Highlights
-
•
High success and pregnancy rates after surgical treatment of cesarean scar pregnancy.
-
•
Shorter time to subsequent pregnancy after surgical treatment when compared with initial conservative management.
-
•
High intervention rate after initial conservative management.
Key words: cesarean scar pregnancy, cesarean delivery, curettage, expectant, fertility, laparoscopic niche resection, medical, methotrexate, reproductive outcomes, surgery
Abstract
BACKGROUND
There is a dramatic rise in cesarean deliveries worldwide, leading to higher complication rates in subsequent pregnancies. One of these complications is a cesarean scar pregnancy. During the last decades, treatment options for cesarean scar pregnancies have changed, and less invasive interventions have been employed to preserve fertility and reduce morbidity. However, the optimal treatment approach and the influence of various treatments on reproductive outcomes have to be determined.
OBJECTIVE
This study aimed to evaluate the short- and long-term outcomes after cesarean scar pregnancy management.
STUDY DESIGN
We conducted a retrospective cohort study of women determined to have a cesarean scar pregnancy from 2010 to 2021 at a tertiary referral center, the Amsterdam University Medical Center, in the Netherlands. Outcomes of the following management strategies were compared: expectant management, methotrexate, curettage with temporary cervical cerclage, or a laparoscopic niche resection. We performed a curettage if the cesarean scar pregnancy did not cross the serosal line of the uterus, and a laparoscopic niche resection was performed if the cesarean scar pregnancy crossed the serosal line. The main outcomes were treatment efficacy and time to subsequent ongoing pregnancy or pregnancy leading to a live birth.
RESULTS
Of the 60 included women, 5 (8.3%) were managed expectantly, 8 (13.3%) were treated with methotrexate, 31 (51.8%) were treated with a curettage, and 16 (26.7%) with a laparoscopic niche resection. The groups were not comparable. The gestational age and human chorionic gonadotropin levels were generally higher in women who received methotrexate or a laparoscopic niche resection. Additional treatment in the conservative group was needed for 4 (80%) women after expectant management and for 7 (87.5%) women after methotrexate treatment. In the surgical group, all 31 women treated with a curettage and all 16 treated with a laparoscopic niche resection did not require additional treatment. The subsequent ongoing pregnancy rate after cesarean scar pregnancy management was 81.1% (30/37) among women who wished to conceive, with a live birth rate of 78.4% (29/37); 1 woman was in her third trimester of pregnancy at the time of analyses. The time between cesarean scar pregnancy management and subsequent ongoing pregnancy was 4 months (interquartile range, 3–6; P=.02) after expectant management, 18 months (interquartile range, 13–22) after initial methotrexate treatment, 5 months (interquartile range, 3–14; P=.01) after a curettage, and 6 months (interquartile range, 4–15; P=.03) after a laparoscopic niche resection.
CONCLUSION
Surgical treatment of a cesarean scar pregnancy led to a high success rate without additional interventions, high pregnancy rates with a short time interval between treatment, and subsequent pregnancy leading to an ongoing pregnancy or live birth. Conservative management, both with expectant management and methotrexate treatment, led to high (re)intervention rates. Different management approaches are indicated for different types of cesarean scar pregnancies.
AJOG Global Reports at a Glance.
Why was this study conducted?
The optimal treatment approach and the influence of various cesarean scar pregnancy (CSP) treatments on reproductive outcomes have yet to be determined. This study was conducted to evaluate the short- and long-term outcomes, including reproductive outcomes, after 4 different CSP management strategies.
Key findings
There were high success rates, ongoing pregnancy, and live birth rates and a short time to a subsequent pregnancy after surgical treatment for CSP.
What does this add to what is known?
Different management approaches are indicated for different types of CSPs.
Introduction
There is a dramatic rise in cesarean deliveries (CDs) worldwide leading to higher complication rates in subsequent pregnancies.1, 2, 3 One of these complications is a cesarean scar pregnancy (CSP), defined by experts as “all pregnancies (gestational sac [GS] and/or placenta) with implantation in, or in close contact with, the niche.”4 If a CSP remains unrecognized it may lead to life-threatening complications, including massive hemorrhage, uterine rupture, placental abnormalities, hysterectomy, fetal death, and even cases of maternal death have been reported.5,6
Knowledge about the consequences of different management strategies employed for CSP on the subsequent reproductive outcomes is essential for both patients and clinicians, because women with a CSP might want to preserve their uterus and fertility after treatment. A CSP is associated with several risks in subsequent pregnancies such as an increased risk for a recurrent CSP, spontaneous miscarriage, uterus rupture, and placenta ccrete spectrum (PAS) disorders.7, 8, 9
Until now, more than 30 different treatments have been reported, including medical treatment, embolization, and various surgical treatments or a combination of these.10 During the last decades, management strategies for CSP have changed and less invasive interventions have been employed to preserve fertility and reduce morbidity.11, 12, 13, 14 These management strategies can be classified into 4 categories, namely (1) expectant management; (2) medical treatment; (3) surgical treatment of CSP without repair of the uterine defect; and (4) surgical treatment with repair of the uterine defect. It has been suggested that resection of the CSP and repair of the uterine defect may aid in improving the reproductive outcomes, including reducing recurrent CSPs.15,16 However, the optimal treatment approach and the influence of various treatments on the reproductive outcomes have yet to be determined. This is mainly because large comparative studies with optimal evaluation and long-term follow-ups are not available. This study aimed to evaluate both the short- and long-term outcomes, including reproductive outcomes, of 4 different CSP management strategies.
Materials and Methods
This study was conducted at the Amsterdam University Medical Center in Amsterdam, the Netherlands (a tertiary referral center for niche-related problems and CSPs), between January 2010 and December 2021. All women diagnosed with a niche in our hospital, including women with a CSP, were included consecutively in a prospective database after giving written informed consent. This study was approved by the ethics committee of the hospital (2018.099). A CSP was defined as a pregnancy (GS and/or placenta) with implantation in the niche.3, 4, 5 Women who previously had a failed curettage for their CSP in other hospitals were excluded because they were treated under the assumption of a nonviable intrauterine pregnancy and the bulk of the pregnancy tissue would already have been removed.
Data collection
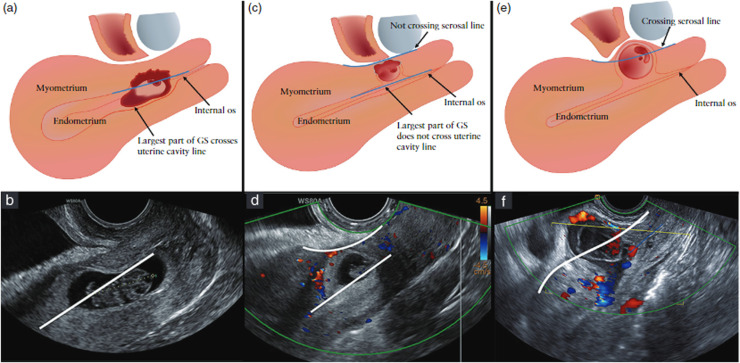
Baseline data included age, smoking status, body mass index (BMI), gestational age (GA), medical and obstetrical history, previously performed conservative therapies in this pregnancy, human chorionic gonadotropin (hCG) serum levels, and physical symptoms at referral. After treatment, symptoms and complications were monitored. All women received a standardized transvaginal ultrasound examination before and after therapy in the sagittal and transverse plane with color Doppler for diagnosis of a CSP. Ultrasound information included the location of the CSP in relation to the niche, the uterine cavity, and the outer contour of the uterus (serosal line), the estimated percentage of protrusion of the GS toward the uterine cavity or toward the bladder, fetal cardiac activity, vascularity, and thickness of the residual myometrium (RMT). In retrospect, we applied the reporting system for CSP that was recently agreed upon among experts.4 This reporting system is based on the location of the GS in relation to the serosal line and the uterine cavity line, shown in Figure 1. This retrospective classification was done by evaluating the CSP recordings, 3-dimensional volumes, and stills of the performed ultrasound examinations and the available magnetic resonance imaging scans by 2 independent researchers (C.V. and R.A.d.L.) who were unaware at that time of the outcomes or the applied therapy. In case women were treated before referral, we asked the referring clinic to provide us with the available imaging to identify the situation before the initial therapy.
Figure 1.
Classification of cesarean scar pregnancies (CSP) as recently consented among experts.4
Schematic (a,c,e) and ultrasound (b,d,f) images of a CSP with the largest part in the uterine cavity (a,b), or with the largest part in the myometrium (c,d) or crossing the seroal line (e,f).
Verberkt. Outcomes after cesarean scar pregnancy management. Am J Obstet Gynecol Glob Rep 2022.
Treatment
Four management strategies were performed in our clinic for CSP, namely expectant management, local and systemic methotrexate (MTX), curettage under transrectal ultrasound guidance with a temporary cervical cerclage, or a laparoscopic niche resection (LNR) with temporary perioperative occlusion of the uterine and ovarian vessels. Detailed information of the applied management strategies with videos are available in the supplemental materials (Table A.1). The procedures were performed by 1 of the 4 surgeons with expertise in performing LNRs, curettage with cerclage, or local MTX application. A uterine artery embolization was used only as co-intervention to treat acute severe hemorrhage.17, 18, 19 Indication for different treatment options changed over time in our center. Until 2017, most women were treated with MTX independent of the location of CSP. However, since the acquired experiences with LNR in nonpregnant women and with curettage in combination with a cervical cerclage for the treatment of CSP, we decided on a more differentiated policy. This led to an adjusted protocol since 2017 in which the management procedure is dictated by the location of the CSP. We generally offered a patient expectant management with weekly follow-ups or a curettage in the case of a CSP (viable and nonviable) that did not cross the serosal line of the uterus (Figure 1) and an LNR in case of a CSP that did cross the serosal line.
Follow-up
Women who were not surgically treated (MTX treatment or expectant management) were followed, and serum hCG levels were determine weekly. An ultrasound examination was performed on indication. After reaching undetectable serum hCG levels, women were seen monthly to evaluate the regression of the vascularity and pregnancy tissue. When a curettage was performed, 1 appointment was scheduled at 6 weeks after the procedure for an ultrasound examination, and after an LNR, women were seen 3 months after the surgery. Women were contacted at 48 months after the diagnosis or in December 2021 to retrieve additional information about their symptoms, hospital visits, re-interventions, fertility, and obstetrical outcomes.
Outcomes
The primary outcome was the efficacy of the therapy, defined as the success of treatment in terms of an uneventful decline in serum hCG levels and resolution of pregnancy tissue without the need for additional treatment. The secondary, short-term outcomes included operative blood loss, operative time, hospital stay, time to undetectable serum hCG levels, infection, or other postoperative complications. The long-term outcome was the time interval between the start of management of CSP and the moment of conception leading to a subsequent ongoing pregnancy. Ongoing pregnancy was defined as a viable intrauterine pregnancy of at least 12 weeks’ gestation confirmed with an ultrasound scan. Time to conception was calculated from the start of management after the diagnosis CSP until the first day of the menstrual cycle in which the conception occurred. In addition, the time interval between the start of management and conception leading to a live birth was also evaluated. Because the total interval is also influenced by the duration of the treatment, we additionally asked women when they stopped contraceptives and calculated the interval between the onset of trying to conceive and the moment of conception leading to a subsequent ongoing pregnancy. We did not use completion of therapy because some women continued their contraceptives for a longer period. The secondary long-term reproductive outcomes included ongoing pregnancy, live birth and miscarriage rates, and pregnancy and delivery complications such as recurrent CSP, PAS, signs of uterine dehiscence, or uterine rupture.
Analyses
All analyses were performed using SPSS (version 26.0) (SPSS Inc, Chicago, IL). To evaluate the efficacy of the different management strategies, the short-term outcomes are described for all interventions for CSP. This also includes the additional LNRs that were performed and not just for the initial treatment. Reproductive data were stratified per applied initial management strategy in our center. This means that if a patient was treated with MTX but also received an additional LNR, she was reported in the MTX group. Thus, in the curettage and the LNR group we only reported on the results of these procedures if given as initial treatment. We performed an intention-to-treat analysis in which we calculated the time from initial management in our hospital to the time of conception leading to a subsequent ongoing pregnancy and time to conception leading to a live birth. But we also calculated the time between the moment they tried to conceive and the time of conception leading to an ongoing pregnancy. A Kaplan-Meier survival curve with a log rank test was constructed to determine the cumulative pregnancy rate and time to next pregnancy after management of CSP. All tests were performed as 2-sided tests, and P values <.05 were considered statistically significant.
Results
Between 2010 and 2021, 63 women with a CSP were diagnosed in our hospital. Three patients were excluded because of an applied (failed) curettage for an undiagnosed CSP before referral to our hospital (Figure A.1). The mean maternal age was 34 (±4.3) years with a median GA (at referral) of 7 weeks (6–8 weeks) (Table 1). Of the 60 women, 34 (56.7%) women had undergone 1 previous CD, 18 (30%) had undergone 2 CDs, and 8 (13.3%) had undergone 3 CDs. In more than half of the pregnancies (55%), fetal cardiac activity was present at the time of diagnosis. The median RMT was 1.9 mm (0.1–3.0 mm) at diagnosis in our center. A total of 28 women were asymptomatic (46.7%) and others reported mild symptoms including irregular blood loss (n=23, 38.3%), abdominal pain (n=5, 8.3%) or a combination (n=4, 6.7%). Eight women (28.2%) received misoprostol before referral to our clinic of which 6 pregnancies were misinterpreted as a miscarriage, whereas the other 2 were misinterpreted as viable intrauterine pregnancies and were unintended. The diagnosis of CSP was made at presentation in our clinic using an ultrasound examination and the indicated treatment for CSP was started.
Table 1.
Characteristics of women with a cesarean scar pregnancy
| Characteristics | Total (n=60) | Expectant (n=5) | MTX (n=8) | Curettage (n=31) | LNR (n=16) |
|---|---|---|---|---|---|
| Maternal age (y) | 34 (±4.3) | 36.0 (±3.1) | 32.8 (±6.0) | 34.7 (±3.6) | 33.2 (±6.0) |
| Body mass index (kg/m2) | 27.3 (±5.5) | 25.6 (±2.6) | 25.7 (±4.8) | 26.3 (±5.4) | 31 (±5.6) |
| Previous CD deliveries | |||||
| 1 | 34 (56.7%) | 4 (80.0%) | 4 (50.0%) | 18 (58.1%) | 8 (50.0%) |
| 2 | 18 (30.0%) | 1 (20.0%) | 3 (37.5%) | 7 (22.6%) | 7 (43.8%) |
| ≥3 | 8 (13.3%) | — | 1 (12.5%) | 6 (19.4%) | 1 (6.3%) |
| Gestational age (wk) | 6.8 (6–8) | 9 (5.5–11.3) | 7.7 (6.7–8.0) | 6 (6.0–7.0) | 7.4 (6.5–9.9) |
| hCG level (U/L) | 6741 (3036–28,217) | 1169 (560–1169) | 58,442 (2833–62,557) | 5208 (3531–20,262) | 27,064 (4632–42,502) |
| Symptoms | |||||
| None | 28 (46.7%) | 4 (80.0%) | 3 (37.5%) | 14 (45.2%) | 7 (43.8%) |
| Blood loss | 23 (38.3%) | 1 (20.0%) | 4 (50.0%) | 14 (45.2%) | 4 (25.0%) |
| Abdominal pain | 5 (8.3%) | — | — | 2 (6.5%) | 3 (18.8%) |
| Combination | 4 (6.7%) | — | 1 (12.5%) | 1 (3.2%) | 2 (12.5%) |
| Cardiac activity | 33 (55.0%) | 3 (60.0%) | 6 (75.0%) | 13 (41.9%) | 11 (68.8%) |
| RMT | 1.9 (0.1–3.0) | 3.3 (1.5–4.4) | 0.4 (0-3.0) | 2.1 (1.1–3.0) | 0 (0–1.0) |
| Crossing serosal line | 22 (36.7%) | — | 6 (75%) | — | 16 (100%) |
| Current smoking | 15 (25%) | — | 3 (37.5%) | 11 (35.5%) | 1 (6.3%) |
Data are presented as mean (±standard deviation), number (percentage), or median (interquartile range).
CD, cesarean delivery; hCG, human chorionic gonadotropin; LNR, laparoscopic niche resection; MTX, methotrexate; RMT, residual myometrium thickness.
Verberkt. Outcomes after cesarean scar pregnancy management. Am J Obstet Gynecol Glob Rep 2022.
Treatment outcomes
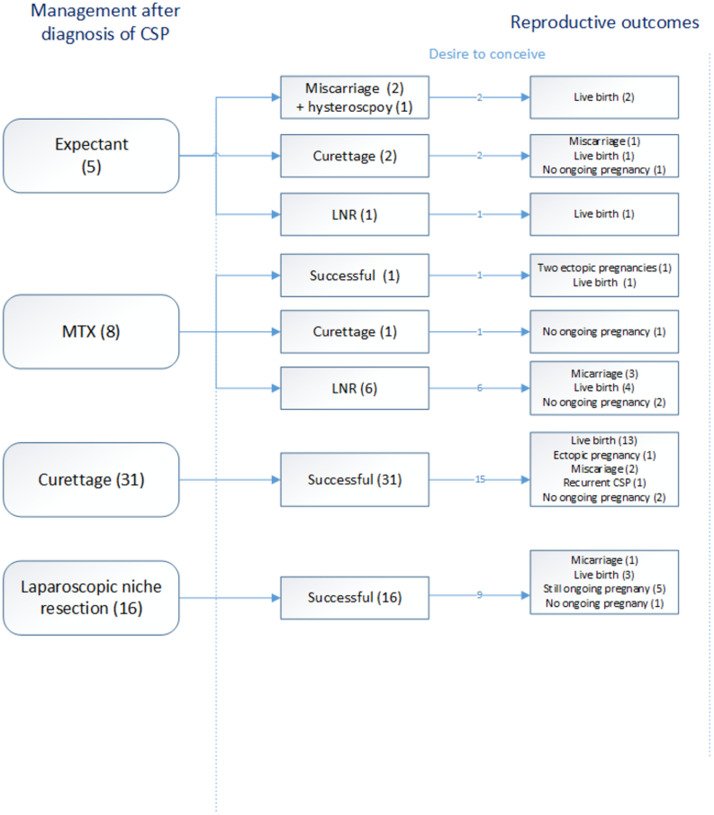
Figure 2 shows an overview of the success rates of each management strategy after a diagnosis of CSP in our center, and Table 2 reports the short-term outcomes. After counseling, the following management strategies were performed: expectant management (n=5, 8.3%), MTX (n=8, 13.3%), curettage (n=31, 51.8%), and LNR (n=16, 26.7%). We will describe the results per management strategy:
Figure 2.
Overview of the success rates and reproductive outcomes per treatment modality after diagnosis of CSP
Some women experienced a pregnancy loss before an ongoing pregnancy, leading to more pregnancies than women with a desire to conceive.
CSP, cesarean scar pregnancy; LNR, laparoscopic niche resection; MTX, methotrexate.
Verberkt. Outcomes after cesarean scar pregnancy management. Am J Obstet Gynecol Glob Rep 2022.
Table 2.
Short term and reproductive outcomes per management strategy for cesarean scar pregnancy
| Short-term outcomes | |||||
|---|---|---|---|---|---|
| Characteristics | Expectant | MTX | Curettage | LNR | |
| Success of therapy (%)a | 20% | 12.5% | 100% | 100% | |
| Blood loss (mL) | — | — | 50 (45–100) | 100 (45–212) | |
| Admission hospital (d) | — | 2 (1–2) | 0 (0–1) | 1 (1–1) | |
| Operation time (min) | — | — | 30 (±13.7) | 154 (±45.6) | |
| hCG resolution (wk) | — | 14 (6–22) | 5 (4–8) | NR | |
| RMT after intervention (mm) | 3.0 (1.3–3.0) | NR | 3.3 (0–2.6) | 7.8 (2–4.2) | |
| Reproductive outcomes | |||||
|---|---|---|---|---|---|
| Total (58) | Expectant | MTX | Curettage | LNR | |
| Attempted to conceive, n (%) | 37 (63.8%) | 5 (100%) | 8 (100%) | 15 (48.4%) | 9 (56.3%) |
| Conceived, n (%) | 32 (86.5%) | 4 (80%) | 5 (62.5%) | 15 (100%) | 8 (88.9%) |
| Ongoing pregnancy | 30 (81.1%) | 4 (80%) | 5 (62.5%) | 13 (86.7%) | 8 (88.9%) |
| Live birth Term birth rate Preterm birth Still pregnant |
29 (78.4%) 26 (89.7%) 3 (12%) 1 (3.3%) |
4 (80%) 4 (80%) — — |
5 (62.5%) 3 (60%) 2 (40%) — |
13 (86.7%) 12 (92.3%) 1 (7.7%) — |
7 (87.5%) 7 (87.5%) — 1 (12.5%) |
| Miscarriage rate Ectopic pregnancy Recurrent CSP |
7 (21.8%) 3 (9.4%) 1 (3.1%) |
1 (25%) — 1 (25%) |
3 (60%) 2 (25%) — |
2 (13.3%) 1 (6.7%) 1 (6.7%) |
1 (12.5%) — — |
| Assisted reproductive technology | 3 (9.4%) | 1 (25%) | 1 (20%) | 1 (6.7%) | — |
| Time to ongoing pregnancy after treatment (mo) | 6 (4–16) | 4 (3–6) | 18 (13–22) | 5 (3–14) | 6 (4–15) |
| Time to ongoing pregnancy per last treatment (mo) | 6 (4–16) | 4 (4–4) | 19 (19–19) | 5 (2–12) | 6 (6–14) |
| Time to ongoing pregnancy from start trying to conceive (mo) | 4 (1–10) | 1 (1–3) | 7 (6–17) | 5 (2–14) | 1 (1–5) |
Data are presented mean (±standard deviation), number (percentage), or median (interquartile range). Assisted reproductive technology involves the manipulation of eggs, sperm, or embryos to achieve pregnancy.
CSP, cesarean scar pregnancy; hCG, human chorionic gonadotropin; LNR, laparoscopic niche resection; MTX, methotrexate; RMT, residual myometrium thickness.
aSuccess defined as uneventful decline of serum hCG levels and resolution of pregnancy tissue without the need for additional treatment.
Verberkt. Outcomes after cesarean scar pregnancy management. Am J Obstet Gynecol Glob Rep 2022.
Expectant management (n=5)
One woman with a vital CSP and an RMT of 5 mm was followed closely but this ended in a fetal death at 9 weeks of gestation, followed by an uneventful curettage with temporary vaginal cerclage after counseling. In 3 women, the pregnancy ended in a spontaneous miscarriage at 7, 8, and 9 weeks’ GA; 2 of these were incomplete and 1 was followed by a curettage, whereas the other underwent a hysteroscopic removal of the placental remnants 3 months after the miscarriage. One woman who presented at 7 weeks’ gestation with a CSP that did not cross the serosal line changed to a CSP that did cross the serosal line after 2 weeks of follow-up. After additional counseling, she underwent an uneventful LNR.
Methotrexate (n=8)
There were 4 women who received systemic MTX treatment for nonvital CSP, 3 who received an intraamniotic MTX injection for vital CSP, and 1 woman received a combination of systemic MTX administration followed by an intraamniotic MTX injection. The median GA was 8 weeks (6.7–8.0). The mean resolution time of hCG levels after MTX treatment was 14 weeks (±6.2). In total, 7 of 8 women (87.5%) received additional treatment with 6 of those undergoing an LNR and 1 undergoing a curettage for removal of retained products of conception. In 1 woman, an emergency LNR was performed because of imminent rupture toward the pelvic sidewall after systemic MTX therapy. In the other women, the LNR was performed because of an insufficient decrease in the hCG levels or persistent gynecologic complaints such as abdominal pain, spotting, and dysmenorrhea. The mean time to additional intervention was 6.8 months (range, 2 weeks–12 months). All these women were treated before the adjusted protocol in 2017.
Curettage (n=31)
A temporary cerclage was placed in all women before the curettage; in 28 cases, this could be removed immediately after the procedure because of minimal blood loss. The cerclage was tightened in 3 women, and in 1 woman, it was combined with a Foley balloon catheter. Both were removed successfully within 6 hours. The median blood loss was 50 mL (45–100 mL). All (31/31) curettages were performed for women with a CSP that did not cross the serosal line and all were effective in terms of complete removal of pregnancy tissue without additional interventions. All women were discharged from the hospital within 24 hours after admission.
Laparoscopic niche resection (n=23)
An LNR was performed in 16 women as primary treatment (CSP crossing the serosal line of the uterus) and in 7 women as secondary treatment after failed initial treatment, and all (23/23) were successful. There were no bladder injuries, a median blood loss of 100 mL (45–212 mL), no conversions to a laparotomy, no infections, and no complications during their convalescence period. All women were discharged 1 day after surgery.
Reproductive outcomes
Long-term follow-up data on reproductive outcomes were available for 58 of 60 (97%) women (Figure 2). Of these, 37 (63.8%) women attempted to conceive after their CSP treatment, whereas 21 (36.2%) women had no desire to conceive or had a wish to conceive in the near future but used contraceptives up to the moment of data analysis. In total, 32 of 37 women (86.5%) became pregnant of which 30 (81.1%) women had an ongoing pregnancy (Table 2). Of the 30 women with an ongoing pregnancy, 8 first experienced 1 or more early pregnancy losses after their CSP treatment. The subsequent ongoing pregnancy rate after initial CSP treatment in women with a desire to conceive was 80% (4/5) for expectant management, 62.5% (5/8) after MTX treatment, 86.7% (13/15) after a curettage, and 88.9% (8/9) after an LNR. The live birth rate was 78.4% (29/37). At the time of writing, 1 woman had an ongoing pregnancy with a GA of 30 weeks. There were 29 live births, 7 miscarriages, 3 tubal pregnancies, and 2 recurrent CSPs. Three women conceived using in vitro fertilization and 29 conceived naturally. There were 3 preterm deliveries; 2 of the women received MTX treatment and 1 of those was followed by an LNR (delivery at 35- and 32-weeks’ gestation). The other preterm delivery was after curettage (delivery at 30 weeks’ gestation). None of the women were diagnosed with PAS in the subsequent pregnancy. The delivery mode was CD in 26 of 29 (89.7%) women, with 1 of those reporting uterine dehiscence during the CD (in the curettage group). The vaginal deliveries were uneventful. Seven women with a desire to conceive did not yet have an ongoing pregnancy. One woman (in the MTX group) stopped after 4 months of trying to conceive. In this study, we observed 2 recurrent CSPs with 1 occurring in the curettage group with subsequent treatment with a curettage, after which she did not wish to conceive anymore. The other recurrent CSP was treated with a curettage after a previous CSP ended in a miscarriage; at time of analyses, she was within the first year after curettage and did not conceive yet. The remaining 4 women had fertility problems after the CSP treatment (1 after curettage, 2 after MTX followed by an LNR, and 1 after initial LNR) with different accompanying diagnoses, including diminished ovarian reserve, male factor, polycystic ovary syndrome, and 1 without a clear diagnosis.
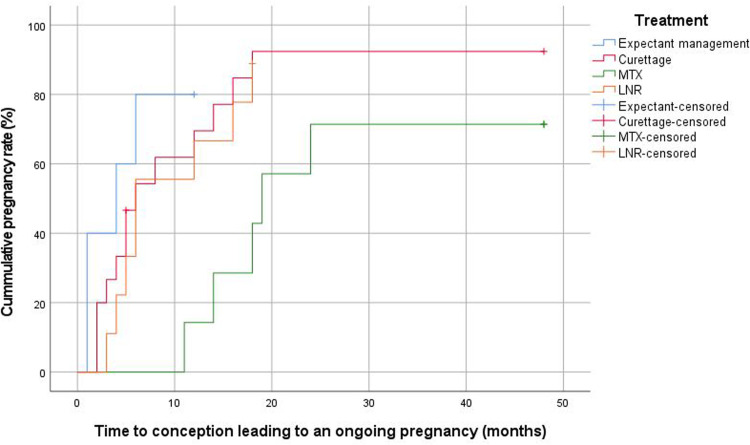
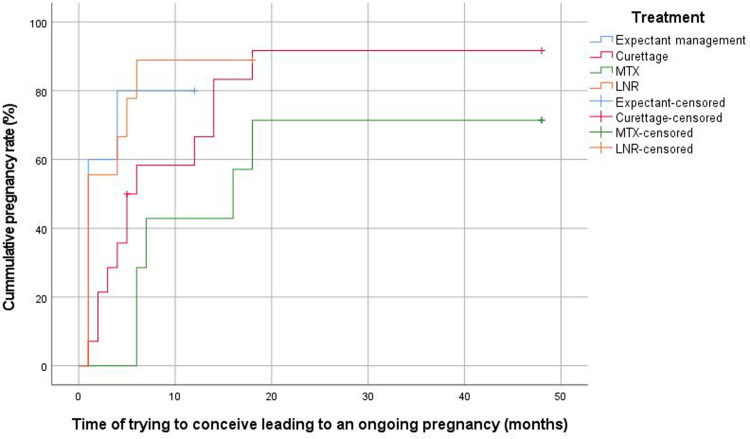
The median overall time interval between management of CSP and subsequent ongoing pregnancy was 6 months (IQR, 4–16). This was 4 months (IQR, 3–6) after expectant management, 18 months (IQR, 13–22) after MTX, 5 months (IQR, 3–14) after curettage, and 6 months (IQR, 4–15) after LNR (Figure 3). This time interval was longer for women who were initially treated with MTX than for those managed expectantly (P=.02), those who underwent curettage (P=.01), or those who underwent an LNR (P=.03). Figure B.1 shows the time from management of CSP to conception leading to a live birth. The median time interval from the last applied therapy to ongoing pregnancy was 4 months (IQR, 4–4) after expectant management, 19 months (IQR, 19–19) after MTX, 5 months (IQR, 2–12) after curettage, and 7 months (IQR, 6–14) after LNR. The interval for those in the expectant management and MTX groups was based only on woman who conceived without additional surgical intervention. The median time from trying to conceive to subsequent ongoing pregnancy was 4 months (IQR, 1–10), and in the different treatment groups, the time to ongoing pregnancy was 1 month (IQR, 1–3) for expectant management, 7 months (IQR, 6–17) for MTX, 5 months (IQR, 2–14) for curettage, and 1 month (IQR, 1–5) for LNR (P=.24) (Figure 4).
Figure 3.
Kaplan-Meier plot for time interval between treatment of CSP and subsequent ongoing pregnancy according to treatment modality
LNR, laparoscopic niche resection; MTX, methotrexate.
Verberkt. Outcomes after cesarean scar pregnancy management. Am J Obstet Gynecol Glob Rep 2022.
Figure 4.
Kaplan-Meier plot for time interval for time of trying to conceive and subsequent ongoing pregnancy according to initial management strategy
LNR, laparoscopic niche resection; MTX, methotrexate.
Verberkt. Outcomes after cesarean scar pregnancy management. Am J Obstet Gynecol Glob Rep 2022.
Discussion
Principal findings
The study demonstrates that both surgical treatments (LNR and curettage) were successful without a need for re-interventions in all women treated in our hospital, whereas MTX treatment was only successful in 12.5% of the women. None of the CSPs that were initially treated with expectant management reached the second trimester. The subsequent ongoing pregnancy rate after CSP treatment in women with a desire to become pregnant was 81.1% with a current live birth rate of 78.4% and a median interval between treatment for the CSP and subsequent ongoing pregnancy of 6 months (IQR, 4–16). This interval was longer for women initially treated with MTX than for those managed expectantly or with surgical interventions. However, the groups were not comparable in that the level of hCG was higher at baseline for those in the MTX and LNR groups than for those in the expectant management or curettage groups.
Results and clinical implications
A curettage is less invasive than an LNR and has the shortest recovery time with high success rates and low complication rates if performed in an expert clinic.14,20 However, some studies reported a higher risk for hemorrhage and uterine perforation after a curettage.21, 22, 23 The systematic review by Birch Peters et al10 describes a high complication rate (21%), and half of the women needed additional treatment after a curettage. An explanation for this might be that the curettage was performed for CSPs at an advanced stage of pregnancy or that the pregnancies crossed the serosal line and the procedures were performed without transrectal guidance or a cerclage, which possibly led to a higher risk for perforation. These types of CSPs may also have a higher risk for trophoblast-invasion toward the large vessels of the uterus, leading to an increased risk for excessive blood loss during a curettage. Patient selection based on advanced ultrasound evaluation by experts is very important to reduce the complication rate during a curettage for a CSP. In patients with a potentially higher risk for complications, that is, in cases in which the CSP crossed the serosal line mostly in an advanced stage of pregnancy and with extensive vascularization, we performed an LNR. However, we developed this experienced-based policy because of a lack of evidence-based guidelines. Given the absence of complications in the curettage group it can be interpreted as a successful selection strategy, however, this may also indicate that we may have overtreated, because we do not know whether a curettage would have led to complications.
Our LNR success rates are consistent with other studies.8,13,24,25 A potential advantage of an LNR in these women is that, in addition to the removal of pregnancy tissue, it also aims to restore the anatomy of the uterus. This may, in theory, be beneficial for future fertility and may reduce niche-related symptoms.9,26,27 An LNR can also be applied as a last resort when other treatments fail or for hemodynamically unstable women.12 Adhesions between the bladder and uterus and increased vascularization owing to gestation lead to a challenging procedure possibly with a higher risk for complications. It is therefore important that only surgeons with extensive experience with LNRs perform these procedures.
This study demonstrates a high ongoing pregnancy rate after surgery (86.7% after curettage and 88.9% after LNR), which is in line with previously reported pregnancy rates of 80.1% after surgical intervention without repair of the uterus and 86.0% after surgical intervention with repair of the uterus.7 After a curettage, women could try to conceive after their first menstruation, whereas women were advised to use contraceptives for 6 months after an LNR and fpr 3 months after MTX treatment. Remarkably, based on the last applied therapy analysis, 11 (11/14, 78.6%) women that underwent an LNR conceived immediately in their first attempt after these 6 months.
After MTX treatment, women experienced a longer time to subsequent pregnancy (18 months; IQR, 13–22). Seven (7/8, 87.5%) women required additional treatment after MTX treatment, leading to prolonged treatment and delayed time to subsequent pregnancy when compared with those treated immediately with surgery. Reported success rates after MTX treatment for CSP range between 57% and 100%.28, 29, 30, 31 The hCG levels among those in the MTX group were higher than among those in the other groups, possibly as a consequence of more advanced pregnancies or because of failed therapy. It has been demonstrated that the efficacy of primary systemic MTX treatment for a CSP was augmented when applied at lower hCG levels and in the absence of fetal cardiac activity.32 This means that we should interpret our results very cautiously, because only 4 women had no cardiac activity and low hCG levels. Early recognition of a CSP is important to administer successful treatment. A recent systematic review also reported a recurrence rate of CSP of up to 19% after MTX treatment.11 Given the high failure rate and higher risk for complications, the Society for Maternal-Fetal Medicine discourages the use of systemic MTX treatment alone in the treatment of CSP.33 However, it could be the only option in settings with a viable CSP when an LNR is not available or at least when further progression of the pregnancy needs to be interrupted until the appropriate surgical intervention can be arranged.
Strengths and limitations
This study was performed at a tertiary hospital in the Netherlands with experience in diagnosing and treating CSP. All women were consecutively included, and information on the location of the CSP was collected and retrospectively classified according to the new consensus among experts.4 Limitations of the study were the retrospective observational design, lack of stratification of the analysis according to the location of CSP, and the heterogeneity in GA, hCG levels, and vitality of the pregnancy at the time of diagnosis. Therefore, the baseline characteristics of the various treatment regimens are not completely comparable, for example, hCG levels were the lowest in the expectant management and curettage groups. Some women were treated with misoprostol before referral, which may have influenced the results. Misoprostol is not indicated for a CSP and is therefore not considered as initial treatment. Although the hCG level is described as a predictor associated with medical treatment failure, the appropriate treatment modality for a specific hCG concentration range remains unclear. The hCG levels reported in the MTX group were high, which may be associated with a higher rate of failure of the medical treatment. Because of our own experiences and supported by more recent studies reporting a low success rate of MTX, we changed our policy and reduced the use of MTX, leading to a relatively low number of patients included in this arm with all the participants in this study arm included before 2017. Because of the skewness of the data related to MTX treatment and to the GA differences among the various groups, this study should not be used to make a 1 to 1 comparison and future randomized controlled trials are needed.
Conclusion and research implications
We observed low success rates after expectant management and initial MTX therapy for CSP, whereas we found high success rates, without re-interventions or complications, after a curettage or an LNR in a selected group with CSP. However, the groups were not comparable with higher hCG levels in the MTX and LNR groups. We observed high ongoing pregnancy rates with short time intervals between the treatment and subsequent pregnancy after these surgical interventions. Based on our experience, curettage and LNR are indicated for different types of CSP. Curettage is recommended for women with a CSP not crossing the serosal line and an LNR is recommended for women with a CSP crossing the serosal line. Future studies are needed to evaluate the efficacy of this policy. Given the low success rates after MTX treatment, we propose to use MTX as first-line treatment only for women with a (relative) contraindication for surgery or for those who do not have access to a referral center with expertise in surgical interventions for CSP. Further prospective research is needed to establish a treatment guideline for CSP, and registration in the central international registry (www.csp-registry.com) would be helpful to obtain reliable data.
Footnotes
The authors report no conflict of interest.
Written consent was obtained from all included patients.
This study did not receive any funding.
Cite this article as: Verberkt C, Lemmers M, de Leeuw RA, et al. Effectiveness, complications, and reproductive outcomes after cesarean scar pregnancy management: a retrospective cohort study. Am J Obstet Gynecol Glob Rep 2022;XX:x.ex–x.ex.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.xagr.2022.100143.
Appendix. Supplementary materials
Women misdiagnosed as a miscarriage before referral and were treated with a curettage. In these women the gestational sac was crossing the serosal line.
References
- 1.Ben Nagi J, Helmy S, Ofili-Yebovi D, Yazbek J, Sawyer E, Jurkovic D. Reproductive outcomes of women with a previous history of caesarean scar ectopic pregnancies. Hum Reprod. 2007;22:2012–2015. doi: 10.1093/humrep/dem078. [DOI] [PubMed] [Google Scholar]
- 2.Boerma T, Ronsmans C. Global epidemiology of use of and disparities in caesarean sections - authors’ reply. Lancet. 2019;394:25. doi: 10.1016/S0140-6736(19)30698-1. [DOI] [PubMed] [Google Scholar]
- 3.Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. Am J Obstet Gynecol. 2012;207:14–29. doi: 10.1016/j.ajog.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Jordans IPM, Verberkt C, de Leeuw RA, et al. Definition and sonographic reporting system for cesarean scar pregnancy in early pregnancy: modified Delphi method. Ultrasound Obstet Gynecol. 2022;59:437–449. doi: 10.1002/uog.24815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silver RM. Implications of the first cesarean: perinatal and future reproductive health and subsequent cesareans, placentation issues, uterine rupture risk, morbidity, and mortality. Semin Perinatol. 2012;36:315–323. doi: 10.1053/j.semperi.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 6.O'Neill SM, Khashan AS, Kenny LC, et al. Caesarean section and subsequent ectopic pregnancy: a systematic review and meta-analysis. BJOG. 2013;120:671–680. doi: 10.1111/1471-0528.12165. [DOI] [PubMed] [Google Scholar]
- 7.Wu J, Ye J, OuYang Z, et al. Outcomes of reproduction following cesarean scar pregnancy treatment: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;262:80–92. doi: 10.1016/j.ejogrb.2021.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Maheux-Lacroix S, Li F, Bujold E, Nesbitt-Hawes E, Deans R, Abbott J. Cesarean scar pregnancies: a systematic review of treatment options. J Minim Invasive Gynecol. 2017;24:915–925. doi: 10.1016/j.jmig.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 9.Morlando M, Buca D, Timor-Tritsch I, et al. Reproductive outcome after cesarean scar pregnancy: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2020;99:1278–1289. doi: 10.1111/aogs.13918. [DOI] [PubMed] [Google Scholar]
- 10.Birch Petersen K, Hoffmann E, Rifbjerg Larsen C, Svarre Nielsen H. Cesarean scar pregnancy: a systematic review of treatment studies. Fertil Steril. 2016;105:958–967. doi: 10.1016/j.fertnstert.2015.12.130. [DOI] [PubMed] [Google Scholar]
- 11.Kaelin Agten A, Cali G, Monteagudo A, Oviedo J, Ramos J. Timor-Tritsch I. The clinical outcome of cesarean scar pregnancies implanted “on the scar” versus “in the niche. Am J Obstet Gynecol. 2017;216 doi: 10.1016/j.ajog.2017.01.019. 510.e1–6. [DOI] [PubMed] [Google Scholar]
- 12.Doroszewska K, Milewicz T, Bereza T, et al. Cesarean scar pregnancy - various methods of treatment. Folia Med Cracov. 2019;59:5–14. [PubMed] [Google Scholar]
- 13.Fei H, Jiang X, Li T, et al. Comparison of three different treatment methods for Cesarean scar pregnancy. Ther Clin Risk Manag. 2019;15:1377–1381. doi: 10.2147/TCRM.S220852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harb HM, Knight M, Bottomley C, et al. Caesarean scar pregnancy in the UK: a national cohort study. BJOG. 2018;125:1663–1670. doi: 10.1111/1471-0528.15255. [DOI] [PubMed] [Google Scholar]
- 15.Donnez O, Donnez J, Orellana R, Dolmans MM. Gynecological and obstetrical outcomes after laparoscopic repair of a cesarean scar defect in a series of 38 women. Fertil Steril. 2017;107:289–296.e2. doi: 10.1016/j.fertnstert.2016.09.033. [DOI] [PubMed] [Google Scholar]
- 16.Tsuji S, Murakami T, Kimura F, et al. Management of secondary infertility following cesarean section: report from the Subcommittee of the Reproductive Endocrinology Committee of the Japan Society of Obstetrics and Gynecology. J Obstet Gynaecol Res. 2015;41:1305–1312. doi: 10.1111/jog.12750. [DOI] [PubMed] [Google Scholar]
- 17.Cao S, Zhu L, Jin L, Gao J, Chen C. Uterine artery embolization in cesarean scar pregnancy: safe and effective intervention. Chin Med J (Engl) 2014;127:2322–2326. [PubMed] [Google Scholar]
- 18.Cosin JA, Bean M, Grow D, Wiczyk H. The use of methotrexate and arterial embolization to avoid surgery in a case of cervical pregnancy. Fertil Steril. 1997;67:1169–1171. doi: 10.1016/s0015-0282(97)81459-8. [DOI] [PubMed] [Google Scholar]
- 19.Lobel SM, Meyerovitz MF, Benson CC, Goff B, Bengtson JM. Preoperative angiographic uterine artery embolization in the management of cervical pregnancy. Obstet Gynecol. 1990;76:938–941. [PubMed] [Google Scholar]
- 20.Liu S, Sun J, Cai B, Xi X, Yang L, Sun Y. Management of cesarean scar pregnancy using ultrasound-guided dilation and curettage. J Minim Invasive Gynecol. 2016;23:707–711. doi: 10.1016/j.jmig.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Cheng LY, Wang CB, Chu LC, Tseng CW, Kung FT. Outcomes of primary surgical evacuation during the first trimester in different types of implantation in women with cesarean scar pregnancy. Fertil Steril. 2014;102:1085–1090.e2. doi: 10.1016/j.fertnstert.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Polat I, Ekiz A, Acar DK, et al. Suction curettage as first line treatment in cases with cesarean scar pregnancy: feasibility and effectiveness in early pregnancy. J Matern Fetal Neonatal Med. 2016;29:1066–1071. doi: 10.3109/14767058.2015.1034100. [DOI] [PubMed] [Google Scholar]
- 23.Timor-Tritsch IE, Khatib N, Monteagudo A, Ramos J, Berg R, Kovács S. Cesarean scar pregnancies: experience of 60 cases. J Ultrasound Med. 2015;34:601–610. doi: 10.7863/ultra.34.4.601. [DOI] [PubMed] [Google Scholar]
- 24.Fuchs N, Manoucheri E, Verbaan M, Einarsson JI. Laparoscopic management of extrauterine pregnancy in caesarean section scar: description of a surgical technique and review of the literature. BJOG. 2015;122:137–140. doi: 10.1111/1471-0528.13060. [DOI] [PubMed] [Google Scholar]
- 25.Lee JH, Kim SH, Cho SH, Kim SR. Laparoscopic surgery of ectopic gestational sac implanted in the cesarean section scar. Surg Laparosc Endosc Percutan Tech. 2008;18:479–482. doi: 10.1097/SLE.0b013e318180f696. [DOI] [PubMed] [Google Scholar]
- 26.Vervoort A, Vissers J, Hehenkamp W, Brölmann H, Huirne J. The effect of laparoscopic resection of large niches in the uterine caesarean scar on symptoms, ultrasound findings and quality of life: a prospective cohort study. BJOG. 2018;125:317–325. doi: 10.1111/1471-0528.14822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vissers J, Hehenkamp W, Lambalk CB, Huirne JA. Post-caesarean section niche-related impaired fertility: hypothetical mechanisms. Hum Reprod. 2020;35:1484–1494. doi: 10.1093/humrep/deaa094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kutuk MS, Uysal G, Dolanbay M, Ozgun MT. Successful medical treatment of cesarean scar ectopic pregnancies with systemic multidose methotrexate: single-center experience. J Obstet Gynaecol Res. 2014;40:1700–1706. doi: 10.1111/jog.12414. [DOI] [PubMed] [Google Scholar]
- 29.Lian F, Wang Y, Chen W, et al. Uterine artery embolization combined with local methotrexate and systemic methotrexate for treatment of cesarean scar pregnancy with different ultrasonographic pattern. Cardiovasc Intervent Radiol. 2012;35:286–291. doi: 10.1007/s00270-011-0097-y. [DOI] [PubMed] [Google Scholar]
- 30.Peng P, Gui T, Liu X, Chen W, Liu Z. Comparative efficacy and safety of local and systemic methotrexate injection in cesarean scar pregnancy. Ther Clin Risk Manag. 2015;11:137–142. doi: 10.2147/TCRM.S76050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang JH, Xu KH, Lin J, Xu JY, Wu RJ. Methotrexate therapy for cesarean section scar pregnancy with and without suction curettage. Fertil Steril. 2009;92:1208–1213. doi: 10.1016/j.fertnstert.2008.07.1780. [DOI] [PubMed] [Google Scholar]
- 32.Bodur S, özdamar Ö, Kiliç S, Gün I. The efficacy of the systemic methotrexate treatment in caesarean scar ectopic pregnancy: a quantitative review of English literature. J Obstet Gynaecol. 2015;35:290–296. doi: 10.3109/01443615.2014.954101. [DOI] [PubMed] [Google Scholar]
- 33.Miller R, Timor-Tritsch IE, Gyamfi-Bannerman C. Society for Maternal-Fetal Medicine (SMFM) Consult Series #49: Cesarean scar pregnancy. Am J Obstet Gynecol. 2020;222:B2–14. doi: 10.1016/j.ajog.2020.01.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Women misdiagnosed as a miscarriage before referral and were treated with a curettage. In these women the gestational sac was crossing the serosal line.