Abstract
We previously reported that the maximal production of Tumor Necrosis Factor (TNF or TNFα) in antigen-activated RBL-2H3 cells (a tumor analog of mucosal mast cells) requires Munc13–4, a regulator of exocytic fusion. In this study, we investigated the involvement of various fusion catalysts in TNF production. We observed a strong correlation between the total TNF level and TNF exocytosis in RBL-2H3 cells. RT-qPCR shows that TNFR1 (TNF receptor 1) is the sole TNFR expressed in these cells, and that its transcription is upregulated upon allergen-mediated activation. Importantly, the addition of soluble TNFR1 inhibits antigen-elicited TNF production in a dosage-dependent fashion. Likewise, TNF production is diminished in the presence of TACE (TNFα Converting Enzyme) inhibitor KP-457, which prevents the generation of soluble TNF (sTNF). Together, these findings indicate that sTNF and TNFR1 function as autocrine agent and receptor respectively at the mast cell surface to boost TNF proliferation during allergic inflammation.
Keywords: inflammation, Munc18, mast cell, degranulation, autocrine signaling, allergy
1. Introduction
Mast cells have recently emerged, besides monocytes/macrophages, NK cells, and T cells, as another key producer of tumor necrosis factor (TNF, TNFα or cachectin) [1–4], with profound physiological and pathological consequences. Mast cell-derived TNF was shown to mediate host defense against viral (e.g., herpes simplex virus) [5] and bacterial infection [6–8]. Mast cell-derived TNF was also found to promote the proliferation and activation of T cells [9, 10]. On the other hand, TNF in excess leads to a variety of inflammatory disorders [11]. For instance, reconstitution of mast cell-deficient mice indicates that mast cell-derived TNF causes allergic airway diseases (e.g., asthma) [12]. Coincidently, persistent elevation of TNF is associated with severe asthma in 15 – 30 million individuals (worldwide) that do not respond to the typical treatment of corticosteroid [13, 14]. To help this difficult-to-treat patient group, it is critical to fully understand the intricate regulation of TNF expression and exocytosis that is unique to mast cells.
Studies of model systems such as RBL-2H3 and BMMC (bone marrow-derived mast cells) have already yielded a great wealth of information regarding the life cycle of TNF in mast cells. In contrast to macrophages that do not express any basal level of TNF [15–17], mast cells pre-store TNF in secretory granules that can be rapidly released upon IgE receptor/FcεRI-dependent activation, in a process called degranulation [18]. This is initiated by FcεRI crosslinking, which triggers phospholipase Cγ-dependent production of secondary messenger inositol 1,4,5-trisphosphate, leading to a sharp rise of intracellular Ca2+ [19], a key signal for regulated fusion of secretory granules in mast cells. In parallel, FcεRI crosslinking boosts the transcription of the TNF gene [2, 20–26], exploiting NFAT (nuclear factor of activated T-cells)-dependent assembly of enhancesomes at the proximal promoter region [27, 28]. Newly synthesized TNF then undergoes constitutive secretion via Golgi-derived vesicles or recycling endosomes.
Like any exocytic event in eukaryotic cells, mast cell exocytosis (degranulation or constitutive secretion) requires membrane-anchored SNAREs (soluble N-ethylmaleimide sensitive factor attachment protein receptors) from apposing membranes (e.g., granule/vesicle vs the plasma membrane) to assemble into a 4-helical bundle (aka the trans-SNARE complex) [29]. The complete assembly of the exocytic trans-SNARE complex, which is coordinated by Munc13 proteins and catalyzed by members of the Munc18 family, causes the mixture of lipid bilayers and subsequently content release. Strikingly, knocking out Munc13–4 from RBL-2H3 mast cells resulted in a severe defect in exocytosis that was accompanied by reduced TNF level [30], demonstrating a probable correlation between exocytosis and TNF synthesis.
In this study, we used knockdown (KD) approaches to connect TNF production specifically with TNF exocytosis. We also uncovered compelling evidence showing that sTNF and TNFR1 serve as autocrine agent and receptor respectively to boost TNF proliferation in allergen-activated mast cells.
2. Materials and Methods
2.1. Cell lines and RNAi
Wild-type RBL-2H3 cells were purchased from ATCC and maintained at 37°C and 5% CO2 in DMEM complete medium (Gibco) that contained 4.5 g/L D-Glucose, 110 mg/L sodium pyruvate, 1x Glutamax (Gibco), and 10% FBS (Gibco; heat inactivated), without any antibiotics. Stable Munc18aKD and Munc18bKD cells were obtained from Sugita’s lab and propagated according to the published protocol [31]. To transiently inhibit VAMP8 expression in RBL-2H3 cells, siGENOME siRNA oligos specific for VAMP8 (Dharmacon) were used to transfect sub-confluent RBL-2H3 cells as described (Adhikari et al., submitted).
2.2. Secretion assays
RBL-2H3 cells and derivatives growing in 6-well plates were sensitized for 3 h with 0.5 μg/mL mouse anti –TNP (trinitrophenol phosphate) IgE (BD Pharmingen) and stimulated with 25 ng/mL of TNP(26)-BSA (Santa Cruz). β-hexosaminidase activities and TNF levels in the supernatant and the cell lysates were quantified as previous described [30]. Wherever indicated, sTNFR1 (Genscript) or KP-457 (Adooq Bioscience) was added along with TNP(26)-BSA during stimulation, and MAP kinase inhibitors (MedChemExpress) including Doramapimod/BIRB 796, AX-15836, Ravoxertinib/GDC-0994, and JNK-IN-8 were added 30 min ahead of TNP(26)-BSA addition.
2.3. Reverse Transcription and PCRs
RBL-2H3 cells (~ 40% confluent) cultured in a 24-well plate were treated with 250 μL of 0.25% Trypsin-EDTA (Gibco) that was subsequently neutralized with 750 μL of complete medium. About 105 cells were collected for RT-qPCR (quantitative PCR) using TaqMan Gene Expression Cells-to-CT Kit (ThermoFisher Scientific) according to the manufacturer’s instructions. Fluorescently labeled, target-specific probes (β-actin: Rn00667869_m1; TNFR1: Rn01492348_m1; and TNFR2: Rn00709830_m1) were purchased from Thermofisher Scientific. The Cq values of TNFRs were standardized against that of β-actin utilizing the Bio-Rad CFX Maestro software and then the relative levels of mRNA expression were determined. For electrophoresis, TNFR1 was amplified from cDNA (prepared with the same TaqMan Gene Expression Cells-to-CT Kit described earlier) using 5’- AAAGAGGTGGAGGGTGAAG −3’ and 5’-GCAGGTTCATGTCGCAAAG-3’ as forward and reverse primers, whereas TNFR2 was amplified using 5’- ACACCCTACAAGCCAGAAC −3’ and 5’-TCCTAACATCAGCAGACCC-3’ as forward and reverse primers. PCR was conducted in a 20 μL mixture containing 1 x One Taq Master Mix (NEB) and 200 nM of primers. Where specified, 1 μL of rat universal cDNA (BioChain) was used as positive control.
3. Results and Discussion
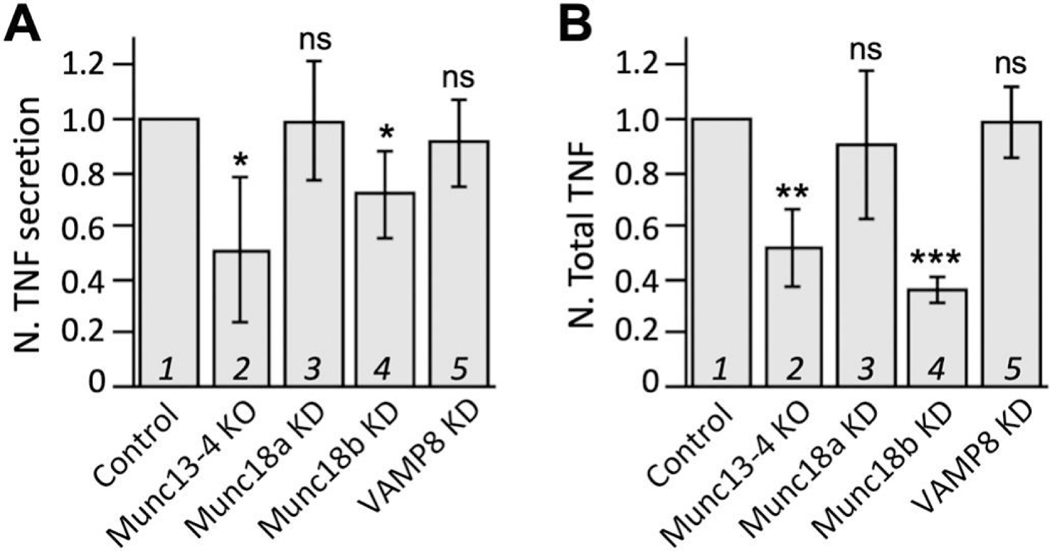
We previously showed that the maximal production of TNF in antigen-activated RBL-2H3 cells requires Munc13–4 [30], a positive regulator of exocytic fusion. This, however, does not prove that exocytosis is connected to TNF production. Munc13–4 could have an unexpected moonlight function in the signaling pathways that promote TNF upregulation. To further investigate the link between exocytosis and TNF production, we decided to knock down each of the three fusion catalysts known for mast cell exocytosis, VAMP8 [32, 33], Munc18a [31], and Munc18b [31]. Like Munc13–4, Munc18b depletion, which caused a reduction of allergen-triggered exocytosis of TNF and other mediators (e.g., β-hexosaminidase, histamine, and serotonin), also diminished the total level of TNF (Fig. 1, compare lanes 1 and 4). In contrast, RBL-2H3 cells depleted with either VAMP8 or Munc18a did not exhibit less TNF protein expression (Fig. 1B, compare lanes 3 and 5 to lane 1). Notably, these cells showed reduced mast cell exocytosis except for TNF (Fig. 1A, compare lanes 3 and 5 to lane 1). These findings argue that an autocrine loop exists in RBL-2H3 cells that couples TNF exocytosis to TNF production.
Figure 1. TNF exocytosis correlates with TNF level.
(A) TNP-BSA/IgE (anti-TNP)-triggered TNF exocytosis from Munc13–4KO cells, stable Munc18aKD cells, stable Munc18bKD cells, or VAMP8KD cells (via siRNA) was measured 1 h after stimulation, and the values were normalized against that of their respective control cells that were treated in the same manner. (B) Likewise, total TNF protein levels were calculated for activated KO or KD cells and normalized against that of the control cells. Averages and standard deviations from at least 4 biological repeats are presented. * p < 0.05; ** p < 0.01; *** p < 0.001
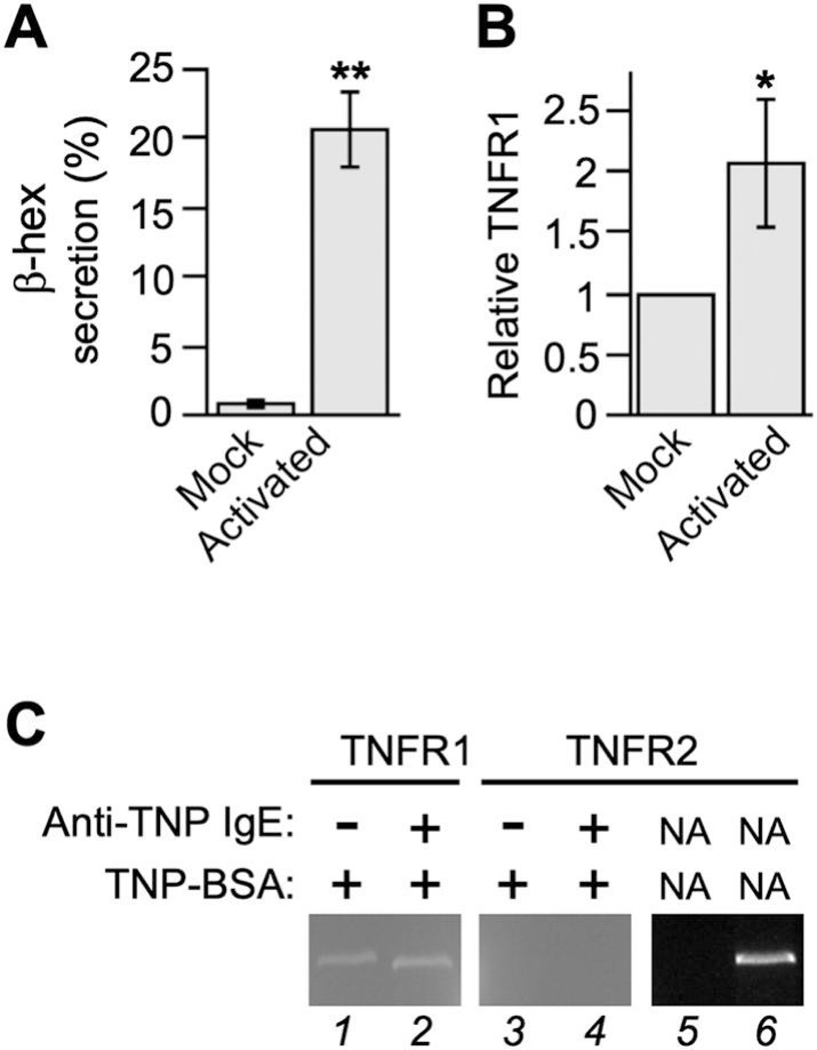
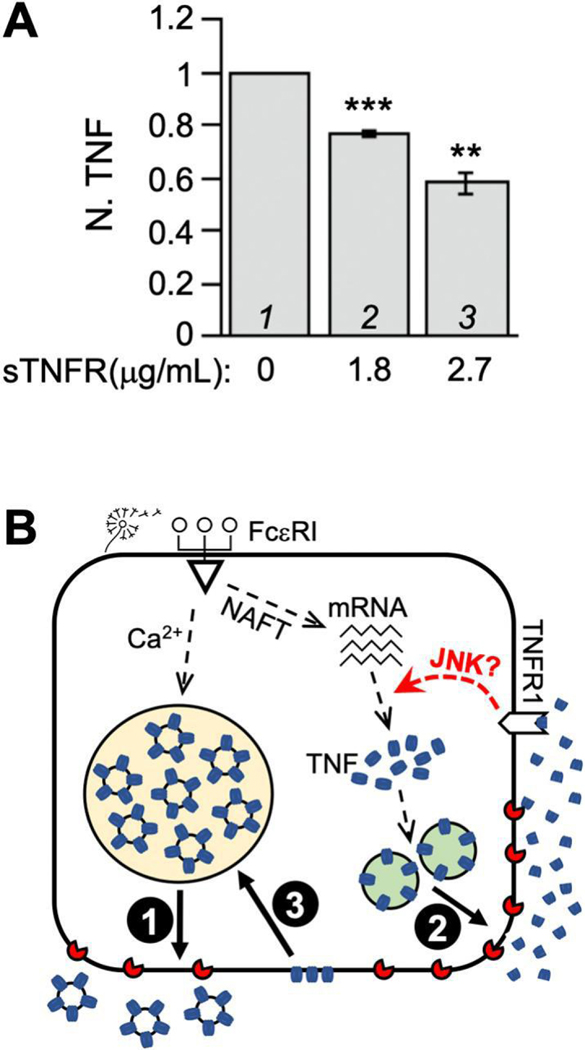
To determine which of the two TNFRs (there are only two mammalian TNFR genes) serves as the autocrine receptor, we exploited RT-qPCR to monitor the mRNA level of TNFR1 and TNFR2 in RBL-2H3 cells. TNFR1 expression was detected in both resting cells (virtually no detectable β-hexosaminidase secretion) and activated cells (20% β-hexosaminidase secretion), but its mRNA level in activated cells was twice as much as that in resting cells (Fig. 2A and B). In comparison, Cook et al [34] reported a 1 – 1.7 fold increase in TNFR1 expression in primary human conjunctival epithelial cells upon the addition of supernatants from allergen–activated conjunctival mast cells or pure IFNγ. TNFR2, on the other hand, was not detected at all in either resting cells or activated cells (Fig. 2C). To assess the functional involvement of TNFR1 in TNF production, we incubated RBL-2H3 cells with sTNFR1 along with TNP-BSA after anti-TNP IgE-dependent sensitization. The purposes of sTNFR1 are to sequester any secreted TNF, preventing it from binding to the TNFR1 on the cell surface, and to interfere with the oligomerization/function of plasma membrane-anchored TNFR1 [35]. We noticed that sTNFR1 inhibits the overall level of TNF in a concentration dependent fashion (Fig. 3A), suggesting that the interaction between secreted TNF and cell surface TNFR1 is responsible for the augmentation of TNF protein levels in allergen-activated RBL-2H3 cells. Because according to our model (Fig. 3B), TNF upregulation in allergen-activated mast cells exploits both FcεRI-mediated signaling and TNFR1-mediated signaling, the disruption of the autocrine loop alone (by sTNFR1) is expected to reduce but not eliminate TNF production, which is consistent with what we had observed.
Figure 2. TNFR1 is expressed and upregulated in activated RBL-2H3 cells.
RBL-2H3 cells sensitized with anti-TNP IgE or buffer were incubated with TNP-BSA for 1 h. (A) Mast cell degranulation was measured and expressed as the percentage of β-hexosaminidase in the supernatant. Meanwhile, lysates from activated (with IgE) or mock treated (with buffer) cells were subject to reverse transcription followed by either qPCR to quantify the relative amount of TNFR mRNA (B) or regular PCR to visually compare the expression of TNFR1 and TNFR2 on 2% agarose gel (lane 1 to 4). In lanes 5 and 6, one μL of H2O and rat universal cDNA were used respectively as template.
Figure 3. TNF feedback loop is mediated by TNFR1.
(A) RBL-2H3 cells sensitized with anti-TNP IgE were challenge with TNP-BSA along with specified amounts of sTNFR1 or buffer (PBS). At 1 h time point, total TNF protein level in all three conditions were measured using ELISA. The total TNF in activated cells subtracted by that in resting cells was set at 1 (lane 1) and used to calculate the relative values of TNF production in the presence of sTNFR1 (lanes 2 and 3). Averages and standard deviations from 3 biological repeats are presented. ** p < 0.01; *** p < 0.001. (B) Model of TNF autocrine loop. Mast cells elicited by allergens undergo calcium dependent exocytosis of full length, membrane-bound TNF (depicted in blue) that is prestored in secretory apparatuses such as MVB (1). Allergens also boost de novo synthesis of TNF, which is deposited into ER (omitted for simplicity) and subsequently delivered onto the plasma membrane via the classical secretory pathway (2). Upon TACE/Adam17 (depicted in red)-dependent cleavage, soluble TNF is released to the extracellular space, where it binds TNFR1 to promote additional TNF production. TNF proteins that have escaped proteolysis can be internalized and delivered to MVB for storage (3).
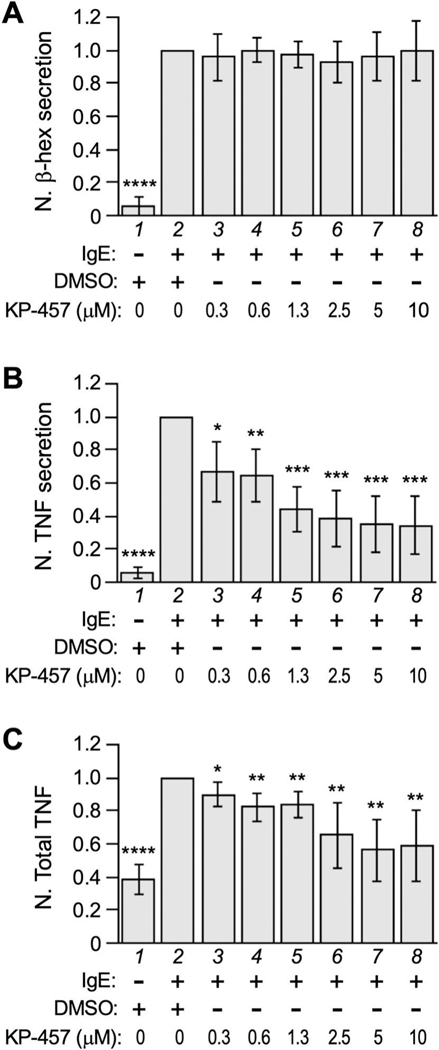
We then turned our attention to the autocrine agent. Although the full-length TNF (mTNF) prestored in multivesicular body (MVB) can be released as the membrane-bound form [36], newly synthesized TNF is delivered to the plasma membrane where it is released as the soluble form (sTNF) via TACE/ADAM17-dependent truncation [37, 38]. To discern whether mTNF or sTNF serves as the autocrine agent, we asked if the inhibition of TNF truncation (and thus release) would affect TNF production by adding KP-457, a specific inhibitor for ADAM17 [39], in our secretion assay. KP-457 is expected to prevent ADAM17-mediated processing of mTNF at the cell surface, but it could also permeate cells to impede mTNF processing in intracellular compartments (if any) [40, 41]. Either way, it would lead to increased mTNF at the cell surface but decreased sTNF in the supernatant. At concentrations between 0.3 μM and 10 μM, KP-457 did not seem to affect the exocytosis of β-hexosaminidase (Fig. 4A), but instead significantly reduced the amount of sTNF protein released into the supernatant (Fig. 4B). Furthermore, KP-457 inhibited the overall level of TNF in allergen-triggered RBL-2H3 cells (Fig. 4C). Together, these findings suggest that it is the specific release of TNF (not the other mast cell mediators) that led to the upregulation of TNF expression. Our study also suggests that any mTNF accumulated on the cell membrane, as a result of ADAM17 inhibition, does not play a role in TNF autoregulation.
Figure 4. TACE inhibitor KP-457 reduces TNF proliferation.
RBL-2H3 cells sensitized with anti-TNP IgE were challenge with TNP-BSA along with specified amounts of KP-457 or solvent (DMSO). At 1 h time point, β-hexosaminidase secretion (A), released TNF (recovered from the supernatant) (B) and total TNF protein level (C) were measured and the values were normalized against that of the solvent control (lane 2). Averages and standard deviations from at least 5 biological repeats are presented. * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001
Although such feedback signaling is conceivable for any TNF producing cell that also expresses TNF receptors (e.g., monocytes and macrophages), the intracellular transduction underlying TNF auto regulation has never been experimentally elucidated or verified, except for the activation of transcription factor NF-κB [42–44]. However, the involvement of NF-κB in TNF expression is unlikely, despite the fact that NF-κB promotes the transcription of many cytokines. This is largely because the putative NF-κB binding sites in TNF promoter do not recognize NF-κB biochemically, or respond to it functionally [27, 45]. We propose that sTNF/TNFR1 interaction at mast cell surface promotes the post-transcriptional regulation of TNF expression via MAP kinase pathways (Fig. 3B), based on the following observations: i) many conventional MAP kinases are activated in TNF triggered cells [46, 47]; ii) MAPK pathways have been shown to target various effector proteins to regulate TNF mRNA stability or translation [48–52]; and iii) inhibiting TNF/TNFR signaling in RBL-2H3 diminishes TNF protein level without reducing TNF mRNA level [30].
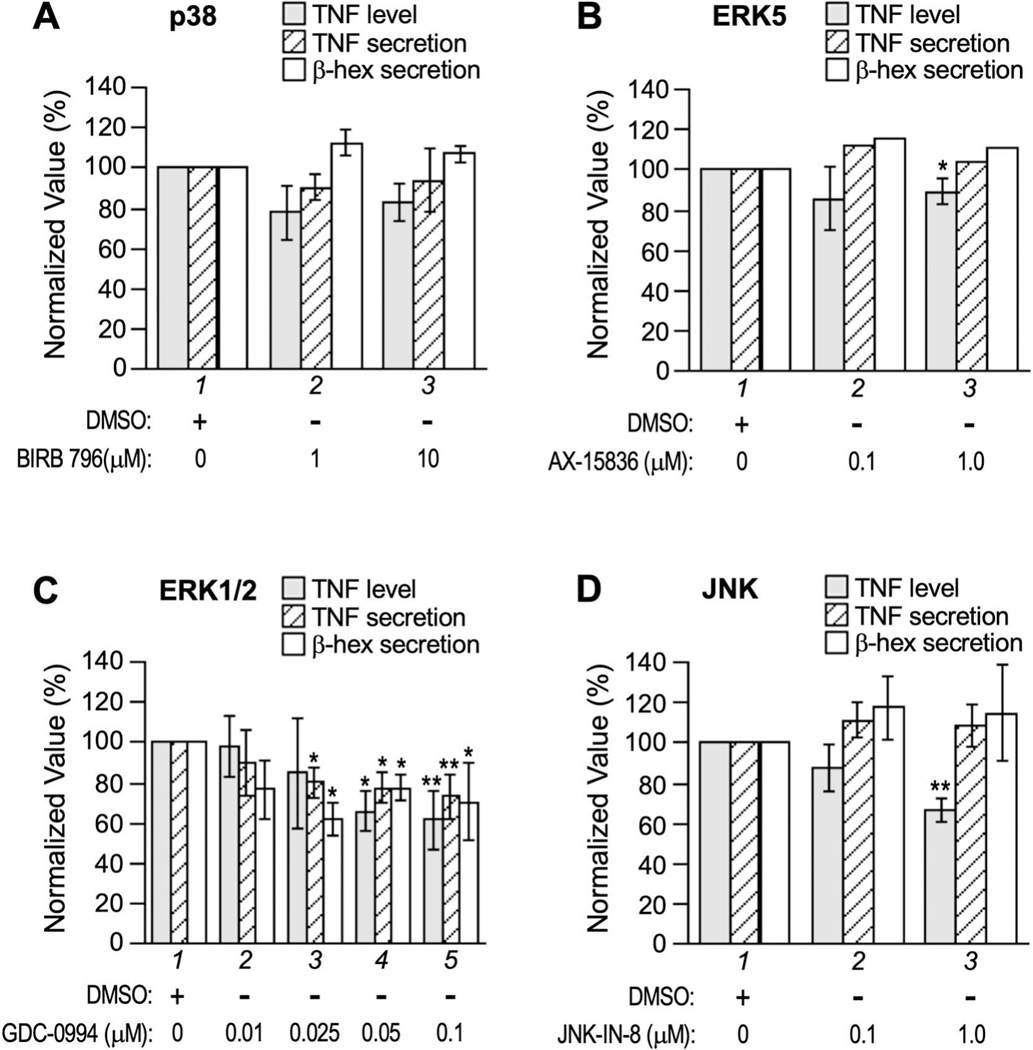
To assess the involvement of MAP kinases in TNF production in allergen-triggered RBL-2H3 cells, we exploited pharmaceuticals that selectively target each of the four conventional MAP kinase families including ERK1/2, p38, JNK, and ERK5 [53, 54]. Under our experimental conditions, p38 inhibitor Doramapimod/BIRB 796 (targeting α, β, γ, and δ isoforms) did not seem to affect exocytosis or significantly reduce TNF proliferation (Fig. 5A), whereas ERK5 inhibitor AX-15836 caused a minor (10%) but statistically significant reduction in total TNF level (Fig. 5B). ERK1/2 inhibitor Ravoxertinib/GDC-0994, on the other hand, inhibited both mast cell exocytosis (judging by the secretion of β-hexosaminidase and TNF) and the overall level of TNF in a seemingly correlated, dose dependent manner (Fig. 5C). Intriguingly, JNK-IN-8 (targeting JNK1, 2, 3) diminished TNF level drastically without impacting TNF or β-hexosaminidase secretion (Fig. 5D), suggesting JNKs specifically regulate the signaling pathway(s) for TNF upregulation in RBL-2H3 cells. Considering the regulation of TNF biosynthesis is both cell-type specific and stimulus-specific, future studies of the autoregulatory loop could lead to the development of powerful new therapeutics to treat human maladies caused by excessive mast cell TNF.
Figure 5. Effect of MAPK inhibitors on exocytosis and TNF production.
Where indicated, MAP kinase inhibitors including (A) Doramapimod/BIRB 796 (IC50 of 38 nM for p38α, 65 nM for p38β, 200 nM for p38γ, and 520 nM for p38δ), (B) AX-15836 (IC50 of 8 nM for ERK5), (C) Ravoxertinib/GDC-0994 (IC50 of 6.1 nM and 3.1 nM for ERK1 and ERK2 respectively), and (D) JNK-IN-8 (IC50 of 4.7 nM, 18.7 nM, and 1 nM for JNK1, JNK2, and JNK3, respectively) were added to sensitized RBL-2H3 cells 30 min before activation by TNP-BSA. Total TNF, TNF secretion (% of total TNF in the supernatant), and β-hexosaminidase secretion (% of total β-hexosaminidase activity in the supernatant) were measured and normalized against that of the mock treatment (DMSO). Averages and standard deviations from 3 to 5 biological repeats are presented. * p < 0.05; ** p < 0.01
Highlights.
TNF exocytosis in allergen-activated mast cells is coupled to increased TNF production
Soluble TNF (but not the full-length, membrane bound TNF) acts as an autocrine agent in TNF upregulation
TNF Receptor 1 (but not TNF Receptor 2) acts as an autocrine receptor in TNF upregulation
c-Jun N-terminal kinase signaling is likely involved in TNF upregulation
Acknowledgements
This work was supported by the National Institute of Allergy and Infectious Diseases Grant R15AI133430 and R03AI169047 to H.X., and by the Mississippi INBRE, which was funded by an Institutional Development Award from the National Institute of General Medical Sciences under grant no. P20GM103476. We thank Jonathan Lindner at MS-INBRE Imaging Facility for excellent technical support.
Footnotes
Authorship
TA and PA conducted experiments, performed data analysis, and edited the manuscript. HX oversaw the entire project, conducted experiments, performed data analysis, and wrote the manuscript.
Conflict of Interest Disclosure
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bischoff SC, et al. , Mast cells are an important cellular source of tumour necrosis factor alpha in human intestinal tissue. Gut, 1999. 44(5): p. 643–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dery RE, et al. , Redundancy or cell-type-specific regulation? Tumour necrosis factor in alveolar macrophages and mast cells. Immunology, 2000. 99(3): p. 427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Izadi D, et al. , Identification of TNFR2 and IL-33 as therapeutic targets in localized fibrosis. Sci Adv, 2019. 5(12): p. eaay0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulz R and Heusch G, Tumor necrosis factor-alpha and its receptors 1 and 2: Yin and Yang in myocardial infarction? Circulation, 2009. 119(10): p. 1355–7. [DOI] [PubMed] [Google Scholar]
- 5.Aoki R, et al. , Mast cells play a key role in host defense against herpes simplex virus infection through TNF-alpha and IL-6 production. J Invest Dermatol, 2013. 133(9): p. 2170–9. [DOI] [PubMed] [Google Scholar]
- 6.Echtenacher B, Mannel DN, and Hultner L, Critical protective role of mast cells in a model of acute septic peritonitis. Nature, 1996. 381(6577): p. 75–7. [DOI] [PubMed] [Google Scholar]
- 7.Liu C, et al. , Tumor Necrosis Factor-alpha Is Required for Mast Cell-Mediated Host Immunity Against Cutaneous Staphylococcus aureus Infection. J Infect Dis, 2018. 218(1): p. 64–74. [DOI] [PubMed] [Google Scholar]
- 8.Malaviya R, et al. , Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature, 1996. 381(6577): p. 77–80. [DOI] [PubMed] [Google Scholar]
- 9.Nakae S, et al. , Mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J Immunol, 2006. 176(4): p. 2238–48. [DOI] [PubMed] [Google Scholar]
- 10.Nakae S, et al. , Mast cells enhance T cell activation: Importance of mast cell-derived TNF. Proc Natl Acad Sci U S A, 2005. 102(18): p. 6467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atretkhany KN, et al. , Distinct modes of TNF signaling through its two receptors in health and disease. J Leukoc Biol, 2020. 107(6): p. 893–905. [DOI] [PubMed] [Google Scholar]
- 12.Reuter S, et al. , Mast cell-derived tumour necrosis factor is essential for allergic airway disease. Eur Respir J, 2008. 31(4): p. 773–82. [DOI] [PubMed] [Google Scholar]
- 13.Brightling C, Berry M, and Amrani Y, Targeting TNF-alpha: a novel therapeutic approach for asthma. J Allergy Clin Immunol, 2008. 121(1): p. 5–10; quiz 11–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russo C and Polosa R, TNF-alpha as a promising therapeutic target in chronic asthma: a lesson from rheumatoid arthritis. Clin Sci (Lond), 2005. 109(2): p. 135–42. [DOI] [PubMed] [Google Scholar]
- 15.Beutler B, Regulation of cachectin biosynthesis occurs at multiple levels. Prog Clin Biol Res, 1990. 349: p. 229–40. [PubMed] [Google Scholar]
- 16.Han J, Thompson P, and Beutler B, Dexamethasone and pentoxifylline inhibit endotoxin-induced cachectin/tumor necrosis factor synthesis at separate points in the signaling pathway. J Exp Med, 1990. 172(1): p. 391–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olszewski MB, et al. , Efficient sorting of TNF-alpha to rodent mast cell granules is dependent on N-linked glycosylation. Eur J Immunol, 2006. 36(4): p. 997–1008. [DOI] [PubMed] [Google Scholar]
- 18.Mukai K, et al. , Mast cells as sources of cytokines, chemokines, and growth factors. Immunol Rev, 2018. 282(1): p. 121–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Draber P, et al. , Signal transduction and chemotaxis in mast cells. Eur J Pharmacol, 2016. 778: p. 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohno I, et al. , Gene expression and production of tumour necrosis factor by a rat basophilic leukaemia cell line (RBL-2H3) with IgE receptor triggering. Immunology, 1990. 70(1): p. 88–93. [PMC free article] [PubMed] [Google Scholar]
- 21.Gon Y, Nunomura S, and Ra C, Common and distinct signalling cascades in the production of tumour necrosis factor-alpha and interleukin-13 induced by lipopolysaccharide in RBL-2H3 cells. Clin Exp Allergy, 2005. 35(5): p. 635–42. [DOI] [PubMed] [Google Scholar]
- 22.Hochdorfer T, et al. , LPS-induced production of TNF-alpha and IL-6 in mast cells is dependent on p38 but independent of TTP. Cell Signal, 2013. 25(6): p. 1339–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Supajatura V, et al. , Differential responses of mast cell Toll-like receptors 2 and 4 in allergy and innate immunity. J Clin Invest, 2002. 109(10): p. 1351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCurdy JD, et al. , Cutting edge: distinct Toll-like receptor 2 activators selectively induce different classes of mediator production from human mast cells. J Immunol, 2003. 170(4): p. 1625–9. [DOI] [PubMed] [Google Scholar]
- 25.Varadaradjalou S, et al. , Toll-like receptor 2 (TLR2) and TLR4 differentially activate human mast cells. Eur J Immunol, 2003. 33(4): p. 899–906. [DOI] [PubMed] [Google Scholar]
- 26.Ohkawara Y, et al. , Human lung mast cells and pulmonary macrophages produce tumor necrosis factor-alpha in sensitized lung tissue after IgE receptor triggering. Am J Respir Cell Mol Biol, 1992. 7(4): p. 385–92. [DOI] [PubMed] [Google Scholar]
- 27.Falvo JV, Tsytsykova AV, and Goldfeld AE, Transcriptional control of the TNF gene. Curr Dir Autoimmun, 2010. 11: p. 27–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein M, et al. , Specific and redundant roles for NFAT transcription factors in the expression of mast cell-derived cytokines. J Immunol, 2006. 177(10): p. 6667–74. [DOI] [PubMed] [Google Scholar]
- 29.Lorentz A, et al. , The SNARE Machinery in Mast Cell Secretion. Front Immunol, 2012. 3: p. 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ayo TE, et al. , TNF Production in Activated RBL-2H3 Cells Requires Munc13–4. Inflammation, 2020. 43(2): p. 744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bin NR, et al. , Crucial role of the hydrophobic pocket region of Munc18 protein in mast cell degranulation. Proc Natl Acad Sci U S A, 2013. 110(12): p. 4610–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tiwari N, et al. , VAMP-8 segregates mast cell-preformed mediator exocytosis from cytokine trafficking pathways. Blood, 2008. 111(7): p. 3665–74. [DOI] [PubMed] [Google Scholar]
- 33.Puri N and Roche PA, Mast cells possess distinct secretory granule subsets whose exocytosis is regulated by different SNARE isoforms. Proc Natl Acad Sci U S A, 2008. 105(7): p. 2580–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cook EB, et al. , Regulation of the receptor for TNFalpha, TNFR1, in human conjunctival epithelial cells. Invest Ophthalmol Vis Sci, 2008. 49(9): p. 3992–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karathanasis C, et al. , Single-molecule imaging reveals the oligomeric state of functional TNFalpha-induced plasma membrane TNFR1 clusters in cells. Sci Signal, 2020. 13(614). [DOI] [PubMed] [Google Scholar]
- 36.Zhang HG, et al. , A membrane form of TNF-alpha presented by exosomes delays T cell activation-induced cell death. J Immunol, 2006. 176(12): p. 7385–93. [DOI] [PubMed] [Google Scholar]
- 37.Sommer A, et al. , Extracellular sphingomyelinase activity impairs TNF-alpha-induced endothelial cell death via ADAM17 activation and TNF receptor 1 shedding. Oncotarget, 2017. 8(42): p. 72584–72596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohan MJ, et al. , The tumor necrosis factor-alpha converting enzyme (TACE): a unique metalloproteinase with highly defined substrate selectivity. Biochemistry, 2002. 41(30): p. 9462–9. [DOI] [PubMed] [Google Scholar]
- 39.Hirata S, et al. , Selective Inhibition of ADAM17 Efficiently Mediates Glycoprotein Ibalpha Retention During Ex Vivo Generation of Human Induced Pluripotent Stem Cell-Derived Platelets. Stem Cells Transl Med, 2017. 6(3): p. 720–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Black RA, et al. , A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature, 1997. 385(6618): p. 729–33. [DOI] [PubMed] [Google Scholar]
- 41.Robertshaw HJ and Brennan FM, Release of tumour necrosis factor alpha (TNFalpha) by TNFalpha cleaving enzyme (TACE) in response to septic stimuli in vitro. Br J Anaesth, 2005. 94(2): p. 222–8. [DOI] [PubMed] [Google Scholar]
- 42.Verma N, et al. , Silencing of TNF-alpha receptors coordinately suppresses TNF-alpha expression through NF-kappaB activation blockade in THP-1 macrophage. FEBS Lett, 2009. 583(17): p. 2968–74. [DOI] [PubMed] [Google Scholar]
- 43.Xaus J, et al. , LPS induces apoptosis in macrophages mostly through the autocrine production of TNF-alpha. Blood, 2000. 95(12): p. 3823–31. [PubMed] [Google Scholar]
- 44.Gane JM, Stockley RA, and Sapey E, TNF-alpha Autocrine Feedback Loops in Human Monocytes: The Pro- and Anti-Inflammatory Roles of the TNF-alpha Receptors Support the Concept of Selective TNFR1 Blockade In Vivo. J Immunol Res, 2016. 2016: p. 1079851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ishizuka T, et al. , Mast cell tumor necrosis factor alpha production is regulated by MEK kinases. Proc Natl Acad Sci U S A, 1997. 94(12): p. 6358–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sabio G and Davis RJ, TNF and MAP kinase signalling pathways. Semin Immunol, 2014. 26(3): p. 237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loupasakis K, et al. , Tumor Necrosis Factor dynamically regulates the mRNA stabilome in rheumatoid arthritis fibroblast-like synoviocytes. PLoS One, 2017. 12(7): p. e0179762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pashenkov MV, et al. , The Role of the p38-MNK-eIF4E Signaling Axis in TNF Production Downstream of the NOD1 Receptor. J Immunol, 2017. 198(4): p. 1638–1648. [DOI] [PubMed] [Google Scholar]
- 49.Buxade M, et al. , The Mnks are novel components in the control of TNF alpha biosynthesis and phosphorylate and regulate hnRNP A1. Immunity, 2005. 23(2): p. 177–89. [DOI] [PubMed] [Google Scholar]
- 50.Carballo E, Lai WS, and Blackshear PJ, Feedback inhibition of macrophage tumor necrosis factor-alpha production by tristetraprolin. Science, 1998. 281(5379): p. 1001–5. [DOI] [PubMed] [Google Scholar]
- 51.Gonzalez-Teran B, et al. , Eukaryotic elongation factor 2 controls TNF-alpha translation in LPS-induced hepatitis. J Clin Invest, 2013. 123(1): p. 164–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gais P, et al. , TRIF signaling stimulates translation of TNF-alpha mRNA via prolonged activation of MK2. J Immunol, 2010. 184(10): p. 5842–8. [DOI] [PubMed] [Google Scholar]
- 53.Cargnello M and Roux PP, Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev, 2011. 75(1): p. 50–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morrison DK, MAP kinase pathways. Cold Spring Harb Perspect Biol, 2012. 4(11). [DOI] [PMC free article] [PubMed] [Google Scholar]