Abstract
The COVID-19 pandemic has brought significant challenges for genomic surveillance strategies in public health systems worldwide. During the past thirty-four months, many countries faced several epidemic waves of SARS-CoV-2 infections, driven mainly by the emergence and spread of novel variants. In that line, genomic surveillance has been a crucial toolkit to study the real-time SARS-CoV-2 evolution, for the assessment and optimization of novel diagnostic assays, and to improve the efficacy of existing vaccines. During the pandemic, the identification of emerging lineages carrying lineage-specific mutations (particularly those in the Receptor Binding domain) showed how these mutations might significantly impact viral transmissibility, protection from reinfection and vaccination. So far, an unprecedented number of SARS-CoV-2 viral genomes has been released in public databases (i.e., GISAID, and NCBI), achieving 14 million genome sequences available as of early-November 2022. In the present review, we summarise the global landscape of SARS-CoV-2 during the first thirty-four months of viral circulation and evolution. It demonstrates the urgency and importance of sustained investment in genomic surveillance strategies to timely identify the emergence of any potential viral pathogen or associated variants, which in turn is key to epidemic and pandemic preparedness.
Keywords: SARS-CoV-2, Viral evolution, Genomic surveillance, Epidemic-pandemic preparedness
1. SARS-CoV-2: an emerging threat of international concern
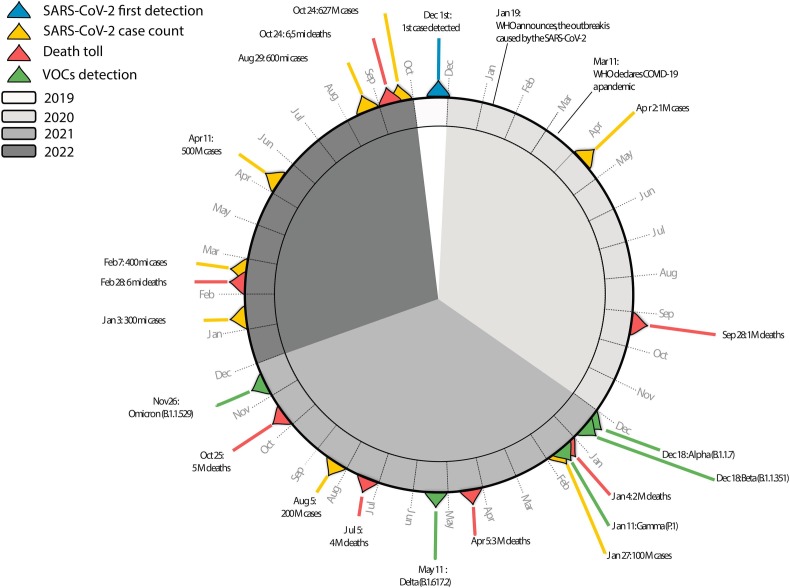
In late December 2019, The World Health Organization (WHO) office in China was informed about a cluster of novel cases of pneumonia of unknown aetiology detected in the city of Wuhan, Hubei province (Huang et al., 2020; Wu et al., 2020) (Fig. 1 ). Shortly afterwards, a new type of coronavirus, later named SARS-CoV-2, was isolated and identified by the Chinese authorities and its genetic sequence was shared with the international community on 10 January 2020 (Zhou et al., 2020) (Fig. 1). The coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 was classified as a pandemic on 11 of March 2020 (Cucinotta and Vanelli, 2020) by The World Health Organization (WHO). Up to early November 2022, more than 627 million confirmed cases of SARS-CoV-2 with exceeding 6.5 million associated deaths have been registered worldwide (WHO, 2022) (Fig. 1). As the pandemic advanced, a variety of methods were applied to understanding viral biology and identifying viral factors that influenced the evolution and global spread of this emerging threat.
Fig. 1.
Timeline of the progression and milestones occurring during the COVID-19 pandemic (December 2019–October 2022). The first COVID-19 case reported is illustrated in blue, the number of reported COVID-19 cases milestones are illustrated in yellow, the total deaths milestones are illustrated in red, the detection of VOCs are illustrated in green. The ring colour indicates different years (2019–2022), as reported in the legend on the top left. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Since the emergence of SARS-CoV-2, genome sequencing has accomplished the function of revealing genetic information, as well as in the development of specific serological and molecular diagnostic tools (Oude Munnink et al., 2021). Diagnostic testing is a critical component of monitoring, preventing and controlling transmission, for which typically the major techniques used have been: i) the detection of viral RNA by nucleic acid amplification tests (NAAT), such as isothermal amplification techniques and the real-time reverse transcription-polymerase chain reaction (RT-qPCR) which is the gold standard for the diagnosis of SARS-CoV-2 acute infection (Mardian et al., 2021); and ii) detection of viral antigens through immunodiagnostic techniques, commonly called rapid diagnostic tests (Ag-RDTs) that offer an opportunity to increase the availability and speed of testing (Mardian et al., 2021). Additionally, the diagnostic screening by NAAT based assays, was also largely used for the early detection of variants of concern (VOCs), of interest (VOI) and under monitoring (VUM), despite if in those cases, the Whole Genome Sequencing (WGS), or at least the partial S-gene sequencing, was described as the best method for characterising potential emerging strains (World Health Organization, 2021).
COVID-19 hindered the healthcare systems of many countries worldwide. During the pandemic, in order to mitigate viral spread, many countries adopted non-pharmaceutical measures such as travel bans, social distancing, school closing and personal protection (Haug et al., 2020). While it partially controlled viral spreading and kept health systems functioning, the magnitude of the impact of the pandemic has varied very differently between countries (Bedford et al., 2020) (Chen et al., 2021) due to underlying local differences such as specific governmental policy responses. Nevertheless, some of those restrictions, such as lockdowns, revealed to be economically and socially unsustainable when applied for long periods of time (Mathieu et al., 2020), with critical side effects with long-lasting societal impacts such as limited access to education (Hoofman and Secord, 2021) and health treatments of other origin (Pley et al., 2021; Sengar et al., 2022).
As part of past and ongoing efforts to contain the COVID-19 pandemic, understanding the role of asymptomatic patients in the transmission chain appeared to be essential for infection control. Asymptomatic and pre-symptomatic transmission seems to be one of the most important features of SARS-CoV-2. Individuals without symptoms can transmit the virus but estimating their influence on outbreaks might be challenging. In this respect the most important evidence comes from epidemiological studies focusing on contact tracing, which helped directly determine whether infections arose in the close contacts of asymptomatic or pre-symptomatic cases, and to what extent this occurred. Recently released data estimated that asymptomatic infections could represent an excess of one-third of all SARS-CoV-2 infections, with a higher prevalence in children compared to elderly people (Oran and Topol, 2021; Sah et al., 2021; Wang et al., 2022). Studies have also revealed that the viral load in symptomatic and asymptomatic infections does not differ substantially, although the period of viral shedding in symptomatic infections appears to be longer (Oran and Topol, 2021; Sah et al., 2021; Zuin et al., 2021). Furthermore, it is still debated the how or the extent by which asymptomatic infection might act as a “silent trigger” for long-term COVID, and what subsequent immune protection might look like (Boyton and Altmann, 2021).
The development of COVID-19 vaccines in a record time has been regarded as a triumph of biomedical research and public health. Existing vaccines have been developed using several different technologies, such as virus-like particles, inactivated viruses, protein subunits, adenoviral vectors and mRNA, leading to several hundred clinical trials and dozens of approved vaccines, representing an extraordinary advance in the field of vaccinology, with the first utilisation, in a high scale of mRNA vaccine technology (Barrett et al., 2022; Heinz and Stiasny, 2021). These technologies, allied with a global effort towards high vaccine coverage has resulted in more than 12 billion vaccine doses administered worldwide up to late October 2022 (WHO, https://covid19.who.int/).
Along the way, several challenges were faced regarding COVID-19 vaccines, such as campaigning for uptake, inequitable worldwide distribution, hesitancy, and the loss of vaccine induced-immunity due to waning and the emergence of antigenically variable and more transmissible VOCs. Despite the latter, vaccine-induced protection against severe disease remains largely preserved, especially with booster doses (Sadarangani et al., 2021; Sitaras et al., 2022). In this respect it is important to note that whereas antibodies physiologically decline after every vaccination, B and T memory cells persist and perform their function, resulting in the prevention of clinical disease (Andrews et al., 2022; Barouch, 2022).
Additionally, during the pandemic, the implementation of an integrated genomics-based surveillance system together with open sharing of genomic surveillance data and collaborative online platforms (e.g. GISAID) have enabled to follow the SARS-CoV-2 real-time evolution, allowing the identification of specific point mutations that have affected the virulence, pathogenesis, host range, immune escape as well as the effectiveness of diagnostics tests, vaccines and therapeutics (Oude Munnink et al., 2021).
In this review, we summarise the global history of SARS-CoV-2 during the first thirty-four months of viral circulation and evolution, focusing particularly on how global genomic surveillance systems enabled the identification of spread, routes and early detection and characterization of variants of concern, and how these findings would eventually direct the global pandemic response.
2. SARS-CoV-2 real-time evolution
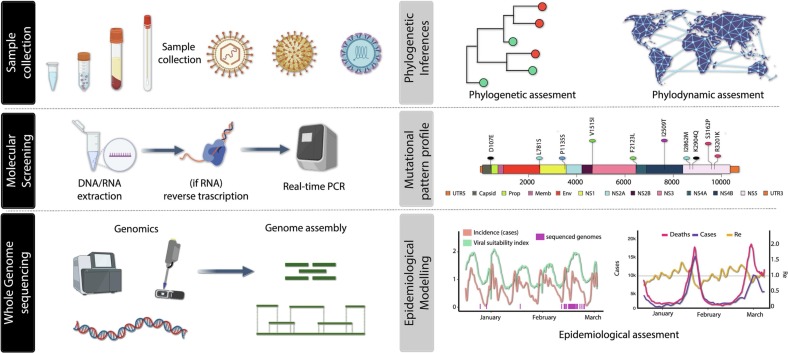
Viruses are continuously changing, and this also includes SARS-CoV-2. Genetic variations occur over time and can lead to the emergence of new variants that might express altered characteristics or phenotypes, some of which may be of public health concern. However, detection and general understanding of these variants may be hampered by limited availability of genomic and epidemiological data. Recently, a novel approach for the surveillance of emerging and re-emerging viral pathogens using High Throughput Sequencing strategies, including portable, real-time nanopore sequencing (often termed ‘genomic epidemiology (Hill et al., 2021)), has impacted the current SARS-CoV-2 pandemic allowing the generation of an unprecedented number of genomic sequences (14 million) (Khare et al., 2021) in a thirty-four months period. The framework described in Fig. 2 includes 6 main steps applied to follow the SARS-CoV-2 real-time evolution. The initial stage of investigation will be sample collection mainly through the nasopharyngeal swabs, followed by molecular screening using real-time PCR. Then, positive samples will be sent for whole genome sequencing, and after that they will either be assembled by mapping reads against a reference genome or by de novo method. The examination of the mutational pattern profile will be used to identify potential genomic changes after novel strains have been subjected to phylogenetic and phylodynamic inferences. The analysis of genomic data along with epidemiological, clinical, meteorological, and mobility data substantially facilitated extensive genomic epidemiological investigations. This allowed early sensitive detection and assignment of circulating viruses into named variants and lineages in a nearly real-time manner (Fig. 2).
Fig. 2.
Genomic surveillance framework for the real-time monitoring of emerging and re-emerging viral pathogens. The framework will include 6 steps. The first step will focus on sample collection. In the second step, outbreak isolates are submitted to molecular screening using real-time PCR. Next (iii), positive samples will be submitted to whole genome sequences and subsequently assembled either using de novo or mapped against a reference genome strategy. Next (iv), novel strains will be submitted to phylogenetic and phylodynamic inferences which in turn will make possible the identification of potential genomic differences through the analysis of the mutational pattern profile (v). In the final step (vi), genomic data will be analysed together with epidemiological, clinical, climatic and mobility data for greatly facilitating large-scale genomic epidemiological investigations.
During the COVID-19 pandemic some emerging viral variants appeared to be of particular concern because they spread more rapidly, caused more severe disease, or also appeared to be capable of immune evasion. The first identified SARS-CoV-2 variant carried a single point mutation in the spike protein (D614G), emerged early in the pandemic and spread rapidly through Europe and North America. Several lines of evidence now suggest that SARS-CoV-2 variants carrying this mutation have increased transmissibility (Korber et al., 2020; Plante et al., 2021; Volz et al., 2021; Yurkovetskiy et al., 2020; Giovanetti et al., 2022a; Nonaka et al., 2021).
For the classification of SARS-CoV-2 genetic variants, WHO uses the Pango numeric nomenclature system. In this system, when an established circulating variant shows signs of changes in the detectability, transmissibility, disease severity, immune escape, or/and susceptibility to available vaccines and treatments in multiple countries, it is categorised as a VOI. When a VOI starts to change its epidemiological behaviour, virulence, clinical symptoms, and/or global public health measures effectiveness, it is classified as a VOC (Boehm et al., 2021). A nomenclature using the Greek alphabet was proposed by the World Health Organization (WHO) and implemented starting in late May 2021 across the globe (Tracking WHO Variants, WHO).
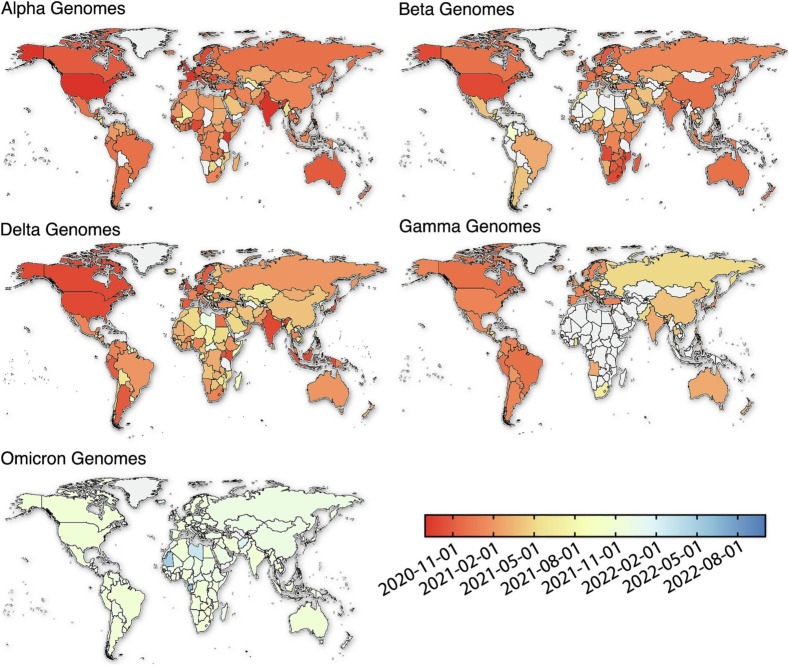
Other variants that emerged in the fall of 2020 are nowadays called previously circulating variant of concern (VOCs) (Fig. 3 ): i) The Alpha variant (alias of B.1.1.7), first detected in the United Kingdom by the end of September 2020 (Fig. 1, Fig. 3), which carries 23 nucleotide substitutions (Galloway, 2021) received WHO designation as a VOC (WHO, 2022) on December 18, 2020 and became predominant in 21 nations as of March 16, 2021; ii) The Beta variant (alias of B.1.351) which emerged independently in South Africa in May 2020 was characterised by seven mutations in the spike protein, including three at key residues in the receptor-binding domain (RDB) (K417N, E484K and N501Y) (Tegally et al., 2021). Also on December 18, 2020, WHO designated Beta as a VOC (WHO, 2022).; iii) The Gamma variant (alias of P.1), first identified in Brazilian travellers in Japan and assigned as VOC in January 2021 (WHO, 2022), which carries 17 unique mutations, including three in the (RDB) of the spike protein (K417T, E484K, and N501Y) (Faria et al., 2021) and iv) in May 2021, the Delta variant (alias of B.1.617.2) first detected in India which carries 29 mutations in several proteins, some of them in RDB in the spike protein (L452R, T478K, P681R)(Cherian et al., 2021; Giovanetti et al., 2022b). Delta was designated as a VOI on April 4, 2021, and as a VOC March 11, 2021(WHO, 2022).
Fig. 3.
VOCs world distribution according to their sequenced genomes. The legend on the bottom right indicates the progression and the spread of each VOC through time at the countrywise level.
The later identification of the Omicron (alias of B.1.1.529) variant in late November 2021 in Botswana and South Africa revealed the identification of more than 50 novel point mutations, 30 of which associated with residue change in the spike protein leading WHO to designate Omicron as a VOC on November 26, 2021 (WHO, 2022). Circulation of Omicron-related lineages (BA.1, BA.2, BA.3, BA.4, and BA.5) rapidly replaced previously circulating variants. More recently, novel Omicron sub-variants BA.4-like and BA.5-like emerged (i.e., BA.5.1, BA.5.2, BA.5.2.1, BE., BF., BN.1, BQ.1 and others) with new defining point mutations which appear to be critical for escape from immune responses generated against older variants (Tegally et al., 2022a, Tegally et al., 2022b; Tuekprakhon et al., 2022; Alcantara et al., 2022). The measurable effects of vaccination and the ongoing sustained transmission of Omicron VOC-derived lineages under no significant changes in disease rates in populations with herd-immunity to previous variants raises questions about future viral adaptation to the human host and the transition from a pandemic to an endemic scenario.
The replacement of dominant lineages during the pandemic reflected the success of SARS-CoV-2 intrinsic mechanisms of adaptation facing the host immune system triggered or not by vaccination and the effects of previous levels of infection by other VOCs across populations. Several studies revealed sporadic evidence of SARS-CoV-2 co-infection cases (Swets et al., 2022). In addition, events of recombination were also described, and appear to be more likely in cases of a sustained long period of infection, especially in immunocompromised or immunosuppressed patients (Turakhia et al., 2022). Recombination may promote phenotypic changes and confer increases in viral fitness. Several recombination events were reported across the VOCs including Alpha-Delta, Beta-Delta, Delta-BA.1 and, BA.1-BA.2 and also across common lineages (Focosi and Maggi, 2022, p. 2). In the Pangolin lineage system (Rambaut et al., 2020), they are classified with the “X” prefix. Currently, WHO tracks the XBB variant (recombinant of BA.2.10.1 and BA.2.75 sublineages) as possible Omicron sub-variants under monitoring (Tracking WHO Variants, WHO).
3. SARS-CoV-2 spillover and spillback
Zoonotic spillover refers to the pathogen transmission from wild animals to humans. A solid percentage of human infectious diseases (70–80%) are from pathogens that originally circulated in non-human animal species (Jelinek et al., 2021). Zoonotic spillover thus has a key role in the emergence of new human infectious diseases (Jelinek et al., 2021). Identifying the factors (e.g., recurrence of interactions between humans, livestock, animals, and wild species) that allow the transmission of pathogens from wild animals to humans is essential to implement strategies focused on preventing or ameliorating new epidemics and pandemics.
Up to now, different zoonoses have emerged from the Coronaviridae family in the past century (n = 7), including three responsible for noteworthy human mortality (SARS-CoV, MERS-CoV, and SARS-CoV-2). Those viruses are believed to have originally derived from a bat reservoir species (Lytras et al., 2022; Pekar et al., 2022), although several intermediate wild animal species (such as dromedary camel, palm civet, and swine) have been suggested to participate in introduction to human populations, e.g., pangolins for SARS-CoV-2 (Schindell et al., 2022) and camels for MERS-CoV (Chu, 2015; Dudas et al., 2018).
SARS-CoV-2 has shown the ability to jump across animal species, such that viral strains might acquire unique mutations to adapt to specific hosts. Spillover (from animals) and spillback (from humans) (Fig. 4 ) thus allows for viral genetic diversity that may be of importance for both animal and human health (Fagre et al., 2021; Larsen et al., 2021; Sharun et al., 2021). Due to factors such as urbanisation and globalisation, there is currently a wide range of wild, farmed, and domesticated species of animals in close contact with humans across the world, within environments with adequate conditions for transmission (e.g., farms, wild-life trade). During the pandemic, spillback was shown to occur e.g., in domesticated animals such as cats and dogs (Bienzle et al., 2022), urban-related animals such as squirrels, and farmed animals such as minks and ferrets (Schindell et al., 2022). A notable example was the mid-2020 mink farm outbreak in Denmark that revealed a mink-specific SARS-CoV-2 variant with a combination of mutations not previously described, but that critically included the mutation D614G which had been shown to play a major role in the maintenance of more transmissible lineages among humans (Larsen et al., 2021). A second example was the finding of sustained transmission in deer US, which were documented to be susceptible to the infection caused by multiple SARS-CoV-2 variants (Hale et al., 2022). To date, however, there has been no evidence of spillback to the human population resulting in the emergence of successful new variants, but awareness of this possibility remains high among policy makers and genomic surveillance specialists (Tegally et al., 2022a, Tegally et al., 2022b).
Fig. 4.
Zoonotic transmission chains. Interactions between humans, and different host species (including wild, domestic animals and vectors), which are important drivers for spillover and spillback events. Different colours indicates different epidemic waves.
4. Genomic surveillance as a tool to track the dynamics of the SARS-CoV-2 pandemic
The COVID-19 pandemic marked a breakpoint for genomic surveillance across the globe, exposing the enormous challenges of implementing genomics in public health surveillance systems (Fig. 2). The first SARS-CoV-2 genomes were shared on January 10th, 2020, with its diagnostic assay available January 13th, 2020, in about less than 15 days after the notification to WHO of an unusual cluster of pneumonia cases in China. The speed of data sharing and pathogen characterization was unprecedented and genomic surveillance started to be consistently used to monitor in real-time evolution and transmission, and to identify genetic variants that could negatively impact both pharmaceutical and non-pharmaceutical countermeasures. However, regional and temporal variation in the intensity of SARS-CoV-2 genome sequencing was primarily driven by socioeconomic disparities, as evidenced by the percentage of COVID-19 cases sequenced in countries around the world. Nearly 80% of high-income countries had more than 0.5% of their COVID-19 cases sequenced, compared to less than 50% of middle and low-income countries (Brito et al., 2022). Recognising the significant opportunities afforded by genomics, there have been since the start of the pandemic major government investments in the development and implementation of whole-genome sequencing (WGS), predominantly within high-income settings. The United Kingdom rapidly established the COVID-19 Genomics UK Consortium (COG-UK) (Marjanovic et al., 2022) and the United States government invested US$1.7 billion to expand genomics capacity for COVID-19 (The White House, 2021). In Australia, the pandemic highlighted the need for an integrated public health genomics surveillance system, resulting in the fast-tracked development of AusTrakka, Australia's current pathogen genomics tool (Hoang et al., 2022). A world-class capacities for genomic surveillance of SARS-CoV-2 and potential viral threats was build up by South Africa aiming to implement the Network for Genomic Surveillance in South Africa (Msomi et al., 2020) within sixty days after the first cases in the region, in May 2020. After this first genome surveillance commitment, the country is implemented tools and efforts to extend the prompt surveillance to other African countries (Tegally et al., 2022a, Tegally et al., 2022b). In South America, Brazil built up a National warning Network led by the Brazilian Ministry of Health and the Pan American Health Organization. Through this initiative a High Throughput Sequencing platform was implemented in all 27 Brazilian states that accounted for the generation of more than 190 k SARS-CoV-2 complete genomes in a 3-years-period.
Across all of these initiatives, genomic surveillance demonstrated crucial knowledge and policy making capacities, including for example: i) genotyping, serotyping, lineage assignment of circulating viral diversity; ii) identifying epidemiologically linked individuals for contact tracing; iii) identifying national and international transmission routes; iv) demonstrating that rapid and universal data sharing is feasible in research; v) democratising access to computational and bioinformatic resources and expertise, in particular from higher income countries to lower income countries; vi) detecting emerging variants of concern before their international expansion; vii) generating knowledge on the mutational landscape of circulating viruses, critical for evaluation and development of treatments and vaccines; viii) advancing methodologies and technology to deal with the never seen before demand for big data, real-time output. In the context of the latter, several inference methods were applied using large-scale models with relatively sparse sampling, as Bayesian inference to estimate the basic reproduction number (R0) (Geidelberg et al., 2021; Stockdale et al., 2022). In addition to that, significant efforts have also been made to complement traditional epidemiological tools with a recent revolution especially using artificial intelligence (AI). AI-based model systems could help to reconstruct outbreak transmission using genomic data, to estimate risk factors, to finely-detect transmission network, to improve pattern recognition of disease spread in populations and predictions of outbreaks in different geographical locations (Eraslan et al., 2019).
5. Prospects and conclusion
A take-home message from the COVID-19 pandemic is how timely generated knowledge has a critical role in the decision making towards containment and control of infectious disease threats. It has long been accepted that genomic data contributes in unique ways to a better understanding of infections in a manner essential to guide and solve issues relevant to public health. However, genomic surveillance is affected by how the sampling is performed, case identification, correct use of diagnostic tests, viral load, and timeframe from case identification to genome sequencing. These pitfalls were exposed early on during the COVID-19 pandemic. Paradoxically, in time and in the presence of investment, the pandemic also provided opportunities to demonstrate that genomics is an indispensable research tool for the real-time surveillance, policy making, control and prevention of emerging infectious diseases. The major technological and infrastructural development that took place due to a public health emergency of unprecedented dimension should not be lost. The new or revamped regional, country, and global level networks that developed and implemented sequencing and bioinformatics of SARS-CoV-2 during the pandemic need to be ensured future sustained funding and complete integration into existing national surveillance, public health, and research systems.
CRediT authorship contribution statement
Stephane Tosta: Methodology, Formal analysis, Data curation, Investigation, Writing – original draft, Writing – review & editing, Visualization. Keldenn Moreno: Investigation, Writing – original draft, Writing – review & editing. Gabriel Schuab: Investigation, Writing – original draft, Writing – review & editing. Vagner Fonseca: Methodology, Formal analysis, Investigation, Visualization. Fátima María Cardozo Segovia: Investigation, Writing – original draft, Writing – review & editing. Simone Kashima: Investigation, Writing – review & editing. Maria Carolina Elias: Investigation, Writing – review & editing. Sandra Coccuzzo Sampaio: Investigation, Writing – review & editing. Massimo Ciccozzi: Investigation, Writing – review & editing. Luiz Carlos Junior Alcantara: Investigation, Writing – review & editing. Svetoslav Nanev Slavov: Investigation, Writing – review & editing. José Lourenço: Validation, Formal analysis, Investigation, Data curation, Writing – review & editing, Visualization. Eleonora Cella: Conceptualization, Validation, Investigation, Writing – original draft, Writing – review & editing. Marta Giovanetti: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization, Supervision.
Declaration of Competing Interest
Not declared.
Acknowledgments
MG is funded by PON “Ricerca e Innovazione” 2014-2020. This work was supported in part by the CRP- ICGEB RESEARCH GRANT 2020 Project CRP/BRA20-03, Contract CRP/20/03. SNS is funded by FAPESP 2017/23205-8. JL was supported by a Principal Investigator research contract by FCiências.ID (Associação para a Investigação e Desenvolvimento de Ciências) of the Faculty of Sciences at the University of Lisbon (UIDP/4046/2020).
Data availability
No data was used for the research described in the article.
References
- Alcantara L.C.J., Nogueira E., Shuab G., Tosta S., Fristch H., Pimentel V., Souza-Neto J.A., Coutinho L.L., Fukumasu H., Sampaio S.C., Elias M.C., Kashima S., Slavov S.N., Ciccozzi M., Cella E., Lourenco J., Fonseca V., Giovanetti M. SARS-CoV-2 epidemic in Brazil: how the displacement of variants has driven distinct epidemic waves. Virus Res. 2022;2(315):198–785. doi: 10.1016/j.virusres.2022.198785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews N., Stowe J., Kirsebom F., Toffa S., Sachdeva R., Gower C., Ramsay M., Lopez Bernal J. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat. Med. 2022;28(4) doi: 10.1038/s41591-022-01699-1. Article 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch, D. H. (2022). Covid-19 VaccinesBashor, L. Gagne, R. B. Bosco-Lauth, A. M. Bowen, R. A. Stenglein, VandeWoude, M. S. (2021) SARS-CoV-2 evolution in animals suggests mechanisms for rapid variant selection. Proc. Natl. Acad. Sci., 118(44), e2105253118. doi: 10.1073/pnas.2105253118. [DOI] [PMC free article] [PubMed]
- Barrett A.D.T., Titball R.W., MacAry P.A., Rupp R.E., von Messling V., Walker D.H., Fanget N.V.J. The rapid progress in COVID vaccine development and implementation. Npj Vaccines. 2022;7(1) doi: 10.1038/s41541-022-00442-8. Article 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford J., Enria D., Giesecke J., Heymann D.L., Ihekweazu C., Kobinger G., Lane H.C., Memish Z., Oh M., Sall A.A., Schuchat A., Ungchusak K., Wieler L.H. COVID-19: towards controlling a pandemic. Lancet. 2020;395(10229):1015–1018. doi: 10.1016/S0140-6736(20)30673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienzle D., Rousseau J., Marom D., MacNicol J., Jacobson L., Sparling S., Prystajecky N., Fraser E., Weese J.S. Risk Factors for SARS-CoV-2 Infection and Illness in Cats and Dogs. Emerg. Infect. Dis. 2022;28(6):1154–1162. doi: 10.3201/eid2806.220423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm E., Kronig I., Neher R.A., Eckerle I., Vetter P., Kaiser L. Novel SARS-CoV-2 variants: the pandemics within the pandemic. Clin. Microbiol. Infect. 2021;27(8):1109–1117. doi: 10.1016/j.cmi.2021.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyton R.J., Altmann D.M. The immunology of asymptomatic SARS-CoV-2 infection: what are the key questions? Nat. Rev. Immunol. 2021;21(12):762–768. doi: 10.1038/s41577-021-00631-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito A.F., Semenova E., Dudas G., et al. Global disparities in SARS-CoV-2 genomic surveillance. Nat. Commun. 2022;13:7003. doi: 10.1038/s41467-022-33713-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Shi Y., Zhang P., Ding C. A cross-country comparison of fiscal policy responses to the COVID-19 global pandemic. J. Comparat. Policy Anal.: Res. Pract. 2021;23(2):262–273. doi: 10.1080/13876988.2021.1878885. [DOI] [Google Scholar]
- Cherian S., Potdar V., Jadhav S., Yadav P., Gupta N., Das M., Rakshit P., Singh S., Abraham P., Panda S., Team N. SARS-CoV-2 spike mutations, L452R, T478K, E484Q and P681R, in the second wave of COVID-19 in Maharashtra, India. Microorganisms. 2021;9(7):1542. doi: 10.3390/microorganisms9071542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D.K.W. Middle East respiratory syndrome coronavirus (MERS-CoV) in dromedary camels in Nigeria. Eurosurveillance. 2015;20(49):30086. doi: 10.2807/1560-7917.ES.2015.20.49.30086. [DOI] [PubMed] [Google Scholar]
- Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Bio-Med.: Atenei Parmensis. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudas G., Carvalho L.M., Rambaut A., Bedford T. MERS-CoV spillover at the camel-human interface. Elife. 2018;16(7) doi: 10.7554/eLife.31257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eraslan G., Avsec Ž., Gagneur J., Theis F.J. Deep learning: new computational modelling techniques for genomics. Nat. Rev. Genet. 2019;20(7):389–403. doi: 10.1038/s41576-019-0122-6. [DOI] [PubMed] [Google Scholar]
- Fagre A., Lewis J., Eckley M., Zhan S., Rocha S.M., Sexton N.R., Burke B., Geiss B., Peersen O., Bass T., Kading R., Rovnak J., Ebel G.D., Tjalkens R.B., Aboellail T., Schountz T. SARS-CoV-2 infection, neuropathogenesis and transmission among deer mice: implications for spillback to New World rodents. PLoS Pathog. 2021;17(5) doi: 10.1371/journal.ppat.1009585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria N.R., Mellan T.A., Whittaker C., Claro I.M., da Candido D.S., Mishra S., Crispim M.A.E., Sales F.C.S., Hawryluk I., McCrone J.T., Hulswit R.J.G., Franco L.A.M., Ramundo M.S., de Jesus J.G., Andrade P.S., Coletti T.M., Ferreira G.M., Silva C.A.M., Manuli E.R.…Sabino E.C. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil. Science. 2021;372(6544):815–821. doi: 10.1126/science.abh2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focosi D., Maggi F. Recombination in coronaviruses, with a focus on SARS-CoV-2. Viruses. 2022;14(6) doi: 10.3390/v14061239. Article 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway S.E. Emergence of SARS-CoV-2 B.1.1.7 Lineage—United States, December 29, 2020–January 12, 2021. MMWR Morb. Mortal. Wkly Rep. 2021;70 doi: 10.15585/mmwr.mm7003e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geidelberg L., Boyd O., Jorgensen D., Siveroni I., Nascimento F.F., Johnson R., Ragonnet-Cronin M., Fu H., Wang H., Xi X., Chen W., Liu D., Chen Y., Tian M., Tan W., Zai J., Sun W., Li J., Li J.…Nie Q. Genomic epidemiology of a densely sampled COVID-19 outbreak in China. Virus Evol. 2021;7(1) doi: 10.1093/ve/veaa102. veaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanetti M., Slavov S.N., Fonseca V., et al. Genomic epidemiology of the SARS-CoV-2 epidemic in Brazil. Nat. Microbiol. 2022;7(1):1490–1500. doi: 10.1038/s41564-022-01191-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanetti M., Fonseca V., Wilkinson E., Tegally H., San E.J., Althaus C.L., Xavier J., Nanev Slavov S., Viala V.L., Ranieri Jerônimo Lima A., Ribeiro G., Souza-Neto J.A., Fukumasu H., Lehmann Coutinho L., Venancio da Cunha R., Freitas C., de Campelo A.E., Melo C.F., de Navegantes Araújo W., Do Carmo Said R.F., Almiron M., de Oliveira T., Coccuzzo Sampaio S., Elias M.C., Covas D.T., Holmes E.C., Lourenço J., Kashima S., de Alcantara L.C.J. Replacement of the gamma by the Delta variant in Brazil: impact of lineage displacement on the ongoing pandemic. Virus Evol. 2022;8(1) doi: 10.1093/ve/veac024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale V.L., Dennis P.M., McBride D.S., Nolting J.M., Madden C., Huey D., Ehrlich M., Grieser J., Winston J., Lombardi D., Gibson S., Saif L., Killian M.L., Lantz K., Tell R.M., Torchetti M., Robbe-Austerman S., Nelson M.I., Faith S.A., Bowman A.S. SARS-CoV-2 infection in free-ranging white-tailed deer. Nature. 2022;602(7897):481–486. doi: 10.1038/s41586-021-04353-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug N., Geyrhofer L., Londei A., Dervic E., Desvars-Larrive A., Loreto V., Pinior B., Thurner S., Klimek P. Ranking the effectiveness of worldwide COVID-19 government interventions. Nat. Hum. Behav. 2020;4(12) doi: 10.1038/s41562-020-01009-0. Article 12. [DOI] [PubMed] [Google Scholar]
- Heinz F.X., Stiasny K. Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action. Npj Vaccines. 2021;6(1) doi: 10.1038/s41541-021-00369-6. Article 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill V., Ruis C., Bajaj S., Pybus O.G., Kraemer M.U.G. Progress and challenges in virus genomic epidemiology. Trends Parasitol. 2021;37(12):1038–1049. doi: 10.1016/j.pt.2021.08.007. [DOI] [PubMed] [Google Scholar]
- Hoang T., da Silva A.G., Jennison A.V., Williamson D.A., Howden B.P., Seemann T. AusTrakka: fast-tracking nationalized genomics surveillance in response to the COVID-19 pandemic. Nat. Commun. 2022;13(1) doi: 10.1038/s41467-022-28529-9. Article 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoofman J., Secord E. The effect of COVID-19 on education. Pediatr. Clin. N. Am. 2021;68(5):1071–1079. doi: 10.1016/j.pcl.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M.…Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek H.F., Mousa M., Alefishat E., Osman W., Spence I., Bu D., Feng S.F., Byrd J., Magni P.A., Sahibzada S., Tay G.K., Alsafar H.S. Evolution, ecology, and zoonotic transmission of Betacoronaviruses: a review. Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.644414. https://www.frontiersin.org/articles/10.3389/fvets.2021.644414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare S., Gurry C., Freitas L., Schultz M.B., Bach G., Diallo A., Akite N., Ho J., Lee R.T., Yeo W., Team G.C.C., Maurer-Stroh S. GISAID’s role in pandemic response. China CDC Weekly. 2021;3(49):1049–1051. doi: 10.46234/ccdcw2021.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korber B., Fischer W.M., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., Hengartner N., Giorgi E.E., Bhattacharya T., Foley B., Hastie K.M., Parker M.D., Partridge D.G., Evans C.M., Freeman T.M., de Silva T.I., Sheffield COVID-19 Genomics Group, McDanal C., Perez L.G.…Montefiori D.C. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182(4):812–827.e19. doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen H.D., Fonager J., Lomholt F.K., Dalby T., Benedetti G., Kristensen B., Urth T.R., Rasmussen M., Lassaunière R., Rasmussen T.B., Strandbygaard B., Lohse L., Chaine M., Møller K.L., Berthelsen A.-S.N., Nørgaard S.K., Sönksen U.W., Boklund A.E., Hammer A.S.…Mølbak K. Preliminary report of an outbreak of SARS-CoV-2 in mink and mink farmers associated with community spread, Denmark, June to November 2020. Eurosurveillance. 2021;26(5) doi: 10.2807/1560-7917.ES.2021.26.5.210009. 2100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytras S., Hughes J., Martin D., Swanepoel P., de Klerk A., Lourens R., Kosakovsky Pond S.L., Xia W., Jiang X., Robertson D.L. Exploring the natural origins of SARS-CoV-2 in the light of recombination. Genome Biol. Evol. 2022;14(2) doi: 10.1093/gbe/evac018. evac018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardian Y., Kosasih H., Karyana M., Neal A., Lau C.-Y. Review of current COVID-19 diagnostics and opportunities for further development. Front. Med. 2021;8 doi: 10.3389/fmed.2021.615099. https://www.frontiersin.org/articles/10.3389/fmed.2021.615099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjanovic S., Romanelli R.J., Ali G.-C., Leach B., Bonsu M., Rodriguez-Rincon D., Ling T. COVID-19 genomics UK (COG-UK) consortium: final report. Rand Health Quart. 2022;9(4):24. [PMC free article] [PubMed] [Google Scholar]
- Mathieu E., Ritchie H., Rodés-Guirao L., Appel C., Giattino C., Hasell J., Macdonald B., Dattani S., Beltekian D., Ortiz-Ospina E., Roser M. Our World in Data; 2020. Coronavirus Pandemic (COVID-19)https://ourworldindata.org/coronavirus [Google Scholar]
- Msomi N., Mlisana K., de Oliveira T., Msomi N., Mlisana K., Willianson C., Bhiman J.N., Goedhals D., Engelbrecht S., Zyl G.V., Preiser W., Hardie D., Hsiao M., Mulder N., Martin D., Christoffels A., York D., Giandhari J., Wilkinson E.…de Oliveira T. A genomics network established to respond rapidly to public health threats in South Africa. The Lancet Microbe. 2020;1(6):e229–e230. doi: 10.1016/S2666-5247(20)30116-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka C.K.V., Franco M.M., Gräf T., de Lorenzo Barcia C.A., de Ávila Mendonça R.N., de Sousa K.A.F., Neiva L.M.C., Fosenca V., Mendes A.V.A., de Aguiar R.S., Giovanetti M., de Freitas Souza B.S. Genomic evidence of SARS-CoV-2 reinfection involving E484K spike mutation, Brazil. Emerg. Infect. Dis. 2021;27(5):1522–1524. doi: 10.3201/eid2705.210191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oran D.P., Topol E.J. The proportion of SARS-CoV-2 infections that are asymptomatic: A systematic review. Ann. Intern. Med. 2021;174(5):655–662. doi: 10.7326/M20-6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oude Munnink B.B., Worp N., Nieuwenhuijse D.F., Sikkema R.S., Haagmans B., Fouchier R.A.M., Koopmans M. The next phase of SARS-CoV-2 surveillance: real-time molecular epidemiology. Nat. Med. 2021;27(9) doi: 10.1038/s41591-021-01472-w. Article 9. [DOI] [PubMed] [Google Scholar]
- Pekar J.E., Magee A., Parker E., Moshiri N., Izhikevich K., Havens J.L., Gangavarapu K., Malpica Serrano L.M., Crits-Christoph A., Matteson N.L., Zeller M., Levy J.I., Wang J.C., Hughes S., Lee J., Park H., Park M.-S., Yan Ching Zi, Lin K., R. T. P…Wertheim J.O. The molecular epidemiology of multiple zoonotic origins of SARS-CoV-2. Science. 2022;377(6609):960–966. doi: 10.1126/science.abp8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plante J.A., Liu Y., Liu J., Xia H., Johnson B.A., Lokugamage K.G., Zhang X., Muruato A.E., Zou J., Fontes-Garfias C.R., Mirchandani D., Scharton D., Bilello J.P., Ku Z., An Z., Kalveram B., Freiberg A.N., Menachery V.D., Xie X.…Shi P.-Y. Spike mutation D614G alters SARS-CoV-2 fitness. Nature. 2021;592(7852) doi: 10.1038/s41586-020-2895-3. Article 7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pley C.M., McNaughton A.L., Matthews P.C., Lourenço J. The global impact of the COVID-19 pandemic on the prevention, diagnosis and treatment of hepatitis B virus (HBV) infection. BMJ Glob. Health. 2021;6(1) doi: 10.1136/bmjgh-2020-004275. Article 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaut A., Holmes E.C., O’Toole Á., Hill V., McCrone J.T., Ruis C., du Plessis L., Pybus O.G. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat. Microbiol. 2020;5(11) doi: 10.1038/s41564-020-0770-5. Article 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadarangani M., Marchant A., Kollmann T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat. Rev. Immunol. 2021;21(8):475–484. doi: 10.1038/s41577-021-00578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P., Fitzpatrick M.C., Zimmer C.F., Abdollahi E., Juden-Kelly L., Moghadas S.M., Singer B.H., Galvani A.P. Asymptomatic SARS-CoV-2 infection: A systematic review and meta-analysis. Proc. Natl. Acad. Sci. U. S. A. 2021;118(34) doi: 10.1073/pnas.2109229118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindell B.G., Allardice M., McBride J.A.M., Dennehy B., Kindrachuk J. SARS-CoV-2 and the missing link of intermediate hosts in viral emergence—what we can learn from other Betacoronaviruses. Front. Virol. 2022;2 https://www.frontiersin.org/articles/10.3389/fviro.2022.875213 [Google Scholar]
- Sengar M., Chinnaswamy G., Ranganathan P., Ashok A., Bhosale S., Biswas S., Chaturvedi P., Dhamne C., Divatia J., D'Sa K., Jain H., Laskar S., Moulik N.R., Mummudi N., Nair S., Nayak L., Nayak P., Patkar S., Pawaskar P., Ramaswamy A., Shetty O., Singh A., Sridhar E., Thorat J., Badwe R., Pramesh C.S. TMH COVID-19 action group. Outcomes of COVID-19 and risk factors in patients with cancer. Nat. Can. 2022;3(5):551. doi: 10.1038/s43018-022-00363-4. [DOI] [PubMed] [Google Scholar]
- Sharun K., Dhama K., Pawde A.M., Gortázar C., Tiwari R., Bonilla-Aldana D.K., Rodriguez-Morales A.J., de la Fuente J., Michalak I., Attia Y.A. SARS-CoV-2 in animals: potential for unknown reservoir hosts and public health implications. Vet. Q. 2021;41(1):181–201. doi: 10.1080/01652176.2021.1921311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaras I., Jacobsen H., Higdon M.M., Dowling W.E., Bar-Zeev N., Deloria Knoll, M. Systematic review of primary and booster COVID-19 sera neutralizing ability against SARS-CoV-2 omicron variant. NPJ Vaccines. 2022;7(1):147. doi: 10.1038/s41541-022-00565-y. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockdale J.E., Liu P., Colijn C. The potential of genomics for infectious disease forecasting. Nat. Microbiol. 2022;7(11) doi: 10.1038/s41564-022-01233-6. Article 11. [DOI] [PubMed] [Google Scholar]
- Swets M.C., Russell C.D., Harrison E.M., Docherty A.B., Lone N., Girvan M., Hardwick H.E., Visser L.G., Openshaw P.J.M., Groeneveld G.H., Semple M.G., Baillie J.K. SARS-CoV-2 co-infection with influenza viruses, respiratory syncytial virus, or adenoviruses. Lancet. 2022;16(399):1463–1464. doi: 10.1016/S0140-6736(22)00383-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegally H., Wilkinson E., Giovanetti M., Iranzadeh A., Fonseca V., Giandhari J., Doolabh D., Pillay S., San E.J., Msomi N., Mlisana K., von Gottberg A., Walaza S., Allam M., Ismail A., Mohale T., Glass A.J., Engelbrecht S., Van Zyl G.…de Oliveira T. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature. 2021;592(7854) doi: 10.1038/s41586-021-03402-9. Article 7854. [DOI] [PubMed] [Google Scholar]
- Tegally H., Moir M., Everatt J., Giovanetti M., Scheepers C., Wilkinson E., Subramoney K., Makatini Z., Moyo S., Amoako D.G., Baxter C., Althaus C.L., Anyaneji U.J., Kekana D., Viana R., Giandhari J., Lessells R.J., Maponga T., Maruapula D.…de Oliveira T. Emergence of SARS-CoV-2 Omicron lineages BA.4 and BA.5 in South Africa. Nat. Med. 2022;28(9) doi: 10.1038/s41591-022-01911-2. Article 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegally H., San J.E., Cotten M., Moir M., Tegomoh B., Mboowa G., Martin D.P., Baxter C., Lambisia A.W., Diallo A., Amoako D.G., Diagne M.M., Sisay A., Zekri A.-R.N., Gueye A.S., Sangare A.K., Ouedraogo A.-S., Sow A., Musa A.O.…Wilkinson E. The evolving SARS-CoV-2 epidemic in Africa: insights from rapidly expanding genomic surveillance. Science. 2022;378(6615) doi: 10.1126/science.abq5358. eabq5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The White House . The White House; 2021, April 16. Fact Sheet: Biden Administration Announces $1.7 Billion Investment to Fight COVID-19 Variants.https://www.whitehouse.gov/briefing-room/statements-releases/2021/04/16/fact-sheet-biden-administration-announces-1-7-billion-investment-to-fight-covid-19-variants/ [Google Scholar]
- Tuekprakhon A., Nutalai R., Dijokaite-Guraliuc A., Zhou D., Ginn H.M., Selvaraj M., Liu C., Mentzer A.J., Supasa P., Duyvesteyn H.M.E., Das R., Skelly D., Ritter T.G., Amini A., Bibi S., Adele S., Johnson S.A., Constantinides B., Webster H.…Screaton G.R. Antibody escape of SARS-CoV-2 omicron BA.4 and BA.5 from vaccine and BA.1 serum. Cell. 2022;185(14):2422–2433.e13. doi: 10.1016/j.cell.2022.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turakhia Y., Thornlow B., Hinrichs A., McBroome J., Ayala N., Ye C., Smith K., De Maio N., Haussler D., Lanfear R., Corbett-Detig R. Pandemic-scale phylogenomics reveals the SARS-CoV-2 recombination landscape. Nature. 2022;609(7929):994–997. doi: 10.1038/s41586-022-05189-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz E., Hill V., McCrone J.T., Price A., Jorgensen D., O’Toole Á., Southgate J., Johnson R., Jackson B., Nascimento F.F., Rey S.M., Nicholls S.M., Colquhoun R.M., da Silva Filipe A., Shepherd J., Pascall D.J., Shah R., Jesudason N., Li K.…Connor T.R. Evaluating the effects of SARS-CoV-2 spike mutation D614G on transmissibility and pathogenicity. Cell. 2021;184(1):64–75.e11. doi: 10.1016/j.cell.2020.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Wu P., Wang J., Lourenço J., Li B., Rader B., Laine M., Miao H., Wang L., Song H., Bharti N., Brownstein J.S., Bjornstad O.N., Dye C., Tian H. Assessing the asymptomatic proportion of SARS-CoV-2 infection with age in China before mass vaccinationJ. R. Soc. Interface. 2022 doi: 10.1098/rsif.2022.0498. [DOI] [Google Scholar]
- WHO WHO Coronavirus (COVID-19) Dashboard. 2022, January 1. https://covid19.who.int
- World Health Organization . World Health Organization; 2021. Recommendations for National SARS-CoV-2 Testing Strategies and Diagnostic Capacities: Interim Guidance, 25 June 2021 (WHO/2019-nCoV/lab_testing/2021.1)https://apps.who.int/iris/handle/10665/342002 [Google Scholar]
- Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G., Hu Y., Tao Z.-W., Tian J.-H., Pei Y.-Y., Yuan M.-L., Zhang Y.-L., Dai F.-H., Liu Y., Wang Q.-M., Zheng J.-J., Xu L., Holmes E.C., Zhang Y.-Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798) doi: 10.1038/s41586-020-2008-3. Article 7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurkovetskiy L., Wang X., Pascal K.E., Tomkins-Tinch C., Nyalile T.P., Wang Y., Baum A., Diehl W.E., Dauphin A., Carbone C., Veinotte K., Egri S.B., Schaffner S.F., Lemieux J.E., Munro J.B., Rafique A., Barve A., Sabeti P.C., Kyratsous C.A.…Luban J. Structural and functional analysis of the D614G SARS-CoV-2 spike protein variant. Cell. 2020;183(3):739–751.e8. doi: 10.1016/j.cell.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X.…Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798) doi: 10.1038/s41586-020-2012-7. Article 7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuin M., Gentili V., Cervellati C., Rizzo R., Zuliani G. Viral load difference between symptomatic and asymptomatic COVID-19 patients: systematic review and Meta-analysis. Infect. Dis. Rep. 2021;13(3):645–653. doi: 10.3390/idr13030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.