Abstract
Spaceflight associated neuro-ocular syndrome (SANS) refers to a distinct constellation of ocular, neurological and neuroimaging findings observed in astronauts during and following long duration spaceflight. These ocular findings, to include optic disc oedema, posterior globe flattening, chorioretinal folds and hyperopic shifts, were first described by NASA in 2011. SANS is a potential risk to astronaut health and will likely require mitigation prior to planetary travel with prolonged exposures to microgravity. While the exact pathogenesis of SANS is not completely understood, several hypotheses have been proposed to explain this neuro-ocular phenomenon. In this paper, we briefly discuss the current hypotheses and contributing factors underlying SANS pathophysiology as well as analogues used to study SANS on Earth. We also review emerging potential countermeasures for SANS including lower body negative pressure, nutritional supplementation and translaminar pressure gradient modulation. Ongoing investigation within these fields will likely be instrumental in preparing and protecting astronaut vision for future spaceflight missions including deep space exploration.
Keywords: optic nerve, imaging, vision
Introduction
When astronauts are exposed to the microgravity environment, multiple physiological changes have been observed including bone density loss, skeletal muscle atrophy, and vision changes.1 2 Spaceflight associated neuro-ocular syndrome (SANS) refers to the unique neuro-ophthalmic findings that have been recently described in astronauts during and after long-duration spaceflight (LDSF). The imaging and clinical findings of SANS include optic disc oedema (ODE), chorioretinal folds, hyperopic refractive shift, posterior globe flattening, and total retinal and retina nerve fibre layer (RNFL) thickening.3
In 2011, Mader et al. first reported the findings of SANS including ODE, choroidal folds, globe flattening, cotton wool spots, and hyperopic shifts in astronauts after LDSF.4 This initial description of what is known today as SANS was subsequently recognised in additional astronauts after LDSF4 (figure 1). After returning to Earth, ODE from SANS may persist for months before complete resolution.5 In addition, the refractive error, choroidal folds, and posterior globe flattening in SANS may persist for years after returning to Earth and may be permanent in some astronauts.3 5 6
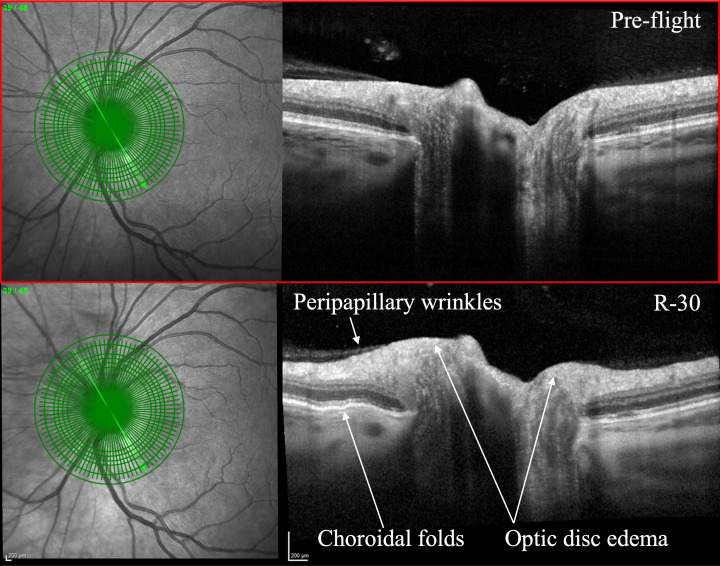
Figure 1.
Pre-flight (top) and 30 days prior to return (R-30, bottom) comparison on optical coherence tomography with R-30 showcasing peripapillary wrinkles, choroidal folds, and optic disc oedema. Courtesy of NASA.
SANS has been assigned a relatively high ‘Likelihood and Consequence’ rating by NASA, indicating potential risk to mission performance and astronaut health for LDSF, especially during extended-duration (>1 year) interplanetary missions. Thus, mitigation strategies and further understanding of SANS are high priorities for future exploratory missions.7 In this article, we review the different hypotheses for the underlying pathogenesis of this unique neuro-ophthalmic phenomenon. We also discuss terrestrial analogues that are being used to further understand SANS, as well as emerging potential countermeasures that are being developed to mitigate the effects of SANS for future spaceflight. Continuation of research in these fields will be critical in reducing the risk of SANS for future spaceflight missions.
Proposed pathogenesis of SANS
Intracranial pressure
The underlying pathogenesis of SANS is not completely understood. However, several hypotheses have been proposed to explain the pathophysiology of this neuro-ocular phenomenon. Initially termed ‘Visual Impairment and Intracranial Pressure’ (VIIP) syndrome, elevated intracranial pressure (ICP) was originally hypothesised to be the primary cause of ODE and other SANS findings.3 8 In the initial 2011 NASA report on seven astronauts following LDSF, five astronauts were documented to have a spectrum of ODE from grade 0 to grade 3 on the Frisén scale.4 In the weightless environment of microgravity, there is a loss of hydrostatic pressure within the human body.9 This peculiar physiological phenomenon causes a cephalad fluid shift which is thought to result in venous stasis within the head and neck.4 Parabolic flight and head-down tilt bed rest studies, which serve as established terrestrial analogues, have demonstrated an increased internal jugular volume, further supporting venous congestion during spaceflight.10 11 This congestion may inhibit normal cerebrospinal fluid (CSF) drainage and cause cerebral venous congestion, both of which may lead to elevated ICP.4 Post-LDSF lumbar puncture (LP) opening pressures in several astronauts with SANS findings, including ODE, have demonstrated mildly elevated ICP values, including 28 cm H2O and 28.5 cm H2O following 12 days and 57 days, respectively, after returning to Earth.4 However, other astronauts with SANS have had LP opening pressures that were normal or borderline, including 21 cm H2O and 22 cm H2O following 19 days and 66 days, respectively, after returning to Earth.4 As an invasive procedure, LPs have not been performed during spaceflight.12 Therefore, the true magnitude of the possible elevation in ICP has not been documented during spaceflight and remains an important knowledge gap.
Apart from ODE, astronauts do not display typical symptoms and signs observed in idiopathic intracranial hypertension (IIH), a terrestrial disease caused by increased ICP. Patients with IIH usually complain of headache, pulsatile tinnitus, blurred vision, diplopia (from non-localising sixth nerve palsy), or transient visual obscurations,13 whereas SANS cases often report difficulty with near vison, and they typically have no additional complaints.4 The clinical presentation of ODE also differs between SANS astronauts and patients with IIH. Most patients with IIH present with bilateral, symmetric ODE, whereas in the first cohort of SANS astronauts, three out of the five astronauts with optic ODE had an asymmetric or unilateral presentation.3 4 These findings suggested that SANS may not be solely due to an elevated ICP, thus the name ‘VIIP’ was subsequently changed to ‘SANS’ to encompass a broader scope for SANS pathogenesis3 14 Other factors including choroidal swelling,15 altered glymphatic drainage at the optic nerve head,16 variations in optic nerve sheath and brain elasticity,17 18 high carbon dioxide levels19, and defects in the vitamin B12–dependent one-carbon transfer pathways20 may also play a role.
Compartmentation of the orbital ONS
As noted above, although ODE in most astronauts has been largely symmetrical following LDSF, a small number of astronauts have been documented with variable degrees of post-mission ODE asymmetry. It is hypothesised that this ODE asymmetry may result from variable increases in CSF pressure within the perioptic subarachnoid space (SAS) caused by a microgravity induced orbital SAS compartmentation.4 One report documented asymmetric ODE and globe flattening for 6 months following LDSF with LP opening pressures of 22 and 16 cm H2O performed 7 days and 12 months post-flight, respectively.5 Although there is thought to be homogeneous pressure and chemical composition within the SAS of the brain and orbit, these findings suggest that an asymmetric increase in CSF pressure within the orbital optic nerve sheath may occur in some astronauts. The tightly confined, densely septated cul-de-sac-like anatomical connection between the intracranial SAS and the SAS surrounding the ON may create a fragile flow equilibrium that may be impacted by the cephalad fluid shifts of LDSF.4 This may lead to an unequal sequestration of CSF surrounding the orbital portion of the ON with or without elevated ICP.
Cytotoxic oedema
It has been hypothesised that there is an inflammatory or oxidative stress pathway mechanism in SANS.21 Galdamez et al. suggest that terrestrial cytotoxic oedema caused by venous stasis may be a contributing mechanism for the optic disc oedema observed in SANS. Venous stasis can lead to a disruption of metabolic activity and cellular nutrient delivery, leading to impaired ATP generation. This ATP depletion can lead to an inability to maintain the low intracellular Na+ composition due to reduced Na+/K+ ATPase activity, leading to axonal oedema.21 22 In addition, a molecular study involving mice during spaceflight observed an upregulation of oxidative and stress response genes in the retina, as well as β-amyloid in the nerve fibres of the optic nerve.23 24 An increase in oxidative free radicals can also inhibit Na+/K+ ATPase, as well as increase expression for proteolytic enzyme matrix metalloprotease-9 (MMP-9), a key enzyme in the disruption of tight junctions and degradation of the basement membrane.21 25 The optic nerve head contains unmyelinated nerve fibres that pass through the lamina cribrosa which requires a relatively high ATP demand.21 26 Therefore, suspected venous stasis during spaceflight may affect this area of high energy demand and subsequently lead to local optic disc oedema seen in SANS.
Ocular glymphatic system
The glymphatic system is a recently discovered brain-wide clearance system in which CSF enters the brain via periarterial channels and exchanges with interstitial fluid within the parenchyma.27 28 Under microgravity conditions, an elevation of orbital optic nerve sheath pressure may occur due to a rise in ICP and/or sequestration of CSF within the orbital SAS caused by long-standing microgravity fluid shifts and variations in optic nerve sheath anatomy and compliance.29–33 This may lead to a reduction or reversal of the normally posteriorly directed translamina cribrosa pressure difference. Wostyn et al. proposed a hypothetical framework by which ODE in astronauts may at least partly result from the forcing of higher pressure perioptic cerebrospinal fluid into the optic nerve and optic disc along perivascular spaces surrounding the central retinal artery.33 Also, glymphatic outflow from the eye into the optic nerve may be impeded under prolonged microgravity conditions leading to fluid stasis within the prelaminar region of the optic nerve head.16
Choroidal changes during spaceflight
With advancing OCT imaging technology, it has been confirmed that choroidal expansion occurs immediately on reaching microgravity9 34 and continues during LDSF.35 Peripapillary choroidal expansion was even found to persist for between 30 and 90 days following LDSF.35 As a possible explanation, Wostyn et al. proposed that besides the rapid pooling of blood in the choroid that occurs on entry into microgravity, there may be a chronic accumulation of choroidal interstitial fluid in the peripapillary region during LDSF.36 This interstitial fluid may take many weeks to exit the eye through the previously discussed glymphatic clearance route and account for the slow recovery of peripapillary choroidal thickness following spaceflight.36 Also, using geometric models of the ONH, Feola et al. proposed that peripapillary choroidal swelling and anatomy may impact the formation of ODE.15 Given this information, Wostyn et al. recently suggested that circumferential peripapillary interstitial choroidal expansion may contribute to the ODE in astronauts.37 Finally, chorioretinal folds in astronauts have been well documented since the original 2011 NASA report and are hypothesised to result from choroidal expansion with hyperextension of choroidal collagen lamella, increased optic nerve sheath pressure and the associated mechanical effects of posterior globe flattening, or a combination of these mechanisms4 36 (figure 2).
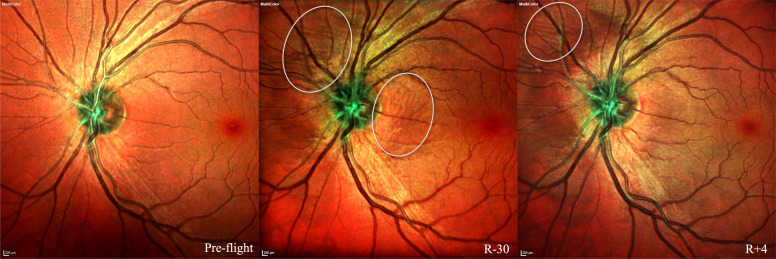
Figure 2.
Multicolor imaging pre-flight (left), 30 days prior to returning to Earth (R-30, middle) and 4 days after returning to Earth (R+4, right). Choroidal folds are noted in R-30 and R+4 (circled). Possible protrusion of optic nerve head is also visible at R-30 and R+4. Courtesy of NASA.
Cerebral blood volume pulsatility and genetic factors
While near-infrared spectroscopy (NIRS) may have future applications for studying the glymphatic system in SANS, this non-invasive modality has been actively used to investigate another SANS hypothesis that proposes that SANS may be due to an increased cerebral pulsatility in microgravity that induces vascular remodelling. In the SPACECOT study, Strangman et al. used NIRS to study cerebral blood volume pulsatility in head-down tilt (HDT) bed rest with a hypercapnic environment.38 The study investigators found an increase in cerebral blood volume pulsatility that increased during head-down tilt. The authors propose that sustained pulsatility during spaceflight may cause a water hammer effect, leading to remodelling of the vasculature and causing persistent SANS findings even after returning to Earth.38
Interestingly, elevated homocysteine levels have been associated with cerebral arterial pulsatility.38 39 Some astronauts with SANS presented with elevated concentrations of homocysteine (as well as 2-methylcitric acid, cystathionine and methylmalonic acid) when compared with astronaut controls without SANS.7 40 These findings suggest that astronauts with certain single nucleotide polymorphisms involved with one-carbon metabolism pathways may increase the risk of developing SANS.20 40 41 Zwart et al. observed that increased 5-methyltetrahydrofolate-homocysteine methyltransferase reductase (MTRR) 66G and serine hydroxymethyltransferase 1 (SHMT1) 1420C alleles increased the odds of visual changes during spaceflight. Lower vitamin B6 (pyridoxine) and B9 (folate) were also associated with ophthalmic changes after spaceflight.41 The genetic findings were followed up with a 30-day HDT bed rest terrestrial analogue study; the authors found that subjects with increased MTRR 66G and 1420C alleles were associated with increased incidence of SANS findings.20 These studies support the association of certain genetic factors in the development of SANS.
Upward brain and optic chiasm displacement
Another hypothesis for SANS revolves around the mechanical upward shift of the brain and optic chiasm that is associated with spaceflight. Roberts et al. conducted a study comparing MRIs of astronaut brains after short-duration and long-duration spaceflight. This study found an upward displacement of the brain in all LDSF astronauts (12 of 12) whereas no astronauts in the short-duration spaceflight group (0 of 6) demonstrated these findings.42 Shinojima et al. theorised that this upward shift of the brain may play a critical role in SANS.43 During upward brain shift, the optic chiasm is shifted upwards which pulls the optic nerve posteriorly. The dura of the optic nerve sheath is connected to periosteum of the orbital bone, which has been theorised to produce an anterior counterforce on the posterior globe in a response to this rearward pull on the optic nerve. This mechanism may produce globe flattening and optic nerve sheath distension that is observed in SANS.
Uncovering the pathogenesis for SANS continues to be area of persistent investigation. The underlying pathophysiology of SANS may be multifactorial that includes some combination of these hypotheses (table 1). One of the natural limitations to studying this neuro-ocular phenomenon is the limited sample size of astronauts. To further understand SANS and its pathogenesis, there is a need for Earth-based analogues. These terrestrial analogues will be instrumental in testing countermeasures to help mitigate SANS for future spaceflight.
Table 1.
Proposed pathogenesis and contributing factors towards the development of spaceflight associated neuro-ocular syndrome (SANS)
| Proposed mechanisms/contributing factors for development of spaceflight associated neuro-ocular syndrome (SANS) | Year(s) | References |
| Elevated intracranial pressure and/or compartmentation of the orbital optic nerve sheath from cephalad fluid shifts during microgravity | 2011 | 4 |
| Genetic risk factors/single nucleotide polymorphisms, androgens and vitamin status | 2012, 2016 | 40 41 |
| Cerebral blood volume pulsatility and vasculature remodelling | 2017 | 38 |
| Upward brain and optic chiasm shift and anterior counterforce on the posterior globe | 2018 | 43 |
| Ocular glymphatic system and cerebral spinal fluid compartmentalisation | 2018 | 30 |
| Cytotoxic oedema/local oxidative stress at the optic nerve head from microgravity-induced venous stasis | 2020 | 21 |
Terrestrial analogues for SANS
Earth-based analogues have been instrumental in simulating microgravity to further understand human physiology during spaceflight. Several established space analogues include dry immersion, HDT bed rest (HDTBR), water immersion, unilateral lower limb suspension and parabolic flight.44 45
Head-down tilt bed rest
HDTBR studies have emerged as a promising terrestrial analogue for studying SANS.44 46 Subjects are tilted downwards to mimic the cephalad fluid shift that is experienced during microgravity. HDTBR have been able to reproduce various SANS findings, including optic nerve sheath distension, retinal nerve layer thickening, increase in choroidal thickness, and optic disc oedema.46–50 Laurie et al. reported the first formation of optic disc oedema after a month of strict HDTBR in a hypercapnic environment.50 The authors had noticed in previous studies that patients with direct ICP monitoring (Ommaya reservoirs) had a reduction in ICP after propping their heads on a pillow.50 51 This suggested that standard pillows and raising the torso to eat during HDTBR may transiently alleviate the cephalad fluid shift that leads to optic disc oedema.50 Since SANS astronauts do not naturally have this fluid shift reduction during LDSF, the authors had subjects undergo HDTBR without raising their head/torso to eat meals or using a standard pillow. This led to optic disc oedema of varying Frisén grades in 5 of 11 subjects (45%) after 30 days. In addition, multiple subjects had an increase in peripapillary total retinal thickness; this increase in retinal thickness was found to be 4–5 times greater than a HDTBR study that used a standard pillow without a hypercapnic environment.50 In addition to further understanding SANS pathogenesis, HDTBR has also been instrumental in testing potential countermeasures which will be discussed in the next section.
Parabolic flight
While SANS is detected during and after LDSF, transient moments of weightlessness during parabolic flight provide important information regarding ICP changes during acute microgravity. The unique parabolic flight research platform consists of an aircraft undergoing repeated cycles of an upward acceleration of 1.8g (ie, hypergravity), followed by approximately 25 s of free fall (ie, microgravity).45 52 Lawley et al. conducted a parabolic flight study with eight individuals each having an Ommaya reservoir which allowed for direct ICP measurement. During acute microgravity, ICP was reduced compared with the terrestrial supine posture, but not to the levels of sitting 90 degrees upright on Earth. The authors suggest that since ICP during acute microgravity is not reduced to the upright levels on Earth, this may lead to remodelling of the eye over longer periods of microgravity.51 While there is no exact replica of SANS on Earth, the use of appropriate terrestrial analogues for SANS becomes increasingly important as countermeasures are being developed for future spaceflight.
Emerging countermeasures for SANS
SANS’ elevated risk for astronaut health and mission performance requires effective countermeasures for planetary and exploratory missions.7 Several promising countermeasures have emerged and are currently being assessed for future spaceflight3; these mitigation strategies arise from various approaches including medical device intervention, nutritional supplementation, artificial gravity, and pre-flight risk assessments.
Lower body negative pressure
Lower body negative pressure (LBNP) has been closely investigated as a countermeasure for SANS.53–56 LBNP is a well-established, non-invasive device that encapsulates the lower body and provides negative pressure to pull fluid away from the cephalad region.57 58 This mechanism can help counteract the cephalad fluid shift from the microgravity environment that is theorised to contribute to SANS. LBNP has been used during spaceflight for cardiovascular physiology, thus establishing itself as a promising countermeasure for SANS.59 Petersen et al. conducted a terrestrial LBNP study with individuals with Ommaya reservoirs and observed a decrease in ICP after LBNP. The authors suggested that 20 mmHg LBNP was the most optimal setting as it was effective in reducing pressure but did not impair perfusion pressure in the brain.53 Regarding SANS clinical findings, LBNP has been observed to reduce the increase of optic nerve sheath distension during head-down tilt.54 The nightly use of terrestrial LBNP during a recent supine bed rest study was documented to mitigate the usual increase in choroidal volume and area.56 Emerging LBNP research includes mobile, flexible LBNP gravity suits, providing an efficient and effective countermeasure for astronauts during future missions.60 Additional countermeasures that also seek to attenuate the cephalad fluid shifts seen during spaceflight include artificial gravity (via centrifugation)61 and venoconstrictive thigh cuffs.62 63
Goggles and modulation of cerebro-ocular hemodynamics
The translaminar pressure gradient (TLPG) refers to the difference between the normally higher intraocular pressure (IOP) and the comparatively lower optic nerve sheath pressure. Spaceflight has been hypothesised to induce a greater increase in optic nerve sheath pressure compared with IOP, leading to a negative TLPG.64 65 Mitigating this microgravity-induced negative TLPG is an area of interest as a countermeasure for SANS. Scott et al. proposed that swimming goggles may serve as a countermeasure for SANS by increasing the IOP and TLPG. The authors used a head-down tilt research platform and observed that TLPG increased with the use of goggles.65 The authors concluded that continued investigation of artificially elevating IOP is needed to further understand goggles as a countermeasure for SANS. Research on pressurised goggles as a countermeasure for SANS is an area of ongoing investigation.66 67
Nutrition and identifying genetic risk factors
As discussed in the pathogenesis section, increased MTRR 66G and SHMT1 1420C alleles were found to be associated with increased SANS findings. In addition, a combination of these risk alleles with low vitamin B2, B6 and B9 status was found to increase the risk of vision deterioration.41 As a result, B vitamin supplementation may be a nutrition-based countermeasure for decreasing the risk of SANS.68 Current metabolic and nutrition research for SANS includes investigating riboflavin, pyridoxine, methylcobalamin, and folate supplementation as a countermeasure for optic disc oedema during HDTBR.69
Promising countermeasures are being developed to reduce the risk of SANS (table 2). In the future, these methods may be combined to provide a greater mitigation effect for astronauts.
Table 2.
Emerging countermeasure strategies for decreasing the risk of spaceflight associated neuro-ocular syndrome (SANS)
| Emerging countermeasure strategies for spaceflight associated neuro-ocular syndrome (SANS) | Rationale | References |
| Lower body negative pressure | Draw fluid from cephalad region towards the lower body | 54 60 |
| Goggles (increased intraocular pressure) | Increase translaminar pressure gradient that is reduced during spaceflight | 65 67 72 |
| B Vitamin supplementation | Relatively low B vitamin status in astronauts with ophthalmic changes | 20 41 68 |
| Venoconstrictive thigh cuffs | Reduce cephalad fluid shift | 62 63 |
| Identifying genetic risk factors | Certain risk alleles are associated with developing SANS | 20 41 68 |
| Artificial gravity (centrifugation) | Maintain hydrostatic pressure to prevent cephalad fluid shift | 61 |
Conclusion and future directions
Given the potential for visual loss and resulting mission compromise during LDSF, understanding and mitigating SANS continues to be an area of intense interest.70 The optimisation of terrestrial analogues and countermeasures for SANS will be critical for astronaut health and mission performance, particularly in future missions that will expose astronauts to longer periods of microgravity than what is currently observed. Additional ophthalmic imaging modalities onboard the International Space Station (ISS) will likely lead to an increased understanding of SANS. In 2018, OCT angiography was brought onboard the ISS.3 The results from this and other noninvasive imaging modality during LDSF may provide a deeper understanding of choroidal involvement and hemodynamics in SANS. SANS research is also focused on developing computational mappings of ocular structure and vision changes to more closely monitor SANS for future spaceflight.71 Ultimately, the ongoing investigations in understanding of the pathophysiology behind SANS will provide stronger support measures for astronauts that embark on planetary missions in the future.
Footnotes
Presented at: This article is published to mark the Festschrift held at the Royal Society of Medicine in London, UK, on 25 and 26 March 2021 to celebrate the retirement of Dr Gordon T Plant. A complete recording of the event is available on the website of the United Kingdom Neuro-Ophthalmology Society (https://uknos.com).
Contributors: JO drafted the initial manuscript. WT, TB, THM, CRG, SSM and AL provided critical feedback and edits for the manuscript. All authors contributed to the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Commissioned; internally peer reviewed.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. Demontis GC, Germani MM, Caiani EG, et al. Human pathophysiological adaptations to the space environment. Front Physiol 2017;8:547. 10.3389/fphys.2017.00547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ong J, Lee AG. An introduction to space medicine and the physiological effects of spaceflight on the human body. In: Lee AG, Ong J, eds. Spaceflight Associated Neuro-Ocular Syndrome. Cambridge, MA: Academic Press, 2022: 1–7. [Google Scholar]
- 3. Lee AG, Mader TH, Gibson CR, et al. Spaceflight associated neuro-ocular syndrome (SANS) and the neuro-ophthalmologic effects of microgravity: a review and an update. NPJ Microgravity 2020;6:7. 10.1038/s41526-020-0097-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mader TH, Gibson CR, Pass AF, et al. Optic disc edema, globe flattening, choroidal folds, and hyperopic shifts observed in astronauts after long-duration space flight. Ophthalmology 2011;118:2058–69. 10.1016/j.ophtha.2011.06.021 [DOI] [PubMed] [Google Scholar]
- 5. Mader TH, Gibson CR, Otto CA, et al. Persistent asymmetric optic disc swelling after long-duration space flight: implications for pathogenesis. J Neuroophthalmol 2017;37:133–9. 10.1097/WNO.0000000000000467 [DOI] [PubMed] [Google Scholar]
- 6. Mader TH, Gibson CR, Barratt MR, et al. Persistent globe flattening in astronauts following long-duration spaceflight. Neuroophthalmology 2021;45:29–35. 10.1080/01658107.2020.1791189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patel ZS, Brunstetter TJ, Tarver WJ, et al. Red risks for a journey to the red planet: the highest priority human health risks for a mission to Mars. NPJ Microgravity 2020;6:33. 10.1038/s41526-020-00124-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee AG, Mader TH, Gibson CR, et al. Space flight-associated neuro-ocular syndrome (SANS). Eye (Lond) 2018;32:1164–7. 10.1038/s41433-018-0070-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nelson ES, Mulugeta L, Myers JG. Microgravity-induced fluid shift and ophthalmic changes. Life (Basel) 2014;4:621–65. 10.3390/life4040621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martin DS, Lee SMC, Matz TP, et al. Internal jugular pressure increases during parabolic flight. Physiol Rep 2016;4:e13068. 10.14814/phy2.13068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marshall-Goebel K, Stevens B, Rao CV, et al. Internal jugular vein volume during head-down tilt and carbon dioxide exposure in the SPACECOT study. Aerosp Med Hum Perform 2018;89:351–6. 10.3357/AMHP.4934.2018 [DOI] [PubMed] [Google Scholar]
- 12. Barr YR. Lumbar puncture during spaceflight: operational considerations, constraints, concerns, and limitations. Aviat Space Environ Med 2014;85:1209–13. 10.3357/ASEM.3674.2014 [DOI] [PubMed] [Google Scholar]
- 13. Thurtell MJ, Kawasaki A. Update in the management of idiopathic intracranial hypertension. Neurol Clin 2021;39:147–61. 10.1016/j.ncl.2020.09.008 [DOI] [PubMed] [Google Scholar]
- 14. Lee AG, Mader TH, Gibson CR, et al. Space flight-associated neuro-ocular syndrome. JAMA Ophthalmol 2017;135:992–4. 10.1001/jamaophthalmol.2017.2396 [DOI] [PubMed] [Google Scholar]
- 15. Feola AJ, Nelson ES, Myers J, et al. The impact of choroidal swelling on optic nerve head deformation. Invest Ophthalmol Vis Sci 2018;59:4172–81. 10.1167/iovs.18-24463 [DOI] [PubMed] [Google Scholar]
- 16. Wostyn P, De Winne F, Stern C, et al. Potential involvement of the ocular glymphatic system in optic disc edema in astronauts. Aerosp Med Hum Perform 2020;91:975–7. 10.3357/AMHP.5670.2020 [DOI] [PubMed] [Google Scholar]
- 17. Wostyn P, De Deyn PP. Optic nerve sheath distention as a protective mechanism against the visual impairment and intracranial pressure syndrome in astronauts. Invest Ophthalmol Vis Sci 2017;58:4601–2. 10.1167/iovs.17-22600 [DOI] [PubMed] [Google Scholar]
- 18. Wostyn P, Mader TH, Gibson CR, et al. The possible role of elastic properties of the brain and optic nerve sheath in the development of spaceflight-associated neuro-ocular syndrome. AJNR Am J Neuroradiol 2020;41:E14–5. 10.3174/ajnr.A6430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Laurie SS, Christian K, Kysar J, et al. Unchanged cerebrovascular CO2 reactivity and hypercapnic ventilatory response during strict head-down tilt bed rest in a mild hypercapnic environment. J Physiol 2020;598:2491–505. 10.1113/JP279383 [DOI] [PubMed] [Google Scholar]
- 20. Zwart SR, Laurie SS, Chen JJ, et al. Association of genetics and B vitamin status with the magnitude of optic disc edema during 30-day strict head-down tilt bed rest. JAMA Ophthalmol 2019;137:1195–200. 10.1001/jamaophthalmol.2019.3124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Galdamez LA, Brunstetter TJ, Lee AG, et al. Origins of cerebral edema: implications for spaceflight-associated neuro-ocular syndrome. J Neuroophthalmol 2020;40:84–91. 10.1097/WNO.0000000000000852 [DOI] [PubMed] [Google Scholar]
- 22. Kahle KT, Simard JM, Staley KJ, et al. Molecular mechanisms of ischemic cerebral edema: role of electroneutral ion transport. Physiology (Bethesda) 2009;24:257–65. 10.1152/physiol.00015.2009 [DOI] [PubMed] [Google Scholar]
- 23. Stenger MB, Tarver WJ, Brunstetter T, et al. Evidence report: risk of spaceflight associated neuro-ocular syndrome (SANS). Human Research Program Human Health Countermeasures Element, 2017. [Google Scholar]
- 24. Zanello SB, Theriot CA, Ponce CMP, et al. Spaceflight effects and molecular responses in the mouse eye: preliminary observations after shuttle mission STS-133. Gravit Space Res 2013;1:29–46. 10.2478/gsr-2013-0003 [DOI] [Google Scholar]
- 25. Heo JH, Han SW, Lee SK. Free radicals as triggers of brain edema formation after stroke. Free Radic Biol Med 2005;39:51–70. 10.1016/j.freeradbiomed.2005.03.035 [DOI] [PubMed] [Google Scholar]
- 26. Morgan JE. Circulation and axonal transport in the optic nerve. Eye (Lond) 2004;18:1089–95. 10.1038/sj.eye.6701574 [DOI] [PubMed] [Google Scholar]
- 27. Denniston AK, Keane PA. Paravascular pathways in the eye: is there an “ocular glymphatic system”? Invest Ophthalmol Vis Sci 2015;56:3955–6. 10.1167/iovs.15-17243 [DOI] [PubMed] [Google Scholar]
- 28. Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 2012;4:147ra111. 10.1126/scitranslmed.3003748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wostyn P, Gibson CR, Mader TH. The glymphatic pathway in the optic nerve: did astronauts already reveal signs of its existence? NPJ Microgravity 2021;7:14. 10.1038/s41526-021-00142-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wostyn P, De Deyn PP. The “ocular glymphatic system”: an important missing piece in the puzzle of optic disc edema in astronauts? Invest Ophthalmol Vis Sci 2018;59:2090–1. 10.1167/iovs.17-23263 [DOI] [PubMed] [Google Scholar]
- 31. Wostyn P, Mader TH, Gibson CR, et al. The escape of retrobulbar cerebrospinal fluid in the astronaut’s eye: mission impossible? Eye (Lond) 2019;33:1519–24. 10.1038/s41433-019-0453-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wostyn P, Mader TH, Gibson CR, et al. The buffering capacity of the brain and optic nerve against spaceflight-associated neuro-ocular syndrome. Proc Natl Acad Sci U S A 2019;116:15770–1. 10.1073/pnas.1908865116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wostyn P, Mader TH, Gibson CR, et al. The perivascular space of the central retinal artery as a potential major cerebrospinal fluid inflow route: implications for optic disc edema in astronauts. Eye (Lond) 2020;34:779–80. 10.1038/s41433-019-0594-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Anderson AP, Swan JG, Phillips SD, et al. Acute effects of changes to the gravitational vector on the eye. J Appl Physiol (1985) 2016;120:939–46. 10.1152/japplphysiol.00730.2015 [DOI] [PubMed] [Google Scholar]
- 35. Macias BR, Patel NB, Gibson CR, et al. Association of long-duration spaceflight with anterior and posterior ocular structure changes in astronauts and their recovery. JAMA Ophthalmol 2020;138:553–9. 10.1001/jamaophthalmol.2020.0673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wostyn P, Gibson CR, Mader TH. Choroidal thickness changes in astronauts during and after spaceflight. RETINA 2021;41:24–6. 10.1097/IAE.0000000000003068 [DOI] [Google Scholar]
- 37. Wostyn P, Gibson CR, Mader TH. Optic disc edema in astronauts from a choroidal point of view. Aerosp Med Hum Perform 2022;93:396–8. 10.3357/AMHP.6010.2022 [DOI] [PubMed] [Google Scholar]
- 38. Strangman GE, Zhang Q, Marshall-Goebel K, et al. Increased cerebral blood volume pulsatility during head-down tilt with elevated carbon dioxide: the SPACECOT study. J Appl Physiol (1985) 2017;123:62–70. 10.1152/japplphysiol.00947.2016 [DOI] [PubMed] [Google Scholar]
- 39. Lim MH, Cho YI, Jeong SK. Homocysteine and pulsatility index of cerebral arteries. Stroke 2009;40:3216–20. 10.1161/STROKEAHA.109.558403 [DOI] [PubMed] [Google Scholar]
- 40. Zwart SR, Gibson CR, Mader TH, et al. Vision changes after spaceflight are related to alterations in folate- and vitamin B-12-dependent one-carbon metabolism. J Nutr 2012;142:427–31. 10.3945/jn.111.154245 [DOI] [PubMed] [Google Scholar]
- 41. Zwart SR, Gregory JF, Zeisel SH, et al. Genotype, B-vitamin status, and androgens affect spaceflight-induced ophthalmic changes. FASEB J 2016;30:141–8. 10.1096/fj.15-278457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roberts DR, Albrecht MH, Collins HR, et al. Effects of spaceflight on astronaut brain structure as indicated on MRI. N Engl J Med 2017;377:1746–53. 10.1056/NEJMoa1705129 [DOI] [PubMed] [Google Scholar]
- 43. Shinojima A, Kakeya I, Tada S. Association of space flight with problems of the brain and eyes. JAMA Ophthalmol 2018;136:1075–6. 10.1001/jamaophthalmol.2018.2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pandiarajan M, Hargens AR. Ground-based analogs for human spaceflight. Front Physiol 2020;11:716. 10.3389/fphys.2020.00716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shelhamer M. Parabolic flight as a spaceflight analog. J Appl Physiol (1985) 2016;120:1442–8. 10.1152/japplphysiol.01046.2015 [DOI] [PubMed] [Google Scholar]
- 46. Ong J, Lee AG, Moss HE. Head-down tilt bed rest studies as a terrestrial analog for spaceflight associated neuro-ocular syndrome. Front Neurol 2021;12:648958. 10.3389/fneur.2021.648958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Laurie SS, Vizzeri G, Taibbi G, et al. Effects of short-term mild hypercapnia during head-down tilt on intracranial pressure and ocular structures in healthy human subjects. Physiol Rep 2017;5:e13302. 10.14814/phy2.13302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Laurie SS, Lee SMC, Macias BR, et al. Optic disc edema and choroidal engorgement in astronauts during spaceflight and individuals exposed to bed rest. JAMA Ophthalmol 2020;138:165–72. 10.1001/jamaophthalmol.2019.5261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Taibbi G, Cromwell RL, Zanello SB, et al. Ocular outcomes comparison between 14- and 70-day head-down-tilt bed rest. Invest Ophthalmol Vis Sci 2016;57:495–501. 10.1167/iovs.15-18530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Laurie SS, Macias BR, Dunn JT, et al. Optic disc edema after 30 days of strict head-down tilt bed rest. Ophthalmology 2019;126:467–8. 10.1016/j.ophtha.2018.09.042 [DOI] [PubMed] [Google Scholar]
- 51. Lawley JS, Petersen LG, Howden EJ, et al. Effect of gravity and microgravity on intracranial pressure. J Physiol 2017;595:2115–27. 10.1113/JP273557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Karmali F, Shelhamer M. The dynamics of parabolic flight: flight characteristics and passenger percepts. Acta Astronaut 2008;63:594–602. 10.1016/j.actaastro.2008.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Petersen LG, Lawley JS, Lilja-Cyron A, et al. Lower body negative pressure to safely reduce intracranial pressure. J Physiol 2019;597:237–48. 10.1113/JP276557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Marshall-Goebel K, Terlević R, Gerlach DA, et al. Lower body negative pressure reduces optic nerve sheath diameter during head-down tilt. J Appl Physiol (1985) 2017;123:1139–44. 10.1152/japplphysiol.00256.2017 [DOI] [PubMed] [Google Scholar]
- 55. Harris KM, Petersen LG, Weber T. Reviving lower body negative pressure as a countermeasure to prevent pathological vascular and ocular changes in microgravity. NPJ Microgravity 2020;6:38. 10.1038/s41526-020-00127-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hearon CM, Dias KA, Babu G, et al. Effect of nightly lower body negative pressure on choroid engorgement in a model of spaceflight-associated neuro-ocular syndrome: a randomized crossover trial. JAMA Ophthalmol 2022;140:59–65. 10.1001/jamaophthalmol.2021.5200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Crystal GJ, Salem MR. Lower body negative pressure: historical perspective, research findings, and clinical applications. J Anesth Hist 2015;1:49–54. 10.1016/j.janh.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 58. Goswami N, Blaber AP, Hinghofer-Szalkay H, et al. Lower body negative pressure: physiological effects, applications, and implementation. Physiol Rev 2019;99:807–51. 10.1152/physrev.00006.2018 [DOI] [PubMed] [Google Scholar]
- 59. Charles JB, Lathers CM. Summary of lower body negative pressure experiments during space flight. J Clin Pharmacol 1994;34:571–83. 10.1002/j.1552-4604.1994.tb02009.x [DOI] [PubMed] [Google Scholar]
- 60. Ashari N, Hargens AR. The mobile lower body negative pressure gravity suit for long-duration spaceflight. Front Physiol 2020;11:977. 10.3389/fphys.2020.00977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Clément G. International roadmap for artificial gravity research. NPJ Microgravity 2017;3:29. 10.1038/s41526-017-0034-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kermorgant M, Sadegh A, Geeraerts T, et al. Effects of venoconstrictive thigh cuffs on dry immersion-induced ophthalmological changes. Front Physiol 2021;12:692361. 10.3389/fphys.2021.692361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Robin A, Auvinet A, Degryse B, et al. DI-5-CUFFS: venoconstrictive thigh CUFFS limit body fluid changes but not orthostatic intolerance induced by a 5-day dry immersion. Front Physiol 2020;11:383. 10.3389/fphys.2020.00383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang LF, Hargens AR. Spaceflight-induced intracranial hypertension and visual impairment: pathophysiology and countermeasures. Physiol Rev 2018;98:59–87. 10.1152/physrev.00017.2016 [DOI] [PubMed] [Google Scholar]
- 65. Scott JM, Tucker WJ, Martin D, et al. Association of exercise and swimming goggles with modulation of cerebro-ocular hemodynamics and pressures in a model of spaceflight-associated neuro-ocular syndrome. JAMA Ophthalmol 2019;137:652–9. 10.1001/jamaophthalmol.2019.0459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Berdahl JP, Yu DY, Morgan WH. The translaminar pressure gradient in sustained zero gravity, idiopathic intracranial hypertension, and glaucoma. Med Hypotheses 2012;79:719–24. 10.1016/j.mehy.2012.08.009 [DOI] [PubMed] [Google Scholar]
- 67. NASA . Equinox balance goggles: the effects of local orbital pressure changes on intraocular pressure (NCC958SMST00012). NASA Life Sciences Data Archive, 2015. [Google Scholar]
- 68. Smith SM, Zwart SR. Spaceflight-related ocular changes: the potential role of genetics, and the potential of B vitamins as a countermeasure. Curr Opin Clin Nutr Metab Care 2018;21:481–8. 10.1097/MCO.0000000000000510 [DOI] [PubMed] [Google Scholar]
- 69. NASA . B complex: 5-methyltetrahydrofolate, riboflavin, pyridoxine, and methylcobalamin supplementation as a non-mechanical countermeasure to mitigate optic disc edema changes during strict 6° head-down tilt bed rest (b_complex_hdt). NASA Life Sciences Data Archive, 2019. [Google Scholar]
- 70. Ong J, Tavakkoli A, Strangman G, et al. Neuro-ophthalmic imaging and visual assessment technology for spaceflight associated neuro-ocular syndrome (SANS). Surv Ophthalmol 2022;67:1443–66. 10.1016/j.survophthal.2022.04.004 [DOI] [PubMed] [Google Scholar]
- 71. NASA . A non-intrusive ocular monitoring framework to model ocular structure and functional changes due to long-term spaceflight (80NSSC20K1831). NASA Life Sciences Data Archive, 2019. [Google Scholar]
- 72. Morgan WH, Cunneen TS, Balaratnasingam C, et al. Wearing swimming goggles can elevate intraocular pressure. Br J Ophthalmol 2008;92:1218–21. 10.1136/bjo.2007.136754 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data are available.