Abstract
TREATgermany is an investigator-initiated prospective disease registry. It investigates physician- and patient-reported disease severity (Eczema Area and Severity Index (EASI), objective Scoring Atopic Dermatitis (oSCORAD), Investigator Global Assessment, Patient-Oriented Eczema Measure (POEM), Patient Global Assessment (PGA)), patient-reported symptoms (itch, sleep loss, depressive symptoms), therapy courses and dermatological quality of life (DLQI) in moderate-to-severe atopic dermatitis with SCORAD > 20. 1,134 atopic dermatitis patients (mean age 41.0 ± 14.7 years, 42.5% females) were enrolled by 40 German recruiting sites (dermatological clinics and practices) between June 2016 and April 2021. The current analysis focuses on itch scores obtained with a numerical rating scale (NRS)) documented for the previous 3 days prior to baseline visit. The results show that 97.2% (1,090 of 1,121) patients experienced itch. Itch severity correlated moderately with severity of atopic dermatitis oSCORAD (rho = 0.44 (0.39–0.48)) and EASI score (rho = 0.41 (0.36–0.46)). A strong correlation was found with self-reported disease severity as PGA (rho = 0.68 (0.65–0.71)), POEM sum score (rho = 0.66 (0.63–0.69)) and dermatological quality of life impairment DLQI (rho = 0.61 (0.57–0.65)). Itch as a subjective complaint is more closely correlated with patient-reported outcomes than with objective assessments by the physician.
Key words: EASI, POEM, pruritus, quality of life, registry
SIGNIFICANCE.
Most people with atopic dermatitis experience itch and, for most of them, it is very important to be free of this complaint. Using data from the TREATgermany registry, this study investigated how patients rated the severity of their itch and how it affected their dermatological quality of life. The results show that patients associated itch more strongly with self-reported outcomes than with physician’s assessments. This indicates that the reduction in itch should be a central focus of patient-centred care for patients with atopic dermatitis.
Treatment and medical care of patients with moderate-to-severe Atopic Dermatitis (TREATgermany) is an investigator-initiated prospective disease registry of patients with moderate to severe affected atopic dermatitis (AD) in Germany and it is part of the European registry family TREAT. It represents a standardized assessment of treatment and outcomes in daily routine care of patients with AD. TREATgermany includes patients 18 years or older with moderate-to-severe AD (objective Scoring Atopic Dermatitis; oSCORAD > 20) consulting a dermatology department or a dermatology practice in Germany (A separate TREATkids for patients under 18 years was started in 2020. This data is not analysed here). First analyses demonstrated a high disease burden and a great need for systemic treatments (1, 2). Dupilumab, the first biologic for AD, which was launched in Germany in December 2017, showed similar effectiveness on clinical signs, symptoms and health-related quality of life (HRQoL) as in clinical trials (3). Itch was significantly reduced 3 months after dupilumab treatment (3).
Itch is the defining symptom of AD and occurs chronically (4–6). It affects patients in severe bouts occurring for minutes to hours, as well as constantly, on a daily basis. Itch constitutes a dominant symptom that characterizes active disease, but patients with AD also report that itch often occurs when the skin looks (macroscopically) non-diseased. Often, itch may be the first symptom of an AD relapse/flair (7). Patient focus groups reported AD-related itching to be the most bothersome AD symptom (8). The German Atopic Dermatitis Intervention Study (GADIS), including 823 children and adolescents, showed significant, but weak, correlations between itch intensity and AD disease severity (9). Another study showed similar relationships (10). Consequentially, studying the symptom of itch in AD remains important and challenging. TREATgermany provides an opportunity to gain more insight into itch in daily routine care. Since 89.0% of patients with AD in TREATgermany stated that it is “very important” for them to be free of itch, it was the aim of this analysis to investigate itch in relation to physician- and patient-reported outcome measures (PROMs).
MATERIAL AND METHODS
TREATgermany (www.treatgermany.org) is a nationwide multicentre academic registry for the long-term observation of patients with moderate-to-severe AD that has been formally approved by the Medical Faculty of Carl Gustav Carus University, Dresden, Germany (number EK 118032016) and the responsible local ethics committees at the other participating sites. Patients are recruited at dermatological practices, clinics and university hospitals. The registry has been described in detail elsewhere (2). Patients with moderate-to-severe AD, current or prior (in the past 2 years) systemic anti-inflammatory treatment and/or objective SCORAD (Scoring Atopic Dermatitis, oSCORAD) > 20 are prospectively followed over the course of at least 24 months. A broad set of physician- and patient-reported outcome measures are documented using validated measurement instruments (1, 2).
In SCORAD, mean itch intensity caused by AD during the past 3 days, was rated by the patients on a numerical rating scale (NRS) ranging from 0 (no itch) to 10 (maximum imaginable itch). The association between various verifiable (documented by the treating physician) and PROMs and itch of patients enrolled in the registry from June 2016 until April 2021 was examined. Investigator-reported outcomes were oSCORAD (11), Eczema Area and Severity Index (EASI) (12) and Investigator Global Assessment (IGA; levels 0 (healed) to 5 (extremely severe)) (13).
PROMs were Patient Global Assessment (PGA), Patient-Oriented Eczema Measure (POEM) (14), Dermatology Life Quality Index (DLQI) (15), Fatigue Severity Scale (FSS) (16) and the Patient Benefit Index (PBI) (17). In addition, pain and sleep loss caused by AD during the past 3 days rated on a numerical rating scale ranging from 0 (no disturbance) to 10 (unbearable disturbance) were examined. This approach is identical to the SCORAD to ensure comparability. The analysis focuses on the baseline visit. Software Package Stata 15 (StataCorp LLC, Texas, USA) was used for descriptive and explorative data analyses: Pearson’s χ2 test, Spearman’s rank sum test and rank correlation including 95% confidence interval (95% CI) were used for describing associations. The qualitative interpretation of the strength of correlation follows Landis and Koch (18).
RESULTS
Characteristics of the study population
As of April 2021, a total of 1,134 patients (mean age 41.0 ± 14.7 years, 42.5% females) were enrolled at 40 recruitment centres (Table I). At baseline registry patients had mean oSCORAD of 40.8 ± 16.0 and EASI scores of 16.2 ± 12.9. 53.5% of them (n = 603) were mildly/ moderately (mild respectively moderate erythema, papule forming and infiltrate) and 39.0% (n = 440) were (very) severely affected (severe erythema, papule forming and infiltrate (with oozing/crusting)) by AD according to the IGA (Table I).
Table I.
Sociodemographic characteristics of the study population including physician and patient-reported outcome measures
| Characteristics | |
|---|---|
| Sex, n (%) | |
| Male | 651 (57.4) |
| Female | 482 (42.5) |
| Divers | 1 (0.1) |
| Age, mean (SD) | 41.0 (14.7) (n = 1,134) |
| Itch, mean (SD) | 5.7 (2.7) (n = 1,121) |
| No itch (0), n (%) | 31 (2.8) |
| Itch (1–6), n (%) | 586 (52.3) |
| Severe itch (≥ 7), n (%) | 504 (45.0) |
| oSCORAD, mean (SD) | 40.8 (16.0) (n = 1,128) |
| Clear (0 to < 8), n (%) | 28 (2.5) |
| Mild (8 to < 24), n (%) | 130 (11.5) |
| Moderate (24 to < 38), n (%) | 328 (29.1) |
| Severe (38–83), n (%) | 642 (56.9) |
| EASI, mean (SD) | 16.2 (12.9) (n = 1,124) |
| Clear (0), n (%) | 15 (1.3) |
| Mild (> 0 to < 6), n (%) | 241 (21.4) |
| Moderate (6 to < 23), n (%) | 599 (53.3) |
| Severe (23–72), n (%) | 269 (23.9) |
| IGA, n (%) | |
| Clear | 16 (1.4) |
| Almost clear | 69 (6.1) |
| Mild | 159 (14.1) |
| Moderate | 444 (39.4) |
| Severe | 350 (31.0) |
| Very severe | 90 (8.0) |
| PGA, n (%) | |
| Clear | 26 (2.3) |
| Almost clear | 90 (8.0) |
| Mild | 222 (19.8) |
| Moderate | 329 (29.4) |
| Severe | 326 (29.1) |
| Very severe | 126 (11.3) |
| DLQI, mean (SD) | 11.9 (7.8) (n = 1,121) |
| No effect at all on patient’s life (0–1), n (%) | 93 (8.3) |
| Small effect on PL (2–5), n (%) | 193 (17.2) |
| Moderate effect on PL (6–10), n (%) | 256 (22.8) |
| Very large effect on PL (11–20), n (%) | 395 (35.2) |
| Extremely large effect on PL (21–30), n (%) | 184 (16.4) |
| POEM, mean (SD) | 16.8 (7.5) (n = 1,123) |
| Clear or almost clear (0–2), n (%) | 51 (4.5) |
| Mild eczema (3–7), n (%) | 105 (9.3) |
| Moderate eczema (8–16), n (%) | 347 (30.9) |
| Severe eczema (17–24), n (%) | 422 (37.6) |
| Very severe eczema (25–28), n (%) | 198 (17.6) |
| Fatigue, mean (SD) | 3.7 (1.6) (n = 1,120) |
| Normal (FSS ≤ 4), n (%) | 673 (60.1) |
| Increased (FSS > 4 and ≤ 5), n (%) | 182 (16.3) |
| High (FSS > 5), n (%) | 265 (23.7) |
SD: standard deviation; oSCORAD: objective Scoring Atopic Dermatitis; EASI: Eczema Area and Severity Index; IGA: Investigator Global Assessment; PGA: Physician global assessment; DLQI: Dermatology Life Quality Life Index; POEM: Patient-Oriented Eczema Measure; FSS: Fatigue Severity Scale.
Itch severity in the study population
A total of 1,121 patients (98.9%) provided data on their itch in the past 3 days prior to baseline visit. 97.2% (n = 1,090 ) of those patients with itch (NRS score > 0). 504 (45.0%) had itch scores of 7 and higher, indicating severe itch (19). Mean itch severity was 5.7 ± 2.7 and was significantly higher in females (6.1 ± 2.8) compared with males (5.5 ± 2.7) (rank-sum test, p < 0.001). There was no relevant (linear) correlation between itch and age (Table II). Compared with pain (3.7 ± 2.8) and sleep loss (4.6±3.4), which were gathered in the same way as itch, itch was the symptom with the highest rating (20). There was no significant association between itch severity and self-reported allergic comorbidities, asthma, allergic rhinitis or food allergy.
Table II.
Mean itch severity depending on age
| Age group | Total |
Male |
Female |
|||
|---|---|---|---|---|---|---|
| n | Itch Mean ± SD | n | Itch Mean ± SD | n | Itch Mean ± SD | |
| ≤ 30 years | 355 | 6.2 ± 2.6 | 168 | 6.0 ± 2.5 | 186 | 6.3 ± 2.7 |
| 31–40 years | 234 | 5.7 ± 2.8 | 145 | 5.3 ± 2.6 | 89 | 6.3 ± 2.9 |
| 41–50 years | 199 | 5.2 ± 2.7 | 127 | 5.0 ± 2.5 | 72 | 5.6 ± 3.0 |
| 51–60 years | 221 | 5.4 ± 2.8 | 135 | 5.3 ± 2.7 | 86 | 5.5 ± 3.0 |
| > 60 years | 112 | 5.9 ± 3.0 | 69 | 5.5 ± 3.1 | 43 | 6.4 ± 2.7 |
SD: standard deviation.
The relationship between disease duration of AD and itch severity was analysed according to age and sex (n = 1,101). As expected, due to the typical manifestation of AD early in life, the mean disease duration increased with increasing age, with the age group 51–60 years having the longest disease duration (n = 219, 39.9 ± 18.0 years). However, the proportion of patients who reported having only had AD for a relatively short time (2 years or less) was higher in the group of patients over 60 years of age than in younger patients (16.1% vs 1.5%). The mean itch according to the disease duration stratified by age and sex is shown in Table III. No correlation between duration of disease and itching is apparent; however, recall bias might reduce the reliability of the data.
Table III.
Association between disease duration of atopic dermatitis (physicians’ questionnaire) and itch stratified by sex (n = 992)
| Disease duration and age groups | Total |
Male |
Female |
|||
|---|---|---|---|---|---|---|
| n | Itch Mean ± SD | n | Itch Mean ± SD | n | Itch Mean ± SD | |
| Disease duration ≤ 2 years | ||||||
| ≤ 30 years old | 8 | 5.9 ± 2.4 | 5 | 6.2 ± 2.4 | 3 | 5.3 ± 2.9 |
| 31–40 years old | 1 | – | 0 | – | 1 | – |
| 41–50 years old | 0 | – | 0 | – | 0 | – |
| 51–60 years old | 6 | 5.7 ± 3.6 | 4 | 4.0 ± 3.2 | 2 | 9.0 ± 1.4 |
| > 60 years old | 18 | 5.8 ± 3.2 | 8 | 4.8 ± 3.2 | 10 | 6.7 ± 3.1 |
| Total | 33 | 5.6 ± 3.1 | 17 | 5.0 ± 2.9 | 16 | 6.3 ± 3.3 |
| Disease duration 3–10 years | ||||||
| ≤ 30 years old | 17 | 7.1 ± 2.3 | 8 | 7.9 ± 2.0 | 9 | 6.3 ± 2.4 |
| 31–40 years old | 6 | 3.2 ± 2.3 | 6 | 3.2 ± 2.3 | 0 | – |
| 41–50 years old | 13 | 5.5 ± 2.6 | 10 | 5.2 ± 2.8 | 3 | 6.3 ± 2.3 |
| 51–60 years old | 19 | 5.9 ± 3.4 | 9 | 6.9 ± 3.6 | 10 | 5.0 ± 3.1 |
| > 60 years old | 21 | 6.2 ± 3.3 | 14 | 5.7 ± 3.1 | 7 | 7.1 ± 3.6 |
| ≤ 30 years old | 76 | 5.9 ± 3.0 | 47 | 5.9 ± 3.1 | 29 | 6.1 ± 2.9 |
| Disease duration ≥ 10 years | ||||||
| ≤ 30 years old | 325 | 6.1 ± 2.6 | 153 | 5.9 ± 2.5 | 171 | 6.3 ± 2.7 |
| 31–40 years old | 219 | 5.8 ± 2.7 | 134 | 5.4 ± 2.5 | 85 | 6.4 ± 2.8 |
| 41–50 years old | 186 | 5.2 ± 2.7 | 117 | 5.0 ± 2.5 | 69 | 5.6 ± 3.0 |
| 51–60 years old | 190 | 5.4 ± 2.7 | 118 | 5.3 ± 2.6 | 72 | 5.6 ± 2.9 |
| > 60 years old | 72 | 5.8 ± 2.9 | 47 | 5.6 ± 3.1 | 25 | 6.0 ± 2.3 |
| ≤ 30 years old | 992 | 5.7 ± 2.7 | 569 | 5.5 ± 2.6 | 422 | 6.0 ± 2.8 |
Table IV shows the influence of itch severity on the importance of being free from itch measured by the PBI. Regardless of sex and reported itch severity, this issue was rated to be “quite” or “very important” in 93.8% and up to 100% of cases. This underlines the high importance of effectively treating this AD symptom and understanding its correlation with other outcomes.
Table IV.
Patient benefit index (PBI): importance of treatment need “be free of itching” stratified by sex and itch severity (n=1,117)
| Sex and itch severity | Patient needs questionnaire – importance of treatment needs: be free of itching |
||||||
|---|---|---|---|---|---|---|---|
| n | Not at all n (%) | Somewhat n (%) | Moderately n (%) | Quite n (%) | Very important n (%) | Does not apply to me n (%) | |
| Male | |||||||
| No itch (0) | 15 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (13.3) | 13 (86.7) | 0 (0.0) |
| Itch (1–6) | 377 | 0 (0.0) | 2 (0.5) | 5 (1.3) | 39 (10.3) | 330 (87.5) | 1 (0.3) |
| Severe itch ≥7) | 251 | 1 (0.4) | 1 (0.4) | 2 (0.8) | 8 (3.2) | 239 (95.2) | 0 (0.0) |
| Female | |||||||
| No itch (0) | 16 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 16 (100.0) | 0 (0.0) |
| Itch (1–6) | 208 | 1 (0.5) | 5 (2.4) | 6 (2.9) | 23 (11.1) | 172 (82.7) | 1 (0.5) |
| Severe itch (≥7) | 250 | 3 (1.2) | 1 (0.4) | 3 (1.2) | 17 (6.8) | 224 (89.6) | 2 (0.8) |
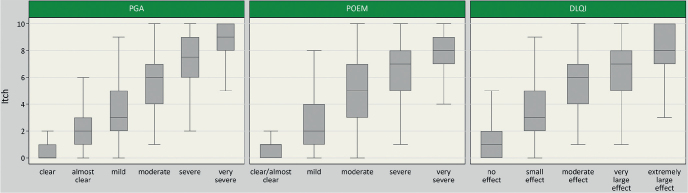
A weak positive correlation was observed between itch severity and physician-rated severity of AD measured by oSCORAD (rho = 0.44 (0.39–0.48)), EASI score (rho = 0.41 (0.36–0.46)) and IGA (rho = 0.46 (0.42–0.51)) (Fig. 1). A strong positive correlation was found between itch and patient-reported disease severity, as assessed by means of the PGA (rho = 0.68 (0.65–0.71)) and POEM sum score (rho = 0.66 (0.63–0.69)). The results are described in detail in Fig. 2. Strong positive correlations were also found for itch and DLQI (rho = 0.61 (0.57–0.65)) (Fig. 2).
Fig. 1.
Boxplots of itch severity over severity by categories of Investigator Global Assessment (IGA) (n = 1,115), Eczema Area and Severity Index (EASI) (n = 1,111) and objective Scoring Atopic Dermatitis (oSCORAD) (n = 1,115). EASI and oSCORAD were categorized as follows: oSCORAD (scale: 0–83): 0–7.9 = clear, 8–23.9 = mild, 24–37.9 = moderate, 38–83 = severe; EASI (scale: 0–72): 0 = clear, 0.1–5.9 = mild, 6–22.9 = moderate, 23–72 = severe (25). Outliers are not shown.
Fig. 2.
Boxplots of itch severity over severity by categories of Patient Global Assessment (PGA) (n = 1,119), Dermatological Life Quality Index (DLQI) (n = 1,120) and (Patient-Oriented Eczema Measure) POEM (n = 1,121). DLQI and POEM were categorized as follows: POEM (scale: 0–28): 0–2 = clear/almost clear, 3–7 = mild, 8–16 = moderate, 17–24 = severe, 25–28 = very severe (26). DLQI (scale: 0–30): 0–1 = no effect at all on patient’s life, 2–5 = small effect, 6–10 = moderate effect, 11–20 = very large effect, 21–30 = extremely large effect.
In total, registry patients had mean total FSS scores of 3.7 ± 1.6 (Table I). 23.7% of patients showed high fatigue (FSS > 5). There was a weak correlation of itch and FSS (rho = 0.32 (0.27–0.38), n = 1,118). Itch was higher in AD patients with higher FSS scores (6.9 ± 2.4 in FSS > 5 compared with 5.2 ± 2.7 in FSS < 4).
In addition, this study examined the association between sleep loss caused by AD during the past 3 days and itch. There was a strong correlation between itch and sleep loss (rho = 0.75 (0.72–0.78), n = 1,121).
DISCUSSION
This study found strong correlations between itch severity and PROMs, such as POEM, DLQI, and PGA. This indicates that itch places substantial burden on patients with AD. On the other hand, physician-rated disease severity was not as closely correlated with itch. This suggests that it is important for clinicians who treat patients with AD to regularly assess itch. If itch and other patient-reported outcomes are not assessed, physicians may underestimate the burden of disease and patient needs.
In a recent analysis in TREATgermany, patients with AD who smoke cigarettes showed a higher disease burden, including higher itch scores compared with non-smokers (21).
The frequency of itch and the impairment of dermatological quality of life in patients with AD have been described previously; however, the current study results demonstrates that itch as a subjective complaint is more closely associated with patient-reported outcomes than with objective assessments by the physician. This reflects the difficulty of assessing AD with its range of phenotypes, clinical pictures and symptoms. HOME (Harmonizing Outcome Measures in Eczema) is an initiative of experts who defined a core outcome set containing clinical signs, patient-reported symptoms, long-term control and dermatological quality of life (www.homeforeczema.org). The peak itch numerical rating scale (NRS) of the past 24 h is the recommended instrument for measuring the intensity of itch in the symptom domain in trials of older children and adults (20). Since the TREATgermany registry was started before this recommendation, this method of measuring itch was only implemented in the data collection in 2021, so it could not yet be used for this analysis. Recap of AD (RECAP) was developed recently and introduced to capture the experience of eczema control over the past week. Two of the 7 questions address itch explicitly within 5 answer options, which are “no days”, “1–2 days”, “3–4 days”, “5–6 days” and “every day”, respectively (22).
In a study with 410 patients with AD, changes from the baseline in NRS, VRS and frequency of itch were weakly to moderately correlated with other patient-reported outcomes (POEM, SCORAD-itch, DLQI) (23). Another study showed a significant decrease in DLQI scores for patients with itch improvement after 16 weeks of treatment (24). Mediation models also showed an indirect effect of AD treatment on DLQI scores via reduction in the severity of pruritus (25).
It is known that patient-reported AD severity appears to be sufficiently valid for assessing AD severity in the clinical and epidemiological setting (26); however, physician-reported outcome measures show low or moderate correlation to itch.
The FSS has been shown to be a valuable tool to assess and quantify fatigue (27). The awareness and assessment of fatigue has also been underestimated previously and remains an under-recognized problem in AD. In addition to being a disease-related symptom, fatigue can be induced by systemic medications. Fatigue may be caused in AD by sedating antihistamines.
The growing list of comorbidities was also confirmed in TREATgermany and reflects the increasing appreciation of its high burden (28). More knowledge about this is necessary to improve the possibilities of medical and psychological interventions.
Pathophysiology and pathogenesis of itching in AD are still not fully understood. A crosstalk between the nervous system, the cutaneous immune system and keratinocyte population explain the origin and persistence of itch in AD (29). Itching in AD is influenced by various factors, such as triggers (e.g. sweat, clothing, allergens, such as dust mites, pollens, food allergens, as well as infections and psychological factors) (4, 5, 30). This explains why treating atopic itch remains a great challenge. Novel approaches have been described, however, with variable effects on itch (29). It is most likely that treating itch sufficiently means targeting inflammation, neural and neuroimmune pathways. Elucidating the roles of peripheral and central sensitization and hypersensitization in AD is a future task (29); however, investigating patient-reported outcomes of atopic itch supports this task.
The strength of these analyses can be summarized as follows: this study investigated itch in a large cohort of patients with AD. As TREATgermany is a registry study the data are obtained in a very similar and uniform pattern in AD clinics. The results attain special importance because the impact of measuring patient-reported outcomes in dermatology has been underestimated in the past, but has also gained increasing significance for drug development (31). As itch is a subjective symptom, which is not easy to score, it is not surprising that itch is more closely associated with patient-reported outcomes than with objective physician assessment.
A limitation of this analysis is measuring itch in only the previous 3 days, as is done in SCORAD, because itch in AD is chronic and long-lasting. The data were obtained in a single country not resembling cultural and ethnic aspects of itch (32). It remains to be seen if these results will be confirmed, for example in TARGET-DERM AD study, a longitudinal cohort study that plans to include 4,000 patients with AD in up to 100 clinical centres in the USA (33). The representativeness of the data is limited by the fact that participation in the registry is voluntary (on the part of both centres and patients).
In the future, TREATgermany may improve our understanding of how AD can be treated more effectively over time to reduce its impact, especially the impact of itch, on all domains of life, including cumulative life impairment.
ACKNOWLEDGEMENTS
We thank all the patients with AD for participating in this study, for their confidence in the study centres and for their great support in providing the data for TREATgermany.
TREATgermany is an academic, investigator-initiated clinical registry financially supported by AbbVie Deutschland GmbH & Co. KG, Galderma S.A., LEO Pharma GmbH, Lilly Deutschland GmbH, Pfizer Inc. and Sanofi-Aventis Deutschland GmbH.
TREATgermany has formally been approved by the Medical Faculty of the Carl Gustav Carus University, Dresden, Germany (No. EK 118032016) and the responsible local ethics committees at the other participating sites.
Conflict of interest
JS is Co-PI of TREATgermany and reports institutional grants for investigator-initiated research from the German GBA, the BMG, BMBF, EU, Federal State of Saxony, Novartis, Sanofi, ALK, and Pfizer. He participated in advisory board meetings as a paid consultant for Sanofi, Lilly, and ALK. KS received honoraria as investigator and/or received speakers’ honoraria and/or received grants and/or has been an advisor for the following companies: AbbVie, Almirall-Hermal, Amgen, Biogen Idec, Boehringer-Ingelheim, Bristol-Myers Squibb, Celgene, Eli-Lilly, Galderma, Janssen, LEO-Pharma, Merck Serono, Morphosys, Novartis, Pfizer, Regeneron, Sanofi, a, und UCB Pharma. LH is research associate of TREATgermany, which is an academic, investigator-initiated clinical registry that is financially supported AbbVie GmbH & Co. KG, Galderma S.A., LEO Pharma GmbH, Lilly Deutschland GmbH, Pfizer Inc. and Sanofi-Aventis Deutschland GmbH. SA received lecture and/or consultancy fees from Novartis, LEO Pharma, Amgen, Lilly, Sanofi, Beiersdorf, Janssen, UCB and AbbVie. EW received consultancy fees from Sanofi/Genzyme and participated in clinical trials of Galderma Ltd and TREVI Ltd. The other authors have no conflicts of interests to declare
REFERENCES
- 1.Heratizadeh A, Haufe E, Stolzl D, Abraham S, Heinrich L, Kleinheinz A, et al. Baseline characteristics, disease severity and treatment history of patients with atopic dermatitis included in the German AD Registry TREATgermany. J Eur Acad Dermatol Venereol 2020; 34: 1263–1272. [DOI] [PubMed] [Google Scholar]
- 2.Schmitt J, Abraham S, Trautmann F, Stephan V, Folster-Holst R, Homey B, et al. Usage and effectiveness of systemic treatments in adults with severe atopic eczema: First results of the German Atopic Eczema Registry TREATgermany. J Dtsch Dermatol Ges 2017; 15: 49–59. [DOI] [PubMed] [Google Scholar]
- 3.Abraham S, Haufe E, Harder I, Heratizadeh A, Kleinheinz A, Wollenberg A, et al. Implementation of dupilumab in routine care of atopic eczema: results from the German national registry TREATgermany. Br J Dermatol 2020; 183: 382–384. [DOI] [PubMed] [Google Scholar]
- 4.Bieber T. Atopic dermatitis. N Engl J Med 2008; 358: 1483–1494. [DOI] [PubMed] [Google Scholar]
- 5.Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet 2020; 396: 345–360. [DOI] [PubMed] [Google Scholar]
- 6.Wollenberg A, Christen-Zach S, Taieb A, Paul C, Thyssen JP, de Bruin-Weller M, et al. ETFAD/EADV Eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereol 2020; 34: 2717–2744. [DOI] [PubMed] [Google Scholar]
- 7.Darsow U., Ripphoff E., J. R. Pathophysiology and clinical manifestation of itch in atopic eczema. In: Ring J., Przybilla B., Ruzicka T., editors. Handbook of Atopic Eczema. 2 ed. Berlin, Heidelberg, New York: Springer; 2006: p. 222–227. [Google Scholar]
- 8.Martin SA, Brown TM, Fehnel S, Deal LS, Katz EG, Chiou CF. The atopic dermatitis itch scale: development of a new measure to assess pruritus in patients with atopic dermatitis. J Dermatolog Treat 2020; 31: 484–490. [DOI] [PubMed] [Google Scholar]
- 9.Weisshaar E, Diepgen TL, Bruckner T, Fartasch M, Kupfer J, Lob-Corzilius T, et al. Itch intensity evaluated in the German Atopic Dermatitis Intervention Study (GADIS): correlations with quality of life, coping behaviour and SCORAD severity in 823 children. Acta Derm Venereol 2008; 88: 234–239. [DOI] [PubMed] [Google Scholar]
- 10.Lei D, Yousaf M, Janmohamed SR, Vakharia PP, Chopra R, Chavda R, et al. Validation of four single-item patient-reported assessments of sleep in adult atopic dermatitis patients. Ann Allergy Asthma Immunol 2020; 124: 261–266. [DOI] [PubMed] [Google Scholar]
- 11.Kunz B, Oranje AP, Labreze L, Stalder JF, Ring J, Taieb A. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology 1997; 195: 10–19. [DOI] [PubMed] [Google Scholar]
- 12.Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol 2001; 10: 11–18. [DOI] [PubMed] [Google Scholar]
- 13.Meurer M, Folster-Holst R, Wozel G, Weidinger G, Junger M, Brautigam M, et al. Pimecrolimus cream in the long-term management of atopic dermatitis in adults: a six-month study. Dermatology 2002; 205: 271–277. [DOI] [PubMed] [Google Scholar]
- 14.Charman CR, Venn AJ, Williams HC. The patient-oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol 2004; 140: 1513–1519. [DOI] [PubMed] [Google Scholar]
- 15.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19: 210–216. [DOI] [PubMed] [Google Scholar]
- 16.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989; 46: 1121–1123. [DOI] [PubMed] [Google Scholar]
- 17.Augustin M, Radtke MA, Zschocke I, Blome C, Behechtnejad J, Schafer I, et al. The patient benefit index: a novel approach in patient-defined outcomes measurement for skin diseases. Arch Dermatol Res 2009; 301: 561–571. [DOI] [PubMed] [Google Scholar]
- 18.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159–174. [PubMed] [Google Scholar]
- 19.Phan NQ, Blome C, Fritz F, Gerss J, Reich A, Ebata T, et al. Assessment of pruritus intensity: prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta Derm Venereol 2012; 92: 502–507. [DOI] [PubMed] [Google Scholar]
- 20.Thomas KS, Apfelbacher CA, Chalmers JR, Simpson E, Spuls PI, Gerbens LAA, et al. Recommended core outcome instruments for health-related quality of life, long-term control and itch intensity in atopic eczema trials: results of the HOME VII consensus meeting. Br J Dermatol 2021; 185: 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pilz AC, Schielein MC, Schuster B, Heinrich L, Haufe E, Abraham S, et al. Atopic dermatitis: disease characteristics and comorbidities in smoking and non-smoking patients from the TREATgermany registry. J Eur Acad Dermatol Venereol 2022; 36: 413–421. [DOI] [PubMed] [Google Scholar]
- 22.Howells LM, Chalmers JR, Gran S, Ahmed A, Apfelbacher C, Burton T, et al. Development and initial testing of a new instrument to measure the experience of eczema control in adults and children: Recap of atopic eczema (RECAP). Br J Dermatol 2020; 183: 524–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silverberg JI, Lai JS, Patel KR, Singam V, Vakharia PP, Chopra R, et al. Measurement properties of the Patient-Reported Outcomes Information System (PROMIS((R)) ) Itch Questionnaire: itch severity assessments in adults with atopic dermatitis. Br J Dermatol 2020; 183: 891–898. [DOI] [PubMed] [Google Scholar]
- 24.Lio PA, Simpson EL, Han G, Soung J, Ball S, Sun L, et al. Improvement in sleep and itch and enhanced quality of life in adult patients with moderate-to-severe atopic dermatitis: results from a phase 3 trial of baricitinib therapy. J Dermatolog Treat 2022; 33: 2057–2062. [DOI] [PubMed] [Google Scholar]
- 25.Simpson EL, Tom WL, Bushmakin AG, Cappelleri JC, Yosipovitch G, Stander S, et al. Relationship among treatment, pruritus, investigator’s static global assessment, and quality of life in patients with atopic dermatitis. Dermatol Ther (Heidelb) 2021; 11: 587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vakharia PP, Chopra R, Sacotte R, Patel N, Immaneni S, White T, et al. Validation of patient-reported global severity of atopic dermatitis in adults. Allergy 2018; 73: 451–458. [DOI] [PubMed] [Google Scholar]
- 27.Valko PO, Bassetti CL, Bloch KE, Held U, Baumann CR. Validation of the fatigue severity scale in a Swiss cohort. Sleep 2008; 31: 1601–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brunner PM, Silverberg JI, Guttman-Yassky E, Paller AS, Kabashima K, Amagai M, et al. Increasing comorbidities suggest that atopic dermatitis is a systemic disorder. J Invest Dermatol 2017; 137: 18–25. [DOI] [PubMed] [Google Scholar]
- 29.Yosipovitch G, Berger T, Fassett MS. Neuroimmune interactions in chronic itch of atopic dermatitis. J Eur Acad Dermatol Venereol 2020; 34: 239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yosipovitch G, Reaney M, Mastey V, Eckert L, Abbe A, Nelson L, et al. Peak Pruritus Numerical Rating Scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br J Dermatol 2019; 181: 761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Copley-Merriman C, Zelt S, Clark M, Gnanasakthy A. Impact of measuring patient-reported outcomes in dermatology drug development. Patient 2017; 10: 203–213. [DOI] [PubMed] [Google Scholar]
- 32.Sutaria N, Parthasarathy V, Roh YS, Choi J, Bordeaux ZA, Trinh P, et al. Itch in skin of colour: a multicentre cross-sectional study. Br J Dermatol 2021; 185: 652–654. [DOI] [PubMed] [Google Scholar]
- 33.Abuabara K, Silverberg JI, Simpson EL, Paller AS, Eichenfield LF, Bissonnette R, et al. International observational atopic dermatitis cohort to follow natural history and treatment course: TARGET-DERM AD study design and rationale. BMJ Open 2020; 10: e039928. [DOI] [PMC free article] [PubMed] [Google Scholar]