Abstract
The limited availability of transplantable organs hinders the success of patient treatment through organ transplantation. In addition, there are challenges with immune rejection and the risk of disease transmission when receiving organs from other individuals. Tissue engineering aims to overcome these challenges by generating functional three-dimensional (3D) tissue constructs. When developing tissues or organs of a particular shape, structure, and size as determined by the specific needs of the therapeutic intervention, a tissue specific oxygen supply to all parts of the tissue construct is an utmost requirement. Moreover, the lack of a functional vasculature in engineered tissues decreases cell survival upon implantation in the body. Oxygen-generating materials can alleviate this challenge in engineered tissue constructs by providing oxygen in a sustained and controlled manner. Oxygen-generating materials can be incorporated into 3D scaffolds allowing the cells to receive and utilize oxygen efficiently. In this review, we present an overview of the use of oxygen-generating materials in various tissue engineering applications in an organ specific manner as well as their potential use in the clinic.
Keywords: Tissue engineering, oxygen distribution, oxygen-generating materials, metal peroxides, scaffolds
Graphical Abstract
Oxygen-generating materials can be incorporated into three-dimensional (3D) scaffolds to enable the cells to receive and utilize oxygen efficiently.

1. Introduction
Organ failure affects people of all ages and demographics, with over 113,000 people in the United States waiting to receive a life extending transplant (1). Due to transplantation being the gold-standard treatment for patients with end-stage organ failure, there are growing concerns about the availability of the lifesaving procedures (2). Currently the demand for transplantable organs is outweighing the available supply, with an average of 17 deaths per day due to the lack of available organs for transplantation (2) (3). As the disparity between available organs for transplant and patients who need them continues to grow, finding alternative therapies to address this hurdle is of special interest for researchers. The discipline of tissue engineering (TE) aims to generate three-dimensional (3D) constructs that can maintain, regenerate, or improve tissue function (4). Not only can TE provide a solution to the issue of organ availability, but also it can reduce the morbidity in recipients of TE interventions (5). These approaches have been quite successful in both in vitro and in vivo models with potential translation into clinical practice (5).
At the intersection of health sciences and engineering, many factors such as size, complexity, and architecture of the tissue to be reconstructed or repaired, types of cells to be used, materials and methods used for developing engineered tissues, as well as the biomolecular cues required for supporting cell proliferation and differentiation, are considered when designing a TE construct for repairing a defective tissue (6). The primary materials used are cells to be seeded, the material from which the scaffold is constructed, and growth factors to direct the growth or differentiation of seeded cells (7, 8).
While a wide variety of cell types are used in TE, the most promising ones are stem cells (e.g., mesenchymal stem cells (MSCs), hematopoietic stem cells (HSCs), and adipose derived stem cells), and progenitor cells (e.g., preosteoblasts, and endothelial progenitor cells) (9). Found in bone marrow, HSCs and MSCs are commonly used in TE due to their ability to differentiate into various cell types provided the presence of required growth factors and proper microenvironment (10) (11). Additionally, MSCs may also be obtained from donor adipose tissue, making these cells easier to obtain than through bone marrow, and therefore more relevant when considering future clinical applications of these therapies (10).
When conceptualizing a possible TE therapy, choosing a suitable scaffolding material is as important as determining which cell types are best to use in the approach. According to Berthiaume and Yarmush, optimal scaffolding materials are characterized by their porosity, biodegradability behavior, and surface chemistry (12). Finding the ideal combination of these characteristics, preferably mimicking extracellular matrix (ECM), is key in promoting the adhesion, growth, and differentiation of seeded cells (13). Such considerations are important when conceptualizing TE therapies with oxygen release, as release kinetics can be affected by a variety of chemical and physical properties of the cell/scaffold complex. For example, hydrophobic scaffolding materials have shown to prolong oxygen release. This approach has seen more success than hydrophilic materials as it takes longer for water to permeate the structure and trigger the release of oxygen from OGMs in the construct.
A significant obstacle in TE is providing adequate vasculature for engineered constructs (14). Currently, implanted scaffolds depend on oxygen diffusion from surrounding vasculature to meet their oxygen needs and remain viable, as the time required to establish adequate vasculature within a scaffold is in the order of weeks (14) (15) (16) (17). This is a major issue especially in engineering large scale constructs, as oxygen diffusion is limited to within about 200 μm from existing vasculature (18). Due to the inability of larger scale constructs to have their oxygen requirements satisfied by simple diffusion, seeded cells in the interior of the scaffold often become necrotic, and the construct is rendered dysfunctional (19).
Oxygen is critical for maintaining the viability of cells due to its critical roles in oxidative phosphorylation and a myriad of other biochemical pathways (20) (21). Limited oxygen supply in hypoxic conditions can cause a variety of physical changes in the cell, mainly reduce the oxygen and energy requirements of cells (21) (22). These changes often cause the hypoxic tissue to become progressively dysfunctional with time, coinciding with greater rates of apoptosis. As such, implanted scaffolds require a steady oxygen supply to retain metabolic functions so they can proliferate and differentiate well within the scaffold.
In response to this challenge, researchers have been investigating the use of oxygen-generating materials as a sustained source of oxygen in engineered tissue constructs. Such approaches are significant since they allow seeded cells to remain viable for longer, in the absence of blood supply (23). Oxygen-generating materials can also enhance cellular differentiation and proliferation behaviors while adequate vasculature is established (24). While there is a variety of oxygen-generating materials that have been used in TE research, the three main classes of materials as seen in literature are liquid peroxides including hydrogen peroxide (H2O2), solid peroxides including magnesium peroxide (MgO2) and calcium (CaO2) peroxide, and fluorinated molecules including perfluorochemicals (PFCs) (25).
Peroxides have seen success in extending construct viability in different TE applications. For instance, solid peroxides have been shown to provide steady oxygen release in vitro for relatively longer periods (26) (27) (28). Through the chemical reaction upon contact with water, solid peroxides (MgO2, CaO2) release H2O2 which is subsequently converted into oxygen (24). Catalase can also be used to hasten the conversion of H2O2 into oxygen. While these compounds have shown promise in resolving oxygen supply issues in TE constructs, they can create cytotoxic reaction intermediates called reactive oxygen species (ROS), which can cause DNA damage and interfere with cellular functions (25). To bypass the production of ROS intermediates and retain cell viability, the use of catalase is often employed to reduce H2O2 before damage occurs to cells (24) (27).
Perfluorocarbons (PFCs) also have been used in biomedical applications due to their inert nature, lending to their inherent biocompatibility. With their long chain structures, PFCs can store large amounts of oxygen, in the typical range of 30–60 mL oxygen per 100 mL PFC used, at near room temperature (25). In addition, these compounds are well suited for oxygen release applications, as they can be used both in aqueous emulsions, and directly embedded into the scaffolding materials (24). Despite the biocompatibility and promising outcomes than traditional direct oxygen-supplementation methods, most of the oxygen-generating materials remain unapproved for the clinical use by the FDA (26) (28).
TE strategies delivering controlled oxygen release over extended periods of time within physiological limits are necessary for the development of suitable biomaterials for clinical translation. Various studies have addressed the biological and chemical properties of the OGMs to design better constructs capable of mitigating the adverse effects of rapid oxygen release. Specifically, the use of hydrophobic polymers to encapsulate oxygen-generating microspheres containing solid peroxides has been evaluated, as the delayed water diffusion into the scaffold provides a sustained and long-term oxygen release (26). Additionally, fluorinated compounds have also been evaluated for the long term release of oxygen (29). Approaches employing materials that can reduce the formation of cytotoxic byproducts have also been evaluated (30).
The primary function of blood vessels is to effectively deliver oxygen and nutrients to the tissues as well as to remove carbon dioxide and other metabolic wastes from them. Therefore, oxygen-rich blood is distributed to each tissue according to its function and need. For example, in lung tissue, the partial pressure of oxygen (pO2) was found to range from 23 to 656 mm Hg (31). The level of oxygen in the liver tissue is between 30- and 50-mm Hg. In short, the need for oxygen varies from organ to organ and tissue to tissue. Therefore, when planning the delivery of oxygen in TE, it should be tailored to each organ and its tissues by considering the physiological distribution of oxygen in native conditions.
Many researchers have reviewed several aspects of O2 generating materials in tissue engineering including, the role of O2 in TE (32) and various O2 release mechanisms from OGMs (24, 33), as well as the need for controlled delivery of O2 (27, 28) and approaches to minimize the toxicity of OGM, potential of OGM in ex vivo, in situ and bioprinting (34). However, in the current review we emphasize how oxygen-generating materials are useful in various TE approaches with a focus on the oxygen requirement of that specific tissue and fulfilling such requirement in engineering tissues.
This article discusses the tissue-specific distribution of oxygen in various organs and the need to mimic such an oxygen distribution in tissue engineered constructs, as well as the attempts to provide a sustained supply of oxygen in different types of engineered tissues.
2. Role of Oxygen in Tissue Homeostasis and Repair
Oxygen and reactive oxygen species play many important roles in a variety of chemical processes, most notably in metabolism and as a signaling molecule (35). Tissue hypoxia typically leads to the expression of Hypoxia Inducible Factors (HIF), which may contribute to apoptosis (36). Alternatively, HIFs may also act as signaling molecules within cells and make changes in cells so that they are better apt to survive in the hypoxic environment (36) (37). The most notable of these changes are a lowered metabolic rate due to the unavailability of oxygen, and higher rates of proliferation and angiogenesis, as the body changes its focus to forming new blood vessels to mitigate the effects of hypoxia on tissue. While this may sound promising for TE applications, the increased rates of angiogenesis seen in hypoxia end after a short period of time. The growth of new blood vessels becomes stagnant, as tissue subjected to long-term hypoxia is unable to generate new vasculature quickly enough to mitigate the effects of hypoxia on the tissue. Additionally, HIFs have been shown to decrease differentiation, therefore interfering with the goals of TE therapies as cells seeded within a tissue construct are hindered from forming functional structures capable of mimicking the native tissue (38) (39).
Long-term hypoxia has been linked to increased prevalence of age-related tissue degeneration due to the reduction in metabolism, as seen in sarcopenia and osteoporosis (40) (41). Decreased metabolism in hypoxia changes the native tissues, leading to reduced mobility and functionality of various tissues. These changes manifest as the progression of fibrosis in cardiac, dermal, hepatic, cartilaginous, and soft tissues (42) (43) (44). As such, the prevention of hypoxia both within a TE scaffold as well as the injury site is of special importance to researchers, as it is critical in the regeneration of functional tissue (39).
Not only does hypoxia affect pre-existing tissue, but it also inhibits the formation of new tissue. Studies describing tissue regeneration in hypoxic and ischemic environments have verified this intriguing effect of hypoxia (45) (46) (47). For instance, researchers used a mouse fracture model and removed the femoral artery to reduce oxygen levels at the fracture site (45). Compared with control rats, those who lost femoral arteries experienced a delay in fracture healing as well as a reduced rate of bone and cartilage formation (45).
Similarly, positive effects of supplemental oxygen therapies in tissue regeneration have been reported in literature. In another murine model, oxygen supplementation through hyperbaric oxygen provided greater degrees of dermal reconstruction, as well as rapid wound healing compared to the non-supplemented controls (48). Interestingly, oxygen supplemented wounds showed the formation of thick dermal layers and faster maturation of stratum corneum after a period of only three days. Such results were not observed to the same degree in non-supplemented controls following the UV radiation, and a distinct stratum corneum was not observed until after five days (48).
Localized oxygen delivery has also proved to be advantageous, as it allows the direct oxygen supplementation at the tissues. TE hydrogels are made by substituting the amino groups on the gelatin backbone with methacrylic anhydride (MAA), resulting in non-toxic and non-immunogenic scaffolds with up to 90% biodegradability (49) (50) (51). An oxygen-generating microsphere containing hydrogel was employed as the oxygen delivery method for cardiomyocytes cultured under hypoxia (52). Compared to the microsphere-free control, cultures containing the oxygen-generating microspheres provided higher rates of cell proliferation while retaining the cell viability (52). Such results confirm the benefits of using localized oxygen-delivery methods in TE applications to facilitate the repair and regeneration of damaged tissue.
3. Impact of Oxygen Generating-Materials in Tissue Engineered Constructs
Besides its general roles in metabolism and homeostasis, oxygen also has tissue-specific roles when it comes to directing the differentiation of stem cells and achieving tissue regeneration. Various studies indicate that the lack of oxygenation causes the expression of hypoxia associated genes, which changes tissue phenotype to mitigate the adverse effects of hypoxia (53). However, a higher expression of Hypoxia Inducible Factor 1-Alpha (HIF-1a) is known to render adipose, cartilage, and muscle tissue fibrotic and brittle as well as making bone porous (40) (54). Such changes render tissue dysfunctional and can lead to hypoxia-induced necrosis. Hypoxia associated outcomes can be seen in engineered tissues where local oxygenation is low and vasculature within the scaffold is unestablished. To resolve this issue, researchers have been studying the use of oxygen-generating materials for TE applications.
3.1. Oxygen-Generating Materials in Bone Tissue Engineering
Bone fractures are one of the most common ailments affecting millions of people across all demographics (74). Such injuries are either healed by natural regeneration processes or corrected by clinical interventions such as caste wrapping, internal fixation, surgeries, or bone grafting (75, 76). Although grafting is the currently used gold standard approach for repairing critical sized bone defects, availability of the grafts is limited, and the demand far outweighs the supply. As such, there is a lot of ongoing research seeking to make oxygen-releasing engineered tissues for grafting out of more readily available materials to solve these challenges (6). Oxygen plays critical role in bone formation and bone degeneration (Fig. 1A). Normoxic condition is favorable for osteoblast activity whereas hypoxia can adversely affect osteoblast proliferation. Similarly, hypoxic conditions favor osteoclast activities and result in bone degeneration.
Figure 1.
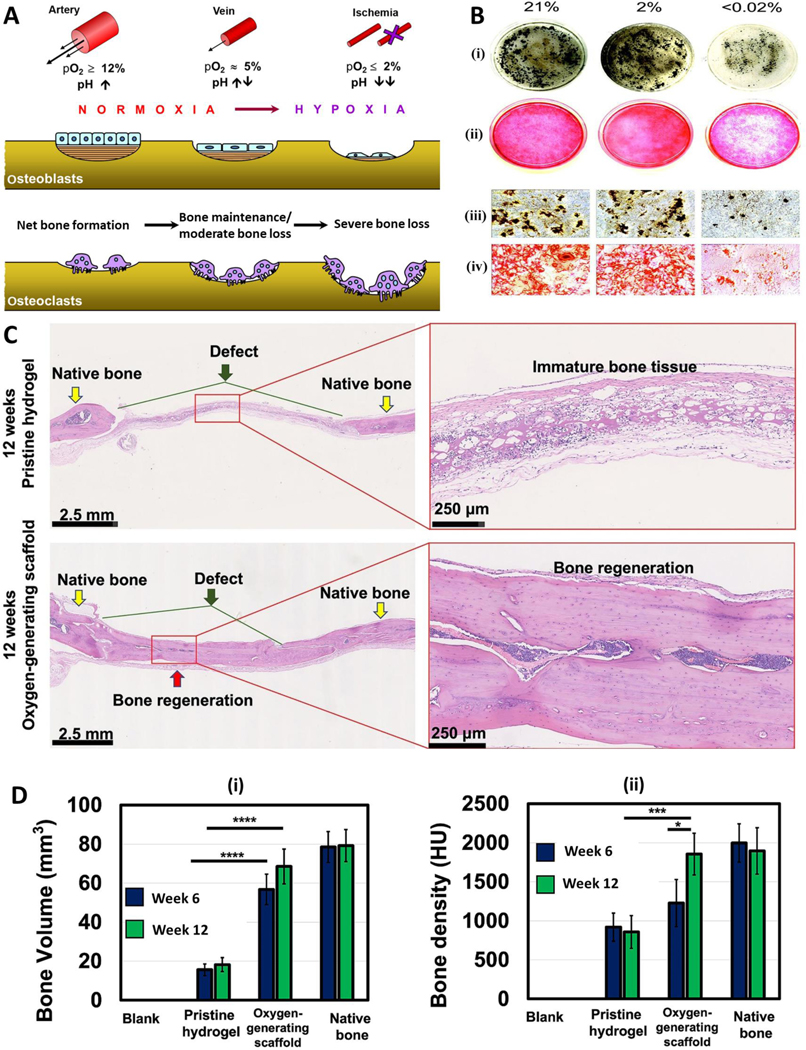
Oxygen-generating materials in bone tissue engineering. A) A scheme showing the effect of oxygen partial pressure in osteoblast and osteoclast activity during bone regeneration. B) Influence of oxygen supply on osteogenic cell survival, maintenance, and differentiation as well as subsequent bone regeneration (studied in terms of before blood vessel formation and after blood vessel formation). C) Mouse osteoblast differentiation following a 24-h exposure to ambient air (21% O2), hypoxia (2% O2), or anoxia (<0.02% O2). Representative low magnification images of (i) von Kossa and (ii) alizarin red stained cells indicated decreased bone nodule formation following less than 0.02% O2 exposure compared with 21 and 2% O2. High magnification images of (ii) von Kossa and (iv) alizarin red stained cells confirmed limited mineralized nodule formation by the anoxia-treated group. D) A scheme showing the fabrication of polycaprolactone (PCL)/CaO2-based oxygen-generating microparticles and oxygen-generating scaffold. E) Bone volume and (ii) Bone density measurements as well as F) H & E-stained histology images of healed bone indicate that the oxygen-generating scaffolds better support bone regeneration in comparison with the pristine scaffolds. Fig. A is reproduced from Ref. (82) with the permission of Springer-Nature, copyright 2013. Fig. B is reproduced from Ref. (83) with the creative common attribution license (CC-BY-0.4), copyright 2004. Fig. C, D are reproduced from Ref. (77) with the permission of Elsevier, copyright 2022.
One study noting the disparity between occurrence of critical bone injuries and these graphs sought to determine the effect that oxygen-generating scaffolds had on bone regeneration at these injury sites. PFO loaded microspheres prolonged the survival and preserved the osteogenic differentiation potency of human periosteal-derived cells (hPDCs) until the new blood vessels were formed and supported rapid bone regeneration. Corroborating the results of an earlier study that showed the decreased osteogenic differentiation of mouse osteoblast under anoxia (<0.02% O2), an optimum level of oxygen is necessary for bone regeneration (Fig. 1B).
In another study, the researchers used gelatin methacryloyl (GelMA) based TE scaffolds which contained calcium peroxide (CaO2) loaded polycaprolactone (PCL) microparticles as an oxygen-generating component (77). Oxygen was supplied to the defect site through the degradation of CaO2 into hydrogen peroxide, and then oxygen. The grafts were then tested in vitro in Sprague-Dawley (SD) rat cranial defect models. Interestingly, the oxygen-generating grafts provided greater degrees of regeneration at 6 and 12 weeks when compared to no treatment and pristine hydrogel controls (77). After 12 weeks, near full bone regeneration within the defects was observed (Fig. 1C). Bone volume and bone density were quantified and found to be comparable to the native bone the oxygen-generating scaffolds (Fig. 1D) (77).
Another study examined the effects of biphasic calcium phosphate (BCP) scaffolds in radial bone defects of New Zealand white rabbits (78). Scaffolds were generated by coating the BCP structures in a CaO2/PCL solution to contain 3% by wt. CaO2. After comparing the CaO2 covered scaffold with an uncovered BCP control, the researchers found that loading of CaO2 lead to greater bone regeneration, as roughly twice the amount of new bone was present in the CaO2/BCP treated injuries. The observed increase in bone regeneration was due to the greater degree of osseointegration found in the CaO2/BCP treated injuries (78). Additionally, a study using mouse models found that upon implantation of a perfluorotributylamine (PFTBA) supplemented engineered tissue composed of mesenchymal stem cells (MSC’s), fibrin, and thrombin, there was a greater degree of ectopic bone formation than in low PFTBA and no PFTBA control groups (63).
Not only do oxygen saturation and requirements differ from tissue to tissue, but they also differ in different parts of the same tissue system. An example of this would be how the bone matrix has an ambient O2 tension of roughly 12%, as the bone matrix lies near vasculature (79). Bone marrow however has oxygen requirements differing from that in bone matrix due to its location within the sinusoidal cavity. Being in the sinusoidal cavity, bone marrow is accustomed to an oxygen concentration of roughly 6% O2 (79). Such observations corroborate previously conducted studies demonstrating that stem cells at low O2 concentrations retain the stem cell phenotype instead of being directed down a differentiation pathway, like the stem cells in bone marrow.
In one study, the researchers were able to determine that cells cultured near typical osteogenic oxygen gradient (12%), and in a 3D environment as opposed to 2D scaffolds better preserved cell viability and pushed seeded MSCs in the direction of osteogenesis (79). Predictably, this combination was most favorable compared to bone marrow oxygenation level (5% O2), as studies would suggest this oxygen level to retain the stem cell phenotype of seeded cells. In normoxia (21% O2), the oxygen concentration is in large excess compared to the natural microenvironment of osteoblasts and would cause unfavorable outcomes (79). Such results stress the need for controlled release of oxygen methods in bone TE.
Similar studies can be improved by attempting to mimic the O2 gradient seen at different points in a tissue and seeing how this gradient would affect seeded cells. An example of such approach would be making a scaffold with a 5% oxygen concentration at one point, which grows across the scaffold to a 12% oxygen point. Following a culture period, it could then be determined whether the system would lead to regions mimicking bone marrow and regions mimicking the bone matrix respectively. Such studies are needed to advance TE therapies and bring them closer to clinical translation or for the maintenance of large tissue constructs in the laboratory. Results of studies like these are crucial for the optimization of bone TE therapies.
A study with a similar concept was conducted concerning the effect that a differing oxygen gradient had on seeded cells when it came to attempting to mimic the structure at the cartilage to bone interface in a single scaffold (80). In this study, researchers constructed a scaffold to have zones of low and high oxygen-generating microparticles (CaO2). The researchers hypothesized that the lower scaffold regions with less oxygen-generating microparticles would favor chondrogenesis, whereas the upper scaffold regions would favor osteogenesis, in accordance with the higher oxygen requirements of the more metabolically active osteoblasts (80). Their hypothesis was well based in fact, as previously conducted studies have shown that chondrogenic differentiation of MSCs takes place better in lower oxygen concentrations (81).
After seeding with MSCs and evaluating the scaffold outcomes in vitro, the high-oxygen concentration zones were shown to increase osteogenic differentiation through increased expression of alkaline phosphatase (ALP), osteocalcin (OCN), and osteopontin (OPN) (80). Increased expression of SRY-Box Transcription Factor 9 (SOX9) in the regions of the scaffold with lower concentrations of the oxygen-generating microparticles validated the hypothesis that lower oxygen generation would favor chondrogenesis over osteogenesis (80). Approaches like these have great potential to be employed in TE settings at the bone-cartilage interface. While not much research exists on the subject, similar approaches may be used to mimic the physiological distribution of oxygen at different points in tissue, for the purpose of improving TE approaches and making them more viable for clinical translation.
3.2. Oxygen-Generating Materials in Cardiovascular Tissue Engineering
Cardiovascular diseases are reported as the highest-ranking cause of death worldwide (6). Despite the common nature of cardiovascular illnesses, they remain difficult to treat due to the limited regeneration capability of the human heart. As such, cell therapies have been investigated with varying degrees of success (84). Oxygen-generating materials are useful in cardiac TE therapies, because various diseases or injuries render the local tissue hypoxic, limiting the delivery of necessary oxygen to the cells. Without a supplemental oxygen source, cellular therapies consume the already scarce oxygen, leading to the failure of the implanted cells (85). Recent investigations into the use of oxygen-generating and carrying materials have shown greater degrees of success than those without such materials, indicating the potential of oxygen supplemented regeneration therapies in cardiac TE.
In one study, researchers constructed a scaffold to mimic the vasculature of the heart by culturing fibroblasts and cardiomyocytes (CMs) on a channeled porous elastomer (29). Subsequently, they tested the effect of oxygen carriers on the culture outcome through supplementation with a perfluorocarbon (PFC) emulsion and compared to an unsupplemented control. Emulsions like these are of interest to researchers due to their ability to supply cultures with generous amounts of oxygen through diffusing oxygen carried by the PFCs. The PFC-emulsion supplemented cultures maintained optimal oxygen partial pressure, higher DNA content, and higher expression of cardiac markers (29). Supplemented cultures also had better contractile properties when compared to the control groups (29).
While most studies concerning cell-based regeneration therapies addressed the need for oxygen in cardiac muscle regeneration, few specifically address the production of reactive oxygen species (ROS), which often arises in TE therapies because of over oxygenation. To address this issue, a group of researchers encapsulated mesenchymal stem cells (MSCs) of 4-week-old male rats in a hydrogel referred as “ROS-scavenging and O2-generating hydrogel (RCGel)”. The gel proved to be capable of eradicating ROS thanks to the inclusion of catalase enzyme, for enhanced MSC survival (Fig. 2A) (30). When compared to pure MSCs, the MSC/RCGel injections presented considerably decreased rates of apoptosis, increased cardiomyocyte survival, and increased formation of new blood vessels following injection in infarcted rat hearts (30).
Figure 2.
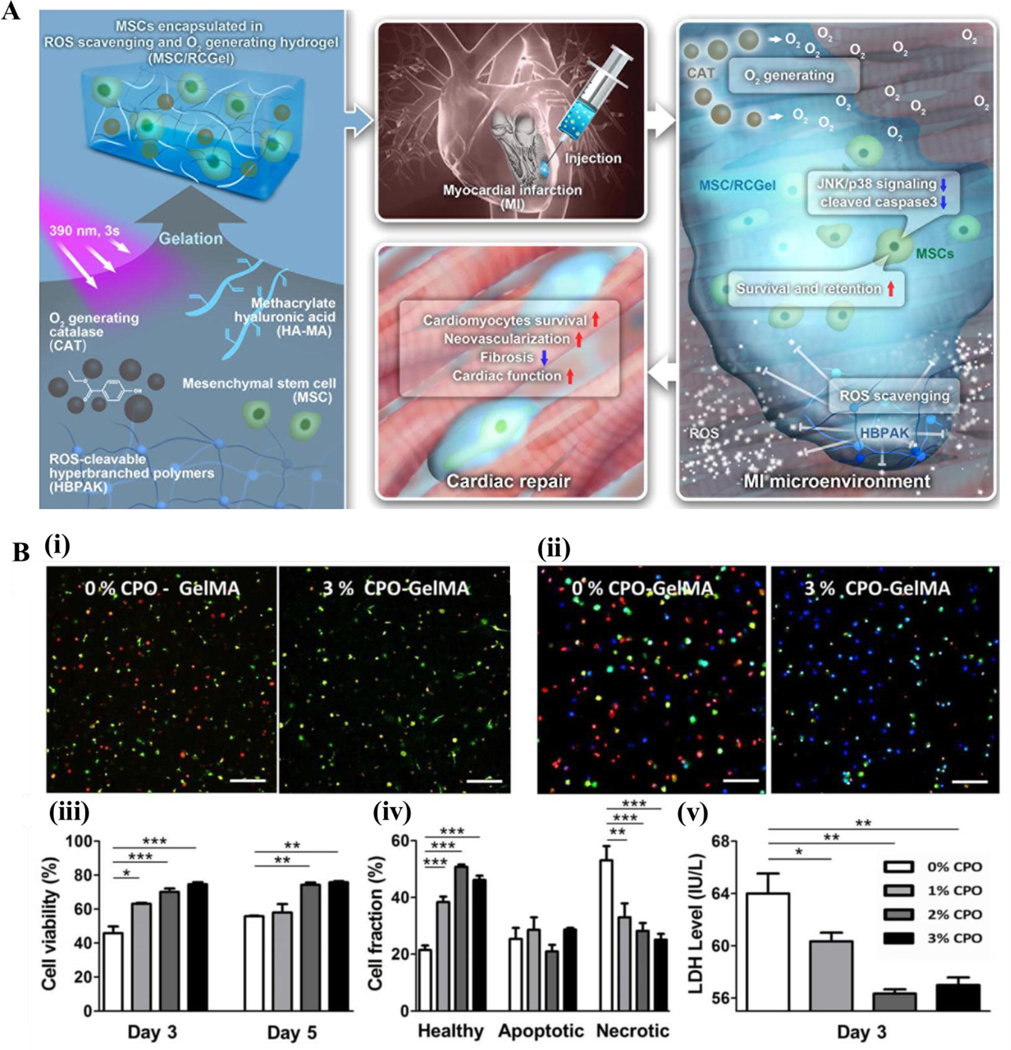
Oxygen-generating materials in cardiac tissue engineering. A) General overview of cell-based therapies employed in cardiac tissue engineering and the importance of oxygen. The scheme shows that an oxygen-generating and ROS scavenging MSC/ hydrogel scaffold (MSC/RCGel), reduces fibrosis and promotes cell survival in an infarcted heart. B) Calcium peroxide (CPO) loaded oxygen generating GelMA hydrogels increase the survival of cardiac side population cells (CSPs) by preventing hypoxia induced necrosis. Confocal microphotographs of (i) live (green)/dead (red) and (ii) healthy (blue), apoptotic (green), and necrotic (red) CSPs cultured under hypoxic conditions in CPO loaded GelMA. Semiquantification of viable cells (iii) as well as (iv) healthy, apoptotic, and necrotic cells. (v) LDH activity of CSP cultured in CPO loaded GelMA hydrogels. Scale bar equals 50 micrometers. 297×150mm (300 × 300 DPI) Fig. A is reproduced from Ref. (30) with the permission of Elsevier, copyright 2022. Fig. B is reproduced from Ref. (67) with the permission of the American Chemical Society, copyright 2017.
Another study showed that an oxygen releasing PVP/PLGA scaffold containing a combination of hydrogen peroxide (H2O2) and catalase supported cardiosphere-derived cells (CDC) proliferation under hypoxic conditions (66). Encapsulated catalase was able to catalyze the degradation of H2O2 into oxygen and provide a sustained supply of oxygen to the cells under hypoxic conditions (66). A similar study examined the effect of oxygen-generating materials on cardiac side population cells (CSPs) (Fig. 2B). The CSPs were suspended in GelMA loaded with CaO2 for oxygen supplementation (67). The researchers found that through the incorporation of at least 2% CaO2 in the hydrogel, the metabolic activity of the supplemented CSPs cultured in 1% oxygen mirrored that of those cultured in normoxia. Additionally, they found that CSP death resulting from hypoxia was attenuated by the addition of CaO2 when compared to non-supplemented CSP/GelMA formulations (67).
Unlike the other tissues reviewed in this article, there is not much known about the effect of oxygen on the differentiation of CM predecessors into functional cardiomyocytes, as researchers are just starting to unpack the mystery of how oxygen gradients in cardiac tissue affect its function. Despite this lack of knowledge, researchers do know that partial pressure of oxygen decreases as one moves from the epicardium to the endocardium (86). While little remains known about why this gradient exists or its functionality, it has been shown that the controlled diffusion of oxygen can mimic these spatial differences in oxygen concentration as seen in vivo (87). Controlled diffusion lends to better engineered cardiac tissue, as seen through differences in contraction rate, protein expression, and aerobic respiration between construct zones with varying oxygen concentrations (87).
Currently, cardiac TE strategies attempt to supply the seeded scaffolds with as much oxygen as necessary to promote differentiated CM survival and proliferation, based on the idea that CMs are highly active regarding aerobic respiration (88) (89). To formulate better TE protocols for the construction of functional cardiac tissue, we first need to have a better understanding of CM progenitor cells themselves, and how oxygen levels over time affect their proliferation and differentiation. Once better understood, this knowledge can then be applied to encourage improved differentiation and viability of progenitor cells. Following this, more studies need to be conducted concerning the effect of oxygen as a signaling molecule for cardiomyocytes. These will lead to more successful scaffolds and improvements in culture and construct designs and protocols and will enhance the ability to form better cardiac tissue constructs in the lab.
3.3. Oxygen-Generating Materials in Skin Tissue Engineering
As our outermost organ, skin is responsible for protecting our bodies from foreign matter (90). Therefore, wounds are some of the most common injuries that the human body is subjected to. As such, most heal on their own and require no medical attention. However, in cases of chronic wounds or in patients with underlying conditions, wounds may not heal properly on their own or healing may be delayed, leading to ulcerations and infections ultimately exacerbating the issue (6) (32) (91).
Cell-based therapies are being investigated for clinical use in wound healing, with the goal of increasing vascularization and closure at the wound site (92). Such goals are challenging, as the development of new vasculature is time-consuming and is quite demanding on metabolic processes, sometimes leading to areas of necrotic tissue at the wound site. Therefore, a myriad of cell types and strategies are currently being studied for potential use in these applications (93).
Across all these strategies, current literature on the topic agrees that supplementation of the therapies with an oxygen source leads to better patient outcomes (Fig. 3A) (94). Supplemental oxygen encourages the production of collagen at wound sites, inducing an increase in the formation of new connective tissue (94). With respect to wound healing, oxygen supplementation is often done by means of different types of dressings, with unique methods of oxygen generation or supplementation across different studies.
Figure 3.
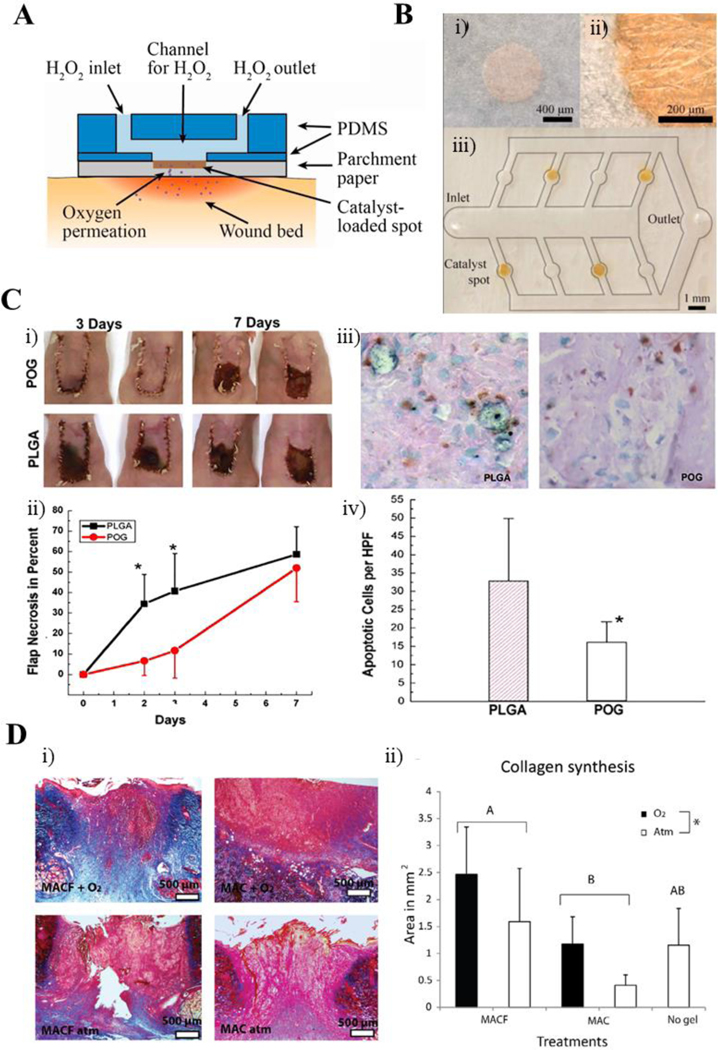
Oxygen-generating materials in wound healing and regeneration. A) Schematic of a catalyzed oxygen-generating paper-based platform to be employed directly on the wound site. B) (i) Top and (ii) magnified views of the catalyst-loaded spots within the parchment paper-based platform as well as (iii) scheme showing the channels for hydrogen peroxide flow. C) Sodium percarbonate (SPO) laden PLGA films were implanted in mice models. Hematoxylin and eosin staining of skin flaps harvested at both 3 and 7 days. Delayed necrosis is seen in the SPO containing PLGA films (POG) through preservation of tissue architecture, hair follicles, epidermis height, and sebaceous glands compared to the non-oxygen-generating control films. D) Perfluorocarbon chains (MACF) based hydrogels were applied on dermal excisional wounds in rats. Representative Masson’s trichrome histology images for MACF and non-fluorinated methacrylamide chitosan (MAC) groups both with and without oxygen, as well as the no-gel control. New collagen synthesis is shown for each group, confirming the benefits of oxygen saturation of perfluorocarbon chains in the MACF group in wound healing. Fig. A, B are reproduced from Ref. (68) with permission from Elsevier, copyright 2014. Fig. C is reproduced from Ref. (69) with the permission of Elsevier, copyright 2007. Fig. D is reproduced from Ref. (70) with the permission of Elsevier, copyright 2016.
One study assessed the ability of oxygenated dressings to reduce the rate of skin flap failure in pig wound models (95). The researchers found that the flaps treated with the oxygenated dressings had significantly slower rates of necrosis, inflammation, and bacterial load compared to the non-oxygenated hydrogel controls (95). An alternate approach employed the use of a parchment paper/PDMS platform with hydrogen peroxide/catalase as the oxygen-generating component, in which they were able to control the flow rate of peroxide into the platform (Fig. 3A,B) (68). Controllable approaches like these are key in fine-tuning the therapies used for tissue regeneration, as with them the production of ROS can be mitigated and the consequences of initial over oxygenation resolved.
A similar study analyzed the effects of sodium percarbonate (SPO)-laden PLGA films on nude mice flaps (Fig. 3C). By placing either the SPO/PLGA and PLGA only control films subcutaneously in 2 cm x 1 cm skin flaps, the researchers were able to verify the positive effects of oxygen release from SPO on the subject outcomes (69). The SPO/PLGA treated flaps featured reduced necrosis in the first few days of the study (69). However, the levels of necrosis present became roughly equal at day 7 of the study (69). In Fig. 3 C, hematoxylin and eosin staining of skin flaps harvested at both 3 and 7 days show delayed necrosis in supplemented scaffolds. Delayed necrosis is seen in the SPO containing PLGA films (POG) through preservation of tissue architecture, hair follicles, epidermis height, and sebaceous glands compared to the non-oxygen-generating control films (69). These results not only verify the positive effects of polymeric oxygen-generating (POG) films on extending the amount of time for which flaps remain viable, but also stress the need for better mechanisms of controlled oxygen release to prevent later stage flap necrosis.
Another study employed the use of perfluorocarbon chains containing oxygen attached to methacrylamide chitosan (MACF) to better understand the effect of oxygen transporting materials on wound healing (Fig. 3D). The researchers’ device facilitated oxygen transport to the wound site in a splintered rat excisional wound model, releasing the dissolved oxygen to promote wound healing (70). They found that through the MACF/oxygen saturated hydrogel, greater degrees of re-epithelialization and collagen synthesis were achieved, as verified through increased concentrations of metabolites within the regeneration pathway (70).
The presence of oxygen acts as a signaling molecule in a variety of ways when it comes to TE applications. While specific oxygen concentrations can direct stem cells down a specific cellular lineage, oxygen, or the lack thereof, can also direct angiogenesis in a concentration dependent manner which can affect the wound healing process (96). Following an injury, wound sites become hypoxic regions. The local hypoxia then acts as a signal for dermal tissue to begin the healing process through an upregulation of VEGF-A, inspiring angiogenesis and creating a dense bed of newly formed capillaries (96) (97). These capillaries are key in delivering much needed nutrients to the wound site to repair the skin ECM. However, studies have shown that the prolonged presence of VEGF-A inspired capillaries in such large magnitudes is not only unnecessary, but responsible for the scarring seen in adult wound healing (96) (97).
Studies have demonstrated that a reduced magnitude of angiogenesis does not affect the closure of wounds, as wound healing was perfectly normal (96). Additionally, studies concerning fetal skin and adult oral mucosa wound healing established links between the lack of hypoxia-induced inflammation and resulting angiogenesis, and the lack of scarring present upon healing of those wounds (96). Aspects of wound healing like these are important to keep in mind when designing and testing a TE therapy for the purpose of wound healing, as a lower level of supplemented oxygen in the initial healing phase can inspire the angiogenesis needed to deliver crucial nutrients to the wound site.
3.4. Oxygen-Generating Materials in Soft Tissue Engineering
Plastic and reconstructive surgeries are performed to repair defects in the soft tissues of patients due to various injuries that occur naturally or due to previous procedures (98). While no sufficient filler material exists for such procedures in clinical settings, adipose tissue transplants are used in these procedures with limited degrees of success. Due to the nature of adipose tissue being highly vascularized, such approaches are challenging in vivo. Adipocytes deep within the transplant cannot remain viable for the extended periods of time required to establish vascularization on their own.
A study concerning the viability of adipocytes in a non-vascularized, non-supplemented fat tissue graft supplemented to ischemic conditions confirmed this (99). Furthermore, the researchers showed that death of transplanted adipocytes started the first day following implantation, and only cells less than 300 μm from the surface of the graft survived and proliferated (99). The desire for better patient outcomes has inspired researchers to create scaffolds either supplemented with oxygen-generating materials, growth factors, or a combination of the two.
Supplementation of transplants with oxygen-generating materials has seen success in laboratory settings (Fig. 4A). One group of researchers created a scaffold consisting of sodium alginate, rat subcutaneous adipose tissue, and calcium peroxide, which was then crosslinked by calcium ions forming cylinder like scaffolds (71). The control was constructed in the same manner but was lacking in the oxygen-generating hydrogen peroxide (71). Following a 7-day culture period in the total absence of oxygen, the researchers found the oxygen-generating scaffold to maintain high cell viability while the unsupplemented scaffold showed highly reduced cell viability (71).
Figure 4.
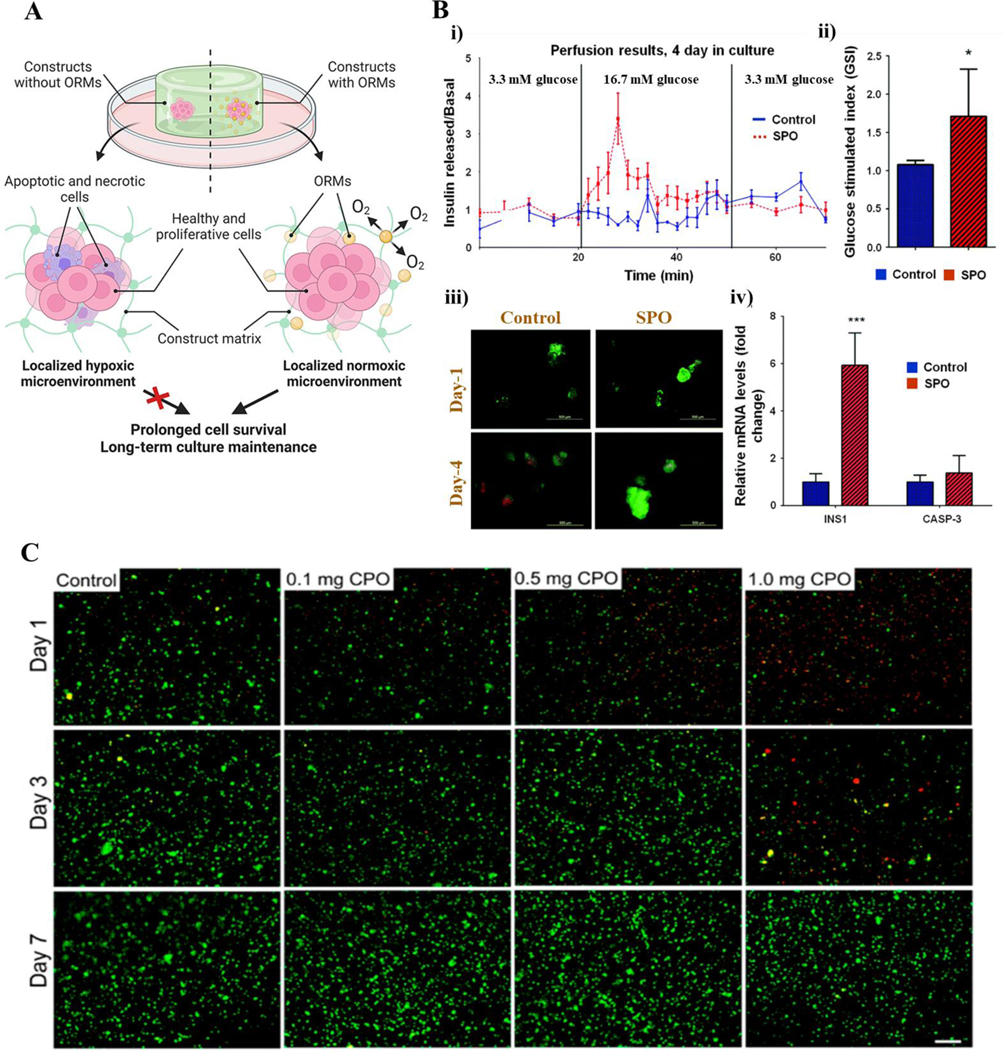
Oxygen-generating materials in soft tissue engineering. A) Schematic showing the role of oxygen-generating/releasing materials (ORMs) and their ability to enhance cell survival in soft tissue engineering applications. B) Incorporation of sodium percarbonate (SPO) on islet cell/PDMS substrate cultures showed greater cell proliferation and viability as seen through i) perfusion results, ii) glucose stimulated index, iii) representative live/dead fluorescent micrographs, and iv) profiling of INS1 and CASP-3 genes of cell cultures, when compared to the non-SPO control cultures. Myosin heavy chain and DAPI (blue) nuclei staining confirms the higher rates of cellular differentiation of SPO-containing cultures. C) Live (green)/dead (red) staining of C2C12 in muscle cells within the GelMA bioinks at different CaO2 concentrations crosslinked with UV light. This study found the optimal CaO2 concentration to be included into the scaffold was 0.5 mg for maintaining and enhancing cell viability and activity. Fig. A is reproduced from Ref. (25) with permission from Elsevier, copyright 2021. Fig. B is modified and reprinted from Ref. (85) with permission from the Royal Society of Chemistry (RSC), copyright 2017. Fig. C is reproduced from Ref. (73) under the creative common attribution license (CC-BY-0.4), copyright 2017.
Another study tested the efficacy of oxygen-generating materials in vivo through use of a fat graft supplemented with oxygen-generating microspheres and adipose derived stem cells (ASCs) in a rat soft tissue defect model (72). After 2 weeks, the oxygen-generating microsphere/ASC supplemented fat graft retained cell viability and encouraged new tissue growth. Positive effects of microsphere incorporation was shown through significant gains in mass and volume, a 2.7-fold increase in adipocyte survival distance from the periphery of the graft, and greater expressions of various growth factors when compared to controls (72).
Both in vitro and in vivo studies analyzing the effects of oxygen-generating materials on adipocytes verify that their supplementation encourages survival and proliferation of seeded cells. OGMs also encouraged tissue regeneration and angiogenesis at rates not seen in unsupplemented scaffolds. Similarly, sufficient supply of oxygen is critical for the survival of pancreatic islets during isolation and encapsulation before and immediately after transplantation. Researchers used SPO as an oxygen source during islet isolation. Then, they used silicon films containing SPO to support cell proliferation in vitro (Figure 4B). Finally, CPO (CaO2) was used as source of oxygen for encapsulated cells in alginate microspheres during implantation. Such results show promise regarding the future implementation of these therapies in clinical settings, as these supplemented grafts have shown repeatedly to proliferate well in unfavorable conditions, be metabolically active, and join with existing tissue in defects, thereby regenerating soft tissues in an effective manner.
The formation of scaffolds capable of generating mass amounts of oxygen for implantation in muscle defects is of special interest to researchers, as active skeletal muscle can show as high as a 100-fold increase in oxygen flux when compared to resting skeletal muscle (100). As such, the incorporation of oxygen-generating materials and their effects have been examined both in cell culture studies as well as in live animal models. One such study conducted through Wake Forest University Researchers examined the activity of particulate oxygen generator (POG) supplemented cell cultures in both hypoxic and normoxic conditions, compared to non-supplemented hypoxic and normoxic cultures (101). The researchers found that the addition of POGs enabled the hypoxic rat muscle precursor cells (MPCs) to multiply and differentiate, whereas in the control hypoxic culture no such differentiation took place (101). They also found that the culture supplemented with POGs featured faster and more defined cell growth compared to the normoxic control, further validating the positive effects of POG supplementation on cultures regardless of oxygenation level (101).
Additionally, oxygen-generating materials have also been applied to 3-D printed tissue constructs via incorporation into the bioinks used to form them. One group of researchers found that addition of calcium peroxide (CaO2) to the GelMA alginate bioinks improved cell viability and proliferation. They also acknowledged its potential to sustain and improve tissue function under hypoxic conditions (Fig. 4C) (73). In addition to promoting cell growth and differentiation, oxygen-generating materials have also been found to preserve cellular homeostasis in cells exposed to hypoxic events.
The use of sodium percarbonate (SPO) was found in one study to reduce HIF-ɑ accumulation, preserve muscle contractility, lessen oxidative stress, and attenuate glycogen depletion in both hypoxic rat extensor digitorum longus (EDL) muscle as well as tibialis anterior (TA) muscle following ligation of the iliac artery when applied in vitro (102). The differences in tetanic contractions and glycogen depletion between supplemented and unsupplemented groups demonstrate the positive effects of SPO on TA muscle (102). SPO is shown to better preserve initial torque and intramuscular glycogen due to its oxygen-generating capabilities compared to the saline injection (102). These findings showing the positive effects of oxygen-generating materials on cell growth, differentiation, and homeostasis in cultures, scaffolds, and injury models validate their potential use in clinical settings when traditional therapies may not deliver the desired outcome or be readily available.
Mimicking the natural tissue in terms of spatiotemporal distribution of oxygen is quite challenging for muscular tissue, however (103). The multitude of contractions performed daily by muscle tissue, as well as it being highly vascularized with variable blood flow and metabolically active, make predicting microenvironment oxygenation in advance quite challenging as such changes can cause oxygen required to fluctuate up to two orders of magnitude above the resting rate (104).
Currently, TE therapies involving self-oxygenation are based on a muscle’s resting oxygen gradient, as stem cells naturally occurring in muscle are highly vascularized as well and these provide the best models to date when it comes to improving seeded stem cell viability in muscle TE scaffolds (105). Because of our inability to accurately predict how muscle oxygenation will change in such an active tissue in advance, our understanding of how oxygen level signaling affects the proliferation and differentiation of satellite cells in muscle leaves much to be desired despite recent advances on the subject. To improve upon current TE strategies employing an oxygen delivery system, more studies need to be done concerning the oxidative and metabolic stresses experienced by the muscle tissue, so that we can form models and better predict the changes and stresses that seeded satellite cells in the scaffold will encounter to supplement them with oxygen accordingly.
TE of soft tissues remains difficult due to the high amount of oxygen needed for cells in the scaffold. Diffusive oxygen supply might remain inadequate due to the thickness of the soft tissue constructs. To combat this, researchers have tried to inspire high rates of oxygen generation through the addition of oxygen-generating materials to tissue constructs (71) (72) (101). However, this approach can lend to over-oxygenation, and fails to oxygenate scaffolds for sufficiently long time to establish vasculature, as oxygen supply usually runs out within days. Through better control of oxygen release kinetics, we can optimize oxygen delivery in soft tissue TE strategies, lending them to be effective alternative therapies.
3.5. Oxygen-Generating Materials in Cartilage Tissue Engineering
Affecting over 527 million people globally, osteoarthritis is a very common disease mostly seen in middle aged and elderly populations (106). Despite its frequency of occurrence and long history of presence in humans, there is no cure for osteoarthritis and cartilage has little to no regenerative capabilities, rendering the cartilage degradation permanent (107) (108). As such, researchers are looking into a plethora of alternative therapies to halt joint degradation and promote regeneration of the cartilage extracellular matrix (ECM), since current therapies are inadequate in producing meaningful impacts on patient outcomes.
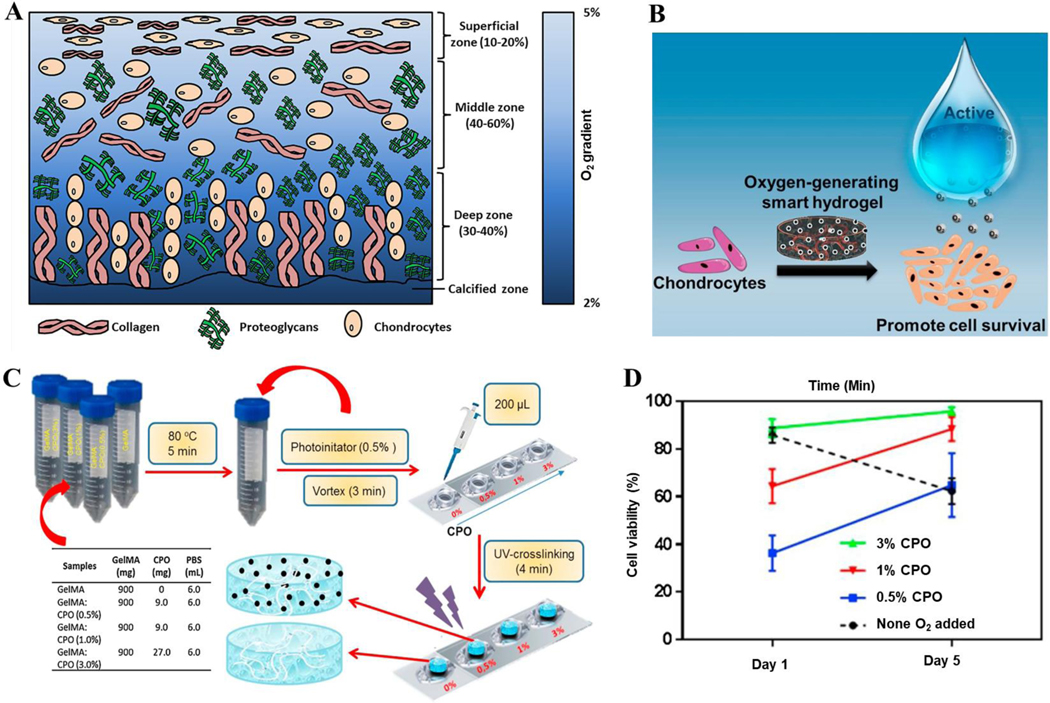
According to data from various studies, supplementation of TE scaffolds with oxygen-generating materials can aid in the proliferation of chondrocytes, thereby regenerating the ECM in various joint and cartilage defects. Therefore, these supplemented scaffolds will likely lead to better patient outcomes upon translation into clinical settings. Residing in an avascular environment, chondrocytes can withstand lower oxygen tensions than other cells (Fig. 5A). As such, there is more importance placed on the porosity of cartilage scaffolds and their ability to allow free diffusion of oxygen and nutrients to seeded cells, with ideal scaffold porosity commonly exceeding 90% (109).
Figure 5.
Oxygen-generating materials in cartilage tissue engineering. A) Schematic showing the oxygen-gradient in various regions of articular cartilage. B) Schematic detailing how oxygen-generating hydrogels supplement chondrocytes with oxygen to promote cell viability. C) Representation of the fabrication process of the oxygen-releasing “smart” hydrogels at various CPO (CaO2) concentrations. GelMA was mixed with CaO2 and the photoinitiator; the mixture was subsequently UV-crosslinked to form the constructs. D) Viability of chondrocytes in GelMA hydrogels from day 1 and day 5 under oxygen-free conditions. Fig. A is reproduced from Ref. (112) under the creative common attribution license (CC-BY-0.4), copyright 2019. Fig. B, C, D are reproduced from Ref. (110) with permission from Elsevier, copyright 2020.
One successful study employed this strategy, using a non-supplemented highly porous scaffold (93.52 ± 3.68% porosity) in a rabbit knee joint cartilage defect. The highly porous silk fibroin (SF)/ chitosan (CS) crosslinked scaffold seeded with bone mesenchymal stem cells (BMSCs) saw full repair of the defect after 12 weeks, compared to a partial repair with the unseeded scaffold, and virtually no repair with pure BMSCs. The researchers attributed the success of their BMSC laced SF/CS scaffold to its ability to easily diffuse oxygen to the seeded cells.
While unsupplemented scaffolds have been investigated in cartilage reconstruction, there exist in vitro studies examining the effects of oxygen-generating materials on cartilage reconstruction (Fig. 5B). Through employment of a GelMA/CaO2 “smart hydrogel”, researchers in one study found that oxygen generation from CaO2 degradation greatly preserved cell viability within their supplemented 3-D scaffolds compared to an unsupplemented control in hypoxic conditions mimicking a cartilage defect (Fig. 5C) (110). In addition, the oxygen release was sustained over the 7-day period due to the two-step nature of oxygen release from CaO2 degradation first into hydrogen peroxide and then into oxygen (110). They also determined the optimal CaO2 concentration to be 3% for keeping the chondrogenic cells viable when compared to lower concentrations (Fig. 5D) (110).
In another study, a similar in vitro approach was tested using PCL/CaO2 and PCL/CaO2/Pluronic F-127 (MP and MP-F respectively) oxygen-generating microparticles. When compared to a PCL-only, the MP-F supplemented hydrogel retained the highest cell viability of the encapsulated chondrocytes (111). They also found the microparticle (MP-F) containing hydrogel with a concentration of 3% to provide the maximum cell viability across all concentrations, as it showed steady oxygen generation across a 7-day period (111).
Mesenchymal stem cells, commonly used in TE applications, can differentiate into a variety of cell lineages. The oxygenation of seeded stem cells is the key factor affecting which cell lineage MSCs differentiate into. In accordance with this idea, it has been shown in studies that increased oxygenation of MSCs pushes them towards osteogenesis, while less intensive oxygenation pushes MSCs down the chondrogenic line of differentiation (80). While chondrocytes are known to inhabit a hypoxic microenvironment of as low as 1% oxygen at points farthest away from established blood vessels, it is also crucial to note that MSCs still need to be supplemented with oxygen-generating materials, as the differentiated cells still require oxygen to carry out their functions (81).
A team of researchers verified this, when they found that micro masses differentiated in markedly low oxygen tension (2% O2), still saw decreased chondrogenesis (81). Despite the decrease in rates of chondrogenesis however, micro masses cultured under 2% O2 still showed higher rates of chondrogenesis than micro masses cultured under 21% O2, validating the harmful effects of over oxygenation and the differing tissue and cell specific needs for oxygen (81). Such results emphasize the need for more studies on the specific distribution of oxygen within tissues and studies to better understand the oxygen directed differentiation of stem cells. Additionally, accurate protocols should be formulated for developing oxygen-generating scaffolds based on tissue type, location, depth, and cellular needs to achieve better outcomes in TE applications and avoid over or under oxygenation of seeded cells.
While there are promising studies analyzing the effects of oxygen-generating material in scaffolds for cartilage regeneration purposes, these approaches have yet to be tested in in vivo applications. Such studies will need to be conducted before translation into clinical practice, as these will test the efficiency of oxygen-generating materials and their effects on chondrocyte proliferation and ECM generation in an actual injury model.
3.6. Oxygen-Generating Materials in Liver Tissue Engineering
There exist a wealth of studies detailing the effect of oxygen supplementation on hepatocyte culture, with various methods used in vitro (113) (114) (115). In one such study, researchers used a more traditional approach, employing a fluorocarbon oxygen carrier in an ECM gel to preserve cell-matrix interactions as well as further promote cell differentiation through the growth factors contained in the gel. They found that implementation of fluorocarbons in the gel to increase the activity and viability of seeded hepatocytes, as determined through heightened rates of cytochrome P450 (CytP450) activity, albumin secretion, and urea production seen in the supplemented cultures (113). The results have shown that the oxygen carrier-collagen scaffolds have a positive effect on hepatocyte survival in culture through direct oxygen supplementation to cells via O2 release from the carrier materials. The researchers of the study determined this through gathering data on AST release, percentage of viable cells, and live/dead staining of hepatocytes (113).
The benefits of oxygen and its role in directing hepatocyte proliferation were further confirmed when another group of researchers compared cells cultured under hyperoxia (95% oxygen) and normoxia (20% oxygen). At day 7 of the study, the researchers found that culture in hyperoxia led to more favorable outcomes with 80% of cells in the 3T3/hyperoxia culture and 60% of cells in the solely hyperoxic culture remaining viable (114). These numbers were much improved compared to the controls, which retained about 35% of cells in both groups cultured in normoxic conditions (114). Additionally, hepatocyte function was preserved in both hyperoxic culture groups, as shown by increased albumin secretion compared to the normoxic cultures (114).
While directly supplementing cultures with oxygen often achieves improved outcomes, bioreactors are another well-studied strategy for supplementing cultures with oxygen, growth factors, and nutrients necessary to proliferate and differentiate. In an interesting study where hepatocyte microtissues were developed and maintained in a bioreactor, the cells were able to retain hepatic functions as shown by urea synthesis and albumin production for the entire duration of the experiment (115). The researchers found that through supplementing the scaffolds with a generous flow of oxygen into the bioreactor, hepatocyte functions were not only preserved but substantially increased (115). One study investigated the possibility to use green algae as a source of O2 in engineered tissues. By incorporating green algae (Chlamydomonas reinhardtii) and common mammalian cell lines into GelMA matrices and providing a light source, the researchers were able to demonstrate the biocompatibility of this new sustainable and plentiful material, and the positive effects of its characteristic photosynthetic oxygen generation on cells (116).
Increased oxygen concentration has been shown to enhance aerobic respiration and cell viability in bioartificial liver as seen through decreased presence of lactate as a function of oxygen and time when compared to lower oxygen concentrations tested. Oxygen concentration was determined using an in-line oxygen electrode. Such studies form an excellent basis to understand the effect of oxygen on hepatocyte metabolism and viability, as well as providing great background from which we can devise new studies to target the specific mechanisms of the role of oxygen in viability, proliferation, and respiration in hepatocytes (117).
While such studies are useful in demonstrating the positive effects that supplemental oxygen in TE therapies can have, if the goal of TE is to produce artificial organoids for transplantation into tissue defects, mimicking the natural spatiotemporal distribution of oxygen within the TE scaffolds is of special interest to produce constructs mirroring the target tissue in vivo. Through different methods, researchers have been able to keep track of the oxygen gradient within the liver and determine how the gradient changes in response to stress. One such study used mathematical modeling of the oxygen gradient in a stem cell derived liver construct (118). Another employed the use of the oxygen-sensitive fluorescent dye Ru(phen)3(2+) and compared its intensity at different points with NADH autofluorescence to demonstrate the reduction in cellular redox metabolism in hypoxia following a major event (119). While such studies do not provide detailed information for the purpose of optimizing current liver TE approaches, they do show that such approaches are great protocols to follow when designing studies to learn more about the oxygen gradient within the liver, and how it affects the viability, proliferation, and differentiation of hepatic progenitor cells.
Despite the still unanswered questions regarding the role of oxygen in the proliferation and differentiation of hepatic progenitor cells, we do know that hepatocytes are one of the most metabolically active cell groups found in the body. As such, have some of the highest rates of oxygen consumption. Given this, the current approach when it comes to the construction of viable bioartificial liver constructs is to embed oxygen-generating materials directly into the serum and/or the scaffolding materials itself and try to supplement seeded hepatocytes with enough oxygen to encourage their proliferation and preserve liver specific functions. Approaches like these have seen success in various studies, as shown by higher rates of albumin and urea secretion in oxygen-supplemented cultures (120) (121). Additionally, the detrimental effects of hypoxia on hepatocyte function and viability are also well documented, with studies demonstrating that lower oxygen percentages in culture correspond with higher rates of cell death and decreased urea and albumin secretion (122).
Despite the increase in the studies of oxygen-generating materials and their effects on hepatocyte scaffolds, there is still more work to be done. Studies examining these materials in vivo have not been conducted yet and will need to be conducted to determine safe-practices and have a better understanding of these materials before implementation in clinical settings can take place.
4. Challenges and Prospects
One engineering challenge in oxygen-generating tissue scaffolds is the spatial distribution of oxygen in tissues. More metabolically active tissue components require high amounts of oxygen, and current TE approaches rely on diffusion to satisfy their oxygen needs. However, this approach can under-oxygenate metabolically active components near the target tissue. This causes an increase in ROS, contributing to oxidative stress and damage. ROS is known to function as a key signaling molecule in triggering apoptosis, and an overload of ROS has been linked to drastically increased rates of apoptosis through normal signaling pathways in addition to oxidative damage (123). Such conclusions are verified by studies examining rates of apoptosis in cells cultured in high ROS environments, such as one study on apoptosis rates of human spermatozoa upon culture in high and low ROS environments (124). Researchers are actively attempting to understand and control diffusion of oxygen in TE approaches through more accurate diffusion models and the use of catalysts. Studies like these will be instrumental in the implementation of these therapies in clinical settings, as oxygen-release control will allow us to best culture cells and reduce apoptosis within TE scaffolds.
Through measure of oxygen partial pressure, we are better able to understand the distribution of oxygen within tissue and how it directs differentiation. Based on literature, we know that cell differentiation is a very sensitive process, and differing levels of oxygen and other nutrients can push cells to differentiate down different pathways. Such results are useful, as when we engineer tissues, we are seeking to regenerate the exact structures that would be present in a normal, healthy organ.
The reliance of current TE therapies on diffusion to meet cell oxygen requirements limits the ability of these therapies to be implemented in clinical settings. One method being studied in kidney tissue engineering is the use of decellularized kidney ECMs. The use of decellularized ECMs is proving fruitful because the normal vasculature of the kidney remains intact, lending it the ability to supply seeded cells with the correct amounts of oxygen directly through diffusion. A possible approach to overcome the vasculature challenge can be to take what is learned from kidney engineering and applying to different tissue types. Researchers can accomplish this by attempting to construct tissue scaffolds in such a way that they mimic the vasculature of the native tissue. The scaffold can then be seeded with stem cells, growth factors, and an oxygen-generating material. Through improved oxygen release kinetics and a structure like that of the native tissue, we may be able to better direct cell differentiations to form the intricate micro-structures that exist within organs and tissues.
Current TE approaches take advantage of reaction control to provide temporal control of oxygen distribution within the scaffold (125) (27) (126) (127) (77). By changing the oxygen releasing mechanism within the scaffold, researchers can regulate how much oxygen will be distributed to tissue at any given point and alter the reaction to shorten or lengthen the period of oxygen delivery (128). The challenge in reaction control in TE applications comes in controlling the oxygen release kinetics to proceed at different rates at different locations in the scaffold. A possible solution to this issue may be to calculate how much catalyst may be needed to alter the oxygen release rate at one part in the scaffold and infuse that amount of catalyst in a specific part within the scaffold.
5. Conclusions
As shown through relevant statistics, the difference between supply of transplantable tissues and patient demand is ever widening and will only continue to grow through time. As such, medical researchers are concerned, leading to a variety of alternate therapies being studied and pursued through research. Of these, TE provides the greatest promise as it can drastically reduce the supply/demand difference in available transplants. TE therapies also have the potential to nearly eliminate waiting time on the organ transplant list given the ability to form viable tissue scaffolds. However, there are still barriers preventing the translation of TE into clinical practice; the main barrier being the need for an oxygen source in tissue scaffolds to ensure metabolic activity, proliferation, and differentiation of seeded cells. Researchers may be able to resolve this issue through the implementation of oxygen-generating materials.
Oxygen-generating materials have shown to provide adequate oxygenation to seeded cells even in hypoxic conditions, both in vitro and in vivo. Not only has the use of these materials been shown to retain scaffold viability, but the implementation of oxygen-generating materials has shown to encourage proliferation and specific differentiations of seeded cells, leading to scaffold cultures demonstrating similar functionalities as the organs for which they are designed to mimic. In some cases, the oxygen generation provided by these materials can extend well through the time needed for angiogenesis to take place within smaller sized scaffolds in vivo. Despite the promise of these materials, more studies have yet to be done to better understand the nature of biocompatibility of these materials, as well as any adverse effects that may present using such materials in TE applications. Upon gaining better understanding of the pharmacology and toxicology associated with such materials, they have the potential to be used in numerous areas of the medical field and advance health sciences.
Table 1.
Oxygen-generating scaffolds for various tissue engineering applications.
| Scaffold material used | Fabrication method | Cell type used | In vivo model used | Oxygen-generating material | Amount of oxygen generating material loaded (Specify w/w%, mg/gm) | Amount of oxygen released | Application | Outcome | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| PCL/nHA porous scaffold, sodium alginate/gelatin hydrogel | 3D printing | BMSCs | New Zealand white rabbits osteonecrosis of femoral head model | CaO2 | 25% w/w | Between 6 and 12.5 mg/L O2 release within 25 days period. | Treatment of osteonecrosis of femoral head | Supported the survival of BMSCs in the construct, and provided bone regeneration in osteonecrosis of femoral head | (55) |
| PCL scaffolds containing PVA-PLGA microtanks | 3D printing | hASCs | Murine calvarial defect model | Pure O2 | ∼300, 400, and 500 psi O2 | ∼250–500 micromoles O2 in 8 h | Calvarial bone regeneration | O2 releasing scaffolds supported both mineralization and osteogenic protein deposition | (56) |
| Beta-tricalcium phosphate (ß-TCP) / Hydroxyapatite (HA) and PCL | 3D printing and PCL/CaO2 coating | Osteoblasts | N/A | CaO2 | 1, 3 and 5% w/w CaO2 in PCL coating | ∼35–55 mmHg within 10 day period. | Bone regeneration | Maintained cell viability under hypoxic conditions | (57) |
| Alginate hydrogel | 3D bioprinting | hBM-MSCs | N/A | CaO2 | 1%, 3% and 5% w/w CaO2 in bioink | ∼15 to 30 mmHg during 20 days period | Bone regeneration | 22% improvement in cell viability when 3% CaO2 was loaded in the scaffolds | (58) |
| Gelatin- SAP/PLGA | Freeze drying | PBMSCs | Sprague Dawley rats | CaO2 | PBMSCs | ∼2–6 mg/L O2 release for in vitro (21 days) and in vivo (28 days. | Bone tissue engineering | CaO2 microparticles supported cell survival under hypoxia both in vitro and in vivo | (59) |
| PLA | Electrospinning | MSCs | N/A | CaO2 | 6.5% w/w and 13% w/w. | ∼30–90 mmHg for 14-days in vitro. | Bone tissue engineering | CaO2 loaded scaffolds supported MSCs proliferation and supported alkaline phosphatase/osteocalcin expression | (60) |
| GelMA | Photocrosslinking | Stem cells from apical papilla | N/A | CaO2 | 0.5%, 1%, and 3% w/w CaO2 | ∼5–25% O2 release undue hypoxia for 7-days. | Endodontic regeneration | Stem cells from apical papilla encapsulated within the scaffolds containing 0.5%, 1%, and 3% CaO2 showed cell survival after 4 and 7 days of cell culture | (61) |
| PLGA-gelatin scaffold | Infiltration of PLGA- SrO2 + MnO2 in gelatin sponge | MC3T3-E1 preosteoblasts and RAW 264.7 macrophages | N/A | SrO2 | - | Up to 7 mg/L O2 release within 72 h | Bone tissue engineering | SrO2 + MnO2@PLGA/gelatin scaffolds promoted the proliferation of preosteoblasts | (62) |
| Fibrin hydrogel | Thrombin crosslinked injectable hydrogel | MSCs | Mice (Ectopic implantation) | Perfluorotributylamine | 5 or 10% PFTB | N/A | Bone tissue engineering, muscle tissue engineering | 2.5-fold increase in bone formation, cell survival, and osteocalcin activity in the O2-generating agent containing scaffold groups | (63) |
| Gelatin-graft-polypyrrole/Periodateoxidized pectin/PLA | Gelation | Human osteosarcoma MG63 cells | N/A | H2O2 | 14% H2O2 | ∼6–10 mg/L during 14-days period. | Bone tissue engineering | Increased cell viability and attachment | (64) |
| PLLA | Microspheres | hASCs | N/A | CaO2 | 0.2% w/w and 0.5% w/w CaO2 in PLLA | ∼6– 7.5% in 15 days period | Bone tissue engineering | hASCs could effectively attach on the surface of microspheres, maintained their characteristic spreading morphology | (65) |
| Porous poly(glycerol-sebacate) scaffold, collagen scaffold | Biorubber was cured in teflon mold in a vacuum oven at 120 deg. C and 100 mTorr. Collagen scaffolds (5 mm diameter) were cut from 2 mm thick sheets | Fibroblasts and cardiomyocytes | N/A | Perfluorocarbons | 440.6 μM | 80.6 μM | Cardiac tissue engineering | PFC supplemented constructs exhibit improved properties, as seen through measures of DNA and cardiac markers connexin-43 and troponin-I | (29) |
| RCGel | RCGel fabricated using hyperbranched polymers (HBPAK), catalase, and methacrylate hylauronic acid. MSCs encapsulated using 3s irradiation treatment | MSCs | Rat myocardial infarction model | RCGel | N/S | N/S | Myocardial infarction | RCGel encapsulated MSCs showed decreased apoptosis and increased angiogenesis | (30) |
| Oxygen-generating microparticles made with PLGA/PVP | Ring-opening polymerization of β-butyrolactone with stannous trifluoromethane sulfonate. Hydrogels were fabricated with NIPAAm, AAc, and HEMA-oHB, by free radical polymerization. | CDCs | N/A | H2O2/Catalase | 3 molar ratios of H2O2/PVP were evaluated: 6/1, 4.5/1, and 3/1 | 6/1 ratio showed most release at over 20% oxygenation | Myocardial infarction model | Inclusion of oxygen generating microparticles ledto enhanced cell proliferation and supported differentiation in ischemia | (66) |
| GelMA hydrogels | Fabricated according to the procedures established by Camci-Unal et al., 2013 | CSPs | N/A | CaO2 | 0, 1, 2, and 3 wt% CaO2 | Oxygen-rich release sustained for 5 days, up to 8% O2 for the 3% CaO2 concentration at day 5 | Ischemic myocardial event therapy | Inclusion of calcium peroxide supported cell metabolism in hypoxia and attenuated seeded cell death | (67) |
| Parchment paper/PDMS | Air plasma treatment used to bind PDMS and parchment paper | NIH 3T3 Fibroblasts | N/A | MnO2 | 4.91 μL/min/spot | 3 mL/h | Wound healing | Dressings demonstrated good biocompatibility and the ability to generate oxygen at clinically relevant concentrations | (68) |
| PLGA films | Solvent casting process | N/A | Ischemic murine wound model | Sodium percarbonate | N/S | 120 mL oxygen/gram sodium percarbonate | Wound healing | Incorporation of sodium percarbonate in PLGA films led to delayed necrosis | (69) |
| Methacrylamide chitosan hydrogel | Fabricated according to the procedures established by Wang et al., 2010 | N/A | Rat excisional wound model | Perfluorocarbon chains | 265.0 ± 9.5 mmHg | Retained release of over 10 mmHg (PO2) over 48 h | Wound healing | Application of fluorinated methacrylamide chitosan hydrogels and oxygen resulted in the highest degree of re-epithelialization among evaluated groups | (70) |
| Sodium alginate, rat subcutaneous adipose tissue | Crosslinking by calcium ions | Adipocytes | Rat model | CaO2 | N/S | N/S | Adipose tissue engineering | Supplemented tissue scaffolds retained cell viability following anoxic culture compared to unsupplemented scaffolds | (71) |
| Fat graft | N/S | ASCs | Rat model | Oxygen-generating microspheres | N/S | N/S | Adipose tissue engineering | Supplementation of the transplant with OGMs and ASCs led to increased cell survival farther from transplant margin | (72) |
| GelMA/alginate bioinks | 3D bioprinting | C2C12 cells | N/A | CaO2 | 0.1, 0.5, and 1.0 mg/mL and 100 μg/mL catalase | N/S | Muscle tissue engineering | Supplemented cell cultures up to 0.5 mg/mL demonstrated enhanced viability and metabolic activity | (73) |
Abbreviations: O2: oxygen, CaO2: calcium peroxide, H2O2: hydrogen peroxide, PCL: polycaprolactone, nHA: nano-hydroxyapatite, SAP: self-assembling peptides, PLGA: poly(lactic-co-glycolic acid, PLLA: poly-L-lactic acid, PLA: polylactic acid, PVA: poly(vinyl)alcohol, PVP: polyvinylpyrrolidone, PDMS: polydimethylsiloxane, GelMA: gelatin methacryloyl, SrO2: strontium peroxide, MnO2: manganese dioxide, MSCs: mesenchymal stem cells, PBMSCs : peripheral blood mononuclear-stem cells, hASCs: human adipose-derived stem cells, CSPs: cardiac side population cells, BMSCs: bone marrow-derived mesenchymal stem cells, CDCs: cardiosphere derived cells, hBM-MSCs: human bone marrow-derived mesenchymal stem cells, rMPCs: rat muscle precursor cells. N/A: not applicable, N/S: not stated.
Funding:
This work was supported by the National Institute of Dental & Craniofacial Research of the National Institutes of Health under Award Number R01DE030129. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Morrison E, Suvarnapathaki S, Blake L, Camci-Unal G. Unconventional biomaterials for cardiovascular tissue engineering. Current Opinion in Biomedical Engineering. 2021;17:100263. [Google Scholar]
- 2.Gómez MP, Pérez B, Manyalich M. International Registry in Organ Donation and Transplantation—2013. Transplantation Proceedings. 2014;46(4):1044–8. [DOI] [PubMed] [Google Scholar]
- 3.Sadeghi F, Ramezani M, Beigee FS, Shadnia S, Moghaddam HH, Zamani N, et al. Organ Procurement From Poisoned Patients: A 14-Year Survey in 2 Academic Centers. Exp Clin Transplant. 2021. [DOI] [PubMed] [Google Scholar]
- 4.Langer R, Vacanti Joseph P. Tissue Engineering. Science. 1993;260(5110):920–6. [DOI] [PubMed] [Google Scholar]
- 5.Bioengineering NioBIa. Tissue Engineering and Regenerative Medicine. In: Services USDoHaH, editor. https://wwwnibibnihgov/sites/default/files/2020-06/Tissue_Engineering_Fact_Sheetpdf. Bethesda, MD: National Institutes of Health; 2019. p. 1–2. [Google Scholar]
- 6.Wu X, Gauntlett O, Zhang T, Suvarnapathaki S, McCarthy C, Wu B, et al. Eggshell Microparticle Reinforced Scaffolds for Regeneration of Critical Sized Cranial Defects. ACS Applied Materials & Interfaces. 2021;13(51):60921–32. [DOI] [PubMed] [Google Scholar]
- 7.Ikada Y. Challenges in tissue engineering. Journal of The Royal Society Interface. 2006;3(10):589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lantigua D, Kelly YN, Unal B, Camci-Unal G. Engineered Paper-Based Cell Culture Platforms. Advanced Healthcare Materials. 2017;6(22):1700619. [DOI] [PubMed] [Google Scholar]
- 9.Augustine R, Dan P, Hasan A, Khalaf IM, Prasad P, Ghosal K, et al. Stem cell-based approaches in cardiac tissue engineering: controlling the microenvironment for autologous cells. Biomedicine & Pharmacotherapy. 2021;138:111425. [DOI] [PubMed] [Google Scholar]
- 10.Pittenger Mark F, Mackay Alastair M, Beck Stephen C, Jaiswal Rama K, Douglas R, Mosca Joseph D, et al. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science. 1999;284(5411):143–7. [DOI] [PubMed] [Google Scholar]
- 11.Kotobuki N, Hirose M, Takakura Y, Ohgushi H. Cultured Autologous Human Cells for Hard Tissue Regeneration: Preparation and Characterization of Mesenchymal Stem Cells from Bone Marrow. Artificial Organs. 2004;28(1):33–9. [DOI] [PubMed] [Google Scholar]
- 12.Berthiaume F, Yarmush ML. Tissue Engineering. In: Meyers RA, editor. Encyclopedia of Physical Science and Technology (Third Edition). New York: Academic Press; 2003. p. 817–42. [Google Scholar]
- 13.Kalva SN, Augustine R, Al Mamun A, Dalvi YB, Vijay N, Hasan A. Active agents loaded extracellular matrix mimetic electrospun membranes for wound healing applications. Journal of Drug Delivery Science and Technology. 2021;63:102500. [Google Scholar]
- 14.Rouwkema J, Rivron NC, van Blitterswijk CA. Vascularization in tissue engineering. Trends in Biotechnology. 2008;26(8):434–41. [DOI] [PubMed] [Google Scholar]
- 15.Papavasiliou G, Cheng M-H, Brey EM. Strategies for vascularization of polymer scaffolds. Journal of investigative medicine : the official publication of the American Federation for Clinical Research. 2010;58(7):838–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen MA, Camci-Unal G. Unconventional Tissue Engineering Materials in Disguise. Trends in Biotechnology. 2020;38(2):178–90. [DOI] [PubMed] [Google Scholar]
- 17.Suvarnapathaki S, Nguyen MA, Goulopoulos AA, Lantigua D, Camci-Unal G. Engineering calcium peroxide based oxygen generating scaffolds for tissue survival. Biomaterials Science. 2021;9(7):2519–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novosel EC, Kleinhans C, Kluger PJ. Vascularization is the key challenge in tissue engineering. Advanced Drug Delivery Reviews. 2011;63(4):300–11. [DOI] [PubMed] [Google Scholar]
- 19.Augustine R, Prasad P, Khalaf IMN. Therapeutic angiogenesis: From conventional approaches to recent nanotechnology-based interventions. Materials Science and Engineering: C. 2019;97:994–1008. [DOI] [PubMed] [Google Scholar]
- 20.Trayhurn P. Oxygen-A Critical, but Overlooked, Nutrient. Frontiers in nutrition. 2019;6:10-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakazawa MS, Keith B, Simon MC. Oxygen availability and metabolic adaptations. Nature reviews Cancer. 2016;16(10):663–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller AT. The role of oxygen in metabolic regulation. Helgoländer wissenschaftliche Meeresuntersuchungen. 1966;14(1):392–406. [Google Scholar]
- 23.Sthijns MMJPE, van Blitterswijk CA, LaPointe VLS. Redox regulation in regenerative medicine and tissue engineering: The paradox of oxygen. Journal of Tissue Engineering and Regenerative Medicine. 2018;12(10):2013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camci-Unal G, Alemdar N, Annabi N, Khademhosseini A. Oxygen-releasing biomaterials for tissue engineering. Polymer International. 2013;62(6):843–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agarwal T, Kazemi S, Costantini M, Perfeito F, Correia CR, Gaspar V, et al. Oxygen releasing materials: Towards addressing the hypoxia-related issues in tissue engineering. Materials Science and Engineering: C. 2021;122:111896. [DOI] [PubMed] [Google Scholar]
- 26.Gholipourmalekabadi M, Zhao S, Harrison BS, Mozafari M, Seifalian AM. Oxygen-Generating Biomaterials: A New, Viable Paradigm for Tissue Engineering? Trends in Biotechnology. 2016;34(12):1010–21. [DOI] [PubMed] [Google Scholar]
- 27.Ashammakhi N, Darabi MA, Kehr NS, Erdem A, Hu S-k, Dokmeci MR, et al. Advances in Controlled Oxygen Generating Biomaterials for Tissue Engineering and Regenerative Therapy. Biomacromolecules. 2020;21(1):56–72. [DOI] [PubMed] [Google Scholar]
- 28.Farris AL, Rindone AN, Grayson WL. Oxygen delivering biomaterials for tissue engineering. Journal of Materials Chemistry B. 2016;4(20):3422–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radisic M, Park H, Chen F, Salazar-Lazzaro JE, Wang Y, Dennis R, et al. Biomimetic Approach to Cardiac Tissue Engineering: Oxygen Carriers and Channeled Scaffolds. Tissue Engineering. 2006;12(8):2077–91. [DOI] [PubMed] [Google Scholar]
- 30.Ding H, Ding J, Liu Q, Lin J, He M, Wu X, et al. Mesenchymal stem cells encapsulated in a reactive oxygen species-scavenging and O2-generating injectable hydrogel for myocardial infarction treatment. Chemical Engineering Journal. 2021:133511. [Google Scholar]
- 31.Carreau A, Hafny-Rahbi BE, Matejuk A, Grillon C, Kieda C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. Journal of Cellular and Molecular Medicine. 2011;15(6):1239–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suvarnapathaki S, Wu X, Lantigua D, Nguyen MA, Camci-Unal G. Breathing life into engineered tissues using oxygen-releasing biomaterials. NPG Asia Materials. 2019;11(1):65. [Google Scholar]
- 33.Sun X, Yao F, Zhang H, Li J. Oxygen-generating materials and their biomedical applications: a review. Journal of Materials Science. 2022;57(20):9077–103. [Google Scholar]
- 34.Augustine R, Gezek M, Bostanci NS, Nguyen A, Camci-Unal G. Oxygen-generating scaffolds: One step closer to the clinical translation of tissue engineered products. Chemical Engineering Journal. 2023;455:140783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Semenza GL. Regulation of Oxygen Homeostasis by Hypoxia-Inducible Factor 1. Physiology. 2009;24(2):97–106. [DOI] [PubMed] [Google Scholar]
- 36.Greijer AE, van der Wall E. The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. Journal of clinical pathology. 2004;57(10):1009–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gala DN, Fabian Z. To Breathe or Not to Breathe: The Role of Oxygen in Bone Marrow-Derived Mesenchymal Stromal Cell Senescence. Stem Cells International. 2021;2021:8899756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sano H, Ichioka S, Sekiya N. Influence of Oxygen on Wound Healing Dynamics: Assessment in a Novel Wound Mouse Model under a Variable Oxygen Environment. PLOS ONE. 2012;7(11):e50212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fathollahipour S, Patil PS, Leipzig ND. Oxygen Regulation in Development: Lessons from Embryogenesis towards Tissue Engineering. Cells, tissues, organs. 2018;205(5–6):350–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li L, Qu Y, Jin X, Guo XQ, Wang Y, Qi L, et al. Protective effect of salidroside against bone loss via hypoxia-inducible factor-1α pathway-induced angiogenesis. Scientific Reports. 2016;6(1):32131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wandrag L, Siervo M, Riley HL, Khosravi M, Fernandez BO, Leckstrom CA, et al. Does hypoxia play a role in the development of sarcopenia in humans? Mechanistic insights from the Caudwell Xtreme Everest Expedition. Redox Biology. 2017;13:60–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li S, Oreffo ROC, Sengers BG, Tare RS. The effect of oxygen tension on human articular chondrocyte matrix synthesis: integration of experimental and computational approaches. Biotechnology and bioengineering. 2014;111(9):1876–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ebert EC. Hypoxic Liver Injury. Mayo Clinic Proceedings. 2006;81(9):1232–6. [DOI] [PubMed] [Google Scholar]
- 44.Bunout D, Barrera G, Hirsch S, Jimenez T, de la Maza MP. Association between activity energy expenditure and peak oxygen consumption with sarcopenia. BMC Geriatrics. 2018;18(1):298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miclau KR, Brazina SA, Bahney CS, Hankenson KD, Hunt TK, Marcucio RS, et al. Stimulating Fracture Healing in Ischemic Environments: Does Oxygen Direct Stem Cell Fate during Fracture Healing? Frontiers in cell and developmental biology. 2017;5:45-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Engin A. Adipose Tissue Hypoxia in Obesity and Its Impact on Preadipocytes and Macrophages: Hypoxia Hypothesis. In: Engin AB, Engin A, editors. Obesity and Lipotoxicity. Cham: Springer International Publishing; 2017. p. 305–26. [DOI] [PubMed] [Google Scholar]
- 47.Utting JC, Robins SP, Brandao-Burch A, Orriss IR, Behar J, Arnett TR. Hypoxia inhibits the growth, differentiation and bone-forming capacity of rat osteoblasts. Experimental Cell Research. 2006;312(10):1693–702. [DOI] [PubMed] [Google Scholar]
- 48.Kawada S, Ohtani M, Ishii N. Increased oxygen tension attenuates acute ultraviolet-B-induced skin angiogenesis and wrinkle formation. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2010;299(2):R694–R701. [DOI] [PubMed] [Google Scholar]
- 49.Wu X, Zhang T, Hoff B, Suvarnapathaki S, Lantigua D, McCarthy C, et al. Mineralized Hydrogels Induce Bone Regeneration in Critical Size Cranial Defects. Advanced Healthcare Materials. 2021;10(4):2001101. [DOI] [PubMed] [Google Scholar]
- 50.Lantigua D, Nguyen MA, Wu X, Suvarnapathaki S, Kwon S, Gavin W, et al. Synthesis and characterization of photocrosslinkable albumin-based hydrogels for biomedical applications. Soft Matter. 2020;16(40):9242–52. [DOI] [PubMed] [Google Scholar]
- 51.Suvarnapathaki S, Nguyen MA, Wu X, Nukavarapu SP, Camci-Unal G. Synthesis and characterization of photocrosslinkable hydrogels from bovine skin gelatin. RSC Advances. 2019;9(23):13016–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fan Z, Xu Z, Niu H, Gao N, Guan Y, Li C, et al. An Injectable Oxygen Release System to Augment Cell Survival and Promote Cardiac Repair Following Myocardial Infarction. Scientific Reports. 2018;8(1):1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loscalzo J. The cellular response to hypoxia: tuning the system with microRNAs. The Journal of Clinical Investigation. 2010;120(11)(1558–8238 (Electronic)):3815–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valle-Tenney R, Rebolledo DL, Lipson KE, Brandan E. Role of hypoxia in skeletal muscle fibrosis: Synergism between hypoxia and TGF-β signaling upregulates CCN2/CTGF expression specifically in muscle fibers. Matrix Biology. 2020;87:48–65. [DOI] [PubMed] [Google Scholar]
- 55.Wang C, Xu H, Liu C, Peng Z, Min R, Zhang Z, et al. CaO2/gelatin oxygen slow-releasing microspheres facilitate tissue engineering efficiency for the osteonecrosis of femoral head by enhancing the angiogenesis and survival of grafted bone marrow mesenchymal stem cells. Biomaterials Science. 2021;9(8):3005–18. [DOI] [PubMed] [Google Scholar]
- 56.Farris AL, Lambrechts D, Zhou Y, Zhang NY, Sarkar N, Moorer MC, et al. 3D-printed oxygen-releasing scaffolds improve bone regeneration in mice. Biomaterials. 2022;280:121318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Touri M, Moztarzadeh F, Osman NAA, Dehghan MM, Mozafari M. 3D–printed biphasic calcium phosphate scaffolds coated with an oxygen generating system for enhancing engineered tissue survival. Materials Science and Engineering: C. 2018;84:236–42. [DOI] [PubMed] [Google Scholar]
- 58.Aghajanpour S, Esfandyari-Manesh M, Ghahri T, Ghahremani MH, Atyabi F, Heydari M, et al. Impact of oxygen-calcium-generating and bone morphogenetic protein-2 nanoparticles on survival and differentiation of bone marrow-derived mesenchymal stem cells in the 3D bio-printed scaffold. (1873–4367 (Electronic)). [DOI] [PubMed] [Google Scholar]
- 59.Zhang Z, Rong Z, Wu G, Wang Y, Tan Z, Zheng J, et al. Gelatin-CaO2/SAP/PLGA composite scaffold enhances the reparation of critical-sized cranial defects by promoting seed cell survival. Applied Materials Today. 2021;22:100960. [Google Scholar]
- 60.Khorshidi S, Karkhaneh A, Bonakdar S. Oxygen-releasing nanofibers for breathable bone tissue engineering application. Journal of Biomaterials Applications. 2020;35(1):72–82. [DOI] [PubMed] [Google Scholar]
- 61.Zou T, Jiang S, Zhang Y, Liu J, Yi B, Qi Y, et al. In Situ Oxygen Generation Enhances the SCAP Survival in Hydrogel Constructs. Journal of Dental Research. 2021;100(10):1127–35. [DOI] [PubMed] [Google Scholar]
- 62.Lin S-J, Huang C-C. Strontium Peroxide-Loaded Composite Scaffolds Capable of Generating Oxygen and Modulating Behaviors of Osteoblasts and Osteoclasts. International Journal of Molecular Sciences [Internet]. 2022; 23(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kimelman-Bleich N, Pelled G, Sheyn D, Kallai I, Zilberman Y, Mizrahi O, et al. The use of a synthetic oxygen carrier-enriched hydrogel to enhance mesenchymal stem cell-based bone formation in vivo. Biomaterials. 2009;30(27):4639–48. [DOI] [PubMed] [Google Scholar]
- 64.Nejati S, Karimi Soflou R, Khorshidi S, Karkhaneh A. Development of an oxygen-releasing electroconductive in-situ crosslinkable hydrogel based on oxidized pectin and grafted gelatin for tissue engineering applications. Colloids and Surfaces B: Biointerfaces. 2020;196:111347. [DOI] [PubMed] [Google Scholar]
- 65.Mohseni-Vadeghani E, Karimi-Soflou R, Khorshidi S, Karkhaneh A. Fabrication of oxygen and calcium releasing microcarriers with different internal structures for bone tissue engineering: Solid filled versus hollow microparticles. Colloids and Surfaces B: Biointerfaces. 2021;197:111376. [DOI] [PubMed] [Google Scholar]
- 66.Li Z, Guo X, Guan J. An oxygen release system to augment cardiac progenitor cell survival and differentiation under hypoxic condition. Biomaterials. 2012;33(25):5914–23. [DOI] [PubMed] [Google Scholar]
- 67.Alemdar N, Leijten J, Camci-Unal G, Hjortnaes J, Ribas J, Paul A, et al. Oxygen-Generating Photo-Cross-Linkable Hydrogels Support Cardiac Progenitor Cell Survival by Reducing Hypoxia-Induced Necrosis. ACS Biomaterials Science & Engineering. 2017;3(9):1964–71. [DOI] [PubMed] [Google Scholar]
- 68.Ochoa M, Rahimi R, Huang TL, Alemdar N, Khademhosseini A, Dokmeci MR, et al. A paper-based oxygen generating platform with spatially defined catalytic regions. Sensors and Actuators B: Chemical. 2014;198:472–8. [Google Scholar]
- 69.Harrison BS, Eberli D, Lee SJ, Atala A, Yoo JJ. Oxygen producing biomaterials for tissue regeneration. Biomaterials. 2007;28(31):4628–34. [DOI] [PubMed] [Google Scholar]
- 70.Patil PS, Fountas-Davis N, Huang H, Michelle Evancho-Chapman M, Fulton JA, Shriver LP, et al. Fluorinated methacrylamide chitosan hydrogels enhance collagen synthesis in wound healing through increased oxygen availability. Acta Biomaterialia. 2016;36:164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang H, Simon T, Jip B, Kramer B, Jake B. Self-oxygenating scaffolds for anoxic culture of adipose tissue. Frontiers in Bioengineering and Biotechnology. 2016;4. [Google Scholar]
- 72.Jung D-W, Kim Y-H, Kim TG, Lee JH, Chung KJ, Lim JO, et al. Improvement of Fat Transplantation: Fat Graft With Adipose-Derived Stem Cells and Oxygen-Generating Microspheres. Annals of Plastic Surgery. 2015;75(4). [DOI] [PubMed] [Google Scholar]
- 73.Seyedmahmoud R, Çelebi-Saltik B, Barros N, Nasiri R, Banton E, Shamloo A, et al. Three-Dimensional Bioprinting of Functional Skeletal Muscle Tissue Using Gelatin Methacryloyl-Alginate Bioinks. Micromachines. 2019;10(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Amin S, Achenbach SJ, Atkinson EJ, Khosla S, Melton Iii LJ. Trends in Fracture Incidence: A Population-Based Study Over 20 Years. Journal of Bone and Mineral Research. 2014;29(3):581–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Teh SW, Koh AE-H, Tong JB, Wu X, Samrot AV, Rampal S, et al. Hypoxia in Bone and Oxygen Releasing Biomaterials in Fracture Treatments Using Mesenchymal Stem Cell Therapy: A Review. Frontiers in Cell and Developmental Biology. 2021;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu X, Stroll SI, Lantigua D, Suvarnapathaki S, Camci-Unal G. Eggshell particle-reinforced hydrogels for bone tissue engineering: an orthogonal approach. Biomaterials Science. 2019;7(7):2675–85. [DOI] [PubMed] [Google Scholar]
- 77.Suvarnapathaki S, Wu X, Zhang T, Nguyen MA, Goulopoulos AA, Wu B, et al. Oxygen generating scaffolds regenerate critical size bone defects. Bioactive Materials. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Touri M, Moztarzadeh F, Abu Osman NA, Dehghan MM, Brouki Milan P, Farzad-Mohajeri S, et al. Oxygen-Releasing Scaffolds for Accelerated Bone Regeneration. ACS Biomaterials Science & Engineering. 2020;6(5):2985–94. [DOI] [PubMed] [Google Scholar]
- 79.Sayin E, Baran ET, Elsheikh A, Mudera V, Cheema U, Hasirci V. Evaluating Oxygen Tensions Related to Bone Marrow and Matrix for MSC Differentiation in 2D and 3D Biomimetic Lamellar Scaffolds. International Journal of Molecular Sciences. 2021;22(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khorshidi S, Karimi-Soflou R, Karkhaneh A. A hydrogel/particle composite with a gradient of oxygen releasing microparticle for concurrent osteogenic and chondrogenic differentiation in a single scaffold. Colloids and Surfaces B: Biointerfaces. 2021;207:112007. [DOI] [PubMed] [Google Scholar]
- 81.Malladi P, Xu Y, Chiou M, Giaccia AJ, Longaker MT. Effect of reduced oxygen tension on chondrogenesis and osteogenesis in adipose-derived mesenchymal cells. American Journal of Physiology-Cell Physiology. 2006;290(4):C1139–C46. [DOI] [PubMed] [Google Scholar]
- 82.Marenzana M, Arnett TR. The Key Role of the Blood Supply to Bone. Bone Research. 2013;1(1):203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Salim A, Nacamuli RP, Morgan EF, Giaccia AJ, Longaker MT. Transient Changes in Oxygen Tension Inhibit Osteogenic Differentiation and Runx2 Expression in Osteoblasts *. Journal of Biological Chemistry. 2004;279(38):40007–16. [DOI] [PubMed] [Google Scholar]
- 84.Ghiroldi A, Piccoli M, Cirillo F, Monasky MM, Ciconte G, Pappone C, et al. Cell-Based Therapies for Cardiac Regeneration: A Comprehensive Review of Past and Ongoing Strategies. International Journal of Molecular Sciences. 2018;19(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McQuilling JP, Sittadjody S Fau - Pendergraft S, Pendergraft S Fau - Farney AC, Farney Ac Fau - Opara EC, Opara EC. Applications of particulate oxygen-generating substances (POGS) in the bioartificial pancreas. (2047–4849 (Electronic)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kirk ES, Honig CR. Nonuniform distribution of blood flow and gradients of oxygen tension within the heart. American Journal of Physiology-Legacy Content. 1964;207(3):661–8. [DOI] [PubMed] [Google Scholar]
- 87.Carrier RL, Rupnick M, Langer R, Schoen FJ, Freed LE, Vunjak-Novakovic G. Effects of oxygen on engineered cardiac muscle. Biotechnology and Bioengineering. 2002;78(6):617–25. [DOI] [PubMed] [Google Scholar]
- 88.Radisic M, Malda J, Epping E, Geng W, Langer R, Vunjak-Novakovic G. Oxygen gradients correlate with cell density and cell viability in engineered cardiac tissue. Biotechnology and Bioengineering. 2006;93(2):332–43. [DOI] [PubMed] [Google Scholar]
- 89.Richards DJ, Li Y, Kerr CM, Yao J, Beeson GC, Coyle RC, et al. Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity. Nature Biomedical Engineering. 2020;4(4):446–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.He JJ, McCarthy C, Camci-Unal G. Development of Hydrogel-Based Sprayable Wound Dressings for Second- and Third-Degree Burns. Advanced NanoBiomed Research. 2021;1(6):2100004. [Google Scholar]
- 91.Augustine R, Kalarikkal N, Thomas S. Advancement of wound care from grafts to bioengineered smart skin substitutes. Progress in Biomaterials. 2014;3(2):103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Augustine R, Dalvi YB, Dan P, George N, Helle D, Varghese R, et al. Nanoceria Can Act as the Cues for Angiogenesis in Tissue-Engineering Scaffolds: Toward Next-Generation in Situ Tissue Engineering. ACS Biomaterials Science & Engineering. 2018;4(12):4338–53. [DOI] [PubMed] [Google Scholar]
- 93.Hendrickx B, Vranckx JJ, Luttun A. Cell-Based Vascularization Strategies for Skin Tissue Engineering. Tissue Engineering Part B: Reviews. 2010;17(1):13–24. [DOI] [PubMed] [Google Scholar]
- 94.Gordillo GM, Sen CK. Evidence-Based Recommendations for the Use of Topical Oxygen Therapy in the Treatment of Lower Extremity Wounds. The International Journal of Lower Extremity Wounds. 2009;8(2):105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zellner S, Manabat R, Roe DF. A dissolved oxygen dressing: A pilot study in an ischemic skin flap model. Journal of International Medical Research. 2014;43(1):93–103. [DOI] [PubMed] [Google Scholar]
- 96.DiPietro LA. Angiogenesis and wound repair: when enough is enough. Journal of leukocyte biology. 2016;100(5):979–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guo S, Dipietro LA. Factors affecting wound healing. Journal of dental research. 2010;89(3):219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gomillion CT, Burg KJL. Stem cells and adipose tissue engineering. Biomaterials. 2006;27(36):6052–63. [DOI] [PubMed] [Google Scholar]
- 99.Eto H, Kato H, Suga H, Aoi N, Doi K, Kuno S, et al. The fate of adipocytes after nonvascularized fat grafting: evidence of early death and replacement of adipocytes. Plast Reconstr Surg. 2012;129(5):1081–92. [DOI] [PubMed] [Google Scholar]
- 100.Radak Z, Zhao Z, Koltai E, Ohno H, Atalay M. Oxygen consumption and usage during physical exercise: the balance between oxidative stress and ROS-dependent adaptive signaling. Antioxidants & redox signaling. 2013;18(10):1208–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ward CL, Corona B, Kesireddy V, Machingal M, Zhao W, Christ G, et al. Oxygen Generating Materials for Muscle Regeneration2010. Available from: http://biomaterials-org.securec7.ezhostingserver.com/abstracts/data/papers/2010/238.pdf.
- 102.Ward CL, Corona BT, Yoo JJ, Harrison BS, Christ GJ. Oxygen Generating Biomaterials Preserve Skeletal Muscle Homeostasis under Hypoxic and Ischemic Conditions. PLOS ONE. 2013;8(8):e72485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mas-Bargues C, Sanz-Ros J, Román-Domínguez A, Inglés M, Gimeno-Mallench L, El Alami M, et al. Relevance of Oxygen Concentration in Stem Cell Culture for Regenerative Medicine. International Journal of Molecular Sciences. 2019;20(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Molé PA, Chung Y Fau - Tran TK, Tran Tk Fau - Sailasuta N, Sailasuta N Fau - Hurd R, Hurd R Fau - Jue T, Jue T. Myoglobin desaturation with exercise intensity in human gastrocnemius muscle. The American Journal of Physiology. 1999;277(1)(0002–9513 (Print)). [DOI] [PubMed] [Google Scholar]
- 105.Richardson RS, Duteil S, Wary C, Wray DW, Hoff J, Carlier PG. Human skeletal muscle intracellular oxygenation: the impact of ambient oxygen availability. The Journal of physiology. 2006;571(Pt 2):415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Long H, Liu Q, Yin H, Wang K, Diao N, Zhang Y, et al. Prevalence Trends of Site-Specific Osteoarthritis From 1990 to 2019: Findings From the Global Burden of Disease Study 2019. Arthritis & Rheumatology. 2022;74(7):1172–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang H-n, Li L, Leng P, Wang Y-z, LÜ C-y. Uninduced adipose-derived stem cells repair the defect of full-thickness hyaline cartilage. Chinese Journal of Traumatology (English Edition). 2009;12(2):92–7. [PubMed] [Google Scholar]
- 108.Malda J, Martens DE, Tramper J, van Blitterswijk CA, Riesle J. Cartilage Tissue Engineering: Controversy in the Effect of Oxygen. Critical Reviews in Biotechnology. 2003;23(3):175–94. [PubMed] [Google Scholar]
- 109.Puppi D, Chiellini F, Piras AM, Chiellini E. Polymeric materials for bone and cartilage repair. Progress in Polymer Science. 2010;35(4):403–40. [Google Scholar]
- 110.Montesdeoca CYC, Afewerki S, Stocco TD, Corat MAF, de Paula MMM, Marciano FR, et al. Oxygen-generating smart hydrogels supporting chondrocytes survival in oxygen-free environments. Colloids and Surfaces B: Biointerfaces. 2020;194:111192. [DOI] [PubMed] [Google Scholar]
- 111.de Sousa Araújo E, Domingues Stocco T, Fernandes de Sousa G, Afewerki S, Marciano FR, Alexandre Finzi Corat M, et al. Oxygen-generating microparticles in chondrocytes-laden hydrogels by facile and versatile click chemistry strategy. Colloids and Surfaces B: Biointerfaces. 2021;205:111850. [DOI] [PubMed] [Google Scholar]
- 112.Pattappa GA-O, Johnstone B, Zellner J, Docheva DA-O, Angele P. The Importance of Physioxia in Mesenchymal Stem Cell Chondrogenesis and the Mechanisms Controlling Its Response. LID - 10.3390/ijms20030484 [doi] LID - 484. (1422–0067 (Electronic)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nahmias Y, Kramvis Y, Barbe L, Casali M, Berthiaume F, Yarmush ML. A novel formulation of oxygen‐carrying matrix enhances liver-specific function of cultured hepatocytes. The FASEB Journal. 2006;20(14):2531–3. [DOI] [PubMed] [Google Scholar]
- 114.Yamada M, Utoh R, Ohashi K, Tatsumi K, Yamato M, Okano T, et al. Controlled formation of heterotypic hepatic micro-organoids in anisotropic hydrogel microfibers for long-term preservation of liver-specific functions. Biomaterials. 2012;33(33):8304–15. [DOI] [PubMed] [Google Scholar]
- 115.Ahmed HMM, Salerno S, Piscioneri A, Khakpour S, Giorno L, De Bartolo L. Human liver microtissue spheroids in hollow fiber membrane bioreactor. Colloids and Surfaces B: Biointerfaces. 2017;160:272–80. [DOI] [PubMed] [Google Scholar]
- 116.Maharjan S, Alva J, Cámara C, Rubio AG, Hernández D, Delavaux C, et al. Symbiotic Photosynthetic Oxygenation within 3D-Bioprinted Vascularized Tissues. Matter. 2021;4(1):217–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jeffries RE, Gamcsik MP, Keshari KR, Pediaditakis P, Tikunov AP, Young GB, et al. Effect of Oxygen Concentration on Viability and Metabolism in a Fluidized-Bed Bioartificial Liver Using 31P and 13C NMR Spectroscopy. Tissue Engineering Part C: Methods. 2012;19(2):93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Leedale JA, Lucendo-Villarin B, Meseguer-Ripolles J, Kasarinaite A, Webb SD, Hay DC. Mathematical modelling of oxygen gradients in stem cell-derived liver tissue. PLOS ONE. 2021;16(2):e0244070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Paxian M, Keller SA, Cross B, Huynh TT, Clemens MG. High-resolution visualization of oxygen distribution in the liver in vivo. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2004;286(1):G37–G44. [DOI] [PubMed] [Google Scholar]
- 120.Cho CH, Park J, Nagrath D, Tilles AW, Berthiaume F, Toner M, et al. Oxygen uptake rates and liver-specific functions of hepatocyte and 3T3 fibroblast co-cultures. Biotechnology and Bioengineering. 2007;97(1):188–99. [DOI] [PubMed] [Google Scholar]
- 121.Kidambi S, Yarmush Rubin S, Novik E, Chao P, Yarmush Martin L, Nahmias Y. Oxygen-mediated enhancement of primary hepatocyte metabolism, functional polarization, gene expression, and drug clearance. Proceedings of the National Academy of Sciences. 2009;106(37):15714–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ghosh A, Onsager C, Mason A, Arriola L, Lee W, Mubayi A. The role of oxygen intake and liver enzyme on the dynamics of damaged hepatocytes: Implications to ischaemic liver injury via a mathematical model. PLOS ONE. 2021;16(4):e0230833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5(5):415–8. [DOI] [PubMed] [Google Scholar]
- 124.Moustafa MH, Sharma RK, Thornton J, Mascha E, Abdel-Hafez MA, Thomas AJ, et al. Relationship between ROS production, apoptosis and DNA denaturation in spermatozoa from patients examined for infertility. Human Reproduction. 2004;19(1):129–38. [DOI] [PubMed] [Google Scholar]
- 125.Aleemardani M, Solouk A, Akbari S, Dehghan MM, Moeini M. Silk-derived oxygen-generating electrospun patches for enhancing tissue regeneration: Investigation of calcium peroxide role and its effects on controlled oxygen delivery. Materialia. 2020;14:100877. [Google Scholar]
- 126.Khorshidi S, Karkhaneh A, Bonakdar S. Fabrication of amine-decorated nonspherical microparticles with calcium peroxide cargo for controlled release of oxygen. Journal of Biomedical Materials Research Part A. 2020;108(1):136–47. [DOI] [PubMed] [Google Scholar]
- 127.Shiekh PA, Singh A, Kumar A. Oxygen-Releasing Antioxidant Cryogel Scaffolds with Sustained Oxygen Delivery for Tissue Engineering Applications. ACS Applied Materials & Interfaces. 2018;10(22):18458–69. [DOI] [PubMed] [Google Scholar]
- 128.Cook CA, Hahn KC, Morrissette-McAlmon JBF, Grayson WL. Oxygen delivery from hyperbarically loaded microtanks extends cell viability in anoxic environments. Biomaterials. 2015;52:376–84. [DOI] [PMC free article] [PubMed] [Google Scholar]