Abstract
Background
Several cases of unusual thrombotic events and thrombocytopenia were described after vaccination with recombinant adenoviral vectors encoding the spike protein antigen of SARS-CoV-2.
Objectives
The objective of this study was to elucidate the impact of a COVID-19 heterologous vaccination schedule, including priming with adenovirus vaccine, on hemostasis profiles.
Methods
The present study is a subanalysis of the CombiVacS clinical trial initiated in April 2021 that included adult participants previously vaccinated with a single dose of ChAdOx1-S. Between 8 and 12 weeks after vaccination, they were randomly assigned (2:1) to receive either BNT162b2 vaccine (intervention group, n = 99) or continue observation (control group, n = 50). Samples drawn before and 28 days after a vaccination with BNT162b2 were analyzed for platelet count and markers of hemostasis (D-dimer, anti-PF4 antibodies, cfDNA, PAI-1, thrombin generation, and serum capacity to activate platelets).
Results
Platelet count from all participants after receiving BNT162b2 was within the normal range. Anti-PF4 antibodies were present in 26% and 18% of the subjects from the control and intervention groups, respectively, at day 28. In most cases, the levels of anti-PF4 antibodies were high before receiving BNT162b2. Serum from these participants did not activate platelets from healthy controls. There were no differences between the groups in PAI-1 and cfDNA plasma levels. According to the D-dimer plasma concentration, the thrombin generation test showed that none of the participants had a procoagulant profile.
Conclusion
Our data suggest that the heterologous vaccination against COVID-19 with ChAdOx1-S and a second dose with BNT162b2 might be safe in terms of haemostasis.
Keywords: anti-PF4 antibodies, coagulation, CombiVacS study, COVID-19 vaccine, hemostasis, heterologous vaccination, thrombocytopenia
Essentials
-
•
Thrombotic events and thrombocytopenia were described after vaccination with recombinant adenoviral vectors.
-
•
A dose of BNT162b2 vaccine after the first dose of ChAdOx1-S did not change the procoagulant profile.
-
•
The presence of high anti-PF4 antibodies does not necessary cause platelet activation.
-
•
This study may help inform the field of safety of heterologous schemes of vaccination.
1. Introduction
The COVID-19 vaccine ChAdOx1-S (AZD1222, Vaxzevria, AstraZeneca) was the first one associated with the development of vaccine-induced immune thrombocytopenia and thrombosis (VITT) between 5 and 20 days following vaccination [[1], [2], [3], [4]]. VITT is due to the high titers of immunoglobulin G (IgG) class antibodies (Abs) directed against the cationic platelet chemokine, platelet factor 4 (PF4), which in turn activates platelets [1,2]. VITT resembles autoimmune heparin-induced thrombocytopenia; however, VITT pathophysiology is independent of heparin exposure [5]. Subsequently, thrombosis with thrombocytopenia was also reported following administration of the Ad26.COV2.S COVID-19 vaccine (Janssen/Johnson & Johnson) [6,7] and, with much lower frequency, the mRNA-based vaccines, BNT162b2 (Comirnaty, BioNTech) and mRNA-1273 (Spikevax, Moderna) [8,9]. Recently, a number of studies have evaluated the immunological effect and reactogenicity of the heterologous vaccination against COVID-19 [10,11], but none of them described the effects of this vaccination schedule strategy on hemostasis. The CombiVacS clinical trial, registered with EudraCT (2021-001978-37) and ClinicalTrials.gov (NCT04860739), tested the immunogenicity and reactogenicity of BNT162b2 (Comirnaty, BioNTech) administered as the second dose in participants primed with ChAdOx1-S, showing that this approach induced a strong immune response with a reactogenicity profile similar to that of each separate vaccine [10]. Notwithstanding, because of the occurrence of several cases of unusual thrombotic events and thrombocytopenia after vaccination against COVID-19, we aimed to assess whether the administration of 1 dose of BNT162b2 in subjects who had received a previous single dose of ChAdOx1-S produces changes in the hemostasis profile or in the occurrence of thrombotic events at day 28 after vaccination compared with both the predosing period and the control group, which received no second vaccine dose within the first 28 days of study. To do so, we have dissected several hemostatic pathways before and after BNT162b2.
2. Methods
2.1. Trial design and participants
The full methodology of the CombiVacS study has been reported elsewhere [10]. Briefly, it is a phase 2, multicenter, open-label, randomized controlled clinical trial that included 676 adults aged 18 to 60 years vaccinated with a single dose of ChAdOx1-S 8 to 12 weeks before screening and with no history of SARS-CoV-2 infection. The main exclusion criteria were the presence of either documented reverse transcription-polymerase chain reaction–confirmed or symptoms compatible with COVID-19 and having received any other vaccine since the prime dose. All participants provided written informed consent before enrollment. The analysis presented here is an ad hoc subanalysis conducted in participants recruited at the La Paz University Hospital (n = 150) as part of the safety analysis planned in the protocol [10]. Race, or ethnicity, or another social determinant data of participants were not collected; hence, the analyses of the impact of these variables on the outcomes could not be performed.
The trial complies with the principles of the Declaration of Helsinki and Good Clinical Practice and was approved by the Spanish Agency of Medicines and Healthcare Products and by the ethics committee at the La Paz University Hospital.
2.2. Randomization
Participants were randomly assigned (2:1) to receive either BNT162b2 (0.3 mL) using a single intramuscular injection (intervention group) or continue observation (control group). The randomization list was centrally generated using SAS software for Windows (version 9.4; SAS Institute Inc.) and imported into the secure Research Electronic Data Capture platform (REDCap version 8.7.4; Vanderbilt University) used for the study electronic case report form (eCRF).
2.3. General study procedures
All participants were clinically assessed and had blood samples drawn for safety and immunology at day 0 (randomization and BNT162b2 dose administration in the intervention group). Follow-up visits on days 7, 14, 28, 90, 180, and 360 were planned to measure vital signs, review any adverse events, update medical and medication records, and collect blood samples.
As to the present analysis, hemostatic parameters in samples at days 0 (V1) and 28 (V4) were evaluated. The range of normality of some variables was determined with values from healthy controls who did not have had COVID-19 and were not vaccinated against SARS-CoV-2, matched with the trial participants in sex (54.1% women), age (37.7 ± 11.1 years), and comorbidities and collected retrospectively from our database [12,13]. Moreover, samples from some of them were analyzed simultaneously with samples from the trial participants.
2.4. Collection and preparation of samples
Human peripheral blood samples were collected in tubes containing 3.2% trisodium citrate (BD Vacutainer) and in tubes for serum (BD Vacutainer). To obtain platelet-poor plasma (PPP), blood was centrifuged at 1500 g for 15 minutes at 23 °C. For serum preparation, tubes were centrifuged at 2500 g for 15 minutes at 23 °C. Citrated PPP and serum aliquots were kept at −80 °C until assayed.
2.5. Plasma and serum determinations
D-dimer was tested using immunoturbidimetry in citrated PPP with an Innovance D-dimer kit in a CS5100 analyzer (Siemens Healthineers), and plasma values above 500 ng/mL are considered pathological by our central laboratory. Anti-PF4 Abs were determined in the serum samples using the ELISA method (00615 Asserachrom HPIA [IgG/A/M], Stago). According to the manufacturer’s instructions, a sample was considered positive if its optic density (OD) was greater than 0.7, value corresponding to 23% of OD of the kit's positive control. The results between 23% and 46% were considered weak positive and ≥46% strong positive. The cell-free DNA (cfDNA) was determined in citrated PPP using the Quant-iT PicoGreen dsDNA assay (Thermo Fisher Scientific). Plasminogen activator inhibitor-1 (PAI-1) in serum was tested with an ELISA kit from Invitrogen (Thermo Fisher Scientific) and range of normality established by the kit manufacturers' instructions.
2.6. Platelet activation test
The ability of anti-PF4–positive sera to stimulate platelets was tested with platelet-rich plasma (PRP) prepared by centrifugation (150 g for 20 minutes at 23 °C) of blood from a group O donor, selected on the basis of not having prothrombotic diseases and not receiving antiplatelet or anticoagulation medication. PRP was diluted 1:5 with HEPES buffer (10 mmol/L HEPES, 145 mmol/L NaCl, 5 mmol/L KCl, and 1 mmol/L MgSO4, pH 7.4). Heat-inactivated serum (56ºC, 30 minutes) from the participants of control and intervention groups whose anti-PF4 Abs levels were above the cutoff value in any of the visits was added in a ratio 1:5 to diluted PRP and incubated with FITC-anti P-selectin mAb (BD Pharmingen). Platelet activation using the participants' serum was also tested on PRP stimulated with a suboptimal concentration of thrombin receptor-activating peptide (TRAP)-6 (Bachem AG) (10 μmol/L, Supplementary Figure 2) at room temperature (RT) before the addition of FITC-anti P-selectin mAb.
After incubation, platelets were diluted in the HEPES buffer for flow cytometry analysis with a FACScan flow cytometer (BD Biosciences) and 10,000 events in the platelet region were acquired and analyzed using BD CellQuest Pro software (BD Biosciences).
2.7. Thrombin generation test
Thrombin generation was measured in citrated PPP using calibrated automated thrombogram (CAT). To evaluate the procoagulant capacity of plasma, coagulation was triggered by proper recalcification and the addition (final concentrations) of 1 pmol/L of recombinant human tissue factor (TF) and 4 μmol/L of phospholipid mixture (PPP-Reagent LOW, Thrombinoscope BV). The procoagulant activity of microparticles (MPs) present in the plasma, associated with their content of either TF or phosphatidylserine (PS), was determined, respectively, in PPP samples with the MP reagent (4 μmol/L phospholipids) or PRP reagent (1 pmol/L of recombinant human TF) by CAT. All CAT reagents were from Diagnostica Stago. Thrombin generation was determined with a Fluoroskan FL instrument (Thermo Labsystems) under the control of Thrombinoscope software, version 3.6 (Thrombinoscope BV), filtered for excitation at 390 nm and emission at 460 nm.
The following parameters were determined: Lag time (LT), or time from the start of the assay until 10 nmol/L thrombin is formed, in min; time to peak (ttPeak) or time required to reach the maximum thrombin concentration in min; peak height (Peak), or maximum thrombin concentration reached, in nmol/L; and endogenous thrombin potential (ETP), or the total amount of thrombin generated over time, in nmol/L.min.
2.8. Statistical analysis
Demographic characteristics of the patients were described by the study groups with mean (SD) values or absolute and relative frequencies. Hemostasis and coagulation profiles and average change between 2 measurements at different times were tested for normality hypothesis assumptions with the Kolmogorov-Smirnov tests. Hemostasis parameters and average change with the assumption of normal distribution were compared using Student’s t-test and described with mean and 95% (95% CI). Otherwise, differences between study groups were analyzed using the Mann–Whitney–Wilcoxon tests and summarized with median, 25th (Q1) and 75th percentiles (Q3). A 2-sided 0.05 significance level was used in all tests. The correlation between variables was analyzed with either the Pearson or Spearman tests depending on the sample distribution. All analyses were conducted using SAS software, version 9.4, of the SAS System for Windows (SAS Institute Inc.).
3. Results
A total of 149 participants were enrolled in the CombiVacS clinical trial at La Paz University Hospital between April 24th and 30th, 2021 and were randomly assigned to receive BNT162b2 vaccine (n = 99) or no vaccine (n = 50). Individuals who had a disease or were taking a medication that could alter coagulation parameters or hemostasis were excluded—5 individuals from the intervention group (1 heterozygous Factor V Leiden mutation, 1 immune thrombocytopenia, 2 treated with atorvastatin, and 1 because he had mumps) and 3 individuals from the control group (1 because of having had a coronary disease treated with acetylsalicylic acid, 1 treated with simvastatin, and 1 confirmed COVID-19 in the first 28 days). Two additional subjects (1 from the intervention group and 1 from the control group) were excluded from the analysis because of the absence of blood sample. Participant disposition is provided in Supplementary Figure 1.
Mean (SD) age of participants was 43 years (9.0), and 78 (56%) participants were female. Most participants (76; 81%) received BNT162b2 vaccine between 8 and 9 weeks after ChAdOx1-S vaccine. Demographic and baseline characteristics were balanced overall in both the study groups, except 6 participants taking contraceptive drugs in the intervention group versus none in the control group (Table).
Table.
Baseline characteristics of the analyzed subset of participants.
| Characteristics of the participants | Control group (n = 46) | Interventional group (n = 93) | Overall (n = 139) |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 21 (46) | 40 (43) | 61 (44) |
| Female | 25 (54) | 53 (57) | 78 (56) |
| Age (y), mean (SD) | 42.4 (9.09) | 43.3 (9.01) | 42.99 (9.01) |
| Age group, n (%) | |||
| 18-49 y | 36 (78) | 66 (71) | 102 (73) |
| 50-59 y | 10 (22) | 27 (29) | 37 (27) |
| Time since prime ChAdOx1-S vaccination, n (%) | |||
| 8-9 wk | 39 (85) | 75 (81) | 114 (82) |
| 10-12 wk | 7 (15) | 18 (19) | 25 (18) |
| Comorbidities, n (%) | |||
| Hypertension | 1 (2) | 1 (1) | |
| Dyslipidaemia | 0 (0) | 1 (1) | |
| Type 1 diabetes mellitus | 1 (2) | 1 (1) | |
| Type 2 diabetes mellitus | 0 (0) | 1 (1) | |
| Hyperparathyroidism | 1 (2) | 0 (0) | |
| Hypothyroidism | 2 (4) | 7 (7) | |
| Cancer | 1 (2) | 2 (2) | |
| Autoimmune disease | 0 (0) | 1 (1) | |
| Viral infection | 2 (4) | 1 (1) | |
| Contraceptive drugs | 0 (0) | 6 (6) |
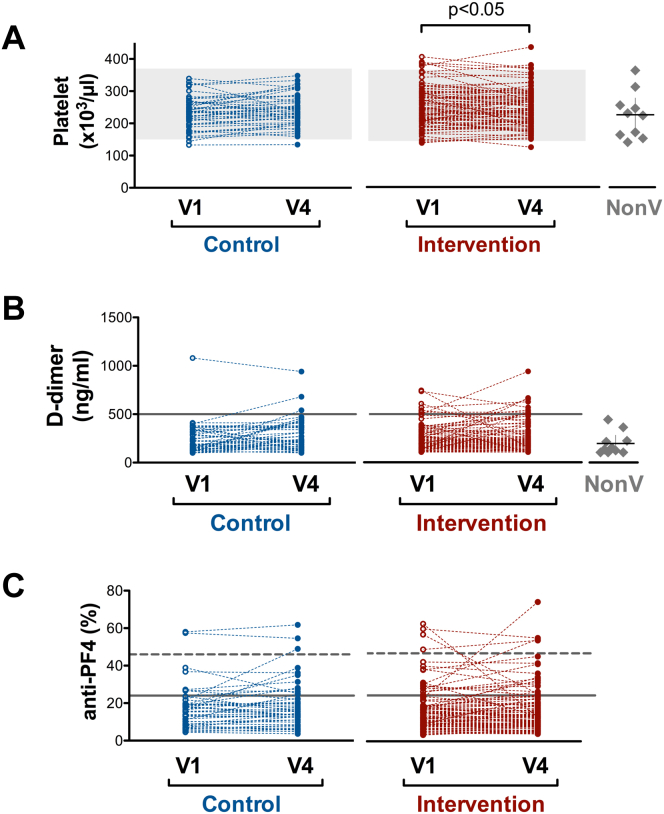
Mean platelet counts in the intervention and control groups were within the normal range but significantly lower in the control group at V1 (Figure 1A and Supplementary Table 1). When change in the platelet count (V4-V1) was evaluated, significant differences were found between the groups: count decrease was observed in the intervention group, whereas the control group showed a slight increase (Supplementary Table 1). Fifty-three (57%) participants from the intervention group had a diminution in their platelet count at V4, and 4 of them had decay above 100 × 109 platelets/L. Nevertheless, their platelet count remained within the normal range, and these changes were not accompanied by an increase in either the level of anti-PF4 or in the procoagulant profile of these patients (data not shown).
Figure 1.
(A) Platelet count (x103/μL), (B) D-dimer (ng/mL), and (C) anti-PF4 antibodies (% of the positive control of the kit), measured in both intervention and control groups on days 0 (V1) and 28 (V4) or in10 healthy controls who did not have COVID-19 and were not vaccinated against SARS-CoV-2 (NonV). Gray zone represents normal range of platelet count (A). Solid gray line represents cutoff reference value of the kits used (B, C) and dashed gray line represents the strong positive cutoff value for anti-PF4 serum levels (C). Scatter dot plots with mean ± 95% CI represent the values obtained for NonV controls (A, B)
Because D-dimer concentration above 500 ng/mL has been reported to be associated to the development of VITT [3], we analyzed the number of participants with values above this threshold. D-dimer concentration was above 500 ng/mL in 1 (2%) participant from the control group and 7 (7.5%) from the intervention group at V1, and in 3 (6.5%) and 10 (10.8%) subjects, respectively, at V4. No significant differences between the groups were observed in plasma D-dimer concentrations at V1 and V4 (Figure 1B and Supplementary Table 1). Indeed, only a 1.2-fold increase in plasma D-dimer at V4 was observed in the control group, which might be explained by the physiological fluctuations of this variable [14]. Strategies used to identify VITT include various immunological and functional assays for PF4 Abs [15].
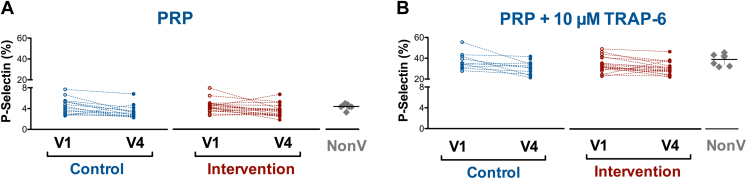
Seven participants (15%) from the control group and 16 (17%) from the intervention group had anti-PF4 Abs above the cutoff value of the technique at V1; 2 (4%) and 4 (4%) of them a strong positive, respectively. At V4, 17 individuals (18%) from the intervention group were positive for anti-PF4 Abs and 12 (26%) in the control group; 3 (6%) and 3 (3%) of them a strong positive, respectively (Figure 1C and Supplementary Table 2). This observation suggests that BNT162b2 vaccination after ChAdOx1-S vaccination does not induce an increase in the anti-PF4 Abs levels. Because the presence of high-titer anti-PF4/heparin Abs can cause platelet activation, we tested the effect of serum from those individuals with the levels of anti-PF4 Abs higher than the cutoff value on platelets from a healthy control not included in either of the 2 groups. As observed in Figure 2 and Supplementary Table 2, none of these sera could activate platelets. This fact was not because of the lack of sensitivity of the assay used because serum from a VITT-positive patient was able to activate platelets even when diluted with serum from a healthy control (Supplementary Figure 3).
Figure 2.
Effect of serum from participants with anti-PF4 antibodies titer beyond cutoff value on either day 0 (V1) or 28 (V4) or both (V1 and V4) on activation (% of P-selectin expression) of unstimulated (A) or 10 μmol/L TRAP-6 stimulated (B) platelets. Scatter dot plots with mean ± 95% CI represent the values obtained with serum from nonvaccinated (NonV) controls analyzed simultaneously with samples from trial participants (A, B). No significant differences in platelet activation were observed between this nonvaccinated group and participants at V1
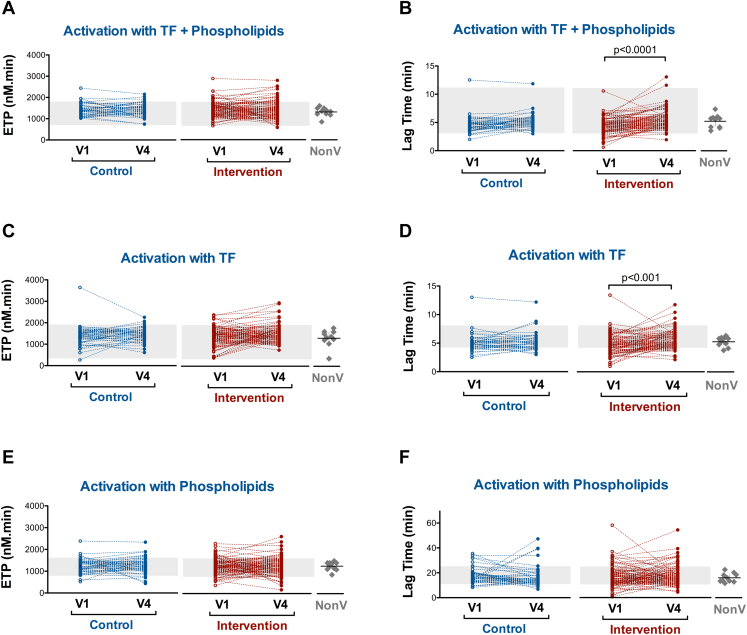
It has been suggested that vaccines may induce a transient hypercoagulable state or increase the hypercoagulable condition in affected persons [16]. We tested the thrombin generation under various conditions so that the generation of thrombin relied on the thrombogenic characteristics of either the plasma or the PS or TF content of the MPs. In all experimental conditions tested, no differences between the groups were observed in ETP at V1 and at V4 (Figure 3A, C, and E and Supplementary Table 3). Neither differences were found in LT (Supplementary Table 4). Nevertheless, significant increases in LT were observed between V1 and V4 within the intervention group, indicating longer time to thrombin generation and consequently delaying the onset of coagulation (Figure 3B, D, and F). The remaining parameters determined by CAT (Peak and ttPeak) did not differ in either inter- or intra-groups (Supplementary Tables 5 and 6).
Figure 3.
Thrombin generation test determined by calibrated automated thrombinography. Endogenous thrombin potential (ETP) and Lag time measured in both intervention and control groups on days 0 (V1) and 28 (V4). Thrombin generation was triggered by 1 pmol/L TF and 4 μmol/L of phospholipids (PPP-Reagent LOW) (A and B); 1 pmol/L TF (PRP reagent) (C and D); and 4 μmol/L phospholipids (MP reagent) (E and F). Gray zone represents in-house reference range (5% and 95% percentiles) obtained from historical control data. Scatter dot plots with mean ± 95% CI represent the values obtained from nonvaccinated (NonV) controls analyzed simultaneously with samples from trial participants. No significant differences were observed in thrombin generation test variables between this nonvaccinated group and participants at V1
At V1 (day 0), the 6 women receiving oral contraceptives in the intervention group showed a hypercoagulable profile in thrombin generation tests compared with women from this group who were not treated with oral contraceptives. Receiving vaccination with BNT162b2 did not increase ETP and peak of thrombin generation at V4 (Supplementary Tables 7 and 8).
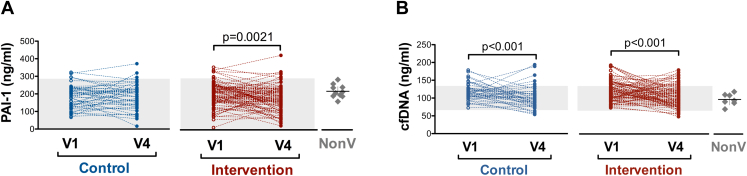
It is known that endothelial damage and hypercoagulability are major players in thrombosis. Thus, we tested the plasma levels of PAI-1, a marker of endothelial activation, in the study participants. We observed no differences in the PAI-1 levels at V1 and V4 between the groups (Supplementary Table 9) but significant decay in the plasma levels between V1 and V4 within the intervention group (Figure 4A).
Figure 4.
(A) Serum PAI-1 and (B) plasma cfDNA concentration measured in both intervention and control groups on days 0 (V1) and 28 (V4). Gray zone represents the range of normality established by kit manufacturers' instructions (A) or in-house reference range (5% and 95% percentiles) obtained from historical control data (B). Scatter dot plots with mean ± 95% CI represent the values obtained for nonvaccinated (NonV) controls analyzed simultaneously with samples from trial participants. Significant differences were obtained in plasma cfDNA levels (P < .05) but not in serum PAI-1 concentration between this nonvaccinated group and participants at V1
Another proposed mechanism for immune thrombosis in VITT is neutrophil activation and systemic activation of leucocytes and circulating cytokines, free nucleic acids, and acute phase reactants. Similar to PAI-1, no differences in cfDNA were observed between the control and intervention groups at any visits (Supplementary Table 9), whereas a significant decrease was observed between V1 and V4 in both groups (Figure 4B). No thrombotic events were reported in any group.
4. Discussion
Recently, the CombiVacS clinical trial [10] has demonstrated that heterologous vaccination schemes elicit a good immune response against SARS-CoV-2. This observation was confirmed by later studies. [11,[17], [18], [19], [20], [21]] The hemostasis subanalysis of the CombiVacS study presented here suggests that this schedule of vaccination does not appear to have an impact on the participant’s hemostatic profile.
On March 18, 2021, the European Medicines Agency (EMA) announced findings of rare thrombosis with thrombocytopenia syndrome occurred after the administration of ChAdOx1-S vaccine, which was named VITT [22]. Later, similar events have also been observed in recipients of the adenoviral vector-based Ad26.COV2.S vaccine and the mRNA-based BNT162b2 and mRNA-1273 vaccines [23]. In this study, we investigated whether COVID-19 heterologous vaccination schedules may change the hemostasis profile of participants included in the CombiVacS trial at La Paz University Hospital.
The age of participants in this trial is within the range reported to have an increased risk of suffering thrombotic episodes and thrombocytopenia after COVID-19 vaccination [24,25]. The exclusion of participants based on certain comorbidities does not seem to affect the representativeness of our results because, to date, no large studies comparing the comorbidities of individuals with VITT with those of the general population have been performed [26], and the results from one of the largest cohorts studied showed that an underlying history of thrombosis or medical comorbidity was not required to explain the occurrence of VITT or associated thrombosis [25].
We studied the hemostatic parameters of participants when randomized to receive 1 intramuscular injection of BNT162b2 or maintain observation (day 0, V1) and at day 28 (V4). Although it is accepted that VITT develops between 5 and 20 days following vaccination, Schönborn et al. [27] followed up 65 patients for at least 36 weeks after developing symptoms of VITT and observed that the platelet activation assay became negative in 73.8% of them with a median time to a negative test result of 15.5 weeks (range, 5-28 weeks), a period that comprise the samples obtained at day 28 (V4). A reduction in platelet count was detected in the intervention group 28 days after receiving the BNT162b2 dose, but this decay did not seem to have a pathological significance because the values remained within the physiological limits. Approximately 16% of individuals were positive at V1 (day 0) for anti-PF4 Abs, and vaccination did not modify this proportion. This percentage is higher than that observed in healthy blood donors (4.3%-6.6%) [28]. Nevertheless, at V1 (day 0), only 4.3% of participants from both groups had a strong titer of anti-PF4 Abs. On the other hand, we cannot rule out the possible increase in anti-PF4 Abs due to the first dose of ChAdOx1-S [29].
When we tested whether the serum from subjects with anti-PF4 Abs higher than the cutoff value of the test was able to stimulate platelets from healthy controls, we observed that none of them modified exposure of P-selectin on the platelet surface. Other authors reported the effect of serum from VITT patients positive to anti-PF4 on platelet activation using the methods different to ours, that is, washed platelets [1] or whole blood [30]. The absence of serum effect on control platelets that we observed was not due to a lack of sensitivity of our method because we did detect a stimulating effect of the serum from patients diagnosed with VITT on platelet activation status (Supplementary Figure 3) but due to the fact that individuals included did not have VITT. In accordance with our findings, Thiele et al. [31] and Barefah et al. [32] reported that the only presence of anti-PF4 Abs by itself was not associated with platelet activation or with thrombocytopenia and that an appropriate antigen and additional inflammatory co-signals are required.
Some studies have reported that VITT is caused by the formation of complexes between vaccine components and PF4, leading to neoantigen exposure on platelet surfaces to which anti-PF4 Abs bind. Moreover, vaccine components might trigger proinflammatory responses that are “danger signals” known to amplify anti-PF4 Abs production [33]. Between days 5 and 20 after vaccination, anti-PF4 Abs from VITT patients activate platelets and granulocytes in the presence of platelets to release procoagulant neutrophil extracellular traps (NETs) that are abundantly found in VITT patients [34]. Cell-free DNA, which can be considered a procoagulant factor and a biomarker for the presence of NETs [35], is elevated in serum from VITT patients [34]. At visit 1, plasma cfDNA is significantly higher in participants who had previously received ChAdOx1-S than in serum from healthy controls who did not have COVID-19 and individuals who were not vaccinated against SARS-CoV-2. We cannot conclude whether this difference might be due to the first ChAdOx1-S dose or to the variability of the population. However, we can affirm that we did not observe an increase in cfDNA in plasma after vaccination with BNT162b2, suggesting an absence of activation of granulocytes by this second heterologous vaccine. Moreover, no vascular damage seemed to be induced by BNT162b2 in individuals primed with ChAdOx1-S because no significant increase in the plasma levels of PAI-1—a marker of endothelial injury—was observed [13].
Regarding the assessment of the potential presence of hypercoagulability after COVID-19 vaccination, D-dimer testing revealed no significant coagulation activation. In addition, after the second vaccine, we did not observe an increase in the procoagulant profile related to either plasma or microparticles or the TF plasma content. This observation was also valid for the group of women treated with oral contraceptives who despite having a procoagulant profile at day 0, vaccination with BNT162b2 did not induce any additional increment in their hemostatic state. Therefore, our study gives support to the fact that the second heterologous vaccination did not increase the hypercoagulability risk in individuals treated with oral contraceptives.
To our knowledge, this study is one of the first studies to evaluate the parameters reported to be altered in VITT [36] developed in individuals included in a heterologous vaccination trial. Two studies that evaluated the hemostatic changes after receiving second doses of COVID-19 vaccines have been recently published. Campello et al. [37] assessed the coagulation profiles in a series of healthy subjects awaiting for the first or second dose after of either the ChAdOx1 or BNT162b2 vaccine. They concluded that there was not significant hypercoagulability after either vaccine but that thrombin generation might increase after the second dose of either vaccine and after the first dose of the ChAdOx1 vaccine. Difference with our results might rely in the time of sampling: they tested thrombin generation in the first 3 to 10 days after vaccination versus 28 days in our trial or to the different TF concentration used as trigger, unknown in Campello’s tests and 1 pmol/L in our experiments. De Laat et al. [38] studied the hemostatic parameters 4 weeks after the second dose of the ChAdOx1-S vaccine in participants who had previously had COVID-19 or not and compared the results from these 2 groups with those from control samples collected before the start of the COVID-19 pandemic. Contrary to us, they found an increased thrombin generation profile in individuals receiving the second dose (with our without previous SARS-CoV-2 infection) compared with control samples. This discrepancy might be explained by the fact that participants received 2 doses of ChAdOx1-S vaccine, which has been associated with an increased risk of causing thrombotic events [39].
We recognize that limitations of our study are the small number of individuals included and the lack of laboratory determinations before the first dose of ChAdOx1-S. Nevertheless, our longitudinal design of the study with biochemical, hemostasis, and coagulation evaluations before and after the vaccination with BNT162b2 and the presence of a control group only vaccinated with ChAdOx1-S may compensate for the lack of prevaccine determinations. The strength of this work relies in the fact that we have evaluated the safety of a heterologous vaccination scheme not only by merely evaluating reactogenicity over the initial days after vaccination or collecting adverse effects, as performed in other studies, but also by a comprehensive assessment of hemostasis through biochemical parameters. This is especially important now that the EMA and the European Centre for Disease Prevention and Control have recommended the possibility of using 2 different COVID-19 vaccines, either for heterologous primary vaccination or for a third dose as a booster 3 to 6 months after a primary vaccination course (heterologous boosting) [40].
5. Conclusion
Our data suggest that the heterologous vaccination against COVID-19 with ChAdOx1-S and a second dose with BNT162b2 might be safe in terms of alteration of coagulation.
Appendices
A complete list of the members of the CombiVacS Study Group (CombiVacS Vaccine Trial Group) from Hospital Universitario La Paz, Madrid, Spain, is as follows: Lucía Martínez de Soto, Amelia Rodríguez Mariblanca, Lucía Díaz García, Elena Ramírez García, Enrique Seco Meseguer, Stefan Mark Stewart Balbás, Alicia Marín Candón, Irene García García, Mikel Urroz Elizalde, Jaime Monserrat Villatoro, Paula de la Rosa, Marta Sanz García, Cristina López Crespo, Vega Mauleón Martínez, Raquel de Madariaga Castell, Laura Vitón Vara, Julio García Rodríguez, Esther Rey Cuevas, Pilar Ayllon García, María Jiménez González, Victoria Hernández Rubio, Paloma Moraga Alapont, Amparo Sánchez, Rocío Prieto, Silvia Llorente Gómez, Cristina Miragall Roig, Marina Aparicio Marlasca, Fernando de la Calle, Marta Arsuaga, and Blanca Duque.
Acknowledgments
The authors thank all trial participants for their involvement. The authors thank Esther Prieto for editorial assistance and writing support (employed by Hospital Universitario La Paz; funded by the Instituto de Salud Carlos III, grant number: PCT20/00018).
Funding
This work is funded by the Instituto de Salud Carlos III (ISCIII), a Spanish public body assigned to the Ministry of Science and Innovation that manages and promotes public clinical research related to public health. The Spanish Clinical Trials Platform (SCReN) is a public network of clinical trial units funded by the Instituto de Salud Carlos III (grant numbers PTC20/00018 and PT17/0017), the State Plan for Research, Development, and Innovation 2013–2016, the State Plan for Scientific and Technical Research and Innovation 2017–2020, and the Subdirectorate General for Evaluation and Promotion of Research, Instituto de Salud Carlos III, cofinanced with FEDER funds. CombiVacS was designed under the umbrella of the VACCELERATE (European Corona Vaccine Trial Accelerator Platform) project. A.M.B., A.J.C., and J.F. are members of the VACCELERATE Network, which aims to facilitate and accelerate the design and implementation of COVID-19 phase 2 and 3 vaccine trials. VACCELERATE received funding from the EU's Horizon 2020 Research and Innovation Programme (grant agreement numbers 101037867 and 860003). The Instituto de Salud Carlos III is the Spanish partner in the VACCELERATE project.
Ethics statement
The trial complies with the principles of the Declaration of Helsinki and Good Clinical Practice and was approved by the Spanish Agency of Medicines and Healthcare Products and by the ethics committee at La Paz University Hospital. This study was registered at EudraCT as #2021-001978-37 and ClinicalTrials.gov as #NCT04860739.
Author contributions
Study conceptualization was done by V.J.Y., J.R.A., A.M.B., N.V.B. and E.G.A.-S. E.M.M., P.A., and A.B.S. developed the study methods. V.J.Y., J.R.A., A.M.B., N.V.B., E.G.A.-S., M.T.A.R., J.F. and A.J.C. were study investigators. A.B.S., J.C.R.R., L.M.d.S., and R.d.M.B. ensured data accuracy. A.M.B., A.J.C., and J.F. were responsible for statistical analysis. V.J.Y., J.R.A., A.M.B., N.V.B., E.G.A.-S., M.T.A.R., C.B.-I., J.F., E.M.M., A.B.S., A.J.C., P.A., J.C.R.R., L.M.d.S., and R.d.M.B. supervised the study. C.B.-I. was responsible for funding acquisition. V.J.Y., J.R.A., A.M.B., N.V.B. and E.G.A.-S. wrote the original draft of the article. All authors reviewed and approved the original draft. All authors reviewed and edited the manuscript, and approved the manuscript for submission. All authors had full access to the full data in the study and accept responsibility to submit for publication. All authors read and approved the final version of the paper.
Relationship Disclosure
C.B.-I. is the general manager of the Instituto de Salud Carlos III. J.R.A. has received fees from Janssen, outside of the submitted work. A.M.B. is principal investigator of clinical trials sponsored by GlaxoSmithKline, Daiichi-Sankyo, Janssen, and Farmalider, outside of the submitted work; and has received fees from Janssen and Pfizer, outside of the submitted work. V.J.Y. and M.T.A.R. are principal investigators of clinical trials sponsored by Pfizer, NovoNordisk, Roche, Sanofi, SOBI, Takeda, and Grifols outside of the submitted work. R.D.M. reports personal fees (speaker fee) and non-financial support from ViiV and Gilead. All other authors declare no competing interests.
Informed patient consent
All participants provided written informed consent before enrolment.
Data availability
Raw data are available upon request directed to the corresponding author; after approval of a proposal, data can be shared through a secure online platform.
Footnotes
Funding information Instituto de Salud Carlos III (ISCIII) The funder designed the trial in cooperation with the Spanish Clinical Trials Platform (SCReN). Trial coordination, participant’s recruitment, and data analysis has been performed by SCReN.
Handling Editor: Dr Henri Spronk
Nora V. Butta and Elena G. Arias-Salgado contributed equally to this study as co-first authors.
The online version contains supplementary material available at https://doi.org/10.1016/j.rpth.2023.100049
Contributor Information
Alberto M. Borobia, Email: alberto.borobia@salud.madrid.org.
José Ramón Arribas, Email: joser.arribas@salud.madrid.org.
Víctor Jiménez Yuste, Email: hemostasia.hulp@gmail.com.
Supplementary material
References
- 1.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schultz N.H., Sorvoll I.H., Michelsen A.E., Munthe L.A., Lund-Johansen F., Ahlen M.T., et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tiede A., Sachs U.J., Czwalinna A., Werwitzke S., Bikker R., Krauss J.K., et al. Prothrombotic immune thrombocytopenia after COVID-19 vaccination. Blood. 2021;138:350–353. doi: 10.1182/blood.2021011958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolf M.E., Luz B., Niehaus L., Bhogal P., Bazner H., Henkes H. Thrombocytopenia and intracranial venous sinus thrombosis after "COVID-19 vaccine AstraZeneca" exposure. J Clin Med. 2021;10:1599. doi: 10.3390/jcm10081599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scully M., Singh D., Lown R., Poles A., Solomon T., Levi M., et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2202–2211. doi: 10.1056/NEJMoa2105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.See I., Su J.R., Lale A., Woo E.J., Guh A.Y., Shimabukuro T.T., et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325:2448–2456. doi: 10.1001/jama.2021.7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegler J.E., Klein P., Yaghi S., Vigilante N., Abdalkader M., Coutinho J.M., et al. Cerebral vein thrombosis with vaccine-induced immune thrombotic thrombocytopenia. Stroke. 2021;52:3045–3053. doi: 10.1161/STROKEAHA.121.035613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cines D.B., Bussel J.B. SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. 2021;384:2254–2256. doi: 10.1056/NEJMe2106315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smadja D.M., Yue Q.Y., Chocron R., Sanchez O., Lillo-Le Louet A. Vaccination against COVID-19: insight from arterial and venous thrombosis occurrence using data from VigiBase. Eur Respir J. 2021;58 doi: 10.1183/13993003.00956-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borobia A.M., Carcas A.J., Perez-Olmeda M., Castaño L., Bertran M.J., García-Pérez J., et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021;398:121–130. doi: 10.1016/S0140-6736(21)01420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nordstrom P., Ballin M., Nordstrom A. Effectiveness of heterologous ChAdOx1 nCoV-19 and mRNA prime-boost vaccination against symptomatic COVID-19 infection in Sweden: a nationwide cohort study. Lancet Reg Health Eur. 2021;11 doi: 10.1016/j.lanepe.2021.100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez-Bello I., Manzano E.M., Rio F.G., Sanz R.J., Cubillos-Zapata C., Casitas R., et al. Procoagulant state of sleep apnea depends on systemic inflammation and endothelial damage. Archivos de Bronconeumologia. 2022;58:117–124. doi: 10.1016/j.arbres.2020.11.017. [DOI] [PubMed] [Google Scholar]
- 13.Monzon Manzano E., Fernandez-Bello I., Justo Sanz R., Robles Marhuenda Á., López-Longo F.J., Acuña P., et al. Insights into the procoagulant profile of patients with systemic lupus erythematosus without antiphospholipid antibodies. J Clin Med. 2020;9:3297. doi: 10.3390/jcm9103297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudnicka A.R., Rumley A., Lowe G.D., Strachan D.P. Diurnal, seasonal, and blood-processing patterns in levels of circulating fibrinogen, fibrin D-dimer, C-reactive protein, tissue plasminogen activator, and von Willebrand factor in a 45-year-old population. Circulation. 2007;115:996–1003. doi: 10.1161/CIRCULATIONAHA.106.635169. [DOI] [PubMed] [Google Scholar]
- 15.Sachs U.J., Cooper N., Czwalinna A., Müller J., Pötzsch B., Tiede A., et al. PF4-dependent immunoassays in patients with vaccine-induced immune thrombotic thrombocytopenia: results of an interlaboratory comparison. Thromb Haemost. 2021;121:1622–1627. doi: 10.1055/a-1535-9002. [DOI] [PubMed] [Google Scholar]
- 16.Klein N.P., Lewis N., Goddard K., Fireman B., Zerbo O., Hanson K.E., et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA. 2021;326:1390–1399. doi: 10.1001/jama.2021.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X., Shaw R.H., Stuart A.S.V., Greenland M., Aley P.K., Andrews N.J., et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398:856–869. doi: 10.1016/S0140-6736(21)01694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munro A.P.S., Janani L., Cornelius V., Aley P.K., Babbage G., Baxter D., et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398:2258–2276. doi: 10.1016/S0140-6736(21)02717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pozzetto B., Legros V., Djebali S., Barateau V., Guibert N., Villard M., et al. Immunogenicity and efficacy of heterologous ChAdOx1-BNT162b2 vaccination. Nature. 2021;600:701–706. doi: 10.1038/s41586-021-04120-y. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt T., Klemis V., Schub D., Mihm J., Hielscher F., Marx S., et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat Med. 2021;27:1530–1535. doi: 10.1038/s41591-021-01464-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw R.H., Stuart A., Greenland M., Liu X., Nguyen Van-Tam J.S., Snape M.D., et al. Heterologous prime-boost COVID-19 vaccination: initial reactogenicity data. Lancet. 2021;397:2043–2046. doi: 10.1016/S0140-6736(21)01115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makris M., Pavord S., Lester W., Scully M., Hunt B. Vaccine-induced Immune thrombocytopenia and thrombosis (VITT) Res Pract Thromb Haemost. 2021;5 doi: 10.1002/rth2.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen P.W., Tsai Z.Y., Chao T.H., Li Y.H., Hou C.J., Liu P.Y. Addressing vaccine-induced immune thrombotic thrombocytopenia (vitt) following COVID-19 vaccination: a mini-review of practical strategies. Acta Cardiol Sin. 2021;37:355–364. doi: 10.6515/ACS.202107_37(4).20210628A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrews N.J., Stowe J., Ramsay M.E., Miller E. Risk of venous thrombotic events and thrombocytopenia in sequential time periods after ChAdOx1 and BNT162b2 COVID-19 vaccines: a national cohort study in England. Lancet Reg Health Eur. 2022;13 doi: 10.1016/j.lanepe.2021.100260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pavord S., Scully M., Hunt B.J., Lester W., Bagot C., Craven B., et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med. 2021;385:1680–1689. doi: 10.1056/NEJMoa2109908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pai M. Epidemiology of VITT. Semin Hematol. 2022;59:72–75. doi: 10.1053/j.seminhematol.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schonborn L., Thiele T., Kaderali L., Günther A., Hoffmann T., Seck S.E., et al. Most anti-PF4 antibodies in vaccine-induced immune thrombotic thrombocytopenia are transient. Blood. 2022;139:1903–1907. doi: 10.1182/blood.2021014214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hursting M.J., Pai P.J., McCracken J.E., Hwang F., Suvarna S., Lokhnygina Y., et al. Platelet factor 4/heparin antibodies in blood bank donors. Am J Clin Pathol. 2010;134:774–780. doi: 10.1309/AJCPG0MNR5NGKNFX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terpos E., Politou M., Ntanasis-Stathopoulos I., Karalis V., Merkouri E., Fotiou D., et al. High prevalence of anti-PF4 antibodies following ChAdOx1 nCov-19 (AZD1222) vaccination even in the absence of thrombotic events. Vaccines. 2021;9:712. doi: 10.3390/vaccines9070712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Handtke S., Wolff M., Zaninetti C., Wesche J., Schönborn L., Aurich K., et al. A flow cytometric assay to detect platelet-activating antibodies in VITT after ChAdOx1 nCov-19 vaccination. Blood. 2021;137:3656–3659. doi: 10.1182/blood.2021012064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thiele T., Ulm L., Holtfreter S., Schönborn L., Kuhn S.O., Scheer C., et al. Frequency of positive anti-PF4/polyanion antibody tests after COVID-19 vaccination with ChAdOx1 nCoV-19 and BNT162b2. Blood. 2021;138:299–303. doi: 10.1182/blood.2021012217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barefah A.S., Radhwi O.O., Alamri S.S., Alahwal H.M., Denetiu I., Almohammadi A.T., et al. Low clinical utility of testing for anti-platelet factor 4 in asymptomatic individuals after ChAdOx1 nCoV-19 vaccine. Int J Lab Hematol. 2022;44:424–429. doi: 10.1111/ijlh.13774. [DOI] [PubMed] [Google Scholar]
- 33.Gong T., Liu L., Jiang W., Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. 2020;20:95–112. doi: 10.1038/s41577-019-0215-7. [DOI] [PubMed] [Google Scholar]
- 34.Greinacher A., Selleng K., Palankar R., Wesche J., Handtke S., Wolff M., et al. Insights in ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia. Blood. 2021;138:2256–2268. doi: 10.1182/blood.2021013231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medeiros S.K., Emery B., Bhagirath V., Parpia S., Dwivedi D.J., Dwivedi N.J., et al. Does cell-free DNA promote coagulation and inhibit fibrinolysis in patients with unprovoked venous thromboembolism? Thromb Res. 2020;186:13–19. doi: 10.1016/j.thromres.2019.11.030. [DOI] [PubMed] [Google Scholar]
- 36.Favaloro E.J. Laboratory testing for suspected COVID-19 vaccine-induced (immune) thrombotic thrombocytopenia. Int J Lab Hematol. 2021;43:559–570. doi: 10.1111/ijlh.13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campello E., Bulato C., Simion C., Spiezia L., Radu C.M., Gavasso S., et al. Assessing clinically meaningful hypercoagulability after COVID-19 vaccination: a longitudinal study. Thromb Haemost. 2022;122:1352–1360. doi: 10.1055/a-1788-5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Laat B., Stragier H., de Laat-Kremers R., Ninivaggi M., Mesotten D., Thiessen S., et al. Population-wide persistent hemostatic changes after vaccination with ChAdOx1-S. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.966028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cari L., Fiore P., Naghavi Alhosseini M., Sava G., Nocentini G. Blood clots and bleeding events following BNT162b2 and ChAdOx1 nCoV-19 vaccine: an analysis of European data. J Autoimmun. 2021;122 doi: 10.1016/j.jaut.2021.102685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.EMA E. ECDC recommendations on heterologous vaccination courses against COVID-19: ‘mix-and-match’ approach can be used for both initial courses and boosters. 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data are available upon request directed to the corresponding author; after approval of a proposal, data can be shared through a secure online platform.