (Hwang et al., 2014). Despite of short period for cultivation, higher contents of ginsenosides in both roots and leaves have been reported. Lee et al. (2020) examined the antioxidant activity and ginsenoside content of hydroponic ginseng (HG) and 5-year soil-cultivated ginseng (SG). HG exhibited 3-times higher ginsenoside contents than normal ginseng. Particularly, ginsenoside Rd, Re, and Rg1 contents in HG were noticeably higher than SG. Although higher diversity and concentration of ginsenosides and functional ingredients have been detected in berries, leaves, and stems of ginseng, they are usually discarded during harvest because of the presence of pathogens and cumulative pesticides that accumulate during the long period of cultivation. However, hydroponic systems may be used to resolve these problems and to enable the use of the indicated parts of ginseng for industrial applications (Song et al., 2019b).
To increase the bioactivity of ginseng, heat, acid, and enzymes treatments, and microbial bioconversion have been attempted (Cui et al., 2016). Red and black ginseng are representative examples of steamed ginseng. These processes destroy the glycosidic bonds within ginsenosides and catalyze their transformation into different forms. For example, unstable glycosyl groups at the C-20 position of ginsenosides Rb1, Rc, and Rd are easily separated, and ginsenoside Rg3 is generated. Furthermore, hydroxyl groups could be hydrolyzed and generate the non-polar and unsaturated ginsenoside Rk1. Additionally, hydrolysis of decarboxylation, ester glycoside and malonic acid monoacyl bonds, and isomerization reactions also occur during repetitive high-temperature treatments (Zhu et al., 2019). Biotransformation is relatively mild processing step occurred by microorganisms, especially probiotics. During biotransformation, deglycosylation, dihydroxylation, demethylation, lactonization, aromatic hydroxylation, and reduction of carbon double bonds may occur (Rupasinghe et al., 2019). Through these reactions, major ginsenosides can be transformed into minor ginsenosides. For instance, ginseng marc was fermented by using Pediococcus acidilactici KCCM 11614P to enhance its antioxidant and NO scavenging activities (Eom et al., 2017). Additionally, Inula britannica was fermented by using Weissella cibaria D30 and Lactobacillus plantarum KCCM 11613P to improve anti-inflammatory and antimicrobial activity respectively (Bae et al., 2019b; Kim et al., 2020).
Ginseng alleviates inflammatory reaction by suppression of interleukin (IL)-6 and tumor necrosis factor (TNF)-α level in lipopolysaccharide (LPS)-induced RAW 264.7. Ginsenoside Rc inhibited the production of pro-inflammatory cytokines, and ginsenoside Re and Rg5 also inhibited the NF-κB signaling pathway by interrupting NF-κB activator-binding kinase-1 or IκB kinase activity (Han et al., 2018; Kim, 2018). Ginsenoside Rh2 also decreases the expression of TNF-α, inducible nitric oxide synthase (iNOS), NF-κB, and cyclooxygenase-2 (COX-2) (Qi et al., 2019).
The aim of this study was to compare the antioxidant and anti-inflammatory activities between non-fermented SG and HG extracts, as well as their fermented extracts.
Materials and methods
Ginseng resources, bacterial and cell culture media, and other chemicals
Six-year cultivated SG and 1- and 2-year cultivated HG (HG1, HG2) were purchased from the regional market at Sangdo-Insamsa Co. (Punggi, Korea) and Chungjung-Saessacksam Co. (Gwangju, Korea), respectively. de Man, Rogosa, and Sharpe (MRS; Difco, Franklin Lakes, NJ, USA) were used for bacterial culture. The Dulbecco’s modified Eagle’s medium (DMEM) was used with the RAW 264.7 murine macrophage cell line, and penicillin/streptomycin (P/S) and fetal bovine serum (FBS) were added to DMEM. These reagents were obtained from HyClone (Logan, UT, USA). Other chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Bacterial culture and cell culture condition
Lactobacillus brevis B7 and B13-2 (B7, B13-2) isolated from cabbage-kimchi was used for fermentation and cultured in MRS broth for 15 h at 37 °C (Song et al., 2019a). RAW 264.7 cells were purchased from the Korean Cell Line Bank (Seoul, Korea) and were used to investigate the anti-inflammatory ability. Cells were cultured in DMEM supplemented with 1% P/S (v/v) and 10% FBS (v/v) at 37 °C under a 5% CO2 atmosphere (MCO-18AIC, SANYO, Japan).
Ginseng extract preparation and fermentation conditions
Preparation of ginseng extracts and fermentation were performed as described by Yoo et al. (2019). The roots were rinsed, chopped, dried for 24 h at 60 °C, and then finely powdered. Each powder (25 g) was blended with 70% ethyl alcohol (500 mL) and extracted for 6 h at 70 °C through reflux system in triplicate. The extracts were filtered using Whatman filter paper No.2 and concentrated using a rotary evaporator (N-1000 V, EYELA, Japan) at 60 °C. The concentrated syrup was diluted using distilled water, filtered through 0.45-µm syringe filters (Advantec, Tokyo, Japan), and stored at − 20 °C until use.
For fermentation, concentrated syrup was diluted to a concentration of 10 mg/mL according to the dried solid content. The pH was adjusted to 6.5 using 5.0 M NaOH. The prepared extracts were pasteurized at 90 °C for 10 min. Proliferated L. brevis B7 (1 × 109 CFU/mL) was centrifuged at 14,000×g for 5 min to remove the culture media, and then resuspended in the same volume of ginseng extract (Song et al., 2019a). The bacterial suspension was inoculated into ginseng extracts with 1% (v/v) concentration, and the fermentation was anaerobically conducted for 24 h at 37 °C. After fermentation, supernatant was collected by centrifugation (14,000×g for 15 min) and then filtered through 0.45-µm syringe filters. Fermented extracts were stored in a refrigerator at − 20 °C.
Total solid, phenolic, and flavonoid contents
The total solid (TS), phenolic (TPC), and flavonoid (TFC) contents were measured by implementing the hot-air drying method, Folin-Ciocalteu (Hwang et al., 2019b), and aluminum chloride colorimetric method, respectively (Bae et al., 2019a).
To evaluate the TS content, 1 mL of ginseng extract was used and dried at 105 °C for 4 h. After the completion of drying, the remaining dry matter was weighed again, and the ratio before and after weight of ginseng extract was calculated.
To measure TPC, 50 µL of sample and 1 mL of 1% sodium carbonate were mixed and incubated for 3 min. After incubation, 50 µL of 50% Folin-Ciocalteu reagent was added and incubated for 30 min. The absorbances were measured at 750 nm and the polyphenolic concentration was calculated using a gallic acid standard curve.
Finally, to measure TFC, 800 µL of 60% ethyl alcohol and 20 µL of 5% sodium nitrite were mixed with 100 µL of sample. After 6 min of incubation, 20 µL of 10% aluminum chloride was added. After additional 6 min incubation, 60 µL of 4% sodium hydroxide was added. The mixtures were incubated for 30 min, and absorbance was measured at 405 nm. Flavonoid content was calculated using a quercetin standard curve.
HPLC analysis
HPLC analysis was performed to determine the ginsenoside composition. Each ginseng extract was diluted 10-fold using HPLC-grade methyl alcohol and filtered through a 0.20-µm syringe filter. Standard ginsenosides (Rb1, Rb2, Rb3, Rc, Rd, Re, Rf, Rg3, F2, and CK) were purchased from Biopurify Phytochemicals Ltd. (Chengdu, China).
Ginsenosides were analyzed using the Waters 600 HPLC (Waters, Milford, MA, USA) and reverse-phase column (Eclipse XDB-C18, 4.6 × 150 mm, 5 μm) (Agilent Technologies, Santa Clara, CA, USA). Two types of solvent, water (A) and acetonitrile (B), were used with the following gradients: 0 min (72% A), 0–7 min (60% A), 7–15 min (50% A), 15–20 min (25% A), and 20–25 min (72% A). The injection volume was 10 µL, and the flow speed was 1 mL/min. The absorbances were detected using a UV detector at 203 nm.
Antioxidant analysis of the ginseng extract
Four-types of antioxidant analysis such as DPPH, ABTS, reducing, and FRAP assays. Each ginseng extract was diluted to 5, 2.5, and 1 mg/mL according to the dried solid matter content.
Radical scavenging activity
DPPH (2,2-diphenyl-1-picryl-hydrazyl) and ABTS (2-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt) assays, were conducted to evaluate the radical scavenging ability described by Yu et al. (2019a) with minor modifications.
Each sample was added to DPPH solution (0.4 mM) at a 1:5 ratio, and the mixtures were incubated for 30 min in the dark. The results were measured at 517 nm.
The ABTS solution was prepared by mixing the same volume of ABTS (14 mM) and potassium persulfate (5 mM) dissolved in 0.1 M potassium phosphate buffer (pH 7.4), and then incubated for 16 h without illumination. The absorbance of the mixture was adjusted to 0.7 ± 0.05 at 734 nm. The sample and the ABTS solution were mixed at a 1:19 ratio and incubated for 15 min without illumination. The absorbances were then measured at 734 nm.
Radical scavenging activity of both the DPPH and ABTS assays was determined as follows:
Reducing power assay
The reducing power assay was performed as reported by Jang and Koh (2018) with minor modifications. The sample (50 µL) was mixed with 250 µL of sodium phosphate buffer (0.2 M, pH 6.6) and 1% potassium ferricyanide, and reacted for 20 min at 50 °C. Subsequently, 250 µL of 10% trichloroacetic acid was added and 500 µL of the mixture was mixed with 400 µL of distilled water and 100 µL of 0.1% ferric chloride. After 30 min, the absorbance was measured at 700 nm. The reducing power was calculated using an L-cysteine standard curve.
Ferric reducing antioxidant power (FRAP) assay
The FRAP assay was performed as described by Eom et al. (2017). FRAP solution was prepared by mixing acetate buffer (300 mM, pH 3.6), 10 mM 2,4,6-tri[2-pyridyl]-s-triazine) suspended in 40 mM HCl, and 20 mM ferric chloride in a 10:1:1 ratio, and the mixture was incubated for 15 min at 50 °C. The sample (50 µL) was mixed with FRAP solution (950 µL), and incubated for 30 min. The absorbance was measured at 593 nm, and the results were calculated using a ferrous sulfate standard curve.
Measurement of cytotoxicity and nitric oxide production
To investigate the cytotoxicity and anti-inflammatory potential of ginseng, MTT and NO assays were performed as described by Yu et al. (2019b).
MTT assay for measurement of cytotoxicity
RAW 264.7 macrophages were seeded into 96-well plates with 100 µL of cell suspension (2 × 106 cells/mL) and incubated for 2 h. Fifty-microliters of diluted samples and DMEM were added and incubated for 24 h at 37 °C. Cell media was discarded and 100 µL of MTT solution (0.5 mg/mL) was added and incubated for 1 h. The solution was discarded, and 100 µL of dimethyl sulfoxide was added to dissolve the generated formazan crystals. The absorbances were estimated at 570 nm, and viability was calculated as follows:
NO assay for nitric oxide production
Cell preparation was same as the previous MTT assay, and LPS (1 µg/mL) was used to induce inflammation. Fifty-microliters of LPS and diluted samples were added to the cells and incubated for 24 h at 37 °C. After incubation, same volume of supernatants and Griess reagent were reacted, and the absorbances were estimated at 540 nm. The amount of NO was measured using a sodium nitrite standard curve.
Enzyme-linked immunosorbent assay (ELISA)
To investigate the down-regulation of pro-inflammatory cytokines, TNF-α, IL-1β, IL-6 (Invitrogen, Vienna, Austria), and prostaglandin E2 (PGE2; Abcam, Cambridge, MA, USA), were assessed in LPS-induced RAW 264.7 cells using ELISA kits. The amounts of cytokines were measured according to the manufacturer’s instructions.
Western blotting
To estimate the down-regulation of inflammatory mediators in RAW 264.7 cells, western blotting was performed (Yu et al., 2019b). Cells were seeded at a density of 1 × 106 cells/well in 6-well plates. After 24 h of incubation at 37 °C, 0.5 mL of sample and LPS (1 µg/mL) were added and incubated again for 24 h. Cells were washed with cold PBS and were lysed using RIPA lysis buffer with a phosphatase/protease inhibitor cocktail solution. The lysates were sonicated and centrifuged at 14,000×g for 30 min. The collected supernatants were stored at − 20 °C.
To quantify the protein concentration, a DC protein assay kit (Bio-Rad, Hercules, CA, USA) was used, and quantified proteins (20 µg) were separated via 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The separated bands were transferred onto polyvinylidene difluoride (PVDF) membranes. Transferred bands were blocked with 5% skim milk and probed with diluted primary antibodies including cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS) (Thermo Scientific Pierce, Rockford, IL, USA), phosphorylated (p)-p65, and IκB-α (Cell Signaling Technology Inc., Beverly, MA, USA) at a 1:2000 ratio. The membranes were incubated overnight at 4 °C with mild agitation. After incubation, membranes were rinsed with Tris-buffered saline with Tween 20 (TBST) buffer, probed with a secondary antibody at a 1:8000 ratio, and incubated at room temperature for 2 h with agitation. Membranes were washed again with TBST, and blotted proteins were visualized using chemiluminescence reagent-processed X-ray films. The intensity of the bands was digitized using the ImageJ software.
Statistical analysis
All experiments were repeated at least 3-times and results have been represented as mean with standard deviation. All results were analyzed by one-way analysis of variance with Duncan’s test to compare the significance. Differences less than 5% (p < 0.05) were considered significant, and all analyses were performed using the SPSS software.
Results and discussion
Fermentation of soil-cultivated and hydroponic ginseng
Before and after fermentation, β-glucosidase activity of L. brevis B7 and B13-2 were analyzed. β-Glucosidase is representative fermentation-involved enzyme and the activity of B7 was approximately 20.31 mU/mL, which was 2-times higher than B13-2 (9.46 mU/mL). Furthermore, preliminary antioxidant assays using non-fermented and fermented ginseng were also conducted. DPPH and ABTS radical scavenging activities of fermented ginseng were presented 2-times higher than non-fermented ginseng (data not shown). Upon comparing the two L. brevis strains, B7 was found to increase antioxidant activity more than B13-2; therefore, L. brevis B7 was selected as the ginseng fermentation strain.
Each ginseng extract was fermented by using B7 strain for 24 h, and the changes in pH and number of viable cells were estimated. As shown in Fig. 1A, the initial pH of all ginsengs was approximately 6.4 and it gradually decreased to 3.50, 3.88, and 3.71 in SG, HG1, and HG2, respectively. The viable number of B7 usually increased until 16–20 h; however, it gradually decreased, as shown in Fig. 1B. The change in pH and cell number might be correlated, because the lowest pH and the highest cell number values were observed in SG. Additionally, the opposite result was observed in HG1. The increased number of B7 during fermentation would produce more lactic acid and metabolites from ginseng compounds through bioconversion (Hwang et al., 2019b). The decrease in the number of B7 might be attributed to the depletion of nutritional sources and generation of antimicrobial components during fermentation. Several lactic acid bacteria have been reported to generate antimicrobial butyric acids and organic acids as metabolites from herbal plants (Kim et al., 2020).
Fig. 1.
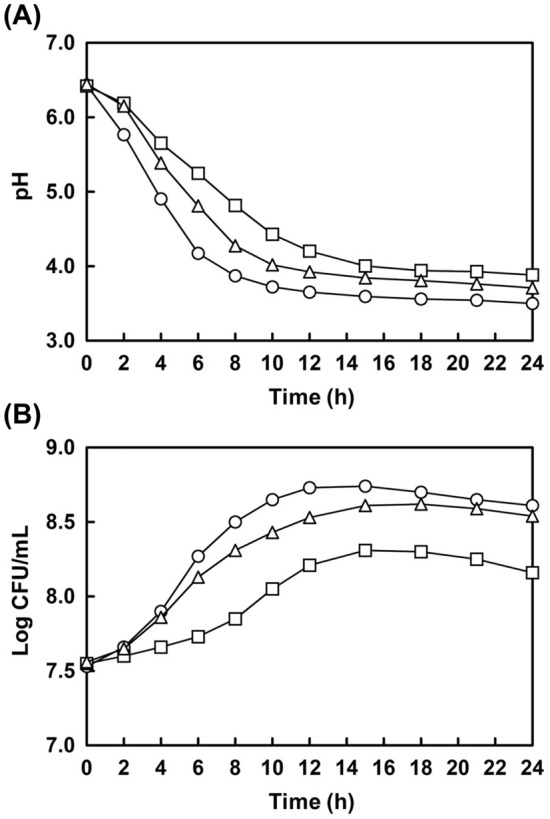
Changes in pH and growth characteristics of L. brevis B7 during fermentation of ginseng extracts. ○, Soil-cultivated ginseng extract; □, 1-year cultivated hydroponic ginseng extract; △, 2-year cultivated hydroponic ginseng extract
Total solid, phenol, and flavonoid content
TS, TPC, and TFC of ginseng were evaluated before and after fermentation, and the results were presented in Table 1. HG consistently showed higher TPC and TFC than SG both before and after fermentation, and similar trends have been reported in previous studies (Hwang et al., 2019b). TS content was slightly decreased by 0.4-0.7% after fermentation in all types of ginseng extracts. Even though TS decreased after fermentation, TPC and TFC were increased in all types of ginsengs. The increased amount of TPC was 2 times higher in HG1 and HG2 than in SG, and in the case of TFC, the increased amount of HG2 seemed to be 2 times higher than SG and HG1. In summary, HG1 showed the highest TPC and TFC; however, better fermentation efficacy was observed in HG2.
Table 1.
Total solid, phenolic, and flavonoid contents of non-fermented and fermented ginseng extracts
| Contents | Ginseng type and fermented condition | |||||
|---|---|---|---|---|---|---|
| NSG | FSG | NH1 | FH1 | NH2 | FH2 | |
| Total solid content (%) | 0.80 ± 0.07 | 0.76 ± 0.02 | 0.80 ± 0.04 | 0.73 ± 0.01 | 0.72 ± 0.01 | 0.67 ± 0.03 |
|
Total phenol content (mg GAE/g) |
39.20 ± 1.38 | 47.34 ± 0.99 | 53.40 ± 0.71 | 67.88 ± 2.16 | 50.06 ± 0.93 | 64.07 ± 2.41 |
|
Total flavonoid content (mg QE/g) |
10.72 ± 0.29 | 13.52 ± 0.25 | 16.98 ± 0.49 | 19.78 ± 0.49 | 13.52 ± 0.34 | 18.63 ± 0.75 |
All values have been presented as mean ± standard deviation of triplicate experiments
N, non-fermented; F, fermented by L. brevis B7; SG, soil-cultivated ginseng; H1, hydroponic ginseng cultivated for 1 year; H2, hydroponic ginseng cultivated for 2 years; GAE, gallic acid equivalent; QE, quercetin equivalent
HPLC analysis for ginsenoside composition
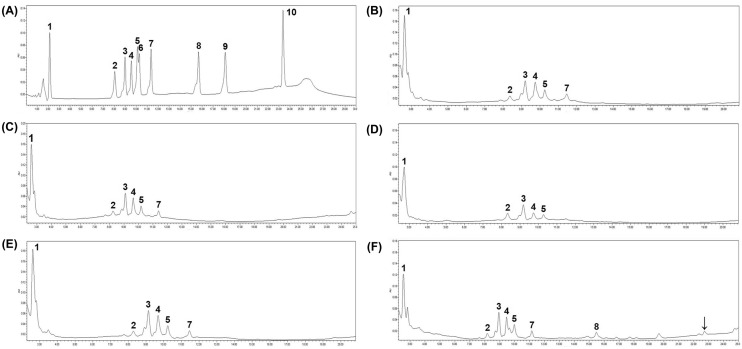
The ginsenoside composition between SG and HG were assessed using HPLC analysis. Since fermented HG2 showed better fermentation efficacy in the preliminary antioxidant assay, TPC, and TFC results, only fermented HG2 was analyzed. These results are shown in Fig. 2. Ginsenoside standards were used for comparison (Fig. 2A), and the results of non-fermented SG, HG1, HG2, fermented HG2 for 1 d, and 7 d were represented in Fig. 2B–F, respectively. Ginsenosides Re, Rf, Rb1, Rc, and Rb2 were commonly detected in all ginsengs. The differences between SG and HG were the existence of Rd and a relatively higher proportion of Rc in HG extracts, and similar ginsenoside composition also have been reported (Hwang et al., 2019b). However, significant differences were not detected between HG1, HG2, and fermented HG2 for 1 day. The concentrations of ginsenoside after fermentation were 14.15 (Re), 3.17 (Rf), 5.92 (Rb1), 5.24 (Rc), 2.36 (Rb2), and 1.06 (Rd) mg/g, respectively. Although a small peak for ginsenoside F2 appeared in the results of 7 days fermentation, the number of B7 decreased from 16 h onward after fermentation. Therefore, it was considered that these results might be attributable to the acidic environment caused by the metabolites of B7 rather than its enzymatic conversion. Additionally, another new peak appeared at a retention time similar to that of CK (black arrow in Fig. 2F); however, it was uncertain because there was a slight gap between the retention time of standard CK and the observed peak. Among the assessed ginsenosides, ginsenosides Rb1, Rb2, and Rc could be transformed into ginsenoside Rd, which was subsequently converted into Rg3, F2, and CK. Additionally, ginsenosides Re and Rf could be converted into ginsenoside Rh1 (Park et al., 2017). Particularly, ginsenosides Rb1, Rb2, Rd, and Re have been reported to exert antioxidant and anti-inflammatory effects (Park et al., 2021).
Fig. 2.
HPLC analysis of non-fermented ginseng and fermented ginseng extracts. (A) Mixtures of ginsenoside standard materials; (B) soil-cultivated ginseng extract; (C) 1-year cultivated hydroponic ginseng extract; (D) 2-year cultivated hydroponic ginseng extract; (E) fermented 2-year cultivated hydroponic ginseng extract by L. brevis B7 for 1 day; (F) fermented 2-year cultivated hydroponic ginseng extract by L. brevis B7 for 7 days. 1. Re; 2, Rf; 3, Rb1; 4, Rc; 5, Rb2; 6, Rb3; 7, Rd; 8, F2; 9, Rg3; 10, CK
Antioxidant activity of non-fermented and fermented ginseng
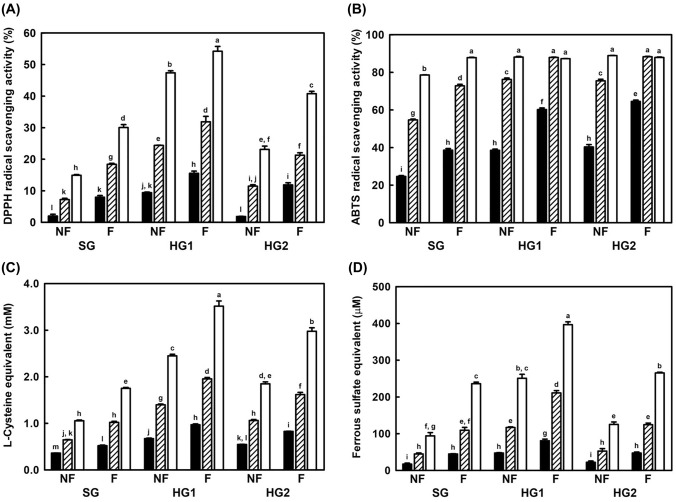
DPPH, ABTS, reducing power, and FRAP assays, were performed to evaluate the antioxidant activities. As shown in Fig. 3, all extracts were diluted to 5, 2.5, and 1 mg/mL, and the results showed a dose-dependent manner. All ginseng samples showed both radical scavenging and ferric-reducing activity. HG consistently showed higher antioxidant activities than SG, and HG1 showed the highest antioxidant activities. The antioxidant activities were increased after fermentation, and the fermentation efficacy was highest in HG2. These results would be correlated with the TPC and TFC results. Therefore, the antioxidant activities of ginseng were considered to be positively correlated with phenolic and flavonoid contents.
Fig. 3.
Antioxidant activity of non-fermented and fermented ginseng extracts. (A) DPPH radical scavenging assay; (B) ABTS radical scavenging assay; (C) reducing power assay; (D) FRAP assay. ■, 1.0 mg/mL;  , 2.5 mg/mL; □, 5.0 mg/mL. NF non-fermented ginseng extract, F fermented ginseng extract by L. brevis B7, SG, soil-cultivated ginseng, HG1 1-year cultivated hydroponic ginseng, HG2 2-year cultivated hydroponic ginseng. All data have been represented as mean ± standard deviation with triplicate experiments. The different letters above error bars indicate significant differences within the same assay (p < 0.05)
, 2.5 mg/mL; □, 5.0 mg/mL. NF non-fermented ginseng extract, F fermented ginseng extract by L. brevis B7, SG, soil-cultivated ginseng, HG1 1-year cultivated hydroponic ginseng, HG2 2-year cultivated hydroponic ginseng. All data have been represented as mean ± standard deviation with triplicate experiments. The different letters above error bars indicate significant differences within the same assay (p < 0.05)
Ginseng could reportedly enhance the activity of antioxidant enzymes such as GSH and SOD in serum, and decrease the accumulation of fibrosis, lipids, and oxidative stress (Jia et al., 2014). Ginsenoside Rg3 suppressed the production of ROS through the regulation of the Nrf-2 pathway, and ginsenoside Re, Rd, and Rh2 increased the activity of SOD, GSH, and CAT, and decreased MDA levels (Chen et al., 2019; Zhang et al., 2019). In another study, uronic acids in ginseng were represented high ferric reducing and radical scavenging activities. Uronic acids are regarded as key compounds for the antioxidant activity of ginseng because of their hydrogen-donating abilities (Zhao et al., 2020). Although ginsenoside Rg1 is known as a strong antioxidant compound, the amount of Rg1 is not related to its antioxidant activity. Likewise, phenolic compounds seemed to exhibit a slightly better proportional relationship with antioxidant activities than the composition of ginsenosides; however, it was also difficult to define it as an absolute correlation (Lee et al., 2016).
Overall, ginseng has been shown to demonstrate antioxidant activities; however, these results cannot be attributed to a single factor. The antioxidant ability of ginseng might be ascribed to a combination of various antioxidant-related factors, such as ginsenosides, phenolic compounds, and oligosaccharides.
NO production
To assess the anti-inflammatory activity, MTT and NO assays were performed to determine the cytotoxicity and inhibition of NO production, respectively. Through the MTT assay, appropriate sample concentrations with more than 95% cell viability were determined (data not shown), and these conditions were considered for further analysis.
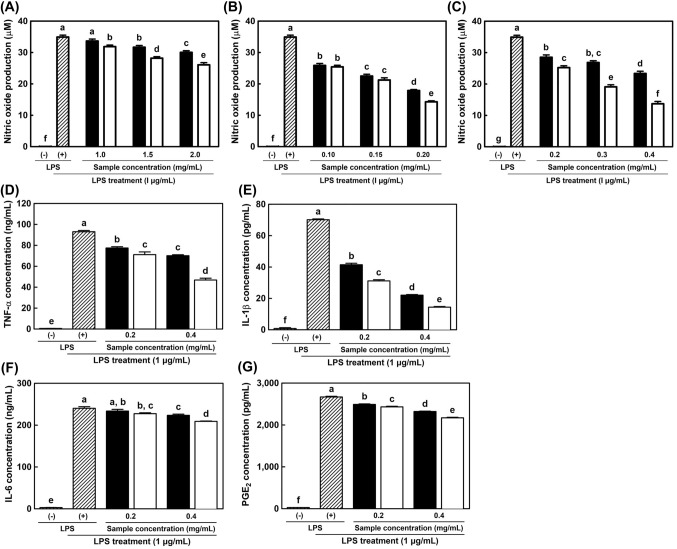
As shown in Fig. 4A–C, the amount of NO was dose-dependently decreased in sample-treated groups. Although the concentration of SG was higher than other samples, they did not effectively inhibit NO production. In contrast, HG1 significantly decreased NO production. NO was produced approximately 34.92 µM in LPS (+) group, and 30.04 (SG), 17.87 (HG1), and 23.35 µM (HG2) of NO were produced in non-fermented sample-treated groups with maximal concentration. In case of fermented sample, 26.06 (SG), 14.28 (HG1), and 13.70 (HG2) µM of NO were generated. The highest anti-inflammatory activity was observed in non-fermented HG1-treated group. However, HG2-treatment exerted the highest inhibitory effect on NO production after fermentation. The common characteristic was that the amount of NO decreased after fermentation in all types of ginsengs. Primarily, the highest efficiency was observed in fermented HG2. For these reasons, HG2 was used for the ELISA and western blotting analysis.
Fig. 4.
Downregulation of nitric oxide (A–C) and pro-inflammatory cytokines (D–G) production by treatment with non-fermented and fermented ginseng extracts in LPS-induced RAW 264.7 cells. (A) Soil-cultivated ginseng; (B) 1-year cultivated hydroponic ginseng; (C) 2-year cultivated hydroponic ginseng; (D) TNF-α; (E) IL-1β; (F) IL-6; (G) PGE2. ELISA analysis (D–G) was performed by treatment with HG2.  , without LPS treatment;
, without LPS treatment;  , LPS treatment with 1 µg/mL concentration; ■, non-fermented ginseng extracts; □, fermented ginseng extracts by L. brevis B7. All data have been presented as mean ± standard deviation with triplicate analysis. The different letters above error bars indicate significant differences within the same ginseng type (p < 0.05)
, LPS treatment with 1 µg/mL concentration; ■, non-fermented ginseng extracts; □, fermented ginseng extracts by L. brevis B7. All data have been presented as mean ± standard deviation with triplicate analysis. The different letters above error bars indicate significant differences within the same ginseng type (p < 0.05)
Assessment of pro-inflammatory cytokines through ELISA kits
To investigate the anti-inflammatory activity, the amounts of pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6, and PGE2 were measured in LPS-induced RAW 264.7 cells using ELISA kits. LPS is a major component of gram-negative bacterial cell wall and induces macrophages to produce secondary mediators, including cytokines and leukotrienes.
These cytokines can stimulate the expression of iNOS, COX-2, chemokines, and intracellular adhesion molecule-1, and differentiation, proliferation, and transmigration of T and B lymphocytes, macrophages, neutrophils, and eosinophils occurred. These immune cells continuously produced more inflammatory cytokines, which results in the damages on proteins and DNA, and lipid oxidation (Ruparelia et al., 2017). PGE2 which relieves the threshold of symptoms is produced through both COX-1 and COX-2; however, COX-2 is mainly involved in PGE2 generation. Furthermore, COX-2 expression is induced by IL-1 and TNF-α (Wang et al., 2014).
In this study, non-fermented and fermented HG2 were used at concentrations of 0.4 and 0.2 mg/mL. As shown in Fig. 4D–G, all mediators were considerably expressed by LPS treatment compared to non-LPS treatment. However, HG2 treatment reduced the production of pro-inflammatory mediators. IL-6 and PGE2 levels were not markedly decreased, however, their differences were statistically significant. Furthermore, both non-fermented and fermented HG2 extracts showed significant reductions in TNF-α and IL-1β levels. All results showed a dose-dependent tendency, and fermented extracts were consistently more effective than those observed in non-fermented ginseng treatment. Downregulation of pro-inflammatory cytokine expression was facilitated by HG2 extract treatment and by using its extract fermented by B7. Therefore, the anti-inflammatory potential of HG2 may be related to the regulation of the NF-κB signaling pathway.
Downregulation of pro-inflammatory mediator expression through western blotting
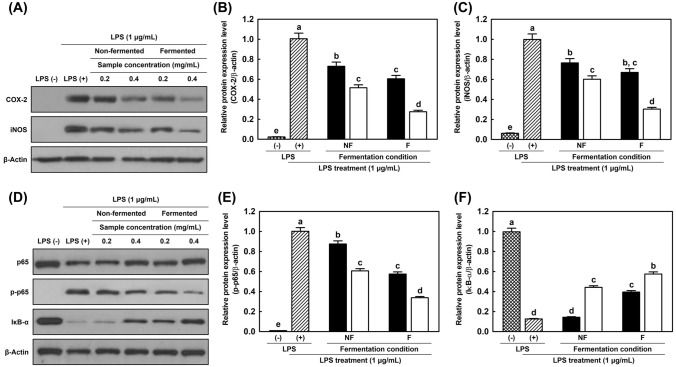
To estimate the anti-inflammatory ability of non-fermented and fermented HG2, the regulation of pro-inflammatory mediators was estimated at the protein level by western blotting. Changes in the levels of COX-2, iNOS, and NF-κB subunits (p65, p-p65, and IκB-α), were determined.
COX is an inflammation-associating enzyme classified as COX-1 and COX-2, whose major function is the production of prostaglandins and thromboxanes from arachidonic acids. Normally, COX-1 is consistently produced in tissues; however, the production of COX-2 is highly induced when the body is subjected to inflammatory responses. Preventing the expression of PGE2 and thromboxane through COX-2 regulation is an essential strategy in the pharmaceutical field because it is directly associated with alleviation of pain. Additionally, COX-2 is also known to play a role in carcinogenesis and inactivation of COX-2 has been shown to present antitumor and anti-angiogenic functions in human cancers (Wang et al., 2014).
iNOS promotes the expression of NO. iNOS is highly stimulated by interferon (IFN)-γ and LPS. NO modulates several immune responses via regulation of apoptosis in tissue cells and function of immune cells, and thus NO has been reported to be an important factor in the development of inflammation (Tripathi et al., 2007).
As shown in Fig. 5A–C, changes in the protein expression of COX-2 and iNOS were observed. When compared to the LPS-treated group, HG2 treatment significantly reduced expression. The highest inhibitory activities were observed in the high-concentration fermented group compared to the other samples. These results correlated with previous results, as shown in Fig. 4A–C Inhibition of NO production in the NO assay was associated with a decrease in iNOS expression. Additionally, the reduction of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 might influence COX-2 expression, and thus PGE2 expression was also affected by these factors.
Fig. 5.
Downregulation of COX-2 and iNOS expression (A–C), and NF-κB signaling pathway (D–F) by treatment with non-fermented and fermented 2-year cultured hydroponic ginseng extracts in LPS-induced RAW 264.7 cells. (A, D) Immunoblotting results; (B) COX-2; (C) iNOS; (E) p-p65; (F) IκB-α. Relative band intensity was measured against β-actin.  , without LPS treatment;
, without LPS treatment;  , LPS treatment with 1 µg/mL concentration; ■, 0.2 mg/mL; □, 0.4 mg/mL. NF, non-fermented ginseng extracts; F, fermented ginseng extracts by L. brevis B7. Cells were subjected to treatments with non-fermented and fermented HG2 extracts (0.2 and 0.4 mg/mL) and inflammatory responses were induced using LPS for 30 min (A–C) and 24 h (D–F). The protein expression levels of pro-inflammatory mediators and β-actin were measured using western blotting. β-Actin was used as a loading control
, LPS treatment with 1 µg/mL concentration; ■, 0.2 mg/mL; □, 0.4 mg/mL. NF, non-fermented ginseng extracts; F, fermented ginseng extracts by L. brevis B7. Cells were subjected to treatments with non-fermented and fermented HG2 extracts (0.2 and 0.4 mg/mL) and inflammatory responses were induced using LPS for 30 min (A–C) and 24 h (D–F). The protein expression levels of pro-inflammatory mediators and β-actin were measured using western blotting. β-Actin was used as a loading control
The anti-inflammatory abilities of HG2 were also evaluated through the regulation of the NF-κB signaling pathway. NF-κB is composed of inducible transcription components (p50/p65) which regulate gene expression associated with immune and inflammatory responses. Normally, inactive hetero- or homo-dimer forms are present in the cytosol, and their activation is impeded by actions of the inhibitors of NF-κB (IκB) family (Liu et al., 2017). Once inflammatory stimuli such as LPS counter NF-κB, inflammatory signaling is activated, IκB is phosphorylated and degraded, and p50 and p65 are also phosphorylated and migrate into the nucleus to activate the transcription of iNOS and pro-inflammatory cytokines. Hence, activation of the NF-κB pathway could be assessed by the detection of p65 and IκB-α levels. Degradation of IκB-α through phosphorylation by the IκB kinase (IKK) complex allows the activation of the NF-κB pathway, and thus investigation of the reduced IκB-α is another inflammatory indicator (Liu et al., 2017). The NF-κB pathway is also triggered by transforming growth factor-β-activated kinase 1 (TAK1) that incorporates the pattern recognition receptor (PRR) pathways for NF-κB activation. Once activated, TAK1 triggers the activation of downstream IKK, which phosphorylates the IκB family, which in turn degrades IκB-α and activates NF-κB (Lu et al., 2008).
The downregulation of non-fermented and fermented HG2 on the NF-κB pathway is illustrated in Fig. 5D–F. The level of p65 was relatively decreased in the LPS-treated group compared to that in the LPS-non-treated group. Both non-fermented and fermented samples with high concentrations showed remarkable intensity. However, generation of p-p65 was inhibited by HG2 treatment and its fermented samples with high concentrations showed the weakest intensity. As mentioned previously, IκB-α is degraded via inflammatory stimulation. LPS treatment and a low concentration of non-fermented sample presented extremely weak intensity of IκB-α because it was degraded by phosphorylation. The LPS-negative group and 0.4 mg/mL of the fermented HG2 sample-treated group showed a high intensity of IκB-α. This indicated that hydroponic ginseng extracts and their ferments by B7 exhibited anti-inflammatory activities through the regulation of the NF-κB pathway.
In conclusion, the functional characteristics of HG and its fermented extracts using L. brevis B7 were investigated in this study and HG demonstrates advantages in accessibility because of its short cultivation periods and lower cost compared to SG. Additionally, leaves, stems, and roots are available and levels of phenolic, flavonoid, and ginsenoside contents are also higher than SG. Through the β-glucosidase assay and preliminary antioxidant assays, L. brevis B7 was selected as the fermentation strain. Three types of ginseng extracts, including SG, HG1, and HG2 and its fermented extracts were used in the present study. After fermentation, total phenolic and flavonoid contents were improved in all types of ginsengs, and HG1 exhibited the highest amount of these compounds. Biotransformation of ginsenosides was expected through fermentation, although remarkable changes were not detected during 1 day of fermentation. Nevertheless, fermented ginseng presented better antioxidant and anti-inflammatory activities than non-fermented ginseng, and the highest fermentation efficacy was observed in HG2. Furthermore, anti-inflammatory activity was assessed using the HG2 extract. Down-regulatory effects of fermented HG2 on expression of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, and PGE2) and iNOS, COX-2, and the NF-κB signaling pathway were assessed through ELISA and western blotting, respectively. These results suggest that fermented HG by probiotic strains possesses health-enhancing properties. Therefore, it may be used in the nutraceutical industry as a functional ingredient.
Acknowledgements
This study did not receive any fund.
Declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Myung Wook Song and Ji-Young Park have contributed equally to this work as first authors.
Contributor Information
Myung Wook Song, Email: carebie251@hanmail.net.
Ji-Young Park, Email: parkgos2@naver.com.
Won-Ju Kim, Email: jootopaz22@naver.com.
Kee-Tae Kim, Email: richard44@hanmail.net.
Hyun-Dong Paik, Email: hdpaik@konkuk.ac.kr.
References
- Bae WY, Kim HY, Choi KS, Chang KH, Hong YH, Eun J, Lee NK, Paik HD. Investigation of Brassica juncea, Forsythia suspensa, and Inula britannica: phytochemical properties, antiviral effects, and safety. BMC Complementary and Alternative Medicine. 2019;19:253. doi: 10.1186/s12906-019-2670-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae WY, Kim HY, Kim KT, Paik HD. Inhibitory effects of Inula britannica extract fermented by Lactobacillus plantarum KCCM 11613P on coagulase activity and growth of Staphylococcus aureus including methicillin-resistant strains. Journal of Food Biochemistry. 2019;43:e12785. doi: 10.1111/jfbc.12785. [DOI] [PubMed] [Google Scholar]
- Chen C, Zhang H, Xu H, Zheng Y, Wu T, Lian Y. Ginsenoside Rb1 ameliorates cisplatin-induced learning and memory impairments. Journal of Ginseng Research. 2019;43:499–507. doi: 10.1016/j.jgr.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Wu S, Zhao C, Yin C. Microbial conversion of major ginsenosides in ginseng total saponins by Platycodon grandiflorum endophytes. Journal of Ginseng Research. 2016;40:366–374. doi: 10.1016/j.jgr.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom SJ, Hwang JE, Kim KT, Paik HD. Increased antioxidative and nitric oxide scavenging activity of ginseng marc fermented by Pediococcus acidilactici KCCM 11614P. Food Science and Biotechnology. 2017;27:185–191. doi: 10.1007/s10068-017-0207-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SY, Kim J, Kim E, Kim SH, Seo DB, Kim JH, Shin SS, Cho JY. AKT-targeted anti-inflammatory activity of Panax ginseng calyx ethanolic extract. Journal of Ginseng Research. 2018;42:496–503. doi: 10.1016/j.jgr.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang CH, Lee SH, Jang GY, Hwang IG, Kim HY, Woo KS, Lee J, Jeong HS. Changes in ginsenoside compositions and antioxidant activities of hydroponic-cultured ginseng roots and leaves with heating temperature. Journal of Ginseng Research. 2014;38:180–186. doi: 10.1016/j.jgr.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JE, Kim KT, Paik HD. Improved antioxidant, anti-inflammatory, and anti-adipogenic properties of hydroponic ginseng fermented by Leuconostoc mesenteroides KCCM 12010P. Molecules. 2019;24:3359. doi: 10.3390/molecules24183359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JE, Suh DH, Kim KT, Paik HD. Comparative study on anti-oxidative and anti-inflammatory properties of hydroponic ginseng and soil-cultured ginseng. Food Science and Biotechnology. 2019;28:215–224. doi: 10.1007/s10068-018-0450-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang YB, Koh EM. Antioxidant content and activity in leaves and petioles of six sweet potato (Ipomoea batatas L.) and antioxidant properties of blanched leaves. Food Science and Biotechnology. 2018;28:337–345. doi: 10.1007/s10068-018-0481-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia C, Xiong M, Wang P, Cui J, Du X, Yang Q, Wang W, Chen Y, Zhang T. Notoginsenoside R1 attenuates atherosclerotic lesions in ApoE deficient mouse model. PLoS One. 2014;9:e99849. doi: 10.1371/journal.pone.0099849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Bae WY, Yu HS, Chang KH, Hong YH, Lee NK, Paik HD. Inula britannica fermented with probiotic Weissella cibaria D30 exhibited anti-inflammatory effect and increased viability in RAW 264.7 cells. Food Science and Biotechnology. 2020;29:569–578. doi: 10.1007/s10068-019-00690-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH. Gut microbiota-mediated pharmacokinetics of ginseng saponins. Journal of Ginseng Research. 2018;42:255–263. doi: 10.1016/j.jgr.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Mo EJ, Choi JE, Jo YH, Jang H, Jeong JY, Jin Q, Chung HN, Hwang BY, Lee MK. Effect of Korean red ginseng extraction conditions on antioxidant activity, extraction yield, and ginsenoside Rg1 and phenolic content: Optimization using response surface methodology. Journal of Ginseng Research. 2016;40:229–236. doi: 10.1016/j.jgr.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Yang H, Lee TK, Lee CH, Seo JW, Kim JE, Kim SY, Park JHY, Lee KW. A short-term, hydroponic-culture of ginseng results in a significant increase in the anti-oxidative activity and bioactive components. Food Science and Biotechnology. 2020;29:1007–1012. doi: 10.1007/s10068-020-00735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduction and Targeted Therapy. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Magwaza ST, Magwaza LS, Odindo AO, Mditshwa A. Hydroponic technology as decentralised system for domestic wastewater treatment and vegetable production in urban agriculture: A review. Science of the Total Environment. 2020;698:134154. doi: 10.1016/j.scitotenv.2019.134154. [DOI] [PubMed] [Google Scholar]
- Park SE, Na CS, Yoo SA, Seo SH, Son HS. Biotransformation of major ginsenosides in ginsenoside model culture by lactic acid bacteria. Journal of Ginseng Research. 2017;41:36–42. doi: 10.1016/j.jgr.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Hyun SH, In G, Park CK, Kwak YS, Jang YJ, Kim B, Kim JH, Han CK. The antioxidant activities of Korean red ginseng (Panax ginseng) and ginsenosides: A systemic review through in vivo and clinical trials. Journal of Ginseng Research. 2021;45:41–47. doi: 10.1016/j.jgr.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z, Li W, Tan J, Wang C, Lin H, Zhou B, Liu J, Li P. Effect of ginsenoside Rh2 on renal apoptosis in cisplatin-induced nephrotoxicity in vivo. Phytomedicine. 2019;61:152862. doi: 10.1016/j.phymed.2019.152862. [DOI] [PubMed] [Google Scholar]
- Ruparelia N, Chai JT, Fisher EA, Choudhury RP. Inflammatory processes in cardiovascular disease: A route to targeted therapies. Nature Reviews Cardiology. 2017;14:133–144. doi: 10.1038/nrcardio.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupasinghe HPV, Parmar I, and Neir SV. Biotransformation of cranberry proanthocyanidins to probiotic metabolites by Lactobacillus rhamnosus enhances their anticancer activity in HepG2 cells in vitro. Oxidative Medicine and Cellular Longevity. 2019: 4750795 (2019) [DOI] [PMC free article] [PubMed]
- Song MW, Jang HJ, Kim KT, Paik HD. Probiotic and Antioxidant Properties of Novel Lactobacillus brevis KCCM 12203P Isolated from Kimchi and Evaluation of Immune-Stimulating Activities of Its Heat-Killed Cells in RAW 264.7 Cells. Journal of Microbiology and Biotechnology. 2019;29:1894–1903. doi: 10.4014/jmb.1907.07081. [DOI] [PubMed] [Google Scholar]
- Song YN, Hong HG, Son JS, Kwon YO, Lee HH, Kim HJ, Park JH, Son MJ, Oh JG, Yoon MH. Investigation of ginsenosides and antioxidant activities in the roots, leaves, and stems of hydroponic-cultured ginseng (Panax ginseng Meyer) Preventive Nutrition and Food Science. 2019;24:283–292. doi: 10.3746/pnf.2019.24.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi P, Tripathi P, Kashyap L, Singh V. The role of nitric oxide in inflammatory reactions. FEMS Immunology and Medical Microbiology. 2007;51:443–452. doi: 10.1111/j.1574-695X.2007.00329.x. [DOI] [PubMed] [Google Scholar]
- Wan Y, Wang J, Xu J, Tang F, Chen L, Tan Y, Rao C, Ao H, Peng C. Panax ginseng and its ginsenosides: Potential candidates for the prevention and treatment of chemotherapy-induced side effects. Journal of Ginseng Research. 2021;45:617–630. doi: 10.1016/j.jgr.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Guo X, Li H, Zhao J, Shao X, Wu C. Prognostic significance of cyclooxygenase-2 protein in pancreatic cancer: A meta-analysis. Tumor Biology. 2014;35:10301–10307. doi: 10.1007/s13277-014-2260-y. [DOI] [PubMed] [Google Scholar]
- Xu QF, Fang XL, Chen DF. Pharmacokinetics and bioavailability of ginsenoside Rb1 and Rg1 from Panax notoginseng in rats. Journal of Ethnopharmacology. 2003;84:187–192. doi: 10.1016/S0378-8741(02)00317-3. [DOI] [PubMed] [Google Scholar]
- Yoo JM, Lee JY, Lee YG, Baek SY, Kim MR. Enhanced production of compound K in fermented ginseng extracts by Lactobacillus brevis. Food Science and Biotechnology. 2019;28:823–829. doi: 10.1007/s10068-018-0504-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HS, Lee NK, Choi AJ, Choe JS, Bae CH, and Paik HD. Antagonistic and antioxidant effect of probiotic Weissella cibaria JW15. Food Science and Biotechnology. 28: 851-855 (2019a) [DOI] [PMC free article] [PubMed]
- Yu HS, Lee NK, Choi AJ, Choe JS, Bae CH, Paik HD. Anti-inflammatory potential of probiotic strain Weissella cibaria JW15 isolated from kimchi through regulation of NF-κB and MAPKs pathways in LPS-induced RAW 264.7 cells. Journal of Microbiology and Biotechnology. 2019;29:1022–1032. doi: 10.4014/jmb.1903.03014. [DOI] [PubMed] [Google Scholar]
- Zhang S, Sun H, Wang C, Zheng X, Jia X, Cai E, and Zhao Y. Comparative analysis of active ingredients and effects of the combination of Panax ginseng and Ophiopogon japonicus at different proportions on chemotherapy-induced myelosuppression mouse. Food and Function. 10: 1563-1570 (2019) [DOI] [PubMed]
- Zhao B, Wang X, Liu H, Lv C, Lu J. Structural characterization and antioxidant activity of oligosaccharides from Panax ginseng C. A. Meyer. International Journal of Biological Macromolecules. 2020;150:737–745. doi: 10.1016/j.ijbiomac.2020.02.016. [DOI] [PubMed] [Google Scholar]
- Zhu L, Luan X, Dou D, Huang L. Comparative analysis of ginsenosides and oligosaccharides in white ginseng (WG), red ginseng (RG) and black ginseng (BG) Journal of Chromatographic Science. 2019;57:403–410. doi: 10.1093/chromsci/bmz004. [DOI] [PubMed] [Google Scholar]