Abstract
The degradation of collagen in different body parts is a critical point for designing collagen-based biomedical products. Here, three kinds of collagens labeled by second near-infrared (NIR-II) quantum dots (QDs), including collagen with low crosslinking degree (LC), middle crosslinking degree (MC) and high crosslinking degree (HC), were injected into the subcutaneous tissue, muscle and joints of the mouse model, respectively, in order to investigate the in vivo degradation pattern of collagen by NIR-II live imaging. The results of NIR-II imaging indicated that all tested collagens could be fully degraded after 35 days in the subcutaneous tissue, muscle and joints of the mouse model. However, the average degradation rate of subcutaneous tissue (k = 0.13) and muscle (k = 0.23) was slower than that of the joints (shoulder: k = 0.42, knee: k = 0.55). Specifically, the degradation rate of HC (k = 0.13) was slower than LC (k = 0.30) in muscle, while HC showed the fastest degradation rate in the shoulder and knee joints. In summary, NIR-II imaging could precisely identify the in vivo degradation rate of collagen. Moreover, the degradation rate of collagen was more closely related to the implanted body parts rather than the crosslinking degree of collagen, which was slower in the subcutaneous tissue and muscle compared to the joints in the mouse model.
Keywords: collagen, degradation rate, NIR-II live imaging, in vivo, crosslinking degree
Graphical abstract
Introduction
Collagen, as a friendly biomaterial for human body, has been widely employed for biomedical applications, such as disc repair, bone defect, tendon damage and drug delivery [1–7], owing to its suitable biological properties and low immunogenicity [3, 8–10]. As an additional implant, an appropriate degradation rate plays a critical role in its clinical performance. Generally, the ideal degradation of a collagen implant is expected to keep the same pace as the regeneration of the host tissue [11–13]. Rapid degradation would not support the regeneration of the injured tissue sufficiently, which would result in re-tear or re-injury [14, 15]. In contrast, the prolonged degradation period of implants could hinder the host tissue reproduction and increase the infection risk of implants and the surrounding tissue [15]. Therefore, the degradation of collagen is an essential factor for the therapeutic results of collagen-based products in the body.
In fact, the collagen degradation mainly depends on the chemical properties of itself and the biological microenvironment of the implantation sites. Firstly, crosslinking degree is one of the most critical properties that would affect the degradation [16] and the function of the collagen-based products [17, 18]. Previously, the effects of crosslinking degree of collagen on its degradation were mainly studied by ex vivo investigation of collagen mass under the exogenous collagenase [8, 17, 19]. Secondly, a key aspect of the microenvironment in the implantation sites is the level of collagenase, which is primarily secreted from macrophages and fibroblasts [20]. A considerable amount of research has investigated in vivo collagen degradation in a single body part such as subcutaneous tissue and knee joint [21–27]. However, since the level of cells and secreted collagenase varies in different body parts, investigation in a single body part is somewhat insufficient, neglecting the varying microenvironment. To date, there is little work that simultaneously takes both the crosslinking degree and the different body parts into account for the in vivo investigation of collagen degradation.
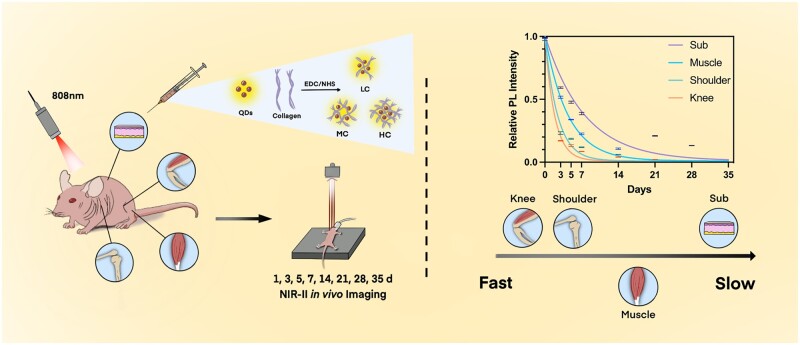
Our study was designed to investigate the role of both the crosslinking degree and the body parts on the collagen degradation (Scheme 1). Notably, second near-infrared (NIR-II) in vivo imaging was utilized as a live animal imaging technique to precisely record the dynamic changes of collagen. Firstly, highly fluorescent NIR-II PbS quantum dots (QDs) were conjugated with low crosslinking degree of collagen (LC), middle crosslinking degree of collagen (MC) and high crosslinking degree of collagen (HC), respectively. Three crosslinking degrees of collagens were injected into the subcutaneous tissue, muscle and joints of the mouse model. Then, the NIR-II fluorescence (FL) intensities of LC, MC and HC in the mouse model were monitored and analyzed by NIR-II live animal imaging from 1 h to 35 days post-injection. Ex vivo imaging and histological analysis were also employed in order to further assess the degradation properties of collagen.
Scheme 1.
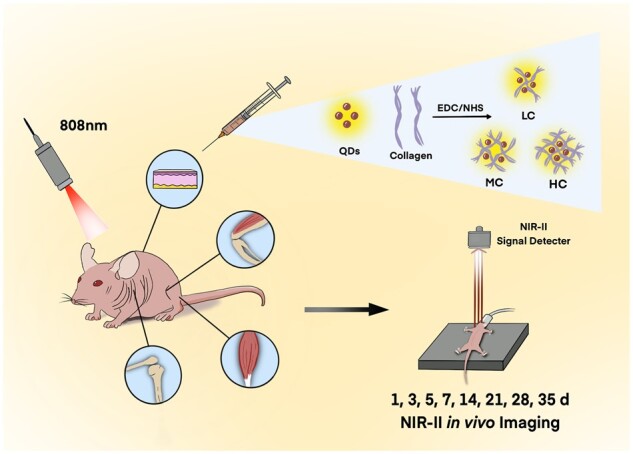
Schematic illustration of monitoring the collagen degradation in vivo.
Methods
Reagents and instrumentation
Lead (II) acetate trihydrate [Pb(CH3CO2)2·3H2O, ≥99%], sodium sulfide and anhydrous (Na2S, ≥90%), were purchased from J&K Scientific (China). Ribonuclease-A from bovine pancreas (>70 U/mg), was purchased from Sigma-Aldrich (USA). Type I collagen from cow calcaneal tendon and sodium hydroxide (NaOH, ≥95%) were bought from Shanghai Macklin Biochemical Co., Ltd. Deionized water was utilized as the working solution. The procedure of microwave synthesis was done with microwave reactor Discover SP (CEM, USA). The NIR-II FL images were obtained from the Artemis Intelligent Imaging system (Shanghai Hengguang Zhiying Medical Technology Co., Ltd.) with 808 nm of excitation source provided by two fiber-coupled lasers. NIR long-pass filter of 1250 nm was used and emitted FL signal was caught by InGaAs camera NIRvana 640 (Princeton, USA).
Preparation of QDs-labeled collagen
Highly fluorescent protein-encapsulated lead sulfide QDs were synthesized according to our previous method [28]. Briefly, 500 µl of 10 mM CH3COOHPb and 500 µl of 50 mg/ml RNase-A were mixed and 1 M NaOH was added to adjust the pH to 9–10. Then 50 µl of 10 mM Na2S was added. The tube was put into the microwave reactor immediately at 70°C for 30 s, and the dark brown product was found. Collagen was dissolved in 0.1 M MES (2-(N-morpholino)ethanesulfonic acid) buffer and mixed with different amounts of crosslinkers at room temperature for 2 h according to the previous study [19] and further conjugated with QDs for another 2 h. Specifically, collagen, EDC (1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrchloride) and NHS (N-Hydroxysuccinimide) (1:1.15:2.76) were utilized in order to prepare HC and set as 100% concentration. Besides, 10% and 50% concentrations were utilized for the same process to form LC and MC, respectively. All freshly prepared QDs-labeled collagen was ultra-centrifugated with 30 kDa ultra-centrifugal filter tube to remove excessive reagents and stored at 4°C. Then, PL (Photoluminescence) spectrum and UV (Ultraviolet) spectrum of QDs-labeled collagen were measured.
In vivo NIR-II live animal imaging
Eight-week-old female BALB/C nude mice with the weight of 23–25 g were provided by Shanghai Jiesijie Laboratory Animal Co., Ltd. Related animal studies were carried out in agreement with the guidelines approved by the Animal Care Committee of the Laboratory Animal from Fudan University. Mice were anesthetized using isoflurane at a flow rate of 1.5 l/min, while oxygen at a flow rate of 0.2 l/min was administered simultaneously, throughout injections and in vivo imaging. Ten microliters of QDs-labeled collagen (LC, MC and HC) and pure QDs were injected to the back (subcutaneous tissue), shoulder joint cavity, knee joint cavity and hind leg muscle of the nude mice. Mutual interference was avoided between the injection sites. Then NIR-II images were obtained at 1 h, 1, 3, 5, 7, 14, 21, 28 and 35 days after injection as appropriate (28 days for joints). The exposure time was 50 ms for subcutaneous tissue and 100 ms for muscle, the shoulder and knee joint. The FL intensity of injection points from each image was measured using Image J and normalized FL intensity was calculated. As collagen degradation is a kind of in vivo metabolism, the exponential decay model (y = span·e−kx + plateau) was utilized for curve fitting by Graphpad Prism 9.3.1 [29, 30], and k, as the degradation rate, was calculated as follows in this work:
Histological analysis and ex vivo NIR-II imaging
Skin, muscle, shoulder and knee joints in different groups were harvested to perform histological analysis. Injection points were marked previously under the guidance of the NIR-II imaging system. The whole layer of skin with a size ∼0.5 × 0.2 cm was collected from subcutaneously injected points. As for shoulder joints, the humerus and scapula as well as muscles and ligaments were kept to ensure that intact shoulder joint capsule was taken. Knee joints were harvested in the same way by keeping the femur and tibia as well as surrounding muscles and ligaments. Muscles with a size ∼0.5 × 0.5 cm were taken from the injection point. Masson’s trichrome (Masson) staining was performed for the specimens mentioned above. The major organs including cerebrum, lung, heart, stomach, kidney, spleen, liver and intestine were harvested and NIR-II images were taken 35 days after injection. Histological analysis of major organs from QDs-injected mice and LC-, MC- and HC-injected mice were performed and hematoxylin–eosin (HE) staining was applied.
Results
Characterization of NIR-II QDs-labeled collagen
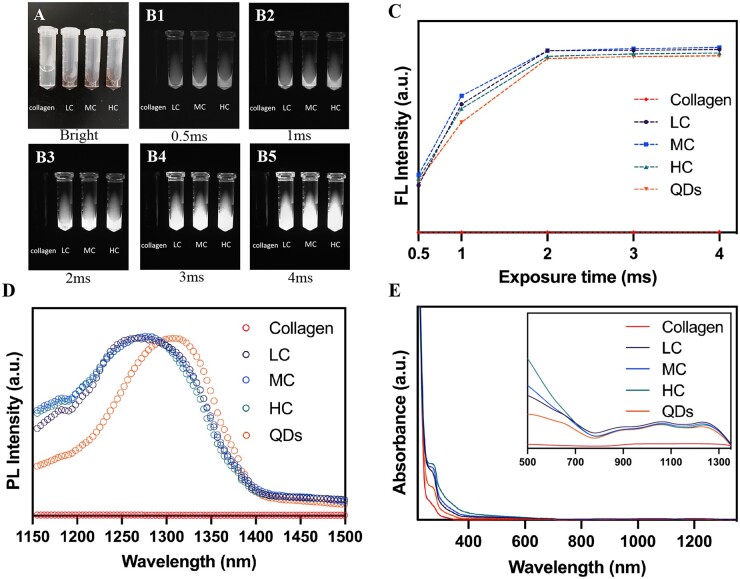
Firstly, fluorescent characteristics of QDs-labeled collagen were measured and shown in Fig. 1. Bright-field images of collagen, LC, MC and HC are demonstrated in Fig. 1A. The color of collagen turned from transparent to dark brown after labeling by NIR-II QDs. NIR-II images of collagen and QDs-labeled collagen with the exposure time ranging from 0.5 to 4 ms were shown in Fig. 1B1–B5, which demonstrates bright NIR-II FL signals of QDs-labeled collagen, while unlabeled collagen was dark. The images of QDs were shown in Supplementary Fig. S1. The values of FL intensity were measured subsequently, which showed rising FL signals with the increase of exposure time. NIR-II FL signals reached the peak at 2 ms of exposure time and persisted with longer exposure times (Fig. 1C), verifying appropriate fluorescent characteristics of QDs-labeled collagen. Longer exposure times such as 15, 25, 50 and 100 ms led to overexposure (Supplementary Fig. S2). This result suggested that the labeling procedure did not affect the FL intensity of QDs. PL spectrum showed that QDs have an emission peak at around 1300 nm by the 808 nm excitation, which was consistent with our previous study [31] (Fig. 1D). The absorbance spectrum of collagen, QDs, LC, MC and HC all showed the peak of protein at around 280 nm [32] and LC, MC and HC had a similar absorbance spectrum (Fig. 1E). Notably, the emission peaks of QDs-labeled collagen had a blue shift in comparison with pure QDs (Fig. 1D), which could be ascribed to the increased interplanar spacing of QDs by labeling the crosslinked collagen, in agreement with our previous results [28, 33]. In summary, all degrees of crosslinked collagens were successfully labeled by NIR-II QDs, and high NIR-II signals of LC, MC and HC could be detected by in vivo NIR-II live imaging.
Figure 1.
Characterization of QDs-labeled collagen. (A) Bright-field and (B1-B5) NIR-II images of collagen, LC, MC and HC at different exposure times. Corresponding FL intensity (C), PL spectrum (D) and absorbance spectrum (E) of collagen, LC, MC, HC and QDs under 808-nm excitation.
The relationship between crosslinking degree and degradation rate of collagen based on in vivo NIR-II live imaging
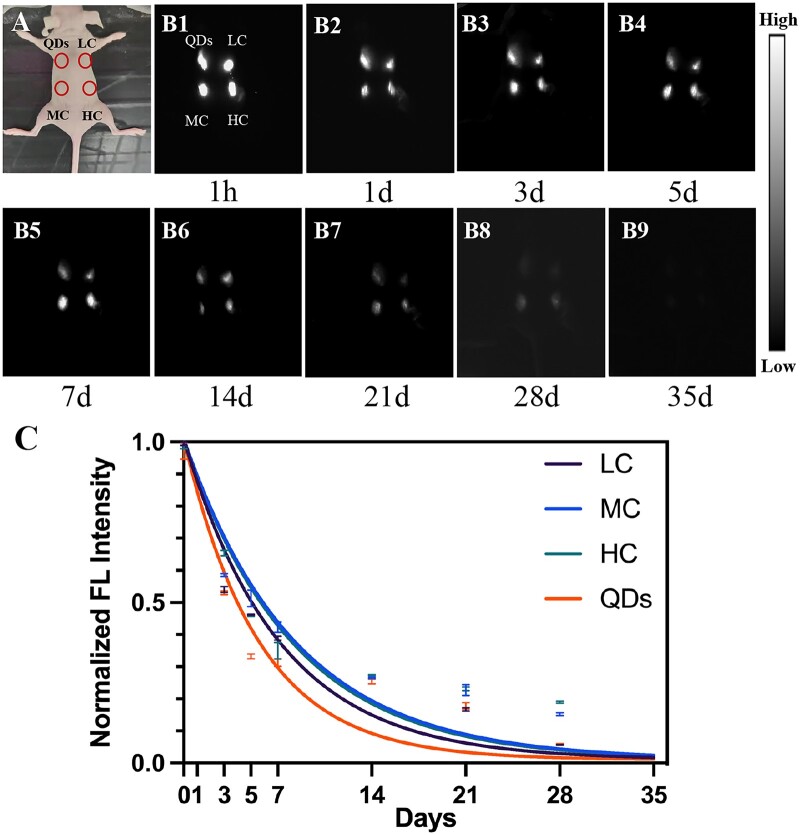
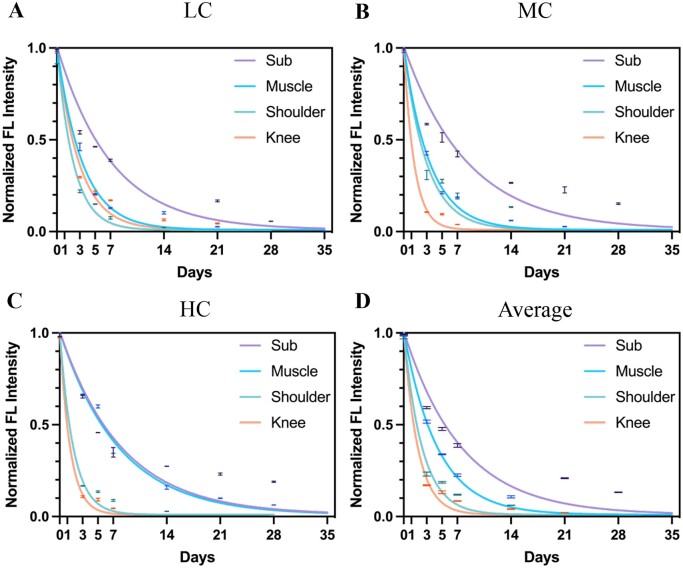
Firstly, the relationship between crosslinking degree and degradation rate of collagen was studied by in vivo NIR-II live imaging. As shown in Fig. 2A, QDs, LC, MC and HC were subcutaneously injected to four points at the back of the nude mouse. Then, NIR-II FL images were obtained from 1 h to 35 days post-injection (Fig. 2B). The bright spots were observed at injection points at 1 h post-injection (Fig. 2B1). The brightness of spots gradually decreased from 1 to 28 days (Fig. 2B2–B8) and no signals were detected in 35 days (Fig. 2B9). Normalized FL intensity was decreased over time (Fig. 2C); however, this attenuation was rapid in the first few days and flattened after 14 days. As shown in Fig. 2C, although MC had the highest FL intensity at the early stage of degradation (from 1 h to 7 days post-injection), HC turned out to have the highest FL intensity at the late stage of degradation (from 14 to 35 days post-injection). Meanwhile, there was no significant difference in the degradation rate of LC (k = 0.14), MC (k = 0.12) and HC (k = 0.12) based on the exponential decay model. Therefore, the change in crosslinking degree of collagen would not affect the degradation rate of collagen in subcutaneous tissue.
Figure 2.
Collagen degradation in subcutaneous tissue. (A) Bright-field image of the subcutaneously injected mouse. (B1-B9) NIR-II fluorescence images were taken at 1 h, 1, 3, 5, 7, 14, 21, 28 and 35 days after injection of QDs, LC, MC and HC. (C) Corresponding FL intensity analysis (B) and colored solid curve representing the degradation rate of QDs, LC, MC and HC, respectively.
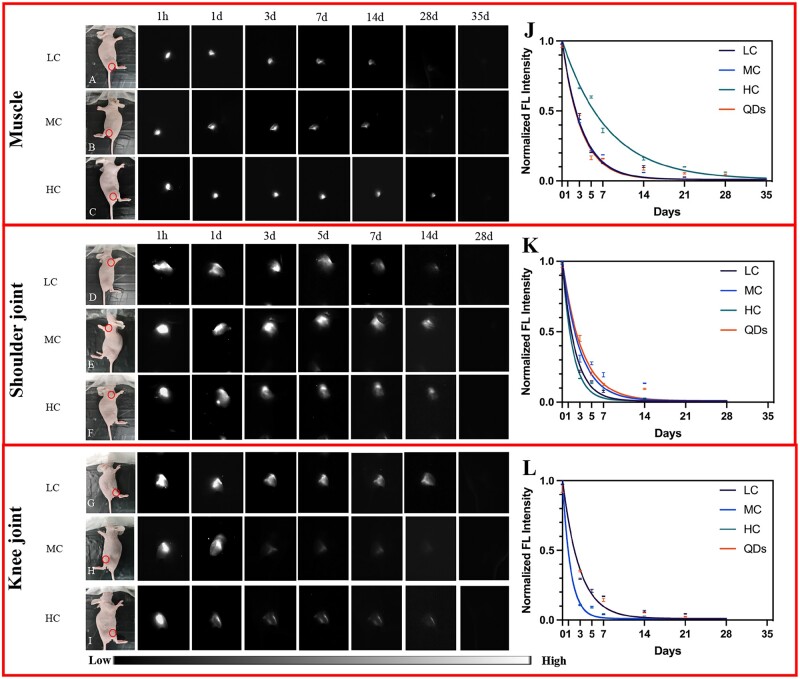
Besides, QDs, LC, MC and HC were also injected into the muscle, shoulder and knee joints and the degradation activity was assessed. As shown in Fig. 3, the NIR-II signals of QDs, LC, MC and HC decreased gradually and no signals were detected at 35 days in muscle (Fig. 3A–C), while the signals disappeared at 28 days in both knee and shoulder joints (Fig. 3D–I). This result suggested that the degradation of collagen in joints was faster than that of subcutaneous tissue and muscle. Moreover, the signals of all three degrees of collagens decreased rapidly at the early stage and gradually flattened at the late stage (Fig. 3J–L), which suggested LC, MC and HC had a similar degradation pattern in the same body parts.
Figure 3.
Collagen degradation in muscle, shoulder joint and knee joint. (A–C) Bright-field and NIR-II fluorescence images of LC (A), MC (B) and HC (C) degradation in muscle. (D–F) Bright-field and NIR-II fluorescence images of LC (D), MC (E) and HC (F) degradation in the shoulder joint. (G–I) Bright-field and NIR-II fluorescence images of LC (G), MC (H) and HC (I) degradation in the knee joint (circles: injection sites). (J–L) Corresponding normalized FL intensity analysis of collagen and degradation rate in muscle (J), shoulder joint (K) and knee joint (L).
Noteworthy, although HC (k = 0.13) in muscle, MC (k = 0.34) in shoulder joint and LC (k = 0.35) in knee joint had a slower degradation rate, respectively, the others had similar degradation rates despite the crosslinking degree in the corresponding body parts (Fig. 3J–L). Thus, the degradation rate of collagen did not change according to the crosslinking degree, when injected to the same body parts.
The relationship between body parts and degradation rate of collagen based on in vivo NIR-II live imaging
The previous result revealed that the total degradation time of collagen in joints was faster than that of subcutaneous tissue and muscle. Therefore, the specific degradation rates in four tested positions were further analyzed to investigate the relationship between the implanted body parts and collagen degradation. As shown in Fig. 4A–C, all kinds of collagen had substantially flatter curves in subcutaneous tissue compared to joints, while the HC in subcutaneous (k = 0.13) and muscle (k = 0.12) had similar degradation rates (Fig. 4C). Specifically, the subcutaneous tissue had the slowest degradation rates with all tested collagen, and the degradation rates of LC, MC and HC were 0.14, 0.12 and 0.12, respectively. Moreover, the degradation rates of HC in the shoulder joint and MC and HC in the knee joints were the fastest, which were 0.56, 0.74 and 0.73. Therefore, it was suggested that collagen degraded at the slowest rate in the subcutaneous tissue and the fastest rate in the knee joint. Likewise, the average degradation rate of collagen also confirmed that subcutaneous tissue (k = 0.13) and muscle (k = 0.23) had a slower degradation rate compared to shoulder (k = 0.42) and knee (k = 0.55) joints, as shown in Fig. 4D. In summary, it was suggested that the microenvironment of implantation markedly affected the degradation pattern of collagen.
Figure 4.
LC, MC and HC degradation in different tissue. Normalized FL intensity and degradation rate of LC (A), MC (B) and HC (C) in subcutaneous tissue, muscle, shoulder joint and knee joint ranging from 1 h to 35 days. (D) Average normalized FL intensity and degradation rate of collagen in subcutaneous tissue, muscle, shoulder joint and knee joint, respectively.
Ex vivo imaging of NIR-II QDs-labeled collagen
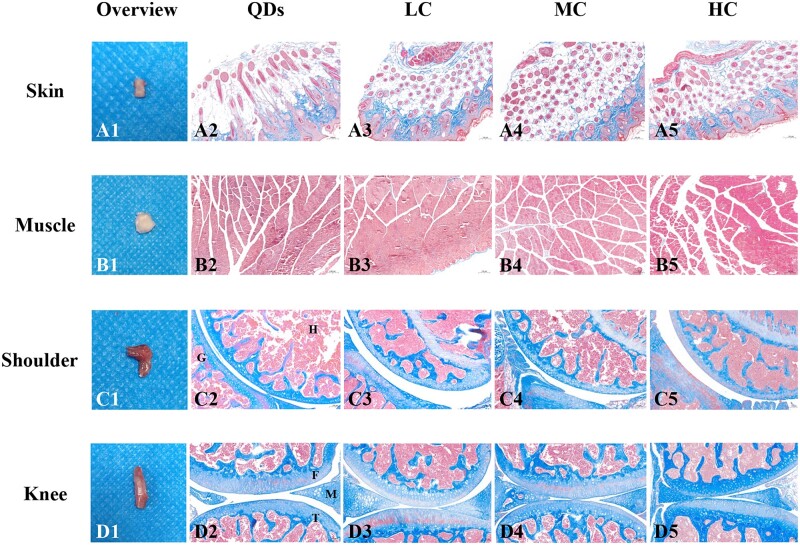
Skin, muscle, shoulder and knee joints were collected from each group to investigate whether the collagen degraded completely as well as the effect of the current method on injected sites. Figure 5A1–D1 showed the macroscopic images of tissues. No redness or edema was found in collected skin and muscle, suggesting there was no obvious inflammation in these tissues (Fig. 5A1 and B1). Shoulder joints and knee joints had normal joint morphology without malformation (Fig. 5C1 and D1). Masson’s trichrome-staining images of tissues injected with LC (Fig. 5A3–D3), MC (Fig. 5A4–D4) and HC (Fig. 5A5–D5) were compared to those that were injected with QDs (Fig. 5A2–D2). Ordered blue-stained collagen was observed in LC, MC and HC groups (Fig. 5A3–A5), which was similar to QDs group (Fig. 5A2), suggesting inherent collagen of host tissue. Red-stained myofiber was found in Fig. 5B3–B5 without blue-stained collagen, suggesting they degraded completely in muscle. Figure 5C3–C5 and D3–D5 showed there were no blue-stained LC, MC or HC in the shoulder joint capsules and knee joint capsules. The morphology of tissue was similar to that of the QDs-injected group, with no exudation or inflammatory cell infiltration. In summary, LC, MC and HC degraded completely in subcutaneous tissue, muscle, shoulder and knee joints without obvious inflammation.
Figure 5.
Macroscopic and microscopic images of collected tissues after the signals disappeared. (A1–D1) Macroscopic images of collected skin (35 days), muscle (28 days for LC, MC; 35 days for HC), shoulder joint (28 days) and knee joint (28 days). (A2–D2) Micrographs of Masson’s trichrome-staining slices of tissues injected with (A2–D2) QDs, (A3–D3) LC, (A4–D4) MC and (A5–D5) HC. H, humerus; G, glenoid; F, femur; T, tibia; M, meniscus. Magnification: ×100 for (A2–A5), (B2–B5), (C2–C5), (D2–D5).
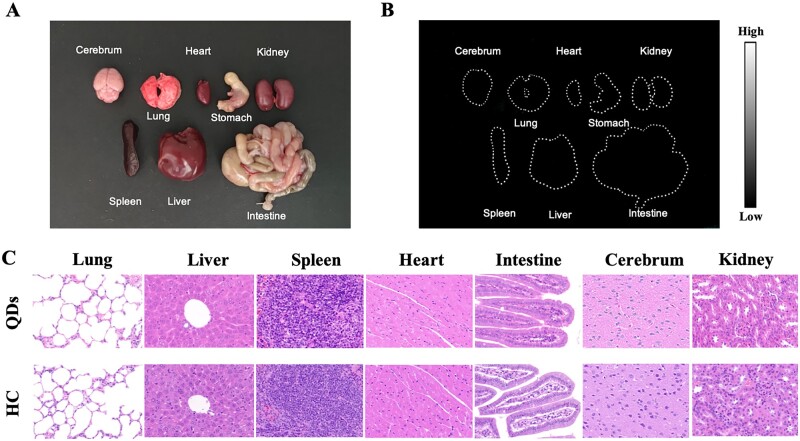
Major organs including the cerebrum, lung, heart, stomach, kidney, spleen, liver and intestine were harvested after no NIR-II FL signals were detected. The bright-field image was shown in Fig. 6A, which demonstrated no significant morphological changes. Then NIR-II FL images of organs were obtained with no obvious signals found in major organs (Fig. 6B). This result indicated that QDs-labeled collagen was metabolized well and did not aggregate in major organs after 35 days of injection. Moreover, histological analysis was performed to investigate the influence of QDs-labeled collagen on the microstructure of these organs, since the previous study confirmed the low biotoxicity of pure QDs [34]. HE staining of organs harvested from QDs-injected BALB/C nude mice and that from HC-injected mice were shown in Fig. 6C. No obvious signs of morphological changes and inflammation were found in the organs of HC-injected mice compared to QDs-injected mice. It could be concluded that QDs-labeled collagen did not deposit to or harm the major organs.
Figure 6.
Biosafety of QDs-labeled collagen. (A) Bright-field image of major organs including cerebrum, lung, heart, stomach, kidney, spleen, liver and intestine from mouse injected with QDs-labeled collagen. (B) NIR-II fluorescence image of major organs. (C) HE staining of organs from QDs-injected mice and HC-injected mice. Magnification: ×400.
Discussion
In recent decades, NIR-II live imaging has emerged as a perfect choice for in vivo live imaging because of its high tissue penetration and low light-scattering characteristics [34–42]. In fact, NIR-II imaging has successfully monitored the degradation of many biomedical implants [22, 43] with high resolution. This study also successfully monitored the collagen degradation patterns by NIR-II in vivo live imaging, confirming its potential for a broader range of biomedical products due to its supreme imaging ability. Therefore, it could be a potential standard in vivo monitoring modality for implanted biomedical products.
It has been previously shown that a higher crosslinking degree of collagen would lead to a slower degradation rate in ex vivo conditions due to the decreased enzyme-reacting points consumed by the crosslinking [16, 17, 44]. However, this in vivo study showed that the crosslinking degree of collagen did not affect the degradation rate in most conditions. This indicates that some biological factors, such as varying degrees of collagenase, might be neglected in the ex vivo study, while they play a critical role in collagen degradation. Interestingly, it has been reported that higher crosslinking degree of collagen would stimulate the dermal fibroblasts to secrete higher level of collagenase [19], and crosslinking would improve the macrophage attachment [44]. A possible explanation is that the collagen would induce an acute reaction which could make the cells to accumulate to produce more collagenase. Although highly crosslinked collagen had a small amount of enzyme reaction points, the high level of collagenase hindered it to remain longer. In contrast, although the lower crosslinking degree of collagen had less amount of enzyme reacting points, it also made the cells secrete a lower level of collagenase. As a result, no significant differences were found between the degradation rates of the three crosslinking degrees of collagens. Therefore, the balance between the enzyme reacting points and the level of collagenase produced by cells would be critical for collagen degradation.
This study showed that three kinds of collagens degraded most slowly in the subcutaneous tissue and most rapidly in the joints regardless of the crosslinking degree, suggesting that the microenvironment of body parts might play a critical role in this process (Scheme 2). As mentioned above, collagenase is a key factor that affects collagen degradation and it might explain the varying degradation rates in different body parts. The synovial fluid in the joint capsule is rich in macrophages [45], the activity of which is correlated with the collagenase activity [44]. In addition, the spaces in the synovial cavity of joints could support more sufficient interactions between collagenase and collagen [44, 46] compared to subcutaneous tissue and muscle. Large amounts of macrophages and a relatively wide interaction space might lead to a quick metabolism of collagen in joints than in subcutaneous tissue and muscle despite the crosslinking degree. Therefore, it could be concluded that the collagen degradation in vivo might be mainly decided by the level of collagenase, and it is the microenvironment of the body parts, which is responsible for the level of collagenase.
Scheme 2.
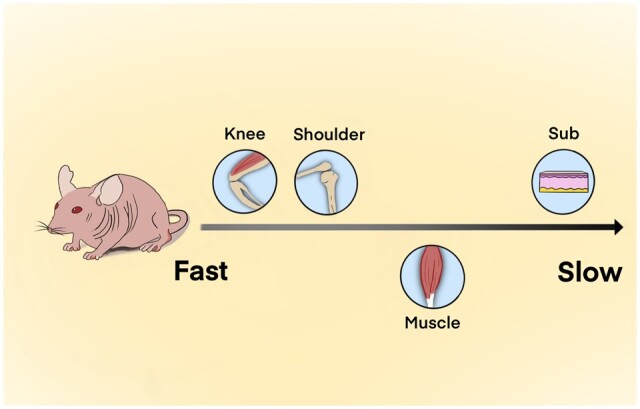
Comparison of collagen degradation rate in different body parts of the mouse model.
Conclusion
In this study, both the crosslinking degree and the implantation sites of collagen have been simultaneously taken into consideration, to monitor and evaluate the in vivo degradation pattern of collagen using NIR-II live imaging. Firstly, three kinds of crosslinking degrees of collagens, including HC, MC and LC, were successfully labeled by NIR-II fluorescent QDs and then were injected into the subcutaneous tissue, muscle, shoulder and knee joints of the mouse model. The NIR-II imaging showed that the implanted collagen had a slower and longer degradation in the subcutaneous tissue and muscle in comparison with that in the joints. Interestingly, the degradation rate of collagen in subcutaneous tissue, muscle, shoulder and knee joints did not depend on the crosslinking degree of collagen. In summary, this study indicated that the degradation pattern of collagen was closely related to the body parts rather than the chemical properties of collagen in the mouse model, implying that the biological microenvironment of the implanted sites should be considered first when designing collagen-based products for biomedical applications.
Supplementary Material
Contributor Information
Huizhu Li, Department of Sports Medicine, Sports Medicine Institute of Fudan University, Huashan Hospital, Fudan University, Shanghai 200040, China.
Xinxian Meng, Department of Plastic and Reconstructive Surgery, School of Medicine, Shanghai Jiao Tong University, Shanghai Ninth People’s Hospital, Shanghai 200011, China.
Huaixuan Sheng, Department of Sports Medicine, Sports Medicine Institute of Fudan University, Huashan Hospital, Fudan University, Shanghai 200040, China.
Sijia Feng, Department of Sports Medicine, Sports Medicine Institute of Fudan University, Huashan Hospital, Fudan University, Shanghai 200040, China.
Yuzhou Chen, Department of Sports Medicine, Sports Medicine Institute of Fudan University, Huashan Hospital, Fudan University, Shanghai 200040, China.
Dandan Sheng, Department of Sports Medicine, Sports Medicine Institute of Fudan University, Huashan Hospital, Fudan University, Shanghai 200040, China.
Liman Sai, Department of Physics, Shanghai Normal University, Shanghai 200234, China.
Yueming Wang, Department of Anatomy and Physiology, School of Medicine, Shanghai Jiao Tong University, Shanghai 200025, China.
Mo Chen, Department of Sports Medicine, Sports Medicine Institute of Fudan University, Huashan Hospital, Fudan University, Shanghai 200040, China.
Yan Wo, Department of Anatomy and Physiology, School of Medicine, Shanghai Jiao Tong University, Shanghai 200025, China.
Shaoqing Feng, Department of Plastic and Reconstructive Surgery, School of Medicine, Shanghai Jiao Tong University, Shanghai Ninth People’s Hospital, Shanghai 200011, China.
Hossein Baharvand, Department of Stem Cells and Developmental Biology, Cell Science Research Center, Royan Institute for Stem Cell Biology and Technology, ACECR, Tehran 1665659911, Iran; Department of Developmental Biology, School of Basic Sciences and Advanced Technologies in Biology, University of Science and Culture, Tehran 1461968151, Iran.
Yanglai Gao, Hexi College, Zhangye, Gansu 73400, China.
Yunxia Li, Department of Sports Medicine, Sports Medicine Institute of Fudan University, Huashan Hospital, Fudan University, Shanghai 200040, China.
Jun Chen, Department of Sports Medicine, Sports Medicine Institute of Fudan University, Huashan Hospital, Fudan University, Shanghai 200040, China.
Supplementary data
Supplementary data are available at Regenerative Biomaterials online.
Funding
This work was supported by National Key R&D Program of China (2021YFA1201303), National Natural Science Foundation of China (82172511, 81972121, 81972129, 82072521, 82011530023 and 82111530200), Sanming Project of Medicine in Shenzhen (SZSM201612078), the Introduction Project of Clinical Medicine Expert Team for Suzhou (SZYJTD201714), Shanghai Talent Development Funding Scheme (2020080), Shanghai Sailing Program (21YF1404100 and 22YF1405200), Shanghai Committee of Science and Technology (22DZ2204900) and Medical Engineering Joint Fund of Fudan University (YG2022-14).
Author contributions
H.L., X.M., H.S. and S.F. performed the experiment and wrote the article. Y.C., D.S., Y.M., M.C., S.F., L.S., Y.L. and Y.W. assisted to analyze the data. H.B., Y.G., Y.L. and J.C. are in charge of the conceptualization and the article revision.
Conflicts of interest statement. The authors declare no conflicts of interest.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Shekhter AB, Fayzullin AL, Vukolova MN, Rudenko TG, Osipycheva VD, Litvitsky PF.. Medical applications of collagen and collagen-based materials. Curr Med Chem 2019;26:506–16. [DOI] [PubMed] [Google Scholar]
- 2. Grabska-Zielińska S, Sionkowska A, Carvalho Â, Monteiro FJ.. Biomaterials with potential use in bone tissue regeneration-collagen/chitosan/silk fibroin scaffolds cross-linked by EDC/NHS. Materials (Basel) 2021;14:1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. An B, Lin YS, Brodsky B.. Collagen interactions: drug design and delivery. Adv Drug Deliv Rev 2016;97:69–84. [DOI] [PubMed] [Google Scholar]
- 4. Makris EA, Gomoll AH, Malizos KN, Hu JC, Athanasiou KA.. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol 2015;11:21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Irawan V, Sung TC, Higuchi A, Ikoma T.. Collagen scaffolds in cartilage tissue engineering and relevant approaches for future development. Tissue Eng Regen Med 2018;15:673–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mozdzen LC, Rodgers R, Banks JM, Bailey RC, Harley BA.. Increasing the strength and bioactivity of collagen scaffolds using customizable arrays of 3D-printed polymer fibers. Acta Biomater 2016;33:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rastogi P, Kandasubramanian B.. Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication 2019;11:042001. [DOI] [PubMed] [Google Scholar]
- 8. Sun L, Li B, Yao D, Song W, Hou H.. Effects of cross-linking on mechanical, biological properties and biodegradation behavior of Nile tilapia skin collagen sponge as a biomedical material. J Mech Behav Biomed Mater 2018;80:51–8. [DOI] [PubMed] [Google Scholar]
- 9. Adamiak K, Sionkowska A.. Current methods of collagen cross-linking: review. Int J Biol Macromol 2020;161:550–60. [DOI] [PubMed] [Google Scholar]
- 10. Taghipour YD, Hokmabad VR, Del Bakhshayesh AR, Asadi N, Salehi R, Nasrabadi HT.. The application of hydrogels based on natural polymers for tissue engineering. Curr Med Chem 2020;27:2658–80. [DOI] [PubMed] [Google Scholar]
- 11. Caballe-Serrano J, Zhang S, Sculean A, Staehli A, Bosshardt DD.. Tissue integration and degradation of a porous collagen-based scaffold used for soft tissue augmentation. Materials (Basel) 2020;13:2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xie X, Xu J, Lin J, Jiang J, Huang Y, Lu J, Kang Y, Hu Y, Cai J, Wang F, Zhu T, Zhao J, Wang L.. A regeneration process-matching scaffold with appropriate dynamic mechanical properties and spatial adaptability for ligament reconstruction. Bioact Mater 2022;13:82–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park SH, Gil ES, Kim HJ, Lee K, Kaplan DL.. Relationships between degradability of silk scaffolds and osteogenesis. Biomaterials 2010;31:6162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. FitzGerald JF, Kumar AS.. Biologic versus synthetic mesh reinforcement: what are the pros and cons? Clin Colon Rectal Surg 2014;27:140–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hakimi O, Mouthuy PA, Carr A.. Synthetic and degradable patches: an emerging solution for rotator cuff repair. Int J Exp Pathol 2013;94:287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gu L, Shan T, Ma YX, Tay FR, Niu L.. Novel biomedical applications of crosslinked collagen. Trends Biotechnol 2019;37:464–91. [DOI] [PubMed] [Google Scholar]
- 17. Davidenko N, Schuster CF, Bax DV, Raynal N, Farndale RW, Best SM, Cameron RE.. Control of crosslinking for tailoring collagen-based scaffolds stability and mechanics. Acta Biomater 2015;25:131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thoma DS, Villar CC, Cochran DL, Hammerle CH, Jung RE.. Tissue integration of collagen-based matrices: an experimental study in mice. Clin Oral Implants Res 2012;23:1333–9. [DOI] [PubMed] [Google Scholar]
- 19. Nair M, Johal RK, Hamaia SW, Best SM, Cameron RE.. Tunable bioactivity and mechanics of collagen-based tissue engineering constructs: a comparison of EDC-NHS, genipin and TG2 crosslinkers. Biomaterials 2020;254:120109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shekhter AB, Balakireva AV, Kuznetsova NV, Vukolova MN, Litvitsky PF, Zamyatnin AA Jr. Collagenolytic enzymes and their applications in biomedicine. Curr Med Chem 2019;26:487–505. [DOI] [PubMed] [Google Scholar]
- 21. Lei K, Chen Y, Wang J, Peng X, Yu L, Ding J.. Non-invasive monitoring of in vivo degradation of a radiopaque thermoreversible hydrogel and its efficacy in preventing post-operative adhesions. Acta Biomater 2017;55:396–409. [DOI] [PubMed] [Google Scholar]
- 22. Zheng L, Liu S, Cheng X, Qin Z, Lu Z, Zhang K, Zhao J.. Intensified stiffness and photodynamic provocation in a collagen-based composite hydrogel drive chondrogenesis. Adv Sci (Weinh) 2019;6:1900099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Campos Y, Fuentes G, Almirall A, Que I, Schomann T, Chung CK, Jorquera-Cordero C, Quintanilla L, Rodríguez-Cabello JC, Chan A, Cruz LJ.. The incorporation of etanercept into a porous tri-layer scaffold for restoring and repairing cartilage tissue. Pharmaceutics 2022;14:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qin W, Li C, Liu C, Wu S, Liu J, Ma J, Chen W, Zhao H, Zhao X.. 3D printed biocompatible graphene oxide, attapulgite, and collagen composite scaffolds for bone regeneration. J Biomater Appl 2022;36:1838–51. [DOI] [PubMed] [Google Scholar]
- 25. Wang M, Chen M, Niu W, Winston DD, Cheng W, Lei B.. Injectable biodegradation-visual self-healing citrate hydrogel with high tissue penetration for microenvironment-responsive degradation and local tumor therapy. Biomaterials 2020;261:120301. [DOI] [PubMed] [Google Scholar]
- 26. Mertens ME, Hermann A, Buhren A, Olde-Damink L, Mockel D, Gremse F, Ehling J, Kiessling F, Lammers T.. Iron oxide-labeled collagen scaffolds for non-invasive MR imaging in tissue engineering. Adv Funct Mater 2014;24:754–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim SH, Lee JH, Hyun H, Ashitate Y, Park G, Robichaud K, Lunsford E, Lee SJ, Khang G, Choi HS.. Near-infrared fluorescence imaging for noninvasive trafficking of scaffold degradation. Sci Rep 2013;3:1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen J, Feng S, Chen M, Li P, Yang Y, Zhang J, Xu X, Li Y, Chen S.. In vivo dynamic monitoring of bacterial infection by NIR-II fluorescence imaging. Small 2020;16:e2002054. [DOI] [PubMed] [Google Scholar]
- 29. Kang YJ, Lee CH, Park SJ, Lee HS, Choi MK, Song IS.. Involvement of organic anion transporters in the pharmacokinetics and drug interaction of rosmarinic acid. Pharmaceutics 2021;13:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ethington J, Goldmeier D, Gaynes BI.. Exponential decay metrics of topical tetracaine hydrochloride administration describe corneal anesthesia properties mechanistically. Cornea 2017;36:363–6. [DOI] [PubMed] [Google Scholar]
- 31. Kong Y, Chen J, Fang H, Heath G, Wo Y, Wang W, Li Y, Guo Y, Evans SD, Chen S, Zhou D.. Highly fluorescent ribonuclease-A-encapsulated lead sulfide quantum dots for ultrasensitive fluorescence in vivo imaging in the second near-infrared window. Chem Mater 2016;28:3041–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Feng S, Chen M, Chen Y, Sai L, Dong S, Li H, Yang Y, Zhang J, Yang X, Xu X, Hao Y, Abdou AMKH, Tran NQ, Chen S, Li Y, Dong J, Chen J.. Seeking and identifying time window of antibiotic treatment under in vivo guidance of PbS QDs clustered microspheres based NIR-II fluorescence imaging. Chem Eng J 2023;451:138584. [Google Scholar]
- 33. Kong Y, Chen J, Gao F, Li W, Xu X, Pandoli O, Yang H, Ji J, Cui D.. A multifunctional ribonuclease-A-conjugated CdTe quantum dot cluster nanosystem for synchronous cancer imaging and therapy. Small 2010;6:2367–73. [DOI] [PubMed] [Google Scholar]
- 34. Yang Y, Chen J, Shang X, Feng Z, Chen C, Lu J, Cai J, Chen Y, Zhang J, Hao Y, Yang X, Li Y, Chen S.. Visualizing the fate of intra-articular injected mesenchymal stem cells in vivo in the second near-infrared window for the effective treatment of supraspinatus tendon tears. Adv Sci (Weinh) 2019;6:1901018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Feng Z, Yang Y, Zhang J, Wang K, Li Y, Xu H, Wang Z, Biskup E, Dong S, Yang X, Hao Y, Chen J, Wo Y.. In vivo and in situ real-time fluorescence imaging of peripheral nerves in the NIR-II window. Nano Res 2019;12:3059–68. [Google Scholar]
- 36. Che Y, Feng S, Guo J, Hou J, Zhu X, Chen L, Yang H, Chen M, Li Y, Chen S, Cheng Z, Luo Z, Chen J.. In vivo live imaging of bone using shortwave infrared fluorescent quantum dots. Nanoscale 2020;12:22022–9. [DOI] [PubMed] [Google Scholar]
- 37. Chen M, Feng S, Yang Y, Li Y, Zhang J, Chen S, Chen J.. Tracking the in vivo spatio-temporal patterns of neovascularization via NIR-II fluorescence imaging. Nano Res 2020;13:3123–9. [Google Scholar]
- 38. Xu H, Chen J, Feng Z, Fu K, Qiao Y, Zhang Z, Wang W, Wang Y, Zhang J, Perdanasari AT, Hanasono MM, Levin LS, Yang X, Hao Y, Li Y, Wo Y, Zhang Y.. Shortwave infrared fluorescence in vivo imaging of nerves for minimizing the risk of intraoperative nerve injury. Nanoscale 2019;11:19736–41. [DOI] [PubMed] [Google Scholar]
- 39. Feng S, Li H, Liu C, Chen M, Sheng H, Huang M, Li Y, Chen J, Zhang J, Hao Y, Chen S.. Real-time in vivo detection and monitoring of bacterial infection based on NIR-II imaging. Front Chem 2021;9:689017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dong S, Feng S, Chen Y, Chen M, Yang Y, Zhang J, Li H, Li X, Ji L, Yang X, Hao Y, Chen J, Wo Y.. Nerve suture combined with ADSCs injection under real-time and dynamic NIR-II fluorescence imaging in peripheral nerve regeneration in vivo. Front Chem 2021;9:676928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Antaris AL, Chen H, Cheng K, Sun Y, Hong GS, Qu CR, Diao S, Deng ZX, Hu XM, Zhang B, Zhang XD, Yaghi OK, Alamparambil ZR, Hong XC, Cheng Z, Dai HJ.. A small-molecule dye for NIR-II imaging. Nat Mater 2016;15:235–42. [DOI] [PubMed] [Google Scholar]
- 42. Yao C, Chen Y, Zhao M, Wang S, Wu B, Yang Y, Yin D, Yu P, Zhang H, Zhang F.. A bright, renal-clearable NIR-II brush macromolecular probe with long blood circulation time for kidney disease bioimaging. Angew Chem Int Ed Engl 2022;61:e202114273. [DOI] [PubMed] [Google Scholar]
- 43. Pei P, Hu H, Chen Y, Wang S, Chen J, Ming J, Yang Y, Sun C, Zhao S, Zhang F.. NIR-II ratiometric lanthanide-dye hybrid nanoprobes doped bioscaffolds for in situ bone repair monitoring. Nano Lett 2022;22:783–91. [DOI] [PubMed] [Google Scholar]
- 44. Yahyouche A, Zhidao X, Czernuszka JT, Clover AJ.. Macrophage-mediated degradation of crosslinked collagen scaffolds. Acta Biomater 2011;7:278–86. [DOI] [PubMed] [Google Scholar]
- 45. Culemann S, Grüneboom A, Krönke G.. Origin and function of synovial macrophage subsets during inflammatory joint disease. Adv Immunol 2019;143:75–98. [DOI] [PubMed] [Google Scholar]
- 46. Steven FS. Polymeric collagen fibrils. An example of substrate-mediated steric obstruction of enzymic digestion. Biochim Biophys Acta 1976;452:151–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.